Abstract
Evolutionarily conserved Notch signaling orchestrates diverse physiological mechanisms during metazoan development and homeostasis. Classically, ligand-activated Notch receptors transduce the signaling cascade through the interaction of DNA-bound CBF1-co-repressor complex. However, recent reports have demonstrated execution of a CBF1-independent Notch pathway through signaling cross-talks in various cells/tissues. Here, we have tried to congregate the reports that describe the non-canonical/CBF1-independent Notch signaling and target gene activation in vertebrates with specific emphasis on their functional relevance.
Keywords: Notch signaling, CBF1-independent Notch, Hes-1, Non-canonical Notch, Neural differentiation, Tumorigenesis
Canonical Notch signaling
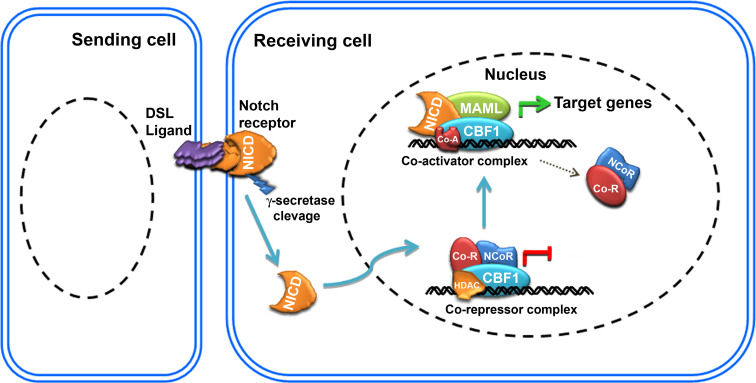
Notch signaling is evolutionarily conserved from Drosophila to higher mammals and involves cell–cell interaction, resulting in various aspects of metazoan development [1, 2]. Notch receptors are activated by transmembrane ligands expressed on neighboring cells which are collectively known as DSL (Delta, Serrate and Lag2). Upon activation, Notch intracellular domain (NICD) is cleaved by γ-secretase and translocated into the nucleus. There, it converts the CSL (CBF1, Su(H), Lag1) co-repressor complex into an activator complex on Notch responsive promoters [3]. The mammalian homologue of CSL is known as C promoter binding factor 1 (CBF1) or recombination signal binding protein for immunoglobulin kappa J region (RBPJk). CBF1 is a DNA binding transcription factor mediating canonical Notch signaling [4]. The constitutively expressed CBF1 is always bound to a specific sequence on promoters of Notch target genes and regulates their expression. In the absence of NICD, CBF1 will recruit transcriptional co-repressors due to its higher affinity, thereby inhibiting the transcription of specific target genes [5]. When canonical Notch signaling is activated through ligand-mediated interaction, cleaved NICD will bind to CBF1 and convert the transcriptional repressor complex into an activator complex together with Mastermind-like (MAML) transcriptional co-activators, and other specific co-activators (Fig. 1) [6, 7]. Activation of Notch signaling triggers the expression of various target genes, such as Hes and the Hes-related (HESR/HEY) family of basic helix-loop-helix (bHLH) transcription factors [8–10]. Hes genes are the mammalian homologue of Drosophila hairy and Enhancer of split proteins, which encode bHLH transcriptional repressors. Even though Hes genes are the major targets of Notch signaling, there are reports on the activation of other tissue-specific targets such as brain lipid binding protein (BLBP) [11]. The Hes family of transcription factors recruit Groucho/TLE co-repressors and regulate the expression of tissue-specific genes such as Mash1 and NeuroD required for various cellular functions [12, 13].
Fig. 1.
Schematic of canonical Notch signaling: Canonical Notch signaling is activated by its ligand leading to γ-secretase cleavage of Notch intracellular domain (NICD) which in turn translocates into the nucleus. There, it converts the transcriptional co-repressor complex into an activator complex by recruiting MAML and specific co-activators (Co-A) following displacement of the co-repressor complex (including NCoR, HDAC and Co-R). Subsequently, the specific co-activator complex will trigger the transcription of Notch target genes (Hes/Herp family)
Canonical Notch signaling plays a central role in diverse cellular tasks such as embryonic development [14–16], stem cell maintenance [17, 18], fate-specific differentiation [19, 20], and adult tissue homeostasis [21]. In addition to their role in development and homeostasis, dysregulation of Notch components are widely and directly implicated in various human disorders [22]. These disorders include developmental syndromes [23–25], and the initiation, progression and maintenance of pancreatic [26] and other cancers [27, 28]. Notch signaling has also emerged as a specific therapeutic target for T-cell acute lymphoblastic leukemia [29] and colon cancer [30]. Even though canonical Notch signaling is very well documented in numerous cell types and diseases, evidence has emerged for the non-canonical activation of Notch signaling or target genes in certain cells/tissues for executing specific functions.
Evidence for non-canonical/CBF1-independent Notch signaling and target gene activation in vertebrates
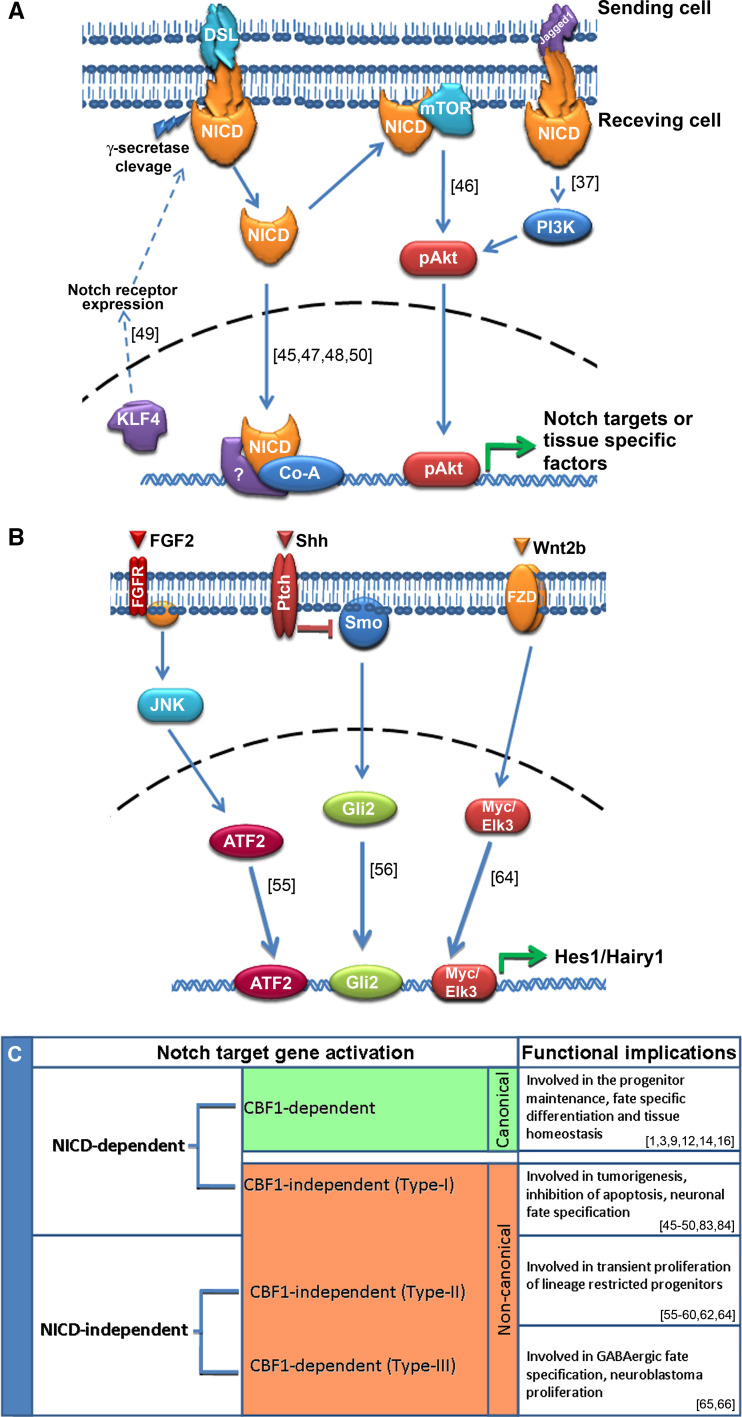
Early evidence for CSL-independent non-canonical Notch signaling originated from studies conducted on Drosophila mutants (both Notch receptor and CSL) [22, 31]. Since CSL mutants are not phenotypically the same as Notch receptor mutants, the possibility of an alternate mechanism of Notch signaling was postulated [32, 33]. CSL-independent Notch signaling has been widely shown in various anatomical/physiological characteristics of Drosophila [34, 35]. Later, reports also emerged from vertebrates for the existence of non-canonical Notch signaling in various tissues [36, 37]. The discrepancy between Notch receptor/ligand expression and Hes genes in neuroepithelial cells also strengthens the notion for the existence of non-canonical signaling in vertebrates. The majority of the initial reports have emerged from the use of either Notch receptors devoid of CBF1 interacting domain or CBF1 null cell lines. Since CBF1 double mutants are embryonically lethal [38], various conditional CBF1 knock-out mouse models were generated for studying the CBF1-mediated signaling in different aspects of mammalian development [39–45]. Upon analysis of reports on non-canonical Notch/CBF1 signaling in vertebrates, we found the existence of two types of CBF1-independent Notch target gene activation, regardless of their function (Fig. 2c). Type-I (non-canonical Notch signaling) involves ligand-mediated activation of Notch receptors, but transduces the pathway independent of CBF1 (Fig. 2a), and Type II involves the activation of Notch target genes completely devoid of either Notch receptor cleavage or CBF1-mediated signal transduction (Fig. 2b; Table 1).
