Abstract
To determine the role that competition plays in a molecular mimic’s capacity to induce autoimmunity, we studied the ability of naïve encephalitogenic T cells to expand in response to agonist altered peptide ligands (APLs), some capable of stimulating both self-directed and exclusively APL-specific T cells. Our results show that although the APLs capable of stimulating exclusively APL-specific T cells are able to expand encephalitogenic T cells in vitro, the encephalitogenic repertoire is effectively outcompeted in vivo when the APL is used as the priming immunogen. Competition as a mechanism was supported by: (i) the demonstration of a population of exclusively APL-specific T cells, (ii) an experiment in which an encephalitogenic T cell population was successfully outcompeted by adoptively transferred naïve T cells, and (iii) demonstrating that the elimination of competing T cells bestowed an APL with the ability to expand naïve encephalitogenic T cells in vivo. In total, these experiments support the existence of a reasonably broad T cell repertoire responsive to a molecular mimic (e.g., a microbial agent), of which the exclusively mimic-specific component tends to focus the immune response on the invading pathogen, whereas the rare cross-reactive, potentially autoreactive T cells are often preempted from becoming involved.
Keywords: experimental autoimmune encephalomyelitis, molecular mimicry, multiple sclerosis, driver clones, autoimmune
T cell recognition is degenerate; a single T cell can react to a heterogeneous assortment of ligands (1 –5). The use of synthetic combinatorial peptide libraries has revealed two key features of this degeneracy: (i) There are a vast number of agonistic ligands for a single clone (possibly >106) each falling along a spectrum of stimulatory potencies and (ii) the native self determinant is often “suboptimal” when compared with many of the synthetic mimic peptides (4). The concept of molecular mimicry describes a situation in which a foreign microbe can initiate an immune response in which a T or B cell component cross-recognizes self. The amino acid sequence in the mimic determinant and the native self-determinant can be very different and in some instances, apparently chemically unrelated. Although several groups have demonstrated degenerate recognition by autoreactive T cells (1, 6, 7), molecular mimicry as a direct cause of autoimmunity has only rarely been shown (6).
We have used the term “driver” to refer to individual self-directed T cell clones that are required to propagate an autoimmune response. In this paper we ask whether APL-specific, non-self-directed T cells can outcompete naïve driver pathogenic clones for activation. The APLs used in our study can be considered analogous to microbial molecular mimics. The ability of these APLs to induce non-self-directed exclusively APL-specific clones was studied in the myelin basic protein (MBP):Ac1-9-induced B10.PL (H-2u) model of experimental autoimmune encephalomyelitis (EAE). EAE is an autoimmune demyelinating disease of the central nervous system mediated predominantly by CD4+ T cells. In the B10.PL model of EAE, Vβ8.2Jβ2.7 T cells expand in each B10.PL mouse primed with MBP or its immunodominant determinant, Ac1-9 (8). The TCR VDJ recombination of the Ac1-9-specific Vβ8.2Jβ2.7 clonotype encodes a CDR3-length of nine aa, GDAGGGYEQ (8). The GDAGGGYEQ clonotype (“DAGGGY,” for short) plays an important role in Ac1-9-induced EAE, “driving” disease progression. This notion was based upon several findings. (i) The Vβ8.2Jβ2.7 DAGGGY T cells are reactive to Ac1-9, the immunodominant encephalitogenic determinant of MBP in H-2u strains of mice (8 –10). (ii) They bear high affinity T cell receptors (11). (iii) They passively induce EAE when transferred into naïve B10.PL H-2u recipients (8). (iv) Mice transgenic for the Vβ8.2Jβ2.7 DAGGGY T cell receptor develop spontaneous EAE (10). (v) Loss of this T cell expansion results in resolution of autoimmunity (8).
Herein we characterize the ability of different agonist APLs of Ac1-9 to expand the DAGGGY clonotype in vivo. By immunizing B10.PL animals with the APLs we were able to demonstrate that certain agonist APLs were effective inducers of APL-specific, non-self-directed T cells. Correlating well with the ability to expand these non-self-directed T cells was an inability to expand the encephalitogenic self-directed repertoire from its naïve state in the mouse. By ruling out the possibility that these APLs exhausted the pathogenic driver response, we proposed an alternative hypothesis: that the non-self-directed APL-specific T cells effectively outcompeted the encephalitogenic repertoire for activation.
Results
The Vβ8.2Jβ2.7 Ac1-9-Specific Response Is Encephalitogenic in the B10.PL Mouse.
To verify the role of the Vβ8.2Jβ2.7 DAGGGY clonotype in actively induced EAE, B10.PL mice were treated with MBP:Ac1-9, pertussis toxin (PTX), and complete Freund’s adjuvant (CFA) so as to induce encephalomyelitis. Mice were then killed during the onset of EAE and draining lymph node cells and spinal cords were removed. Fig. 1A shows that after in vivo priming with Ac1-9-CFA, a Vβ8.2Jβ2.7 expansion is present in the lymph nodes and spinal cord of mice during the initial stages of EAE. This expansion was Ac1-9 specific, based upon several criteria. (i) It arose only after priming with Ac1-9 and not after priming with CFA alone (Fig. 1A). (ii) It was found only in wells cultured with Ac1-9 and not found when cells were cultured with medium alone or purified protein derivative (PPD) of mycobacteria (Fig. 1A). (iii) The direct sequencing of the expansion revealed the characteristic Vβ8.2Jβ2.7 encoded DAGGGY CDR3 sequence, known to be Ac1-9 specific (8). (iv) The expansion was found in the spinal cord of mice suffering from EAE (Fig. 1A). Thus, it was hypothesized that the ability of a molecular mimic to expand this encephalitogenic clone in vivo might correlate with its ability to induce EAE.
