Introduction
The operative technique of repair of intrasynovial lacerations of the digital flexor tendons aims to 1) minimize secondary iatrogenic trauma to the tendon and the surrounding gliding surface during repair; 2) coapt tendon ends with sufficient strength to resist physiological tensile loads applied during early postoperative rehabilitation; 3) achieve a smooth, non-bulbous repair site to minimize the work required for digital flexion. Combined transection of both the flexor digitorum superficialis (FDS) and the flexor digitorum profundus (FDP) tendons distal to the A2 pulley is particularly challenging due to the FDS-FDP relationship at that level.
Different techniques are required to repair distal FDS tendon transections due to the structure of the two slips in the region of the proximal interphalangeal joint. This report describes our operative technique for repair of combined FDS and FDP transections following decussation of the FDS tendon.
Indications
Combined repair of the FDS and FDP tendons distal to the A2 pulley is indicated in clean transections. Restoration of FDS tendon function allows for independent proximal interphalangeal joint flexion and helps provide for a smooth gliding surface for the FDP tendon at this level.
Contraindications
Combined repair of FDS and FDP tendons distal to the A2 pulley is not attempted in all cases. Both the severity of associated injuries to the digit and presence of active infection are considered on planning repair. Presentation greater than 4 weeks following injury may be a contraindication to attempted primary repair of either tendon. In addition, tendon lacerations with ‘ragged’ ends, such as those sustained following saw injuries, might not allow for primary repair that is amenable to the application of controlled early rehabilitation to maximize intrasynovial tendon excursion.
In cases that require prolonged immobilization to protect neurovascular repair, soft tissue coverage or fracture healing, the early motion rehabilitation that is necessary to minimize adhesions in combined FDS and FDP repair may pose risks to the repair site. Nonetheless, we advocate a careful early motion (short-arc) protocol even for severe combined injuries.1
Surgical Anatomy
Proximal to the distal edge of A2 pulley the FDS tendon flattens and divides into radial and ulnar slips. These slips rotate dorsally around the FDP tendon as they extend distally. At this level, the FDP tendon lies volarly. Portions of the two slips decussate as they meet directly dorsal to the FDP tendon (Camper’s chiasm) although the radial and ulnar slips remain distinct as they insert into the base of the middle phalanx. The decussation of the slips produces a central (rather than radial or ulnar) longitudinal flexor moment at the PIP joint. The FDP tendon is comprised of radial and ulnar bundles marked by the longitudinal groove between them. It extends distally to insert onto the base of the distal phalanx.
The extra-synovial portion of the digital flexor tendons is covered by adventitia known as paratenon. As the tendon enters the fibro-osseous tunnel, a parietal layer of synovium lines the deep surface of the tunnel. The A2 pulley, which extends over the proximal phalanx, measures an average of 17 mm in length. The first cruciate pulley (C1) lies immediately distal to it, and the small, biomechanically inconsequential A3 pulley lies directly over the PIP joint.
In addition to the diffusion within the synovial environment, intrasynovial flexor tendons receive direct vascularization from three sources.2 The paratenon contains longitudinally oriented vessels proximally. Also, the vinculae bring vascularity directly from the digital arteries and bear consideration in the surgical field (Figure 1). A digital arterial transverse (ladder) branch is noted at the level of the neck of the proximal phalanx. This vessel is an important vascular supply to the vinculum brevis superficialis that supplies the terminal portion of the FDS tendon slips, and the vinculum longus profundus that supplies the dorsal aspect of the FDP. The vinculum longus superficialis arises from the digital arteries at the proximal aspect of the proximal phalanx and travels to one or both FDS slips proximal to or at the division of the FRS into two slips. Finally, the bony insertion of the FDS contributes to the vascularity of the terminal FDS tendon.