Fig. 2.
Schematic of non-canonical Notch signaling/target gene activation. a The non-canonical Notch signaling (Type-I) pathway requires ligand mediated cleavage of Notch receptor but transduces the signals independent of CBF1 interaction. Here, the cleaved NICD interacts with tissue-specific co-activators (Co-A) and other undefined factors to activate downstream targets/functions. The cleaved NICD can also interact with components of other signaling pathways and activate downstream components of Notch signaling. b Non-canonical Notch target gene activation (Type-II) is completely devoid of either ligand-mediated NICD-release or CBF1-interaction, and hence target genes are activated through alternate signaling mechanisms. Downstream effectors of JNK, Shh and Wnt are known to directly activate the expression of Hes-1/Hairy-1 independent of Notch/CBF1 interaction. c Classification of Notch signaling and its functional implication. References for each signaling pathway are given in square brackets
Table 1.
List of reports for non-canonical Notch signaling/target gene activation in vertebrates
| Sl. No. | Cells/tissues | Interacting pathways | Reference |
|---|---|---|---|
| Type-I | |||
| 1 | EMT in human cervical tumor derived cell line | Jagged1-PI3K/pAKt | [37] |
| 2 | Breast tumor progression | KLF4 | [49] |
| 3 | B-lymphocyte lineage commitment | – | [40] |
| 4 | Mammary gland tumorigenesis | Notch4/Int3 | [45] |
| 5 | Endothelial cell maintenance/anti apoptosis | Bcl2 | [68] |
| 6 | Inhibition of neglect-induced apoptosis and thereby cell survival | NICD-mTOR-Akt | [46] |
| 7 | c-myc expression in human erythroleukemia cells | NICD-YY1 | [48] |
| 8 | Neoplastic transformation | – | [83] |
| 9 | Neoplastic transformation of RKE cells | – | [84] |
| 10 | Inhibition of muscle cell differentiation | – | [36, 47] |
| 11 | Generation of neuronal restricted intermediate neural progenitors | – | [50] |
| 12 | Proliferation of hematopoietic progenitors | solD4-Notch | [51] |
| Type-II | |||
| 13 | Maintenance of ES cell derived neural progenitors | FGF2-JNK-ATF2-Hes1 | [55] |
| 14 | Proliferation of Retinal progenitors | Shh-Gli2-Hes1 | [56] |
| 15 | Hes-1 expression in growth arrested human endothelial cells | JNK-Hes1 | [62] |
| 16 | Maintenance of retinal ciliary marginal zone progenitors | Wnt2b-Hairy1 | [64] |
| 17 | Inhibition of retinal progenitor cell differentiation | Wnt2b | [90] |
| 18 | Maintenance of hematopoietic stem cells | – | [58] |
| 19 | Hes-1 regulation in multipotent mesodermal and neural cells | Shh-Hes1 | [63] |
| 20 | Hes-1 expression in hematopoietic progenitors | E2A | [57] |
| 21 | Hes-1/Hes-5 expression in DN thymocytes | – | [60] |
| 22 | Hes-1 expression in Pax-5 deficient pro-B cells | – | [59] |
| 23 | Hes-1/Hes-3 expression in neuro-epithelial cells | – | [53] |
| 24 | Hey-2 expression in pillar cells of organ of corti | FGF-Hey-2 | [70] |
| Type-III | |||
| 25 | Specification of GABAergic neuron differentiation | bHLH-RBPJk | [66] |
| 26 | Hes-1 expression in neuroblastoma cells | TGFα-Ras-Hes1 | [65] |
| 27 | Hes-5 expression in retinal progenitors | Shh | [56] |
Cells/tissues and interacting pathways with individual references are listed
– Pathway not mentioned
In CBF1-independent non-canonical Notch signaling, cleaved NICD interacts with components of other signaling pathways and activates the downstream targets. Both canonical Notch targets and other tissue-specific transcription factors are activated through this non-canonical Notch signaling and are basically concentrated on the tissue-specific promoters. However, recent reports have shown the execution of non-canonical Notch signaling through membrane-tethered NICD outside the nucleus (Fig. 2a) [46]. Thus, irrespective of the known transcriptional regulatory function, cleaved/activated Notch receptors are involved in the various aspects of cellular function in a context-dependent manner. In vertebrates, the CBF1-independent non-canonical Notch signaling was initially reported in proliferating myoblasts in vitro [36, 47]. Subsequently, in vivo models/tissues concerning the involvement of CBF1-independent non-canonical pathways in tumor cells/tumor progression have also been extensively shown [37, 45, 46, 48, 49]. Various effects such as proliferation, neoplastic transformation, tumor progression, and apoptosis have been implicated as the result of such a non-canonical pathway in cancer. The involvement of CBF1-independent Notch signaling in specifying the fate during early neural differentiation has been well documented by Mizutani et al. [50]. Consistent with neural fate specification, CBF1-independent Notch signaling is implicated in B-lymphocyte lineage commitment [40] and proliferation of hematopoietic progenitors [51].
