INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) has been successfully used in patients receiving myeloablative chemotherapy and irradiation for the treatment of a variety of hematological malignancies. Initially, dose intensification to induce a maximal anti-tumor effect was considered essential. Since allogeneic HSCT can be accompanied by harmful immunologic complications including graft-versus-host disease (GVHD) early speculation favored the use of autologous hematopoietic stem cells or stem cells derived from a homozygous twin as the best source of cells for transplantation. However, subsequent clinical studies illustrated that the positive counterbalance of GVHD was a decreased likelihood of relapse after transplantation, compensating for the immune complications of allogeneic HSCT, leading to better disease-free survival. Apparently, donor-derived immune effector cells, primarily T cells, were not only responsible for the development of GVHD but also for the reduced risk of relapse. As such, depletion of T cells from the graft to prevent GVHD resulted in an increased incidence of relapse after transplantation indicating that donor T cells were capable of mediating a graft-versus-leukemia/lymphoma (GVL) effect. Since transplantation with a T-cell replete graft from a homozygous twin did not result in GVL reactivity, it was concluded that the mere presence of T cells in the graft expressing a broad repertoire of specificities was not sufficient to mediate GVL reactivity but that alloreactive T cells per se were responsible for the anti-tumor effect. Further proof of the capacity of donor derived T cells to mediate a curative GVL effect came from the administration of donor lymphocytes to patients with recurrence of their malignancy after allogeneic HSCT. Donor lymphocyte infusion (DLI) as a treatment for recurrence of the malignant disease has resulted in 20-90% complete remissions depending on the malignancy. Chronic myeloid leukemia (CML) in chronic phase was found to be most susceptible to DLI but also patients treated for relapsed acute myeloid leukemia (AML), multiple myeloma (MM), chronic lymphocytic leukemia (CLL) or non-Hodgkin's lymphoma (NHL), and a few patients with acute lymphoblastic leukemia (ALL), showed clinically evident responses.
Although T cells mediate an anti-tumor effect following allogeneic HSCT, in HLA mismatched transplantations complete removal of T cells of donor origin did not result in full abrogation of the GVL effect. Alloreactive NK cells of donor origin present in the graft or developing from donor stem cells following transplantation were also found to be capable of mediating an anti-tumor effect. Thus, T cells and NK cells make use of the genetic disparity between donor and recipient, resulting in better disease-free survival in patients with high risk hematological malignancies. In addition, the role of donor anti-host antibodies developing after allogeneic HSCT may also play a role in anti-tumor effects and GVHD.
From these observations it can be concluded that the main advantage of allogeneic HSCT over autologous HSCT is the exploitation of the immune response to further eradicate the malignancy. This insight into the immunobiology of allogeneic HSCT has led to the development of strategies to reduce the intensity of the conditioning regimens to limit the toxicity of the transplant procedure. Reduced intensity conditioning using profound immunodepletion of the recipient instead of full myeloablation has greatly increased the applicability of allogeneic HSCT allowing treatment of elderly patients with limited transplant related morbidity and mortality. Reduced intensity conditioning has also changed the balance between donor and recipient cells following transplantation. This may change the balance between GVHD and GVL in favor of the anti-tumor response that can improve the outcome of allogeneic HSCT with a better quality of life.
To allow appropriate execution of the immune mediated anti-tumor effects, several immunological phenomena have to take place. First, immune cells have to be activated in vivo or in vitro leading to the appropriate production and expansion of T cells, NK cells or antibody-producing cells. Cells and allo-antibodies have to localize to the tumor sites and mediate effector mechanisms resulting in tumor destruction. Preferentially, following a contraction phase of the immune response, a memory response should develop capable of sustained anti-tumor control. In this report we will discuss several aspects known to play a role in the development and execution of the immune response, and also try to identify missing links in understanding the biology of the immune reaction. Insight in these reactivities will lead to more specifically directed immune responses following transplantation leading to increased anti-tumor reactivity with decreased immunological complications following allogeneic HSCT.
ADAPTIVE T-CELL IMMUNE RESPONSES
T-cell Phenotype: Naïve/Effector/Memory
Naïve T cells develop in vivo following an education and selection process in the thymus, and circulate and migrate to lymphatic tissues to be activated upon encountering their specific target antigen. Following activation, they expand and execute their function, and following a contraction phase, a subset of T cells becomes memory cells, ready to be activated upon renewed antigen exposure. During life, an expanding repertoire of antigen experienced T cells develops, depending on the exposure to foreign antigens. Thus, the donor T-cell repertoire infused into the patient will be heterogeneous and will depend on the immunological history of the donor. The donor T cells can be divided into naïve (TN; never antigen-activated), effector (TE; recently activated) and memory T cells (TM), which can be subsetted further into effector memory (TEM) and central memory cells (TCM)1,2. Due to the fact that the TM subset only contains T cells that have been activated in the past, the T cell receptor (TCR) repertoire of TM is more narrow than TN. However, since TM have a lower threshold for activation than TN, if the donor T cell repertoire contains TM that can recognize recipient tissues, these cells may be readily activated after infusion leading to an in vivo immune response. Donor T cells have diverse activation histories, which can impact on their ability to mediate GVL and GVHD. The T cell subsets differ in various ways, including how they traffic, become activated, expand, and function after infusion. These repertoire dependent and independent features of T-cell subsets impact on GVL mediated by polyclonal unmanipulated donor T cell infusions. TN, TEM and TCM cells can all potentially mediate GVL3-5, although it is likely that TEM are the least potent in curing tumors due to their reduced ability to expand after transfer4. It is not likely that simple separation of the T cell subsets before infusion will lead to induction of GVL without GVHD.
Separation of GVHD from GVL and improvement of effectivity and specificity of GVL may be obtained by in vitro selection or expansion of antigen specific T cells, or with genetically modified T cells with redirected specificities. For adoptive transfer of in vitro selected and expanded T cells with defined specificities, TCM cells may be ideal 6,7. Yet some studies indicate that TN rather than TCM cells are superior after gene modification and adoptive transfer8. Despite this progress in understanding T-cell biology, the relative contributions of TN or TM alloreactive T cells to controlling relapse after transplantation is unknown, and may depend on the repertoire of the donor, and the genetic disparity between donor and recipient.
Antigen presentation and Antigen Presenting Cells
In allogeneic HSCT, CD4+ and CD8+ T cells that mediate GVL must interact with MHC/peptide complexes present on leukemia target cells9-11. Since in the context of HLA matched transplantation most leukemia-reactive, minor histocompatibility antigen (mHA) specific T cells are TN. These T cells must likely be primed by professional antigen presenting cells (APC) to be appropriately activated in vivo, although the precise roles of APC subsets [dendritic cells (DC), macrophages, B cells] are not well defined. Therefore an effective GVL response may require that professional APC presenting the antigens expressed by leukemia cells or that the leukemic cells themselves acting as professional APC, as may be the case in CML, are present to provoke a T-cell response of a sufficient magnitude. Early after transplant, when there are residual conditioning regimen-resistant host APC, direct presentation of antigens derived from genes endogenously co-expressed by APC and the malignant cells likely provides a sufficient source of antigen to drive effective GVL; and data in mouse models support this hypothesis9,12. The kinetics of an appropriate mHA-reactive T-cell response show early activation, expansion, and then a decline, typically mimicking T cell responses in pathogen systems13,14. Since a maximal GVL effect may require a sustained response this may be achieved by repeated stimulation of initially activated cells, new recruitment of T cells directed against these specificities, or subsequent activation of T cells directed against other antigens.
If host hematopoiesis after transplantation is completely replaced by cells of donor origin, including donor-derived APC, and the immune response cannot be directly triggered by leukemic APC, the recipient (malignancy) associated antigens can not be endogenously presented by professional APC. Under those circumstances, donor T cell activation has to be triggered by host antigens that are cross-presented by donor APC15. Mouse models have demonstrated that indirect presentation occurs, although how effective this process is after transplant is less well studied10,15-17. Late onset GVHD in clinical allogeneic HSCT, when full donor chimerism has developed, may indicate that this also occurs in humans. If at this time, indirectly presented host antigens are derived from non-hematopoietic cells, it is possible that the host mHAs targeted may not be expressed on leukemia cells, leading to GVHD, but not GVL. If allo-immunity is directed against ubiquitously expressed host antigens both GVHD and GVL can be expected. This type of immune response may be sustained due to continuous antigen access for donor-derived APC; although the continuous host antigen/peptide exposure to donor T cells can also lead to trapping or consumption of alloreactive and potentially GVL-inducing T cells in tissues18 and T cell exhaustion19,20. To induce cross-presentation, activation signals are likely necessary. In mouse models, CD4 “help” by CD40L-expressing T cells, type I interferons (IFN) and Toll-like receptor (TLR) ligation all promote cross-presentation21-26. How to exploit these mechanisms in patients who relapse after transplantation is less clear. In a patient who has relapsed, the standard approach is to first withdraw immunosuppressive drugs, and if this is ineffective to administer DLI. These are both T cell directed interventions and little attention is paid to the APC component of the response. Possible strategies that would target the APC component could include adding vaccination against hematopoietically expressed host mHAs or stimuli that promote cross-presentation of host antigens by donor APC.
T cell Allo-activation (signal 1)
If allogeneic HSCT is performed over HLA barriers, a profound immune response is likely to occur. Allogeneic T-cell responses against HLA antigens are comprised of multiple responses directed against a variety of peptides presented in the context of non-self HLA molecules. These T-cell responses are caused by normal T cells educated to recognize foreign peptides in the context of self MHC, but are cross-reactive with unrelated antigens when presented in the context of non-self MHC molecules. As a consequence, these T-cell responses are present in both the TN and the TM compartments of the donors. Therefore, following HLA-mismatched HSCT, TM cells, capable of recognizing a variety of antigens presented in various tissues of the recipient, will be infused. Since TM cells can be activated in the absence of professional APC, severe GVHD is more likely to occur if patients are transplanted with HLA-mismatched donor grafts. On the other hand, if patients are transplanted with a donor graft from a very young individual or umbilical cord blood, the likelihood of large numbers of donor TM cells capable of recognizing allo-HLA antigens is more limited, and therefore grafts from umbilical cord blood or very young donors are less likely to cause this severe type of GVHD since the allo-HLA directed TN cells must be activated by professional APC. If allo-HLA responses develop, they will be directed against both normal and malignant target cells leading to both GVL and GVHD. After HLA-matched transplantation, the mHA presented and recognized in the context of self MHC are less likely to be previously encountered by donor T cells, and therefore professional APC with additional co-stimulatory molecules play a more crucial role in the initiation of the alloimmune response. If primary immune responses against hematopoiesis-associated host mHA (discussed in detail below) expressed by the APC develop, these T-cell responses may lead to separation of GVL and GVHD, whereas transplantation over HLA barriers are likely to cause immune responses leading to both GVHD and GVL reactivity.
T cell Allo-activation (Signal 2 and 3)
Co-stimulatory molecules that deliver a second signal to donor T cells can play pivotal roles in the donor TN cell activation required for GVHD induction. The most well-studied pathway, CD28/CTLA-4:B7 involves homologues that can deliver positive (CD28/B7) or negative (CTLA-4/B7) interactions. Specific blockade of the positive pathway reduces GVHD, whereas blockade of the inhibitory pathway increases GVHD lethality27. Other CD28 family members include inducible costimulator (ICOS) and the programmed death-1 (PD-1) pathways. ICOS is present on activated and TM cells, binds ICOS-ligand (a.k.a. B7h), and promotes TE cell responses28,29. Precluding ICOS signals on donor T cells diminishes donor T-cell mediated alloresponses, especially of the gastrointestinal tract and liver30,31, which proved to be CD4+ T cell-dependent disease in some32, but not in other studies33. Loss of ICOS signaling worsens CD8+ T-cell-mediated GVHD, as a consequence of increased expansion of donor CD8+ T cells33. PD-1, an inhibitor of activated T cells, also is expressed in the cytoplasm of CD4+CD25+ T regulatory (Treg) cells32. Blockade or absence of PD-1 on donor cells accelerates both CD4+- and CD8+-mediated GVHD34.
Members of the TNF receptor (TNFR) family function as costimulatory molecules and modulate GVHD and GVL. OX40 is present on both activated CD4+ and CD8+ T cells as well as on activated APC. Despite the presence of the receptor on both T cell populations, activation of OX40 has been reported to promote CD4+ but not CD8+ T cell-mediated GVHD35. CD40 ligand (CD40L) is expressed on activated CD4+ T cells, and CD40:CD40L interaction increases acute GVHD36 by promoting CD4+ T cell-mediated tissue destruction and CD8+ T cell expansion37. All four of the aforementioned costimulatory pathway members (CD28, ICOS, OX40, and CD40L) act independently, as inhibition of any single pathway does not eliminate GVHD, and co-blockade results in greater protection38,39. Two other TNFR members include 4-1BB and glucocorticoid-induced tumor necrosis factor receptor (GITR). 4-1BB is expressed on activated CD4+ and CD8+ T cells and on NK cells40,41. Inhibition of 4-1BB binding to 4-1BB ligand reduces CD8+ T cell-mediated GVHD lethality and CD4+ Th1 generation in GVHD42,43. GITR is expressed constitutively on CD4+CD25+ Treg cells and activated CD4+CD25− and CD8+CD25− T cells44. Stimulating GITR on Treg cells or removal of GITR+ cells reverses suppression, leading to the development of autoimmune disease44. GITR stimulation on CD4+CD25− T cells reduced GVHD in MHC II-disparate recipients, whereas stimulation of GITR of CD8+CD25− T cells increased proliferation and GVHD in a MHC I-disparate murine model45.
In addition to signal 1 and 2, activation of naive CD8+ T cells to undergo clonal expansion and develop effector function requires a third signal that can be delivered by IL-12, type I interferon (IFN), or adjuvant. The third signal, most important when antigen levels are low46, results in the upregulation of bcl347 and granzyme B expression48. Even at high antigen levels, the third signal, while not needed for proliferation, is necessary for the full development of cytolytic effector function46. The third signal is critical in determining whether stimulation by antigen results in tolerance versus development of effector function and establishment of a responsive memory population. Prolonged exposure to antigen and costimulation, along with a third signal are required for CD8+ T cell clonal expansion. Importantly signal 3 availability at the tumor site can influence CD8+ T cell responses to a solid tumor49.
