Abstract
Heparanase is a promising anticancer target because of its involvement in cancer invasion and metastasis. Heparanase cleaves heparan sulfate (HS), a sulfated polysaccharide, and activates a series of HS-mediated cell proliferation and angiogenesis processes. Understanding the substrate specificity of heparanase will aid the discovery of heparanase inhibitors. Here, we sought to determine the specificity of heparanase using synthetic polysaccharide substrates. The substrates were prepared using purified HS biosynthetic enzymes. Using these substrates, we were able to dissect the structural moieties required for heparanase. Our data suggest that heparanase cleaves the linkage between a GlcA unit and an N-sulfo glucosamine unit carrying either a 3-O-sulfo or a 6-O-sulfo group. In addition, heparanase cleaves the linkage of a GlcA unit and N-sulfo glucosamine unit with a 2-O-sulfated GlcA residue, not a 2-O-sulfated IdoA residue, in proximity. We also discovered that the polysaccharide with repeating disaccharide units of IdoA2S-GlcNS inhibits the activity of heparanase. Our findings advance the understanding of the substrate specificity of heparanase and identify a lead compound for developing polysaccharide-based heparanase inhibitors.
Keywords: Carbohydrate Biosynthesis, Glycosaminoglycan, Heparan Sulfate, Heparin, Polysaccharide
Introduction
Heparanase cleaves heparan sulfate (HS),3 which is expressed in a variety of cells and tissues. The degradation by heparanase results in smaller HS fragments ranging in size from 10 to 20 sugar units, and these fragments modulate the functions of growth factors and growth factor receptors (1). Consequently, heparanase plays regulatory roles in several pathophysiological processes such as tissue remodeling, inflammation, angiogenesis, metastasis, and tumor progression (2–4). The contribution of heparanase to tumor formation, invasion, and metastasis has been noted. The level of heparanase is up-regulated in a number of primary tumors such as head and neck, pancreatic carcinoma, hepatocellular carcinoma, and several cultured human tumor cell lines (5–9). The overexpression of heparanase has been correlated to an invasive phenotype in experimental animals (10). Furthermore, heparanase has been linked to tumorigenesis in a wide array of cancers, such as breast, prostate, and colon (11–21). Thus, there is considerable interest to find the inhibitor of heparanase for anticancer drug development.
HS is comprised of repeating disaccharide units of glucuronic (GlcA)/iduronic (IdoA) acid 1→4 linked to glucosamine of which the residue is N-acetylated (GlcNAc), N-sulfated (GlcNS), or N-unsubstituted (GlcNH2) (22). The biosynthesis of HS is carried out by an array of biosynthetic enzymes to decorate the end products with sulfo groups and IdoA units. HS biosynthesis begins with the alternating addition of GlcA and N-acetyl glucosamine by glycosyltransferases, which are encoded by EXT1 and EXT2 genes. Concomitantly with chain elongation, the polysaccharide is modified by N-deacetylase/N-sulfotransferase, which converts GlcNAc to a GlcNS unit. The GlcA unit is then converted to IdoA by altering the configuration at the C5 position by C5-epimerase (23). Further O-sulfations are carried out by 2-O-sulfotranferase (2-OST), 6-O-sulfotransferase (6-OST), and 3-O-sulfotransferase (3-OST) to form the highly sulfated products. The sulfation patterns and the position of the IdoA residue of HS give rise to the selectivity that allows HS to bind to a variety of ligands and play a regulatory role in physiological processes such as embryogenesis (24) and pathological roles such as tumorigenesis (25).
To date, many efforts have been made to elucidate the substrate recognition mechanism of heparanase. It has been perceived that heparanase acts on specific sulfated saccharide sequences (26). Pikas et al. (27) examined the specificity of heparanase utilizing an octasaccharide and a capsular polysaccharide of Escherichia coli K5 strain, as well as its chemically modified derivatives. This study revealed that O-sulfo groups are important for heparanase recognition but not N-sulfation. The Pikas report also indicated that 2-O-sulfation on the IdoA or GlcA was essential for recognition by heparanase. Several years later, Okada et al. (28) further detailed the substrate specificity of heparanase using tetra- and hexasaccharides. The data from this report suggest the following: 1) the minimum heparanase recognition site is a trisaccharide; 2) heparanase cleaves the linkage of GlcA and GlcNS that is 6-O-sulfated or 3,6-O-disulfated; and 3) the 2-O-sulfated iduronic acid (IdoA2S) is nonessential for heparanase cleavage. Despite their success, the results from these reports cannot provide a clear picture of which monosaccharide sequences are recognized by heparanase beyond a disaccharide/trisaccharide level.
In this manuscript, we sought to define the substrate specificity of heparanase utilizing synthetic polysaccharide substrates. The polysaccharides were prepared by purified HS biosynthetic enzymes, and each polysaccharide substrate has unique sulfation patterns (29). Utilizing this approach, we synthesized a library of structures. To investigate the effects of certain saccharide structures that are present in low abundance, we also prepared the polysaccharides with repeating units of GlcA-GlcNS3S and GlcA2S-GlcNS. The polysaccharide substrates were exposed to heparanase, and the extent of the degradation was monitored by gel permeation chromatography. Instead of recognizing specific sulfated monosaccharide sequences, our data suggest that heparanase reads certain sulfation types. It cleaves the linkage of GlcA-GlcNS6S (or -3S) or the linkage of GlcA-GlcNS with a GlcA2S residue in the vicinity. Furthermore, the polysaccharide containing IdoA2S-GlcNS is a potent inhibitor of heparanase. Our data provide a systematic view of the effects of sulfation pattern on the susceptibility to heparanase and uncover a lead compound for developing a substrate-like inhibitor of heparanase.