Type II CBF1-independent Notch target gene activation is completely devoid of γ-secretase-mediated cleavage of Notch receptor. Here, the expression of Notch target genes is activated by alternate signaling pathways or factors (Fig. 2b). Though Hes-1 is considered as one of the principal Notch target genes, it shows the maximum non-canonical activation in various contexts. As evidenced from various mutants of Notch receptor [39], ligand [52] or effectors [41], it is clear that Hes-1 is not always under the strict control of Notch/CBF1 interaction. The observation that Hes-1 and Hes-3 are expressed prior to Notch receptor or ligand expression in neuroepithelial cells has strengthened the above statements [53, 54]. Moreover, the latest reports from our laboratory and others have shown the existence of Notch/CBF1-independent pathways for the activation of Hes-1 expression in various cells/tissues [55]. Notch-independent Hes-1 expression is mainly reported in neural progenitors [55], retinal progenitors [56], hematopoietic progenitors [57, 58], T/B-cell precursors [59–61], endothelial cells [62], and cancer cells [63]. In addition to Hes-1, other Notch targets have also been reported to express independently of canonical Notch signaling. Kubo et al. [64] have shown that Hairy1 can be activated non-canonically by Wnt2b in the ciliary marginal zone (CMZ) of chick retina.
In addition to the above, there are reports for the Notch-independent, CBF1-dependent signaling in neurons and cancer cells (Type-III) [65, 66]. Reports have indicated that Hes-1 can be regulated without Notch receptor cleavage and NICD release in a CBF1-dependent manner in cancer cells [65]. Consistent with Hes-1, another strict Notch target gene Hes-5 can also express without γ-secretase cleavage of Notch receptor in a CBF1-dependent manner in retinal stem cells [56]. A non-canonical PTF1-CBF1 transcription factor complex is also reported in the generation of GABAergic neurons, and this mechanism is independent of canonical Notch signaling [66, 67]. However, both CBF1-dependent and -independent mechanisms work together to perform particular cellular functions such as maintenance of neural progenitors [55], endothelial cells maintenance [68], neurite outgrowth in PC12 cells [69], organ of corti pillar cell maintenance [70], and T-cell specification of hematopoietic progenitors [57].
Cross-talk between various signaling pathways executes non-canonical Notch signaling and target gene activation
The cross-talk of Notch signaling with other signaling pathways has been reported in various cellular functions, such as cell fate specification, proliferation, stem cell maintenance, and oncogenesis [71–75]. Therefore, it can be assumed that these different pathways might be involved in the activation of non-canonical Notch signaling and Notch target genes. These signaling pathways, which include Hedgehog, Jak/STAT, RTK, TGF-β, Wnt and Notch [74, 76], network together and execute various cellular processes starting from developmental fate specification to higher complex organogenesis and tissue homeostasis [77–79]. In addition to this, various factors such as growth factors are also involved in activating/triggering the CBF1-independent Notch target gene activation (Table 1). Growth factors are mainly implicated in activation of Notch target genes non-canonically in various tissues [55]. The molecular mechanism of activation/triggering of non-canonical Notch signaling via the interaction with Wnt and abl tyrosine kinase pathways have been clearly demonstrated in Drosophila [22, 31, 80, 81]. Similarly, interaction of Notch receptor/NICD with other molecular pathways or components has also been demonstrated in vertebrates. The mechanism of activation or implementation of non-canonical Notch signaling is context-dependent and the interacting molecules/pathway will vary according to the tissue or function. Deltex1-mediated non-canonical activation of Notch signaling through Jagged1 has been reported in human cervical tumor-derived cells (Fig. 2a) [37]. Here, Jagged1–Notch interaction further activates phosphoinositide 3-kinases (PI3K)-Akt signaling and triggers the pro-oncogenic induction which is completely independent of CBF1. The involvement of Akt signaling for the execution of a non-canonical Notch pathway is also evidenced in neural stem cells and neglect-induced cells. Ligand (Dll4/Jagged1)-induced activation of Notch receptor directly phosphorylates and activates the PI3K-Akt, mTOR (mammalian target of rapamycin) pathway and subsequently induces the expression of Hes-3 and Shh which leads to the proliferation of neural stem cells [73]. Similarly, in neglect-induced HeLa cells, membrane-tethered NICD interacts with Rictor and mTOR to trigger Akt phosphorylation leading to inhibition of apoptosis [46]. Moreover, the NICD-mTOR-Akt pathway does not require interaction with CBF1 and transduces the pathway non-canonically. The direct interaction of ligand-activated Notch receptor with other factors such as Ying Yang1 (YY1) and Bcl2 has been shown to instigate the non-canonical pathway in human erythroleukemia and endothelial cells, respectively [48, 68]. Krüppel-like family of transcription factor 4, KLF4, is another transcription factor activating the expression of Notch1 mRNA and its cleavage to active form in human mammary epithelial cells (Fig. 2a). However, the KLF4-mediated transformation of epithelial cells is independent of canonical Notch signaling which is demonstrated by the use of dnCBF1 and dnMAML constructs [49]. In general, canonical or non-canonical Notch signaling is initiated through the interaction of membrane-bound ligands (Dll4/Jagged1) with Notch receptor; however, soluble forms of ligands are also reported in activating the Notch signaling [82]. In proliferating hematopoietic stem cells, the soluble form of Delta4 ligand (solD4) activates non-canonical Notch cascade and enhances its proliferation [51]. solD4 carries out this mechanism in a CBF1-independent manner and does not induce any significant increase in the expression of principal Notch target genes. Although there are many more reports for the non-canonical activation of Notch signaling in various contexts, the exact molecular mechanism has not been illustrated [36, 40, 47, 50, 83, 84].
As mentioned earlier, Notch-independent activation of HES/HEY family of transcription factors is achieved through the cross-talk with other signaling pathways. Common regulatory pathways such as Wnt, Shh, FGF and MAPK are reported to directly activate the expression of various Notch target genes in different contexts. Hes-1 is the principal Notch target which can be activated non-canonically; our recent report has shown that FGF2 is able to transactivate Hes-1 expression independent of CBF1/Notch in neural progenitors [55]. Here, FGF2 activates c-Jun N-terminal kinase (JNK) through Cdc42-Ras pathway, and the activated JNK further phosphorylates ATF2. Further, phospho-ATF2 in turn binds to Hes-1 promoter and activates Hes-1 expression independent of CBF1 pathway (Fig. 2b). Involvement of JNK signaling in non-canonical Hes-1 expression is also reported in human endothelial cells [62]. In addition to this, Shh is known to activate Hes-1 expression non-canonically in retinal progenitors [56] and multipotent mesodermal/neural stem cells [63]. In retinal progenitors, Shh mediates non-canonical activation of Hes-1 through Gli2 which in turn binds to Hes-1 promoter [56]. The direct activation of Notch-independent Hes-1 expression is also shown in hematopoietic progenitors which is carried out through the direct binding of E2A transcription factor on Hes-1 promoter [57]. Similarly, in human neuroblastoma cells (SK-N-BE(2)c), TGFα activates Hes-1 expression non-canonically [65]. Though Hes-1 activation by TGFα is independent of Notch receptors, it requires CBF1 as in the case of canonical Notch signaling. TGFα transduces the pathway through EGF receptor/Ras and finally phosphorylates ERK1/2, and, thus, activated phospho-ERK triggers the expression of Hes-1 in a CBF1-dependent manner. Even in the absence of externally administrated TGF-α, SK-N-BE(2)c cells are maintained by the expression of non-canonical Hes-1 through MEK-ERK pathway. In addition to this, undefined factors present in the serum may also activate MEK/ERK leading to the expression of Hes-1 [65].
