Abstract
To infect their host cells the Microsporidia use a unique invasion organelle, the polar tube complex. During infection, the organism is injected into the host cell through the hollow polar tube formed during spore germination. Currently, three proteins, PTP1, PTP2, and PTP3 have been identified by immunological and molecular techniques as being components of this structure. Genomic data suggests that Microsporidia are capable of O-linked, but not N-linked glycosylation as a post-translational protein modification. Cells were infected with Encephalitozoon cunicuili, labeled with radioactive mannose or glucosamine, and the polar tube proteins were examined for glycosylation. PTP1 was clearly demonstrated to be mannosylated consistent with 0-glycosylation. In addition, it was evident that several other proteins were mannosylated, but no labeling was seen with glucosamine. The observed post-translational mannosylation of PTP1 may be involved in the functional properties of the polar tube, including its adherence to host cells during penetration.
Introduction
Microsporidia are obligate intracellular parasitic protists which are widely distributed in vertebrates and invertebrates. They are found in numerous mammals including humans and have emerged as pathogens in immune compromised hosts, such as those with AIDS or organ transplantation (Weber and Bryan 1994; Wittner and Weiss 1999). While the majority of reported cases of micro-sporidiosis have involved the gastrointestinal track, keratoconjunctivitis, disseminated infection, and encephalitis are also well-described outcomes of infection (Wittner and Weiss 1999). Infection occurs, in most cases, due to the ingestion of environmentally resistant spores which are then activated and germinated in the gastrointestinal tract. During germination, the spore ruptures at its anterior end and the polar filament is extruded forming a hollow polar tube through which the sporoplasm and nucleus of the organism travel to reach the cytoplasm of the host cell (Keohane and Weiss 1998). It is not clear if the polar tube penetrates the host cell or merely causes an invagination of the host cell membrane delivering the contents of the spore into this microenvironment, but it does appear that the final penetration of the host cell membrane is due to an interaction of the host cell and the microsporidian sporoplasm (Foucault and Drancourt 2000). If a spore is phagocytosed, it can also germinate delivering the spore contents to the original cell or to surrounding cells (Couzinet et al. 2000; Hayman et al. 2005; Weidner and Sibley 1985).
The composition of the polar tube is an area of active investigation in order to understand how this structure functions during germination and its subsequent interaction with the host cell. Studies have demonstrated that polar tube needs to interact with the host cell membrane in order for infection to occur. Glycoproteins have been shown to be involved in the adherence of spores to host cells and the subsequent invasion of these cells by microsporidia (Hayman et al. 2005; Southern et al. 2007). Xu et al. (2004), studying glycosylation, demonstrated that Encephalitozoon hellem polar tube protein 1 (PTP1) bound to concanavalin A (ConA), that the polar tube of both Anncaliia algerae and E. hellem cou6ld be labeled by ConA-gold, and that this binding was inhibited by mannose. Biochemical studies suggested that this was due to the presence of O-linked mannosylation on PTPs. To directly demonstrate the presence of glycoprotein modifications in Encephalitozoon cuniculi, we used radiolabeled mannose and glucosamine along with immunoprecipitation to prove that EcPTP1 is modified by mannosylation.
Methods
Tissue culture
E. cuniculi was cultured in T25 flasks at 37° C with 5% CO2 in RK13 cells (CCL37; American Type Culture Collection, Rockville, MD). Infected RK13 cells were maintained in continuous culture in minimum essential medium (MEM, Invitrogen, Carlsbad, CA) supplemented with 7% heated inactive fetal calf serum and 1% penicillin–streptomycin–amphotericin B (Invitrogen, Carlsbad, CA). Cultures were subpassaged every 3 weeks. Supernatants from infected flasks containing microsporidian spores were collected twice weekly and replaced with fresh medium. Spores concentrations were determined by counting spores using an Improved Neubauer hemocytometer.
Glycoprotein labeling
Infected and uninfected cells were incubated at 37°C in cell culture media for 4 days containing either 5 μCi of [2]-3H-Mannose (ART-120 Mannose-[2–3H(N)], 1 mCi/ml, American Radiolabeled Chemicals, Inc., St. Louis, MO) or [2]-3H-Glucosamine (ART-110 Glucosamine HCL,D-[6–3H], 1 mCi/ml, American Radiolabeled Chemicals, Inc., St. Louis, MO). At 8 days post-infection, cells were lysed with 25-μm-gauge needle to release spores. Spores were purified by filtration through 0.2-μm filter, and PTP protein lysates were prepared as described (Keohane et al. 1998).