T-Cell Trafficking
Manipulation of T cell trafficking may be an attractive strategy to separate GVL and GVHD. Trafficking and migration involve a complex series of events mediated by integrins, chemokine receptors, and selectins on the surface of lymphocytes and the interaction of these molecules with cognate extracellular ligands. Selectins (addressins) affecting lymphocyte migration may also be expressed on endothelial cells of lymphoid and target tissues50. The biologic roles of homing molecules can be pleiotropic, for example, contributing to the binding of T cells and APC at the immunological synapse51.
The CC-chemokine receptor-7 (CCR7) is thought to play an important role in the entry of naïve lymphocytes into secondary lymphoid organs prior to their activation by APC, after which it is down-regulated as activated T cells migrate to target tissues to execute effector functions52,53. Individual selectins, integrins, and chemokines/chemokine receptors have all been implicated in the pathogenesis of GVHD50.
Chemokine receptor CCR2 knockout donor T cells and β7 integrin knockout donor T cells have both demonstrated decreased homing to the liver and GI tract and decreased GVHD, but intact GVL responses54-56. A humanized antibody to the α4 subunit of certain integrin heterodimers, natalizumab, has been tested for use in inflammatory bowel disease and multiple sclerosis57-61. Heterodimers with the α4 subunit are important for homing to Peyer's patches and gut mucosa, and may thus be useful for blocking migration of activated T cells to GVHD target sites while still permitting activation of GVL effectors in lymphoid tissue62. This hypothesis remains to be tested, but it is supported by the knowledge that the α4 subunit forms heterodimers with the β7 subunit described above. Broad questions remain regarding the function of homing molecules and T cell trafficking during GVL, but an assessment of the homing molecules implicated in GVHD presents at least an initial road map50. Such studies are necessary to distinguish molecules critical for GVL from those that may be blocked to prevent GVHD without impairing GVL.
T-Cell Cytolysis
Cytotoxic T cell lymphocytes (CTL) execute their function via use of the perforin/granzyme system and death receptor ligands [FasL, tumor necrosis factor (TNF), TNF-related apoptosis inducing ligand (TRAIL), tumor necrosis factor-like weak inducer of apoptosis (TWEAK)], which utilize the target cell's own machinery to initiate apoptosis63. As with mechanisms of lymphocyte homing, the role of cytotoxicity has been studied in greater detail in GVHD than in GVL. Most studies have emphasized preservation of GVL cytotoxicity while suppressing GVHD. Studies with patient samples have demonstrated varying levels of leukemic sensitivity to FasL and perforin, the two classic cytotoxic effector molecules64.
Murine models have suggested the perforin pathway is important for GVL, whereas FasL appears to have a greater role in GVHD63. However, both molecules have been implicated in both GVHD and GVL63,65,66. TNFα, which plays a central role in GVHD pathogenesis, has also been implicated in GVL, although it is possible that the GVL effect of TNFα may be related to an effect on donor T cells rather than on host neoplastic cells10,63,67. The role of TWEAK in GVL is unknown, however TRAIL may have a role limited to GVL without potential for induction of GVHD63.
THE ROLE OF MINOR HISTOCOMPATIBILITY ANTIGENS IN THE GVL EFFECT
Minor Histocompatibility Antigens
GVHD and GVL activity occur in the majority of allogeneic HSCT recipients who receive T-replete grafts and/or DLI from genotypically MHC-matched donors. Thus, GVHD and GVL in this setting are triggered by donor-recipient genetic disparity at polymorphic loci outside the MHC, which are consequently referred to as minor histocompatibility loci. Studies in both mice and humans have demonstrated that most minor histocompatibility loci encode short peptides, termed minor histocompatibility antigens (mHAs), which are encoded by polymorphic genes. These are presented on the cell surface by MHC class I and II molecules, where they can be recognized by donor CD8+ and CD4+ T cells, respectively. CD8+ and CD4+ T cell clones specific for recipient mHAs can be isolated from most HSCT recipients of T-replete grafts or after DLI.
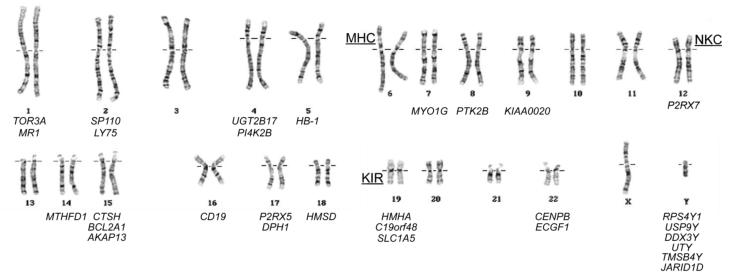
Molecular characterization of mHAs recognized by T cells after MHC-matched HSCT has demonstrated that the genetic loci encoding mHAs can be divided into two broad categories based on their chromosomal location (Figure 1). The first category comprises a small class of Y chromosome genes that encode male-specific mHAs (H-Y antigens), which are targets for female T cells in sex-mismatched transplants. The genes encoding all of the known H-Y antigens have X chromosome homologues that encode proteins that are 81-99% identical in sequence with the Y chromosome-encoded isoforms, and are expressed both in and outside the testis. The extensive sequence disparity between the Y and X chromosome-encoded isoforms creates a large number of male-specific peptides that could potentially serve as targets for female T cells in MHC-matched transplants. The immunologic significance of H-Y antigens is enhanced by the fact that the Y chromosome is inherited as a single functional genetic element, and therefore all H-Y antigens are in complete linkage disequilibrium. At least twelve H-Y antigens encoded by six different Y chromosome genes have been identified to date, and many more remain to be identified.
Figure 1. Chromosomal distribution of the 30 genes known to encode mHAs.
The chromosomal locations of the Major Histocompatibility Complex (MHC), Natural Killer Complex (NKC), and Killer-cell Immunoglobulin-like Receptor (KIR) locus are also indicated.
A heterogeneous group of polymorphic autosomal loci widely distributed throughout the genome comprise the second major class of mHA-encoding genes (Figure 1). Although some of these loci encode well-characterized proteins with known functions, there are several whose protein products are as yet completely uncharacterized. The polymorphism in autosomal minor histocompatibility loci is generated by variation in human genome sequences, which creates most of the autosomal mHAs identified to date. Variation in genome structure, which creates at least four known mHAs, likely creates other mHAs that have yet to be characterized. Nonsynonymous single nucleotide polymorphisms (SNPs) in the coding sequence of normal self proteins that create amino acid polymorphisms in the sequence of these proteins create most of the known autosomal mHAs. Such polymorphisms can alter how peptides are generated from self-proteins inside the proteasome. Peptides are then transported into the endoplasmic reticulum by the transporter associated with antigen processing, bind to MHC molecules, or are recognized by T cells when presented on the cell surface in a complex with MHC. Both mHAs encoded within normal, as well as alternative open reading frames are frequently found68,69. Alternative mRNA splicing due to SNPs in the introns of protein-coding genes70 and single nucleotide deletions leading to frameshift polymorphisms71 can also create mHAs. Large-scale variation in the human genome structure creates mHAs through deletion of entire protein-coding sequences. A common deletion polymorphism on chromosome 4q that spans 117 kb and includes the entire UGT2B17 locus creates at least three different mHAs72-74.
Expression of mHAs in Normal and Malignant Tissues
Evaluation of the expression of mHAs in normal and malignant tissues has provided insight into the potential contributions of mHA-specific T-cell responses to GVL activity. Many mHAs are expressed in both hematopoietic and nonhematopoietic cells in vitro, suggesting that T-cell responses to these antigens could contribute to both GVL and GVHD. However, a significant fraction of mHAs show expression that is limited to cells of hematopoietic origin, raising the prospect that T-cell responses against these mHAs might contribute selectively to GVL activity. Since HLA class II molecules under most conditions are preferentially expressed on hematopoietic as compared with nonhematopoietic cells, HLA-class II restricted mHA have a higher likelihood of being selectively expressed by malignant hematopoietic cells75-77. CD4+ and CD8+ T cell clones specific for mHAs that are selectively expressed in hematopoietic cells recognize lymphoid and myeloid leukemic cells in vitro and inhibit the growth of clonogenic leukemic cells in in vitro culture78,79. CD8+ mHA-specific CTL clones also inhibit the engraftment of human acute leukemia into NOD/SCID mice, demonstrating that leukemic stem cells80,81 express mHAs and can be targeted by mHA-specific CTL82,83, which can be active against established hematopoietic tumors in vivo 84.
Contributions of mHA-specific T-cell Responses to GVL
Cellular and molecular dissection of immune responses occurring in recipients after HSCT or DLI has provided compelling evidence that both autosomal and H-Y mHAs expressed on recipient tumor cells are targets of GVL responses in MHC-matched HSCT. Flow cytometric analysis with mHA peptide/MHC tetramers has shown that mHA-specific T cells with in vitro reactivity against recipient leukemic cells increase in frequency in peripheral blood before and during clinical regression of malignancy68,69,71,85,86. Analysis of the T-cell repertoire in patients with persistent or recurrent disease who responded to DLI showed that the T cell response associated with GVL is polyclonal and likely directed at a significant number of distinct antigens87. Comprehensive application of single-cell cloning has directly confirmed this hypothesis and demonstrated that GVL reactions involving T cells with diverse antigen specificity occur in patients with CML88,89 and CLL90.
Retrospective analyses of transplant outcome have attempted to evaluate the contribution of mHA-specific T cells to GVL activity by correlating donor-recipient disparity at specific minor histocompatibility loci with post-transplant relapse or remission of disease. Studies that focused on disparity at autosomal mHAs have had inconsistent results, but the largest such studies showed no significant correlation between donor-recipient disparity at specific loci with tumor control after HSCT. In contrast, several large retrospective analyses have identified a significantly lower rate of relapse in male recipients of female hematopoietic cell grafts (F→M HSCT) than in any other donor/recipient gender combination, suggesting that T cell responses to H-Y antigens have a clinically measurable anti-leukemic effect91,92. Thus, T cell responses against multiple H-Y antigens are thus likely to be elicited in any F→M HSCT recipient.
Human Genomic Diversity and the Number of mHAs
At least thirty different autosomal and Y chromosome genes have been shown to encode mHAs (Figure 1), but it is likely that many more mHAs remain to be identified. The human genome is characterized by an enormous quantity of sequence and structural variation. To date more than 150,000 SNPs that cause amino acid polymorphisms in the sequence of known or predicted proteins (www.hapmap.org), and common deletion polymorphisms that span several dozen known and predicted genes have been identified. Thus, there are a very large number of polymorphic protein and peptide sequences that, in the setting of MHC-matched allogeneic HSCT, could potentially encode mHAs. Recognition of the enormous diversity in the human genome demonstrates that there is abundant polymorphism that could potentially be exploited to enhance GVL activity after MHC-matched HSCT, but also suggests that whole-genome approaches to understanding GVL will likely provide the key to its successful exploitation.
T-cell Responses to Nonpolymorphic Antigens
In addition to its direct anti-tumor action, the mHA-specific T-cell response that occurs in recipients of allogeneic HSCT can enable and recruit anti-tumor T-cell responses to nonpolymorphic antigens that are derived from normal self proteins and are over or aberrantly expressed in recipient tumor cells. CD8+ T cells specific for the HLA-A*0201-restricted PR1 peptide that is contained within both proteinase-3 and elastase, the major constituents of the primary azurophil granules of normal promyelocytes as well as AML and CML blasts, can be detected in the blood of many HLA-A*0201+ CML patients who achieve remission after MHC-matched HSCT93,94. PR1-specific CTL recognize myeloid leukemia cells in vitro95 and inhibit the growth of CML colony-forming units96. The protein product of the WT1 gene is also a target of spontaneous post-transplant T-cell responses that could contribute to GVL activity against myeloid and lymphoid leukemias. CD8+ HLA-A*0201-restricted responses to WT1 have been observed in HLA-A*0201+ ALL patients after HSCT and their appearance was correlated with clearance of molecular markers of residual disease97.
Increasing evidence also suggests that donor T-cell responses against recipient tumor-specific antigens frequently occur in allogeneic HSCT recipients and could make significant contributions to GVL activity. Regression of multiple myeloma in several patients who received DLI with CD4+ cells was associated with the appearance and selective expansion of myeloma-specific CD8+ T cells98. Antigens encoded by cancer-testis (C-T) genes including NY-ESO-1 and the MAGE and SSX gene families are often expressed in advanced or poor-prognosis myeloma, and B- and T-cell responses to these C-T antigens are preferentially detected in patients who have undergone allogeneic HSCT99. CML cells express several tumor-specific antigens that can elicit T-cell responses in cancer patients, including BCR-ABL junctional peptides, and BCRABL-specific T cells have been detected in CML patients both before and after HSCT94. In recent studies remission of CLL after nonmyeloablative MHC-matched HSCT was closely associated with appearance of tumor-specific CD8+ T cells90,100.
Role of HLA-DP Mismatch in Unrelated Donor Transplantation
Recent genetic and clinical studies suggest that donor/recipient mismatch at loci within the classical MHC, most notably at HLA-DP, may play a uniquely important role in preventing or treating relapse after transplantation from unrelated donors101. High-density SNP genotyping of 1,500 HSCT donor/recipient pairs at the Fred Hutchinson Cancer Research Center in Seattle has shown that sibling donor/recipient pairs who are identical at the HLA-A, -B, and -DR loci are invariably also identical at HLA-C, HLA-DQ, and HLA-DP, and in fact are identical throughout the entire ~4 Mb region encompassing the MHC. This is presumably due to inheritance of the same two copies of chromosome 6 from their parents (S. Li and E.H. Warren, in preparation). However, the same is not true for unrelated donor/recipient pairs, even those who are matched at the DNA sequence level at the HLA-A, HLA-B, and HLA-DR loci. Significant genetic nonidentity within the classical MHC exists within unrelated donor/recipient pairs who are otherwise identical at the DNA sequence at the HLA-A, -B, and -DR, loci, and this nonidentity is particularly prominent at the HLA-DP locus (S. Li and E.H. Warren, in preparation). Donor/recipient mismatch for HLA-DP represents a major histocompatibility mismatch that would be expected to stimulate a polyclonal HLA-DP-restricted, donor anti-recipient T-cell response in most, if not all, mismatched pairs, and an HLA-DP-restricted response could potentially have a significant anti-tumor effect101,102. Two retrospective analyses collectively including more than 10,000 unrelated donor transplants facilitated by the National Marrow Donor Program and the Japan Marrow Donor Program have, in fact, confirmed that donor/recipient mismatch at HLA-DP is associated with a statistically significant decrease in the rate of post-transplant relapse103,104. A similar effect associated with donor/recipient mismatch at HLA-C was also identified, but the clinical significance of HLA-C mismatch is not as great as that as that associated with HLA-DP mismatch because it is observed in far fewer unrelated donor/recipient pairs (S. Li and E.H. Warren, in preparation).
