Abstract
The CA125 antigen, recognized by the OC125 antibody, is a tissue-specific, circulating antigen expressed in ovarian cancer. The CA125 antigen is encoded by the MUC16 gene, cloned by Lloyd and Yin. The full-length gene describes a complex tethered mucin protein present primarily in a variety of gynecologic tissues, especially neoplasms. OC125 and other related antibodies react with glycosylation-dependent antigens present exclusively in the cleaved portion of the molecule. These antibodies are not useful as screening tools, nor can they detect the proximal residual MUC16 protein fragment after cleavage. This has limited its diagnostic and therapeutic applications. Using synthetic peptides we raised novel-specific antibodies to the carboxy-terminal portion of MUC16, retained by the cell, proximal to the putative cleavage site. These antibodies were characterized using fluorescence-activated cell-sorting analysis, enzyme-linked immunoassay, Western blot analysis, and immunohistochemistry. Each of the selected monoclonal antibodies was reactive against recombinant GST-ΔMUC16c114 protein and the MUC16 transfected SKOV3 cell line. Three antibodies, 4H11, 9C9, and 4A5 antibodies demonstrated high affinities by Western blot analysis and saturation-binding studies of transfected SKOV3 cells, and displayed antibody internalization. Immunohistochemical positivity with novel antibody 4H11 was similar to OC125, but with important differences, including diffuse positivity in lobular breast cancer and a small percentage of OC125-negative ovarian carcinomas that showed intense and diffuse 4H11. Development of such antibodies may be useful for the characterization of MUC16 biology and allow for future studies in targeted therapy and diagnostics.
Keywords: MUC16, antibodies, immunohistochemistry, CA125, OC125, tissue microarray
INTRODUCTION
A serum assay can detect elevated levels of the circulating CA125 antigen in many epithelial ovarian cancer patients, and this antigen, derived using the ovarian cell line OVCA433, is recognized by the OC125 antibody.1,2 The detection of circulating CA125 in serum has proven to be a useful tool for the management of ovarian cancer patients and clinical trials.3,4 However, CA125 is neither sufficiently sensitive nor specific for general cancer screening.5,6 A variety of CA125-linked antibodies including VK8 and M11 have subsequently been defined as present on ovarian cancer cells.7–9 Although these antibodies have been used to develop serum assays and various other studies in ovarian cancer, they have significant shortcomings for clinical use in screening or tissue delivery.
The sequence of the cDNA-encoding MUC16/CA125 was described by Yin and Lloyd in 2001 and completed by O'Brien in 2002.10–12 The complete MUC16 protein has various components consisting of a cytoplasmic tail with potential phosphorylation sites, a transmembrane domain, and an external domain proximal to an apparent cleavage site. Distal to the cleavage site, the released external domain contains 16–20 tandem repeats of 156 amino acids, each with many potential glycosylation sites.11 The overall repeat structure is well conserved across mammals, but the repeats are not completely identical in exact amino acid composition.
The MUC16 protein is part of a family of complex tethered mucins that includes both MUC1 and MUC4.13 MUC1 is present in a variety of tissues and appears to signal through a beta catenin pathway, interact with EGF receptor and mediate drug resistance, and can act as an oncogene.14–17 The MUC4 protein is also expressed in a variety of tissues but is common on neoplasms of the gastrointestinal track.18–20 In contrast, the CA125 antigen has been more restricted in its distribution and is present primarily in gynecologic tissues and overexpressed in Müllerian neoplasms.21 However, the CA125 antigen, recognized by the OC125 antibody, is a heavily glycosylated antigen expressed in the tandem repeat region of the larger MUC16 protein. This glycoprotein is typically shed from a putative cleavage site in the extracellular domain of the MUC16 peptide backbone. The vast majority of MUC16-reactive antibodies, including OC125, react with the glycosylation-dependent antigen present exclusively in the cleaved portion of the molecule, so the true distribution of MUC16 expression is not known.21 There is currently no antibody available to track the fate of the remaining MUC16 protein fragment after cleavage and CA125 release. Such antibodies could be useful for diagnostic and therapeutic applications as well as biologic studies. Furthermore, studies of membrane receptor trafficking and intracellular events have also been limited by the lack of a specific antibody against the proximal portion of MUC16.
In order to better explore the biology of human MUC16, we have derived monoclonal antibodies against the extracellular portion of the MUC16-carboxy terminus, proximal to the putative cleavage site, as well as one monoclonal antibody against the internal cytoplasmic domain. In contrast to prior antibodies, these are derived against the peptide backbone of MUC16 and are not directed at complex glycoprotein epitopes. These antibodies have potential for use in enzyme-linked immunosorbent assay (ELISA), fluorescence-activated cell sorting (FACS), Western blot analysis, immunoprecipitation, and immunohistochemistry. Since these epitopes are proximal to the cleavage site, they are unlikely to be found in the circulation and may be novel targets for therapeutic interventions. Herein, we demonstrate the identification and characterization of antibodies developed against the MUC16 peptide backbone.
