Cytomegalovirus (CMV) is a hardy and adept virus that has succeeded in maintaining its presence for many centuries. CMV has a consistent ability to circumvent human immune defenses and is able to do so in a variety of ways; its ability to thwart our defenses makes infection with CMV a challenge to prevent and treat. Specific populations affected by an infection with CMV include newborns and immunocompromised patients. Recipients of solid organ transplants (SOT) also have an exceptionally high risk of CMV infection, which may ultimately place the patient and transplanted organ at risk for loss.
Traditional prophylaxis treatments are often used to prolong the time between a possible CMV infection post transplantation. Another agent, CMV immune globulin, intravenous (CMVIG) (Cytogam®), is an effective treatment that may be used concurrently with traditional prophylaxis to help limit infection with CMV. This Product Profiler will review CMV, current therapeutic options for CMV prophylaxis, and the pharmacology, efficacy, and safety of CMVIG.
DISEASE OVERVIEW
Cytomegalovirus Host Evasion and Subsequent Pathology
CMV is a ubiquitous herpes virus that is composed of double-stranded DNA, a protective protein capsid, a tegument that houses enzymes and proteins, and a lipoprotein membrane envelope (Baden 2008, Cytomegalovirus Infections 2008, McDevitt 2006).
Like other herpes viruses, CMV has the intricate ability to establish tenure within a host cell’s DNA and is subsequently capable of establishing latency and/or causing an active infection. This opportunistic pathogen often creates latent infections in various cells including monocytes, neutrophils, lymphocytes, endothelial cells, and smooth muscle cells (AJT 2004, Froberg 2004, Sia 2000). CMV is capable of causing severe illness in newborns, transplant recipients, and patients with HIV (Froberg 2004).
CMV is a highly evolved virus in that it has developed numerous mechanisms to evade host defenses. These evasion techniques, often referred to as “immuno evasion,” have allowed the virus to develop camouflage and sabotage techniques to effectively disrupt normal immune responses. The cellular and genetic changes induced by CMV can be divided into three categories: immune escape, impaired replication, and impaired signaling (Fisher 2009).
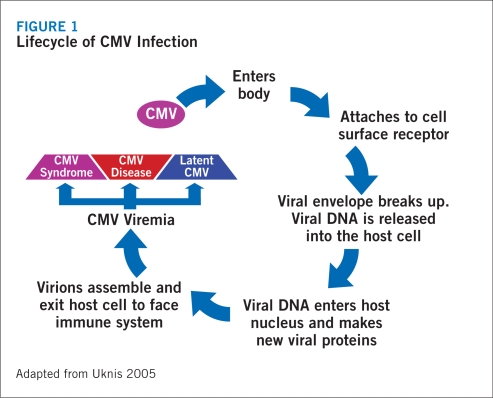
The virus may establish a latent infection by using immuno evasion to camouflage its presence from immune detection (Froberg 2004). During a latent infection, the virus intercalates its DNA into the host genome, ensuring its survival each time the cell divides. Because little to no virions are produced during this process, the apoptotic pathway is not activated, allowing the infected cell to survive indefinitely (Froberg 2004). With a latent infection, the virus remains in a reversible, nonproductive infective state (Froberg 2004). Although CMV may establish a latent infection and remain inactive in many cells, it may simultaneously cause active and chronic infections in the salivary glands (Froberg 2004). This allows the virus to facilitate its transmission to other hosts (Froberg 2004) (Figure 1).
FIGURE 1.
Lifecycle of CMV Infection
CMV is capable of disrupting or activating cellular immune signaling and replication (Froberg 2004). This type of activity can be seen in steps involved in the antigen presentation process common to Major Histocompatibility Complex (MHC) Class I and II cells (Froberg 2004). CMV is able to disrupt MHC class presentation on the surface of cells (Froberg 2004).