EXPERIMENTAL PROCEDURES
Preparation of Heparanase Expression Plasmid
The bacteria clone (clone 6250265) containing the full-length human heparanase was purchased from Open Biosystems (Huntsville, AL). Two primers, 5′-ATATTAGAATTCATG CTGCTGCGCTCGAAGCCT-3′ and 5′-ATAAATTCTAGAGATGCAAGCAGCAACTTTGGC-3′, were synthesized, where the restriction sites of EcoRI and XbaI are underlined. The PCR reaction was performed to amplify heparanase using the conditions as follows: 95 °C for 2 min, followed by 30 cycles of 95 °C for 30 s, 55 °C for 30 s, 68 °C for 2 min, and 68 °C for 10 min. The resulting PCR product was cloned into the pcDNA3.1-A vector (Invitrogen) using EcoRI and XbaI sites to form a heparanase Myc-tagged protein. The plasmid (heparanase/pcDNA3.1) was confirmed by sequencing analysis (Genomic Analysis Core Facility, University of North Carolina).
Expression of Recombinant Heparanase in CHO Cells
Wild type Chinese hamster ovary (CHO) cells were transiently transfected with heparanase/pcDNA3.1 according to a standard protocol. Briefly, CHO cells were cultured in F-12 medium supplemented with 10% fetal bovine serum and maintained at 37 °C in a 5% CO2 humidified incubator. About 2 × 105 cells were seeded per well (diameter, 3.5 cm) in a 6-well plate. Upon 90–95% confluence, transfection was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After 48 h, cells were harvested with a cell scraper and centrifuged (2,000 rpm for 8 min). The cells were lysed in 100 μl of lysis buffer (1% Triton X-100, 1.5 m sucrose, and 1 mm phenylmethylsulfonyl fluoride) on ice for 1 h. The lysate was centrifuged (at 12,000 rpm for 10 min at 4 °C), and the supernatant was collected. The expression level of heparanase in both cell lysate and medium was assessed by Western analysis using 10% SDS-PAGE blotted with mouse anti-Myc monoclonal antibody and anti-mouse horseradish peroxidase (Invitrogen). We only detected heparanase in cell extract.
Preparation of Metabolically 35S-labeled HS from CHO Cells
Confluent cells (T75 flask) were incubated with 0.5 mCi/ml of sodium [35S]sulfate (MD Biomedicals) for 16 h at 37 °C in 5 ml of F-12 medium supplemented with 10% dialyzed fetal bovine serum (26). To the medium, 1 ml of solution containing 1 mg/ml Pronase (Sigma), 240 mm sodium acetate, pH 6.5, and 1.92 m NaCl was added and incubated at 37 °C overnight. The sample was then purified by a DEAE column that was equilibrated with a buffer containing 20 mm NaAcO, pH 5, 150 mm NaCl. The HS was then eluted with a buffer containing 20 mm NaAcO, pH 5, 1 m NaCl. The purified [35S]HS was dialyzed against 50 mm ammonium bicarbonate using a 3,500 molecular weight cut-off membrane. The sample was treated with 0.1 m NaOH and 9 mm sodium borohydride at 46 °C for 16 h to cleave the linkage between HS and the core protein. The sample was finally treated with chondroitinase ABC (20 units/ml; Sigma) to remove chondroitin sulfate. The 35S-labeled HS was dried and ready for the subsequent analysis.
Assaying for Heparanase Enzymatic Activity
Enzymatic reactions were carried out in 50 mm MES, pH 6.0, containing 50,000 counts per min of [35S]HS and 0.2 μg of heparanase. The reaction was incubated for 24 h at 37 °C followed by subsequent heating (100 °C, 5 min) and centrifugation (13,200 rpm for 5 min) to precipitate the insoluble material. The products were analyzed by gel permeation chromatography (GPC)-HPLC. Briefly, the reaction was injected into a TSK-gel G3000SWXL column (Tosoh Biosciences), which was equilibrated by a buffer containing 20 mm MOPS, 400 mm NaCl, pH 7.0, at a flow rate of 1.0 ml/min. The performance of the GPC-HPLC was assessed using a set of protein standards, including blue dextran (2000 kDa), β-amylase (200 kDa), bovine serum albumin (66 kDa), carbonic anhydrase (29 kDa), cytochrome c (12.4 kDa), and sodium azide (0.065 kDa). All these standards were purchased from Sigma. A shift of the 35S-labeled peak toward the lower molecular range indicated heparanase cleavage.
Preparation of 35S-labeled HS Polysaccharide Substrates
All synthetic polysaccharides started from heparosan, which was purified from the media of E. coli K5 using a DEAE column (30). 6-O-Sulfo heparosan (compound 2) was prepared by incubating heparosan and 6-OST-3. Briefly, 1 μg of heparosan, 0.3 mg/ml of 6-OST-3 and 50 μm [35S]PAPS (1.3 × 103 cpm/pmol) were mixed in 100 μl of reaction buffer containing 50 mm MES, 0.5% Triton X-100, pH 7.0. The reaction was incubated at 37 °C for 16 h. The product was purified by a DEAE column.
Alternatively, heparosan was converted to N-sulfo heparosan (compound 3) through chemical deacetylation and enzymatic N-sulfation. To prepare deacetylated heparosan, heparosan (20 mg/ml) was incubated in 0.1 m NaOH and 0.1 m NaBH4 and incubated at 50 °C for 3 h. The reaction was neutralized with acetic acid and then dialyzed against 50 mm ammonium bicarbonate using a 3500 molecular weight cut-off membrane. The polysaccharide was then incubated in 2 m NaOH at 68 °C for 3 h and then neutralized by concentrated HCl (37%, w/w) followed by dialysis using a 3500 molecular weight cut-off membrane. The deacetylated heparosan (10 μg/ml) was N-sulfated using 0.075 mg/ml of NST and 50 μm [35S]PAPS (4.8 × 102 cpm/pmol) in 100 μl of reaction buffer containing 50 mm MES, 0.5% Triton X-100, pH 7.0. The reaction was incubated at 37 °C for 16 h. The product was purified by a DEAE column.