Hairy-1, another Notch target gene, can also be regulated independent of canonical Notch signaling. Retinal stem cell-like progenitors in the chick CMZ are maintained through activation of Hairy-1, mediated by Wnt signaling [64]. Kubo et al. [64] have clearly demonstrated the involvement of Wnt2b effectors such as ELK3, LMO4 and Zic2 in the Notch-independent expression of Hairy-1 in CMZ progenitors. Consistent with Hes-1 and Hairy-1, other Notch target gene such as Hey-2 is expressed independently of canonical Notch signaling for maintaining pillar cell fate in the Organ of Corti [70]. The Notch-independent Hey-2 expression in pillar cells is triggered through FGF signaling, and both Notch and FGF signaling are together involved in the maintenance of these cells.
Functional implications of non-canonical Notch signaling/target gene activation
The requirement of a non-canonical/CBF1-independent Notch signaling and target gene activation during embryonic development and normal homeostasis is becoming extremely imperative, since it is required for various cellular functions ranging from transcriptional repression, tissue regeneration, cell fate decision, proliferation, differentiation, and tumorigenesis. As discussed previously, eukaryotes perform all these diverse physiological tasks through the cross-talk of limited number of signaling pathways, which are evolutionarily conserved [74, 76]. Canonical Notch pathway is a cell–cell-mediated signaling mechanism and requires a proper niche where cell–cell interactions occur, thereby activating the Notch targets. Moreover, activation of canonical Notch signaling triggers the expression of wide variety of downstream target genes simultaneously. Therefore, tissue-specific activation of certain transcription factor(s) may not be possible. To activate specific Notch target genes/transcription factors exclusively in a tissue-specific manner, canonical activation of Notch signaling may not be a good choice. Thus, differential Notch signaling or non-canonical activation of Notch target genes may become a pre-requisite for the tissue-specific activation. Also, it is observed that certain cells/tissues will not express/express reduced levels of Notch components (both receptor and ligand) [35] or become resistant to canonical Notch activation through CBF1 for specifying particular fate/function during the course of development [50]. This is affected by making cells impervious to canonical Notch/CBF1 activation as a result of tissue-specific chromatin re-modeling [85, 86]. Therefore, cells/tissues recruit multiple signaling cross-talks and non-canonical signaling cascades context-dependently for executing their precise functions. The non-canonical/CBF1-independent Notch target activation in vertebrates is mainly reported in the proliferation of lineage-restricted progenitors, fate-specific differentiation, and oncogenic transformation.
Proliferation and transient amplification of lineage restricted progenitors
Although Notch signaling maintains various tissue-specific stem cells, the mechanism involved in the derivation of lineage-restricted progenitors from stem cells is not very clear. The differential regulation (CBF1-independent/dependent) of Notch signaling has been reported in the maintenance/generation of various lineage-restricted progenitors during development. The differential regulation of a specific set of transcription factors is attained through the cross-talk of various growth factors or signaling pathways involved in the activation of Notch target genes non-canonically. These activated target genes will directly help in the proliferation of progenitors either by transcriptionally repressing the classical pro-neural genes or by activating the cell cycle regulators. This kind of differential regulation of a subset of progenitors along with their prolonged proliferation was mainly reported in neural progenitors, especially in retinal as well as ES cell-derived neural progenitors [55, 56, 64]. Wnt2b-mediated Notch-independent activation of Hairy-1 in CMZ of chick retina would help in the prolonged proliferation and maintenance of Rdh10-positive retinal progenitors [64]. Wnt signaling is able to activate the expression of CMZ-specific transcription factors and their maintenance, but fails to do so in the absence of Hairy-1 [64]. Therefore, non-canonical activation of Hairy-1 differentially maintains Rdh10-positive retinal progenitors; on the other hand, canonical Notch signaling maintains retinal progenitors in the central region that finally differentiates into Müller cells through the activation of Hes-5 as reported earlier [87–89]. Shh signaling is also reported in the proliferation and maintenance of a specific subset of retinal progenitors through the expression of Hes-1 independent of Notch/CBF1 signaling [56]. Here, non-canonical Notch target activation maintains Müller glial and bipolar progenitors in the postnatal retina. This is in contradiction to the previous reports [64], and we assume that the ultimate effect of these differential signaling pathways may be temporally regulated and context-dependent. Non-canonical Hes-1 activation is also involved in the maintenance of a subset of ES cell-derived neural progenitors [55] and hematopoietic stem cell proliferation [57, 58]. Though non-canonical Notch signaling is reported in the maintenance of hematopoietic stem cells, there are contradictory reports to this which claim that canonical Notch signaling is required for hematopoietic stem cell maintenance [72]. In retinal progenitors, Wnt2b is also known to inhibit pro-neural genes directly without the involvement of Notch target gene activation [90].
However, CBF1-independent Notch signaling (Type-I) is also reported in the maintenance and proliferation of lineage-restricted progenitors. Recently, it has been shown that neural stem cells are differentially regulated by Notch signaling in developing the telencephalic ventricular zone, where two different stem cell populations co-exist [50]. Neural stem cells (NSCs) are maintained through CBF1-dependent canonical Notch activation, whereas a CBF1-independent pathway is executed for the proliferation and maintenance of intermediate neural progenitors (INPs). In agreement with in vivo reports, evidence has reported for CBF1-independent Notch signaling in the regulation of myoblasts cells in vitro [36, 47]. Here, activated Notch receptor transduces the signaling cascade independent of CBF1 and inhibits myogenesis and osteogenesis with the help of factors other than MyoD. However, CBF1-dependent Notch signaling is also reported in the inhibition of myoblast differentiation through MyoD activation in a cell-specific manner [91]. The soluble form of Delta4 (solD4) has been shown to be involved in the transient amplification of hematopoietic stem cells non-canonically while membrane-bound Delta4 maintains the hematopoietic stem cells through canonical Notch signaling [51]. solD4 non-canonically activates the proliferation of hematopoietic stem cells which are independent of CBF1 and would not induce a significant increase in target gene expression.
Fate specific differentiation
As discussed in the previous session, non-canonical activation of the Notch signaling/target gene is involved in the generation of lineage-restricted progenitors in various tissues during development. Subsequently, these lineage-restricted progenitors will differentiate into their respective fates with the help of their niche factors. The non-canonical/CBF1-independent mechanism of lineage restriction and fate-specific differentiation is mainly reported in the developing nervous system and also lymphoid progenitor fate specification.
