Abstract
Objective
Adipokines (cytokines produced by adipose tissue) play a major role in the control of body weight and energy distribution. Retinol-binding protein (RBP) 4, only recently recognized as an adipokine, has been proposed to modulate systemic insulin sensitivity. The goal of this study was to determine whether there is an association between maternal plasma RBP4 concentration and the birth of a large-for-gestational-age (LGA) newborn in women with and without gestational diabetes mellitus (GDM).
Study design
This cross-sectional study included pregnant women at term in the following groups: 1) normal pregnancy with an appropriate-for-gestational-age (AGA) neonate (n=64); 2) normal pregnancy with an LGA neonate (n=44); 3) GDM with an AGA neonate (n=55); and 4) GDM with an LGA neonate (n=42). Maternal plasma RBP4 concentration was determined by ELISA. Parametric and non-parametric statistics were used for analyses.
Results
1) Patients with GDM, either with AGA or LGA neonates, had a higher median plasma concentration of RBP4 than normal pregnant women who delivered an AGA neonate (p=0.01 and p=0.008, respectively); 2) mothers without GDM but with LGA neonates had a higher median plasma concentration of RBP4 than those with normal pregnancy and AGA newborns (p=0.001); 3) these findings remained significant after adjusting for maternal age, BMI and gestational age at blood sampling.
Conclusion
GDM is characterized by alterations in maternal circulating RBP4 concentrations akin to those of Type 2 DM. Retinol binding protein 4 concentrations in maternal plasma may play a role in accelerated fetal growth in the absence of overt carbohydrate intolerance.
Keywords: metabolism, pregnancy, appropriate-for-gestational-age (AGA), adipokine, cytokine, adipose tissue, BMI, overweight, obesity, insulin resistance, insulin sensitivity
Introduction
Gestational diabetes mellitus (GDM) is defined as a carbohydrate intolerance of varying severity, with onset, or first recognition, during pregnancy [12, 26, 71, 78]. The clinical importance of GDM stems from the fact that this adverse metabolic state affects 1–10% of all pregnancies [7, 9, 14, 45], as well as from its association with maternal, fetal and neonatal complications [11, 16, 19, 23, 40, 70].
Several mechanisms of disease have been implicated in GDM including autoimmunity [51, 105], single gene mutations [24, 88], insulin resistance, and β cells dysfunction [10, 13, 34, 87], supporting the notion of GDM as a syndrome. In contrast to other “obstetrical syndromes”[85], GDM has a well-recognized non-gestational counterpart, namely Type-2 diabetes mellitus (Type 2 DM). This view is supported by the observation that these two metabolic complications share common risk factors such as advanced age [20] and ethnic origin [7]. One of the major common risk factors for GDM and Type 2 DM is obesity [39], and the term “diabesity” has been coined to highlight the strong association between excess fat accrual and carbohydrate intolerance [1]. Importantly, similar mechanisms have been implicated in GDM and Type 2 DM [8, 15], supporting the notion that these two condition are closely related.
Adipose tissue is now recognized as a highly active organ that secretes endocrine, paracrine and autocrine proteins termed adipokines [2, 28, 29, 94]. These bioactive molecules play an important role in the regulation of appetite, energy balance, insulin resistance, lipid metabolism and inflammation [22, 86]. The physiological importance of adipokines has led to the notion that alterations in the expression and secretion of these bioactive molecules are causatively linked to obesity-related diseases such as diabetes [37, 48], atherosclerosis [79, 81], and dyslipidemia [25]. Recently, retinol-binding protein-4 (RBP4), a 21 kDa protein predominantly synthesizes in the liver, was described as a novel adipokine [89, 97, 106, 107]. In addition to its well-established role as the major blood carrier of retinol, RBP4 has been implicated in the regulation of systemic insulin sensitivity [31, 74, 82, 106]. Consistent with this view, increased circulating RBP4 concentrations have been reported in several metabolic complications such as obesity [5, 6, 31, 33, 47, 72, 99], insulin resistance [18, 31, 72, 84, 103], metabolic syndrome [5, 35, 83, 104], polycystic ovary syndrome [32, 98] and cardiovascular disease [35, 104].
Only a few studies have addressed maternal circulating RBP4 concentration in normal gestation [100] and complications of pregnancy [36, 90, 95, 101, 102], and reports regarding plasma RBP4 concentrations in patients with GDM are scarce and yielded conflicting results [17, 41, 42, 50]. Furthermore, there are no data regarding the association between maternal plasma RBP4 and either overweight/obesity or the delivery of a large-for-gestational-age (LGA) neonate. Thus, the aim of this study was to determine whether there is an association between maternal plasma RBP4 concentration, GDM, and the delivery of an LGA newborn.
Materials and Methods
A cross-sectional study was conducted by searching our clinical database and bank of biological samples, and included pregnant women at term in the following groups: 1) normal pregnant women who delivered an appropriate-for-gestational-age (AGA) newborn (n=64); 2) normal pregnant women who delivered an LGA newborn (n=44); 3) women with GDM who delivered an AGA newborn (n=55); and 4) women with GDM who delivered an LGA newborn (n=42). Women with multiple pregnancies or fetal chromosomal and/or congenital anomalies were excluded.
All participating women provided a written informed consent prior to the collection of maternal blood samples. The utilization of samples for research purposes was approved by the institutional review boards of Sotero del Rio Hospital (Santiago, Chile) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/ NIH/DHHS). Many of these samples have been employed to study the biology of inflammation, hemostasis, and growth factor concentrations in normal pregnant women, and those with pregnancy complications.
Clinical Definitions
The definition of a normal pregnancy included all of the following: (1) no medical, obstetrical or surgical complications; (2) no labor and intact membranes; (3) delivery of a term neonate (>37 weeks) with a birth weight above the 10th percentile;[30] and (4) a normal oral 75-g oral glucose tolerance test (OGTT) between 24 and 28 weeks of gestation[4].
Diagnosis of GDM was based on the World Health Organization (WHO) criteria of fasting plasma glucose ≥126 mg/dl (≥7.0 mmol/L) or plasma glucose ≥140 mg/dl (≥7.8 mmol/L) two hours after the 75-g OGTT [4]. LGA newborn was defined as an infant with a birth weight above the 90th percentile and AGA neonate as a birthweight between the 10th and 90th percentile [30]. First trimester body mass index (BMI) was calculated according to the following formula: weight (kg)/height (m2). A normal weight was defined as a BMI between 18.5 and 25 kg/m2 and overweight/obese as a BMI ≥25 kg/m2 based on the WHO criteria [3].
