Abstract
Two-component systems regulate critical cellular processes in microorganisms, and each comprises a homodimeric histidine kinase receptor and a cytoplasmic response regulator. Histidine kinases, often membrane associated, detect environmental input at sensor domains and propagate resulting signals to catalytic cytoplasmic transmitter domains. Recent studies on the great diversity of sensor domains reveal patterns of domain organization and biochemical properties that provide insight into mechanisms of signaling. Despite the enormous sequence variability found within sensor input domains, they fall into a relatively small number of discrete structural classes. Subtle rearrangements along a structurally labile dimer interface, in the form of possible sliding or rotational motions, are propagated from the sensor domain to the transmitter domain to modulate activity of the receptor.
Introduction
The ability of microorganisms to adapt to changes in the environment, by modifying gene expression levels in response to various stimuli, is conferred by two-component signal transduction systems. Signals are perceived by histidine kinase receptors and then transmitted to response regulator proteins, which typically act as transcription factors to elicit genetic responses [1]. Each histidine kinase sensor contains a variable input domain that is adapted for the detection of a specific stimulus, typically a chemical ligand, and a conserved transmitter domain that transfers the signal to its cognate response regulator through a phosphorylation cascade that begins on a conserved histidine within the transmitter domain [2]. Through genome sequencing, thousands of different histidine kinases have been identified in bacteria, archaea, fungi, yeast, and some plants, by virtue of high sequence conservation of the catalytic kinase domain.
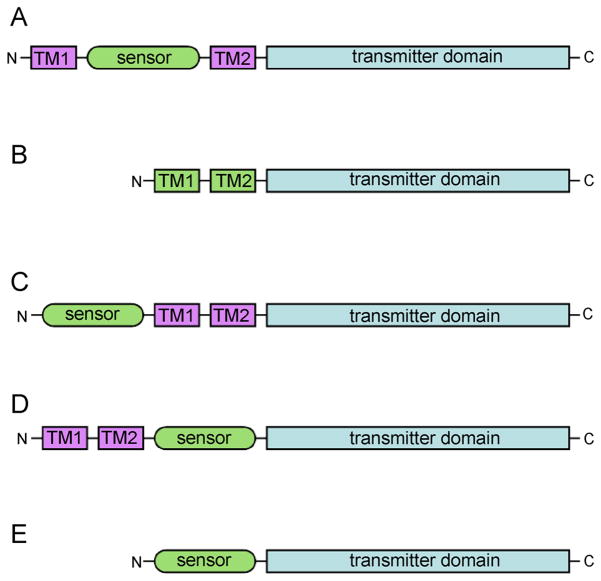
The protoypical histidine kinase sensor is a homodimeric integral membrane protein in which the sensor domain is formed by an extracellular loop contained between two membrane-spanning segments, and the transmitter domain follows the last transmembrane segment and is localized within the cytoplasm. A similar sensor topology exists in chemotaxis receptors, which signal through separate histidine kinase proteins and typically activate flagella rather than genes [3]. Some histidine kinase receptors deviate from the prototypical model and may have their sensor domains within the membrane or fully cytoplasmic. Therefore, depending on the subcellular localization of the specific system and properties of the detected stimulus, input domains may be extracellular, membrane-embedded, or intracellular (Figure 1).
Figure 1. Histidine kinase Domain Organization.
A schematic showing some basic examples of sensor domain organization in context with the full length histidine kinase receptor: (A) The sensor domain is often formed as a folded extracellular loop between usually two transmembrane segments in a membrane-spanning histidine kinase. (B) The sensor domain may be embedded within the membrane, composed from transmembrane helices as if having a truncated extracellular loop truncated to a stub. (C) The cytoplasmic sensor domain may be located N-terminal to two or more transmembrane segments in a membrane-anchored histidine kinase. (D) The cytoplasmic sensor domain may be located C-terminal to two or more transmembrane segments in a membrane-anchored histidine kinase. (E) The cytoplasmic sensor domain may reside N-terminal to the C-terminal transmitter domain in a soluble histidine kinase that lacks transmembrane segments.
Transmembrane segments designated TM1 and TM2 may be composed of more than one transmembrane segment, but always an odd number.
A recent surge in the structural study of sensor domains has revealed greater detail than ever before, and we begin to see discrete structural classes and global structural properties that provide insight into histidine kinase signaling. In this review we survey results from the study of sensor domains, concentrating on the last two years during which the emphasis has been mainly structural and biochemical in nature. We hope to elicit an appreciation for the functional diversity of sensor domains, and hope to provide a larger context of structural classification within which specific systems may be placed. We also describe how common structural properties contribute to the mechanisms of signaling, specifically between the sensor and transmitter domains of histidine kinase receptors.
Extracellular Sensor Domains
Recent structural study reveals three structural classes of extracellular sensor domain: mixed alpha-beta folds, all-alpha folds, and sensor domains that show a similar fold to periplasmic binding proteins. Sequence analysis reveals a potential additional class of all-beta sensors for which no determined structures exist. The term PDC (PhoQ-DcuS-CitA) domain has been given to an α+β-structural class of sensor domains, in reference to the initial three extracellular sensor domains PhoQ [4•,5], DcuS [6•,7], and CitA [8,9•] for which structures were first determined. PhoQ, DcuS, and CitA sense divalent ions, certain C4-dicarboxylates, and citrate respectively. PDC sensors, which appear to be most prevalent, are distinguished by a central five-stranded anti-parallel β-sheet scaffold that is flanked by α-helices on either side, beginning with a long N-terminal helix and often ending with a short C-terminal helix. The five-stranded β-sheet in PDC sensor domains has the same topology as in PAS (Per/ARNT/Sim) domains [10], but other structural features are distinctive [4]. Recent additions to the collection of PDC sensor domain structures include the oligosaccharide sensor AbfS [11] and the PhoR periplasmic domain deposited in the PDB (ID: 3cwf) by the Midwest Center for Structural Genomics (MCSG).