Fig. 1.
Characterization of the Ac1-9-specific Vβ8.2Jβ2.7 clonotype. (A) Mice were immunized with CFA alone or Ac1-9-CFA and draining lymph nodes were removed for analysis. Vβ8.2Jβ2.7 spectra are shown. Arrows indicate a TCR CDR3 length of 9 aa. Significant expansions within the Vβ8.2Jβ2.7 spectra were not seen when B10.PL animals were immunized with CFA alone. Likewise, when lymph node cells isolated from Ac1-9-CFA primed animals were incubated with medium alone, no significant expansions were found. In contrast, when lymph node cells from Ac1-9-CFA primed animals were cultured with Ac1-9, an expansion correlating to a CDR3 length of 9 aa was seen. This 9 aa expansion was also seen directly without the need of an in vitro culturing step when mononuclear cells were obtained from spinal cords isolated from mice suffering from EAE. This figure shows one of several similar experiments. (B) The MBP Ac1-9-specific Vβ8.2Jβ2.7 T cell hybridoma 172.10 can be stimulated by Ac1-6(M4), Ac1-9, and LDVM1-9(Y4). Stimulatory responses are measured in units of IL-2 production. LDVM1-9(Y4) (∆) is superior to Ac1-9 (□) in its ability to stimulate the Ac1-9-specific hybridoma, 172.10. In contrast Ac1-6(M4) (◊) is less effective when compared to Ac1-9. This figure shows one of several similar experiments.
The Vβ8.2Jβ2.7 Clonotype Responds in Vitro to LDVM1-9(Y4) and Ac1-6(M4).
To compare the ability of different APLs of Ac1-9 to stimulate the characteristic Vβ8.2Jβ2.7 clonotype, a Vβ8.2Jβ2.7 Ac1-9-specific DAGGGY T cell hybridoma (9), was incubated with different APLs in the presence of splenic APCs. Fig. 1B shows that LDVM1-9(Y4) was superior to Ac1-9 in its ability to stimulate the public clonotype in vitro. Thus, the natural ligand, Ac1-9, is a suboptimal agonist for the Vβ8.2Jβ2.7 clonotype. In contrast, Ac1-6(M4) (open diamonds) was inferior to Ac1-9 in its ability to stimulate the Ac1-9-specific Vβ8.2Jβ2.7 T cell clone.
Ac1-9(Y4) Is Able to Expand the Encephalitogenic Vβ8.2Jβ2.7 Clonotype in Vivo.
One might propose several possible mechanisms for failure of an APL agonist to expand a pathogenic self-directed repertoire from its naïve state within an animal. As mentioned earlier, in addition to stimulating self-directed T cells, an APL agonist might be able to stimulate a large number of exclusively APL-specific, non-self-directed T cells. These exclusively APL-specific T cells in turn may outcompete pathogenic self-directed clones for activation. Alternatively, an APL agonist may induce an antiinflammatory cytokine profile (12) or antagonize pathogenic T cells (13). Lastly, it may induce an active exhaustion of the pathogenic repertoire. In favor of the latter explanation, a published report demonstrated negative selection during the peripheral immune response to an APL (14). These results, however, are somewhat controversial. A more recent study conducted in the same experimental system reported enhanced antigen-specific T cell responses rather than negative selection. The authors of the latter study concluded that immunizing with a high concentration of an APL agonist resulted in an antiinflammatory feedback loop involving IFN-γ (15).
To exclude immunologic exhaustion as a possible mechanism for our results, we immunized B10.PL animals with differing amounts of Ac1-9(Y4) and then characterized the intensity of the driver Vβ8.2Jβ2.7 clonotypic expansion. Like Ac1-9 itself (Fig. 1A), Ac1-9(Y4) was an excellent inducer of the Vβ8.2Jβ2.7 Ac1-9-specific public response (Fig. S1 A and B). However, at a very high concentration of antigen (200 μg/mouse), the expansion of the Vβ8.2Jβ2.7 clonotype decreased but was not entirely eliminated (Fig. S1C). Therefore, to avoid immunologic exhaustion and to focus on the competitive activities among T cell clones, an eightfold lower amount of 25 μg was used for all subsequent APL immunizations. These results correspond with the findings that Ac1-9(Y4) induces severe EAE at low but not high concentrations (15).
The Driver Vβ8.2Jβ2.7 Clonotype Is Highly Susceptible to T Cell Competition in Vivo.