MATERIALS AND METHODS
Cell Cultures
OVCAR3, SKOV3, and A2780 cell lines were obtained through the American Type Culture Collection (ATCC, Manassas, VA) and sustained in culture according to the ATCC literature. For the creation of MUC16+ transfected cell lines, the carboxyterminus portion of the MUC16 cDNA was introduced as green fluorescent protein fusion proteins using the Vitality phrGFP vector expression system (Stratagene, LaJolla, CA). Stable cell lines were selected using geneticin (G418, Invitrogen, Grand Island, NY) in their respective culture media and isolated by expression of Green Fluorescence Protein. Stable transfectants were routinely maintained in G418 in their culture media respectively. The characteristics of the MUC16 transfectants are described elsewhere and summarized in the supplemental information. The ΔMUC16c114 transfectants have cell surface expression of MUC16 protein from the putative cleavage site to the carboxyterminus (AA 1776 to 1890).12
Monoclonal Preparation
Using the MUC16 sequence, peptide sequences encoding elements of the ΔMUC16c114 amino acid sequence were synthesized at the Memorial Sloan-Kettering Cancer Center (MSKCC) Microchemistry Core Facility. We synthesized 3 polypeptides (Figure 1) and modified Peptide 1 and Peptide 2 with a cysteine at the N-terminus for better conjugation to KLH. Equal concentrations of the KLH-conjugated peptides were mixed and then used as the immunogen for 5 BALB/c mice. We selected 1 of the 5 mice whose serum showed the highest reactivity to individual peptides by ELISA, and the MSKCC Monoclonal Antibody Core Facility performed the fusion and selected the antibodies using standard protocols. After 10 days of fusion, supernatants were selected and screened for reactivity by ELISA against the individual synthetic peptides.
Figure 1.

Three MUC16 carboxy terminus peptides were synthesized at the MSKCC Microchemistry Core Facility. Peptide 1 is near the putative cleavage site, peptide 2 is before the transmembrane, and peptide 3 is the internal peptide, which is inside the transmembrane.
ELISA
Sandwich ELISA was performed to see the positivity of the antibodies to individual peptides and GST-ΔMUC16c 114 fusion protein following routine core facility protocol for ELISA assay.
FACS Analyses
Adherent target cells were removed by 0.05% Trypsin and 0.1% EDTA, washed, and counted by a hemocytometer. Cells were distributed into multiple Eppendorf tubes with at least 0.5–1 × 106 cells per tube. Cells were washed with phosphate buffered saline (PBS) containing 1% FCS and 0.025% Sodium Azide (FACS buffer). For internal FACS staining, cells in the Eppendorf tubes were permeabilized with 1:10 diluted FACS Permeabilizing Solution 2 (BD BioSciences, San Jose, CA) for 10 minutes at room temperature and then washed twice with ice cold FACS buffer. Then they were incubated either without (for second antibody control) or with 1 μg/tube of bioreactive supernatants of mouse MUC16 monoclonals for 30 minutes on ice. For surface FACS staining, cells were incubated either without (for second antibody control) or with 1 μg/tube of bioreactive supernatants of MUC16 monoclonals (9B11.20.16, 9C9.21.5.13 and 4H11.2.5), Mouse anti-human OC125 (M3519), Mouse anti-human M11 (M3520) (DakoCytomation, Dako North America Inc., Carpinteria, CA) or VK8 (kindly provided by Dr. Beatrice Yin and Dr. Ken Lloyd, MSKCC, New York, NY) for 30 minutes on ice. Cells in Eppendorf tubes were also surface stained with 1 μg/tube of non-specific isotype matched control mouse antibodies (13C4 for IgG1 and 4E11 for IgG2b monoclonals obtained from MSKCC Monoclonal Core Facility) and incubated on ice for 30 minutes. All cells were washed three times with FACS buffer. Cells were incubated with 1 μg/tube of second antibody Goat anti-mouse IgG1-PE or IgG2b-PE for 30 minutes on ice and then washed three times with FACS buffer. The cells were analyzed by a FACS Calibur machine at the MSKCC Flow Cytometry Core Facility.
Western Blot Analysis
Stable cell lines were cultured in 10 cm dishes in their respective culture media and incubated with 5% CO2 at 37°C for 3 days. They were washed twice with ice cold PBS to remove the serum-containing media. Adherent cells were scraped with 1–2 ml of ice cold PBS, and the cells were spun down in an Eppendorf tube at 4°C in an Eppendorf centrifuge. Supernatant was discarded, and the cells were lysed with 0.2 ml of modified Ripa lysis buffer (20 mM Tris-HCL; pH 7.4; 150 mM NaCl; 1% NP-40; 1 mM Na3VO4; 1 mM PMSF; 1 mM DTT; 10 ug/ml leupeptin; and 10 ug/ml aprotinin) for 30 minutes on ice and spun at 4°C for 10 minutes. The soluble solution was separated into a tube and the debris pellet was discarded. Protein concentration was measured using the Bio-Rad Protein Assay (BioRaD Laboratories, Hercules, CA). Equal amounts of proteins (GST-MUC16-CD-fusion protein or stable cell line extracts) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane using a BioRad transfer apparatus in a cold room at 4°C. The membranes were blocked with 3% bovine serum albumin (BSA) in PBS with 0.1% Tween-20 (PBST) at 4°C overnight. Membranes were probed with primary antibody (1:1000 dilution) for 1 hr at room temperature and then washed three times with PBST. Then the membranes were stained with corresponding second antibody, anti-Mouse IgG Horse Radish Peroxidase (HRP) linked whole antibody from sheep (GE Healthcare, UK) (1:5000 dilution), for 1 hr at room temperature. Membranes were washed three times with PBST and developed with a Western Lightning® chemiluminescence reagent (ECL, Perkin Elmer, Waltham, MA) for 1–5 minutes at room temperature, and the signals were developed on Kodak BioMax Film.