To confirm labeling of spore lysate, spore coat and polar tube preparations were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and after electrophoresis, the gel was immersed in Autofluor (National Diagnostics, Atlanta, GA) for 1 h and dried overnight. The gel was imaged at −80°C for up to 15 days on high performance autoradiographic film (Hyperfilm, GE Healthcare Life Sciences, Piscataway, NJ) that was first sensitized with a pre-flash unit (Sensitize, GE Healthcare Life Sciences, Piscataway, NJ).
Polar tube protein purification, detection, and immunoprecipitation
PTPs were isolated from other proteins in the spores using a modification of our published protocol (Weidner 1976; Keohane et al. 1998). Spores (3×107) in 1% SDS were disrupted with 0.5-μm acid-washed glass beads (Sigma, St. Louis, MO) for 4 min on a Mini-Beadbeater (Biospec Products, Bartlesville, OK). The disrupted spores suspension was separated from the glass beads and washed five times with 1% SDS followed by a brief wash with 9 M urea to remove soluble spore coat proteins. The pellet was then incubated with 0.5 ml of 2% dithiothreitol (DTT) in 25 mM of Tris (pH 7.4), 10 μg/ml of aprotinin and leupeptin (Sigma,St. Louis, MO), and 5 mM of ethylene glycol tetraacetic acid at room temperature for 2 h to solubilize the PTPs. The solution was spun at 13,500 xg in a microcentrifuge, and the supernatant was removed (solubilized PTP proteins). The pellet fraction contained spore coat proteins. Protein concentrations were determined by Coomassie brilliant Blue G-250 dye binding procedure (BioRad, Richmond, CA) using an albumin standard.
PTP extracts from cells labeled with radioactive sugars were then immunoprecipitated using a Seize-X Mammalian Immunoprecipitation Kit (Pierce, Rockford, IL) employing rabbit antibody to E. hellem (anti-EhPTP1) following the manufacturer’s protocols to produce an immobilized anti-EhPTP1 column. We have previously determined that anti-EhPTP1 cross reacts with E. cuniculi PTP1 (Keohane et al. 1999). Immune complexes were then separated by SDS electrophoresis, and the gel was immersed in Autofluor for 1 h and dried overnight. The gel was then imaged on sensitized autoradiographic film by exposure at −80°C for up to 15 days.
For immunoblot analysis, proteins were separated by SDS-PAGE using 10% polyacrylamide gels and the gel was then transferred to an Immobilon-P membrane (Millipore, Bedford, MA) using standard methods (Keohane et al. 1999). Immunoblot detection was then carried out using the ECL Western Blotting Detection Reagent (GE Healthcare Life Sciences, Piscataway, NJ) according to the manufacturer’s protocols. Anti-EhPTP1 was used at a 1:6000 dilution, and a peroxidase-conjugated anti-rabbit IgG secondary antibody was used at a 1:5000 dilution.
Results and discussion
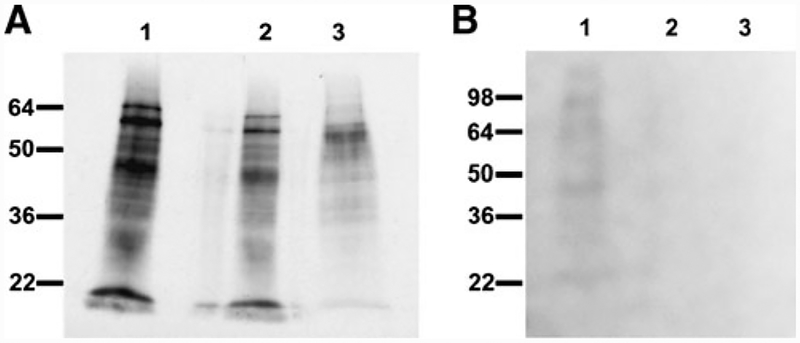
Labeling of E. cuniculi with radioactive 3H-Mannose demonstrated that many proteins incorporated this sugar, consistent with the presence of mannose containing glycoproteins in the spore coat as well as the polar tube (Fig. 1a). No labeling of any E. cuniculi proteins was seen with 3H-Glucosamine (Fig. 1b, lanes 2 and 3); however, as expected, labeling could be demonstrated in uninfected RK13 cell lysate (Fig. 1b, lane 1). This is consistent with an absence of N-linked glycosylation in E. cuniculi and the presence of O-linked glycosylation (mannosylation) as suggested by genome analysis (Vivares and Metenier 2004).