The N-sulfo heparosan (compound 3) was 6-O-sulfated using a mixture of 6-OST-1 and 6-OST-3 to prepare N-sulfo 6-O-sulfo heparosan (compound 4). In brief, 1 μg of N-sulfo heparosan (compound 3) was incubated with 0.2 mg/ml of 6-OST-1, 0.3 mg/ml of 6-OST-3 and 50 μm [35S]PAPS in 100 μl reaction buffer. After incubation at 37 °C for 16 h, the product was purified by DEAE chromatography. N-sulfo 3-O-sulfo heparosan (compound 5) was prepared similar to compound 4 by replacing 6-OST-1 and 6-OST-3 with 0.1 mg/ml of 3-OST-1.
To prepare N-sulfo 2-O-sulfo heparosan (compound 6), N-sulfo heparosan (compound 3) was modified with 0.15 mg/ml of 2-OST and 50 μm [35S]PAPS in 100 μl of reaction buffer. N-sulfo 2-O-sulfo heparan (compound 7) was prepared using a mixture of 0.1 mg/ml of C5-epimerase and 0.15 mg/ml of 2-OST with 50 μm [35S]PAPS in 100 μl of reaction buffer. The reactions were incubated at 37 °C for 16 h, and the products were purified by a DEAE column.
Analytical Procedures for Synthetic Polysaccharides
To verify the structural composition of the enzymatically synthesized substrates, disaccharide analysis was performed using two distinct techniques: heparin lyase digestion and nitrous acid degradation. For heparin lyase digestion, sulfated heparosan (1–10 μg) was degraded with a mixture of heparin lyase I, II, and III (0.1 mg/ml each) in 300 μl of 50 mm sodium phosphate, pH 7.0 (31). The digestion was incubated at 37 °C overnight. The resultant disaccharides were isolated using a BioGel P-2 column followed by disaccharide analysis using reverse phase ion-pairing HPLC as described (31).
To determine the presence of GlcA2S versus IdoA2S, nitrous acid degradation was performed by deaminative cleavage (pH 1.5) at the glycosidic linkage containing N-sulfo glucosamine units (32). After degradation, the disaccharides were isolated using a BioGel P-2 column and analyzed using a C18 column (Grace Davison Discovery Sciences) under reverse phase ion-pairing-HPLC conditions.
Inhibition of the Activity of Heparanase
The enzymatic reactions were assembled in a same way as described under “Experimental Procedures” except with the addition of compound 2, 3, 7, or PI-88 (0.5–50 μg/ml). Complete inhibition was indicated by the identical elution profiles of 35S-labeled substrate with or without heparanase cleavage on GPC-HPLC.
RESULTS
Expression of Heparanase in CHO Cells
Recombinant heparanase was transiently expressed in CHO cells by transfecting the plasmid expressing Myc-tagged heparanase. The level of heparanase was determined by Western analysis (Fig. 1A). As expected, heparanase is synthesized as a 65-kDa protein (pro-heparanase), and a processed heparanase appeared at 50 kDa. It was reported that the N terminus is cleaved by cathepsin L to yield an active enzyme with a molecular mass of 50 kDa (3, 26, 33). The recombinant enzyme was incubated with 35S-labeled HS isolated from CHO cells to confirm the enzymatic activity. Indeed, the size of the degraded [35S]HS migrated toward smaller molecular mass range after heparanase treatment as determined by GPC-HPLC (Fig. 1B). Using protein molecular mass standards, we estimated the molecular mass of uncleaved HS was 69 kDa, whereas the molecular mass of heparanase-degraded HS was 10 kDa (Fig. 1c). Further analysis of the size of heparanase-digested HS using BioGel P-10 suggested that >80% of the product are larger than decasaccharide (supplemental Fig. 1). We also observed a slight shift in the chromatogram of the 35S-labeled HS treated with pcDNA3.1-transfected cell extract (Fig. 1B). This observation is not unanticipated because CHO cells express endogenous levels of heparanase (34). We also observed the extent of the cleavage of 35S-labeled HS correlated with the amount of cell extracts in the reaction (data not shown). We estimated that the level of heparanase from the overexpressed CHO cells was 60-fold higher than from the wild type CHO cells.
FIGURE 1.
Preparation and characterization of recombinant heparanase. Plasmid expressing C-terminal Myc-tagged heparanase (Hep.) was transiently transfected into CHO cells. Heparanase was harvested from cell extracts. Panel A shows the presence of the 65-kDa unprocessed heparanase protein (top band) and the active 50 kDa protein after proper intracellular processing (bottom band). Panel B indicates that the expressed recombinant heparanase protein possessed enzymatic activity. This result is shown by the shift in the GPC chromatogram. The molecular mass of the cleavage products was measured by gel filtration chromatography using a TSK-gel G3000SWXL column. The molecular mass standards used were β-amylase (200 kDa), bovine serum albumin (66 kDa), carbonic anhydrous (29 kDa), and cytochrome c (12.4 kDa). The void volume (V0) and total column volume (Vt) were determined by resolving blue dextran (2000 kDa) and sodium azide (65 Da), respectively. Panel C shows the size of the uncleaved material (69 kDa) versus the cleaved material (10 kDa) as indicated by arrows. cpm, counts per min.
Preparation of Polysaccharide Substrates with Different Sulfation Patterns
We employed a library of polysaccharides to interrogate the substrate specificity of heparanase. The polysaccharides were prepared using different combinations of HS biosynthetic enzymes, allowing us to engineer the sulfation pattern in the polysaccharides. A total of seven polysaccharide constructs were prepared as shown in Fig. 2. The level of sulfations for each polysaccharide construct was estimated based on the number of [35S]sulfo groups transferred to the polysaccharide (Table 1). Disaccharide analysis of the polysaccharides was also conducted, confirming the presence of the desired sulfation types and levels (supplemental Fig. 2).
FIGURE 2.
