Abstract
Purpose
To report the first use of in vitro fertilization (IVF) and preimplantation genetic diagnosis to achieve an unaffected pregnancy in an autosomal-recessive retinal dystrophy.
Design
Case report.
Methods
An affected male with Stargardt disease and his carrier wife underwent IVF. Embryos obtained by intracytoplasmic sperm injection underwent single-cell DNA testing via polymerase chain reaction and restriction enzyme analysis to detect the presence of ABCA4 mutant alleles. Embryos were diagnosed as being either affected by or carriers for Stargardt disease. A single carrier embryo was implanted.
Results
Chorionic villus sampling performed during the first trimester verified that the fetus possessed only one mutant paternal allele and one normal maternal allele, thus making her an unaffected carrier of the disease. A healthy, live-born female was delivered.
Conclusion
IVF and preimplantation genetic diagnosis can assist couples with an affected spouse and a carrier spouse with recessive retinal dystrophies to have an unaffected child.
Introduction
Stargardt disease is the most common type of hereditary recessive macular dystrophy. The estimated incidence for the disease is 1 in 10,000 live births, and it characteristically presents in juveniles and young adults.1 Patients begin to experience a bilateral, gradual decline in their vision between the ages of 6 and 20 years, and can present with visual acuity ranging from 20/20 to 20/200. Their prior visual acuity is often normal, though their final acuity is often 20/200 or worse.2
Stargardt disease, similar to age-related macular degeneration (AMD), causes central visual impairment. Though the clinical picture is highly variable, some characteristics can include the presence of orange-yellow flecks distributed around the macula and the mid-retinal periphery and localized to the retinal pigment epithelium (RPE) on fundus photography.1-4 These changes may not be visible on initial presentation, however, as the first sign can often be disappearance of the foveolar reflex. Later stages can include an area of atrophic RPE ranging from 2 disc diameters horizontally to 1.5 disc diameters vertically in the macula, giving the appearance of “bulls-eye” maculopathy.2
Stargardt disease is caused by mutations in the photoreceptor-specific ABCA4 (formerly known as ABCR) gene on human chromosome 1p13-p21 (STGD1).3 Variants in this gene have also been linked to the development of autosomal recessive cone-rod dystrophy and retinitis pigmentosa.5 Over 500 disease-associated alleles have been identified, with exceptional allelic heterogeneity, making genetic analyses of the disease challenging. Conventional mutation detection techniques have been able to detect only 25 to 60% of all expected mutations, while direct sequencing could identify 66 to 80%.6 A study by Jaakson et al.6 demonstrated the benefits of using an ABCR400 genotyping microarray, which was shown to be over 99% effective in screening for all known ABCR gene mutations and was able to detect approximately 70-75% of all expected mutations.6,7
Preimplantation genetic diagnosis is a technique introduced in the early-1990s that involves analysis of embryos created in vitro for genetic defects and only implanting those embryos that are free of defects.8 Traditionally, groups with indications for such interventions have included those with high risk of having children with genetic disease who have opted to terminate pregnancies based on prenatal tests, have had recurrent miscarriages, have objections to abortion, and/or who have concurrent infertility and those who are being treated with in-vitro fertilization (IVF) and in whom low rate of success with this method can be attributed to chromosomal aneuploidies in the embryos.8 The uses of this technology have expanded, and reports of its successful use in cases of retinoblastoma and X-linked retinoschisis have been published.9,10
Preimplantation genetic diagnosis involves collection of single cells (blastomeres) through biopsies that can be performed on polar bodies, at the cleavage-stage, and at the blastocyst stage, which is the latest stage at which the embryo can be biopsied. After cells are obtained, there are several techniques available for DNA analysis. PCR is used to amplify DNA from the polar body or blastomere while fluorescence in situ hybridization (FISH) is used to analyze the chromosomal complement of blastomeres. Embryos without aberrances are then isolated and used for the pregnancy. The first application of this technique was for patients with carriers of an X-linked disease, but advancements in single-cell analysis expanded the technique for use in cases of many monogenic diseases.8 Here, we present a couple’s experience with preimplantation genetic diagnosis for Stargardt disease, resulting in the successful delivery of a live-born child without the disease. To our knowledge, this is the first report of the application of preimplantation genetic diagnosis in the case of an autosomal-recessive retinal dystrophic disease.