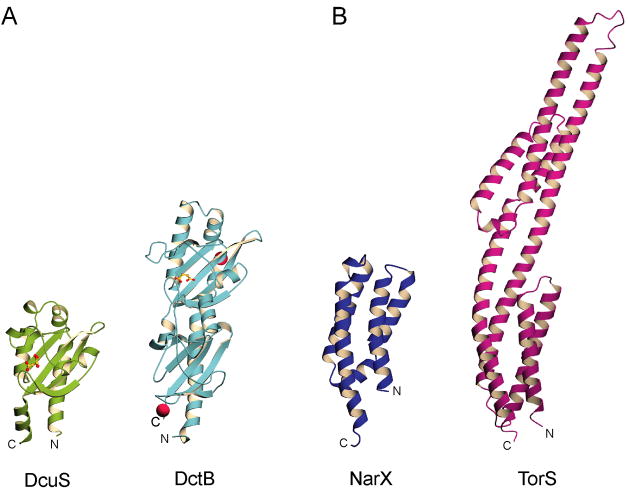
A recurring theme observed in extracellular sensor domains are domain insertions in which a second membrane-distal PDC domain is inserted between the first and second helices of the membrane-proximal PDC domain to form a double-PDC domain (Figure 2A). There is very low sequence similarity between the two domains in these cases, despite relatively high structural agreement. Domain insertion was first reported in the quorum sensor LuxQ [12,13], which forms a complex with the periplasmic binding protein LuxP to sense autoinducer-2). Domain insertion was later reported in DctB [6•,14•], a C4-dicarboxylate sensor containing a ligand binding pocket in the membrane-distal domain. More recent examples include the double-PDC sensors HK1s-Z2, -Z3, -Z6, -Z8, and -Z16 (Zhang and Hendrickson, unpublished), which have been grouped into the same family by a structural genomics analysis, and the sensing domain of sporulation factor KinD, which was deposited in the PDB (code 3fos) by MCSG. It should be noted that PDC and double-PDC folds are also found in input domains of chemotaxis sensors and this fact is exemplified by structures in the PDB with codes 3c8c and 2qhk that have been deposited by the New York SGX Research Center for Structural Genomics and MCSG, respectively. It would therefore be misleading to represent all chemotaxis receptor input domains as being helical like Tar [15]. Although some families of PDC and double-PDC sensor domains have been identified and grouped through common sequence motifs and homology as Cache [16,17] and CHASE [18,19] domains, we believe such methods of domain classification are neither comprehensive nor rigorous on a large scale considering the extreme sequence diversity found within sensor domains.
Figure 2. Sensor Domain Insertions.
Ribbon diagrams of (A) PDC sensors DcuS and DctB, and (B) all-alpha sensors NarX and Tors showing domain insertions in the fold. Non-protein moieties are shown in ball-and-stick representation with carbon in yellow, oxygen in red, and calcium in magenta.
All-helical extracellular sensor domains are represented by NarX [20••], TorS [21], and a structure in the PDB with code 3kkb deposited by MCSG, which form four-helix bundles that are similar in some extent to Tar [15]. A domain insertion, reminiscent of those in double-PDC sensor domains, is seen in TorS. Here, a membrane-distal left-handed four-helix bundle is stacked against a membrane-proximal right-handed four-helix bundle (Figure 2B). Unlike NarX, which senses nitrite and nitrate through direct binding, TorS forms a complex with the accessory periplasmic binding protein TorT and detects trimethylamine-N-oxide [22].
A third class of extracellular sensor domain has been predicted to be similar in fold to periplasmic binding proteins, and is exemplified by the single structural representative HK29s [23] for which the stimulus has yet to be determined. In comparison to true periplasmic binding proteins, there is an apparent lack of flexibility in the hinge region between the two domain lobes of HK29s. Sequence analysis of extracellular sensor domains reveals that tandem repeat motifs exist in this structural class as well.
Membrane-Embedded Sensor Domains
Histidine kinase receptors that contain multiple transmembrane segments often lack obvious extracellular domains, and in subsets of such cases, the sensory region may lie within the transmembrane segments (Figure 1B). Biochemical and structural studies of the DesK temperature sensor shows that the transmembrane regions are required for a thermal response [24,25]. In the membrane-embedded SenS redox sensor, regulation requires binding of the secreted octameric heme-binding protein HbpS to its N-terminal transmembrane region [26]. In addition, evidence suggests that the plant Etr1 histidine kinase detects ethylene within the hydrophobic N-terminal transmembrane region [27], and the AgrC quorum sensor detects an autoinducing peptide via two short extracellular loops in proximity to the transmembrane region [28,29]. Although no structures exist for the transmembrane regions of histidine kinase receptors, the structure of the phototaxis sensory rhodopsin II-transducer complex (HtrII-SrII) [30], in which the transducer protein forms a four-helix bundle in the membrane, provides insight into the transmembrane helical arrangement of histidine kinases.