In conclusion, genetic disparity between donor and recipient is the key to the therapeutic efficacy of HSCT but is also the root of GVHD, its primary limitation. Cellular and molecular dissection of GVL responses have demonstrated that GVL after MHC-matched HSCT is initiated by donor CD8+ and CD4+ T cells that recognize recipient mHAs encoded by polymorphic genes. This alloresponse likely recruits additional effector cells recognizing tumor-specific or tumor-associated antigens encoded by nonpolymorphic genes that are over or aberrantly expressed in recipient tumor cells. The GVL response in most HSCT recipients is therefore likely to comprise a broadly focused immune response directed at a large number of polymorphic and nonpolymorphic target antigens. Deeper understanding of the breadth and the antigenic specificity of the GVL response will be required to exploit it successfully. Selective expression of some GVL target antigens in recipient hematopoietic cells and recipient tumor cells, with limited expression in nonhematopoietic tissues, may allow the development of therapeutic strategies for enhancing GVL activity without inducing or aggravating GVHD. Characterization of the extensive sequence and structural variation in the human genome suggests that there are likely to be a large number of polymorphic protein and peptide sequences at which immunotherapy to augment GVL could potentially be directed.
IMMUNE CONTRACTION AND SUPPRESSION
Survival of the Organism from a Potentially Overzealous Immune Response
Analogous to our understanding of tumoriogenesis in which the initial emphasis on oncogenes became supplanted with the characterization of tumor suppressors as critical checkpoints that need to be over-ridden, there has been an increasing realization that merely stimulating the immune system in cancer will not be sufficient. Immune responses to any stimuli eventually undergo a contraction phase and also induce potent suppressive pathways, as biologic responses are geared to end. Immunotherapy in cancer has shown great promise but has been lacking with sustained responses and overall survival being impacted. In part, this can be attributed to contraction and suppression pathways inherent to the immune response itself and in part due to manipulation of the suppressive pathways by the tumor. It has become clear that either augmenting the immune response in cancer or providing adoptive immunotherapy will need to overcome these hurdles in order to maintain the response. This represents a key pathway that, despite the successful generation of anti-tumor effectors will limit responses.
Naive T-cell Expansion, Differentiation, Contraction and Memory T-cell Generation
Whereas cytoreductive anti-tumor therapies can reduce disease burden, residual tumor cells must be eradicated or held in check by the immune system. The most compelling evidence for a T-cell mediated immune mediated anti-tumor response can be derived from the adoptive transfer of allogeneic donor lymphocytes used to treat leukemia recurrence following transplantation105. Assays for minimal residual disease in CML have shown a gradual reduction in tumor burden, often requiring months to years to achieve the full biological effect. These data are consistent with a critical threshold number of anti-tumor reactive T cells that is needed to achieve and sustain an anti-leukemia effect.
Both TN and TM cells can contribute to anti-tumor responses. With age and repetitive radiation or chemotherapy courses, fewer näive T-cells are produced106. Under those conditions, TM cells may be the dominant anti-tumor reactive T cell population. Naive T cells encountering immunogeneic tumor peptides or antigens can be skewed toward an anti-leukemia response, especially during periods of lymphopenia induced by cytoreductive therapies. This results in cytokine accumulation (e.g. IL-7, IL-15) and substantial T-cell homeostatic expansion and differentiation of naive T cells into TE cells that reduce tumor burden107. Typically within the first week, a second and profound contraction phase occurs due to activation-induced cell death, cytokine consumption, and/or inhibitory mechanisms (e.g. exposure to IFN-gamma or TGFβ immunosuppressive cytokines, upregulation of CTLA-4 or PD-1 inhibitory coreceptors or TRAIL or Fas pathway death receptors). Following the contraction phase, a small subset of antigen-reactive TEM, TCM, and putative memory stem cells survive, each of which may retain an anti-tumor response capacity. In the presence of CD4+ T-cell help, post-homeostatic proliferation-induced memory-like cells are produced which can have similar responses to conventional CD8+ TM cells108 The precise mechanisms by which CD4+ T cells maintain CD8+ T cell memory are still poorly understood and this may impact the efficacy of CD8+ T cell transfer.
If tumor antigen levels are sufficient to trigger their TCR, memory cells may receive survival signals that promote their persistence and function, thereby permitting further reductions in tumor burden and continued immune surveillance. If antigen levels are too high, T cells may become exhausted and are known to express inhibitory molecules such as PD-1, which upon engagement by PD-1 ligand (PD-L) render antigen-reactive T cells hyporesponsive in terms of proliferation, cytokine production, and killing capacity20. If antigen levels are too low, these memory populations would become quiescent until they are re-engaged by antigens that trigger their TCR and sufficient adjuvant signals such as cytokines or costimulatory pathway receptors that can re-awaken cells to proliferate and lyse tumor cells.
The Tumor Microenvironment
A key factor that regulates the size and activation status of the memory cell pool is tissue location. Anti-tumor reactive TM cells may reside in secondary lymphoid organs, parenchymal organs, bone marrow, or the tumor microenvironment itself. Some but not all of these environments are conducive to an anti-tumor response by providing positive signals (e.g. costimulation, stimulatory cytokines) and only a limited number of inhibitory signals or cell populations that restrain memory cell activation, expansion or acquisition of lytic function. Another key factor in the naive and memory T-cell response is the capacity of anti-tumor reactive T cell to migrate to the tumor site. Therefore, T-cell populations not residing at the tumor site and lacking necessary addressins, selectins or chemokine receptors for migration to tumor site are unlikely to substantively contribute to the immune response.
The immune environment in which TN or TM cells reside may suppress the anti-tumor immune response by affecting T-cell activation, proliferation, pro- or anti-inflammatory cytokine expression, cytolytic molecule expression, pro- or anti-apoptotic molecule expression or the regulation of receptors involved in migration. The size and activation status of the TM cell compartment is determined by the balance between positive and negative signals delivered to the memory T-cell. The positive and negative immune pathways work in concert but suppression tends to trump over stimulation. The lack of the stimulatory factors may also predispose the immune cells to the suppressive pathways. These suppressive pathways may predominate even more in the aged individual which may significantly limit immune interventions in this population. Typically, the tumor highjacks its microenvironment to foster tumor growth and discourage a productive immune response. Tumor cells such as those seen in Hodgkin's lymphoma cells can produce immunosuppressive cytokines such as IL-10 or TGFβ that suppress T-cell proliferation or induce Tregs that constrain TE-cell responses in the tumor microenvironment109. Tumor cells can actively attack the attackers by expressing fasL and PD-L110.
Tumor cells may express high levels of the intracellular tryptophan catabolic pathway, indoleamine 2,3 dioxygenase (IDO), as has been demonstrated for AML cells111 In addition, CD11c+CD19+ plasmacytoid dendritic cells (pDC) present in draining lymph nodes or the tumor microenvironment of rodents and humans can express high levels of IDO, which may be upregulated by tumor cells (e.g. melanoma, breast cancer), Toll-like receptor-9 (TLR-9) ligation from exposure to bacteria products, or the T-cell response itself via the elaboration of IFN-gamma or surface expression of CTLA-4112. High IDO levels act in the local microenvironment to stimulate integrated stress response pathways in T cells activated by amino acid starvation resulting in cell cycle arrest or apoptosis. In rodents, IDO expression in pDC has been shown to augment mature Tregs and upon TLR-9 ligation blocks the conversion of Tregs into Th17-like cells113, while in humans, TLR-9 activated pDC can convert naive T cells into Tregs114.
Tumors can cause T-cell dysfunction by altering TCR signaling mechanisms115. Depletion of the amino acid L-arginine by cytoplasmic arginase I profoundly inhibits CD4+ and CD8+ T-cell functions116. L-arginine depletion is associated with the down-regulation of the TCR zeta chain in T cells that infiltrate in the tumor microenvironment. Arginase I is expressed at high levels in myeloid-derived suppressor cells (MDSC) that infiltrate the tumor microenvironment116. Murine MDSC have been shown to have an increased uptake of L-arginine due to high levels of a cationic amino acid transporter, 2B117. In addition, prostaglandin-E2 is synthesized by MDSC, which most likely contributes to immune suppression in the tumor microenvironment118. In humans, MDSC are CD34+CD33+CD13+, indicative of a myeloid lineage119. In mice, Gr-1lowCD11bhighLy-6ChighSSClow and Gr-1highCD11blow cell fractions possessed suppressive potential119. The latter cells co-expressed F4/80, high levels of Ly6C, and low levels of CSF-1 receptor (CD115). Suppression was observed, albeit at as lesser potency on a cell-by-cell basis for Gr-1hiCD11bhi cells. IFN-gamma or lipopolysaccharide not only activates MDSC, but suppresses the differentiation of DC from bone marrow myeloid progenitors. Therefore, an inflammatory response to tumor cells can result in the generation of MDSC in the tumor microenvironment, offsetting the host anti-tumor immune response. Intriguingly, in patients with renal cell carcinoma, IL-2 therapy markedly increased MDSC percentages and arginase I levels120. Thus, IL-2 has a dual effect on the immune response by increasing TE cells and NK cells as well as Tregs and MDSC. MDSC also can suppress T-cell function via other mechanisms such as the elaboration of nitric oxide or regulation of CD80 and PD-L expression. Vascular endothelial cell growth factor (VEGF), known to be produced by almost all tumor cells, has been shown to inhibit the development of DC from the bone marrow, which was associated with an increase in immature, immune suppressive Gr1+ myeloid cells121. In some types of cancer patients, immature DC in fact are increased in the peripheral blood of patients.
A functional state of tumor-mediated suppression may occur as a result of deviation of the immune response toward a non-favorable TE cell phenotype (e.g. Tregs type I; anti-inflammatory cytokine producing Th2 cells; TGFβ-secreting Th3 cells). Alternatively, tumor cells may inhibit antigen recognition by a variety of mechanisms. For example, some tumors down-regulate major histocompatibility complex antigens (e.g. MHC class I in neuroblastoma cells) or potentially immunodominant antigens (e.g. EBV nuclear antigens, 3A, B, C in Hodgkin's disease). Tumor cells can also secrete “decoy” molecules such as soluble MICA which can inhibit NKG2D-mediated killing by NK cells. Defective APC function has been reported in cancer patients as a consequence of low expression of costimulatory molecules (e.g. B7 ligands), exposure to high levels of IL-10 that result in tolerogenic DC, or high expression of inhibitory co-receptors (e.g. PD-L).
Therapeutic Strategies to Overcome Immune Contraction and Suppression
Bypassing activation-induced cell death (AICD) is critical for sustaining activated immune cell engraftment and function. However, many cytokines and pathways can exert opposing effects. IFN-gamma is a major mediator that is critical for many anti-tumor effector functions as well as a major mediator limiting T-cell responses, particularly CD4+ T cells via AICD122. During T-cell activation, CD25 (IL-2Rα chain) is upregulated followed by the down-regulation of CD127 (IL-7Rα chain) at the time of TE cell to effector/memory transition. As such, it is reasonable to consider the use of IL-2 or IL-7 to expand TE cells. However, the biology of IL-7 and other cytokines such as IL-15 is complicated due to the effects of presentation of the cytokine by DC's potentially yielding opposing effects on the T cell123. Moreover, the consumption of cytokines that can occur during the expansion phase, particularly following lymphopenia and homeostatic expansion, may result in a relative deficiency of cytokines to support activated TE cells. Exogenous IL-2 administration has been shown to increase TE cells as well as NK cells and Tregs. Of note, high doses of IL-2 can cause AICD of activated T cells, and IL-2 withdrawal can cause cytokine-deprivation induced apoptosis. Therefore the available levels of IL-2 for IL-2R engagement are critical in determining the ultimate T cell fate. Recent data suggest that the binding of IL-2 with an antibody can result in supraphysiologic effects in vivo and even bypass the need of NK cells for IL-15124,125. In rodents, IL-7, the second member of the IL-2R common gamma chain family of cytokines, has been shown to be a critical growth factor for CD8+ TE and TM cell survival. In humans, exogenous IL-7 supported a sustained increase in peripheral blood CD4+ and CD8+ T cells with broadening of the TCR repertoire diversity, induced T cell cycling and anti-apoptotic protein (bcl2) upregulation, and expanded TN cells, with a proportional diminution in Treg frequency126. Since IL-7 was well-tolerated in this first in-human study, IL-7 administration may prove especially useful in situations in which IL-7 levels are limiting such as following homeostatic expansion during the resolving lymphopenia phase.
IL-15, the third member of the IL-2R common gamma chain family of cytokines, has been shown to be essential for memory cell survival. In vivo studies in rodent and non-human primates have demonstrated the capacity of IL-15 administration to facilitate T-cell survival, especially antigen-specific central memory T cells. Studies of human T cells indicate that IL-15 favors the in vitro generation of TCM versus TE cells and also appears to be critical for NK cell survival. IL-15 is being readied for first-in-human testing in the near future. However, the “trans” presentation that IL-15 appears to require for optimal effects suggests that simple administration of the cytokine may not be sufficient127. IL-21, the fourth member of this family that is closely related to IL-2, also promotes the function of CD8+ TE cells128. In contrast to IL-2, IL-21 inhibits the maturation of CD8+ TN cells into granzyme B- and CD44-expressing effector CD8+ CTL129. At the same time, IL-21 increased the anti-tumor regressive capacity of adoptively transferred CTL. These data are consistent with the paradigm that cytokines, such as IL-21, which limit both the stage of TE cell differentiation and preserve the expression of key cell surface homing receptors such as L-selectin. Thus, IL-7, IL-15 and IL-21 are new candidate cytokines that may favor the persistence of TM cells with improved anti-tumor properties, as compared to IL-2. New approaches for cytokine delivery, such as cytokine-anti-cytokine antibody complexes may provide a superior methodology to achieve more pronounced in vivo biological effects by increasing their otherwise short serum half-life.
Thus far, the success of bypassing negative regulators (e.g. CTLA-4 or PD-1 pathway blockade for T cells, killer inhibitory receptor blockade for NK cells) may result in augmented anti-tumor effects but may also result in possible auto-reactive toxicities. For example, antibodies directed against CTLA-4 have shown promising results in clinical trials in augmenting endogenous anti-tumor reactive T cells while at the same time inducing autoimmune-mediated destruction of non-tumor cells in some patients130. Anti-PD-1 and anti-PD-L1 antibodies are in clinical trials in cancer patients; anti-PD-L1 antibody may be particularly useful in preventing the T-cell exhaustion state that chronic antigen activation may cause20. However, in vivo studies have suggested that targeting multiple members of the PD1/PD-L family may be needed for optimal effects. Agonistic antibodies against costimulatory pathway members (e.g. CD40/CD40L, OX40/OX40L, 4-1BB/4-1BBL) alone or combined with blockade of inhibitory co-receptors may prove to be particularly efficacious in patients with both APC and T-cell dysfunction.