Methods
Patient Description
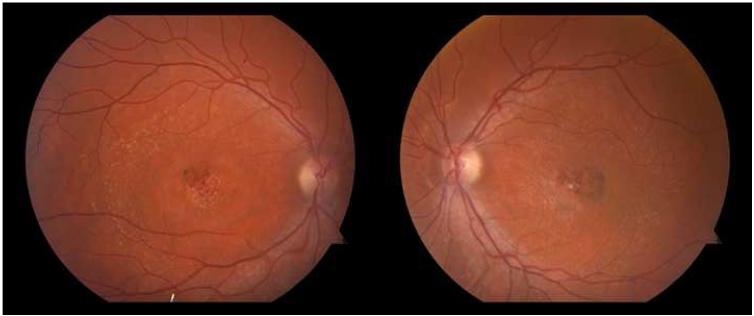
A 29-year-old man affected with Stargardt Disease (Visual Acuity 20/70 in both eyes, Figures 1 and 2) and his 29–year-old wife sought genetic counseling regarding their desire to conceive in the midst of concerns over having an affected child. Although the frequency of disease-causing mutations in the general population is low (approximately 5%),11 the couple were advised to have genetic testing for the wife. Genetic testing performed on both the patient and his wife confirmed his possession of two ABCA4 mutations (c5018+2C>T and c5882G>A). The c5018+2C>T variant is a relatively rare splice site mutation that has been associated with Stargardt Disease in at least two studies).12,13 The c5882G>A substitution, resulting in the G1961E amino acid change, is an extensively characterized and one of the most frequent STGD mutations.
Figure 1. Bilateral color fundus photographs of patient with Stargardt disease.
The classic yellow-white flecks associated with Stargardt disease can be evidenced in both eyes, extending peripherally, along with pigmentary changes in the macula.
Figure 2. Bilateral autofluorescence images of patient with Stargardt disease.
Increased levels of autofluorescence with peripapillary sparing can be noted bilaterally, characteristic of Stargardt disease.
Testing of his wife revealed one possibly pathogenic ABCA4 mutation (c455G>A). The c455G>A variant, resulting in R152Q protein change, has been described in at least 3 studies.11-13 In one,11 the variant was described as a ‘rare variant” since it was also detected in controls. In the two others,12,13 it was defined as a disease-causing mutation. This variant exemplifies the difficulty of unambiguous assignment of pathogenicity to very rare ABCA4 missense alleles where supportive evidence, such as segregation with the disease in families and frequency in the general population, is hard, if not impossible, to obtain.
In this particular case, the R152Q variant was considered possibly pathogenic to avoid the potential disease expression in compound heterozygous state. Because the risk of having an affected child was now 50%, the couple was offered and accepted the option of undergoing IVF with PGD.
Mutation Analyses
A blood sample was obtained from the husband, affected with Stargardt disease, and his wife and an ABCA4 mutation screening with ABCR500 chip6 was performed. Specifically, polymerase chain reaction (PCR)-amplification of the genomic DNA with specific primers for Exons 1 through 49, and flanking intronic sequences, followed by purification of PCR products and array-based primer extension with dideoxy terminators was performed, using primers to detect, at that time, 441 known mutations for Stargardt Disease. The current array detects 519 mutations (Asper Biotech, Tartu, Estonia, www.asperbio.com). Results showed that she was a carrier of the the c455G>A (R152Q) ABCA4 variant. The same testing on the affected husband found two Stargardt disease-associated mutations, the splice site-affecting c5018+2 C>T mutation and the c5882G>A (G1961E) mutation, thereby confirming the clinical diagnosis of STGD.