Scheme for the synthesis of polysaccharide substrate. Heparosan, a capsular polysaccharide isolated from E. coli K5, was used as the starting material for substrate design. The synthesis was carried out using purified recombinant HS biosynthetic enzymes. The procedures for the enzymatic synthesis of polysaccharide substrates are described under “Experimental Procedures.” The polysaccharide substrates were purified using DEAE columns, and the desired structures were confirmed by disaccharide analysis. C5-epi, C5-epimerase; NST, N-sulfotransferase.
TABLE 1.
Summary of the modification to synthetic polysaccharides and the results of their heparanase degradation
| Polysaccharide substrates | Repeating disaccharide unita | O-35S sulfation level (nmol/μg of polysaccharide) | Heparanase cleavageb |
|---|---|---|---|
| 1 | GlcA-GlcNAc6S | 0.4 | − |
| 2 | GlcA-GlcNS | 2.4 | − |
| 3 | GlcA-GlcNS6S | 1.2 | + |
| 4 | GlcA-GlcNS3S | 0.4 | + |
| 5 | GlcA2S-GlcNS | 1.8 | + |
| 6 | IdoA2S-GlcNS | 2.3 | − |
a The boldface font represents the enzymatic modification within the substrate.
b The + represents those structures that were cleaved, whereas (−) represents those structures that are resistant to cleavage.
The Contribution of Sulfation on the Glucosamine Residue to the Cleavage by Heparanase
We examined the effect of N- and O-sulfation of glucosamine residue on the susceptibility to heparanase digestion. Two groups of substrates were prepared. Group I contains a single type of sulfation of the polysaccharides, including 6-O-sulfo heparosan (2) and N-sulfo heparosan (3). Group II contains two types of sulfation on the glucosamine units, including N-sulfo 6-O-sulfo heparosan (4) and N-sulfo 3-O-sulfo heparosan (5). To facilitate the analysis, 35S-labels were introduced to the substrates. Because heparosan (1) was known to be resistant to the digestion (27), we did not test its susceptibility to heparanase digestion.
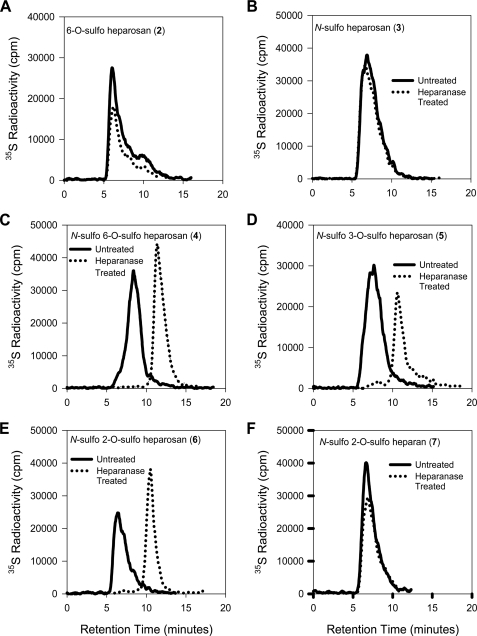
Both 6-O-sulfo heparosan and N-sulfo heparosan were exposed to heparanase, and the products were analyzed by GPC-HPLC. The elution profiles of the 35S-labeled substrates with or without heparanase from GPC-HPLC nearly overlapped, suggesting that neither of the substrates was susceptible to the digestion of heparanase (Fig. 3, A and B). It has also been reported that N-sulfo heparosan is not a substrate for heparanase (27, 28). Thus, our data confirmed the previous findings. Previous substrate studies also suggested that heparanase is unable to cleave the linkage of GlcA-GlcNAc6S using the model oligosaccharide substrates (28). Thus, our results are consistent with the reported conclusion derived from using oligosaccharide substrates.
FIGURE 3.
GPC-HPLC profiles of polysaccharide substrates with or without heparanase digestions. Site-specifically 35S-labeled polysaccharide substrates were incubated with 0.2 μg/ml of heparanase to determine the reactivity to the cleavage by the enzyme. The samples were then resolved by GPC-HPLC. Panels A–F show the profiles of substrates 2, 3, 4, 5, 6, and 7 with or without heparanase treatment. cpm, counts per min.
We next tested the substrates containing two types of sulfation, namely N- and O-sulfation on glucosamine units (4 and 5). Both 4 and 5 differed in the position of O-sulfo group on glucosamine units. N-sulfo 6-O-sulfo heparosan (4) contained the O-sulfo group at the 6-OH position, whereas N-sulfo 3-O-sulfo heparosan (5) contained the O-sulfo group at the 3-OH position (Fig. 2). The GPC-HPLC analysis of 4 and 5 (with or without heparanase treatment) revealed that the molecular mass of the polysaccharides were reduced, suggesting that both substrates were susceptible to the digestion of heparanase (Fig. 3, C and D). Because substrate 4 was an excellent substrate for heparanase and can be prepared readily in a large quantity, we used it as a substrate for the subsequent inhibition experiments as described below. It should be noted that substrate 5 contains the domain that is part of antithrombin-binding site of the HS carrying anticoagulant activity (35). It has been demonstrated that heparanase cleaves the oligosaccharide containing a linkage of -GlcA-GlcNS3S6S- (27); however, whether heparanase degrades the linkage of -GlcA-GlcNS3S- has not been reported. Taken together, our results demonstrate that both N- and O-sulfations (either 6-O-sulfation or 3-O-sulfation) are required for the cleavage by heparanase.
Effects of 2-O-Sulfation on the Susceptibility of Heparanase Digestion
The contribution of O-sulfation on the hexuronic acid unit to heparanase digestion was also investigated. Here, we prepared the substrate containing the -GlcA2S-GlcNS- (6) domain and the substrate containing the -IdoA2S-GlcNS- (7) domain. Substrate 6 was found to be cleaved by heparanase as demonstrated by the GPC analysis (Fig. 3E), suggesting introducing 2-O-sulfo groups to the GlcA unit conferred the susceptibility to the digestion of heparanase. The results of the analysis of heparanase-treated substrate 7 revealed a starkly different situation. The 35S-labeled substrate 7 that carries the repeating unit of IdoA2S-GlcNS is completely resistant to the heparanase digestion (Fig. 3F).