Sample collection and determination of RBP4 in maternal plasma
Maternal blood samples were collected at clinical visit. The gestational age at blood sampling was >37 weeks for all women included in the study. Maternal blood was collected into vacutainer tubes and samples were centrifuged at 1300 g for 10 minutes at 4°C. The plasma obtained was stored at −80°C until analysis. Maternal plasma concentration of RBP4 was determined by sensitive enzyme-linked immunoassays (Millipore Corporation, St. Charles, MO, USA). The RBP4 immunoassay was validated for human plasma in our laboratory, prior to the conduction of this study. Immunoassays were carried out according to the manufacturer’s recommendations. The calculated inter- and intra-assay coefficients of variation for RBP4 immunoassays in our laboratory were 5% and 5.1%, respectively. The sensitivity was calculated to be 0.1 ng/mL.
Statistical analysis
The Shapiro–Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution of the data. Non-parametric methods were used to perform the statistical analysis for parameters not normally distributed such as RBP4 concentrations. Kruskal–Wallis test with post hoc analysis and Mann–Whitney U tests were used for comparison of continuous variable among groups. Comparison of proportions was performed by Fisher’s exact test. Linear regression analysis was employed to determine which factors were significantly and independently correlated with maternal plasma RBP4 concentration (after log transformation). The following parameters were included in the model: maternal age, first trimester BMI, gestational age at blood collection (as a contentious variable), and the presence of GDM or an LGA neonate (as categorical variables). A p-value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 14 (SPSS Inc., Chicago, IL, USA).
Results
The demographic and clinical characteristics of the study groups are presented in Table 1. Patients with GDM either with an AGA neonate or an LGA neonate had a higher median maternal age than normal pregnant women with an AGA neonate. (p<0.001 for both comparisons). The rate of overweight/obese pregnant women was significantly higher in the GDM+LGA group than in patients with a normal pregnancy and an AGA neonate (69% vs. 45.3%, respectively; p=0.01). RBP4 was detected in the plasma of all subjects. There was a significant difference in the median maternal RBP4 concentration among the groups (p=0.005, Kruskal-Wallis).
Table 1.
Demographic and clinical characteristics of the study population.
| Normal pregnancy | GDM | |||
|---|---|---|---|---|
| AGA neonate (n = 64) |
LGA neonate (n = 44) |
AGA neonate (n = 55) |
LGA neonate (n = 42) |
|
| Maternal age (years)*, # | 26 (22–29) | 28 (22–32) | 34 (28–39) | 32 (30–38) |
| First trimester BMI (kg/m2) | 24 (23–31) | 25 (23–29) | 26 (24–30) | 28 (24–32) |
| BMI ≥ 25& | 45.3% (29) | 54.5% (24) | 67.2% (37) | 69.0% (29) |
| Smoking | 14.0% (9) | 13.6% (6) | 5.4% (3) | 9.5% (4) |
| Gestational age at blood sampling (weeks) | 39.0 (38.0–39.0) | 39.5 (38.6–40.0) | 38.8 (38.2–40.0) | 38.5 (38.0–39.7) |
| Gestational age at delivery (weeks) | 39.0 (38.2–39.7) | 39.6 (38.7–40.1) | 39.1 (38.5–40.0) | 39.0 (38.1–39.8) |
| Birth weight (g)*, $ | 3330 (3157–3555) | 4155 (4050–4425) | 3455 (3225–3725) | 4195 (4030–4405) |
Values are expressed as median (interquartile range) or as percentage (number).
AGA – Appropriate for gestational age; LGA – Large-for-gestational-age; GDM – Gestational Diabetes Mellitus; BMI – Body Mass Index
Kruskal-Wallis p<0.001
p=0.01 – Normal pregnancy+AGA vs. GDM+AGA; Normal pregnancy+AGA vs. GDM+LGA
p=0.01 – Normal pregnancy+AGA vs. GDM+LGA
p<0.001 – Normal pregnancy+AGA vs. Normal pregnancy+LGA; Normal pregnancy+AGA vs. GDM+LGA
Maternal plasma RBP4 concentration in women with a normal pregnancy: AGA vs. LGA
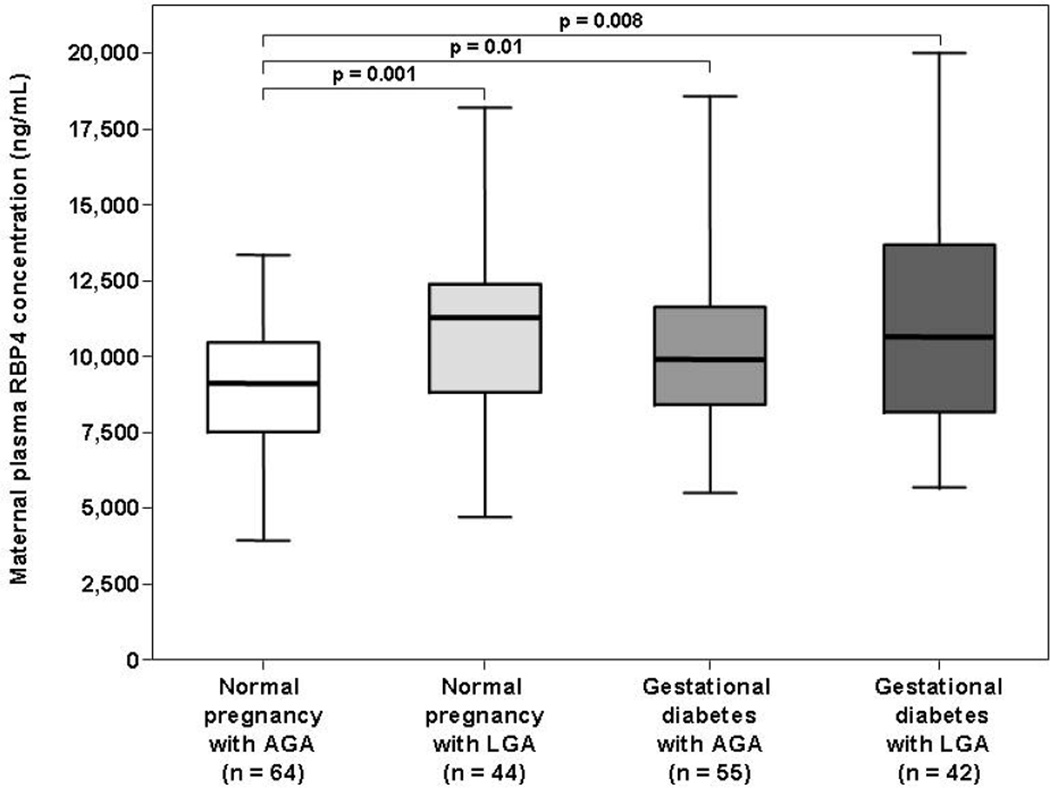
Among women with a normal pregnancy, the median maternal plasma RBP4 concentration was higher in pregnant women with an LGA neonate than in those with an AGA newborn (11,248 ng/mL, interquartile range [IQR]: 8,804 –12,364 vs. 9,094 ng/mL, IQR: 7,490–10,445, respectively, p=0.001; Figure 1).