The generation of neuronal-restricted INPs from neural stem cells through differential Notch signaling is well documented in developing telencephalon [50]. The neuronal INPs and neural stem cells are differentially maintained through CBF1-independent and -dependent mechanisms, respectively. Upon differentiation, the CBF1-independent INPs predominantly generates neurons and is further resistant to CBF1 activation. However, CBF1-dependent neural stem cells are able to differentiate into neuronal, glial, and oligodendrocyte lineages. Therefore, it can be assumed that the neuronal restriction of progenitors is specifically attained through the non-canonical CBF1-independent Notch signaling. In addition to this, Shh-mediated non-canonical/CBF1-independent expression of Hes-1 in retinal progenitor cells (RPC) will confer their Müller glial and bipolar fates [56]. This non-canonical pathway will selectively enhance the proliferation of these progenitors at the expense of rod photoreceptors, whereas perturbation of Shh/Gli will result in the reduction of Müller and bipolar cells. Notch-independent expression of Hey-2 through FGF signaling is reported in the maintenance of pillar cell fate in the organ of corti. Both canonical Notch signaling and FGF-regulated Hey-2 together maintain the pillar cell fate by inhibiting Math1. Perturbation of both Notch and FGF signaling resulted in the transdifferentiation of pillar cells into hair cells through math1 expression [70]. The non-canonical Notch signaling is also reported in the in vitro differentiation of adipose-derived stem cells. Schwann cell differentiation from adipose-derived stem cells occurs through non-canonical Notch signaling [92]. A recent report from Hori et al. [66] has also shown the involvement of the Notch-independent CBF1-Ptf1a complex in the specification of GABAergic neurons during spinal cord development.
As mentioned above, non-canonical/CBF1-independent Notch target gene activation is also implicated in the fate specification of lymphoid progenitors. Common lymphoid progenitors (CLP) derived from hematopoietic stem cells can give rise to both T-cells and B-cells during development, and Notch signaling plays a key role in the fate specification of these progenitors [93, 94]. CBF1-mediated canonical Notch signaling is required for the maintenance and differentiation of T-lymphocytes through the activation of T-lineage-specific target genes by CBF1/NICD complex [40, 93, 95]. At the same time, it will activate a CBF1-independent pathway through Deltex and inhibit the expression of E47 transcription factor required for the commitment of B-cell lineage [40, 96, 97]. E proteins mediate T-cell lineage specification at the expense of NK and myeloid cell maturation through activating Notch target gene expression in concert with Notch signaling during CLP differentiation [57]. The conditional knock-out of CBF1 also confirms the role of CBF1-mediated Notch signaling in the T versus B lineage fate specification [40]. CBF1 knock-out in bone marrow cells inhibited the differentiation of T-cells along with generation of B-cells [98].
Tumorigenesis
Notch signaling has been considerably associated with tumorigenesis, proliferation, and progression of cancers. Initially, it was shown that translocation in T-cell acute lymphoblastic lymphoma (T-ALL) is associated with the up-regulation of the activated form of Notch1 [99]. Later, the oncogenic role of Notch has been shown in a wide range of cancers including neuronal, hematopoietic, and epithelial cancers [16, 27, 100, 101]. Notch-regulated cancers are involved in promoting proliferation or inhibition of apoptosis and tumorigenicity of the tumor cells. Inhibition through γ-secretase inhibitor reverted some of these characteristics [102, 103].
There are many recent reports regarding CBF1-independent Notch signaling in the regulation/maintenance of various cancers. Persistent proliferation (through the dysregulation of Notch target genes), neoplastic transformation, and inhibition of apoptosis are the main consequences of non-canonical/CBF1-independent Notch signaling in cancer cells/tissues. CBF1-independent Notch signaling is mainly reported in the various aspects of cancer induction and progression. Although CBF1-mediated Notch4/Int3 signaling is essential for the mammary alveolar development [104], tumorigenesis and tumor growth in mammary cells are triggered through a CBF1-independent Notch4/Int3 signaling [45]. Targeted deletion of CBF1 in Int3-expressing mammary cells did not show any significant change in its hyper-proliferative effect but reduced apoptotic activity. Thus, Notch4/Int3 induces tumor formation and its progression without activating classical downstream targets. Along with the in vivo tumorigenic role of non-canonical Notch signaling, their involvement in in vitro transformation process through viral oncogenes has been documented in different cell lines. CBF1-independent Notch signaling has been implicated in the E1A-mediated neoplastic transformation of RK3E kidney cells. Notch receptor without the CBF1-interacting domain is able to translocate into the nucleus and induces neoplastic transformation [83, 84]. Jagged1-mediated Notch signaling has been shown in the progression of HPV-driven human cervical cancer in a CBF1-independent manner. The oncogenic properties like anoikis resistance and induction of epithelial-mesenchymal transformation are mediated through the activation of PI3K signaling via CBF1-independent non-canonical Notch pathway [37]. Consistent with this observation, the epithelial transformation by KLF4 is mediated through a CBF1-independent Notch pathway during breast tumor progression. KLF4 directly activates the transcription of Notch1 gene and transduces signaling cascade through a CBF1-MAML-independent pathway resulting in the transformation of epithelial cells (Fig. 2a) [49].
Besides hyper-proliferation and transformation of cancer cells/tissues, non-canonical/CBF1-independent Notch signaling is known to inhibit apoptosis and, thereby contribute to cancer progression. Endothelial cells are usually resistant to various apoptotic signaling in vivo [68], and Notch signaling has been reported in the maintenance of endothelial cells through CBF1-dependent and -independent anti-apoptotic pathway. CBF1-independent pathway is mediated through the activation of Bcl-2 and inhibits pro-apoptotic pathways together with CBF1-dependent mechanism [68]. Perumalsamy et al. [46] have clearly shown the involvement of a non-canonical Notch signaling for the inhibition of neglect-mediated apoptosis in mammalian cells including cancerous cells. The CBF1-independent pathway is executed via the activation of mTOR-Akt/PKB kinase through the membrane-tethered NICD (Fig. 2a) [46]. This is the first report which examines the Notch-mediated mTOR-Akt pathway in inhibiting the neglect-mediated apoptosis and, thus, survival in a non-canonical manner.
As mentioned earlier, various Notch target genes are implicated in the proliferation and progression of cancer cells [71]. Hes-1, one of the major Notch target genes, is activated independently of Notch in various cancer cells/tissues. Different regulatory pathways such as Notch, Ras/MAPK and Shh are dysregulated in diverse cancers, and Hes-1 is the converging point between all these signaling pathways. Therefore, Notch/CBF1-independent non-canonical activation of Hes-1 may be involved in the activation or maintenance of cancer cells [63, 71]. This hypothesis was strengthened by the observations of Stockhausen et al. [65] where they could also show the non-canonical activation of Hes-1 in neuroblastoma cells. They showed the rapid activation of Hes-1 in neuroblastoma cells through TGFα-mediated Ras/MAPK pathway. This activation is independent of Notch receptor cleavage and is able to inhibit Hash-1, which would in turn induce proliferation of these cells [65]. Previous reports also suggested that Notch signaling mediates the TGFα-induced oncogenic Ras/MAPK signaling in different human tumors [105, 106]. The CBF1-independent Notch signaling has been shown to activate the expression of YY1 target gene c-myc in cancer cells [48]. Here, the NICD directly interacts with YY1 transcription factor on c-myc promoter region. However, the CBF1-independent c-myc expression is not able to induce tumorigenesis in K562 cells. Therefore, it could be assumed that various signaling mechanisms can cross-talk and trigger the expression of specific mitogenic agents in a context-dependent manner which leads to the consistent proliferation of cells during pathological conditions.