A chemical inhibitor of the IDO pathway (1-methyl-tryptophan) is being studied in phase I trials in cancer patients. COX-2 inhibitors have been used successfully, especially in patients with solid tumors, to reverse PGE2-mediated suppression and the frequency of inducible Tregs that can occur as a result of the tumor microenvironment131. MDSC-mediated inhibition of anti-tumor T-cell or NK cell immune responses may be overcome by sunitinib malate132, a receptor kinase inhibitor, TGFβ neutralization133, modulators of arginine metabolism (see above) or nitric-oxide synthase inhibitor [e.g. N(G) nitro-L-arginine methyl ester (L-NAME)]134. Anti-VEGF antibody in rodent but not human studies decreases suppressive DC numbers120,121.
Cellular depletion approaches such as those targeting Tregs (e.g. IL-2 diphtheria toxin, a.k.a. denileukin diftitox or Ontak®) have been variably efficacious in augmenting endogenous T-cell responses by depleting Tregs. The transfer of ex vivo expanded and activated anti-tumor reactive T cells, including those that are forced to express an anti-tumor reactive TCR by gene transfer, have been reported to be efficacious in patients with melanoma, generally an immune responsive disease135. Rodent studies have indicated that combined in vivo Treg depletion with the adoptive transfer of syngeneic CTL136 may be a useful strategy that circumvents the in vivo dysfunction of anti-tumor reactive T-cells (e.g. TCR zeta chain down-modulation) and inhibits the suppression conferred by Tregs.
The role of CD4+ T cells and the ratio of their subsets may be critical in maintaining CD8+ T-cell responses (survival and function) also needs to be explored. More attempts at combination approaches need to be undertaken. Recent data also suggests that activated DC may do more than simply initiate the adaptive response but may also play a role in maintaining antigen-specific CD8+ T-cell function and survival137. These data indicate that cytoreductive conditioning in HSCT may do much more than simply promote T-cell expansion via homeostasis but instead activates DC via TLR. Understanding the mechanisms involved may have a profound impact on the use of HSCT and adoptive immunotherapy. Finally, it has been shown in numerous models that peripheral immune readouts may have no bearing on anti-tumor responses. There needs to be studies that understand how normal contraction processes occur within any attempt to stimulate or provide immune responses. AICD coupled with immune suppression and deprivation of supportive cells and cytokines may prematurely diminish responses and make the host resistant to further attempts at stimulation. Targeting immune responses to the tumor site and, importantly, assessing immune effects within the tumor itself may offer the best indicators of success as well as limit systemic toxicities. Understanding normal immune contractions/suppression and how the tumor can further subvert it is critical if the immune system is to be applied in cancer therapies.
ADAPTIVE B-CELL IMMUNE RESPONSES IN GVL
The role of B cells and Antibodies in GVL
Evidence that B cells contribute to tumor immunity after allogeneic HSCT comes primarily from studies that have attempted to characterize immunologic events in patients with documented effective GVL responses in vivo. For example, Wu et al. studied patients with relapsed CML who responded to DLI138. Antibodies reactive with CML cells were identified after DLI response, and specific protein targets were identified using serological analysis of recombinant cDNA expression libraries (SEREX). Several target proteins were characterized in greater detail, and it was further shown that the generation of antibodies specific for these targets coincided with the disappearance of CML cells in vivo. Similar antibodies were not present before transplant, before DLI, or in patients who developed chronic GVHD after transplant. Antibodies to these CML-associated antigens were also not present in healthy donors or in patients who did not respond to DLI. Further studies demonstrated that several of these target antigens were preferentially expressed in both normal and CML progenitor cells139.
Antibody responses to tumor-associated antigens have also been identified in patients with myeloma after allogeneic HSCT. Compared with CML, graft-versus-myeloma (GVM) responses after allogeneic HSCT occur less frequently and often only result in partial responses. Patients with myeloma who received prophylactic DLI early after transplant were found to have increased numbers of normal polyclonal CD20+ B cells in peripheral blood140. Using a similar SEREX approach Bellucci et al. identified a panel of myeloma-associated antigens that were targets of antibody responses in patients with myeloma who responded to DLI141. Antibody responses were not detected before DLI or in patients who did not receive DLI after transplant. These target proteins were highly expressed in myeloma cells and antibodies to some of these antigens were detected in different DLI responders. Taken together, these studies in different hematologic malignancies suggest that polyclonal antibody responses to a variety of tumor-associated antigens frequently occur in patients with effective graft-versus-tumor responses in vivo. Nevertheless, the mechanisms whereby these antibody responses contribute to elimination of tumor cells in vivo have not been established.
Target Antigens and Potential Mechanisms of Action of Antibodies
Several studies have reported that post-transplant patient antibodies can recognize cell membrane proteins expressed on tumor cell targets. This was recently shown for a polymorphic protein, ILT5, which is expressed on the cell membrane of normal DC142. This antigen is also expressed on myeloid leukemia cells, and antibodies in patient serum were able to induce both complement-mediated cytotoxicity of AML cells as well as antibody dependent cellular cytotoxicity (ADCC). In this instance, immunogenicity was due to a polymorphism in the ILT5 gene. As a result of this polymorphism, donor B cells were able to recognize a variant of this protein in the transplant recipient that was not present in the HLA identical transplant donor. Overall, ILT5 reactive antibodies were found in 5.4% of HSCT patients, but not in solid organ transplant recipients, patients with autoimmune diseases, multiparous women, or healthy individuals.
Antibodies to a B cell membrane antigen, BCMA, have also been found in patients with myeloma143. BCMA is a transmembrane receptor of the TNF superfamily that is selectively expressed by mature B cells. In this setting anti-BCMA antibodies were also able to induce both complement-mediated cytotoxicity of myeloma cells, as well as ADCC. BCMA antibodies were only detected in patients with myeloma who received DLI. However, in this case, no genetic polymorphism in the BCMA gene was detected to account for the immunogenicity of this specific protein. Although few antibodies to cell membrane proteins have been described, few studies have specifically attempted to identify these types of antibodies as current methods that have been used to determine the specificity of antibodies in patient sera such as SEREX are not well suited to detect membrane proteins. It is therefore possible that antibodies to cell membrane proteins are a more common phenomenon in patients with GVL.
Most antigens that have been identified as targets of post-transplant antibodies are intracellular proteins. As with cell membrane proteins described above, the immunogenicity of some of these proteins is due to genetic polymorphisms that distinguish recipient and donor. For example, Y chromosome encoded proteins are immunogenic in male patients who receive stem cell grafts from female donors. Males are tolerant to these “self” antigens, but HY proteins are highly immunogenic in females and elicit both B- and T-cell responses after HSCT. HY proteins are widely expressed in normal tissues as well as tumor cells. B-cell responses to HY proteins have been associated with chronic GVHD, but patients with antibodies to HY proteins also have a significantly lower risk of relapse after transplant than patients without HY antibodies144. In addition to HY proteins, a number of other intracellular proteins have been identified as targets of antibody responses in patients with GVL138,141. In most of these cases, polymorphisms that distinguish recipient from donor have not been identified and the immunogenicity of these proteins has not been explained. Nevertheless, the persistence of high titer IgG antibodies for long periods after transplant suggests that these proteins are highly immunogenic. It is also possible that specific T-cell responses directed against peptide epitopes derived from the same proteins are also present in these patients. Coordinated B- and T-cell responses have been reported for HY proteins and similarly coordinated responses may also exist for many of these other proteins145. In these cases, the presence of specific antibodies reactive with soluble proteins or protein fragments may facilitate antigen presentation and the development of specific CD4+ and CD8+ T-cell responses to distinct peptide epitopes contained within these larger fragments.
Although these have not been studied as extensively, antibodies directed against soluble or secreted proteins have also been detected after allogeneic HSCT. Antibodies specific for secreted proteins may significantly deplete these proteins in vivo resulting in profound functional effects. For example, some solid tumor patients who respond to tumor vaccines and infusions of anti-CTLA4 antibody have been found to develop high titer antibodies specific for soluble MICA146. MICA is expressed on tumor cell membranes in response to DNA damage and is a known ligand for NKG2D expressed on NK cells and CD8+ TE cells. However, soluble MICA secreted by tumor cells results in the down-regulation of NKG2D on NK cells and TE cells with subsequent loss of cytolytic function. Antibodies to MICA induce the clearance of this soluble protein resulting in the re-expression of NKG2D and restoration of cytolytic effector cell function. In patients with AML after allogeneic HSCT, Ho et al recently reported a decrease in soluble NKG2D ligands following vaccination with autologous leukemia cells genetically engineered to secrete GM-CSF147. Elevated levels of soluble MICA have also been found after allogeneic HSCT148. In this study, elevated levels of anti-MICA were associated with decreased chronic GVHD and increased risk of relapse after allogeneic HSCT. In many patients, the effectiveness of the GVL response is limited by immune suppressive factors secreted by the tumor cells themselves. The generation of specific antibodies directed against immune suppressive molecules may therefore be an indirect mechanism whereby donor B cells can promote GVL responses mediated by other effector cells. Further studies are needed to explore this novel mechanism whereby antibodies can potentially contribute to the GVL response in vivo.
Although the development of B-cell responses after allogeneic HSCT appears to be associated with effective GVL responses, it has also been noted that antibodies to tumor cell antigens are frequently found in patients with solid tumors. In contrast to the post-transplant setting, the presence of antibodies is not clearly associated with effective autologous tumor immunity in patients with solid tumors149. A very large database of antigens identified by SEREX is now available in the Cancer Immunome Database (http://ludwig-sun5.unil.ch/CancerImmunomeDB). In some cases such as NY-ESO-1, T-cell responses to distinct epitopes within these proteins have also been identified, but the clinical relevance of these endogenous antibody responses has not been established150. In mouse models that have examined the role of B cells in tumor immunity, it has been found that the presence of B cells can actually inhibit effective tumor immunity151.
Methods to Identify Target Antigens
Thus far, most studies that have attempted to define the specificity of antibodies in the post-transplant setting have relied on the SEREX approach to identify and clone specific target proteins. This has been a very useful tool and has allowed the broad characterization of antibody specificity. However, the SEREX method also has limitations and may not be able to identify all relevant target antigens. Since target genes are identified in a cDNA expression library, this method favors the identification of highly expressed genes and protein targets represented by low copy number mRNAs are difficult to detect. This method also does not favor the identification of cell membrane proteins. Protein microarrays that include very large numbers of targets are now available and these can provide an alternative approach for identification of antibody specificity after allogeneic HSCT and especially in the setting of GVL. Protein microarrays have the advantage of providing an unbiased approach to target identification and also greatly simplify and standardize the procedure for characterizing the specificity of complex polyclonal antibody responses. Although current protein microarrays now contain several thousand proteins, this approach also has limitations. Many relevant proteins may not be included in these arrays and this approach is not designed to detect responses specific for protein polymorphisms. The cost of these arrays may be prohibitive for large-scale studies but it is likely that this will also be a useful approach for defining antibody specificities in the setting of GVL responses in vivo.
As methods for high throughput genotyping and whole genome sequencing continue to evolve rapidly, it is likely that these methods will also facilitate the ability to identify antibody targets. For example, the identification of genetic disparities that distinguish recipients and stem cell transplant donors has already been used to predict candidate immunogenic targets for GVHD responses. Using current bioinformatic tools, it is also possible to identify genetic disparities or mutations that predict for the creation of immunogenic epitopes. Once specific epitopes are identified in silico, the immunogenicity of these epitopes can be validated in patients who have undergone allogeneic HSCT. In the future, this approach can be used to validate both B- and T-cell epitopes and to examine whether they are associated with either GVL or GVHD (or both).
Mechanisms That Promote Antibody Responses and Implications for Therapy
The administration of myeloablative or intensely immune suppressive therapy prior to allogeneic HSCT leads to profound lymphopenia that often persists for prolonged periods. The physiologic response to lymphopenia facilitates the homeostatic proliferation of donor B and T cells and supports the recovery of these cells to normal levels. In this environment, recent studies have suggested that B cell recovery following HSCT is supported, at least in part, by high levels of B-cell activating factor (BAFF)152,153. This homeostatic cytokine promotes B cell survival and differentiation and thus supports the secretion of antibodies generated by donor B cells. As noted above, these antibodies may contribute to the GVL response, and the persistence of high levels of BAFF may therefore be an important mechanism to support this functional activity. Other cytokines and microenvironmental factors likely also play important roles in the reconstitution of B cell numbers and repertoire following transplant. These factors have not been well defined in the clinical setting, and further studies to identify factors that modulate B cell reconstitution are warranted. As we develop a better understanding of the role of B cells in the GVL response in vivo, the characterization of these factors may provide novel opportunities for modulating B cell reconstitution to promote GVL after allogeneic HSCT.
Although B-cell responses noted above have been associated with GVL, it is important to acknowledge that B cells also likely contribute to the development of chronic GVHD after allogeneic HSCT144,152,154. This is further supported by the finding that B-cell-directed therapy with anti-CD20 monoclonal antibody (rituximab) can provide effective therapy for some patients with steroid-resistant chronic GVHD155-157. Despite many attempts to distinguish GVL and GVHD, it is evident that there is substantial overlap between immune responses directed against either cancer cells or normal tissues in the transplant recipient. In this setting, new approaches to enhance B-cell responses to promote GVL may also have the unwanted toxicity of increasing chronic GVHD. Similarly, new and more effective strategies to treat or prevent chronic GVHD by targeting donor B cells may also have the unwanted effect of reducing GVL and increasing the risk of relapse after allogeneic HSCT. These issues will need to be carefully considered in the design and evaluation of any new clinical strategies to modulate B cell reconstitution following transplant.