Figure 1. Box and whisker plot of the comparison of the median maternal plasma RBP4 concentrations between women with and without GDM and/or an LGA fetus.
The median maternal plasma RBP4 concentration was higher in patients with GDM, either with an AGA or with an LGA neonate, than in those with a normal pregnancy and an AGA fetus. Similarly, pregnant women with a normal pregnancy and an LGA neonate had a higher median maternal plasma RBP4 concentration than those with a normal pregnancy and an AGA newborn.
Maternal plasma RBP4 concentration in women with a gestational diabetes mellitus: AGA vs. LGA
Among women with gestational diabetes mellitus, the median maternal plasma RBP4 concentration did not differ significantly between patients with an AGA neonate and those with an LGA newborn (9,884 ng/mL, IQR: 8,402 –11,589 vs. 10,618 ng/mL, IQR: 8,168–13,530, respectively, p=0.3; Figure 1).
Maternal plasma RBP4 concentration in women with a normal pregnancy vs. patients with gestational diabetes mellitus
Among women who delivered an AGA neonate, the median maternal plasma RBP4 concentration was higher in patients with GDM than in those with a normal pregnancy (p=0.01; Figure 1). Among women who delivered an LGA neonate, the median maternal plasma RBP4 concentration did not differ significantly between patients with a normal pregnancy and those with GDM (p=0.9; Figure 1).
Maternal plasma RBP4 concentration in normal weight vs. overweight/obese pregnant women
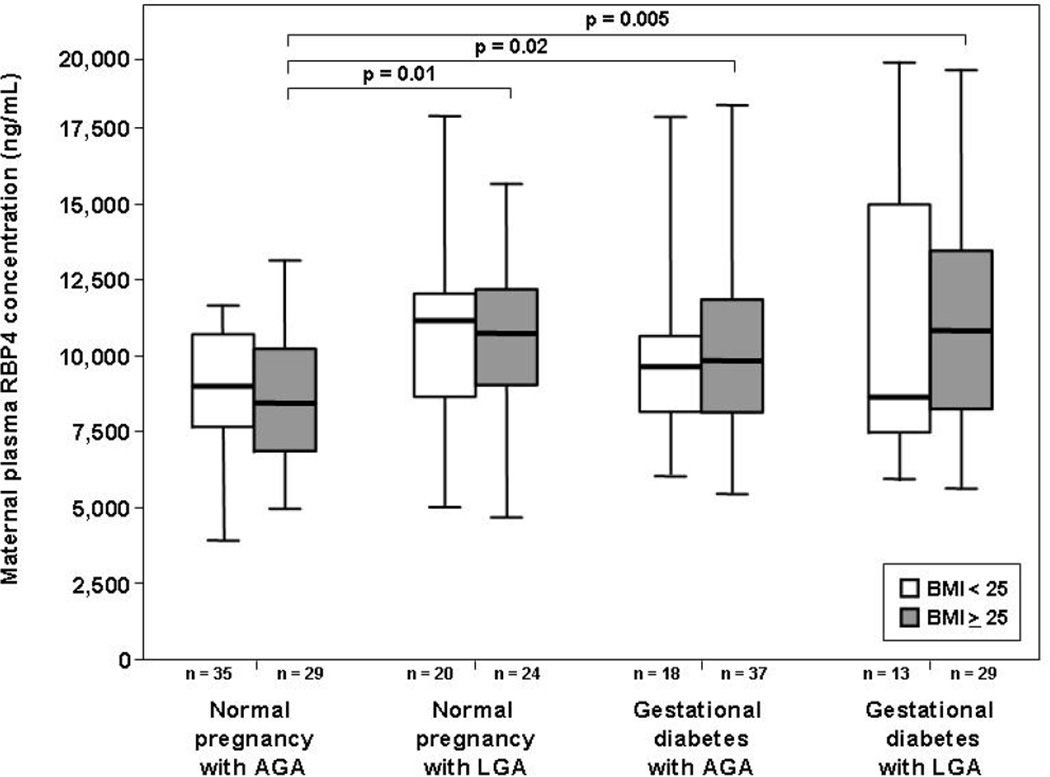
Among normal weight pregnant women, the median maternal plasma RBP4 concentration did not differ significantly between the different study groups (Kruskal-Wallis, p=0.2; Figure 2). In contrast, there was a significant difference in the median maternal RBP4 concentration among the groups when the analysis was restricted to overweight/obese patients (Kruskal-Wallis, p=0.015; Figure 2). The median maternal plasma RBP4 concentration in overweight/obese patients with GDM, either with an AGA or an LGA neonate, was higher than in overweight/obese pregnant women with an AGA newborn in the absence of GDM (p=0.02 and p=0.005, respectively; Figure 2). Similarly, the median maternal plasma RBP4 concentration in overweight/obese pregnant women with an LGA neonate was higher than in those with an AGA newborn (p=0.01; Figure 2).
Figure 2. Box and whisker plot of the comparison of the median maternal plasma RBP4 concentrations between normal weight and overweight/obese pregnant women, with and without GDM and/or an LGA fetus.
Among overweight/obese patients, the median maternal plasma RBP4 concentration was significantly higher in patients with GDM, either with an AGA or with LGA neonate, than in those with a normal pregnancy and an AGA neonate. Similarly, overweight/obese pregnant women with a normal pregnancy and an LGA newborn had a higher median maternal plasma RBP4 concentration than those with a normal pregnancy and an AGA neonate. In contrast, among normal weight pregnant women, there was no significant difference in the median maternal plasma RBP4 concentration between the different study groups. Within each study group, the median maternal plasma RBP4 concentration did not differ significantly between normal weight and overweight/obese pregnant women.