Cytoplasmic Sensor Domains
Many histidine kinase receptors are entirely cytoplasmic, including the sensor domain. In the case of membrane-anchored histidine kinases, cytoplasmic sensor domains may be found either at the N-terminus before the first transmembrane segment (Figure 1C), or after the last transmembrane segment before the C-terminal kinase domain (Figure 1D). Studies have revealed that many cytoplasmic sensor domains adopt a true PAS fold, distinct from the PDC-fold of many extracellular sensor domains. Examples include the Bradyrhizobium japonicum and Rhizobium meliloti FixL sensor domains, which were among the first structures of histidine kinase sensor domains determined, and they continue to be subject to ongoing study [31•–38•,39]. PAS domains consist of a five-stranded anti-parallel β-sheet core flanked by α-helices, and are part of a broad family of signaling domains that may be involved with cofactor binding and protein-protein interaction. FixL senses oxygen through heme, the NreB sensor detects oxygen through an iron-sulfur ([4Fe-4S]2+) cluster [40], and the LovK photosensor domain forms a flavin adduct upon absorption of blue light [41]. In the case of the MmoS redox-sensor, two tandem PAS domains (PAS-A and PAS-B) are found in the sensor domain with a FAD cofactor bound to the N-terminal PAS domain [42]. In the extensively studied ArcB redox-sensor, a putative cytoplasmic PAS sensor domain contains redox-active cysteine residues and is regulated by changes in redox states of quinone and menaquinone pools [43,44]. Although extracellular PDC sensors are PAS-like in that they contain a central β-sheet scaffold of the same topology, we believe they should not be classified as PAS domains based simply on having a similar core because the additional N-terminal, C-terminal, and transverse helices present in PDC sensors defines a larger discrete domain such that fold-recognition algorithms used by the DALI server [45] detect greater similarities between various PDC sensors with each other than with other PAS sensors. The classification of PDC sensors as PAS domains may also imply having similar evolutionary relationships when their extreme sequence diversities suggest independent parallel evolution instead.
A second structural family of cytoplasmic sensor domains are those that adopt a GAF fold [46], another ubiquitous protein signaling domain. GAF sensor domains consists of a six-stranded anti-parallel β-sheet core, are related in structure to PAS domains, and are often found in tandem in cytoplasmic histidine kinase sensors. The structures of the first GAF domain (GAF-A) of Mycobacterium tuberculosis DosS [47] and DosT [48] sensor domains have been recently determined, and are heme-containing redox sensors that specifically detect changes in the redox state of a bound iron, or in binding of oxygen, respectively. The Mycobacterium smegmatis GAF-A redox-sensing domain of the DevS histidine kinase is also heme-bound and controlled through the binding of oxygen; however, the structure of the DevS GAF-B domain yields little clue about its function [49]. The structure of two additional GAF domains from histidine kinases of unknown function have also been deposited into the PDB (codes 3hcy and 3cit) by MCSG.
A third family of cytoplasmic sensor domains are found in phytochromes, soluble histidine kinase photoreceptors found in bacteria, fungi, and plants. Structural studies of the Pseudomonas aeruginosa bacteriophytochrome photosensory core domain (PaBphP-PCD) reveals a tripartite PCD that contains a PAS, GAF, and phytochrome (PHY) domain arranged along an extended α–helix [50,51•]. Similar to the PAS and GAF domains, the PHY domain also contains a five-stranded anti-parallel β-sheet scaffold. Together the PAS and GAF domains form a chromophore binding domain (CBD) that is covalently linked to a bilin chromophore which photoconverts between red- and far-red absorbing states. Additional structural studies include the entire PCD of Synechocystis 6803 Cph1 [52], and CBD’s of Dienococcus radiodurans DrBphP-CBD [53] and Rhodopseudomonas palustris RpBphP-CBD [54]. The aforementioned structures of histidine kinase sensor domains are summarized in Table 1.
Table 1.