For the embryos, DNA obtained from biopsied cells underwent PCR-amplification of the genomic DNA with primers for known mutations for Stargardt disease, just as the procedure was performed on blood samples from the parents. Controls included maternal and paternal genomic DNA as well as standard DNA with blanks to demonstrate lack of exogenous DNA contamination of the samples.
The patient’s wife underwent chorionic villus sampling (CVS) at 11 weeks gestation. Direct DNA sequence analysis was performed on the sample to test for mutations in exons 5, 35, and 42 of the ABCA4 gene. The Amplistr Identifiler kit (Applied Biosystems) was used to compare 16 microsatellite markers in the maternal DNA with the chorionic villus sample in order to evaluate for maternal DNA contamination of the CVS sample. Results demonstrated that the fetus was a carrier for Stargardt disease, as expected, possessing only the paternal c5018+2C>T mutation.
In Vitro Fertilization and Embryo Biopsy
The experimental nature of the procedure was discussed with the couple, who agreed verbally and in writing that they wished to complete the procedure in order to reduce the risk of their fetus having Stargardt disease. Controlled ovarian stimulation was carried out using 1 mg/d of leuprolide acetate initiated in the mid-luteal phase. Injectable gonadotropins (follicle-stimulating hormone (FSH) and human menopausal gonadotropin (hmG)) were administered in a 2:1 dosing ratio starting on day 3 of the ensuing period, with a concurrent halving of the leuprolide acetate dose to 0.5 mg/d. Results of the methods described above for oocyte retrieval, fertilization, and biopsy of embryos are presented in the Table. This cycle yielded 17 oocytes, 16 of which were fertilized by intracytoplasmic sperm injection (ICSI). Of these, 15 were biopsied, with 6 demonstrating one maternal and one paternal mutant allele and thus labeled as affected with Stargardt disease. For 3 of the 15, molecular data was inconclusive, leaving 6 that were identified as having normal (unaffected) maternal alleles. Of these 6, 2 were cryopreserved, 3 were discarded, and 1 was transferred into the uterus on day 5, resulting in a singleton pregnancy.
Table.
Results of IVF with PGD for Stargardt Disease
| IVF/PGD Process | Cycle 1 |
|---|---|
| Retrieved oocytes (post-ovarian stimulation) | 17 |
| Oocytes fertilized by ICSI | 16 |
| Embryos biopsied for ABCA4 mutation | 15 |
| Unaffected embryos | 6 |
| Cryopreserved embryos | 2 |
| Embryos transferred to uterus | 1 |
| Pregnancy outcome | Singleton pregnancy |
Results of IVF with PGD for Stargardt disease. IVF = in vitro fertilization; PGD = pre-implantation genetic diagnosis; ICSI = intracytoplasmic sperm injection
Results
Chorionic villus sampling was performed during the first trimester, verifying that the fetus possessed only one mutant paternal allele and one normal maternal allele, thus making her an unaffected carrier of the disease. A healthy, live-born female was delivered.