It is very important to note that a partial cleavage of substrate 7 was observed if the substrate was prepared using wild type 2-OST (supplemental Fig. 3). We conclude that the cleavage is due to the fact that a low level of -GlcA2S-GlcNS- is present in the substrate because wild type 2-OST sulfates both IdoA and GlcA (36). To overcome the contamination of GlcA2S in substrate 7, we utilized 2-OST mutant (2-OST Y94I), which exclusively sulfates the IdoA but not GlcA (37).
Heparanase Cleaves the Linkage That Contains Nonsulfated Glucuronic Acid
We then proved that the linkage of -GlcA-GlcNS (3S or 6S)- was indeed susceptible to the heparanase cleavage. To this end, we prepared two site-specific, 35S-labeled substrates 4 and 5, where the 35S-label was located at 6-OH position or 3-OH position, respectively. The polysaccharides were digested with heparanase followed by nitrous acid degradation. The degraded products were analyzed for the presence of 35S-labeled monosaccharide as illustrated in Fig. 4. If heparanase cleaves the linkage of -GlcA-GlcNS3S- or GlcA-GlcNS6S-, we should observe a 35S-labeled monosaccharide in the heparanase-treated substrates followed by nitrous acid degradation, whereas the monosaccharide should be absent in the substrate without heparanase treatment. As expected, 35S-labeled monosaccharide in heparanase-treated substrates 4 and 5 was observed as shown in Fig. 4, whereas no monosaccharide can be detected in the control sample. Our data suggest that heparanase cleaves the linkage between GlcA and GlcNS6S (or -3S).
FIGURE 4.
Determination of the heparanase cleavage sites within substrates 4 and 5. 35S-labeled substrates 4 and 5 were degraded with nitrous acid with or without pretreatment of heparanase. The products were analyzed by BioGel P-2. Panel A shows the profile of substrate 4 with or without pretreatment of heparanase. Panel B shows the profiles of substrate 5 with or without pretreatment of heparanase. Panel C depicts the reactions involved in the nitrous acid degradation with or without pretreatment of heparanase. cpm, counts per min.
Our next goal was to determine whether heparanase cleaves the linkage of -GlcA2S-GlcNS-, given the fact that substrate 6 is susceptible to heparanase cleavage. We prepared 2-O-35S sulfated substrate 6 and utilized the same method as described above with an intention to observe the 35S-labeled monosaccharide. However, we did not detect any 35S-labeled monosaccharide, suggesting that heparanase does not cleave the linkage of -GlcA2S-GlcNS- directly (supplemental Fig. 4). We then hypothesized that heparanase cleaves the linkage of GlcA-GlcNS, whereas a GlcA2S unit is located in proximity. To this end, N-35S sulfated substrate 6 was prepared. Substrate 6 was treated with heparanase followed by the degradation with a mixture of heparin lyases, and the products were analyzed by BioGel P-2 (Fig. 5C). As shown in Fig. 5, a 35S-labeled monosaccharide ([35S]GlcNS) was observed in the heparanase-treated sample, while this monosaccharide was absent in the substrate without heparanase treatment. Taken together, our results confirmed that heparanase does not cleave the linkage of -GlcA2S-GlcNS- but rather cleaves the linkage of -GlcA-GlcNS- nearby.
FIGURE 5.
Determination of the heparanase cleavage sites within substrate 6. 35S-labeled substrate 6 was degraded with heparin lyases I, II, and III with or without pretreatment of heparanase. The products were analyzed by BioGel P-2. It is worth noting that substrate 6 contains 35S-labeled N-sulfation and unlabeled 2-O-sulfation. Panel A shows the profile of substrate 6 without pretreatment of heparanase. Panel B shows the profiles of substrate 6 with pretreatment of heparanase. Panel C shows the reactions involved in the heparin lyase digestion with or without pretreatment of heparanase.
Polysaccharide 7, Containing -IdoA2S-GlcNS- Repeating Unit, Inhibits the Activity of Heparanase
As demonstrated above, polysaccharide 7, containing the repeating unit of -IdoA2S-GlcNS-, is not a substrate for heparanase, whereas substrate 6, containing -GlcA2S-GlcNS-, is a substrate. We hypothesized that polysaccharide 7 could serve as an inhibitor for heparanase. The reactions were then carried out in the presence of a range of concentrations (0.5–50 μg/ml) of substrate 7. To demonstrate the structural selectivity, both substrates 3 and 2 were also used. Substrate 3, with repeating disaccharide units of GlcA-GlcNAc, did not inhibit the enzyme activity even at the concentration of 50 μg/ml (Fig. 6A). Similarly, the same result was shown for substrate 2 with the repeating units of GlcA-GlcNAc6S (data not shown). However, the activity of heparanase was clearly affected by polysaccharide 7 in a dose-responsive manner (Fig. 6B). In fact, the addition of 50 μg/ml of polysaccharide 7 caused complete inhibition of the enzymatic activity. PI-88, a known inhibitor of heparanase, also exhibited the inhibition effect under these conditions (Fig. 6C). The inhibition potency of polysaccharide 7 was comparable to PI-88. Collectively, these data concluded that the polysaccharide with the repeating disaccharide structure IdoA2S-GlcNS could serve as a heparanase inhibitor.
FIGURE 6.
Inhibition of the activity of heparanase. Panel A shows the GPC-HPLC profiles of heparanase-treated 35S-labeled substrate in the presence of different concentrations of N-sulfo heparosan (polysaccharide 3) as well as the untreated 35S-labeled substrate. Panel B shows the GPC-HPLC profiles of heparanase-treated 35S-labeled substrate in the presence of different concentrations of N-sulfo 2-O-sulfo heparan (polysaccharide 7) as well as the untreated 35S-labeled substrate. Panel C shows the GPC-HPLC profiles of heparanase-treated 35S-labeled substrate in the presence of different concentrations of PI-88 as well as the untreated 35S-labeled substrate. cpm, counts per min.