Under normal circumstances, this disruption would initiate natural killer (NK) cell responses. However, through unique short (US) genetic segments, CMV effectively replicates an MHC Class I homolog that is capable of binding to host peptides (Froberg 2004). Peptides are continually expressed on the cell surface, inhibiting NK activity and curbing their normal cytotoxic effects (Froberg 2004). CMV also prevents apoptosis by binding specific viral proteins, ensuring its survival in the host cell (Froberg 2004). Lastly, CMV can replicate a G-protein-coupled receptor homolog (Froberg 2004). The CMV generated homolog binds to chemokines and initiates a sequence of complications, including vascular and atheromatous lesions in the affected host (Froberg 2004).
In addition, the presence of US genetic segments in the CMV genome allows the virus to (Froberg 2004, Pereyra 2004):
Degrade immune complexes
Inhibit peptide translocation (Class I)
Bind to certain MHC molecules (Class I)
Dislocate complexes for rapid degradation (Class I)
Destroy proteins involved in presentation (Class II)
Downregulate cellular responses (Class II)
Replicate a cytokine homolog to inhibit cytokine production (Class II)
Epidemiology
CMV is widely prevalent and can be found in all geographic and socioeconomic groups (CDC 2008). However, it is more frequently found in developing countries and in lower socioeconomic conditions (Beers 2006, CDC 2008). It is common for 60%–90% of the general population to have been exposed to and to be infected by CMV because of its wide predominance in many geographical locations (Beers 2006). Naturally, as patients age, they have a higher risk of CMV infection (Beers 2006).
RISK FACTORS FOR CMV INFECTION IN SOLID ORGAN TRANSPLANT RECIPIENTS
Many factors are involved that can cause complications during and after SOT procedures. However, CMV has been noted as the most common pathogen to cause complications leading to morbidity and mortality in post-SOT recipients (EBCG 2001, Razonable 2004). True to its nature, CMV is an opportunistic virus that thrives on factors that favor infection and subsequent disease.
CMV Serostatus
The predictive risk for subsequent CMV infection lies heavily in the serostatus of both the organ donor (D) and recipient (R) (Razonable 2005). CMV serostatus is in fact, the most important risk factor for CMV infection and disease in organ transplant recipients. The pattern for CMV infection in transplant recipients is categorized by three types of infection: primary, reactivation/recurrent, and superinfection/reinfection (NIAID 2005).
Primary infection is the leading cause of CMV infection in SOT recipients (NIAID 2005, Razonable 2005). A primary infection is most prevalent when a seronegative recipient (R−) receives an organ from a seropositive (D+) donor. This type of transplant confers a high risk for developing a primary infection (Razonable 2005).
The second pattern of CMV infection is called reactivation or recurrent infection (NIAID 2005). This infection carries an intermediate risk for CMV infection and occurs through the reactivation of a latent CMV infection already present in the recipient (McDevitt 2006, Razonable 2005).
The final type of infection is the superinfection or reinfection pattern of CMV (NIAID 2005). Superinfections occur when both the donor and recipient are positive for CMV but are infected with two distinct strains (McDevitt 2006, NIAID 2005): the recipient’s original latent strain and the donor’s latent or active strain (NIAID 2005). This infection carries an intermediate risk for CMV infection post transplant. Table 1 lists levels of risk of CMV infection post transplant.
TABLE 1. Risk Factors for the Development of Post-transplant CMV Infection.
| CMV serostatus (donor/recipient) | Risk of primary infection, reactivation, superinfection |
|---|---|
| D+/R− | High risk (primary) |
| D+/R+ | Intermediate risk (reactivation/superinfection) |
| D–/R+ | Intermediate risk (reactivation) |
| D–/R− | Low risk (primary) |
Source: McDevitt 2006
Additional Risk Factors for Post-transplant CMV Infection
The type of organ transplant also plays a role in the risk of acquiring CMV infection (McDevitt 2006). Liver, lung, and pancreas transplant recipients have a high risk of acquiring a CMV infection while heart, small bowel, and kidney transplantation carry a lower risk (McDevitt 2006; Razonable 2005). In most instances, CMV infection often develops within 3 months of transplantation if prophylactic viral therapy is not initiated (McDevitt 2006). CMV may cause a variety of illnesses during infection, although, tissue invasive diseases involving the transplanted organ are more likely (McDevitt 2006). The presence of CMV may also add to the risk of organ rejection. Risk factors include retransplantation, fulminant hepatitis, bacterial infections, contaminated blood products, cadaveric allograft transplantation, Human Leukocyte Antigen (HLA) mismatch, and the amount of virus present in the donor organ (McDevitt 2006, Sia 2000).