INNATE NK CELL IMMUNE RESPONSES
NK Cell Biology
The GVL effect goes beyond adaptive immune responses and a role for NK cells has been shown in man and mouse. Yet there are striking species differences between mouse and man that need to be considered when attempting to translate results. Dominant among these differences is the expression of CD56 as a marker on human NK cells and the existence of CD56bright and CD56dim subsets. CD56bright NK cells are present in the lymph node and blood in man, are highly proliferative and produce cytokines but are weakly lytic suggesting they exert immunoregulatory functions. Mice lack both CD56 as a marker on their NK cells and have few NK cells in the lymph nodes. The MHC binding receptors in mouse and human are also fundamentally different. In man, Killer-immunoglobulin-like receptors (KIR) are the dominant HLA-binding molecules that regulate NK cell activity whereas mice express the lectin-binding Ly49 family. Additionally, mice are housed under specific pathogen free (SPF) conditions and their baseline NK cell killing is relatively poor which correlates with very low granzyme/perforin expression unless activated whereas human NK cells readily express these lytic molecules and exhibit de novo function without activation. In addition, human NK cells survive better ex vivo while murine NK cells inevitably die after two weeks in culture. Despite these differences, there are many similarities in biologic paradigms between human and mouse NK cells including the expression of regulatory molecules (NKG2D, CD94/NKG2A and others), lytic pathways, and effects of cytokines (IL-15, IL-2, Flt3L) critical for their development. Attempts to use xenograft models to study human NK cell development and activity has been inconsistent but progress has been made by the discovery that IL-15 trans-presentation by species specific IL-15Rα is required and warrants further study.
Although both human and murine inhibitory receptors (KIR and Ly49, respectively) recognize MHC molecules, the natural ligands for many KIR are unknown, including all of the activating KIR, even though some may bind MHC molecules at low affinity. In the homologous murine Ly49 system, the activating receptor Ly49H recognizes the murine cytomegalovirus (CMV) glycoprotein m157, providing “proof of principle” that activating receptors may recognize viral proteins. Until recently, it had been presumed that only cells of the adaptive immune system have memory. Recently, the Lanier lab has shown that Ly49H+ (the receptor for muCMV) NK cells dramatically expand and then contract after viral control, yet amazingly these cells can be found in the recipient 3 months after infection suggesting that “NK cell memory” exists158. A role for human activating receptors in infection is supported by studies of patients with HIV showing an association between AIDS progression and in transplantation by association with human CMV, but memory NK cells are not well studied in man.
Development of NK cell Self Tolerance
The mechanism by which NK cells acquire self-tolerance and alloreactivity has been referred to as NK cell education or licensing. This is one of the most widely debated topics in NK cell biology over the past several years. Self-tolerance is the process by which NK cell function is suppressed after ligation of “self” through class I recognizing inhibitory receptors. Although alloreactivity is determined by a lack of inhibition and a positive balance of activating signals, how an NK cell acquires effector function is more complicated. Several models have been proposed to explain why inhibitory receptor expression correlates with the acquisition of effector function. These concepts differ in their implied mechanisms and whether the process is one of activation or loss of function. What is agreed upon between these and other models is that human NK cells lacking inhibitory receptors are hyporesponsive159-161. Although the exact mechanism remains unknown, self-tolerance may be the result of coordinated developmental pathways whereby mature NK cell function is synchronized with the acquisition of self-inhibitory receptors and their subsequent ligation. The complexity of these interactions is highlighted by the expression of multiple inhibitory receptors capable of recognizing self-MHC, the net summation of activating receptor signals and the variable expression of ligands resulting in a “rheostat”162 or a continuum “tunable rheostat” model integrating a cadre of different signals on the NK cell itself and its potential targets163. In humans, the best evidence for NK cell education is the finding of enhanced function of self-KIR expressing NK cells (KIR in an individual who also expresses its cognate HLA ligand) where NK cells expressing non-self KIR are hyporesponsive. Although KIR interactions dominate the clinical literature, receptors beyond KIR are operant in rheostat models and include class I recognizing NKG2A (recognizes HLA-E), LIR-1 (recognizes HLA-A, B, and G) and class I MHC independent interactions including but not limited to: CD16 (FcRIII), natural cytotoxicity receptors (NKp30, 44, 46), DNAM-1, LFA-1, NKG2D, and CD244 (2B4).
Above and beyond the control of NK cytotoxicity by MHC class I expression on tumor cells, we are beginning to appreciate tissue-specific differences in the ability of NK cells to kill targets. Most notably, transplant data suggest that myeloid malignancies are more susceptible to NK cell control than lymphoid malignancies164,165. Why myeloid cells are more susceptible is not known. One possibility is that myeloid leukemias share with normal myeloid APC the activation-induced C-type lectin (AICL) ligand for NKp80, another C-type lectin NK cell recetor, which could license the NK cell for cytotoxicity166. AICL is a myeloid-specific activating receptor that is upregulated by TLR stimulation suggesting that inflammation may prime NK cells to kill targets. Other differences in receptor-ligand interaction strength have also been proposed and are presumed to be target specific. For example, in CML NKG2D interacting with MICA/B, abundantly expressed on leukemic cells, appears to be the mechanism of NK cell-target engagement167. A critical question in determining their clinical potential is whether NK cells exhibit cytotoxicity against leukemia stem cells. Some data suggest that, at least in CML, NK cells can engage early leukemic CD34+ progenitors although the cytotoxicity is weak. NK cells exert cytotoxicity via perforin-granzyme pathways, but recently a role for TRAIL engagement with TRAIL receptor on the NK cell has been shown to be important. Strategies to exploit this mechanism may be of therapeutic importance. Notably the proteosomal inhibitor, bortezomib, upregulates TRAIL and can increase cytotoxicity of NK cells to renal cell cancer and CML progenitors168. Studies aimed at sensitizing targets to NK cell killing warrant further study.
The Role of NK Cells in the GVL Response
NK cells are attractive to exploit in the setting of hematopoietic transplantation because they are the first lymphocytes to reconstitute after transplantation at a time when the adaptive immune system is impaired. A number of groups have been interested in harnessing the activity of NK cells in the setting of autologous HSCT to prevent relapse, the biggest cause of treatment failure from this procedure. These studies employed low-dose IL-2 administration, which was capable of expanding NK cells in vivo with a modest increase in cytolytic activity. However, even higher doses of IL-2 and autologous hematopoietic cell infusions were not strong enough to result in significant clinical benefit when evaluating definitive clinical endpoints such as time to disease progression and relapse. The biology of NK cells has now explained this result to be partially a result of 1) a number of class I MHC “self” recognizing inhibitory receptors displayed on the surface of NK cells and 2) a consistent finding that low-dose IL-2 administration may lead to blunting of the immune response by increasing Tregs. These two issues underpin current strategies to exploit NK cells in the clinic. The first major advance was reported by Ruggeri et al who found a reduced risk of relapse of leukemia in AML patients who received transplants from donors who were mismatched at HLA-B or HLA-C ligands for KIR164. The advantages of KIR ligand mismatch appear to be specific to AML and dependent on potent graft T-cell depletion suggesting that the platform is critically important to see beneficial effects of early post transplant therapy. The potential benefits include: 1) decreased GVHD as host DC are killed by donor NK cells, 2) better anti-tumor activity via direct cytotoxicity, 3) improved engraftment mediated by NK cell release of hematopoietic cytokines, and 4) ultimately better survival. Additional clinical trials have either supported or failed to find relapse protection and a survival benefit169-176, which may be explained by important differences in transplant platforms between studies including preparative regimen, extent of T-cell depletion, graft source, donor, stem cell dose, disease and disease status. Taken together, these results suggest that NK cells play a role in allogeneic HSCT and myeloid leukemia therapy; however, the complexities of the KIR system and the presence of other functional receptors on NK cells may explain some of the confusion in interpreting published studies.
KIR Genotyping: Implications for Donor Selection
The role of KIR immunogenetics is complicated by 16 genes and over 100 allelic polymorphisms, some of which define distinct functional differences. Despite these complexities, some simple associations have been made in determining clinical outcome after HSCT. Unrelated donors and recipients from 209 HLA- matched and 239 mismatched T-replete URD transplantations for AML were analyzed by KIR genotyping177. Based on gene content, donors were stratified as having a B/x haplotype if they contained one or more B-defining genes (KIR2DS1, 2, 3, 5 and KIR2DL5), while those lacking these genes were classified as having a KIR A/A haplotype. Three-year overall survival was significantly higher after transplantation from a KIR B/x donor (31% [95% CI: 26-36] vs. 20% [95% CI: 13-27]; P = 0.007). Multivariate analysis demonstrated a 30% improvement in the relative risk of relapse-free survival with B/x donors. This demonstrates that unrelated donors with KIR B haplotypes confer significant survival benefit to patients undergoing T-replete HSCT for AML. Certain B haplotype KIR groups have also been found to favorably affect outcome after T-cell depleted HLA identical sibling transplants165. Thus, it seems likely that the genetic characteristics of a stem cell donor could affect transplant outcome, however data on the relative impact of specific KIR genes or groups of genes needs further study to understand why effects are not applicable to diseases beyond AML.
NK Cell Adoptive Transfer
Much of what is known about lymphocyte adoptive transfer comes from T-cell infusion literature, which may extrapolate to NK cell therapy. Emerging concepts suggested that in vivo expansion of lymphocytes were dependent on a potent lymphodepleting regimen to 1) create space, 2) increase endogenous cytokines, and 3) to eliminate immune rejection of adoptively transferred cells. This has been well established in mouse models and the infusion of carboxy-fluorescein diacetate, succinimidyl ester (CFSE, a fluorescence dye which gets diluted out in proliferating cells) labeled lymphocytes which did not expand in the absence of a lymphodepleting regimen178. This lymphodepletion strategy was first translated into humans by Dudley et al who demonstrated in vivo expansion of melanoma specific T-cells after a preparative regimen of high dose cyclophosphamide and fludarabine179. Since HSCT always uses a lymphodepleting regimen, whether fully ablative or not, the same biologic concepts for lymphocyte adoptive transfer may be extrapolated.
In 2005, Miller et al were the first to show in vivo expansion of NK cells after adoptive transfer of haploidentical related donor NK cells180. The success of this regimen was dependent on a lymphodepleting preparative regimen that included cyclophosphamide and fludarabine. NK cells were infused with IL-2 administration for the 2 week interval after adoptive transfer. Chimerism studies established unequivocal proof of concept that donor derived haploidentical NK cells persisted and expanded in vivo in some patients. However, this strategy was limited by inadequate efficacy which may correlate with failure to expand donor NK cells in vivo. This could be explained by inadequate potency of the preparative regimen, insufficient cytokines acting in vivo (endogenous IL-15 induced by lymphodepletion), or contraction of the immune response by increasing Treg cells stimulated by IL-2. In an attempt to overcome some of these barriers several groups have been exploring increased intensity of a lymphodepleting regimen with the addition of total body irradiation approaching that used in a fully ablative preparative regimen. Although these studies are ongoing, there are suggestions with both T-cell and NK cell adoptive transfer that a more potent lymphodepleting regimen may be beneficial. Therefore, to maximize the effects of adoptive transfer, combination with hematopoietic cell transplantation may be warranted to avoid prolonged neutropenia.
One of the challenges in NK cell therapy is how to maximize the effector to target ratio in vivo which likely correlates with efficacy. IL-15 has recently shown promise in primates181 and will be made available through the National Cancer Institute for clinical testing. Although in vivo expansion of NK cells is attractive and allows little ex vivo manipulation, an alternative approach which could also avoid the potential toxicities of a potent lymphodepleting regimen is to expand NK cells ex vivo to increase numbers. One method proposed by Campana et al uses K562 stimulators transduced with 41BBligand and membrane-bound IL-15182. Another approach is described by Berg et al who developed GMP compatible methods using B-cell feeders to promote NK cell expansion over 500-fold183. As one contemplates comparative studies between in vivo and ex vivo expansion approaches, several questions need to be answered. Does in vivo expansion lead a more efficient NK cell education and resultant anti-tumor function than ex vivo expansion? How do in vivo and ex vivo expansion methods affect NK cell activation, receptor expression, survival, homing, and durability of response? All of these questions warrant further study.
In conclusion, our understanding of NK cell target interactions requires more detailed exploration of the potency of specific NK molecules and their ligands on specific target cells (including malignant stem cells), the stages of NK maturation and the effect of the recipient milieu on NK cell maturation and NK clonal selection. Also, relatively little is known about the homing of NK cells to their targets and whether NK cells are restricted in the tissues they patrol. Importantly we do not yet have a clear idea about the ultimate potency of NK cells in their most favorable context to permanently eliminate malignancy and to best apply them to the problem of post-transplant relapse.
CONCLUSION
Immune responses following allogeneic HSCT and DLI have illustrated the robust potential of allogeneic T-cell, NK cell and/or antibody responses to treat patients with hematological malignancies. Several issues, however, have limited the successful application of these immune responses. Key amongst these are GVHD, which has been difficult to separate from GVL, and either upfront resistance of tumors to GVL or late relapse. How to deliver GVL and promote immune reconstitution from donor T cells with acceptable GVHD remains a central mantra in the field. Overcoming these challenges would be facilitated by a detailed mechanistic understanding of GVL resistance and GVHD. Identification of relevant target structures, the type of effector cells necessary for optimal and specific responses, and mechanisms of action are among prerequisites for successful “next steps”. We also need to understand the induction, expansion and trafficking of immune responses which may allow specific recognition of hematopoietic tissues from the host and not target organs of GVHD. Knowledge of the factors that limit the effectiveness of alloimmune responses to execute a specific anti-tumor effect is essential to increase the likelihood of success with no or limited toxicity. These factors include escape mechanisms of tumor cells to prevent recognition and elimination by alloimmune responses which may include down-regulation of the target structures to be recognized, suppression of homing or interaction at the site of the tumor, or the development of suppressive factors preventing the development of the immune responses in vivo.