Binding and internalization studies with monoclonal antibodies and OVCAR3 and SKOV3 stable transfectants
Purified monoclonal antibodies were labeled with 131I using the iodogen method and purified by size exclusion chromatography.22 Saturation binding studies were performed with radiolabeled antibodies using substrates of intact OVCAR-3 cells. Briefly, 10 test solutions were prepared (in triplicate) and they contained increasing amounts of the radioiodinated antibodies, 3–500 000 cells in a total volume of 500 μL of PBS (0.2 % BSA; pH 7.4). The cells were isolated by rapid filtration through a glass fiber membrane and washed with ice cold tris buffered saline. Cells were counted in a gamma counter with standards of total activity added. For each concentration of radiolabeled antibody, non-specific binding was determined in the presence of 100 nM of the unmodified antibody. The data were analyzed with a least squares regression method (Origin, Microcal, Software Inc., Northampton, MA) to determine the Kd and Bmax values, and a Scatchard transformation was performed.
Antibody cell internalization studies were performed with 131I-4H11 and 131I-OC125 monoclonal antibodies and SKOV3-phrGFP-ΔMUC16c334 stable transfected cells. Briefly, radiolabeled antibody (370 MBq/mg, 100 kcpm) in 2 mL of medium was added to SKOV3 cells plated in a 6-well plate. The plates were incubated at 37°C for up to 24 hours. At various time points, the medium was removed from three wells and the cells washed with 2 × 2 mL PBS. Cell surface bound activity was then stripped and collected with 2 × 2 mL of an ice cold acid wash (100 mM acetic acid 100 mM glycine; pH 3.0). The cells were then dissolved with 2 × 1 ml 1 M NaOH and collected. At the end of the study all samples were counted with a gamma counter together with standards, representing the initial amount of radioactivity added. All the media samples were analyzed by ITLC-SG with mobile phases of 5% TCA to determine unbound 131I.
Tissue microarray (TMA)
Tissue microarrays were either constructed within our institution or bought from a commercial laboratory if not available internally. Briefly, core-needle biopsies of pre-existing paraffin-embedded tissue were obtained from the so-called donor blocks and then relocated into a recipient paraffin-arrayed “master” block by using the techniques by Kononen et al. and subsequently modified by Hedvat et al.23,24 A manually operated Tissue Arrayer MTA-1 from Beecher Instruments Inc. (Sun Prairie, WI) was used to produce sample circular spots (cores) that measured 0.6 to 1.0 mm in diameter. The cores were arrayed 0.3 to 0.4 mm apart from each other. A layer of control tissues was strategically laid around the actual tissue microarrays in order to avoid edging effects. The specific composition of each tissue microarray is delineated below. Slides of tissue microarrays for ovarian cancer, prostate cancer, adenocarcinoma of the lung, mucinous neoplasms of the pancreas, and invasive ductal and invasive lobular breast carcinoma were prepared by cutting 4 um sections from formalin-fixed paraffin-embedded tissue. Normal adult and fetal tissue microarrays were obtained from a commercial source (Biomax, US). OVCAR3 cells were used as positive controls.
Immunohistochemistry
Immunohistochemistry was performed on the tissue microarrays with both standard OC125 (Ventana, Tuscon, AZ) and the novel monoclonal antibodies. Sections of the tissue microarrays were cut at 4 microns, placed on Superfrost/Plus microscope slides (Fisher brand) and baked in a 60° oven for at least 60 minutes. The slides were then deparaffinized and hydrated to distilled water, soaked in citrate buffer at pH 6.00 for 30 minutes at 97° C, washed in running water for 2–5 minutes, incubated for 5 minutes in 3% hydrogen peroxide diluted in distilled water. Slides were washed in distilled water for 1 minute, transferred to a bath of phosphate buffered saline (PBS), pH 7.2, for two changes of 5 minutes each and placed in 0.05% BSA diluted in PBS for a minimum of 1 minute. After drying around tissue sections, normal serum was applied at a 1:20 dilution in 2% BSA/PBS and incubated for a minimum of 10 minutes at room temperature in a humidity chamber. The serum was then suctioned off without allowing the sections to dry, and approximately 150 lambda of novel antibody at a dilution of 1:1000 was placed on the tissue. The slide was incubated overnight (approximately 15–18 hours) at 4° C in a humidity chamber. Primary antibody was washed off using three changes of PBS for 10 minutes each. Secondary antibody, biotinylated α-mouse from Vector laboratories (Burlingame, Ca), was applied at 1:500 dilution in 1% BSA/PBS and incubated for 45–60 minutes at room temperature in humidity chamber. The antibody was washed off again using three changes of PBS as above. Slides were then transferred to a bath of diaminobenzidine (DAB), diluted in PBS for 5–15 minutes. The slides were then washed in tap water for 1 minute, counterstained using Harris modified hematoxylin (Fisher), decolorized with 1% acid alcohol and blue in ammonia water, dehydrated with 3 changes each of 95% ethanol, 100% ethanol and xylene for 2 minutes each and coverslipped with permanent mounting medium.