Structures of Histidine Kinase Sensor Domains
| Extracellular Sensors | ||
|---|---|---|
| Name | Fold | Reference(s) |
| NarX | all-helical | [20••] |
| 3kkb | all-helical | PDB code 3kkb |
| TorS | double all-α | [21] |
| PhoQ | PDC | [4•,5] |
| CitA | PDC | [8,9•] |
| DcuS | PDC | [6•,7] |
| AbsF | PDC | [11] |
| PhoR | PDC | PDB code 3cwf |
| LuxQ | double-PDC | [12,13] |
| DctB | double-PDC | [6•,14•] |
| HK1s-Z2 | double-PDC | Unpublished |
| HK1s-Z3 | double-PDC | Unpublished |
| HK1s-Z6 | double-PDC | Unpublished |
| HK1s-Z8 | double-PDC | Unpublished |
| HK1s-Z16 | double-PDC | Unpublished |
| KinD | double-PDC | PDB code 3fos |
| HK29s | PBP-like | [23] |
| Membrane-embedded Sensors | ||
| Name | Fold | Reference(s) |
| HtrII-SrII | all-helical | [30] |
| Cytoplasmic Sensors | ||
| Name | Fold | Reference(s) |
| FixL | PAS | [31•–38•,39] |
| MmoS | PAS | [42] |
| DosS | GAF | [47] |
| DosT | GAF | [48] |
| DevS | GAF | [49] |
| BphP-PCD | PAS, GAF, PHY | [50,51•,52–54] |
Sensor Domain Dimerization and Insights into Mechanisms of Function
It is widely accepted that histidine kinases exist as homodimers, and that signal transduction exists in the context of a dimer. One might blindly expect all sensor domains to form dimers in solution when expressed as truncated protein constructs in relation to the full length intact receptor; however, where reported, many sensor domains (NarX, DcuS, DctB, CitA, HK29s, HK1s-Z2, -Z3, -Z6, -Z8, and -Z16) are largely monomeric in solution during purification [6•,9•,20••,23]; Zhang and Hendrickson, unpublished]. In cases where self-association has been measured by equilibrium analytical centrifugation (TorS and DcuS), Kd values can be relatively high [20••,21] and high protein concentrations are needed to observe dimerization. Light scattering experiments have also shown that propensities for dimerization in solution may depend on the signaling state (LuxPQ sensor complex), and that changes in affinity at the interface may have functional relevance [13]. Although self associations are weak for many sensor domains when studied in isolation, these associations are expected to be enhanced by several orders of magnitude for intact receptors in membranes [55] and with intrinsically dimeric cytoplasmic transmitter domains [56] whereby the local concentration is extremely high.
Transmembrane segments in membrane-embedded histidine kinases are expected to form dimeric four-helical bundles, as seen in the structure of the functionally related chemotaxis transducer protein of the HtrII-SrII complex [30], where N- and C-terminal ends of extracellular sensor domains must necessarily be oriented in the same direction, in a roughly parallel arrangement in close proximity to each other in order to establish connection (Figure 3). The prevailing model of sensor domain dimerization is similar to that seen in the chemotaxis receptor Tar [15], in which interactions along the length of the first extracellular N-terminal helix form a significant part of the interface. Functional in vivo evidence for a similar dimeric arrangement in histidine kinase sensor domains was initially seen in the structural study of Escherichia coli PhoQ [4•]. Additional examples of extracellular sensor domains that show similar apparent physiologically relevant dimeric associations in the crystal lattice include NarX [20••], DcuS [6•], DctB (Sinorhizobium meliloti) [14•], CitA (citrate-bound) [9•], LuxQ [13], HK1s-Z2 and -Z3 (Zhang and Hendrickson, unpublished), and KinD (PDB code: 3fos). In the case of cytoplasmic sensors, a similar arrangement is observed in both the wild type and Q188L mutant forms of the P. aeruginosa BphP-PCD and helical connections to the kinase transmitter domain have been modeled [50,51•].
Figure 3. Dimeric Sensor Domains with Modeled Transmembrane Helices.
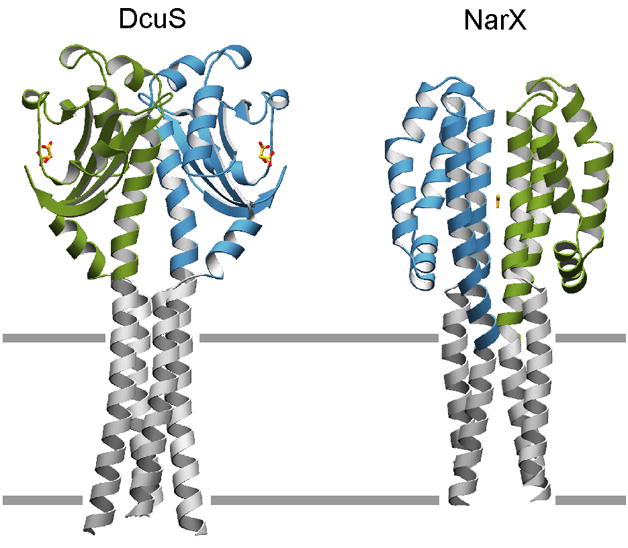
Dimeric sensor domain structures of DcuS and NarX have been modeled with transmembrane helices based on those of the phototaxis transducer [30]. Drawings are as ribbon diagrams with residues of the sensor domains colored green and blue and the modeled transmembrane residues colored grey. Non-protein moieties malate and nitrate, bound to DcuS and NarX respectively, are depicted in ball-and-stick representation. Putative plane of the lipid bilayer is depicted as grey lines.
As self-association between individual sensor domain subunits is relatively weak, crystal lattice packing forces or minor electrostatic and structural differences in sensor domains due to ligand binding, mutations, and variations in sequence between different homologues may result in different modes of self-association during crystallization. PhoQ (E. coli acid-mutant) [4•], NarX (apo state) [20••], CitA (apo state) [9•], TorS [21], MmoS [42], HK1s-Z8 and –Z16 (Zhang and Hendrickson, unpublished), DctB (Vibrio cholerae) [6•], AbfS [11], PhoR (PDB code 3cwf), and the Synechocystis 6803 BphP-PCD [52] are examples for which pairs of sensor domain subunits do not form physiologically relevant dimers even though a variant may exist in a different crystal lattice in which physiologically relevant dimers are observed. In fact, PhoQ (E. coli acid-mutant), HK1s-Z8 and –Z16, and Synechocystis 6803 BphP-PCD actually form head-to-tail dimers in which N- and C-terminal ends of sensor domain subunits face opposite directions, and although a relatively large surface area is buried within the interface, it is clear that buried surface area is not a reliable determinant of biologically relevant dimerization.