Future initiatives should be explored to identify and overcome roadblocks preventing successful application of allogeneic therapy for the treatment of hematological malignancies to exploit T cells, NK cells or antibody mediated mechanisms. Specific strategies in mouse and in man may include 1) Studies to better understand mechanisms of trafficking, target recognition, and in vivo expansion of tumor specific responses, 2) Studies of mechanisms that suppress immune responses systemically or locally at the site of the tumor, 3) Studies on in vitro generation, expansion and engineering of specific immune cells for adoptive transfer, 4) Studies on the susceptibility of tumor stem cells to immune attack, 5) Induction of effective anti-tumor immunity with vaccines or inhibition of negative regulatory pathways and 6) High throughput screening for antigens involved in anti-tumor responses using genome wide analysis. Support for collaborative laboratory studies and clinical trials as well as prospective banking of human samples and collection of data will facilitate translation of basic research into successful clinical research strategies. Given the wealth of talent within the transplantation community, promoting interactive program grants between institutions will provide synergy, broaden the use of existing institution core resources and build on individual strengths to move the field forward and systematically address strategies needed to overcome posttransplant relapse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. PMID: 15032595. [DOI] [PubMed] [Google Scholar]
- 2.Hand TW, Kaech SM. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunol Res. 2009;45:46–61. doi: 10.1007/s12026-008-8027-z. PMID: 18629449. [DOI] [PubMed] [Google Scholar]
- 3.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L-memory T cells without graft-versus-host disease. Blood. 2004;103:1534–1541. doi: 10.1182/blood-2003-08-2987. PMID: 14551132. [DOI] [PubMed] [Google Scholar]
- 4.Zheng H, Matte-Martone C, Li H, Anderson BE, Venketesan S, Sheng Tan H, Jain D, McNiff J, Shlomchik WD. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111:2476–2484. doi: 10.1182/blood-2007-08-109678. PMID: 18045967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng H, Matte-Martone C, Jain D, McNiff J, Shlomchik WD. Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia. J Immunol. 2009;182:5938–5948. doi: 10.4049/jimmunol.0802212. PMID: 19414745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. PMID: 15980149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. PMID: 18060041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, Palmer DC, Ji Y, Reger RN, Leonard WJ, Danner RL, Rosenberg SA, Restifo NP. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. PMID: 19805141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shlomchik WD, Matte-Martone C, Gilliland DG, Chen LP. Mechanisms of GVL against a murine blast crisis CML. Blood. 2006;108:60A–61A. PMID: [Google Scholar]
- 10.Matte CC, Cormier J, Anderson BE, Athanasiadis I, Liu J, Emerson SG, Pear W, Shlomchik WD. Graft-versus-leukemia in a retrovirally induced murine CML model: mechanisms of T-cell killing. Blood. 2004;103:4353–4361. doi: 10.1182/blood-2003-10-3735. PMID: 14982874. [DOI] [PubMed] [Google Scholar]
- 11.Matte-Martone C, Liu J, Jain D, McNiff J, Shlomchik WD. CD8+ but not CD4+ T cells require cognate interactions with target tissues to mediate GVHD across only minor H antigens, whereas both CD4+ and CD8+ T cells require direct leukemic contact to mediate GVL. Blood. 2008;111:3884–3892. doi: 10.1182/blood-2007-11-125294. PMID: 18223170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11:1244–1249. doi: 10.1038/nm1309. PMID: 16227991. [DOI] [PubMed] [Google Scholar]
- 13.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. PMID: 17892848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi EY, Christianson GJ, Yoshimura Y, Jung N, Sproule TJ, Malarkannan S, Joyce S, Roopenian DC. Real-time T-cell profiling identifies H60 as a major minor histocompatibility antigen in murine graft-versus-host disease. Blood. 2002;100:4259–4265. doi: 10.1182/blood-2002-05-1299. PMID: 12393464. [DOI] [PubMed] [Google Scholar]
- 15.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. PMID: 17438575. [DOI] [PubMed] [Google Scholar]
- 16.Jones SC, Murphy GF, Friedman TM, Korngold R. Importance of minor histocompatibility antigen expression by nonhematopoietic tissues in a CD4+ T cell-mediated graft-versus-host disease model. J Clin Invest. 2003;112:1880–1886. doi: 10.1172/JCI19427. PMID: 14679183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. PMID: 15522961. [DOI] [PubMed] [Google Scholar]
- 18.Meunier MC, Roy-Proulx G, Labrecque N, Perreault C. Tissue distribution of target antigen has a decisive influence on the outcome of adoptive cancer immunotherapy. Blood. 2003;101:766–770. doi: 10.1182/blood-2002-04-1032. PMID: 12393700. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. PMID: 19043418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. PMID: 16382236. [DOI] [PubMed] [Google Scholar]
- 21.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. PMID: 14502286. [DOI] [PubMed] [Google Scholar]
- 22.Kurts C, Carbone FR, Barnden M, Blanas E, Allison J, Heath WR, Miller JF. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. PMID: 9396776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuchtey J, Chefalo PJ, Gray RC, Ramachandra L, Harding CV. Enhancement of dendritic cell antigen cross-presentation by CpG DNA involves type I IFN and stabilization of class I MHC mRNA. J Immunol. 2005;175:2244–2251. doi: 10.4049/jimmunol.175.4.2244. PMID: 16081792. [DOI] [PubMed] [Google Scholar]
- 24.Ackerman AL, Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol. 2004;5:678–684. doi: 10.1038/ni1082. PMID: 15224093. [DOI] [PubMed] [Google Scholar]
- 25.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. PMID: 15233723. [DOI] [PubMed] [Google Scholar]
- 26.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e, Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. PMID: 15711573. [DOI] [PubMed] [Google Scholar]
- 27.Yu XZ, Bidwell SJ, Martin PJ, Anasetti C. CD28-specific antibody prevents graft-versus-host disease in mice. J Immunol. 2000;164:4564–4568. doi: 10.4049/jimmunol.164.9.4564. PMID: 10779758. [DOI] [PubMed] [Google Scholar]
- 28.Nurieva RI, Mai XM, Forbush K, Bevan MJ, Dong C. B7h is required for T cell activation, differentiation, and effector function. Proc Natl Acad Sci U S A. 2003;100:14163–14168. doi: 10.1073/pnas.2335041100. PMID: 14615582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. PMID: 11343121. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard VM, Eng JM, Ramirez-Montagut T, Tjoe KH, Muriglan SJ, Kochman AA, Terwey TH, Willis LM, Schiro R, Heller G, Murphy GF, Liu C, Alpdogan O, van den Brink MR. Absence of inducible costimulator on alloreactive T cells reduces graft versus host disease and induces Th2 deviation. Blood. 2005;106:3285–3292. doi: 10.1182/blood-2005-01-0410. PMID: 15956289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, Sharpe AH, Noelle RJ, Rudensky AY, Mak TW, Serody JS, Blazar BR. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105:3372–3380. doi: 10.1182/blood-2004-10-3869. PMID: 15618467. [DOI] [PubMed] [Google Scholar]
- 32.Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J Immunol. 2006;176:2808–2816. doi: 10.4049/jimmunol.176.5.2808. PMID: 16493037. [DOI] [PubMed] [Google Scholar]
- 33.Yu XZ, Liang Y, Nurieva RI, Guo F, Anasetti C, Dong C. Opposing effects of ICOS on graft-versus-host disease mediated by CD4 and CD8 T cells. J Immunol. 2006;176:7394–7401. doi: 10.4049/jimmunol.176.12.7394. PMID: 16751384. [DOI] [PubMed] [Google Scholar]
- 34.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. PMID: 12874215. [DOI] [PubMed] [Google Scholar]
- 35.Blazar BR, Sharpe AH, Chen AI, Panoskaltsis-Mortari A, Lees C, Akiba H, Yagita H, Killeen N, Taylor PA. Ligation of OX40 (CD134) regulates graft-versus-host disease (GVHD) and graft rejection in allogeneic bone marrow transplant recipients. Blood. 2003;101:3741–3748. doi: 10.1182/blood-2002-10-3048. PMID: 12521997. [DOI] [PubMed] [Google Scholar]
- 36.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Buhlman J, Xu J, Flavell RA, Korngold R, Noelle R, Vallera DA. Blockade of CD40 ligand-CD40 interaction impairs CD4+ T cell-mediated alloreactivity by inhibiting mature donor T cell expansion and function after bone marrow transplantation. J Immunol. 1997;158:29–39. PMID: 8977172. [PubMed] [Google Scholar]
- 37.Buhlmann JE, Gonzalez M, Ginther B, Panoskaltsis-Mortari A, Blazar BR, Greiner DL, Rossini AA, Flavell R, Noelle RJ. Cutting edge: sustained expansion of CD8+ T cells requires CD154 expression by Th cells in acute graft versus host disease. J Immunol. 1999;162:4373–4376. PMID: 10201970. [PubMed] [Google Scholar]
- 38.Saito K, Sakurai J, Ohata J, Kohsaka T, Hashimoto H, Okumura K, Abe R, Azuma M. Involvement of CD40 ligand-CD40 and CTLA4-B7 pathways in murine acute graft-versus-host disease induced by allogeneic T cells lacking CD28. J Immunol. 1998;160:4225–4231. PMID: 9574523. [PubMed] [Google Scholar]
- 39.Taylor PA, Lees C, Noelle R, Yagita F, Killeen N, al. E. Superior GVHD prevention by combined costimulatory blockade: comparison of single and combined pathway blockade of CD28/CTLA4:B7, CD40L:CD40 and OX40:OX40L pathways. Blood. 2002;100:A818–819. PMID: [Google Scholar]
- 40.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A. 1989;86:1963–1967. doi: 10.1073/pnas.86.6.1963. PMID: 2784565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. PMID: 9878117. [DOI] [PubMed] [Google Scholar]
- 42.Nozawa K, Ohata J, Sakurai J, Hashimoto H, Miyajima H, Yagita H, Okumura K, Azuma M. Preferential blockade of CD8(+) T cell responses by administration of anti-CD137 ligand monoclonal antibody results in differential effect on development of murine acute and chronic graft-versus-host diseases. J Immunol. 2001;167:4981–4986. doi: 10.4049/jimmunol.167.9.4981. PMID: 11673505. [DOI] [PubMed] [Google Scholar]
- 43.Blazar BR, Kwon BS, Panoskaltsis-Mortari A, Kwak KB, Peschon JJ, Taylor PA. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J Immunol. 2001;166:3174–3183. doi: 10.4049/jimmunol.166.5.3174. PMID: 11207270. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. PMID: 11812990. [DOI] [PubMed] [Google Scholar]
- 45.Muriglan SJ, Ramirez-Montagut T, Alpdogan O, Van Huystee TW, Eng JM, Hubbard VM, Kochman AA, Tjoe KH, Riccardi C, Pandolfi PP, Sakaguchi S, Houghton AN, Van Den Brink MR. GITR activation induces an opposite effect on alloreactive CD4(+) and CD8(+) T cells in graft-versus-host disease. J Exp Med. 2004;200:149–157. doi: 10.1084/jem.20040116. PMID: 15249593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. PMID: 12732656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valenzuela JO, Hammerbeck CD, Mescher MF. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J Immunol. 2005;174:600–604. doi: 10.4049/jimmunol.174.2.600. PMID: 15634875. [DOI] [PubMed] [Google Scholar]
- 48.Curtsinger JM, Lins DC, Johnson CM, Mescher MF. Signal 3 tolerant CD8 T cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J Immunol. 2005;175:4392–4399. doi: 10.4049/jimmunol.175.7.4392. PMID: 16177080. [DOI] [PubMed] [Google Scholar]
- 49.Curtsinger JM, Gerner MY, Lins DC, Mescher MF. Signal 3 availability limits the CD8 T cell response to a solid tumor. J Immunol. 2007;178:6752–6760. doi: 10.4049/jimmunol.178.11.6752. PMID: 17513722. [DOI] [PubMed] [Google Scholar]
- 50.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. PMID: 15701715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sims TN, Dustin ML. The immunological synapse: integrins take the stage. Immunol Rev. 2002;186:100–117. doi: 10.1034/j.1600-065x.2002.18610.x. PMID: 12234366. [DOI] [PubMed] [Google Scholar]
- 52.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. PMID: 9419363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potsch C, Vohringer D, Pircher H. Distinct migration patterns of naive and effector CD8 T cells in the spleen: correlation with CCR7 receptor expression and chemokine reactivity. Eur J Immunol. 1999;29:3562–3570. doi: 10.1002/(SICI)1521-4141(199911)29:11<3562::AID-IMMU3562>3.0.CO;2-R. PMID: 10556810. [DOI] [PubMed] [Google Scholar]
- 54.Terwey TH, Kim TD, Kochman AA, Hubbard VM, Lu S, Zakrzewski JL, Ramirez-Montagut T, Eng JM, Muriglan SJ, Heller G, Murphy GF, Liu C, Budak-Alpdogan T, Alpdogan O, van den Brink MR. CCR2 is required for CD8-induced graft-versus-host disease. Blood. 2005;106:3322–3330. doi: 10.1182/blood-2005-05-1860. PMID: 16037386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrovic A, Alpdogan O, Willis LM, Eng JM, Greenberg AS, Kappel BJ, Liu C, Murphy GJ, Heller G, van den Brink MR. LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood. 2004;103:1542–1547. doi: 10.1182/blood-2003-03-0957. PMID: 14563643. [DOI] [PubMed] [Google Scholar]
- 56.Waldman E, Lu SX, Hubbard VM, Kochman AA, Eng JM, Terwey TH, Muriglan SJ, Kim TD, Heller G, Murphy GF, Liu C, Alpdogan O, van den Brink MR. Absence of beta7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood. 2006;107:1703–1711. doi: 10.1182/blood-2005-08-3445. PMID: 16291587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, Dube R, Cohen A, Steinhart AH, Landau S, Aguzzi RA, Fox IH, Vandervoort MK. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. PMID: 15958805. [DOI] [PubMed] [Google Scholar]
- 58.Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, Lublin FD, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA, Sandrock AW. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–923. doi: 10.1056/NEJMoa044396. PMID: 16510745. [DOI] [PubMed] [Google Scholar]
- 59.Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. PMID: 16510744. [DOI] [PubMed] [Google Scholar]
- 60.Hyams JS, Wilson DC, Thomas A, Heuschkel R, Mitton S, Mitchell B, Daniels R, Libonati MA, Zanker S, Kugathasan S. Natalizumab therapy for moderate to severe Crohn disease in adolescents. J Pediatr Gastroenterol Nutr. 2007;44:185–191. doi: 10.1097/01.mpg.0000252191.05170.e7. PMID: 17255829. [DOI] [PubMed] [Google Scholar]