Discussion
We have presented a new application of preimplantation genetic diagnosis in the case of Stargardt disease, to our knowledge the first reported case for an autosomal recessive retinal dystrophic disease. Preimplantation genetic diagnosis uses single-cell embryonic biopsies in prenatal diagnosis and can aid couples in the avoidance of pregnancy termination by detecting inherited diseases before implantation of the embryo.8 Single cells are aspirated and probed using fluorescence in situ hybridization to determine ploidy status, leading to transfer of euploid embryos and discarding of aneuploid embryos. Though its use has broadened, the suggested indications for preimplantation genetic diagnosis have been advanced maternal age (over 37 or 38 years), repeated implantation failure (three or more failed transfers), repeated miscarriage in patients with normal karyotypes (history of at least three miscarriages), or severe male factor infertility (based on semen analysis).14 The latest report on the use of preimplantation genetic diagnosis from the European Society of Human Reproduction and Embryology showed an overall increase in its use, with the most common indications for autosomal recessive diseases being beta-thalassemia and/or sickle cell syndromes, cystic fibrosis, and spinal muscular atrophy.15
Although many advances have occurred in the understanding of Stargardt disease since ABCA4 was implicated as the causal gene, treatment options remain limited. Current recommendations include limiting sunlight exposure through the use of ultraviolet-blocking sunglasses and the avoidance of vitamin A, but these options do not address the underlying etiology of the disease, which is the accumulation of A2E leading to destruction of cell membranes, increased susceptibility to blue light-associated damage, and altered lysosomal function. Future therapeutic options could include gene therapy and compounds specifically targeting the visual cycle, but these options are still being explored.7,16 At present, patients with Stargardt disease will go on to experience progressive, bilateral, gradual decline in their vision beginning between the ages of 6 and 20 years leading to a final visual acuity of 20/200 or worse with associated central scotoma.2 Given the lack of curative therapies available, PGD can provide a ready alternative for at-risk and/or affected couples who do not wish to pass on this disease to their offspring.
The advent of ABCA4 genotyping microarray makes it possible to screen for all known (>500) ABCA4 mutations in affected or unaffected parents followed by direct sequencing of DNA obtained by single-cell embryonic biopsies for specific parental variants. The ABCA4 array detects all known disease-associated mutations with >99% reliability, and is currently able to find ~75% of all disease-associated mutations.6,7 This method was successfully employed in the present case to screen parents and resulted in detecting two disease-associated variants in the affected father and one in the asymptomatic mother. The embryos obtained by IVF were screened for these three specific mutations by direct sequencing of PCR-amplified DNA from a single blastomere after which only an embryo carrying one mutant paternal allele was selected for subsequent implantation.
Despite the successful case presented herein, preimplantation genetic diagnosis remains challenging and costly, with an associated risk of misdiagnosis. In a recent survey of IVF clinics in the United States, 21% reported inconsistencies between the results of embryonic genetic analysis and subsequent genetic testing, which is why 96% of clinics surveyed recommended follow-up testing through amniocentesis or chorionic villus sampling. Causes of these errors include embryonic mosaicism, contaminating DNA, allele drop-out or preferential amplification, and mislabeling of samples in the clinic.17 Lack of post-natal blood testing is a limitation of our study, given the risk of errors in embryonic genetic analysis associated with preimplantation genetic diagnosis.
Additionally, although the couple in our case was fortunate enough to achieve a successful delivery after one cycle, in vitro fertilization often requires several cycles to achieve success, making it an expensive treatment option.18 According to one review of the results of IVF worldwide, the average pregnancy rates reported per aspiration for in vitro fertilization and intracytoplasmic sperm injection were 26.7% and 27.7%, respectively.19 However, the couple in our case was healthy, young, and without any history of reproductive challenges, seeking IVF and preimplantation genetic diagnosis only as a means of reducing the risk of having a child with Stargardt disease, whereas traditional applications of IVF and preimplantation genetic diagnosis have been in cases of couples with a history of reproductive challenges who seek these assistive technologies as a last resort for conception. Thus, it would be expected that the success rate in young, healthy couples like the one in our case who use these technologies for reducing disease risk in their offspring would be higher than in those who use these technologies after prior failed attempts at conception in order to have healthy deliveries.
The couple in our case report was aware of the risks associated with the procedure, but chose to undergo preimplantation genetic diagnosis because the male partner has been living with Stargardt disease and, given that his wife was a carrier of a mutation in the ABCA4 gene, they did not wish to pass this disease on to their child. The fetus was found to carry the paternal mutation along with an unaffected maternal allele, leading to the healthy delivery of an unaffected female. Without the use of preimplantation genetic diagnosis, the risk of their child having Stargardt disease would have been 50%. As such, we recommend that preimplantation genetic diagnosis be offered to those couples who are at risk of having a child with Stargardt disease as a way to potentially reduce the risk of having an affected child, particularly given the limited treatment options available once this disease is inherited. Here, we presented a successful case in which preimplantation genetic diagnosis led to the delivery of a healthy girl with only one mutant ABCA4 allele, thus unaffected by Stargardt disease.