Sometimes self-associations can be misleading and certain caveats must be made in the interpretation of apparent dimers in sensor domain crystal structures. In the case of the initial structure of citrate-bound CitA, crystallized in the presence of molybdenum, a convincing physiologically relevant “head-to-head” dimer formed from G and J subunits was reported [8]; however, subsequent crystal studies of CitA in the absence of molybdenum show a different and more plausible dimeric arrangement [9•]. In the initial structure of PhoQ (Salmonella typhimurium) [5], the N- and C-terminal ends of sensor domain subunits in a putative dimer are located relatively far apart, despite the fact that there are close associations along some regions of the N-terminal helices in a manner that points the N- and C-terminal ends outwards from the same face of the dimer. In light of the more recent E. coli PhoQ [4•] structure, which shows a functional dimeric arrangement in which N- and C-terminal ends of different subunits are more closely positioned, it is unclear whether the S. typhimurium PhoQ structure represents a valid signaling state. HK1s-Z6 (Zhang and Hendrickson, unpublished) reveals a dimeric association between membrane-distal regions of the N-terminal helices, such that the helical N- and C-terminal regions of opposing subunits are located relatively far apart. The biological relevance of such an association is questionable.
It is apparent from the wealth of recent study of sensor domains that a certain plasticity exists along the dimer interface, and presumably dimerization cannot be absolutely rigid as conformational changes must be transmitted along the interface to the kinase transmitter domain upon detection of stimulus. There is evidence to suggest that such changes may be subtle. Structural studies and comparisons of the cytoplasmic FixL sensor in various signaling states reveals five different dimeric arrangements along a conserved interface in which certain subgroups of such arrangements are closely related [31•]. It is believed that distortions in the β–sheet due to ligand binding cause quaternary changes that can be transmitted along the dimer interface towards the kinase transmitter domain. A similar mechanism is proposed for CitA [9•], in which citrate binding causes a β-sheet distortion that results in a piston-like sliding motion between N- and C-terminal helices that are propagated into the cytoplasmic kinase domain. In NarX, nitrate binding at the dimer interface is also believed to effect signaling through a similar piston sliding motion between terminal helices [20••]. Such mechanisms have been proposed in the related chemotaxis signaling of Tar, where asymmetric ligand binding leads to relative helical displacements in only one subunit [57]. Asymmetry and plasticity along the dimer interface has also been observed in bacteriophytochrome photosensory core domains [50]. Structural studies of the LuxPQ signaling complex reveal ligand-induced asymmetry at the dimer interface but through a significant rotation [13]. Rotational events are also thought to occur within the transmembrane signaling helices of the phototaxis receptor complex [30], but there they are less pronounced. Although there is an apparent lack of uniformity between the specific conformational changes that take place within sensor domains upon changes in stimulus, the recent structural evidence suggests that communication between sensor and transmitter domains in histidine kinase signaling is mediated by subtle structural changes along the dimer interface and that there may be related aspects of symmetry and asymmetry.
Conclusion
The wealth of data from recent studies of sensor domains allows for their global structure-based classification, and has revealed how common structural themes are involved in mechanisms of signal transduction. Perhaps future study of full-length intact proteins, rather than isolated domains, would provide greater insight into signaling. Considering the fact that histidine kinase receptors regulate many critical cellular processes in microorganisms, such study also has pharmaceutical relevance towards understanding and finding potential new treatments for disease.
Unanswered Questions and Future Directions.
What is the full range of sensor domain structural diversity?
What if any correlates exist between structural type and ligand selectivity?
How does ligand binding initiate signal transmission in different systems?
Are mechanisms for transmembrane signal transmission conserved?
What parallels exist between histidine kinase and chemotactic sensors?
What are the structures of full-length, intact histidine kinase receptors?