Immunohistochemistry scoring
Commercially available antibodies, such as OC125 and M11, target complex glycosylation-dependent epitopes. Our hypothesis is that glycosylation may be tissue specific; therefore, it was important to examine the utility of the peptide-directed antibodies in paraffin-fixed tissues and survey the prevalence of MUC16 expression. The three candidate antibodies, 4H11, 9C9 and 4A5, were characterized using OVCAR3 cell line pellets. Of the three, the 4H11 antibody showed the strongest, most diffuse and consistent staining pattern at multiple dilutions, with the least amount of background staining and, therefore, was optimized for use in human tissues in the pathology core facility.
Using 4H11, we stained and scored positivity using tissue microarrays from high-stage, high-grade ovarian serous carcinomas (Figure 2), these tumors being the most common type of ovarian cancer, representing approximately 80–85% of all ovarian carcinomas in Western industrialized nations.25 To test the specificity of the novel antibody, we also stained tissue microarrays of cancers of the prostate, lung, breast, and pancreas and compared their staining intensities with that of OC125 monoclonal antibody (Supplemental Figure 1A–D). To determine whether there would be any cross-reactivity with normal human tissues, the antibodies were also tested on normal human adult and fetal TMAs.
Figure 2.
Comparison staining of high-grade serous ovarian carcinomas using OC125 (left panel) and 4H11 (right panel)
All of the stained sections were reviewed by a reference pathologist (KJP). A subset of cores for which there was equivocal staining was also independently scored by a second pathologist (RAS) to ensure consistency in scoring methods. Only cytoplasmic and/or membranous staining was considered positive. If a portion of the cell showed membranous staining, that was considered partial staining. A scoring system was devised to provide a semiquantitative assessment of staining distribution and intensity in individual cores. At the same time, it was designed to be useful for comparing the staining distribution and intensity between OC125 and the novel antibodies. The score incorporated the percentage of cells, the intensity and pattern of the staining according to the following standards: score 0: no staining; score 1: <5% strong or weak; score 2: 5–50% strong or weak; score 3: 51–75% strong or 51–100% weak; score 4: 76–99% strong; and score 5: 100% strong staining (Figure 3). The pathologist first reviewed all tissue microarrays stained with OC125 and scored each core. Then the same cores stained with the novel antibodies were scored one to several days after OC125 without reference to the previous results. Direct comparison of the scoring between the stains for each core was made only after all of the scoring was completed. The same process was used for all non-ovarian tissue microarrays. After comparison, core staining was determined to be concordant, equivocal, or discordant based on the point differentials. Concordant cores differed by 0 to 1 point, equivocal cores differed by 2 points, and discordant cores differed by 3 to 5 points. The one exception to this rule was when the difference of 1 point was between a score of 0 and 1, in which case, the differences were considered equivocal. This was in order to truly separate negative cases from even focally positive ones.
Figure 3.

Immunohistochemical scoring of OC125 and 4H11 on tissue microarrays of high-grade ovarian serous carcinoma. Only membranous and/or cytoplasmic staining was considered positive.
Score 0: No staining; Score 1: <5% strong or weak; Score 2: 5–50% strong or weak; Score 3: 51–75% strong or 51–100% weak; Score 4: 76–99% strong; Score 5: 100% strong Figure 3A: OC125 (Score 0); Figure 3B: OC125 (Score 1); Figure 3C: OC125 (Score 2); Figure 3D: OC125 (Score 3); Figure 3E: OC125 (Score 4); Figure 3F: OC125 (Score 5); Figure 3G: 4H11 (Score 0); Figure 3H: 4H11 (Score 1); Figure 3I: 4H11 (Score 2); Figure 3J: 4H11 (Score 3); Figure 3K: 4H11 (Score 4); Figure 3L: 4H11 (Score 5)
RESULTS
MUC16-directed monoclonal antibodies were isolated by ELISA-based screening using both the individual peptides and recombinant GST-ΔMUC16c114 protein followed by sequential subcloning for single cell clones. The identified monoclonal antibodies are listed in Table 1A and include those derived from peptide 1 (9B11), peptide 2 (9C9, 4H11, 9C7, 5C2, 28F7 and 4A5), and peptide 3 (31A3). Each of the selected monoclonal antibodies was reactive against GST-ΔMUC16c114. As expected, the commercial MUC16-directed antibodies (OC125, M11, or VK8) did not bind to GST-ΔMUC16c114 in ELISA or Western blotting. The clones were tested in FACS against OVCAR3 ovarian cancer cells and in Western blot analysis against GST-ΔMUC16c114 (Table 1B), and selected purified monoclonal antibodies were isolated.
Table 1A and 1B.