- 61.Sands BE, Kozarek R, Spainhour J, Barish CF, Becker S, Goldberg L, Katz S, Goldblum R, Harrigan R, Hilton D, Hanauer SB. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn's disease not in remission while receiving infliximab. Inflamm Bowel Dis. 2007;13:2–11. doi: 10.1002/ibd.20014. PMID: 17206633. [DOI] [PubMed] [Google Scholar]
- 62.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. PMID: 7511642. [PubMed] [Google Scholar]
- 63.van den Brink MR, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol. 2002;2:273–281. doi: 10.1038/nri775. PMID: 12001998. [DOI] [PubMed] [Google Scholar]
- 64.Otten HG, van Ginkel WG, Hagenbeek A, Petersen EJ. Prevalence and clinical significance of resistance to perforin- and FAS-mediated cell death in leukemia. Leukemia. 2004;18:1401–1405. doi: 10.1038/sj.leu.2403414. PMID: 15215873. [DOI] [PubMed] [Google Scholar]
- 65.Hsieh MH, Korngold R. Differential use of FasL- and perforin-mediated cytolytic mechanisms by T-cell subsets involved in graft-versus-myeloid leukemia responses. Blood. 2000;96:1047–1055. PMID: 10910921. [PubMed] [Google Scholar]
- 66.Baker MB, Altman NH, Podack ER, Levy RB. The role of cell-mediated cytotoxicity in acute GVHD after MHC-matched allogeneic bone marrow transplantation in mice. J Exp Med. 1996;183:2645–2656. doi: 10.1084/jem.183.6.2645. PMID: 8676085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hill GR, Teshima T, Gerbitz A, Pan L, Cooke KR, Brinson YS, Crawford JM, Ferrara JL. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest. 1999;104:459–467. doi: 10.1172/JCI6896. PMID: 10449438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slager EH, Honders MW, van der Meijden ED, van Luxemburg-Heijs SA, Kloosterboer FM, Kester MG, Jedema I, Marijt WA, Schaafsma MR, Willemze R, Falkenburg JH. Identification of the angiogenic endothelial-cell growth factor-1/thymidine phosphorylase as a potential target for immunotherapy of cancer. Blood. 2006;107:4954–4960. doi: 10.1182/blood-2005-09-3883. PMID: 16497972. [DOI] [PubMed] [Google Scholar]
- 69.van Bergen CA, Kester MG, Jedema I, Heemskerk MH, van Luxemburg-Heijs SA, Kloosterboer FM, Marijt WA, de Ru AH, Schaafsma MR, Willemze R, van Veelen PA, Falkenburg JH. Multiple myeloma-reactive T cells recognize an activation-induced minor histocompatibility antigen encoded by the ATP-dependent interferon-responsive (ADIR) gene. Blood. 2007;109:4089–4096. doi: 10.1182/blood-2006-08-043935. PMID: 17234742. [DOI] [PubMed] [Google Scholar]
- 70.Kawase T, Akatsuka Y, Torikai H, Morishima S, Oka A, Tsujimura A, Miyazaki M, Tsujimura K, Miyamura K, Ogawa S, Inoko H, Morishima Y, Kodera Y, Kuzushima K, Takahashi T. Alternative splicing due to an intronic SNP in HMSD generates a novel minor histocompatibility antigen. Blood. 2007;110:1055–1063. doi: 10.1182/blood-2007-02-075911. PMID: 17409267. [DOI] [PubMed] [Google Scholar]
- 71.de Rijke B, van Horssen-Zoetbrood A, Beekman JM, Otterud B, Maas F, Woestenenk R, Kester M, Leppert M, Schattenberg AV, de Witte T, van de Wiel-van Kemenade E, Dolstra H. A frameshift polymorphism in P2X5 elicits an allogeneic cytotoxic T lymphocyte response associated with remission of chronic myeloid leukemia. J Clin Invest. 2005;115:3506–3516. doi: 10.1172/JCI24832. PMID: 16322791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murata M, Warren EH, Riddell SR. A human minor histocompatibility antigen resulting from differential expression due to a gene deletion. J Exp Med. 2003;197:1279–1289. doi: 10.1084/jem.20030044. PMID: 12743171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Terakura S, Murata M, Warren EH, Sette A, Sidney J, Naoe T, Riddell SR. A single minor histocompatibility antigen encoded by UGT2B17 and presented by human leukocyte antigen-A*2902 and -B*4403. Transplantation. 2007;83:1242–1248. doi: 10.1097/01.tp.0000259931.72622.d1. PMID: 17496542. [DOI] [PubMed] [Google Scholar]
- 74.Kamei M, Nannya Y, Torikai H, Kawase T, Taura K, Inamoto Y, Takahashi T, Yazaki M, Morishima S, Tsujimura K, Miyamura K, Ito T, Togari H, Riddell SR, Kodera Y, Morishima Y, Kuzushima K, Ogawa S, Akatsuka Y. HapMap scanning of novel human minor histocompatibility antigens. Blood. 2009;113:5041–5048. doi: 10.1182/blood-2008-07-171678. PMID: 18809759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vogt MH, van den Muijsenberg JW, Goulmy E, Spierings E, Kluck P, Kester MG, van Soest RA, Drijfhout JW, Willemze R, Falkenburg JH. The DBY gene codes for an HLA-DQ5-restricted human male-specific minor histocompatibility antigen involved in graft-versus-host disease. Blood. 2002;99:3027–3032. doi: 10.1182/blood.v99.8.3027. PMID: 11929796. [DOI] [PubMed] [Google Scholar]
- 76.Griffioen M, van der Meijden ED, Slager EH, Honders MW, Rutten CE, van Luxemburg-Heijs SA, von dem Borne PA, van Rood JJ, Willemze R, Falkenburg JH. Identification of phosphatidylinositol 4-kinase type II beta as HLA class II-restricted target in graft versus leukemia reactivity. Proc Natl Acad Sci U S A. 2008;105:3837–3842. doi: 10.1073/pnas.0712250105. PMID: 18316730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stumpf AN, van der Meijden ED, van Bergen CA, Willemze R, Falkenburg JH, Griffioen M. Identification of 4 new HLA-DR-restricted minor histocompatibility antigens as hematopoietic targets in antitumor immunity. Blood. 2009;114:3684–3692. doi: 10.1182/blood-2009-03-208017. PMID: 19706888. [DOI] [PubMed] [Google Scholar]
- 78.Falkenburg JH, Goselink HM, van der Harst D, van Luxemburg-Heijs SA, Kooy-Winkelaar YM, Faber LM, de Kroon J, Brand A, Fibbe WE, Willemze R, et al. Growth inhibition of clonogenic leukemic precursor cells by minor histocompatibility antigen-specific cytotoxic T lymphocytes. J Exp Med. 1991;174:27–33. doi: 10.1084/jem.174.1.27. PMID: 2056279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faber LM, van der Hoeven J, Goulmy E, Hooftman-den Otter AL, van Luxemburg-Heijs SA, Willemze R, Falkenburg JH. Recognition of clonogenic leukemic cells, remission bone marrow and HLA-identical donor bone marrow by CD8+ or CD4+ minor histocompatibility antigen-specific cytotoxic T lymphocytes. J Clin Invest. 1995;96:877–883. doi: 10.1172/JCI118134. PMID: 7635982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. PMID: 7509044. [DOI] [PubMed] [Google Scholar]
- 81.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. PMID: 9212098. [DOI] [PubMed] [Google Scholar]
- 82.Bonnet D, Warren EH, Greenberg PD, Dick JE, Riddell SR. CD8(+) minor histocompatibility antigen-specific cytotoxic T lymphocyte clones eliminate human acute myeloid leukemia stem cells. Proc Natl Acad Sci U S A. 1999;96:8639–8644. doi: 10.1073/pnas.96.15.8639. PMID: 10411928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosinski KV, Fujii N, Mito JK, Koo KK, Xuereb SM, Sala-Torra O, Gibbs JS, Radich JP, Akatsuka Y, Van den Eynde BJ, Riddell SR, Warren EH. DDX3Y encodes a class I MHC-restricted H-Y antigen that is expressed in leukemic stem cells. Blood. 2008;111:4817–4826. doi: 10.1182/blood-2007-06-096313. PMID: 18299450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hambach L, Nijmeijer BA, Aghai Z, Schie ML, Wauben MH, Falkenburg JH, Goulmy E. Human cytotoxic T lymphocytes specific for a single minor histocompatibility antigen HA-1 are effective against human lymphoblastic leukaemia in NOD/scid mice. Leukemia. 2006;20:371–374. doi: 10.1038/sj.leu.2404056. PMID: 16357839. [DOI] [PubMed] [Google Scholar]
- 85.Marijt WA, Heemskerk MH, Kloosterboer FM, Goulmy E, Kester MG, van der Hoorn MA, van Luxemburg-Heys SA, Hoogeboom M, Mutis T, Drijfhout JW, van Rood JJ, Willemze R, Falkenburg JH. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci U S A. 2003;100:2742–2747. doi: 10.1073/pnas.0530192100. PMID: 12601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takami A, Sugimori C, Feng X, Yachie A, Kondo Y, Nishimura R, Kuzushima K, Kotani T, Asakura H, Shiobara S, Nakao S. Expansion and activation of minor histocompatibility antigen HY-specific T cells associated with graft-versus-leukemia response. Bone Marrow Transplant. 2004;34:703–709. doi: 10.1038/sj.bmt.1704583. PMID: 15322566. [DOI] [PubMed] [Google Scholar]
- 87.Claret EJ, Alyea EP, Orsini E, Pickett CC, Collins H, Wang Y, Neuberg D, Soiffer RJ, Ritz J. Characterization of T cell repertoire in patients with graft-versus-leukemia after donor lymphocyte infusion. J Clin Invest. 1997;100:855–866. doi: 10.1172/JCI119601. PMID: 9259585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zorn E, Wang KS, Hochberg EP, Canning C, Alyea EP, Soiffer RJ, Ritz J. Infusion of CD4+ donor lymphocytes induces the expansion of CD8+ donor T cells with cytolytic activity directed against recipient hematopoietic cells. Clin Cancer Res. 2002;8:2052–2060. PMID: 12114403. [PubMed] [Google Scholar]
- 89.Kloosterboer FM, van Luxemburg-Heijs SA, van Soest RA, van Egmond HM, Barbui AM, Strijbosch MP, Willemze R, Falkenburg JH. Minor histocompatibility antigen-specific T cells with multiple distinct specificities can be isolated by direct cloning of IFNgamma-secreting T cells from patients with relapsed leukemia responding to donor lymphocyte infusion. Leukemia. 2005;19:83–90. doi: 10.1038/sj.leu.2403572. PMID: 15526024. [DOI] [PubMed] [Google Scholar]
- 90.Nishida T, Hudecek M, Kostic A, Bleakley M, Warren EH, Maloney D, Storb R, Riddell SR. Development of tumor-reactive T cells after nonmyeloablative allogeneic hematopoietic stem cell transplant for chronic lymphocytic leukemia. Clin Cancer Res. 2009;15:4759–4768. doi: 10.1158/1078-0432.CCR-09-0199. PMID: 19567591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103:347–352. doi: 10.1182/blood-2003-07-2603. PMID: 12969970. [DOI] [PubMed] [Google Scholar]
- 92.Stern M, Brand R, de Witte T, Sureda A, Rocha V, Passweg J, Baldomero H, Niederwieser D, Gratwohl A. Female-versus-male alloreactivity as a model for minor histocompatibility antigens in hematopoietic stem cell transplantation. Am J Transplant. 2008;8:2149–2157. doi: 10.1111/j.1600-6143.2008.02374.x. PMID: 18828773. [DOI] [PubMed] [Google Scholar]
- 93.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–1023. doi: 10.1038/79526. PMID: 10973322. [DOI] [PubMed] [Google Scholar]
- 94.Rezvani K, Grube M, Brenchley JM, Sconocchia G, Fujiwara H, Price DA, Gostick E, Yamada K, Melenhorst J, Childs R, Hensel N, Douek DC, Barrett AJ. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood. 2003;102:2892–2900. doi: 10.1182/blood-2003-01-0150. PMID: 12829610. [DOI] [PubMed] [Google Scholar]
- 95.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, Fukushima P, Barrett AJ. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–2457. PMID: 8839835. [PubMed] [Google Scholar]
- 96.Molldrem JJ, Clave E, Jiang YZ, Mavroudis D, Raptis A, Hensel N, Agarwala V, Barrett AJ. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997;90:2529–2534. PMID: 9326217. [PubMed] [Google Scholar]
- 97.Rezvani K, Yong AS, Savani BN, Mielke S, Keyvanfar K, Gostick E, Price DA, Douek DC, Barrett AJ. Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes after allogeneic stem-cell transplantation for acute lymphoblastic leukemia. Blood. 2007;110:1924–1932. doi: 10.1182/blood-2007-03-076844. PMID: 17505014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Orsini E, Bellucci R, Alyea EP, Schlossman R, Canning C, McLaughlin S, Ghia P, Anderson KC, Ritz J. Expansion of tumor-specific CD8+ T cell clones in patients with relapsed myeloma after donor lymphocyte infusion. Cancer Res. 2003;63:2561–2568. PMID: 12750280. [PubMed] [Google Scholar]
- 99.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, Schilling G, Faltz C, Wolschke C, Dierlamm J, Ritter G, Eiermann T, Hossfeld DK, Zander AR, Jungbluth AA, Old LJ, Bokemeyer C, Kroger N. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109:1103–1112. doi: 10.1182/blood-2006-04-014480. PMID: 17023585. [DOI] [PubMed] [Google Scholar]
- 100.Hoogendoorn M, Jedema I, Barge RM, van Luxemburg-Heijs SA, Beaumont F, Marijt EW, Willemze R, Falkenburg JH. Characterization of graft-versus-leukemia responses in patients treated for advanced chronic lymphocytic leukemia with donor lymphocyte infusions after in vitro T-cell depleted allogeneic stem cell transplantation following reduced-intensity conditioning. Leukemia. 2007;21:2569–2574. doi: 10.1038/sj.leu.2404838. PMID: 17611558. [DOI] [PubMed] [Google Scholar]
- 101.Rutten CE, van Luxemburg-Heijs SA, Griffioen M, Marijt EW, Jedema I, Heemskerk MH, Posthuma EF, Willemze R, Falkenburg JH. HLA-DP as specific target for cellular immunotherapy in HLA class II-expressing B-cell leukemia. Leukemia. 2008;22:1387–1394. doi: 10.1038/leu.2008.90. PMID: 18418406. [DOI] [PubMed] [Google Scholar]
- 102.Rutten CE, van Luxemburg-Heijs SA, van der Meijden ED, Griffioen M, Oudshoorn M, Willemze R, Falkenburg JH. Both permissive and nonpermissive HLA-DPB1 mismatches can induce polyclonal HLA-DPB1 specific immune responses in vivo and in vitro. Blood. 115:151–153. doi: 10.1182/blood-2009-10-249821. PMID: 20056799. [DOI] [PubMed] [Google Scholar]
- 103.Shaw BE, Gooley TA, Malkki M, Madrigal JA, Begovich AB, Horowitz MM, Gratwohl A, Ringden O, Marsh SG, Petersdorf EW. The importance of HLA-DPB1 in unrelated donor hematopoietic cell transplantation. Blood. 2007;110:4560–4566. doi: 10.1182/blood-2007-06-095265. PMID: 17726164. [DOI] [PubMed] [Google Scholar]
- 104.Kawase T, Matsuo K, Kashiwase K, Inoko H, Saji H, Ogawa S, Kato S, Sasazuki T, Kodera Y, Morishima Y. HLA mismatch combinations associated with decreased risk of relapse: implications for the molecular mechanism. Blood. 2009;113:2851–2858. doi: 10.1182/blood-2008-08-171934. PMID: 18997170. [DOI] [PubMed] [Google Scholar]
- 105.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. PMID: 19029455. [DOI] [PubMed] [Google Scholar]
- 106.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. PMID: 7800006. [DOI] [PubMed] [Google Scholar]
- 107.Asavaroengchai W, Kotera Y, Mule JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci U S A. 2002;99:931–936. doi: 10.1073/pnas.022634999. PMID: 11792864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. PMID: 12690202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dotti G. Blocking PD-1 in cancer immunotherapy. Blood. 2009;114:1457–1458. doi: 10.1182/blood-2009-05-223412. PMID: 19696208. [DOI] [PubMed] [Google Scholar]