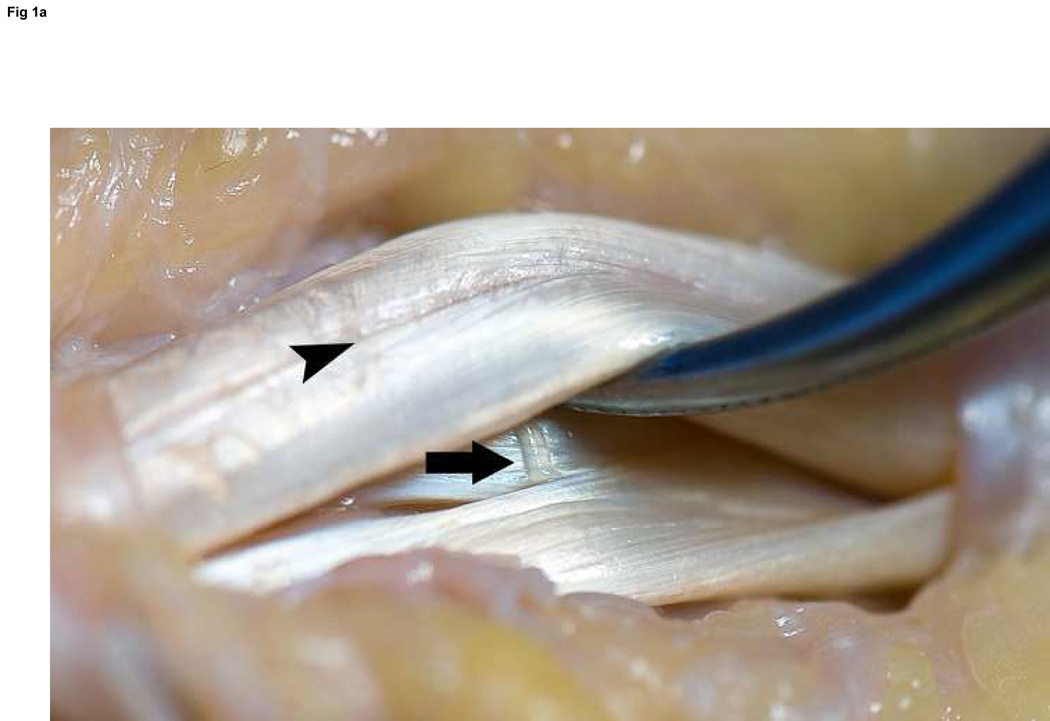
Figure 1. Flexor tendon anatomy exposed between the A2 and A4 pulleys.
A: Oblique view without laceration; note the vinculum longus profundus emerging just distal to the FDS decussation (arrow) and the radial and ulnar bundles of the FDP tendon clearly marked by the longitudinal groove between them (arrowhead). B: Volar perspective after laceration. C: Axial perspective demonstrating the ovoid shape of the FDP tendon, and the flat shape to the FDS slips.
Technique
Preparation
Repair of flexor tendons is performed in the operating room with loupe magnificationunder brachial tourniquet control and either regional or general anesthesia.
Exposure and Retrieval of Tendon Ends
Exposure is achieved using midaxial, Bruner, or a combination of midaxial and Bruner incisions. Existing skin transections are incorporated when possible. Once the neurovascular bundles are identified and protected, full thickness flaps including subcutaneous fat are raised to expose the fibro-osseous flexor sheath. Sweeping the sheath with a moist sponge aids in identifying the pulleys. A minimum amount of fibro-osseus sheath is opened. Even though preservation of at least 50% of the A2 pulley maintains its biomechanical function, a minimum of sheath is opened to provide windowed access to the transected tendons.
Milking the sheath from proximal to distal and then grasping the deep fibers of the tendon with fine-toothed forceps can be effective in retrieving the proximal tendon end. If this is unsuccessful, a midpalmar incision (such as one made to release the A1 pulley for trigger finger) is made to identify the tendon, and is followed by antegrade passage of the tendon shuttled on the end of a pediatric feeding tube.3 Once delivered into the wound it is secured by passing a 25-gauge needle transversely through the proximal flexor sheath, transfixing the tendon for repair.
Repair of the FDS Tendon
At the distal margin of the A2 pulley the FDS tendon has divided into radial and ulnar slips, which move dorsally as they rotate around the FDP tendon. At this level, initial repair of the FDP tendon is recommended as the FDS slips are easily handled following FDP repair. However, if the location of the transection is at the level of the proximal interphalangeal joint where the FDS slips lie dorsal to the FDP tendon. It is therefore is usually easier to repair the FDS slips at the outset.
The flat, thin structure of the slips precludes a robust core suture technique such as those advocated for FDP repair. We use 5-0 or 6-0 polypropylene suture (Prolene: Ethicon Inc., Somerville, NJ) on a small, tapered needle placed in a Becker configuration (Figure 2).4 We construct either a two-strand or a four-strand repair for each slip of the FDS, resulting in a four-strand or an eight-strand repair.