Acknowledgements/Disclosures
a. Funding/Support: Supported by grants from The New York Community Trust (New York, NY), National Eye Institute/NIH EY015520 and EY013545 (Bethesda, MD), Foundation Fighting Blindness (Owings Mills, MD) and unrestricted funds from Research to Prevent Blindness (New York, NY). The funding organizations had no role in the design or conduct of this research.
e. Other Acknowledgements: None of the funding organizations has a public stance on prenatal diagnosis, in vitro fertilization, and/or selective implantation involving the discard of embryos.
Biography
 Mahsa A. Sohrab, B.A., is a fourth-year medical student at Columbia University College of Physicians and Surgeons and was a recipient of the 2008 Research to Prevent Blindness Medical Student grant, which funded her year of research at the Harkness Eye Institute between her third and fourth years of medical school. She plans on pursuing a career in ophthalmology and is continuing her work as a clinical investigator in Dr. Smith’s lab.
Mahsa A. Sohrab, B.A., is a fourth-year medical student at Columbia University College of Physicians and Surgeons and was a recipient of the 2008 Research to Prevent Blindness Medical Student grant, which funded her year of research at the Harkness Eye Institute between her third and fourth years of medical school. She plans on pursuing a career in ophthalmology and is continuing her work as a clinical investigator in Dr. Smith’s lab.
 R. Theodore Smith, M.D., Ph.D. is Associate Clinical Professor of Ophthalmology and Biomedical Engineering and director of the NIH supported Retinal Image Analysis Lab at Columbia. Dr. Smith is clinical investigator of the NEI-supported Columbia Macular Genetics Study.
R. Theodore Smith, M.D., Ph.D. is Associate Clinical Professor of Ophthalmology and Biomedical Engineering and director of the NIH supported Retinal Image Analysis Lab at Columbia. Dr. Smith is clinical investigator of the NEI-supported Columbia Macular Genetics Study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
b. Financial Disclosures: None.
d. Conformity with Author Information: All participants provided signed, informed consent for participation in the study and for the publication of the data obtained. Per Columbia University IRB guidelines, approval was waived since the case report involved less than 3 patients, and the official stance of the IRB on this issue has been provided as an attachment.
References
- 1.Anderson KL, Baird L, Lewis RA, et al. A YAC Contig Encompassing the Recessive Stargardt Disease Gene (STGD) on Chromosome 1p. Am J Hum Genet. 1995;57:1351–63. [PMC free article] [PubMed] [Google Scholar]
- 2.Westerfeld C, Mukai S. Stargardt’s Disease and the ABCR Gene. Seminars in Ophthalmology. 2008;23:59–65. doi: 10.1080/08820530701745249. [DOI] [PubMed] [Google Scholar]
- 3.Allikmets R, Singh N, Sun H, et al. A Photoreceptor Cell-Specific ATP-Binding Transporter Gene (ABCR) is Mutated in Recessive Stargardt Macular Dystrophy. Nat Genet. 1997;15:236–46. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 4.Allikmets R, Shroyer NF, Singh N, et al. Mutation of the Stargardt Disease Gene (ABCR) in Age-Related Macular Degeneration. Science. 1997;277:1805–7. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 5.Cremers FP, van de Pol DJ, van Driel M, et al. Autosomal Recessive Retinitis Pigmentosa and Cone-Rod Dystrophy Caused By Splice Site Mutations in the Stargardt’s Disease Gene ABCR. Hum Mol Genet. 1998;7:355–62. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- 6.Jaakson K, Zernant J, Kulm M, et al. Genotyping Microarray (Gene Chip) for the ABCR (ABCA4) Gene. Hum Mut. 2003;22:395–403. doi: 10.1002/humu.10263. [DOI] [PubMed] [Google Scholar]