Can sensor domains be engineered into custom bacterial sensor systems?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended Reading
Papers of particular interest, published within the period of the review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu Rev Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutta R, Qin L, Inouye M. Histidine kinases: diversity of domain organization. Mol Microbiol. 1999;34:633–640. doi: 10.1046/j.1365-2958.1999.01646.x. [DOI] [PubMed] [Google Scholar]
- 3.Kirby JR. Chemotaxis-Like Regulatory Systems: Unique Roles in Diverse Bacteria. Annu Rev Microbiol. 2009;63:45–59. doi: 10.1146/annurev.micro.091208.073221. [DOI] [PubMed] [Google Scholar]
- 4•.Cheung J, Bingman CA, Reyngold M, Hendrickson WA, Waldburger CD. Crystal structure of a functional dimer of the PhoQ sensor domain. J Biol Chem. 2008;283:13762–13770. doi: 10.1074/jbc.M710592200. The structure of a biologically relevant PDC sensor domain dimer is characterized for the first time with supporting in vivo data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho US, Bader MW, Amaya MF, Daley ME, Klevit RE, Miller SI, Xu WQ. Metal bridges between the PhoQ sensor domain and the membrane regulate transmembrane signaling. J Mol Biol. 2006;356:1193–1206. doi: 10.1016/j.jmb.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 6•.Cheung J, Hendrickson WA. Crystal Structures of C4-Dicarboxylate Ligand Complexes with Sensor Domains of Histidine Kinases DcuS and DctB. J Biol Chem. 2008;283:30256–30265. doi: 10.1074/jbc.M805253200. Similar mechanisms of C4-dicarboxylate ligand binding are revealed by high resolution structures of PDC sensors DcuS and DctB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappalardo L, Janausch IG, Vijayan V, Zientz E, Junker J, Peti W, Zweckstetter M, Unden G, Griesinger C. The NMR structure of the sensory domain of the membranous two-component fumarate sensor (histidine protein kinase) DcuS of Escherichia coli. J Biol Chem. 2003;278:39185–39188. doi: 10.1074/jbc.C300344200. [DOI] [PubMed] [Google Scholar]
- 8.Reinelt S, Hofmann E, Gerharz T, Bott M, Madden DR. The structure of the periplasmic ligand-binding domain of the sensor kinase CitA reveals the first extracellular PAS domain. J Biol Chem. 2003;278:39189–39196. doi: 10.1074/jbc.M305864200. [DOI] [PubMed] [Google Scholar]
- 9•.Sevvana M, Vijayan V, Zweckstetter M, Reinelt S, Madden DR, Herbst-Irmer R, Sheldrick GM, Bott M, Griesinger C, Becker S. A ligand-induced switch in the periplasmic domain of sensor histidine kinase CitA. J Mol Biol. 2008;377:512–523. doi: 10.1016/j.jmb.2008.01.024. An important study detailing the mechanism of citrate binding in the CitA sensor, revealing conformational changes that occur between the ligand-bound and apo states. [DOI] [PubMed] [Google Scholar]
- 10.Moglich A, Ayers RA, Moffat K. Structure and Signaling Mechanism of Per-ARNT-Sim Domains. Structure. 2009;17:1282–1294. doi: 10.1016/j.str.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emami K, Topakas E, Nagy T, Henshaw J, Jackson KA, Nelson KE, Mongodin EF, Murray JW, Lewis RJ, Gilbert HJ. Regulation of the Xylan-degrading Apparatus of Cellvibrio japonicus by a Novel Two-component System. J Biol Chem. 2009;284:1086–1096. doi: 10.1074/jbc.M805100200. [DOI] [PubMed] [Google Scholar]
- 12.Neiditch MB, Federle MJ, Miller ST, Bassler BL, Hughson FM. Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol Cell. 2005;18:507–518. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Neiditch MB, Federle MJ, Pompeani AJ, Kelly RC, Swem DL, Jeffrey PD, Bassler BL, Hughson FM. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Zhou YF, Nan BY, Nan J, Ma QJ, Panjikar S, Liang YH, Wang YP, Su XD. C4-Dicarboxylates Sensing Mechanism Revealed by the Crystal Structures of DctB Sensor Domain. J Mol Biol. 2008;383:49–61. doi: 10.1016/j.jmb.2008.08.010. Structures of the DctB sensor domain in the apo state and in various ligand-bound states reveals mechanisms of ligand binding, stereospecificity, and dimerization. [DOI] [PubMed] [Google Scholar]
- 15.Yeh JI, Biemann HP, Prive GG, Pandit J, Koshland DE, Kim SH. High-resolution structures of the ligand binding domain of the wild-type bacterial aspartate receptor. J Mol Biol. 1996;262:186–201. doi: 10.1006/jmbi.1996.0507. [DOI] [PubMed] [Google Scholar]
- 16.Anantharaman V, Aravind L. Cache - a signaling domain common to animal Ca2+ channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem Sci. 2000;25:535–537. doi: 10.1016/s0968-0004(00)01672-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhulin IB, Nikolskaya AN, Galperin MY. Common extracellular sensory domains in transmembrane receptors for diverse signal transduction pathways in Bacteria and Archaea. J Bacteriol. 2003;185:285–294. doi: 10.1128/JB.185.1.285-294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anantharaman V, Aravind L. The CHASE domain: a predicted ligand-binding module in plant cytokinin receptors and other eukaryotic and bacterial receptors. Trends Biochem Sci. 2001;26:579–582. doi: 10.1016/s0968-0004(01)01968-5. [DOI] [PubMed] [Google Scholar]
- 19.Mougel C, Zhulin IB. CHASE: an extracellular sensing domain common to transmembrane receptors from prokaryotes, lower eukaryotes and plants. Trends Biochem Sci. 2001;26:582–584. doi: 10.1016/s0968-0004(01)01969-7. [DOI] [PubMed] [Google Scholar]