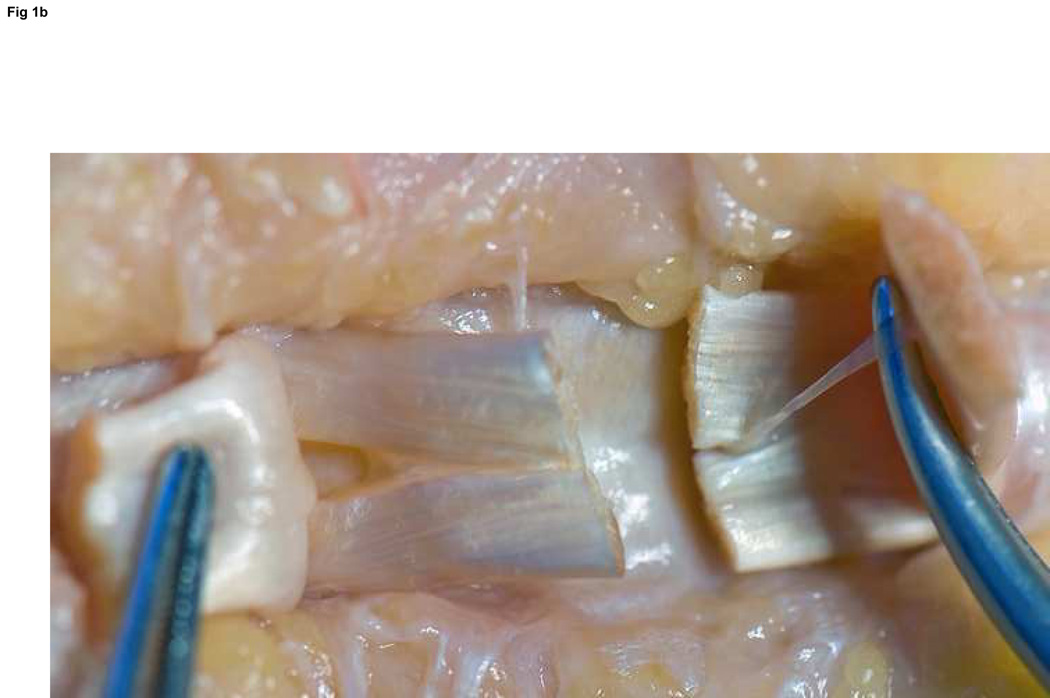
Figure 2. FDS repair.
Becker suture of the ulnar one-half of an FDS slip using 5-0 Prolene. This is repeated for each half of FDS slips on both sides of the laceration. This