MUC16-carboxyterminus monoclonal antibodies showing their reactivity to GST-ΔMUC16c114 western, FACS analysis on OVCAR3 wild type cells

|
Characterization of Anti-MUC16 monoclonal antibodies
We used the OVCAR3 wild type and the SKOV3 cells transduced with phrGFP-ΔMUC16c114 to characterize the selected antibodies by FACS analysis. All of the selected monoclonal antibodies bound to both cell lines while commercial VK8, M11 and OC125 antibodies bound to the OVCAR3 cells but not to the SKOV3-phrGFP-ΔMUC16c114 cell line. The antibodies against peptide 3 required permeabilization since it is an internal epitope (Supplemental Figure 2).
Western blot analysis using the GST-ΔMUC16c114 purified protein showed strong binding with 4H11 and 9C9 antibodies (Figure 4A), while the other selected antibodies showed less binding. The SKOV3-phrGFP-ΔMUC16c114 transfectant was also positive by Western blot analysis using 4H11 and 9C9 antibodies (Figure 4B). As before, the commercial antibodies did not interact with the GST-ΔMUC16c114 purified protein or cell lysates of the SKOV3-phrGFP-ΔMUC16c114 cell line.
Figure 4A.

Western blot analysis of GST-ΔMUC16c114 fusion protein with monoclonal antibodies 9C9.21.5.13 and 4H11.2.5
Figure 4B.

Western blot analysis of SKOV3-phrGFP-ΔMUC16c114 and SKOV3-phrGFP-ΔMUC16c334 protein extract and probed with monoclonal antibodies 9C9.21.5.13 and 4H11.2.5
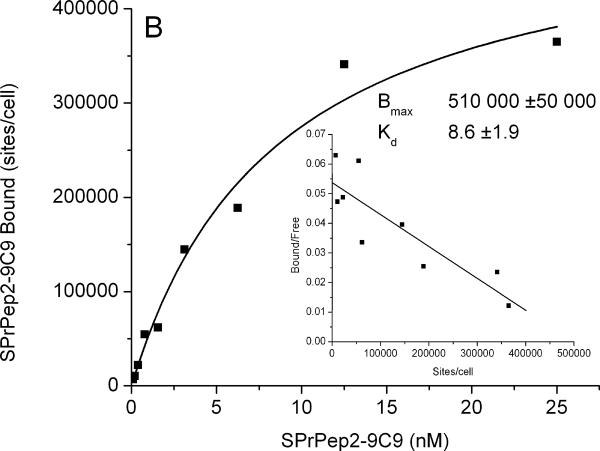
The binding of six monoclonal antibodies against OVCAR3 MUC16 were examined in affinity binding studies. Three antibodies—9C7, 5C2 and 28F7—showed only modest levels of binding compared to the nonspecific binding of these antibodies to the OVCAR3 cells, which carry large numbers of MUC16 binding sites. In contrast, 4H11, 9C9, and 4A5 monoclonal antibodies showed highly specific binding affinity, as shown in Figure 5A, with binding affinities of 6.8–8.6 nM against the cell surface epitopes of OVCAR3 cells. We also examined the internalization of antibody bound to cell surface MUC16 protein. We examined internalization in the transfected SKOV3-phrGFP-ΔMUC16c334 cell line which bears the carboxy terminus of MUC16, including the 4H11 epitope and a single degenerate tandem repeat sequence to interact with the OC125 antibody. The commercial antibodies OC125, M11, and VK8 all bind to the cell surface of this transduced cell line. The 131I-labeled 4H11 showed rapid internalization at a high level, whereas 131I-labeled OC125 antibody was internalized at a much lower rate (Figure 5B).
Figure 5A.
MUC16 carboxy terminus monoclonal antibodies binding affinity on OVCAR3 cells (Panels A–D)
Figure 5B.
Internalization of radio-labelled 4H11 and OC125 monoclonal antibodies on SKOV3-phrGFP-ΔMUC16c334 stable transfected cells
Immunohistochemistry results
Given their highly specific binding affinities, the antibodies 9C9, 4A5, and 4H11 were characterized for utility in immunohistochemistry using OVCAR3 cell lines. Of the three, the 4H11 antibody was selected to be optimized for use in human tissues based on its robust sensitive and specific staining pattern as compared to the other two antibodies.
Ovary
Two high-stage, high-grade ovarian serous carcinoma tissue microarray slides composed of 419 cores, representing primary, metastatic and recurrent tumors from 40 patients were stained with both OC125 and 4H11 monoclonal antibodies (Figure 2). The OC125 tissue microarrays showed 279 (66%) cores with 3–5 staining, 99 (24%) with 1–2 staining, and 41 (10%) with no staining. The 4H11 tissue microarrays showed 236 (56%) with 3–5 staining, 91 (22%) with 1–2 staining, and 92 (22%) with no staining. The two antibodies were concordant in 233 (56%) cores, equivocal in 161 (38%), and discordant in 25 (6%). Of the 25 discordant cores, 12 (48% of discordant cases, 3% of all cases) showed greater 4H11 positivity than OC125. Nine were discordant by a difference of 4 points, and 3 were discordant by a difference of 5 points. There was a total of 186 discordant and equivocal cores together, 48 (26%) of which showed greater staining with 4H11 than OC125. The staining pattern of both 4H11 and OC125 was cytoplasmic and membranous, although the membranous pattern of OC125 was stronger and better defined than 4H11 in the majority of cases. Discordant cases demonstrated higher levels of 4H11 than other cases.