The median maternal plasma RBP4 concentrations did not differ significantly between normal weight and overweight/obese patients in normal pregnant women with an AGA neonate (normal weight: 9,107 ng/mL, IQR: 7,745–10,838 vs. overweight/obese: 8,541 ng/mL, IQR: 6,939–10,354, respectively, p=0.4; Figure 2), in normal pregnant women with an LGA neonate (normal weight: 11,290 ng/mL, IQR: 8,727–12,363 vs. overweight/obese: 10,866 ng/mL, IQR: 8,979–12,364, respectively, p=0.9; Figure 2), in patients with GDM and an AGA neonate (normal weight: 9,756 ng/mL, IQR: 8,325–10,691 vs. overweight/obese: 9,957 ng/mL, IQR: 8,238–11,986, respectively, p=0.4; Figure 2) and in patients with GDM and an LGA neonate (normal weight: 8,735 ng/mL, IQR: 7,416–16,688 vs. overweight/obese: 10,963 ng/mL, IQR: 8,348–13,620, respectively, p=0.6; Figure 2).
Multiple linear regression analysis was employed to examine the relationship between GDM, delivery of an LGA neonate and maternal plasma RBP4 concentrations while adjusting for maternal age, gestational age at blood sampling and BMI (as a continuous variable). The final regression model suggested that the presence of GDM and delivery of an LGA neonate were independently associated with high maternal plasma RBP4 concentrations (p<0.001).
Discussion
Principal findings of the study
1) Patients with GDM, either with AGA or LGA neonates, had a higher median plasma concentration of RBP4 than normal pregnant women who delivered an AGA neonate; 2) among normal pregnant women, patients with LGA neonates had a higher median plasma concentration of RBP4 than those with AGA newborns; 3) these findings remained significant after adjusting for maternal age, BMI and gestational age at blood sampling.
RBP4 is a novel adipokine with metabolic properties
Retinol-binding protein 4, the major blood carrier of retinol, is produced predominantly by the liver [97]. Recently, RBP4 was characterized as a novel adipokine, linking adipocytes glucose metabolism with systemic insulin resistance [89, 97, 106, 107]. The following findings suggest that this adipokine has a regulatory role in glucose homeostasis: 1) circulating concentration of RBP4 are increased in knockout mice for adipocytes glucose-transporter 4 (GLUT4) [106]; 2) over expression of RBP4, or injection of recombinant RBP4 in normal mice induces insulin resistance [106]; 3) mice heterozygous or homozygous for knockout of the gene encoding RBP4 have increased insulin sensitivity, as compared to with wild-type mice [106]; 4) treatment with an insulin-sensitizing drug reduces the elevated concentrations of RBP4 in both adipose tissue and serum of mice [106]; 5) polymorphysims in the RBP4 gene are associated with insulin resistance in humans [21, 73]; 6) overweight/obese patients have higher circulating RBP4 concentrations than normal weight individuals [5, 6, 31, 33, 47, 72, 99]; and 7) increased plasma concentrations of RBP4 was reported in several metabolic complications such as insulin resistance [18, 31, 72, 84, 103], metabolic syndrome [5, 35, 83, 104], polycystic ovary syndrome [32, 98] and cardiovascular disease [35, 104].
RBP4 in human pregnancy
There is only a limited number of reports regarding circulating maternal RBP4 concentration [36, 90, 95, 100–102]. Ueland et al.[100] found a significant increase in maternal fasting RBP4 concentration as a function of gestational age in normal pregnant women which was correlated with the decline in insulin sensitivity. Inuou et al. [36] and Shangguan et al.[90] reported that RBP4 is increased in patients with gestational hypertension and preeclampsia, whereas Stepan et al.[95] found no significant difference in maternal circulating RBP4 between patients with and without preeclampsia. Recently, we have reported that early-onset preeclampsia, but not late-onset preeclampsia, pregnancy with a small-for-gestational-age neonate or a fetal death, is associated with a higher median maternal plasma concentration of RBP4 than normal pregnancy [102]. In addition, we reported that RBP4 is a physiologic constituent of the amniotic fluid and the that median amniotic fluid concentration of RBP4 is elevated in pregnancies complicated by intra-amniotic infection/inflammation [101]. Taken together, these data suggest that RBP4, like other adipokines such as adiponectin, visfatin and resistin [43, 52–69, 75–77, 92], may play a role in normal human gestation, as well as in complications of pregnancy.
Gestational diabetes mellitus is characterized by a high maternal plasma RBP4 concentration
The findings of the present study indicate that patients with GDM, either with an AGA or an LGA neonate, have a higher median plasma RBP4 concentration than normal pregnant women with an AGA neonate. Our findings are in agreement with those of Lewandowski et al.[50], Chan et al.[17] and Kim [41] who all reported higher concentrations of RBP4 in patients with GDM but in contrast to those report by Krzyzanowska et al.[42] in which serum RBP4 concentrations were lower in patients with GDM. Differences in study design can account for the discrepancy between the studies. Specifically, gestational age at sampling, ethnic origin, and the ELISA kit used for RBP4 determination differed between the study conducted by Krzyzanowska et al. [42] and the present report.
The findings reported herein extend the aforementioned observations by demonstrating that GDM is associated with high circulating RBP4 concentrations in patients who delivered either an AGA or an LGA neonate. In addition, our findings suggest that the overweight/obese patients with GDM, but not those with normal weight, have increased maternal RBP4 concentrations. The latter finding may help to reconcile the inconsistency in the literature regarding maternal circulating RBP4 concentrations in patients with GDM. Finally, this is the first study to compare RBP4 concentrations in normal pregnant women and GDM patients at term.
Why is gestational diabetes mellitus is associated with high maternal plasma RBP4 concentrations?
Selective down-regulation of GLUT4 in adipocytes is almost an universal feature of insulin resistance states [91, 106]. Increased concentrations of RBP4 have been proposed as the molecular mechanism linking GLUT4 down-regulation with systemic insulin resistance [106]. Specifically, RBP4 impairs insulin signaling in muscle and induces the expression of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase in the liver [106]. Importantly, adipocytes GLUT4 content is decreased by approximately 45–60% in patients with GDM [27, 80], suggesting that similar mechanisms of disease can be applied to GDM and other insulin resistance states. The latter observation can also explain why the differences in maternal circulating RBP4 concentrations reported herein were confined to overweight/obese individuals. We recognize that the cross-sectional design of our study limited our ability to infer a causal relationship between elevated maternal circulating RBP4 concentrations and GDM. Nevertheless, it is tempting to speculate that the explanation for the high maternal RBP4 concentration is similar to that reported in Type 2 DM and other conditions characterized by insulin resistance.