DISCUSSION
The journey for identifying and characterizing heparanase has been long and less straightforward. The first mention of heparanase in literature occurred in 1975 when Ogren and Lindahl (38) reported the cleavage of heparin by an enzyme isolated from the mastocytomas of tumor bearing mice. Due to the lack of a reliable assay for monitoring the degradation of HS/heparin and a low abundance of heparanase, the cDNA of heparanase remained unidentified for another two decades (21, 39–41). The substrate specificities of heparanase were confounded by the structural heterogeneity of HS polysaccharide substrates. It largely was assumed that heparanase cleaves a very specific sulfated monosaccharide sequence. Previously, Pikas et al. (27) reported that N-sulfo groups and IdoA units are not required for substrate recognition, but O-sulfation on the hexuronic acid is essential for the enzyme action. This study also suggested that heparanase recognizes 2-O-sulfated hexuronic units that may be either IdoA or GlcA. However, Okada et al. (28) reported that the 2-O-sulfate group on the hexuronic residue was not essential for enzyme action. Using an impressive array of hexa- and tetrasaccharides, Okada et al. (28) concluded that heparanase cleaves the linkage of -GlcA-GlcNS6S-. However, it is still difficult to decipher the precise contribution of each O-sulfation type to the susceptibility of heparanase, largely due to the fact that the choice of oligosaccharides contained multiple types of modifications. Furthermore, these oligosaccharides did not cover all possible heparanase cleavable linkages. To this end, whether heparanase recognizes single or multiple monosaccharide sequences remains elusive.
In this article, we utilized synthesized polysaccharide substrates with systematically varied sulfations to determine the effects of N-sulfation, 6-O-sulfation, 2-O-sulfation, and epimerization on the susceptibility of heparanase cleavage. Synthetic polysaccharides offer several advantages. First, the sulfation type is highly homogeneous due to the exclusive regioselectivity of the biosynthetic enzymes. Such sulfation selectivity was confirmed by the disaccharide analysis. Second, the sulfation patterns in the synthetic polysaccharides are present in nearly all the HS isolated from natural sources. Thus, the synthetic polysaccharides represent the domain structures of HS isolated from natural sources. Third, the length of the synthetic polysaccharides is comparable to cellular HS. The results of our substrate specificity studies should represent the action of heparanase on cellular HS. In fact, the products of heparanase-cleaved HS are mainly larger than decasaccharide (supplemental Fig. 1). Thus, studies with polysaccharide substrates should eliminate the potential artifacts using oligosaccharide substrates. In our study, we found that the polysaccharides containing -GlcA-GlcNS6S- and -GlcA-GlcNS3S- are excellent substrates for heparanase. The effect of 6-O-sulfation is consistent with previous findings using oligosaccharide substrates (28). We also found that the polysaccharide with a repeating unit of -GlcA-GlcNAc6S- is not a substrate, consistent with the observation reported by Okada et al. (28) using an oligosaccharide substrate that contains the linkage of GlcA-GlcNAc6S. The effect of 2-O-sulfation on the hexuronic acid (GlcA and IdoA) is somewhat surprising. Our data suggested that the polysaccharide bearing the repeating unit of -GlcA2S-GlcNS- is cleaved by heparanase. Although the structure of -GlcA2S-GlcNS- has been suggested by Pikas et al. (42) using a chemically sulfated N-sulfo heparosan, their conclusion could be complicated by the presence of other types of sulfation in the polysaccharide. Starkly different from -GlcA2S-GlcNS-, the polysaccharide bearing IdoA2S-GlcNS is not a substrate and serves as an inhibitor of heparanase.
Our results revealed the substrate specificity of heparanase, which is summarized in Fig. 7. Heparanase cleaves the linkage of a nonsulfated glucuronic acid and glucosamine carrying N-sulfo or both N-sulfo and O-sulfo groups. Our model suggests that the enzyme recognizes certain sulfation patterns rather than specific sulfated monosaccharide sequences. Heparanase recognizes the N-sulfo group as well as the O-sulfo group at the glucosamine unit at +1 site. In the case that O-sulfation of glucosamine is absent, heparanase recognizes the GlcA2S unit located at the +2 site for cleaving the substrate. Alternatively, heparanase possibly recognizes the GlcA2S residue located at the −2 site. When replacing a GlcA2S unit at the +2 (or −2) position with IdoA2S, the site is no longer a cleavage site for heparanase. In addition, replacing the N-sulfo group with N-acetyl at the +1 position becomes resistant to heparanase degradation. It should be noted that whether the GlcA2S located beyond +2 or −2 site affects the susceptibility to the heparanase cleavage remains to be determined.
FIGURE 7.
Proposed heparanase substrate recognition sites. Heparanase cleaves the linkage between a nonsulfated GlcA and GlcNS. If the +1 site is 3-O-sulfated or 6-O-sulfated, the enzyme cleaves. If the +1 site is not O-sulfated, the enzyme searches for the structure beyond the GlcNS at −1 or +1 site. It is possible to recognize the 2-O-sulfo group located at either the +2 site or at the −2 site.
The active role of heparanase in tumor metastasis has attracted considerable interests in searching for an inhibitor of heparanase via different mechanisms of action (11). A number of chemical entities are known to inhibit the activity of heparanase. Among them, sulfated oligosaccharides like PI-88 have reached phase III clinical trials. Heparin is also a potent inhibitor of heparanase (43, 44). However, the anticoagulant activity of heparin could cause an undesired bleeding effect when used as an anticancer drug. Furthermore, heparin is capable of displacing the growth factors that are sequestered by the HS located in the extracellular matrix and consequently stimulates cell proliferation. Thus, there is a strong demand to maintain the activity for inhibiting heparanase while eliminating the anticoagulant or cell proliferation activities. Toward this goal, a glycol split heparin-like polysaccharide was discovered by Naggi et al. (45). Because the glycol split polysaccharide contains unnatural saccharide units and there is no clear pathway to degrade such molecule, it is a concern if it is accumulated in the body as a long term anticancer therapeutic. Here, we reported that a polysaccharide with the repeating unit of -IdoA2S-GlcNS- can also serve as an inhibitor. It is known that the polysaccharide carrying -IdoA2S-GlcNS- repeating sequence has no anticoagulant activity or the activity in stimulating cell proliferation (46). In fact, polysaccharide with -IdoA2S-GlcNS- serves as an inhibitor for fibroblast growth factor/HS-mediated cell proliferation activity (47), a favorable property as an anticancer agent. Our next goal is to identify the minimum saccharide structure carrying the -IdoA2S-GlcNS repeat for inhibiting the activity of heparanase.
