Abstract
Oncogenes usually increase their normal function when activated. But seemingly oncogenic mutations in IDH1 and IDH2 reduce their native enzyme activity. In this issue of Cancer Cell, Ward et al. pin down a neomorphic enzyme activity as a possible oncogenic function for these alterations.
IDH1 and IDH2 are the NADP+-dependent isocitrate dehydrogenases, which catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate(α-KG). IDH1 resides in the cytoplasm and peroxisome whereas IDH2 resides in the mitochondria. The IDH1 R132 mutations were found to occur frequently in gliomas in a whole-genome exon-sequencing analysis (Parsons et al., 2008), and a high frequency of the same mutations were later discovered in AML (Mardis et al., 2009). Interestingly, some gliomas contain IDH2 R172 mutations, albeit less frequently than IDH1 mutations (Yan et al., 2009). IDH2 R172 is analogous to IDH1 R132, and both play a role in binding isocitrate in the enzyme active site. A spectrum of missense mutations is observed at IDH1 R132 and IDH2 R172 in cancer. For instance, common IDH1 mutations include R132H and R132C, and common IDH2 mutations include R172K and R172M. Almost all reported cases of IDH1 and IDH2 mutation have been heterozygous, and inactivating alterations such as frameshifts, deletions, and nonsense mutations have not been observed for these genes in cancer. This genetic evidence led to early speculation that the IDH mutations confer the enzymes with an oncogenic gain of function.
Initial functional studies of IDH1 and IDH2 mutations appeared to stand in contrast to the gain of function hypothesis. These data revealed that the mutations reduce the ability of IDH1 and IDH2 to convert isocitrate to α-KG (Yan et al., 2009). Furthermore, IDH1 R132 mutants can dominant negatively inhibit wild-type IDH1 activity in vitro (Zhao et al., 2009). This led to the speculation that IDH1 and IDH2 are tumor suppressors with a propensity to develop dominant negative point mutations. Recently, Dang et al. reignited the debate over the nature of the IDH mutations by showing that the IDH1 R132 mutants gain the neomorphic enzymatic activity to reduce α-KG to R(-)-2-hydroxyglutarate (2HG, Fig. 1) (Dang et al., 2009). So which functions of the IDH mutations – dominant negative activity or neomorphic enzyme activity - are important in cancer?
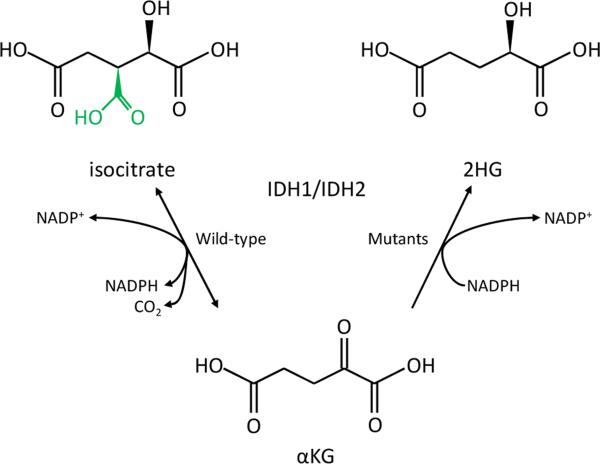
Figure 1. Mutations in the active site of IDH1 and IDH2 lead to a neomorphic enzyme activity.
Wild-type IDH1 and IDH2 normally catalyze the conversion of isocitrate to α-KG (left reaction), and at the same time reduce NADP+ to NADPH and produce CO2. R132 in wild-type IDH1, as well as R140 and R172 in wild-type IDH2, form hydrogen bonds with the β-carboxyl (green) of isocitrate. Cancer-derived mutations affecting these residues cause the enzymes to instead convert α-KG to 2HG, while at the same time oxidizing NADPH to NADP+ (right reaction). 2HG and isocitrate share an identical chemical backbone but differ solely in the presence of the β-carboxyl on isocitrate, but not 2HG. IDH1 R132, IDH2 R140, and IDH2 R172 mutation apparently favors conversion to 2HG rather than isocitrate since 2HG lacks this group.
In this issue, Ward et al. find clues to help answer this question by turning to IDH2. Given their structural resemblance and similar distribution in cancer, the IDH2 R172 mutations likely function similarly to the IDH1 R132 mutations. But IDH2 mutations have not previously been found in leukemias. Furthermore, neither dominant negative activity nor 2HG production have been investigated extensively for these IDH2 mutants.
Ward and colleagues are among the first to show that IDH2 R172 mutations also occur in AMLs (Green and Beer, 2010; Gross et al., 2010; Ward et al., 2010), and that a novel IDH2 mutation, R140Q, also repeatedly occurs in this type of cancer (Green and Beer, 2010; Ward et al., 2010). Together with the IDH1 R132 and IDH2 R172 mutations, the IDH mutations occur in a significantly larger proportion of AMLs than previously thought, with 23% of AML cases mutated in this study. Curiously, IDH2 R140 mutations or the equivalent IDH1 R100 mutations had not previously been found in a large number of gliomas (Yan et al., 2009). This underscores a striking, but unexplained, difference in the IDH mutation spectrum between gliomas and leukemias.