Fig. 1.
Analysis of glycosylation in E. cuniculi. a Incorporation of[2]-3H-Mannose. Lane 1 spore wall (DTT insoluble fraction as described in methods), lane 2 E. cuniculi spore lysate, and lane 3 PTP preparation (DTT soluble fraction as described in methods). b. Incorporation of [2]-3H-Glucosamine. Lane 1 RK13 (host cell) lysate, lane 2 E. cuniculi spore lysate, and lane 3 PTP preparation (DTT soluble fraction as described in materials and methods). After SDS-PAGE the gel was dried overnight and used for signal detection. The gel was imaged at −80°C for 15 days as described in the methods
To examine further if the mannosylated proteins seen in the DTT solubilized PTP preparation were PTP proteins, immunoprecipitation and immunoblot analyses were performed using antibody to EcPTP1. Immunoblot confirmed the immunoprecipitation of EcPTP1 from the purified PTP extract (Fig. 2a). Autoradiography confirmed that the immunoprecipitated EcPTP1 was labeled with radioactive mannose (Fig. 2b), consistent with post-translational O-mannosylation.
Fig. 2.
Analysis of EcPTP1 Glycosylation. a Immunoblots using anti-EcPTP1. EcPTP1 is clearly detected in both the E. cuniculi spore lysate (lane1) and the immunoprecipitated material (lane 2). Immunoprecipitation was done using anti-EcPTP1 as described in methods. EcPTP1 migrates at ~50 kDa. The EcPTP1 antiserum also recognized a diffuse band at ~35 kDa which may be due to proteolysis of EcPTP1 during sample preparation. b. Autoradiography of pull-down assay using [2]-3H-Mannose-labeled E. cuniculi. Lane 1 RK13 lysate (negative control), lane 2 immunoglobin control, lane 3 residual PTP preparation bound to anti-EcPTP1 beads after washing and elution, and lane 4 eluted PTP material from anti-EcPTP1 beads after washing as described in methods. After SDS-PAGE, the gel was dried overnight and used for signal detection. The gel was imaged at −80°C for 15 days as described in the methods. This demonstrates that PTP1 is labeled by [2]-3H-Mannose
Glycan modifications of proteins are involved in host pathogen interactions and are known to contribute to adherence and invasion in many eukaryotes. These post-translational modifications play important roles in the stability, conformation of protein, differentiation, development, and intracellular communication (Varki 1993; Rademacher et al. 1988). Little is known about the glycosylation processes of many intracellular pathogens including Microsporidia. Chitin, a polysaccharide, is found in the spore wall of the microsporidia (Vavra and Larson 1999). Staining with periodic acid-Schiff and other carbohydrate reactive histology reagents suggests that in addition to the spore wall, the anterior end of the spore (containing the anchoring disk complex) and the polar tube have glycan modifications (Vavra and Larson 1999). This was initially reported in Stempellia spores (Vavra 1972), but has since been reported in many Microsporidia including all of the Encephalitzoonidae. Many obligate intracellular parasites have a reduced ability to perform classical O-linked and N-linked glycosylation (Ernst and Prill 2001). Based on its genome sequence, the microsporidium E. cuniculi lacks the enzymes required for N-linked glycosylation but has an intact pathway for O-linked mannosylation (Katinka et al. 2001). The absence of N-glycosylation is supported by its absence in glycopeptide extracts of E. cuniculi and Antonospora locustae analyzed by gas chromatography coupled to mass spectrometry (MS) (Taupin et al. 2007). Lectin-binding experiments on spore extracts of Glugea plecoglossi (Kim et al. 1999) and E. intestinalis (Hayman et al. 2001) have demonstrated binding to ConA and wheat germ agglutinin. EhPTP1 was demonstrated to bind ConA, and this reaction was due to O-mannosylation rather than N-glycosylation (Xu et al. 2004). O-mannosylation is present in Saccharomyces (Sentrandreu and Northcote 1969) and other fungi (Gentzsch and Tanner 1997), and is known to bind ConA. Similar binding of ConA and Galanthus nivalis agglutinin to PTP1 and other spore proteins has been seen in E. cuniculi, E. intestinalis, Anncaliia algerae, A. locustae, Paranosema grylii, and Glugea americanus (Taupin et al. 2007; Polonais et al. 2005; Dolgikh et al. 2007; Weiss unpublished data). A study of the O-glycan fraction of E. cuniculi and A. locustae using MS techniques established that 70% was due to mannose and that this mannose was present as linear polymers of two to eight mannose molecules in an α−1,2 linkage (Taupin et al. 2007).