Increased maternal plasma RBP4 concentrations is a feature of patients with an LGA neonate
The findings of the present study which indicating that normal pregnant women with an LGA neonate have a higher median maternal plasma RBP4 concentrations than normal pregnant women who delivered an AGA neonate are novel. Of note, delivery of an LGA neonate was independently associated with elevated maternal plasma RBP4 concentrations after adjusting for possible confounding factors. The explanation for this association is not clear. Since the data of the present study pertain only to maternal circulation, we cannot determine whether the increased RBP4 concentration in maternal circulation is a result of high maternal secretion, increased transplacental transport of RBP4 from the LGA fetus, or enhanced secretion from the larger placentas of LGA fetuses. Moreover, no conclusive data exist as to whether RBP4 can cross the human placenta, although such transport has been demonstrate in rats.[93, 96] In addition, there is paucity of information regarding cord blood RBP4 concentrations. Only two studies have address this question [17, 46], and only one [17] reported positive correlation between cord blood RBP4 and birthweight suggesting that LGA neonates might have higher circulating RBP4 concentrations than AGA neonates.
Another possible explanation that can account for the high maternal plasma RBP4 concentrations in patients with an LGA neonate is the presence of subtle carbohydrates intolerance in this subset of pregnant women. Minor abnormalities of glucose metabolism in patients with an LGA neonate have been reported in the absence of overt GDM [38, 44, 49, 108]. Thus, similarly to patients with GDM, it is possible that higher maternal plasma RBP4 concentrations relates to the maternal glucose metabolism rather than transfer from the fetal circulation or increase placental RBP4 secretion to the maternal circulation.
In conclusion, GDM is characterized by alterations in maternal circulating RBP4 concentrations akin to those of Type 2 DM. Pregnant women with an LGA neonate have a higher median plasma concentration of RBP4 that those of normal pregnant women with an AGA neonate, suggesting subclinical glucose metabolism in the former group. Collectively, these finding support the notion that adipokines play an important role obesity-related complications of pregnancy.
Acknowledgment
Supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.From the NIH: Successful diet and exercise therapy is conducted in Vermont for "diabesity". JAMA. 1980;243:519–520. [PubMed] [Google Scholar]
- 2.Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech.Rep.Ser. 2003;916:i-149. backcover. [PubMed] [Google Scholar]
- 3.Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech.Rep.Ser. 2003;916:i-149. backcover. [PubMed] [Google Scholar]
- 4.Prevention of diabetes mellitus. Report of a WHO Study Group. World Health Organ Tech.Rep.Ser. 1994;844:1–100. [PubMed] [Google Scholar]
- 5.Aeberli I, Biebinger R, Lehmann R, L'allemand D, Spinas GA, Zimmermann MB. Serum retinol-binding protein 4 concentration and its ratio to serum retinol are associated with obesity and metabolic syndrome components in children. J Clin.Endocrinol.Metab. 2007;92:4359–4365. doi: 10.1210/jc.2007-0468. [DOI] [PubMed] [Google Scholar]
- 6.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin.Endocrinol.Metab. 2007;92:1971–1974. doi: 10.1210/jc.2006-2712. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet.Med. 2004;21:103–113. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J.Clin.Endocrinol.Metab. 2001;86:989–993. doi: 10.1210/jcem.86.3.7339. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J.Clin.Invest. 2005;115:485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J.Clin.Invest. 2005;115:485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchanan TA, Xiang AH, Kjos SL, Trigo E, Lee WP, Peters RK. Antepartum predictors of the development of type 2 diabetes in Latino women 11–26 months after pregnancies complicated by gestational diabetes. Diabetes. 1999;48:2430–2436. doi: 10.2337/diabetes.48.12.2430. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am.J.Obstet.Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 13.Catalano PM, Bernstein IM, Wolfe RR, Srikanta S, Tyzbir E, Sims EA. Subclinical abnormalities of glucose metabolism in subjects with previous gestational diabetes. Am.J.Obstet.Gynecol. 1986;155:1255–1262. doi: 10.1016/0002-9378(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 14.Catalano PM, Kirwan JP. Clinical utility and approaches for estimating insulin sensitivity in pregnancy. Semin.Perinatol. 2002;26:181–189. doi: 10.1053/sper.2002.33977. [DOI] [PubMed] [Google Scholar]
- 15.Catalano PM, Kirwan JP, Haugel-de MS, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. J.Nutr. 2003;133:1674S–1683S. doi: 10.1093/jn/133.5.1674S. [DOI] [PubMed] [Google Scholar]
- 16.Catalano PM, Vargo KM, Bernstein IM, Amini SB. Incidence and risk factors associated with abnormal postpartum glucose tolerance in women with gestational diabetes. Am.J.Obstet.Gynecol. 1991;165:914–919. doi: 10.1016/0002-9378(91)90438-w. [DOI] [PubMed] [Google Scholar]
- 17.Chan TF, Chen HS, Chen YC, Lee CH, Chou FH, Chen IJ, et al. Increased serum retinol-binding protein 4 concentrations in women with gestational diabetes mellitus. Reprod.Sci. 2007;14:169–174. doi: 10.1177/1933719106298407. [DOI] [PubMed] [Google Scholar]
- 18.Choi SH, Kwak SH, Youn BS, Lim S, Park YJ, Lee H, et al. High plasma retinol binding protein-4 and low plasma adiponectin concentrations are associated with severity of glucose intolerance in women with previous gestational diabetes mellitus. J Clin.Endocrinol.Metab. 2008;93:3142–3148. doi: 10.1210/jc.2007-1755. [DOI] [PubMed] [Google Scholar]
- 19.Coustan DR, Carpenter MW, O'Sullivan PS, Carr SR. Gestational diabetes: predictors of subsequent disordered glucose metabolism. Am.J.Obstet.Gynecol. 1993;168:1139–1144. doi: 10.1016/0002-9378(93)90358-p. [DOI] [PubMed] [Google Scholar]