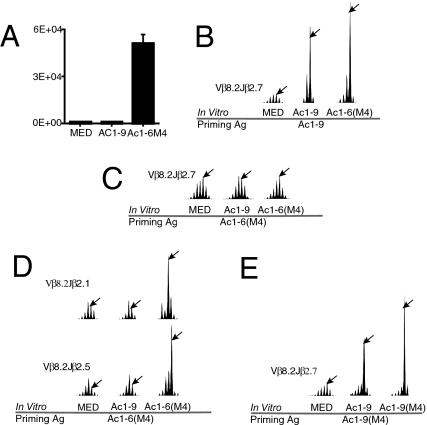
Previous reports have indicated that residues flanking a minimal core of amino acids can have profound effects on T cell activation, either enhancing or interfering with the activation of particular T cells (16, 17). Ac1-9’s interaction with I-Au is unique in that when bound within the MHC binding groove it is predicted that the first MHC pockets remain unoccupied. Thus, of our panel of agonistic ligands for the Vβ8.2Jβ2.7 DAGGGY clonotype (Fig. 1B), we hypothesized that the APL LDVM1-9(Y4), which was composed of additional flanking residues, LDVM, might expand a unique population of APL-specific T cells focused on the N-terminal LDVM residues. In addition, a similar phenomenon was predicted to exist with the very short agonist Ac1-6(M4). To investigate the latter, B10.PL animals were immunized with 25 μg of Ac1-6(M4). Lymphocytes from these animals were then fused with BW5147 (α−β−) to make Ac1-6(M4)-specific T cell hybridomas. Twenty Ac1-6(M4)-responsive hybridomas were screened, none of which could recognize Ac1-9 (one representative hybridoma is shown in Fig. 2A). Thus, the majority of the Ac1-6(M4)-specific T cells were not cross-reactive to Ac1-9. In contrast, when B10.PL animals were initially immunized with Ac1-9, Ac1-6(M4) was very effective at expanding the public Vβ8.2Jβ2.7 driver clonotype in vitro (Fig. 2B). To test whether Ac1-6(M4) could expand the Vβ8.2Jβ2.7 clonotype in vivo, B10.PL animals were immunized with 25 μg of Ac1-6(M4) and T cell repertoire analysis was conducted 10 days later on draining lymph node cells. Although Ac1-6(M4) was effective at expanding the Vβ8.2Jβ2.7 encephalitogenic repertoire in vitro (Figs. 1B and 2 B), Fig. 2C reveals that active immunization with Ac1-6(M4) failed to expand the naïve Vβ8.2Jβ2.7 Ac1-9-specific repertoire. In addition, when B10.PL mice were primed with Ac1-6(M4) there was only a marginal in vitro recall response to Ac1-9 (data not shown). In agreement with these findings, TCR repertoire analysis of samples obtained from Ac1-6(M4)-immunized animals revealed Ac1-6(M4)-specific expansions, even within the Vβ8 family, which did not cross-recognize the longer peptide, Ac1-9 (Fig. 2D). The expansions seen in Fig. 2D are believed to be Ac1-6(M4) specific because they arise from Ac1-6(M4)-immunized animals and are seen when lymphocytes are incubated with Ac1-6(M4) but not when lymphocytes are incubated with Ac1-9 or medium alone (Fig. 2D). Thus, one plausible explanation for the ability of Ac1-6(M4) to stimulate the Vβ8.2Jβ2.7 Ac1-9-specific T cell repertoire in vitro (Figs. 1B and 2 B) but not in vivo (Fig. 2C) is that upon priming a naïve B10.PL animal with Ac1-6(M4), the non-Ac1-9-specific Ac1-6(M4)-specific T cells (Fig. 2 A and D) outcompete the encephalitogenic Vβ8.2Jβ2.7 T cells.
Fig. 2.
Ac1-9-specific or APL-specific T cell expansions following immunization with Ac1-9 vs. Ac1-6(M4). (A) B10.PL mice were primed with 25 μg of Ac1-6(M4). Draining lymph nodes were removed 10 days later and cells were cultured with Ac1-6(M4) for 3 days and then fused with the 5147 (α−/β−) T cell fusion partner to make Ac1-6(M4)-specific T cell hybridomas. Of the 20 hybridomas isolated, none were able to cross-recognize Ac1-9. One representative example is shown here. B10.PL animals were then primed with (B) Ac1-9, (C and D) Ac1-6(M4), or (E) Ac1-9(M4) emulsified in CFA. Ten days later, animals were killed and draining lymph nodes were removed for immunoscope analysis. (B) Lymphocytes isolated from Ac1-9-primed animals showed strong expansions of the public Vβ8.2Jβ2.7 clonotype when cultured in vitro with either Ac1-9 or Ac1-6(M4). (C) Although Ac1-6(M4) is capable of stimulating the Vβ8.2Jβ2.7 clonotype in vitro, priming with Ac1-6(M4) failed to expand the public Vβ8.2Jβ2.7 Ac1-9-specific clonotype. (D) Priming with Ac1-6(M4) expanded a population of Ac1-6(M4)-specific T cells, which did not cross-recognize Ac1-9. (E) In contrast to Ac1-6(M4), Ac1-9(M4) was effective at expanding the naïve Vβ8.2Jβ2.7 clonotype when used as an immunogen. This represents one of several similar experiments.
Although we have suggested competition as the mechanism accounting for these effects, several other potential mechanisms remain possible. For example, the K4M substitution could somehow interfere with the activation of naïve Vβ8.2Jβ2.7 T cells. To address this possibility, we immunized B10.PL animals with 25 μg of the longer peptide, Ac1-9(M4), capable of strong stimulation of Ac1-9-specific T cells (18). Fig. 2E shows that lengthening Ac1-6(M4) to Ac1-9(M4) to include an additional three residues of MBP, effectively restored the ability to expand the Ac1-9-specific public Vβ8.2Jβ2.7 response in vivo. Importantly, we were unable to identify any exclusively Ac1-9(M4)-specific T cells that did not cross-recognize Ac1-9. Thus, there was no T cell competition evident when mice were immunized with Ac1-9(M4). Still other possibilities for the failure of Ac1-6(M4) to expand the characteristic Vβ8.2Jβ2.7 Ac1-9-specific response in vivo could not be excluded. For example, in some experiments (Fig. 1B), but not all (Fig. 2B), the short Ac1-6(M4) was an inferior in vitro activator of the characteristic Vβ8.2Jβ2.7 Ac1-9-specific clonotype. Therefore, additional evidence was sought in favor of the T cell competition hypothesis.