- 20••.Cheung J, Hendrickson WA. Structural Analysis of Ligand Stimulation of the Histidine Kinase NarX. Structure. 2009;17:190–201. doi: 10.1016/j.str.2008.12.013. A difference distance matrix analysis of structures of the all-helical NarX sensor domain in both nitrate-bound and apo states reveals a piston-sliding conformational change between the N- and C-terminal helices. This study brings new light to a well-studied two-component system and reveals similar mechanisms of function to chemotaxis receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore JO, Hendrickson WA. Structural Analysis of Sensor Domains from the TMAO-Responsive Histidine Kinase Receptor TorS. Structure. 2009;17:1195–1204. doi: 10.1016/j.str.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Baraquet C, Theraulaz L, Guiral M, Lafitte D, Mejean V, Jourlin-Castelli C. TorT, a member of a new periplasmic binding protein family, triggers induction of the tor respiratory system upon trimethylamine N-oxide electron-acceptor binding in Escherichia coli. J Biol Chem. 2006;281:38189–38199. doi: 10.1074/jbc.M604321200. [DOI] [PubMed] [Google Scholar]
- 23.Cheung J, Le-Khac M, Hendrickson WA. Crystal structure of a histidine kinase sensor domain with similarity to periplasmic binding proteins. Proteins. 2009;77:235–241. doi: 10.1002/prot.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albanesi D, Martin M, Trajtenberg F, Mansilla MC, Haouz A, Alzari PM, de Mendoza D, Buschiazzo A. Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc Natl Acad Sci USA. 2009;106:16185–16190. doi: 10.1073/pnas.0906699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin M, Albanesi D, Alzari PM, de Mendoza D. Functional in vitro assembly of the integral membrane bacterial thermosensor DesK. Prot Expr Purif. 2009;66:39–45. doi: 10.1016/j.pep.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Bogel G, Schrempf H, Lucana DO. The heme-binding protein HbpS regulates the activity of the Streptomyces reticuli iron-sensing histidine kinase SenS in a redox-dependent manner. Amino Acids. 2009;37:681–691. doi: 10.1007/s00726-008-0188-5. [DOI] [PubMed] [Google Scholar]
- 27.Voet-van-Vormizeele J, Groth G. Ethylene Controls Autophosphorylation of the Histidine Kinase Domain in Ethylene Receptor ETR1. Mol Plant. 2008;1:380–387. doi: 10.1093/mp/ssn004. [DOI] [PubMed] [Google Scholar]
- 28.Geisinger E, George EA, Muir TW, Novick RP. Identification of ligand specificity determinants in AgrC, the Staphylococcus aureus quorum-sensing receptor. J Biol Chem. 2008;283:8930–8938. doi: 10.1074/jbc.M710227200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen RO, Winzer K, Clarke SR, Chan WC, Williams P. Differential recognition of Staphylococcus aureus quorum-sensing signals depends on both extracellular loops 1 and 2 of the transmembrane sensor AgrC. J Mol Biol. 2008;381:300–309. doi: 10.1016/j.jmb.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Gordeliy VI, Labahn J, Moukhametzianov R, Efremov R, Granzin J, Schlesinger R, Buldt G, Savopol T, Scheidig AJ, Klare JP, et al. Molecular basis of transmembrane signalling by sensory rhodopsin II-transducer complex. Nature. 2002;419:484–487. doi: 10.1038/nature01109. [DOI] [PubMed] [Google Scholar]
- 31•.Ayers RA, Moffat K. Changes in Quaternary Structure in the Signaling Mechanisms of PAS Domains. Biochem. 2008;47:12078–12086. doi: 10.1021/bi801254c. Analysis of high resolution structures of the FixL sensor crystallized in different space groups and in various ligand-bound states reveal a relatively plastic conserved dimer interface with implications for mechanisms of signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunham CM, Dioum EM, Tuckerman JR, Gonzalez G, Scott WG, Gilles-Gonzalez MA. A distal arginine in oxygen-sensing Heme-PAS domains is essential to ligand binding, signal transduction, and structure. Biochemistry. 2003;42:7701–7708. doi: 10.1021/bi0343370. [DOI] [PubMed] [Google Scholar]
- 33.Gilles-Gonzalez MA, Caceres AI, Sousa EHS, Tomchick DR, Brautigam CA, Gonzalez C, Machius M. A proximal arginine R206 participates in switching of the Bradyrhizobium japonicum FixL oxygen sensor. J Mol Biol. 2006;360:80–89. doi: 10.1016/j.jmb.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 34.Gong W, Hao B, Mansy SS, Gonzalez G, Gilles-Gonzalez MA, Chan MK. Structure of a biological oxygen sensor: a new mechanism for heme-driven signal transduction. Proc Natl Acad Sci USA. 1998;95:15177–15182. doi: 10.1073/pnas.95.26.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong WM, Hao B, Chan MK. New mechanistic insights from structural studies of the oxygen-sensing domain of Bradyrhizobium japonicum FixL. Biochemistry. 2000;39:3955–3962. doi: 10.1021/bi992346w. [DOI] [PubMed] [Google Scholar]
- 36.Hao B, Isaza C, Arndt J, Soltis M, Chan MK. Structure-based mechanism of O-2 sensing and ligand discrimination by the FixL heme domain of Bradyrhizobium japonicum. Biochemistry. 2002;41:12952–12958. doi: 10.1021/bi020144l. [DOI] [PubMed] [Google Scholar]
- 37.Key J, Moffat K. Crystal structures of deoxy and CO-bound bjFixLH reveal details of ligand recognition and signaling. Biochemistry. 2005;44:4627–4635. doi: 10.1021/bi047942r. [DOI] [PubMed] [Google Scholar]