This group provides support for the idea that IDH1 and IDH2 are proto-oncogenes, with neomorphic enzyme activity as their shared oncogenic function. They show that, like IDH1 R132 mutations, IDH2 R140 and R172 mutations lead to the production of 2HG. In the wild-type enzymes, the IDH1 R132, IDH2 R172, and IDH2 R140 residues all form hydrogen bonds with the β-carboxyl of isocitrate. Mutation of these residues presumably favors binding to 2HG, which resembles isocitrate but lacks this β-carboxyl (Fig. 1). This finding points to the neomorphic enzyme activity to convert α-KG to 2HG as one possible oncogenic mechanism for all of the IDH1 and IDH2 mutations observed in cancer, rather than an unimportant side effect of the IDH1 mutations. Moreover, Ward et al. suggest that IDH1 and IDH2 act as oncogenes rather than tumor suppressors by showing that siRNA knockdown of IDH1 or IDH2 results in lower glioma cell growth in vitro, the opposite of what might be expected for knockdown of a tumor suppressor.
Though the neomorphic enzyme activity of the IDH mutants probably influences cancer or precancer cell biology, the downstream effects of this activity remain unknown. Most attention has been focused on the possibility that 2HG acts as an oncometabolite, either as a general mutagen or by modulating a specific cellular process. For instance, IDH1 R132H can upregulate the cancer-associated transcription factor HIF-1α in vitro (Zhao et al., 2009). It has been speculated that 2HG could mediate this upregulation by inhibiting prolyl hydroxylases and releasing HIF-1α from prolyl hydroxylase-dependent downregulation (Frezza et al., 2010). On the other hand, other potential effects of neomorphic IDH1 and IDH2 enzyme activity, such as metabolic flux away from α-KG, a central cellular metabolite, and alteration of the cellular NADP+/NADPH balance, could also have far-reaching metabolic effects on the cell. Future studies involving animal models, rigorous enzyme study, and interrogation of relevant cell lines will be needed to determine the consequences of IDH mutations on cancer cell development.
Already, 2HG has shown promise as a cancer biomarker: Ward and colleagues used 2HG as a marker to identify AML samples to screen for novel IDH1 and IDH2 mutations. This approach led to their discovery of the IDH2 R140 alteration, and may find a role in the diagnosis of patients with new or relapsed cases of AML or glioma. If 2HG levels are high in the serum, urine, or cerebrospinal fluid of patients with IDH-mutated cancers, measurement of this metabolite could be used as an adjunct to histopathological analysis or even in place of a more invasive procedure.
Several features of the IDH1 and IDH2 mutations make their study exciting for the future development of therapeutics. First, in contrast to oncogenic signaling molecules that have proven difficult to target with small compounds, the active sites of metabolic enzymes are likely amenable to such targeting. Second, IDH mutations appear early in cancer development compared to other genetic alterations, and they are found in cancers that are composed of relatively undifferentiated cells. Based on this, Ward and colleagues speculate that the mutations could mediate a block in cellular differentiation that leads to carcinogenesis. If true, a therapeutic strategy aimed at modulating cell differentiation pathways may aid in the treatment of these cancers. Finally, though the mutations occur early in cancer, the same IDH mutation is always retained as gliomas progress to higher-grade tumors (Yan et al., 2009) and in relapses of AML (Chou et al., 2010), indicating that the mutant enzymes could serve as stable therapeutic targets. If so, small molecules that target mutated IDH enzymes or yet-to-be-identified players in their oncogenic network may be very successful in treating these challenging cancers.
References
- Chou WC, Hou HA, Chen CY, Tang JL, Yao M, Tsay W, Ko BS, Wu SJ, Huang SY, Hsu SC, et al. Distinct clinical and biological characteristics in adult acute myeloid leukemia bearing isocitrate dehydrogenase 1 (IDH1) mutation. Blood. doi: 10.1182/blood-2009-11-253070. [DOI] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009 doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C, Tennant DA, Gottlieb E. IDH1 mutations in gliomas: when an enzyme loses its grip. Cancer cell. 17:7–9. doi: 10.1016/j.ccr.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Green A, Beer P. Somatic mutations of IDH1 and IDH2 in the leukemic transformation of myeloproliferative neoplasms. The New England journal of medicine. 362:369–370. doi: 10.1056/NEJMc0910063. [DOI] [PubMed] [Google Scholar]
- Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, Sasaki M, Jin S, Schenkein DP, Su SM, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. The Journal of experimental medicine. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, et al. Recurring Mutations Found by Sequencing an Acute Myeloid Leukemia Genome. The New England journal of medicine. 2009 doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010 doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. IDH1 and IDH2 Mutations in Gliomas. The New England journal of medicine. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, Yu W, Li Z, Gong L, Peng Y, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]