Conclusions and perspectives
The widespread distribution of Notch components and their ability to interact with other signaling pathways provide the maximum flexibility for Notch signaling to execute a wide range of functions. Classical activation of Notch signaling through ligand-mediated interaction triggers the expression of different downstream effectors in a specific tissue. This kind of non-specific activation of factors may not be desirable for specifying a particular fate/function. Therefore, non-canonical activation of Notch signaling becomes an imperative step to specifically perform tissue-specific tasks. Collectively, non-canonical/CBF1-independent Notch target gene activation is involved in the generation of lineage-restricted progenitors and their fate-specific differentiation. Since multipotent progenitors have to generate different lineage-restricted progenitors, various signaling cross-talk mechanisms are carried out together in a precise and time-dependent manner. Therefore, both canonical and non-canonical Notch signaling may interact to maintain the multipotent progenitors as well as generation and differentiation of fate-specific progenitors. The involvement of non-canonical Notch signaling during tumorigenesis and tumor progression may be due to the dysregulation of Notch components and thereby the difference in their affinity to specific transcriptional activators/inhibitors. Therefore, in view of recent reports on non-canonical/CBF1-independent Notch target gene activation, it can be concluded that cross-regulatory mechanisms work together in a context-dependent manner to execute specific tasks.
References
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 4.Honjo T. The shortest path from the surface to the nucleus: RBP-J kappa/Su(H) transcription factor. Genes Cells. 1996;1:1–9. doi: 10.1046/j.1365-2443.1996.10010.x. [DOI] [PubMed] [Google Scholar]
- 5.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 6.Barrick D, Kopan R. The Notch transcription activation complex makes its move. Cell. 2006;124:883–885. doi: 10.1016/j.cell.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 7.Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Structural basis for cooperativity in recruitment of MAML coactivators to Notch transcription complexes. Cell. 2006;124:973–983. doi: 10.1016/j.cell.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Kageyama R, Ishibashi M, Takebayashi K, Tomita K. bHLH transcription factors and mammalian neuronal differentiation. Int J Biochem Cell Biol. 1997;29:1389–1399. doi: 10.1016/s1357-2725(97)89968-2. [DOI] [PubMed] [Google Scholar]
- 9.Kageyama R, Ohtsuka T. The Notch-Hes pathway in mammalian neural development. Cell Res. 1999;9:179–188. doi: 10.1038/sj.cr.7290016. [DOI] [PubMed] [Google Scholar]
- 10.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 11.Anthony TE, Mason HA, Gridley T, Fishell G, Heintz N. Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes Dev. 2005;19:1028–1033. doi: 10.1101/gad.1302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis RL, Turner DL. Vertebrate hairy and Enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene. 2001;20:8342–8357. doi: 10.1038/sj.onc.1205094. [DOI] [PubMed] [Google Scholar]
- 13.Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci. 2009;66:1631–1646. doi: 10.1007/s00018-009-8668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol. 2003;23:543–553. doi: 10.1161/01.ATV.0000060892.81529.8F. [DOI] [PubMed] [Google Scholar]
- 15.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 16.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto N, Tanigaki K, Han H, Hiai H, Honjo T. Notch/RBP-J signaling regulates epidermis/hair fate determination of hair follicular stem cells. Curr Biol. 2003;13:333–338. doi: 10.1016/s0960-9822(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 18.Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 19.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 20.Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20:2739–2753. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwanbeck R, Schroeder T, Henning K, Kohlhof H, Rieber N, Erfurth ML, Just U. Notch signaling in embryonic and adult myelopoiesis. Cells Tissues Organs. 2008;188:91–102. doi: 10.1159/000113531. [DOI] [PubMed] [Google Scholar]
- 22.Talora C, Campese AF, Bellavia D, Felli MP, Vacca A, Gulino A, Screpanti I. Notch signaling and diseases: an evolutionary journey from a simple beginning to complex outcomes. Biochim Biophys Acta. 2008;1782:489–497. doi: 10.1016/j.bbadis.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Gridley T (2003) Notch signaling and inherited disease syndromes. Hum Mol Genet 12 Spec No 1:R9–R13 [DOI] [PubMed]
- 24.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 25.Louvi A, Arboleda-Velasquez JF, Artavanis-Tsakonas S. CADASIL: a critical look at a Notch disease. Dev Neurosci. 2006;28:5–12. doi: 10.1159/000090748. [DOI] [PubMed] [Google Scholar]
- 26.Mysliwiec P, Boucher MJ. Targeting Notch signaling in pancreatic cancer patients—rationale for new therapy. Adv Med Sci. 2009;54:136–142. doi: 10.2478/v10039-009-0026-3. [DOI] [PubMed] [Google Scholar]
- 27.Pierfelice TJ, Schreck KC, Eberhart CG, Gaiano N. Notch, neural stem cells, and brain tumors. Cold Spring Harb Symp Quant Biol. 2008;73:367–375. doi: 10.1101/sqb.2008.73.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose SL. Notch signaling pathway in ovarian cancer. Int J Gynecol Cancer. 2009;19:564–566. doi: 10.1111/IGC.0b013e3181a12ed2. [DOI] [PubMed] [Google Scholar]
- 29.Weng AP, Aster JC. Multiple niches for Notch in cancer: context is everything. Curr Opin Genet Dev. 2004;14:48–54. doi: 10.1016/j.gde.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 30.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 31.Martinez Arias A, Zecchini V, Brennan K. CSL-independent Notch signalling: a checkpoint in cell fate decisions during development? Curr Opin Genet Dev. 2002;12:524–533. doi: 10.1016/s0959-437x(02)00336-2. [DOI] [PubMed] [Google Scholar]
- 32.Rusconi JC, Corbin V. Evidence for a novel Notch pathway required for muscle precursor selection in Drosophila . Mech Dev. 1998;79:39–50. doi: 10.1016/s0925-4773(98)00170-1. [DOI] [PubMed] [Google Scholar]
- 33.Zecchini V, Brennan K, Martinez-Arias A. An activity of Notch regulates JNK signalling and affects dorsal closure in Drosophila . Curr Biol. 1999;9:460–469. doi: 10.1016/s0960-9822(99)80211-5. [DOI] [PubMed] [Google Scholar]
- 34.Barolo S, Walker RG, Polyanovsky AD, Freschi G, Keil T, Posakony JW. A notch-independent activity of suppressor of hairless is required for normal mechanoreceptor physiology. Cell. 2000;103:957–969. doi: 10.1016/s0092-8674(00)00198-7. [DOI] [PubMed] [Google Scholar]
- 35.Neves A, Priess JR. The REF-1 family of bHLH transcription factors pattern C. elegans embryos through Notch-dependent and Notch-independent pathways. Dev Cell. 2005;8:867–879. doi: 10.1016/j.devcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Nofziger D, Miyamoto A, Lyons KM, Weinmaster G. Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development. 1999;126:1689–1702. doi: 10.1242/dev.126.8.1689. [DOI] [PubMed] [Google Scholar]