- 20.Coustan DR, Nelson C, Carpenter MW, Carr SR, Rotondo L, Widness JA. Maternal age and screening for gestational diabetes: a population-based study. Obstet.Gynecol. 1989;73:557–561. [PubMed] [Google Scholar]
- 21.Craig RL, Chu WS, Elbein SC. Retinol binding protein 4 as a candidate gene for type 2 diabetes and prediabetic intermediate traits. Mol.Genet.Metab. 2007;90:338–344. doi: 10.1016/j.ymgme.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 23.Ecker JL, Greenberg JA, Norwitz ER, Nadel AS, Repke JT. Birth weight as a predictor of brachial plexus injury. Obstet.Gynecol. 1997;89:643–647. doi: 10.1016/s0029-7844(97)00007-0. [DOI] [PubMed] [Google Scholar]
- 24.Ellard S, Beards F, Allen LI, Shepherd M, Ballantyne E, Harvey R, et al. A high prevalence of glucokinase mutations in gestational diabetic subjects selected by clinical criteria. Diabetologia. 2000;43:250–253. doi: 10.1007/s001250050038. [DOI] [PubMed] [Google Scholar]
- 25.Farvid MS, Ng TW, Chan DC, Barrett PH, Watts GF. Association of adiponectin and resistin with adipose tissue compartments, insulin resistance and dyslipidaemia. Diabetes Obes.Metab. 2005;7:406–413. doi: 10.1111/j.1463-1326.2004.00410.x. [DOI] [PubMed] [Google Scholar]
- 26.Gabbe SG. Gestational diabetes mellitus. N.Engl.J.Med. 1986;315:1025–1026. doi: 10.1056/NEJM198610163151609. [DOI] [PubMed] [Google Scholar]
- 27.Garvey WT, Maianu L, Zhu JH, Hancock JA, Golichowski AM. Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes. Heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters. Diabetes. 1993;42:1773–1785. doi: 10.2337/diab.42.12.1773. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J.Clin.Endocrinol.Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J.Clin.Endocrinol.Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, et al. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev.Med.Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 31.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N.Engl.J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 32.Hahn S, Backhaus M, Broecker-Preuss M, Tan S, Dietz T, Kimmig R, et al. Retinol-binding protein 4 levels are elevated in polycystic ovary syndrome women with obesity and impaired glucose metabolism. Eur.J Endocrinol. 2007;157:201–207. doi: 10.1530/EJE-07-0143. [DOI] [PubMed] [Google Scholar]
- 33.Haider DG, Schindler K, Prager G, Bohdjalian A, Luger A, Wolzt M, et al. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin.Endocrinol.Metab. 2007;92:1168–1171. doi: 10.1210/jc.2006-1839. [DOI] [PubMed] [Google Scholar]
- 34.Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J.Clin.Endocrinol.Metab. 2001;86:568–573. doi: 10.1210/jcem.86.2.7137. [DOI] [PubMed] [Google Scholar]
- 35.Ingelsson E, Lind L. Circulating retinol-binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care. 2009;32:733–735. doi: 10.2337/dc08-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue S, Takamoto N, Akahori Y, Masumoto A, Nakatsukasa H, Msuyama H, et al. Elevated level of serum retinol-binding protein 4 in pregnancy-induced hypertension. J Obstet.Gynaecol.Res. 2009;35:293–300. doi: 10.1111/j.1447-0756.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- 37.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann RC, McBride P, Amankwah KS, Huffman DG. The effect of minor degrees of glucose intolerance on the incidence of neonatal macrosomia. Obstet.Gynecol. 1992;80:97–101. [PubMed] [Google Scholar]
- 39.Khine ML, Winklestein A, Copel JA. Selective screening for gestational diabetes mellitus in adolescent pregnancies. Obstet.Gynecol. 1999;93:738–742. doi: 10.1016/s0029-7844(98)00550-x. [DOI] [PubMed] [Google Scholar]
- 40.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Choi HJ, Im JA. Retinol-binding protein 4 responses during an oral glucose tolerance testing in women with gestational diabetes mellitus. Clin.Chim.Acta. 2008;391:123–125. doi: 10.1016/j.cca.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 42.Krzyzanowska K, Zemany L, Krugluger W, Schernthaner GH, Mittermayer F, Schnack C, et al. Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia. 2008;51:1115–1122. doi: 10.1007/s00125-008-1009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21:902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langer O, Anyaegbunam A, Brustman L, Divon M. Management of women with one abnormal oral glucose tolerance test value reduces adverse outcome in pregnancy. Am.J.Obstet.Gynecol. 1989;161:593–599. doi: 10.1016/0002-9378(89)90361-x. [DOI] [PubMed] [Google Scholar]
- 45.Langer O, Anyaegbunam A, Brustman L, Guidetti D, Mazze R. Gestational diabetes: insulin requirements in pregnancy. Am.J.Obstet.Gynecol. 1987;157:669–675. doi: 10.1016/s0002-9378(87)80026-1. [DOI] [PubMed] [Google Scholar]
- 46.Laudes M, Oberhauser F, Bilkovski R, Schubert M, Udelhoven M, Wassmer G, et al. Human fetal adiponectin and retinol-binding protein (RBP)-4 levels in relation to birth weight and maternal obesity. Exp.Clin.Endocrinol.Diabetes. 2009;117:146–149. doi: 10.1055/s-0028-1100379. [DOI] [PubMed] [Google Scholar]
- 47.Lee JW, Lee HR, Shim JY, Im JA, Lee DC. Abdominal visceral fat reduction is associated with favorable changes of serum retinol binding protein-4 in nondiabetic subjects. Endocr.J. 2008;55:811–818. doi: 10.1507/endocrj.k08e-030. [DOI] [PubMed] [Google Scholar]
- 48.Lee YY, Lee NS, Cho YM, Moon MK, Jung HS, Park YJ, et al. Genetic association study of adiponectin polymorphisms with risk of Type 2 diabetes mellitus in Korean population. Diabet.Med. 2005;22:569–575. doi: 10.1111/j.1464-5491.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- 49.Leikin EL, Jenkins JH, Pomerantz GA, Klein L. Abnormal glucose screening tests in pregnancy: a risk factor for fetal macrosomia. Obstet.Gynecol. 1987;69:570–573. [PubMed] [Google Scholar]
- 50.Lewandowski KC, Stojanovic N, Bienkiewicz M, Tan BK, Prelevic GM, Press M, et al. Elevated concentrations of retinol-binding protein-4 (RBP-4) in gestational diabetes mellitus: negative correlation with soluble vascular cell adhesion molecule-1 (sVCAM-1) Gynecol.Endocrinol. 2008;24:300–305. doi: 10.1080/09513590802141052. [DOI] [PubMed] [Google Scholar]