- 110.Bollard CM, Rossig C, Calonge MJ, Huls MH, Wagner HJ, Massague J, Brenner MK, Heslop HE, Rooney CM. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. doi: 10.1182/blood.v99.9.3179. PMID: 11964281. [DOI] [PubMed] [Google Scholar]
- 111.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli I, Horenstein AL, Fiore F, Massaia M, Colombo MP, Baccarani M, Lemoli RM. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. PMID: 17164341. [DOI] [PubMed] [Google Scholar]
- 112.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80. doi: 10.1038/nri2233. PMID: 18064049. [DOI] [PubMed] [Google Scholar]
- 113.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. PMID: 19635913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. PMID: 18832696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mizoguchi H, O'Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. PMID: 1465616. [DOI] [PubMed] [Google Scholar]
- 116.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. PMID: 18364002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. PMID: 19197294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. PMID: 17483367. [DOI] [PubMed] [Google Scholar]
- 119.Bronte V. Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol. 2009;39:2670–2672. doi: 10.1002/eji.200939892. PMID: 19757440. [DOI] [PubMed] [Google Scholar]
- 120.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. PMID: 19201693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. PMID: 9834220. [PubMed] [Google Scholar]
- 122.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, Back TC, Longo DL, Blazar BR, Wiltrout RH, Welniak LA, Redelman D, Murphy WJ. IFN-gamma mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nat Med. 2007;13:354–360. doi: 10.1038/nm1554. PMID: 17334371. [DOI] [PubMed] [Google Scholar]
- 123.Guimond M, Veenstra RG, Grindler DJ, Zhang H, Cui Y, Murphy RD, Kim SY, Na R, Hennighausen L, Kurtulus S, Erman B, Matzinger P, Merchant MS, Mackall CL. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–157. doi: 10.1038/ni.1695. PMID: 19136960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. PMID: 16484453. [DOI] [PubMed] [Google Scholar]
- 125.Prlic M, Kamimura D, Bevan MJ. Rapid generation of a functional NK-cell compartment. Blood. 2007;110:2024–2026. doi: 10.1182/blood-2007-04-086108. PMID: 17554057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. PMID: 18573906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. PMID: 19103877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. PMID: 17953510. [DOI] [PubMed] [Google Scholar]
- 129.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. PMID: 18276844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. PMID: 16204013. [DOI] [PubMed] [Google Scholar]
- 131.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. PMID: 15958566. [DOI] [PubMed] [Google Scholar]
- 132.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. PMID: 19276342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. PMID: 19109155. [DOI] [PubMed] [Google Scholar]
- 134.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. PMID: 18364001. [DOI] [PubMed] [Google Scholar]
- 135.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. PMID: 16946036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou Q, Bucher C, Munger ME, Highfill SL, Tolar J, Munn DH, Levine BL, Riddle M, June CH, Vallera DA, Weigel BJ, Blazar BR. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. 2009;114:3793–3802. doi: 10.1182/blood-2009-03-208181. PMID: 19724059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wrzesinski C, Paulos CM, Gattinoni L, Palmer DC, Kaiser A, Yu Z, Rosenberg SA, Restifo NP. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. PMID: 17273561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu CJ, Yang XF, McLaughlin S, Neuberg D, Canning C, Stein B, Alyea EP, Soiffer RJ, Dranoff G, Ritz J. Detection of a potent humoral response associated with immune-induced remission of chronic myelogenous leukemia. J Clin Invest. 2000;106:705–714. doi: 10.1172/JCI10196. PMID: 10974024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang XF, Wu CJ, Chen L, Alyea EP, Canning C, Kantoff P, Soiffer RJ, Dranoff G, Ritz J. CML28 is a broadly immunogenic antigen, which is overexpressed in tumor cells. Cancer Res. 2002;62:5517–5522. PMID: 12359762. [PMC free article] [PubMed] [Google Scholar]
- 140.Bellucci R, Alyea EP, Weller E, Chillemi A, Hochberg E, Wu CJ, Canning C, Schlossman R, Soiffer RJ, Anderson KC, Ritz J. Immunologic effects of prophylactic donor lymphocyte infusion after allogeneic marrow transplantation for multiple myeloma. Blood. 2002;99:4610–4617. doi: 10.1182/blood.v99.12.4610. PMID: 12036895. [DOI] [PubMed] [Google Scholar]
- 141.Bellucci R, Wu CJ, Chiaretti S, Weller E, Davies FE, Alyea EP, Dranoff G, Anderson KC, Munshi NC, Ritz J. Complete response to donor lymphocyte infusion in multiple myeloma is associated with antibody responses to highly expressed antigens. Blood. 2004;103:656–663. doi: 10.1182/blood-2003-07-2559. PMID: 14563636. [DOI] [PubMed] [Google Scholar]
- 142.Pfistershammer K, Lawitschka A, Klauser C, Leitner J, Weigl R, Heemskerk MH, Pickl WF, Majdic O, Bohmig GA, Fischer GF, Greinix HT, Steinberger P. Allogeneic disparities in immunoglobulin-like transcript 5 induce potent antibody responses in hematopoietic stem cell transplant recipients. Blood. 2009;114:2323–2332. doi: 10.1182/blood-2008-10-183814. PMID: 19617579. [DOI] [PubMed] [Google Scholar]
- 143.Bellucci R, Alyea EP, Chiaretti S, Wu CJ, Zorn E, Weller E, Wu B, Canning C, Schlossman R, Munshi NC, Anderson KC, Ritz J. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood. 2005;105:3945–3950. doi: 10.1182/blood-2004-11-4463. PMID: 15692072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, Hochberg EP, Wu CJ, Alyea EP, Cutler C, Ho V, Soiffer RJ, Antin JH, Ritz J. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. PMID: 15613541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zorn E, Miklos DB, Floyd BH, Mattes-Ritz A, Guo L, Soiffer RJ, Antin JH, Ritz J. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med. 2004;199:1133–1142. doi: 10.1084/jem.20031560. PMID: 15096539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR, Dranoff G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A. 2008;105:1285–1290. doi: 10.1073/pnas.0711293105. PMID: 18202175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ho VT, Vanneman M, Kim H, Sasada T, Kang YJ, Pasek M, Cutler C, Koreth J, Alyea E, Sarantopoulos S, Antin JH, Ritz J, Canning C, Kutok J, Mihm MC, Dranoff G, Soiffer R. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc Natl Acad Sci U S A. 2009;106:15825–15830. doi: 10.1073/pnas.0908358106. PMID: 19717467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boukouaci W, Busson M, Peffault de Latour R, Rocha V, Suberbielle C, Bengoufa D, Dulphy N, Haas P, Scieux C, Amroun H, Gluckman E, Krishnamoorthy R, Toubert A, Charron D, Socie G, Tamouza R. MICA-129 genotype, soluble MICA and anti-MICA antibodies as biomarkers of chronic graft versus host disease. Blood. 2009;114:5216–5224. doi: 10.1182/blood-2009-04-217430. PMID: 19786616. [DOI] [PubMed] [Google Scholar]
- 149.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. PMID: 19562338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E, Knuth A, Chen YT, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. PMID: 16860654. [DOI] [PubMed] [Google Scholar]
- 151.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. PMID: 9585241. [DOI] [PubMed] [Google Scholar]
- 152.Sarantopoulos S, Stevenson KE, Kim HT, Bhuiya NS, Cutler CS, Soiffer RJ, Antin JH, Ritz J. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. PMID: 17947475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, Ho VT, Alyea EP, Koreth J, Blazar BR, Soiffer RJ, Antin JH, Ritz J. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874. doi: 10.1182/blood-2008-09-177840. PMID: 19168788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114:4919–4927. doi: 10.1182/blood-2008-10-161638. PMID: 19749094. [DOI] [PubMed] [Google Scholar]
- 155.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, Klickstein LB, Levin J, Miller K, Reynolds C, Macdonell R, Pasek M, Lee SJ, Ho V, Soiffer R, Antin JH, Ritz J, Alyea E. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. PMID: 16551963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zaja F, Bacigalupo A, Patriarca F, Stanzani M, Van Lint MT, Fili C, Scime R, Milone G, Falda M, Vener C, Laszlo D, Alessandrino PE, Narni F, Sica S, Olivieri A, Sperotto A, Bosi A, Bonifazi F, Fanin R. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40:273–277. doi: 10.1038/sj.bmt.1705725. PMID: 17549053. [DOI] [PubMed] [Google Scholar]
- 157.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15:1005–1013. doi: 10.1016/j.bbmt.2009.04.003. PMID: 19660713. [DOI] [PubMed] [Google Scholar]
- 158.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. PMID: 19136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. PMID: 16079848. [DOI] [PubMed] [Google Scholar]
- 160.Gasser S, Raulet DH. Activation and self-tolerance of natural killer cells. Immunol Rev. 2006;214:130–142. doi: 10.1111/j.1600-065X.2006.00460.x. PMID: 17100881. [DOI] [PubMed] [Google Scholar]
- 161.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. PMID: 16901727. [DOI] [PubMed] [Google Scholar]
- 162.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182:4572–4580. doi: 10.4049/jimmunol.0803900. PMID: 19342631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30:143–149. doi: 10.1016/j.it.2009.01.006. PMID: 19282243. [DOI] [PubMed] [Google Scholar]
- 164.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, Urbani E, Negrin RS, Martelli MF, Velardi A. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. PMID: 10381530. [PubMed] [Google Scholar]
- 165.Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, Zeilah J, Kurlander R, Srinivasan R, Childs R, Hensel N, Barrett AJ. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–2152. doi: 10.1038/sj.leu.2404892. PMID: 17673900. [DOI] [PubMed] [Google Scholar]
- 166.Welte S, Kuttruff S, Waldhauer I, Steinle A. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat Immunol. 2006;7:1334–1342. doi: 10.1038/ni1402. PMID: 17057721. [DOI] [PubMed] [Google Scholar]
- 167.Sconocchia G, Lau M, Provenzano M, Rezvani K, Wongsena W, Fujiwara H, Hensel N, Melenhorst J, Li J, Ferrone S, Barrett AJ. The antileukemia effect of HLA-matched NK and NKT cells in chronic myelogenous leukemia involves NKG2D-target-cell interactions. Blood. 2005;106:3666–3672. doi: 10.1182/blood-2005-02-0479. PMID: 16046526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yong AS, Keyvanfar K, Hensel N, Eniafe R, Savani BN, Berg M, Lundqvist A, Adams S, Sloand EM, Goldman JM, Childs R, Barrett AJ. Primitive quiescent CD34+ cells in chronic myeloid leukemia are targeted by in vitro expanded natural killer cells, which are functionally enhanced by bortezomib. Blood. 2009;113:875–882. doi: 10.1182/blood-2008-05-158253. PMID: 18922853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, Maccario R, Bonetti F, Wojnar J, Martinetti M, Frassoni F, Giorgiani G, Bacigalupo A, Holowiecki J. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. PMID: 12689936. [DOI] [PubMed] [Google Scholar]
- 170.Beelen DW, Ottinger HD, Ferencik S, Elmaagacli AH, Peceny R, Trenschel R, Grosse-Wilde H. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood. 2005;105:2594–2600. doi: 10.1182/blood-2004-04-1441. PMID: 15536148. [DOI] [PubMed] [Google Scholar]
- 171.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, Stern M, Pende D, Perruccio K, Burchielli E, Topini F, Bianchi E, Aversa F, Martelli MF, Velardi A. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. PMID: 17371948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Davies SM, Ruggieri L, DeFor T, Wagner JE, Weisdorf DJ, Miller JS, Velardi A, Blazar BR. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100:3825–3827. doi: 10.1182/blood-2002-04-1197. PMID: 12393440. [DOI] [PubMed] [Google Scholar]
- 173.Bishara A, De Santis D, Witt CC, Brautbar C, Christiansen FT, Or R, Nagler A, Slavin S. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004;63:204–211. doi: 10.1111/j.0001-2815.2004.00182.x. PMID: 14989709. [DOI] [PubMed] [Google Scholar]
- 174.Bornhauser M, Schwerdtfeger R, Martin H, Frank KH, Theuser C, Ehninger G. Role of KIR ligand incompatibility in hematopoietic stem cell transplantation using unrelated donors. Blood. 2004;103:2860–2861. doi: 10.1182/blood-2003-11-3893. author reply 2862.PMID: 15033884. [DOI] [PubMed] [Google Scholar]
- 175.Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, Boudreau C, Nelson G, Oudshoorn M, van Rood J, Velardi A, Maiers M, Setterholm M, Confer D, Posch PE, Anasetti C, Kamani N, Miller JS, Weisdorf D, Davies SM. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. PMID: 16864058. [DOI] [PubMed] [Google Scholar]
- 176.Kroger N, Binder T, Zabelina T, Wolschke C, Schieder H, Renges H, Ayuk F, Dahlke J, Eiermann T, Zander A. Low number of donor activating killer immunoglobulin-like receptors (KIR) genes but not KIR-ligand mismatch prevents relapse and improves disease-free survival in leukemia patients after in vivo T-cell depleted unrelated stem cell transplantation. Transplantation. 2006;82:1024–1030. doi: 10.1097/01.tp.0000235859.24513.43. PMID: 17060849. [DOI] [PubMed] [Google Scholar]
- 177.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, Marsh SG, Guethlein LA, Parham P, Miller JS, Weisdorf DJ. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. PMID: 18945962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. PMID: 12122110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. PMID: 12242449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. PMID: 15632206. [DOI] [PubMed] [Google Scholar]
- 181.Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, Riddell SR. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. PMID: 19605850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. PMID: 19383914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Berg M, Lundqvist A, McCoy P, Jr., Samsel L, Fan Y, Tawab A, Childs R. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11:341–355. doi: 10.1080/14653240902807034. PMID: 19308771. [DOI] [PMC free article] [PubMed] [Google Scholar]