- 37.Veeraraghavalu K, Subbaiah VK, Srivastava S, Chakrabarti O, Syal R, Krishna S. Complementation of human papillomavirus type 16 E6 and E7 by Jagged1-specific Notch1-phosphatidylinositol 3-kinase signaling involves pleiotropic oncogenic functions independent of CBF1;Su(H);Lag-1 activation. J Virol. 2005;79:7889–7898. doi: 10.1128/JVI.79.12.7889-7898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak TW, Honjo T. Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development. 1995;121:3291–3301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- 39.de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- 40.Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- 41.Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa RM, Honjo T, Silva AJ. Learning and memory deficits in Notch mutant mice. Curr Biol. 2003;13:1348–1354. doi: 10.1016/s0960-9822(03)00492-5. [DOI] [PubMed] [Google Scholar]
- 43.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 44.Nakhai H, Siveke JT, Klein B, Mendoza-Torres L, Mazur PK, Algul H, Radtke F, Strobl L, Zimber-Strobl U, Schmid RM. Conditional ablation of Notch signaling in pancreatic development. Development. 2008;135:2757–2765. doi: 10.1242/dev.013722. [DOI] [PubMed] [Google Scholar]
- 45.Raafat A, Lawson S, Bargo S, Klauzinska M, Strizzi L, Goldhar AS, Buono K, Salomon D, Vonderhaar BK, Callahan R. Rbpj conditional knockout reveals distinct functions of Notch4/Int3 in mammary gland development and tumorigenesis. Oncogene. 2009;28:219–230. doi: 10.1038/onc.2008.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perumalsamy LR, Nagala M, Banerjee P, Sarin A. A hierarchical cascade activated by non-canonical Notch signaling and the mTOR-Rictor complex regulates neglect-induced death in mammalian cells. Cell Death Differ. 2009;16:879–889. doi: 10.1038/cdd.2009.20. [DOI] [PubMed] [Google Scholar]
- 47.Shawber C, Nofziger D, Hsieh JJ, Lindsell C, Bogler O, Hayward D, Weinmaster G. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development. 1996;122:3765–3773. doi: 10.1242/dev.122.12.3765. [DOI] [PubMed] [Google Scholar]
- 48.Liao WR, Hsieh RH, Hsu KW, Wu MZ, Tseng MJ, Mai RT, Wu Lee YH, Yeh TS. The CBF1-independent Notch1 signal pathway activates human c-myc expression partially via transcription factor YY1. Carcinogenesis. 2007;28:1867–1876. doi: 10.1093/carcin/bgm092. [DOI] [PubMed] [Google Scholar]
- 49.Liu Z, Teng L, Bailey SK, Frost AR, Bland KI, LoBuglio AF, Ruppert JM, Lobo-Ruppert SM. Epithelial transformation by KLF4 requires Notch1 but not canonical Notch1 signaling. Cancer Biol Ther. 2009;8:1840–1851. doi: 10.4161/cbt.8.19.9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- 51.Lahmar M, Catelain C, Poirault S, Dorsch M, Villeval JL, Vainchenker W, Albagli O, Lauret E. Distinct effects of the soluble versus membrane-bound forms of the notch ligand delta-4 on human CD34+CD38low cell expansion and differentiation. Stem Cells. 2008;26:621–629. doi: 10.1634/stemcells.2007-0428. [DOI] [PubMed] [Google Scholar]
- 52.Grandbarbe L, Bouissac J, Rand M, Hrabe De Angelis M, Artavanis-Tsakonas S, Mohier E. Delta-Notch signaling controls the generation of neurons/glia from neural stem cells in a stepwise process. Development. 2003;130:1391–1402. doi: 10.1242/dev.00374. [DOI] [PubMed] [Google Scholar]
- 53.Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, Kageyama R. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004;131:5539–5550. doi: 10.1242/dev.01436. [DOI] [PubMed] [Google Scholar]
- 54.Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–348. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Sanalkumar R, Indulekha CL, Divya TS, Divya MS, Anto RJ, Vinod B, Vidyanand S, Jagatha B, Venugopal S, James J. ATF2 maintains a subset of neural progenitors through CBF1/Notch independent Hes-1 expression and synergistically activates the expression of Hes-1 in Notch-dependent neural progenitors. J Neurochem. 2010;113:807–818. doi: 10.1111/j.1471-4159.2010.06574.x. [DOI] [PubMed] [Google Scholar]
- 56.Wall DS, Mears AJ, McNeill B, Mazerolle C, Thurig S, Wang Y, Kageyama R, Wallace VA. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic hedgehog/Hes1 activity. J Cell Biol. 2009;184:101–112. doi: 10.1083/jcb.200805155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikawa T, Kawamoto H, Goldrath AW, Murre C. E proteins and Notch signaling cooperate to promote T cell lineage specification and commitment. J Exp Med. 2006;203:1329–1342. doi: 10.1084/jem.20060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F, Pear WS. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2:356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoflinger S, Kesavan K, Fuxa M, Hutter C, Heavey B, Radtke F, Busslinger M. Analysis of Notch1 function by in vitro T cell differentiation of Pax5 mutant lymphoid progenitors. J Immunol. 2004;173:3935–3944. doi: 10.4049/jimmunol.173.6.3935. [DOI] [PubMed] [Google Scholar]
- 60.Tanigaki K, Tsuji M, Yamamoto N, Han H, Tsukada J, Inoue H, Kubo M, Honjo T. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 61.Harman BC, Jenkinson WE, Parnell SM, Rossi SW, Jenkinson EJ, Anderson G. T/B lineage choice occurs prior to intrathymic Notch signaling. Blood. 2005;106:886–892. doi: 10.1182/blood-2004-12-4881. [DOI] [PubMed] [Google Scholar]
- 62.Curry CL, Reed LL, Nickoloff BJ, Miele L, Foreman KE. Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest. 2006;86:842–852. doi: 10.1038/labinvest.3700442. [DOI] [PubMed] [Google Scholar]
- 63.Ingram WJ, McCue KI, Tran TH, Hallahan AR, Wainwright BJ. Sonic Hedgehog regulates Hes1 through a novel mechanism that is independent of canonical Notch pathway signalling. Oncogene. 2008;27:1489–1500. doi: 10.1038/sj.onc.1210767. [DOI] [PubMed] [Google Scholar]
- 64.Kubo F, Nakagawa S. Hairy1 acts as a node downstream of Wnt signaling to maintain retinal stem cell-like progenitor cells in the chick ciliary marginal zone. Development. 2009;136:1823–1833. doi: 10.1242/dev.029272. [DOI] [PubMed] [Google Scholar]
- 65.Stockhausen MT, Sjolund J, Axelson H. Regulation of the Notch target gene Hes-1 by TGFalpha induced Ras/MAPK signaling in human neuroblastoma cells. Exp Cell Res. 2005;310:218–228. doi: 10.1016/j.yexcr.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Hori K, Cholewa-Waclaw J, Nakada Y, Glasgow SM, Masui T, Henke RM, Wildner H, Martarelli B, Beres TM, Epstein JA, Magnuson MA, Macdonald RJ, Birchmeier C, Johnson JE. A nonclassical bHLH Rbpj transcription factor complex is required for specification of GABAergic neurons independent of Notch signaling. Genes Dev. 2008;22:166–178. doi: 10.1101/gad.1628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ. PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol. 2006;26:117–130. doi: 10.1128/MCB.26.1.117-130.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacKenzie F, Duriez P, Wong F, Noseda M, Karsan A. Notch4 inhibits endothelial apoptosis via RBP-Jkappa-dependent and -independent pathways. J Biol Chem. 2004;279:11657–11663. doi: 10.1074/jbc.M312102200. [DOI] [PubMed] [Google Scholar]