The findings of this study revealed the recognition pattern of heparanase. The structural moiety recognized by heparanase includes a GlcA unit and GlcNS unit carrying O-sulfations. The enzyme proved to be promiscuous in the aspect of the type and location of O-sulfation required for recognition. Furthermore, the polysaccharide containing GlcA2S renders the susceptibility of heparanase cleavage. Our study also discovered that IdoA2S-GlcNS may serve as an inhibitor of heparanase enzymatic activity. The results should provide the substrate specificity of heparanase needed for the design of an effective inhibitor.
Supplementary Material
Acknowledgments
We greatly appreciate the gift of PI-88 from Dr. Bob Linhardt (Rensselaer Polytechnic Institute). We thank Ms. Heather Bethea and Dr. Kai Li (both are at University of North Carolina) for providing us with 2-OST Y94I protein for this study. We also thank Dr. Ulf Lindahl (Uppsala University) for constructive discussion on the cleavage specificity of heparanase and Heather Bethea for reviewing the manuscript.
This work was supported in part by National Institutes of Health Grants R01AI050050, R01HL094463, and R21AI074775. This work was also supported by Grant-in-aid 0855424E from the American Heart Association, Mid-Atlantic Affiliate.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- HS
- heparan sulfate
- GlcA
- glucuronic acid
- IdoA
- iduronic acid
- GlcNAc
- N-acetylated glucosamine
- GlcNS
- N-sulfated glucosamine
- 2-OST
- 2-O-sulfotransferase
- IdoA2S
- 2-O-sulfated iduronic acid
- CHO
- Chinese hamster ovary
- GPC
- gel permeation chromatography
- MES
- 4-morpholineethanesulfonic acid
- HPLC
- high pressure liquid chromatography
- MOPS
- 4-morpholinepropanesulfonic acid
- PAPS
- adenosine 3′-phosphate,5′-phosphosulfate.
REFERENCES
- 1.Vreys V., David G. (2007) J. Cell Mol. Med. 11, 427–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dempsey L. A., Brunn G. J., Platt J. L. (2000) Trends Biochem. Sci. 25, 349–351 [DOI] [PubMed] [Google Scholar]
- 3.Parish C. R., Freeman C., Hulett M. D. (2001) Biochim. Biophys. Acta 1471, M99–108 [DOI] [PubMed] [Google Scholar]
- 4.Vlodavsky I., Friedmann Y. (2001) J. Clin. Invest. 108, 341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen-Kaplan V., Doweck I., Naroditsky I., Vlodavsky I., Ilan N. (2008) Cancer Res. 68, 10077–10085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doweck I., Kaplan-Cohen V., Naroditsky I., Sabo E., Ilan N., Vlodavsky I. (2006) Neoplasia 8, 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quiros R. M., Rao G., Plate J., Harris J. E., Brunn G. J., Platt J. L., Gattuso P., Prinz R. A., Xu X. (2006) Cancer 106, 532–540 [DOI] [PubMed] [Google Scholar]
- 8.Simizu S., Ishida K., Osada H. (2004) Cancer Sci. 95, 553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao Y., Kleeff J., Shi X., Büchler M. W., Friess H. (2003) Hepatol. Res. 26, 192–198 [DOI] [PubMed] [Google Scholar]
- 10.Ilan N., Elkin M., Vlodavsky I. (2006) Int. J. Biochem. Cell Biol. 38, 2018–2039 [DOI] [PubMed] [Google Scholar]
- 11.Davidson B., Shafat I., Risberg B., Ilan N., Trope' C. G., Vlodavsky I., Reich R. (2007) Gynecol. Oncol. 104, 311–319 [DOI] [PubMed] [Google Scholar]
- 12.Friedmann Y., Vlodavsky I., Aingorn H., Aviv A., Peretz T., Pecker I., Pappo O. (2000) Am J. Pathol. 157, 1167–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gohji K., Hirano H., Okamoto M., Kitazawa S., Toyoshima M., Dong J., Katsuoka Y., Nakajima M. (2001) Int. J. Cancer 95, 295–301 [DOI] [PubMed] [Google Scholar]
- 14.Ikuta M., Podyma K. A., Maruyama K., Enomoto S., Yanagishita M. (2001) Oral. Oncol. 37, 177–184 [DOI] [PubMed] [Google Scholar]
- 15.Koliopanos A., Friess H., Kleeff J., Shi X., Liao Q., Pecker I., Vlodavsky I., Zimmermann A., Büchler M. W. (2001) Cancer Res. 61, 4655–4659 [PubMed] [Google Scholar]
- 16.Marchetti D., Nicolson G. L. (2001) Adv. Enzyme. Regul. 41, 343–359 [DOI] [PubMed] [Google Scholar]
- 17.Mikami S., Ohashi K., Usui Y., Nemoto T., Katsube K., Yanagishita M., Nakajima M., Nakamura K., Koike M. (2001) Jpn. J. Cancer Res. 92, 1062–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogishima T., Shiina H., Breault J. E., Tabatabai L., Bassett W. W., Enokida H., Li L. C., Kawakami T., Urakami S., Ribeiro-Filho L. A., Terashima M., Fujime M., Igawa M., Dahiya R. (2005) Clin. Cancer Res. 11, 1028–1036 [PubMed] [Google Scholar]