- 51.Mauricio D, de LA. Autoimmune gestational diabetes mellitus: a distinct clinical entity? Diabetes Metab Res.Rev. 2001;17:422–428. doi: 10.1002/dmrr.237. [DOI] [PubMed] [Google Scholar]
- 52.Mazaki-Tovi S, Romero R, Kim SK, Vaisbuch E, Kusanovi JP, Erez O, et al. Could alterations in maternal plasma visfatin concentration participate in the phenotype definition of preeclampsia and SGA? The Journal of Maternal-Fetal and Neonatal Medicine. 2009 doi: 10.3109/14767050903301017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazaki-Tovi S, Romero R, Vaisbuch E, Chaiworapongsa T, Erez O, Mittal P, et al. Low circulating maternal adiponectin in patients with pyelonephritis: adiponectin at the crossroads of pregnancy and infection. Journal Of Perinatal Medicine. 2009 doi: 10.1515/JPM.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Chaiwaropongsa T, Mittal P, et al. Maternal Plasma Visfatin in Preterm Labor. J Matern.Fetal Neonatal Med. 2009;22:693–704. doi: 10.1080/14767050902994788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiwaropongsa T, et al. Dysregulation of maternal serum adiponectin in preterm labor. J.Matern.Fetal Neonatal Med. 2009;22:887–904. doi: 10.1080/14767050902994655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiwaropongsa T, et al. Maternal Serum Adiponectin Multimers in Gestational Diabetes. Journal Of Perinatal Medicine. 2009 doi: 10.1515/JPM.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiwaropongsa T, et al. Maternal Serum Adiponectin Multimers in Patients with a Small-For-Gestational-Age Newborn. Journal Of Perinatal Medicine. 2009 doi: 10.1515/JPM.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Mittal P, et al. Adiponectin in amniotic fluid in normal pregnancy, spontaneous labor at term, and preterm labor: A novel association with subclinical intrauterine infection/inflammation. J.Matern.Fetal Neonatal Med. 2009 doi: 10.3109/14767050903026481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Efraty Y, Schiff E, et al. Determining the source of fetal adiponectin. J.Reprod.Med. 2007;52:774–778. [PubMed] [Google Scholar]
- 60.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Schiff E, Sivan E. Cord blood adiponectin in large-for-gestational age newborns. Am.J.Obstet.Gynecol. 2005;193:1238–1242. doi: 10.1016/j.ajog.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 61.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Wiser A, Schiff E, et al. Maternal serum adiponectin levels during human pregnancy. J.Perinatol. 2007;27:77–81. doi: 10.1038/sj.jp.7211639. [DOI] [PubMed] [Google Scholar]
- 62.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Yinon Y, Wiser A, et al. Adiponectin and leptin concentrations in dichorionic twins with discordant and concordant growth. J Clin.Endocrinol.Metab. 2009;94:892–898. doi: 10.1210/jc.2008-2118. [DOI] [PubMed] [Google Scholar]
- 63.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Yinon Y, Wiser A, et al. Adiponectin and leptin concentrations in dichorionic twins with discordant and concordant growth. J Clin.Endocrinol.Metab. 2009;94:892–898. doi: 10.1210/jc.2008-2118. [DOI] [PubMed] [Google Scholar]
- 64.Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Curr.Diab.Rep. 2005;5:278–281. doi: 10.1007/s11892-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 65.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat.Med. 2008;36:485–496. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F, et al. Adiponectin multimers in maternal plasma. J.Matern.Fetal Neonatal Med. 2008;21:796–815. doi: 10.1080/14767050802266881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than NG, et al. Maternal visfatin concentration in normal pregnancy. J Perinat.Med. 2009;37:206–217. doi: 10.1515/JPM.2009.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than NG, et al. Visfatin in human pregnancy: maternal gestational diabetes vis-a-vis neonatal birthweight. J Perinat.Med. 2009;37:218–231. doi: 10.1515/JPM.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Gotsch F, et al. Maternal serum adiponectin multimers in preeclampsia. J Perinat.Med. 2009;37:349–363. doi: 10.1515/JPM.2009.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Metzger BE, Cho NH, Roston SM, Radvany R. Prepregnancy weight and antepartum insulin secretion predict glucose tolerance five years after gestational diabetes mellitus. Diabetes Care. 1993;16:1598–1605. doi: 10.2337/diacare.16.12.1598. [DOI] [PubMed] [Google Scholar]
- 71.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–B167. [PubMed] [Google Scholar]
- 72.Mills JP, Furr HC, Tanumihardjo SA. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp.Biol.Med (Maywood.) 2008;233:1255–1261. doi: 10.3181/0803-RM-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munkhtulga L, Nakayama K, Utsumi N, Yanagisawa Y, Gotoh T, Omi T, et al. Identification of a regulatory SNP in the retinol binding protein 4 gene associated with type 2 diabetes in Mongolia. Hum.Genet. 2007;120:879–888. doi: 10.1007/s00439-006-0264-4. [DOI] [PubMed] [Google Scholar]
- 74.Muoio DM, Newgard CB. Metabolism: A is for adipokine. Nature. 2005;436:337–338. doi: 10.1038/436337a. [DOI] [PubMed] [Google Scholar]
- 75.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J.Perinat.Med. 2007;35:522–531. doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, et al. Adiponectin in severe preeclampsia. J.Perinat.Med. 2007;35:503–512. doi: 10.1515/JPM.2007.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nien JK, Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, et al. Resistin: a hormone which induces insulin resistance is increased in normal pregnancy. J.Perinat.Med. 2007;35:513–521. doi: 10.1515/JPM.2007.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Sullivan JB, Mahan CM. CRITERIA FOR THE ORAL GLUCOSE TOLERANCE TEST IN PREGNANCY. Diabetes. 1964;13:278–285. [PubMed] [Google Scholar]