The Results Obtained with Ac1-6(M4) Hold True for Another APL of Ac1-9.
Because the Vβ8.2Jβ2.7 response to Ac1-9 arises after priming with Ac1-9, Ac1-20, MBP, or whole spinal cord homogenate, where the amino terminus is relatively available, we synthesized LDVM1-9(Y4), which includes the adjacent 5′ Golli (genes of the oligodendrocyte lineage) residues, LDVM. In this peptide, the 4K-to-4Y substitution is required for induction of 1–9 reactivity because it allows the peptide to bind in the appropriate register to the MHC class II I-Au molecule (19). Fig. 3A shows that, like Ac1-6(M4), LDVM1-9(Y4) was excellent at recalling the public Vβ8.2Jβ2.7 response from Ac1-9-primed animals. Similarly, it was an excellent stimulator of the Vβ8.2Jβ2.7 hybridoma, 172.10 (Fig. 1B). To characterize the LDVM1-9(Y4)-specific T cell repertoire, B10.PL animals were immunized with LDVM1-9(Y4) and draining lymph nodes were harvested on day 10. T cell repertoire analysis revealed a strong “public” LDVM1-9(Y4)-specific expansion within the Vβ7Jβ2.5 spectrum that was non-cross-reactive with Ac1-9 (Fig. 3B). As additional evidence that this Vβ7Jβ2.5 expansion was non-cross-reactive with Ac1-9, B10.PL mice were immunized with Ac1-9 and draining lymph nodes were analyzed for expansions within the Vβ7Jβ2.5 spectrum. The Gaussian distributions seen in Fig. 3C are evidence in support of the non-cross-reactive nature of the Vβ7Jβ2.5 LDVM1-9(Y4)-specific response. Fig. 3D shows the response of an LDVM1-9(Y4)-specific T cell hybridoma that is unable to recognize Ac1-9. We then asked whether the ability to expand a population of non-cross-reactive T cells correlated with an inability to expand the Ac1-9-specific Vβ8.2Jβ2.7 DAGGGY clonotype in vivo. Fig. 3E shows that, when used as the initial immunogen, LDVM1-9(Y4) was only able to minimally expand the public encephalitogenic repertoire in two out of six animals. In summary, there is no direct relationship between the in vitro stimulatory potency of a peptide agonist and its ability to expand a self-reactive clone from its naïve state within the animal. In addition, the ability to expand non-cross-reactive, nonpathogenic T cells correlates well with an antigen’s inability to expand a pathogenic population. Other factors such as antigen dose and the MHC binding affinity of the mimic agonist may also play a role in the ability/inability to expand self-reactive pathogenic T cells.
Fig. 3.
LDVM1-9(Y4) is effective at expanding the Vβ8.2Jβ2.7 clonotype from Ac1-9-primed animals but not when used as an immunogen. (A) B10.PL mice were primed with Ac1-9 and in vitro recall responses to medium, Ac1-9, and LDVM1-9(Y4) were analyzed. Cultures incubated with Ac1-9 or LDVM1-9(Y4) showed significant expansion of the characteristic Vβ8.2Jβ2.7 clonotype. (B) Mice were then primed with LDVM1-9(Y4). After a preliminary survey of all VβJβ combinations, a “public” Vβ7Jβ2.5 LDVM1-9(Y4)-specific expansion was detected. This expansion was seen in all B10.PL animals primed with LDVM1-9(Y4), one of six replicates shown here. (C) B10.PL animals were then immunized with Ac1-9 and the Vβ7Jβ2.5 spectrum was inspected. No significant expansions were seen. (D) An LDVM1-9(Y4)-specific hybridoma (1 × 104 cells) incubated with medium or MBP:Ac1-9 in the presence of splenic APCs resulted in no IL-2 production (as measured in this HT-2 cell assay). The hybridoma was, however, able to strongly respond to LDVM1-9(Y4). This clone is an example of an LDVM1-9(Y4)-specific, non-Ac1-9-specific T cell clone. (E) Immunization with LDVM1-9(Y4) failed to strongly expand the public Vβ8.2Jβ2.7 clonotype. Six individual mice are shown. Very small expansions were seen in two animals.
The Ability of a Peptide to Induce EAE Correlates Well with Its Ability to Expand the Encephalitogenic Clonotype.
Because Ac1-6(M4) was capable of expanding a population of Ac1-9-specific T cells in vitro (Fig. 2) but was incapable of expanding the naïve Vβ8.2Jβ2.7 clonotype in vivo, it was of interest to determine the encephalitogenic potential of this peptide. Table 1 shows that Ac1-6(M4) was incapable of inducing EAE in naïve wild-type animals (0/6). Similarly, when EAE induction was attempted with the longer 13-mer peptide, LDVM1-9(Y4), only a fraction (4/10) of the animals developed EAE and disease severity was limited to minimal tail paralysis (EAE scores = 1). On the other hand, correlating well with their ability to expand the naïve encephalitogenic Vβ8.2Jβ2.7 repertoire (Figs. 1A and 2 E), both Ac1-9 and Ac1-9(M4) were able to induce severe EAE (Table 1). Lastly, like Ac1-9M4, we have previously shown that 25–75 μg of Ac1-9(Y4) is also an effective inducer of EAE (19). Thus, the ability of an APL to expand autoreactive T cells in vitro does not correlate with its ability to induce autoimmunity in vivo. This experiment also highlights the role of the driver response in autoimmunity.