Breast Cancer
A variety of other tissues were also examined for 4H11 staining to test the antibody's specificity. Of the 50 cores of invasive ductal carcinomas of the breast (number of patients unavailable), only 2 (4%) showed a score of 4 or greater 4H11 staining and none had scores of 3–5 for OC125 staining. The staining pattern with OC125 was mostly apical/luminal with some granular cytoplasmic staining. Some tumors with intracytoplasmic lumina also picked up the OC125 stain. 4H11 showed a more diffuse cytoplasmic blush without membranous accentuation.
In contrast, the invasive lobular breast carcinoma tissue microarray (composed of 179 cores with viable tumor, number of patients unavailable) had frequent MUC16 staining with 4H11. In this tissue microarray, 168 cores (94%) showed no staining for OC125, 5 (3%) showed 1–2 staining, and only 6 (3%) showed a staining intensity of 3. 4H11 staining was different in its distribution pattern, with 49 (27%) showing no staining, 81 (45%) showing 1–2 staining, and 49 (27%) showing 3–4 staining. Neither OC125 nor 4H11 had cores with a staining intensity of 5. The staining pattern was of cytoplasmic, luminal/membranous, or intraluminal for both OC125 and 4H11. The intraluminal pattern was strong and intense for both stains and highlighted the intracytoplasmic lumen that is commonly present in lobular carcinomas. The concordance rates were 34% concordant, 43% equivocal, and 23% discordant. Of the equivocal and discordant cases, there was none in which the OC125 was greater than the 4H11. All 42 discordant cases and 76 of 77 equivocal cases had 4H11 greater than OC125. There was also focal luminal staining with 4H11 in benign breast ducts and lobular carcinoma in situ.
Lung, pancreatic and prostatic adenocarcinomas
Tumors from other organs were not reactive with either antibody. The lung adenocarcinoma TMA had 237 cores from 86 patients containing viable tumor. In the pancreatic TMA there were 92 cores from 21 patients containing pancreatic mucinous tumors, including intraductal papillary mucinous neoplasms (IPMN) and invasive ductal carcinomas. In the prostate cancer TMA there were 169 cores (number of patients not available). None of these cancer tissue microarrays had significant binding to either OC125 or 4H11. This information is summarized in Table 2.
Table 2.
Staining intensity of OC125 as compared to 4H11 in tissue microarrays
| OC125 vs. 4H11 staining intensity score (%) | ||||||
|---|---|---|---|---|---|---|
| Site | 0 | 1–2 | 3–5 | |||
| Ovary high grade serous | OC125 | 4H11 | OC125 | 4H11 | OC125 | 4H11 |
| 10 | 28 | 24 | 22 | 66 | 56 | |
| Breast invasive ductal | 68 | 78 | 32 | 18 | 0 | 4 |
| Breast invasive lobular | 94 | 27 | 3 | 45 | 3 | 27 |
| Lung adenocarcinoma | 63 | 77 | 24 | 18 | 13 | 5 |
| Pancreas mucinous neoplasms | 98 | 88 | 2 | 10 | 0 | 2 |
| Prostate adenocarcinoma | 0 | 0 | 0 | 0 | 0 | 0 |
Score 0: 0% staining; 1: <5% strong or weak; 2: 5–50% strong or weak; 3: 51–75% strong or 51–100% weak; 4: 76–99% strong; 5: 100% strong
Normal Tissues
There was no staining with OC125 or 4H11 in normal adult colon, rectum, ectocervix, small intestine, ovary, liver, pancreatic ducts, spleen, kidney, and skin. OC125 and 4H11 both stained endocervical glands (OC125 luminal, 4H11 weak cytoplasmic), esophageal glands (luminal), bronchial epithelium (OC125 luminal, 4H11 intracytoplasmic granules), and thymic corpuscles (cytoplasmic). 4H11 demonstrated weak to moderate staining of the gastric glands, particularly at the crypts, with an intracytoplasmic granular pattern. Other organs that showed punctuate intracytoplasmic staining with 4H11 only were prostate, seminiferous tubules of the testes, and the islet cells of the pancreas. The staining in the pancreatic islets cells was particularly strong and consistent. There was also nonspecific staining of liver, kidney and brain with 4H11. There were no cases that stained with OC125 and not 4H11.
Similarly, there was no staining with either OC125 or 4H11 in fetal heart, gallbladder, colon, small intestine, liver, rectum, adrenal, thyroid, spleen, skin, bone, epididymis, brain, lung, muscle, smooth muscle, kidney, eye, umbilical cord, and placenta. OC125 only stained thymic corpuscles in a pattern similar to that in adult tissue. 4H11 stained both fetal pancreatic endocrine cells and endocervical glands in a similar pattern to that of their adult counterparts. Islet cells showed a granular cytoplasmic pattern, and endocervical glands showed a linear luminal pattern, which was more similar to the OC125 pattern in the adult tissue.