PTP1 is the major polar tube (by percentage of the total protein mass) in DTT soluble polar tube extracts from Microsporidia (Keohane et al. 1998). PTP1 migrates aberrantly on SDS-PAGE (Keohane et al. 1994; Keohane et al. 1996; Keohane et al. 1998), and MS analysis has demonstrated that EhPTP1 was larger than predicted, consistent with glycosylation but not with other defined post-translational modifications (e.g. phosphorylation, myristylation, etc.) (Xu et al. 2004). Previous results from our laboratory (Xu et al. 2004) had suggested that EhPTP1 was O-glycosylated and that N-glycosylation was not present. The results in the current paper provide direct biochemical evidence for the presence of mannose residues on PTP1. The E. cuniculi genome encodes an α−1,2 mannosyltransferase (KTR family, Ecu04_1130) and two O-mannosyltransferases (PMT family, Ecu02_1300 and Ecu06_0950) consistent with this biochemical labeling (Katinka et al. 2001; Vivares and Metenier 2004; Xu et al. 2004). It is likely that O-glycosylation of PTP1 plays a role in the function of the polar tube which is protecting the microsporidian polar tube from degradation in the gastrointestinal tract of their hosts, and may interact with host cell membrane mannose receptors facilitating infectivity and adherence of the polar tube to the host cell membrane (Xu et al. 2004). Further understanding of the role of O-mannosylation in the Microsporidia may ultimately lead to new therapeutic approaches for the treatment of these ubiquitous pathogenic protists as has been demonstrated for other pathogenic organisms (Zhang et al. 2002).
Acknowledgement
This work was supported by Grant AI31788 from the National Institute of Allergy and Infectious Diseases.
References
- Couzinet S, Cejas E, Schittny J, Deplazes P, Weber R, Zimmerli S (2000) Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes. Infect Immun 68:6939–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgikh VV, Semenov PB, Beznusenko GV (2007) Protein glycosylation in the spores of the microsporidia Paranosema (Antonospora) grylli. Tsitologiia 49:607–613 [PubMed] [Google Scholar]
- Ernst JF, Prill SK (2001) O-glycosylation. Med Mycol 39:67–74 [PubMed] [Google Scholar]
- Foucault C, Drancourt M (2000) Actin mediates Encephalitozoon intestinalis entry into the human enterocyte-like cell line, Caco-2. Microb Pathog 28:51–58 [DOI] [PubMed] [Google Scholar]
- Gentzsch M, Tanner W (1997) Protein-O-glycosylation in yeast: protein-specific mannosyltransferases. Glycobiology 7:481–486 [DOI] [PubMed] [Google Scholar]
- Hayman JR, Hayes SF, Amon J, Nash TE (2001) Developmental expression of two spore wall proteins during maturation of the microsporidian Encephalitozoon intestinalis. Infect Immun 69:7057–7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman JR, Southern TR, Nash TE (2005) Role of sulfated glycans in adherence of the microsporidian Encephalitozoon intestinalis to host cells in vitro. Infect Immun 73:841–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinka MD, Duprat S, Cornillot E, Metenier G, Thomarat F, Prensier G, Barbe V, Peyretaillade E, Brottier P, Wincker P, Delbac F, El Alaoui H, Peyret P, Saurin W, Gouy M, Weissenbach J, Vivares CP (2001) Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414:450–453 [DOI] [PubMed] [Google Scholar]
- Keohane EM, Weiss LM (1998) Characterization and function of the microsporidian polar tube: a review. Folia Parasitol 45:117–127 [PubMed] [Google Scholar]
- Keohane EM, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM (1994) The identification and characterization of a polar tube reactive monoclonal antibody. J Eukaryot Microbiol 41:48S. [PubMed] [Google Scholar]
- Keohane EM, Takvorian KEM, PM CA, Tanowitz HB, Wittner M, Weiss LM (1996) Identification of a microsporidian polar tube protein reactive monoclonal antibody. J Eukaryot Microbiol 43:26–31 [DOI] [PubMed] [Google Scholar]