Table 1.
Ac1-6(M4) cannot induce EAE
| Exp. no. | Antigen | Dose, μg | Sequence | Incidence, % | Mean day of onset | Mean severity |
| 1 | Ac1-11 | 50 | Ac-ASQKRPSQRSK | 3/3 (100) | 13.0 | 4.3 |
| (1-9) Y4 | 100 | ASQYRPSQR | 4/4 (100) | 14.0 | 2.5 | |
| LDVM1-9(Y4) | 100 | LDVMASQYRPSQR | 2/4 (50) | 13.0 | 1.0 | |
| 2 | Ac1-9 | 75 | Ac-ASQKRPSQR | 6/6 (100) | 13.0 | 2.8 |
| Ac1-9 (M4) | 25 | Ac-ASQMRPSQR | 6/6 (100) | 15.2 | 3.0 | |
| Ac1-6 (M4) | 25 | Ac-ASQMRP | 0/6 (0) | |||
| LDVM1-9(Y4) | 25 | LDVMASQYRPSQR | 2/6 (33.3) | 12.0 | 1.0 |
The clinical severity of EAE was scored daily as follows: 1, toss of tail tonus; 2, hind limb weakness or forelimb involvement alone; 3, total hind limb paralysis; 4, hind and forelimb paralysis; and 5, moribund/death. Mean severity was calculated using scores from only those mice exhibiting clinical signs of EAE. Bold indicates altered amino acid from the native sequence.
An alternative explanation of these findings might simply be that the APL, LDVM1-9(Y4), failed to induce a strong cytokine response (12, 13). To investigate this possibility, draining lymph node cells from both Ac1-9-immunized and LDVM1-9(Y4)-immunized B10.PL animals were analyzed for IFN-γ production. Fig. 4 reveals that LDVM1-9(Y4) was excellent at generating an IFN-γ cytokine response regardless of the priming antigen.
Fig. 4.
IFN-γ production in response to Ac1-9 or LDVM1-9. B10.PL mice were immunized with either Ac1-9 (open bars) or LDVM1-9(Y4) (closed bars) and draining lymph node cells were harvested and assayed for IFN-γ secretion when cultured with either medium, Ac1-9, or LDVM1-9(Y4). Culture with LDVM1-9(Y4) induced a strong IFN-γ response when mice were immunized with either Ac1-9 or LDVM1-9(Y4). In contrast, IFN-γ secretion was greatly reduced when animals were immunized with LDVM1-9(Y4) and draining lymph node cells were cultured with Ac1-9.
T Cell Adoptive Transfer Experiments Confirm the High Sensitivity of the Driver Response to T Cell Competition.
To seek conclusive evidence for T cell competition as a mechanism preventing the expansion of naïve pathogenic T cells, T cell transfer experiments were performed in which naïve Ac1-9-specific T cells were transferred into wild-type B10.PL recipients before priming with Ac1-9. These naïve T cells were isolated from the Ac1-9-specific TCR Tg B10.PL mouse developed by Juan Lafaille and bear a unique Vβ8.2Jβ2.4 TCR (20). Thus, in this experiment the endogenous naïve Ac1-9-specific repertoire is asked to respond in the presence of differing numbers of competing T cells of similar specificity. Staining with Ac1-9-I-Au tetramers shows that the transferred transgenic T cells are of lower affinity than the endogenous Vβ8.2Jβ2.7 driver clone (Fig. 5A). However, the Vβ8.2Jβ2.4 T cells are proinflammatory, encephalitogenic T cells similar to the Vβ8.2Jβ2.7 DAGGGY clonotype and mice transgenic for this TCR also develop spontaneous EAE (20). Fig. 5B shows that upon adoptive transfer without antigen, the naïve transgenic cells do not proliferate and a portion of the T cells can be found in the lymph nodes. After in vivo priming with Ac1-9 these cells become activated and proliferate (Fig. 5C).
Fig. 5.
Demonstration of T cell competition using adoptive transfer strategies. (A) Splenocytes from Ac1-9-specific Vβ8.2Jβ2.4 and Vβ8.2Jβ2.7 transgenic mice were isolated and stained with PerCP-labeled anti-CD4 and either PE-labeled anti-TCRβ (H57-597) or PE-labeled, tetrameric MBP1-9[4Y]:I-Au complexes. Stained cells were analyzed by flow cytometry and data shown were gated on CD4+ T cells. (B) B10.PL animals received an adoptive transfer of 5 × 106 CFSE-labeled CD4+ T cells obtained from the Vβ8.2Jβ2.4 Tg mouse. After the transfer, if the animals were not primed with Ac1-9 the T cells did not proliferate. (C) One day after transfer of 5 × 106 Vβ8.2Jβ2.4 CFSE-labeled Tg T cells, animals were primed with Ac1-9, which resulted in proliferation of the transferred T cells. (D) Adoptive transfer of competing naïve Ac1-9-specific transgenic T cells effectively prevents the expansion of the public Vβ8.2Jβ2.7 driver clone. Mice received 0, 5 × 103, 5 × 104, and 5 × 105 competing T cells isolated from the Vβ8.2Jβ2.4 Tg mouse. Twenty-four hours later, they were primed with 75 μg of Ac1-9 emulsified in CFA. The expansion of the endogenous Ac1-9-specific Vβ8.2Jβ2.7 clone was lost in a dose-dependent fashion. The strength of individual Vβ8.2Jβ2.7 expansions corresponding to a CDR3 length of 9 aa are shown numerically as a relative index of stimulation. (E) Actual Vβ8.2Jβ2.7 spectra seen after transfer of 5 × 104 Vβ8.2Jβ2.4 Ac1-9-specific T cells. In the presence of competing T cells, the endogenous Vβ8.2Jβ2.7 clone failed to expand. (F) Vβ8.2Jβ2.4 spectra showing expansion of the adoptively transferred Vβ8.2Jβ2.4 T cells after immunization with Ac1-9. (G) Depletion of the exclusively LDVM1-9(Y4)-specific competing T cells allows the Ac1-9-specific Vβ8.2Jβ2.7 clonotype to expand following priming with LDVM1-9(Y4). Competing T cells were depleted via i.p. injection of anti-CD3 and anti-Vβ7 antibody. Following this depleting protocol mice were reconstituted with noncompeting T cells by adoptive transfer of unexpanded bulk Vβ8 T cells. Mice were finally immunized with 25 μg of LDVM1-9(Y4) and euthanized 10 days later for analysis. Following this regimen the naïve Vβ8.2Jβ2.7 Ac1-9-specific clonotype strongly responded to priming with LDVM1-9(Y4).