DISCUSSION
The expression of the MUC16/CA125 antigen has long been associated with gynecologic tissues. CA125, though it is not sensitive or specific enough to be used as a general screening tool, is routinely used to monitor patients with ovarian carcinoma. The tests used to measure CA125 are antibody-based detection methods, as are the immunohistochemical stains routinely performed for diagnostic purposes. The epitope specificity of 26 antibodies to MUC16 was studied in the first report from the International Society of Oncodevelopmental Biology and Medicine (ISOBM) TD-1 Workshop, and the application of 22 antibodies to immunohistochemistry was reported in the second report from the TD-1 workshop.7,21 The existing antibodies were grouped as OC125-like, M11-like, or OV197-like and all of the known antibodies recognized CA125 epitopes in the repeating, glycosylated elements in the external domain of the tethered mucin MUC16, distal to the putative cleavage site.
This released external domain contains 16–20 tandem repeats of 156 amino acids, each with potential glycosylation sites. An apparent cysteine-based disulfide loop of 19 amino acids is present in all repeats, and the N-terminal end contains a hairbrush structure that is heavily O-glycosylated.11 The deduced size would be 2.5 MD for the protein part, and with added carbohydrates, this could increase to 5 MD.10,26 OC125, M11, and most other antibodies prepared against ovarian cancer cell extracts are directed at these complex, glycosylation-dependent antigens. These antigens are exclusively present in the shed portion of MUC16 and cannot be employed to follow the biology of the proximal portion of MUC16 and may not accurately reflect tissue distribution since the glycosylation patterns can vary substantially among tissues. The identification of antibodies directed against proximal sequences in the peptide backbone of MUC16 provides important new tools for the exploration of ovarian cancer biology.
We have developed novel antibodies that are directed at that non-cleaved, non-glycosylated peptide backbone of MUC16. Both 4H11 and 9C9 react with peptide sequences in the non-cleaved ectodomain of MUC16 and are detectable on the surface of ovarian cancer cell lines and in paraffin-fixed tissues from human ovarian cancer surgical specimens. The antibodies show high affinity and are readily internalized by ovarian cancer cells when bound to the ectodomain of MUC16. This suggests that the proximal portion of MUC16 has an independent biology from the more distal, cleaved portion of the mucin. It also suggests that the proximal portions of MUC16 could provide convenient targets for diagnostic and therapeutic interventions. Targeting the peptide backbone of MUC16 could provide highly specific tissue delivery for genetically engineered cells, liposomes, or antibody conjugates.
Antibodies like 4H11 may also prove to be useful as tools in immunohistochemistry. 4H11 appears to be relatively specific to high-grade ovarian serous carcinoma. Invasive lobular breast carcinoma is the major exception and shows extensive MUC16 protein as detected by 4H11. Lobular carcinoma of the breast has unique biology which is characterized by a propensity to metastasize to serosal surfaces.27 Since MUC16 is the cognate binding partner of mesothelin, this may have important implications for lobular cancer.28 The discordance rates for OC125 and 4H11 also suggest that 4H11 might provide additional, independent information from OC125 in a small subset of ovarian carcinomas. Some tumors that are negative with OC125 retain cytoplasmic and extracellular portions of the MUC16 glycoprotein, portions of the molecule that are likely involved in transduction of signals potentially important in the malignant phenotype.
In conclusion, we have demonstrated the development and characterization of novel monoclonal antibodies that react to the tethered peptide backbone of the complex MUC16 protein. This could have important diagnostic and therapeutic implications in the management of human ovarian carcinoma.
Supplementary Material
Supplemental Figure 1A–D: Comparison staining intensities of OC125 and 4H11 monoclonal antibodies on tissue microarrays containing cancers of the prostate (2A, concordant), lung (2B, discordant), breast (2C, discordant), and pancreas (2D, discordant).
Supplemental Figure 2: FACS analysis as described in the Material and Methods section was performed with commercial antibodies and MUC16 carboxy terminus monoclonal antibodies on OVCAR3 wt, SKOV3-phrGFP-ΔMUC16c114 and SKOV3-phrGFP-ΔMUC16c334 stable transfected cell lines.
ACKNOWLEDGMENTS
The authors greatly acknowledge the help and support of the MSKCC core facilities, especially the Microchemistry, DNA Sequencing, Monoclonal Antibody, Flow Cytometry, Pathology, and Molecular Cytology cores.