- Keohane EM, Orr GA, Zhang HS, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM (1998) The molecular characterization of the major polar tube protein gene from Encephalitozoon hellem, a microsporidian parasite of humans. Mol Biochem Parasitol 94:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane EM, Orr GA, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM (1999) Polar tube proteins of microsporidia of the family Encephalitozoonidae. J Eukaryot Microbiol 46:1–5 [DOI] [PubMed] [Google Scholar]
- Kim JH, Ogawa K, Wakabayashi H (1999) Lectin-reactive components of the microsporidian Glugea plecoglossi and their relation to spore phagocytosis by head kidney macrophages of ayu Plecoglossus altivelis. Dis Aquat Organ 39:59–63 [DOI] [PubMed] [Google Scholar]
- Polonais V, Prensier G, Méténier G, Vivarès CP, Delbac F (2005) Microsporidian polar tube proteins: highly divergent but closely linked genes encode PTP1 and PTP2 in members of the evolutionarily distant Antonospora and Encephalitozoon groups. Fungal Genet Biol 42:791–803 [DOI] [PubMed] [Google Scholar]
- Rademacher TW, Parekh RB, Dwek RA (1988) Glycobiology. Annu Rev Biochem 57:785–838 [DOI] [PubMed] [Google Scholar]
- Sentrandreu R, Northcote DH (1969) The characterization of oligosaccharides attached to threonine and serine in a mannan glycopeptide obtained from the cell wall of yeast. Carbohydr Res 10:584–585 [Google Scholar]
- Southern TR, Jolly CE, Lester ME, Hayman JR (2007) EnP1, a microsporidian spore wall protein that enables spores to adhere to and infect host cells in vitro. Eukaryot Cell 6:1354–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin V, Garenaux E, Mazet M, Maes E, Denise H, Prensier G,Vivarès CP, Guérardel Y, Méténier G (2007) Major O-glycans in the spores of two microsporidian parasites are represented by unbranched manno-oligosaccharides containing alpha-1, 2 linkages. Glycobiology 17:56–67 [DOI] [PubMed] [Google Scholar]
- Varki A (1993) Biological roles of oligosccharides: all of theories are correct. Glycobiology 3:97–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavra J (1972) Detection of polysaccharides in microsporidian spores by means of the periodic acid-thiosemicarbazide-sliver proteinate test. J Microsc 14:357–360 [Google Scholar]
- Vavra J, Larson J (1999) Structure of the microsporidia In: Wittner M,Weiss LM (eds) The microsporidida and microsporidiosis. Washington D.C, ASM Press, pp 7–84 [Google Scholar]
- Vivares CP, Metenier G (2004) The Microsporidia genome: living with minimal genes as an intracellular eukaryote In: Lindsay DS, Weiss LM (eds) Opportunistic infections: Toxoplasma, Sarcocystis, and Microsporidia, vol. 8 Kluwer Academic Press, Norwell, Mass, pp 215–242 [Google Scholar]
- Weber R, Bryan RT (1994) Microsporidial infections in immunodeficient and immunocompetent patients. Clin Infect Dis 19:517–521 [DOI] [PubMed] [Google Scholar]
- Weidner E (1976) The microsporidian spore invasion tube. The ultrastructure, isolation, and characterization of the protein comprising the tube. J Cell Biol 71:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E, Sibley LD (1985) Phagocytized intracellular micro-sporidian blocks phagosome acidification and phagosomelysosome fusion. J Protozool 32:311–317 [DOI] [PubMed] [Google Scholar]
- Wittner M, Weiss LM (1999) The Microsporidia and microsporidiosis.ASM Press, Washington DC [Google Scholar]
- Xu Y, Takvorian PM, Cali A, Orr G, Weiss LM (2004) Glycosylation of the major polar tube protein of Encephalitozoon hellem, a micro-sporidian parasite that infects humans. Infect Immun 72:6341–6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Duchene M, Stanley SL (2002) A monoclonal antibody to the amebic lipophosphoglycan-proteophosphoglycan antigens can prevent disease in human intestinal xenografts infected with Entamoeba histolytica. Infect Immun 70:5873–5876 [DOI] [PMC free article] [PubMed] [Google Scholar]