Can these naïve adoptively transferred Vβ8.2Jβ2.4 Ac1-9-specific T cells outcompete the encephalitogenic Vβ8.2Jβ2.7 DAGGGY clonotype? Fig. 5 D and E show that the adoptive transfer of Ac1-9-specific TCR Tg T cells effectively eliminated the driver Vβ8.2Jβ2.7 expansion to Ac1-9 in a dose-dependent fashion. Thus, the Vβ8.2Jβ2.7 DAGGGY clonotype can be outcompeted by T cells of similar specificity and cytokine production even if the cells bear lower avidity TCRs (Fig. 5A). Evidently, the relative T cell precursor frequencies appear to be an important factor in this example.
As a final definitive proof that T cell competition was responsible for the inability of LDVM1-9(Y4) to expand the Vβ8.2Jβ2.7 clonotype in vivo, we depleted the endogenous exclusively LDVM1-9(Y4)-specific competing T cells and then measured the expansion of the endogenous Vβ8.2Jβ2.7 DAGGGY clonotype in response to LDVM1-9(Y4) priming. In this experiment B10.PL animals were first treated with depleting antibodies directed against CD3 (25 μg per mouse) and Vβ7 (100 μg per mouse) to eliminate the exclusively LDVM1-9(Y4)-specific Vβ7Jβ2.5 public repertoire (depicted in Fig. 3B). This protocol also deleted ≈80% of all T cells (data not shown). Thus, the non-Vβ7 exclusively LDVM1-9(Y4)-specific T cells were also severely depleted. Following this depletion the mice were reconstituted with a naïve unexpanded population of bulk Vβ8 T cells to restore the Vβ8.2Jβ2.7 public clonotype in its naïve state at a relatively low frequency among other nonexpanded Vβ8 T cells. After undergoing this depletion and reconstitution protocol, the mice in theory maintain a relative low frequency of resident naïve, unexpanded Vβ8.2Jβ2.7 Ac1-9-specific clones but lack the dominant exclusively LDVM1-9(Y4)-specific Vβ7Jβ2.5 competing T cells. The animals were then immunized with 25 μg of LDVM1-9(Y4). Consistent with our other findings, in the absence of competing T cells, the naïve Vβ8.2Jβ2.7 clonotype expanded robustly to priming with LDVM1-9(Y4) in vivo (Fig. 5G). This experiment provides conclusive evidence that T cell competition can prevent expansion of autoreactive T cells in vivo.
Discussion
The theory of molecular mimicry is based upon the idea that T cells specific to a foreign antigen can induce autoimmunity by cross-recognizing a self-determinant. However, there are clear constraints on the relationships that have to exist among the foreign and self-determinants if a state of autoimmunity is to be induced via this pathway. Most importantly, the foreign mimic-determinant must be readily processed from the foreign antigen to ensure that it can be presented to autoreactive T cells. Because only a fraction of the potential determinants are ever effectively processed and presented, a large proportion of mimicking peptides will remain poorly displayed, unable to engage a potentially autoreactive repertoire. Most studies characterizing molecular mimicry have not identified naturally processed, dominant molecular mimics. Instead, these studies have focused on identifying any microbial peptide capable of stimulating autoreactive T cells.