- 69.Levy OA, Lah JJ, Levey AI. Notch signaling inhibits PC12 cell neurite outgrowth via RBP-J-dependent and -independent mechanisms. Dev Neurosci. 2002;24:79–88. doi: 10.1159/000064948. [DOI] [PubMed] [Google Scholar]
- 70.Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, Russell TL, Ellenbogen RG, Bernstein ID, Beachy PA, Olson JM. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64:7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 72.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 73.Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 74.Hurlbut GD, Kankel MW, Lake RJ, Artavanis-Tsakonas S. Crossing paths with Notch in the hyper-network. Curr Opin Cell Biol. 2007;19:166–175. doi: 10.1016/j.ceb.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 75.Nagao M, Sugimori M, Nakafuku M. Cross talk between Notch and growth factor/cytokine signaling pathways in neural stem cells. Mol Cell Biol. 2007;27:3982–3994. doi: 10.1128/MCB.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerhart J. 1998 Warkany lecture: signaling pathways in development. Teratology. 1999;60:226–239. doi: 10.1002/(SICI)1096-9926(199910)60:4<226::AID-TERA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 77.Barolo S, Posakony JW. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev. 2002;16:1167–1181. doi: 10.1101/gad.976502. [DOI] [PubMed] [Google Scholar]
- 78.Pires-daSilva A, Sommer RJ. The evolution of signalling pathways in animal development. Nat Rev Genet. 2003;4:39–49. doi: 10.1038/nrg977. [DOI] [PubMed] [Google Scholar]
- 79.Cummings FW. On the origin of pattern and form in early Metazoans. Int J Dev Biol. 2006;50:193–208. doi: 10.1387/ijdb.052058fc. [DOI] [PubMed] [Google Scholar]
- 80.Le Gall M, De Mattei C, Giniger E. Molecular separation of two signaling pathways for the receptor, Notch. Dev Biol. 2008;313:556–567. doi: 10.1016/j.ydbio.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanders PG, Munoz-Descalzo S, Balayo T, Wirtz-Peitz F, Hayward P, Arias AM. Ligand-independent traffic of Notch buffers activated Armadillo in Drosophila . PLoS Biol. 2009;7:e1000169. doi: 10.1371/journal.pbio.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen N, Greenwald I. The lateral signal for LIN-12/Notch in C. elegans vulval development comprises redundant secreted and transmembrane DSL proteins. Dev Cell. 2004;6:183–192. doi: 10.1016/s1534-5807(04)00021-8. [DOI] [PubMed] [Google Scholar]
- 83.Dumont E, Fuchs KP, Bommer G, Christoph B, Kremmer E, Kempkes B. Neoplastic transformation by Notch is independent of transcriptional activation by RBP-J signalling. Oncogene. 2000;19:556–561. doi: 10.1038/sj.onc.1203352. [DOI] [PubMed] [Google Scholar]
- 84.Jeffries S, Capobianco AJ. Neoplastic transformation by Notch requires nuclear localization. Mol Cell Biol. 2000;20:3928–3941. doi: 10.1128/mcb.20.11.3928-3941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lessard J, Wu JI, Ranish JA, Wan M, Winslow MM, Staahl BT, Wu H, Aebersold R, Graef IA, Crabtree GR. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron. 2007;55:201–215. doi: 10.1016/j.neuron.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Namihira M, Kohyama J, Semi K, Sanosaka T, Deneen B, Taga T, Nakashima K. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev Cell. 2009;16:245–255. doi: 10.1016/j.devcel.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 87.Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–394. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 88.Honjo M, Tanihara H, Kido N, Inatani M, Okazaki K, Honda Y. Expression of ciliary neurotrophic factor activated by retinal Muller cells in eyes with NMDA- and kainic acid-induced neuronal death. Invest Ophthalmol Vis Sci. 2000;41:552–560. [PubMed] [Google Scholar]
- 89.Scheer N, Groth A, Hans S, Campos-Ortega JA. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001;128:1099–1107. doi: 10.1242/dev.128.7.1099. [DOI] [PubMed] [Google Scholar]
- 90.Kubo F, Takeichi M, Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development. 2005;132:2759–2770. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- 91.Kopan R, Nye JS, Weintraub H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994;120:2385–2396. doi: 10.1242/dev.120.9.2385. [DOI] [PubMed] [Google Scholar]
- 92.Kingham PJ, Mantovani C, Terenghi G. Notch independent signalling mediates Schwann cell-like differentiation of Adipose derived stem cells. Neurosci Lett. 2009;467:164–168. doi: 10.1016/j.neulet.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 93.MacDonald HR, Wilson A, Radtke F. Notch1 and T-cell development: insights from conditional knockout mice. Trends Immunol. 2001;22:155–160. doi: 10.1016/s1471-4906(00)01828-7. [DOI] [PubMed] [Google Scholar]
- 94.Dallman MJ, Smith E, Benson RA, Lamb JR. Notch: control of lymphocyte differentiation in the periphery. Curr Opin Immunol. 2005;17:259–266. doi: 10.1016/j.coi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 95.De Smedt M, Reynvoet K, Kerre T, Taghon T, Verhasselt B, Vandekerckhove B, Leclercq G, Plum J. Active form of Notch imposes T cell fate in human progenitor cells. J Immunol. 2002;169:3021–3029. doi: 10.4049/jimmunol.169.6.3021. [DOI] [PubMed] [Google Scholar]
- 96.Bain G, Robanus Maandag EC, te Riele HP, Feeney AJ, Sheehy A, Schlissel M, Shinton SA, Hardy RR, Murre C. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. doi: 10.1016/s1074-7613(00)80421-5. [DOI] [PubMed] [Google Scholar]
- 97.Ordentlich P, Lin A, Shen CP, Blaumueller C, Matsuno K, Artavanis-Tsakonas S, Kadesch T. Notch inhibition of E47 supports the existence of a novel signaling pathway. Mol Cell Biol. 1998;18:2230–2239. doi: 10.1128/mcb.18.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 99.Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 100.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, Brat DJ, Perry A, Eberhart CG. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 101.Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, Maric D, Eberhart CG, Fine HA. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 103.Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, Pieper RO. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106:417–427. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- 104.Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K, Honjo T, Hennighausen L. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol. 2006;293:565–580. doi: 10.1016/j.ydbio.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 105.Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, Rudolf M, Siziopikou K, Kast WM, Miele L. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- 106.Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]