- 38•.Key J, Srajer V, Pahl R, Moffat K. Time-resolved crystallographic studies of the heme domain of the oxygen sensor FixL: Structural dynamics of ligand rebinding and their relation to signal transduction. Biochemistry. 2007;46:4706–4715. doi: 10.1021/bi700043c. Time-resolved structural studies reveals dynamics and conformational changes between ligand-bound and ligand-free states in the well-studied FixL sensor domain, with implications for mechanisms of function. [DOI] [PubMed] [Google Scholar]
- 39.Miyatake H, Mukai M, Park SY, Adachi S, Tamura K, Nakamura H, Nakamura K, Tsuchiya T, Iizuka T, Shiro Y. Sensory mechanism of oxygen sensor FixL from Rhizobium meliloti: Crystallographic, mutagenesis and resonance Raman spectroscopic studies. J Mol Biol. 2000;301:415–431. doi: 10.1006/jmbi.2000.3954. [DOI] [PubMed] [Google Scholar]
- 40.Mullner M, Hammel O, Mienert B, Schlag S, Bill E, Unden G. A PAS domain with an oxygen labile [4Fe-4S](2+) cluster in the oxygen sensor kinase NreB of Staphylococcus carnosus. Biochemistry. 2008;47:13921–13932. doi: 10.1021/bi8014086. [DOI] [PubMed] [Google Scholar]
- 41.Purcell EB, Siegal-Gaskins D, Rawling DC, Fiebig A, Crosson S. A photosensory two-component system regulates bacterial cell attachment. Proc Natl Acad Sci USA. 2007;104:18241–18246. doi: 10.1073/pnas.0705887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ukaegbu UE, Rosenzweig AC. Structure of the Redox Sensor Domain of Methylococcus capsulatus (Bath) MmoS. Biochemistry. 2009;48:2207–2215. doi: 10.1021/bi8019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bekker M, Alexeeva S, Laan W, Sawers G, Teixeira de Mattos J, Hellingwerf K. The ArcBA two-component system of Escherichia coli is regulated by the redox state of both the ubiquinone and the menaquinone pool. J Bacteriol. 2010;192:746–754. doi: 10.1128/JB.01156-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malpica R, Franco B, Rodriguez C, Kwon O, Georgellis D. Identification of a quinone-sensitive redox switch in the ArcB sensor kinase. Proc Natl Acad Sci USA. 2004;101:13318–13323. doi: 10.1073/pnas.0403064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holm L, Sander C. Protein-Structure Comparison by Alignment of Distance Matrices. J Mol Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- 46.Ho YSJ, Burden LM, Hurley JH. Structure of the GAF domain, a ubiquitous signaling motif and a new class of cyclic GMP receptor (vol 19, pg 5288, 2000) EMBO J. 2001;20:1483–1483. doi: 10.1093/emboj/19.20.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho HY, Cho HJ, Kim YM, Oh JI, Kang BS. Structural Insight into the Heme-based Redox Sensing by DosS from Mycobacterium tuberculosis. J Biol Chem. 2009;284:13057–13067. doi: 10.1074/jbc.M808905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Podust LM, Ioanoviciu A, Ortiz de Montellano PR. 2.3 A X-ray structure of the heme-bound GAF domain of sensory histidine kinase DosT of Mycobacterium tuberculosis. Biochemistry. 2008;47:12523–12531. doi: 10.1021/bi8012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JM, Cho HY, Cho HJ, Ko IJ, Park SW, Baik HS, Oh JH, Eom CY, Kim YM, Kang BS, et al. O-2- and NO-sensing mechanism through the DevSR two-component system in Mycobacterium smegmatis. J Bacteriol. 2008;190:6795–6804. doi: 10.1128/JB.00401-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang X, Kuk J, Moffat K. Crystal structure of Pseudomonas aeruginosa bacteriophytochrome: Photoconversion and signal transduction. Proc Natl Acad Sci USA. 2008;105:14715–14720. doi: 10.1073/pnas.0806718105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Yang XJ, Kuk J, Moffat K. Conformational differences between the Pfr and Pr states in Pseudomonas aeruginosa bacteriophytochrome. Proc Natl Acad Sci USA. 2009;106:15639–15644. doi: 10.1073/pnas.0902178106. Structural studies of the entire dimeric photosensory core of a bacteriophytochrome in different signaling states reveal global conformational changes as well as conformational changes near the chromophore binding site and of the chromophore itself. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Essen LO, Mailliet J, Hughes J. The structure of a complete phytochrome sensory module in the Pr ground state. Proc Natl Acad Sci USA. 2008;105:14709–14714. doi: 10.1073/pnas.0806477105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner JR, Zhang JR, Brunzelle JS, Vierstra RD, Forest KT. High resolution structure of Deinococcus bacteriophytochrome yields new insights into phytochrome architecture and evolution. J Biol Chem. 2007;282:12298–12309. doi: 10.1074/jbc.M611824200. [DOI] [PubMed] [Google Scholar]
- 54.Yang X, Stojkovic EA, Kuk J, Moffatt K. Crystal structure of the chromophore binding domain of an unusual bacteriophytochrome, RpBphP3, reveals residues that modulate photoconversion. Proc Natl Acad Sci USA. 2007;104:12571–12576. doi: 10.1073/pnas.0701737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grasberger B, Minton AP, Delisi C, Metzger H. Interaction between Proteins Localized in Membranes. Proc Natl Acad Sci USA. 1986;83:6258–6262. doi: 10.1073/pnas.83.17.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marina A, Waldburger CD, Hendrickson WA. Structure of the entire cytoplasmic portion of a sensor histidine-kinase protein. EMBO J. 2005;24:4247–4259. doi: 10.1038/sj.emboj.7600886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chervitz SA, Falke JJ. Molecular mechanism of transmembrane signaling by the aspartate receptor: A model. Proc Natl Acad Sci USA. 1996;93:2545–2550. doi: 10.1073/pnas.93.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]