- 19.Shinyo Y., Kodama J., Hongo A., Yoshinouchi M., Hiramatsu Y. (2003) Ann. Oncol. 14, 1505–1510 [DOI] [PubMed] [Google Scholar]
- 20.Uno F., Fujiwara T., Takata Y., Ohtani S., Katsuda K., Takaoka M., Ohkawa T., Naomoto Y., Nakajima M., Tanaka N. (2001) Cancer Res. 61, 7855–7860 [PubMed] [Google Scholar]
- 21.Vlodavsky I., Friedmann Y., Elkin M., Aingorn H., Atzmon R., Ishai-Michaeli R., Bitan M., Pappo O., Peretz T., Michal I., Spector L., Pecker I. (1999) Nat. Med. 5, 793–802 [DOI] [PubMed] [Google Scholar]
- 22.Rabenstein D. L. (2002) Nat. Prod. Rep. 19, 312–331 [DOI] [PubMed] [Google Scholar]
- 23.Feyerabend T. B., Li J. P., Lindahl U., Rodewald H. R. (2006) Nat. Chem. Biol. 2, 195–196 [DOI] [PubMed] [Google Scholar]
- 24.Perrimon N., Bernfield M. (2000) Nature 404, 725–728 [DOI] [PubMed] [Google Scholar]
- 25.Sasisekharan R., Shriver Z., Venkataraman G., Narayanasami U. (2002) Nat. Rev. Cancer 2, 521–528 [DOI] [PubMed] [Google Scholar]
- 26.Bame K. J. (2001) Glycobiology 11, 91R–98R [DOI] [PubMed] [Google Scholar]
- 27.Pikas D. S., Li J. P., Vlodavsky I., Lindahl U. (1998) J. Biol. Chem. 273, 18770–18777 [DOI] [PubMed] [Google Scholar]
- 28.Okada Y., Yamada S., Toyoshima M., Dong J., Nakajima M., Sugahara K. (2002) J. Biol. Chem. 277, 42488–42495 [DOI] [PubMed] [Google Scholar]
- 29.Peterson S., Frick A., Liu J. (2009) Nat. Prod. Rep. 26, 610–627 [DOI] [PubMed] [Google Scholar]
- 30.Vann W. F., Schmidt M. A., Jann B., Jann K. (1981) Eur. J. Biochem. 116, 359–364 [DOI] [PubMed] [Google Scholar]
- 31.Duncan M. B., Liu M., Fox C., Liu J. (2006) Biochem. Biophys. Res. Commun. 339, 1232–1237 [DOI] [PubMed] [Google Scholar]
- 32.Shively J. E., Conrad H. E. (1976) Biochemistry 15, 3932–3942 [DOI] [PubMed] [Google Scholar]
- 33.Abboud-Jarrous G., Rangini-Guetta Z., Aingorn H., Atzmon R., Elgavish S., Peretz T., Vlodavsky I. (2005) J. Biol. Chem. 280, 13568–13575 [DOI] [PubMed] [Google Scholar]
- 34.Bame K. J. (1993) J. Biol. Chem. 268, 19956–19964 [PubMed] [Google Scholar]
- 35.Liu J., Shworak N. W., Fritze L. M., Edelberg J. M., Rosenberg R. D. (1996) J. Biol. Chem. 271, 27072–27082 [DOI] [PubMed] [Google Scholar]
- 36.Rong J., Habuchi H., Kimata K., Lindahl U., Kusche-Gullberg M. (2001) Biochemistry 40, 5548–5555 [DOI] [PubMed] [Google Scholar]
- 37.Li K., Bethea H. N., Liu J. (2010) J. Biol. Chem. 285, 11106–11113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogren S., Lindahl U. (1975) J. Biol. Chem. 250, 2690–2697 [PubMed] [Google Scholar]
- 39.Hulett M. D., Freeman C., Hamdorf B. J., Baker R. T., Harris M. J., Parish C. R. (1999) Nat. Med. 5, 803–809 [DOI] [PubMed] [Google Scholar]
- 40.Kussie P. H., Hulmes J. D., Ludwig D. L., Patel S., Navarro E. C., Seddon A. P., Giorgio N. A., Bohlen P. (1999) Biochem. Biophys. Res. Commun. 261, 183–187 [DOI] [PubMed] [Google Scholar]
- 41.Toyoshima M., Nakajima M. (1999) J. Biol. Chem. 274, 24153–24160 [DOI] [PubMed] [Google Scholar]
- 42.Lindahl U., Li J. P., Kusche-Gullberg M., Salmivirta M., Alaranta S., Veromaa T., Emeis J., Roberts I., Taylor C., Oreste P., Zoppetti G., Naggi A., Torri G., Casu B. (2005) J. Med. Chem. 48, 349–352 [DOI] [PubMed] [Google Scholar]
- 43.Bar-Ner M., Eldor A., Wasserman L., Matzner Y., Cohen I. R., Fuks Z., Vlodavsky I. (1987) Blood 70, 551–557 [PubMed] [Google Scholar]
- 44.Ishai-Michaeli R., Svahn C. M., Weber M., Chajek-Shaul T., Korner G., Ekre H. P., Vlodavsky I. (1992) Biochemistry 31, 2080–2088 [DOI] [PubMed] [Google Scholar]
- 45.Naggi A., Casu B., Perez M., Torri G., Cassinelli G., Penco S., Pisano C., Giannini G., Ishai-Michaeli R., Vlodavsky I. (2005) J. Biol. Chem. 280, 12103–12113 [DOI] [PubMed] [Google Scholar]
- 46.Chen J., Jones C. L., Liu J. (2007) Chem. Biol. 14, 986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDowell L. M., Frazier B. A., Studelska D. R., Giljum K., Chen J., Liu J., Yu K., Ornitz D. M., Zhang L. (2006) J. Biol. Chem. 281, 6924–6930 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.