Disclosures This work was supported by PO1-CA52477-16 and Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center. Dr. Smith Jones is supported by PO1-CA033049-25 and P50-CA086438-08
REFERENCES
- 1.Bast RC, Jr, Feeney M, Lazarus H, et al. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bast RC, Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 3.Rustin GJ, Bast RC, Jr, Kelloff GJ, et al. Use of CA-125 in clinical trial evaluation of new therapeutic drugs for ovarian cancer. Clin Cancer Res. 2004;10:3919–3926. doi: 10.1158/1078-0432.CCR-03-0787. [DOI] [PubMed] [Google Scholar]
- 4.Rosen DG, Wang L, Atkinson JN, et al. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005;99:267–277. doi: 10.1016/j.ygyno.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 5.Bast RC, Jr, Badgwell D, Lu Z, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(Suppl 3):274–281. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 6.Moore RG, Maclaughlan S, Bast RC., Jr Current state of biomarker development for clinical application in epithelial ovarian cancer. Gynecol Oncol. 2010;116:240–245. doi: 10.1016/j.ygyno.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nustad K, Lebedin Y, Lloyd KO, et al. Epitopes on CA125 from cervical mucus and ascites fluid and characterization of six new antibodies. Third report from the ISOBM TD-1 workshop. Tumour Biol. 2002;23:303–314. doi: 10.1159/000068570. [DOI] [PubMed] [Google Scholar]
- 8.Fendrick JL, Konishi I, Geary SM, et al. CA125 phosphorylation is associated with its secretion from the WISH human amnion cell line. Tumour Biol. 1997;18:278–289. doi: 10.1159/000218041. [DOI] [PubMed] [Google Scholar]
- 9.Fendrick JL, Staley KA, Gee MK, et al. Characterization of CA125 synthesized by the human epithelial amnion WISH cell line. Tumour Biol. 1993;14:310–318. doi: 10.1159/000217844. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien TJ, Beard JB, Underwood LJ, et al. The CA 125 gene: a newly discovered extension of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumour Biol. 2002;23:154–169. doi: 10.1159/000064032. [DOI] [PubMed] [Google Scholar]
- 11.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98:737–740. doi: 10.1002/ijc.10250. [DOI] [PubMed] [Google Scholar]
- 12.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 13.Hollingsworth M, Swanson B. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 14.Huang L, Ren J, Chen D, et al. MUC1 cytoplasmic domain coactivates Wnt target gene transcription and confers transformation. Cancer Biol Ther. 2003;2:702–706. [PubMed] [Google Scholar]
- 15.Li Q, Ren J, Kufe D. Interaction of human MUC1 and beta-catenin is regulated by Lck and ZAP-70 in activated Jurkat T cells. Biochem Biophys Res Commun. 2004;315:471–476. doi: 10.1016/j.bbrc.2004.01.075. [DOI] [PubMed] [Google Scholar]
- 16.Ren J, Agata N, Chen D, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5:163–175. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren J, Bharti A, Raina D, et al. MUC1 oncoprotein is targeted to mitochondria by heregulin-induced activation of c-Src and the molecular chaperone HSP90. Oncogene. 2006;25:20–31. doi: 10.1038/sj.onc.1209012. [DOI] [PubMed] [Google Scholar]
- 18.Ramsauer VP, Pino V, Farooq A, et al. Muc4-ErbB2 complex formation and signaling in polarized CACO-2 epithelial cells indicate that Muc4 acts as an unorthodox ligand for ErbB2. Mol Biol Cell. 2006;17:2931–2941. doi: 10.1091/mbc.E05-09-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bafna S, Singh AP, Moniaux N, et al. MUC4, a multifunctional transmembrane glycoprotein, induces oncogenic transformation of NIH3T3 mouse fibroblast cells. Cancer Res. 2008;68:9231–9238. doi: 10.1158/0008-5472.CAN-08-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponnusamy MP, Singh AP, Jain M, et al. MUC4 activates HER2 signaling and enhances the motility of human ovarian cancer cells. Br J Cancer. 2008;99:520–526. doi: 10.1038/sj.bjc.6604517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nap M, Vitali A, Nustad K, et al. Immunohistochemical characterization of 22 monoclonal antibodies against the CA125 antigen: 2nd report from the ISOBM TD-1 Workshop. Tumour Biol. 1996;17:325–331. [PubMed] [Google Scholar]
- 22.Markwell MA, Fox CF. Surface - specific iodination of membrane proteins of viruses and eucarytic cells using 1,3,4, 6-tetrachloro-3alpha,6alpha-diphenylglycouril. Biochemistry. 1978;17:4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- 23.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 24.Hedvat CV, Hegde A, Chaganti RS, et al. Application of tissue microarray technology to the study of non-Hodgkin's and Hodgkin's lymphoma. Hum Pathol. 2002;33:968–974. doi: 10.1053/hupa.2002.127438. [DOI] [PubMed] [Google Scholar]
- 25.Soslow RA. Histologic subtypes of ovarian carcinoma: an overview. Int J Gynecol Pathol. 2008;27:161–174. doi: 10.1097/PGP.0b013e31815ea812. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien TJ, Beard JB, Underwood LJ, et al. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol. 2001;22:348–366. doi: 10.1159/000050638. [DOI] [PubMed] [Google Scholar]
- 27.Harris M, Howell A, Chrissohou M, et al. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br J Cancer. 1984;50:23–30. doi: 10.1038/bjc.1984.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneko O, Gong L, Zhang J, et al. A binding domain on mesothelin for CA125/MUC16. J Biol Chem. 2009;284:3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1A–D: Comparison staining intensities of OC125 and 4H11 monoclonal antibodies on tissue microarrays containing cancers of the prostate (2A, concordant), lung (2B, discordant), breast (2C, discordant), and pancreas (2D, discordant).
Supplemental Figure 2: FACS analysis as described in the Material and Methods section was performed with commercial antibodies and MUC16 carboxy terminus monoclonal antibodies on OVCAR3 wt, SKOV3-phrGFP-ΔMUC16c114 and SKOV3-phrGFP-ΔMUC16c334 stable transfected cell lines.