- 79.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 80.Okuno S, Akazawa S, Yasuhi I, Kawasaki E, Matsumoto K, Yamasaki H, et al. Decreased expression of the GLUT4 glucose transporter protein in adipose tissue during pregnancy. Horm.Metab Res. 1995;27:231–234. doi: 10.1055/s-2007-979946. [DOI] [PubMed] [Google Scholar]
- 81.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 82.Polonsky KS. Retinol-binding protein 4, insulin resistance, and type 2 diabetes. N.Engl.J Med. 2006;354:2596–2598. doi: 10.1056/NEJMe068091. [DOI] [PubMed] [Google Scholar]
- 83.Qi Q, Yu Z, Ye X, Zhao F, Huang P, Hu FB, et al. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin.Endocrinol.Metab. 2007;92:4827–4834. doi: 10.1210/jc.2007-1219. [DOI] [PubMed] [Google Scholar]
- 84.Ribel-Madsen R, Friedrichsen M, Vaag A, Poulsen P. Retinol-binding protein 4 in twins: regulatory mechanisms and impact of circulating and tissue expression levels on insulin secretion and action. Diabetes. 2009;58:54–60. doi: 10.2337/db08-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Romero R. The child is the father of the man. Prenat Neonat Med. 1996:8–11. [Google Scholar]
- 86.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ryan EA, Imes S, Liu D, McManus R, Finegood DT, Polonsky KS, et al. Defects in insulin secretion and action in women with a history of gestational diabetes. Diabetes. 1995;44:506–512. doi: 10.2337/diab.44.5.506. [DOI] [PubMed] [Google Scholar]
- 88.Saker PJ, Hattersley AT, Barrow B, Hammersley MS, McLellan JA, Lo YM, et al. High prevalence of a missense mutation of the glucokinase gene in gestational diabetic patients due to a founder-effect in a local population. Diabetologia. 1996;39:1325–1328. doi: 10.1007/s001250050577. [DOI] [PubMed] [Google Scholar]
- 89.Sell H, Eckel J. Regulation of retinol binding protein 4 production in primary human adipocytes by adiponectin, troglitazone and TNF-alpha. Diabetologia. 2007;50:2221–2223. doi: 10.1007/s00125-007-0764-3. [DOI] [PubMed] [Google Scholar]
- 90.Shangguan X, Liu F, Wang H, He J, Dong M. Alterations in serum adipocyte fatty acid binding protein and retinol binding protein-4 in normal pregnancy and preeclampsia. Clin.Chim.Acta. 2009;407:58–61. doi: 10.1016/j.cca.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 91.Shepherd PR, Kahn BB. Glucose transporters and insulin action--implications for insulin resistance and diabetes mellitus. N.Engl.J Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 92.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, et al. Adiponectin in human cord blood: relation to fetal birth weight and gender. J.Clin.Endocrinol.Metab. 2003;88:5656–5660. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 93.Sklan D, Shalit I, Lasebnik N, Spirer Z, Weisman Y. Retinol transport proteins and concentrations in human amniotic fluid, placenta, and fetal and maternal sera. Br.J Nutr. 1985;54:577–583. doi: 10.1079/bjn19850144. [DOI] [PubMed] [Google Scholar]
- 94.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 95.Stepan H, Ebert T, Schrey S, Reisenbuchler C, Bluher M, Stumvoll M, et al. Preliminary report: Serum levels of retinol-binding protein 4 in preeclampsia. Metabolism. 2009;58:275–277. doi: 10.1016/j.metabol.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 96.Takahashi YI, Smith JE, Goodman DS. Vitamin A and retinol-binding protein metabolism during fetal development in the rat. Am.J Physiol. 1977;233:E263–E272. doi: 10.1152/ajpendo.1977.233.4.E263. [DOI] [PubMed] [Google Scholar]
- 97.Tamori Y, Sakaue H, Kasuga M. RBP4, an unexpected adipokine. Nat.Med. 2006;12:30–31. doi: 10.1038/nm0106-30. [DOI] [PubMed] [Google Scholar]
- 98.Tan BK, Chen J, Lehnert H, Kennedy R, Randeva HS. Raised serum, adipocyte, and adipose tissue retinol-binding protein 4 in overweight women with polycystic ovary syndrome: effects of gonadal and adrenal steroids. J Clin.Endocrinol.Metab. 2007;92:2764–2772. doi: 10.1210/jc.2007-0091. [DOI] [PubMed] [Google Scholar]
- 99.Tschoner A, Sturm W, Engl J, Kaser S, Laimer M, Laimer E, et al. Retinol-binding protein 4, visceral fat, the metabolic syndrome: effects of weight loss. Obesity.(Silver.Spring) 2008;16:2439–2444. doi: 10.1038/oby.2008.391. [DOI] [PubMed] [Google Scholar]
- 100.Ueland T, Dalsoren T, Voldner N, Godang K, Henriksen T, Bollerslev J. Retinol-binding protein-4 is not strongly associated with insulin sensitivity in normal pregnancies. Eur.J Endocrinol. 2008;159:49–54. doi: 10.1530/EJE-07-0682. [DOI] [PubMed] [Google Scholar]
- 101.Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Than GN, Kim SK, et al. Retinol Binding Protein 4: An Adipokine Associated with Intra-amniotic Infection / Inflammation. J Matern Fetal Neonatal Med. 2009 doi: 10.3109/14767050902994739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vaisbuch E, Romero R, Mazaki-Tovi S, Erez O, Kim SK, Chaiworapongsa T, et al. Retinol binding protein 4 - a novel association with early-onset preeclampsia. J Perinat.Med. 2009 doi: 10.1515/JPM.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.von EM, Humpert PM. Retinol-binding protein-4 in experimental and clinical metabolic disease. Expert.Rev.Mol.Diagn. 2008;8:289–299. doi: 10.1586/14737159.8.3.289. [DOI] [PubMed] [Google Scholar]
- 104.von EM, Lepper PM, Liu D, Lang K, Baumann M, Nawroth PP, et al. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia. 2007;50:1930–1937. doi: 10.1007/s00125-007-0743-8. [DOI] [PubMed] [Google Scholar]
- 105.Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes. Diabetes. 1999;48:848–854. doi: 10.2337/diabetes.48.4.848. [DOI] [PubMed] [Google Scholar]
- 106.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 107.Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin.Endocrinol.Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yogev Y, Langer O, Xenakis EM, Rosenn B. The association between glucose challenge test, obesity and pregnancy outcome in 6390 non-diabetic women. J.Matern.Fetal Neonatal Med. 2005;17:29–34. doi: 10.1080/14767050400028766. [DOI] [PubMed] [Google Scholar]