In addition, when a self-antigen is used to prime an animal, T cells with unique TCRs can potentially expand. Each of these autoreactive T cells will fall along a spectrum of inherent pathogenic potential. This is evidenced by the fact that different self-directed TCR transgenic animals, with specificities for the same determinant, will display a characteristic incidence of autoimmunity (10, 11, 20, 21). Although such animals may also differ in their regulatory T cell repertoires, it is likely that the difference in their TCR affinity is also responsible for the varied disease incidences. In addition, different autoreactive T cell clones with specificities for the same self-determinant will often induce unique disease courses when passively transferred into naïve recipients (22). In fact, several autoreactive clones induce disease only when transferred in high numbers. It is likely that among the diverse sets of T cells potentially expandable to a self-antigen, only a small proportion are able to induce and drive states of autoimmunity, a group we have called driver clones (7, 8, 19). Thus, even if a mimic can activate self-directed T cells, it may not stimulate the pathogenic subset; individual T cells may respond to unique sets of molecular mimics. Lastly, as shown here, being able to stimulate the driver subset in vitro, is still not sufficient to induce autoimmunity. The mimic also needs to be able to activate and expand this subset from its naïve state, which exists among a group of highly competitive memory and naïve T cells (23 –25). Studies linking molecular mimicry to autoimmunity have relied heavily upon deductive reasoning; in most cases, ligands have been characterized that are capable of stimulating already primed autoreactive T cells in vitro. The reasoning behind such studies is as follows: if molecular mimic X can stimulate autoreactive T cells specific to self-determinant Y, then a microbially induced immune response to mimic X should result in autoimmunity directed to self-determinant Y. There are several caveats to such reasoning. For example, due to a wide array of TCR specificities, it is likely that many (possibly most) T cells raised to mimic X will be specific to X and non-cross-reactive to self-determinant Y. Thus, immunizing with mimic X will expand a large population of nonautoreactive clones. Owing to competitive forces among T cells for activation, this potentially large population of nonautoreactive T cells may interfere with the activation of the self-reactive pathogenic driver T cell repertoire; in fact, we have just described such a case. Ligands capable of stimulating primed encephalitogenic T cells in vitro can be ineffective at expanding these same T cells as they reside in a naïve state within the animal. We find a strong correlation between an APL’s ability to expand APL-specific, non-self-directed T cells with its inability to expand the encephalitogenic self-directed repertoire in vivo. In our study, we used APLs that differed only slightly from the native sequence, Ac1-9. We predict that differences between a microbial molecular mimic and the corresponding self-determinant will allow for a mimic-specific non-cross-reactive repertoire to be expanded in the setting of infection. Our experiments argue that this mimic-specific, non-self-directed repertoire, through competitive means, will focus the immune response on the pathogen, thereby preempting the induction of autoimmunity. These relationships help to explain why, given such a largely degenerate T cell repertoire, that there are relatively few cases of molecular mimicry. They also have direct implications on the design of APLs for the treatment of cancer and autoimmunity and for the use of APLs in vaccines.
Materials and Methods
Mice.
B10.PL/J mice were purchased from The Jackson Laboratory. Clone 19 and clone 172.10 Ac1-9-specific Tg B10.PL animals were obtained from Juan Lafaille and Joan Goverman, respectively (10, 20). Experiments were approved by the LIAI Animal Care and Use Committee.
Hybridomas.
The Ac1-9 specific hybridoma, 172.10, has been maintained in this laboratory and was generated originally at California Institute of Technology. Other T cell hybridomas were created as described in the text.
Peptides.
Peptides were purchased from Macromolecular Resources. Purity was >95% as determined by mass spectrometry and capillary electrophoresis. The following peptides were used in this study: Ac1-9 (Ac-ASQKRPSQR), Ac1-9(Y4) (Ac-ASQYRPSQR), Ac1-9(M4) (Ac-ASQMRPSQR), Ac1-6(M4) (Ac-ASQMRP), and LDVM1-9(Y4) (LDVMASQYRPSQR). LDVM1-9(Y4) was also synthesized at University of California, Davis.
Induction of EAE.
For induction of EAE, mice were immunized s.c. with 100 μL of a CFA emulsion containing 200 μg of Mycobacterium tuberculosis H37Ra (Difco) and the indicated amount and type of antigen s.c.. One and 3 days later, mice were injected i.p. with 150 ng of purified PTX (List Biological) as described (8).
T Cell Repertoire Analysis (Immunoscope).
Repertoire analyses were performed using a modified protocol similar to that described by Pannetier et al. (26). Total RNA was isolated from cell suspensions of individual samples using the RNeasy kit (Qiagen). cDNA syntheses were then performed using an oligo-dT primer according to the manufacturer’s instructions (Life Technologies). From each cDNA, PCR reactions were then performed using a Vβ8.2 primer (cattattcatatggtgctggc) and a common Cβ primer (cactgatgttctgtgtgaca). Run-off reactions were performed with a single internal fluorescent primer for each Jβ tested. These products were then denatured in formamide and analyzed on an ABI PRISM 310 genetic analyzer using GeneScan 2.0 software (Perkin-Elmer). The relative intensity of signal (RIS) values were calculated as the area under the experimental peak divided by the area under the control peak found within a Gaussian distribution. Peaks were normalized before division. RIS values >4 are considered significant.
Adoptive Transfer of Naïve TCR Transgenic T Cells.
Spleen cells were isolated from Ac1-9-specific Vβ8.2Jβ2.4 TCR transgenic mice. CD4+ T cells were then purified using MACS beads (Miltenyi Biotec). T cells were then diluted in PBS, warmed to room temperature, and injected i.v. into 6-week-old wild-type B10.PL animals. Twenty-four hours later, animals were injected s.c. with 75 μg of Ac1-9 emulsified in CFA. Ten days later, draining lymph node cells were analyzed by TCR repertoire analysis.
Flow Cytometry.
Splenocytes were isolated from transgenic mice. Cells were incubated with Fc-block (BD Biosciences) before staining. Briefly, cells were incubated at 12 °C with biotinylated, recombinant MBP1-9(4Y):I-Au tetramers that were generated as described in Radu et al. (27). Following a wash, tetramer-stained cells were incubated with PerCP labeled-anti-CD4 (BD Biosciences) on ice and then washed and analyzed; cells were incubated with PE-labeled H57-597 (BD Biosciences) on ice and then washed and analyzed.
Supplementary Material
Acknowledgments
We thank Drs. J. Lafaille and J. Goverman for providing transgenic mice. E.M. is supported by career awards from the Howard Hughes Medical Institute and Burroughs Wellcome Fund. This work was also supported by grants from the National Institutes of Health (AI-42396 and AI-40877), the National Multiple Sclerosis Society (RG-2643).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914508107/DCSupplemental.