- 7.Allikmets Rando. Stargardt Disease: From Gene Discovery to Therapy. In: Tombran-Tink J, Barnstable CJ, editors. Retinal Degenerations: Biology, Diagnostics, and Therapeutics. Humana Press, Inc.; Totowa, NJ: 2007. pp. 105–114. Tombran-Tink and Barnstable. [Google Scholar]
- 8.Sermon K, Steirteghem AV, Liebaers I. Preimplantation Genetic Diagnosis. Lancet. 2004;363:1633–41. doi: 10.1016/S0140-6736(04)16209-0. [DOI] [PubMed] [Google Scholar]
- 9.Xu K, Rosenwaks Z, Beaverson K, et al. Preimplantation Genetic Diagnosis for Retinoblastoma: The First Reported Liveborn. Am J Ophthalmol. 2004;137:18–23. doi: 10.1016/s0002-9394(03)00872-9. [DOI] [PubMed] [Google Scholar]
- 10.Morales R, Lledo B, Ortiz JA, et al. Preimplantation Genetic Diagnosis of X-Linked Retinoschisis Using Multiple Displacement Amplification (MDA) Reprod Biomed Online. 2008;16:886–92. doi: 10.1016/s1472-6483(10)60157-5. [DOI] [PubMed] [Google Scholar]
- 11.Rivera A, White K, Stöhr H, et al. A Comprehensive Survey of Sequence Variation in the ABCA4 (ACBR) Gene in Stargardt Disease and Age-Related Macular Degeneration. Am J Hum Genet. 2000;67:800–13. doi: 10.1086/303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonelli F, Testa F, Crecchio G, et al. New ABCR Mutations and Clinical Phenotype in Italian Patients with Stargardt Disease. Invest Ophthalmol Vis Sci. 2000;41:892–7. [PubMed] [Google Scholar]
- 13.Fumagalli A, Ferrari M, Soriani N, et al. Mutational scanning of the ABCR gene with double-gradient denaturing-gradient gel electrophoresis (DG-DGGE) in Italian Stargardt disease patients. Hum Genet. 2001;109:326–38. doi: 10.1007/s004390100583. [DOI] [PubMed] [Google Scholar]
- 14.Harper J, Sermon K, Geraedts J, et al. What next for preimplantation genetic screening? Human Reproduction. 2008:478–80. doi: 10.1093/humrep/dem424. [DOI] [PubMed] [Google Scholar]
- 15.Goossens V, Harton G, Moutou C, et al. ESHRE PGD Consortium data collection IX: cycles from January to December 2006 with pregnancy follow-up to October 2007. Human Reproduction. 2009:1–25. doi: 10.1093/humrep/dep059. [DOI] [PubMed] [Google Scholar]
- 16.Cideciyan AV, Swider M, Aleman TS, et al. ABCA4 disease progression and a proposed strategy for gene therapy. Human Molecular Genetics. 2009:931–41. doi: 10.1093/hmg/ddn421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baruch S, Kaufman D, Hudson K. Genetic testing of embryos: practices and perspectives of US in vitro fertilization clinics. American Society for Reproductive Medicine. 2008:1053–8. doi: 10.1016/j.fertnstert.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 18.Omurtag KR, Toth TL. The Cost Effectiveness and Health Outcomes of In Vitro Fertilization (IVF) as a Mandated Benefit. Fertility and Sterility. 2007;88:S122. [Google Scholar]
- 19.Adamson GD, de Mouzon J, Lancaster P, et al. World Collaborative Report on In Vitro Fertilization, 2000. Fertility and Sterility. 2006;85:1586–622. doi: 10.1016/j.fertnstert.2006.01.011. [DOI] [PubMed] [Google Scholar]