The degree of immunosuppression may influence the rate or risk of CMV infection as well (Sia 2000). For example, the type, dose, duration, and sequence of immunosuppressive therapy may dictate the level and speed in which CMV may flourish (NIAID 2005, Sia 2000). Many anti-rejection drugs used for prophylaxis limit the body’s natural ability to mount responses by suppressing cell-mediated immune responses. They may also induce blunt antibody responses, inhibit proliferation, suppress T- and B-cell lymphocytes, and cause leukopenia (Sia 2000).
The extent of viral replication is also a considerable concern in the development of CMV infection (Razonable 2005). The reactivation and subsequent replication of latent CMV may dictate the pathogenesis and severity of an infection (Sia 2000). In addition, the rate and ability of the virus to infect and progress has been linked to the organ type and the efficiency of the immune system at the time of infection and/or transplantation (McDevitt 2006). The severity of CMV is usually categorized as either CMV infection or CMV disease. Unfortunately, these two categories are not universally defined and discrepancies may occur (McDevitt 2006). In many studies, CMV infection is defined as the isolation of the virus in any body fluid or tissue specimen (Ljungman 2002), while the disease refers to the presence of CMV in blood or body tissues with the presence of clinical symptoms (McDevitt 2006). The severity of CMV infection and its sublime opportunity to progress into disease depends on the host’s immune system and the type of SOT. It is important to note that although a percentage of patients may become infected with CMV, not all will progress to CMV disease (McDevitt 2006) (Table 2).
TABLE 2. Frequency of CMV Infection and CMV Disease in Solid Organ Transplant Recipients.
| Organ | CMV Infection (%)a | CMV Disease (%)b |
|---|---|---|
| Kidney | 8–32 | 8 |
| Liver | 22–29 | 29 |
| Heart | 9–35 | 25 |
| Lung or heart–lung | 39–41 | 39 |
| Pancreas or kidney–pancreas | 50 | 50 |
CMV infection refers to viremia without clinical symptoms.
CMV disease refers to viremia with clinical symptoms.
Source: McDevitt 2006
DIRECT AND INDIRECT CONSEQUENCES OF CMV INFECTION
Any organ transplant procedure carries inherent risks for complications. CMV infection specifically can often cause systemic and tissue invasive diseases in this population subtype (Razonable 2005). Infection with CMV acquired post transplantation often predisposes patients to both direct and indirect consequences such as febrile syndrome, myelosuppression, hepatitis, pneumonitis, gastrointestinal disease, and encephalitis (Razonable 2005). This patient population is often prone to opportunistic infections which may often lead to acute or chronic rejection of the allograft (Razonable 2005).
Active or recurrent infections with CMV after transplantation affect patient outcomes as well. For example, liver transplant patients 1 year after surgery were found to have higher mortality rates, increased medical expenses, and longer hospital stays than those of their CMV-negative transplant counterparts (Sia 2000). Similar decreased survival rates were observed when heart transplant patients were followed over a 5-year period. Patients positive for CMV disease had a 32% survival rate compared to 68% of their CMV-negative counterparts (Sia 2000). Lung transplant recipients who received CMV-positive lungs also developed severe complications related to the graft and had lower survival rates than CMV negative graft recipients (Sia 2000). Direct and indirect consequences of CMV infection in patients post transplant are summarized in Table 3.
TABLE 3. Direct and Indirect Consequences of CMV Infection.
| Direct consequences | Indirect consequences |
|---|---|
|
|
Sources: Preiksaitis 2005, Razonable 2005, Sia 2000