References
- 1.Bhardwaj V, Kumar V, Geysen HM, Sercarz EE. Degenerate recognition of a dissimilar antigenic peptide by myelin basic protein-reactive T cells. Implications for thymic education and autoimmunity. J Immunol. 1993;151:5000–5010. [PubMed] [Google Scholar]
- 2.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: Viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagerty DT, Allen PM. Intramolecular mimicry. Identification and analysis of two cross-reactive T cell epitopes within a single protein. J Immunol. 1995;155:2993–3001. [PubMed] [Google Scholar]
- 4.Hemmer B, et al. Identification of high potency microbial and self ligands for a human autoreactive class II-restricted T cell clone. J Exp Med. 1997;185:1651–1659. doi: 10.1084/jem.185.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loftus C, Huseby E, Gopaul P, Beeson C, Goverman J. Highly cross-reactive T cell responses to myelin basic protein epitopes reveal a nonpredictable form of TCR degeneracy. J Immunol. 1999;162:6451–6457. [PubMed] [Google Scholar]
- 6.Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: Autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- 7.Maverakis E, van den Elzen P, Sercarz EE. Self-reactive T cells and degeneracy of T cell recognition: evolving concepts—from sequence homology to shape mimicry and TCR flexibility. J Autoimmun. 2001;16:201–209. doi: 10.1006/jaut.2000.0493. [DOI] [PubMed] [Google Scholar]
- 8.Menezes JS, et al. A public T cell clonotype within a heterogeneous autoreactive repertoire is dominant in driving EAE. J Clin Invest. 2007;117:2176–2185. doi: 10.1172/JCI28277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urban JL, et al. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988;54:577–592. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- 10.Goverman J, et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 11.Garcia KC, Radu CG, Ho J, Ober RJ, Ward ES. Kinetics and thermodynamics of T cell receptor- autoantigen interactions in murine experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2001;98:6818–6823. doi: 10.1073/pnas.111161198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson LB, Greer JM, Sobel RA, Lees MB, Kuchroo VK. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 13.Franco A, et al. T cell receptor antagonist peptides are highly effective inhibitors of experimental allergic encephalomyelitis. Eur J Immunol. 1994;24:940–946. doi: 10.1002/eji.1830240424. [DOI] [PubMed] [Google Scholar]
- 14.Anderton SM, Radu CG, Lowrey PA, Ward ES, Wraith DC. Negative selection during the peripheral immune response to antigen. J Exp Med. 2001;193:1–11. doi: 10.1084/jem.193.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minguela A, Pastor S, Mi W, Richardson JA, Ward ES. Feedback regulation of murine autoimmunity via dominant anti-inflammatory effects of interferon gamma. J Immunol. 2007;178:134–144. doi: 10.4049/jimmunol.178.1.134. [DOI] [PubMed] [Google Scholar]
- 16.Moudgil KD, Grewal IS, Jensen PE, Sercarz EE. Unresponsiveness to a self-peptide of mouse lysozyme owing to hindrance of T cell receptor-major histocompatibility complex/peptide interaction caused by flanking epitopic residues. J Exp Med. 1996;183:535–546. doi: 10.1084/jem.183.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carson RT, Vignali KM, Woodland DL, Vignali DA. T cell receptor recognition of MHC class II-bound peptide flanking residues enhances immunogenicity and results in altered TCR V region usage. Immunity. 1997;7:387–399. doi: 10.1016/s1074-7613(00)80360-x. [DOI] [PubMed] [Google Scholar]
- 18.Kumar V, et al. Major histocompatibility complex binding affinity of an antigenic determinant is crucial for the differential secretion of interleukin 4/5 or interferon gamma by T cells. Proc Natl Acad Sci USA. 1995;92:9510–9514. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maverakis E, et al. Autoreactive T cells can be protected from tolerance induction through competition by flanking determinants for access to class II MHC. Proc Natl Acad Sci USA. 2003;100:5342–5347. doi: 10.1073/pnas.0936151100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 21.Waldner H, Whitters MJ, Sobel RA, Collins M, Kuchroo VK. Fulminant spontaneous autoimmunity of the central nervous system in mice transgenic for the myelin proteolipid protein-specific T cell receptor. Proc Natl Acad Sci USA. 2000;97:3412–3417. doi: 10.1073/pnas.97.7.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abromson-Leeman S, Bronson R, Dorf ME. Experimental autoimmune peripheral neuritis induced in BALB/c mice by myelin basic protein-specific T cell clones. J Exp Med. 1995;182:587–592. doi: 10.1084/jem.182.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kedl RM, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge Q, Bai A, Jones B, Eisen HN, Chen J. Competition for self-peptide-MHC complexes and cytokines between naive and memory CD8+ T cells expressing the same or different T cell receptors. Proc Natl Acad Sci USA. 2004;101:3041–3046. doi: 10.1073/pnas.0307339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troy AE, Shen H. Cutting edge: Homeostatic proliferation of peripheral T lymphocytes is regulated by clonal competition. J Immunol. 2003;170:672–676. doi: 10.4049/jimmunol.170.2.672. [DOI] [PubMed] [Google Scholar]
- 26.Pannetier C, et al. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radu CG, Anderton SM, Firan M, Wraith DC, Ward ES. Detection of autoreactive T cells in H-2u mice using peptide-MHC multimers. Int Immunol. 2000;12:1553–1560. doi: 10.1093/intimm/12.11.1553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.