allows for a four-strand repair of each slip.
Repair of the FDP Tendon
CORE SUTURE TECHNIQUE
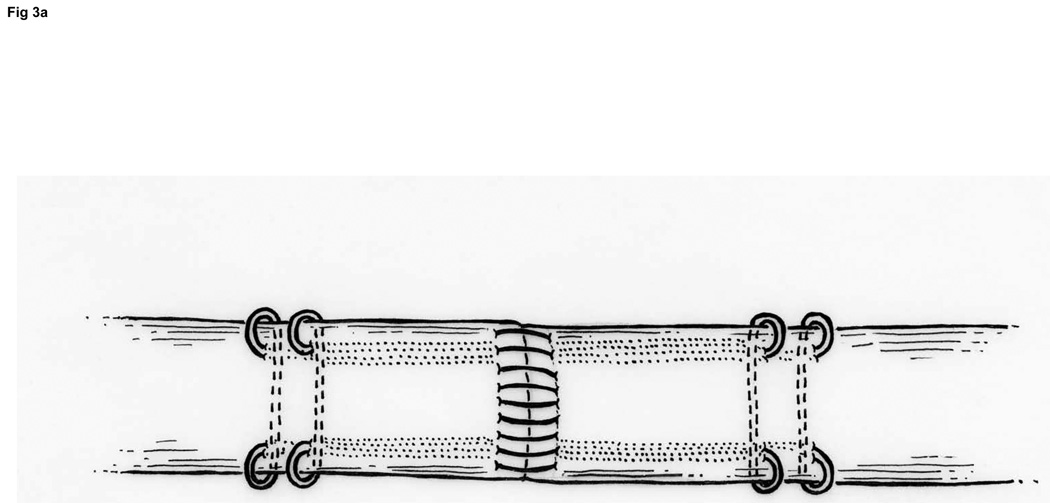
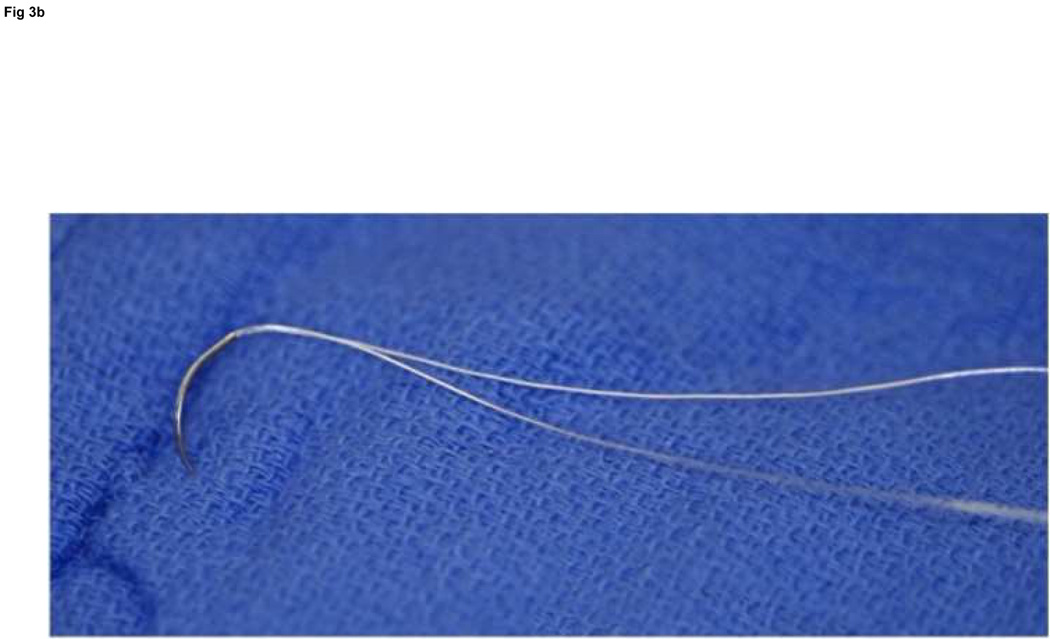
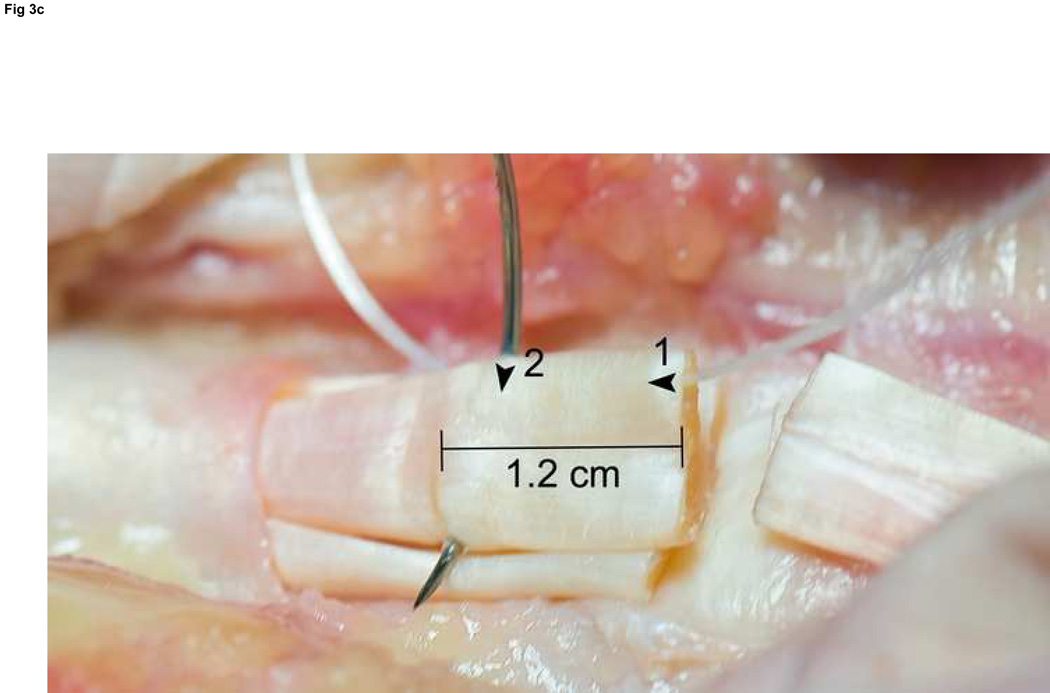
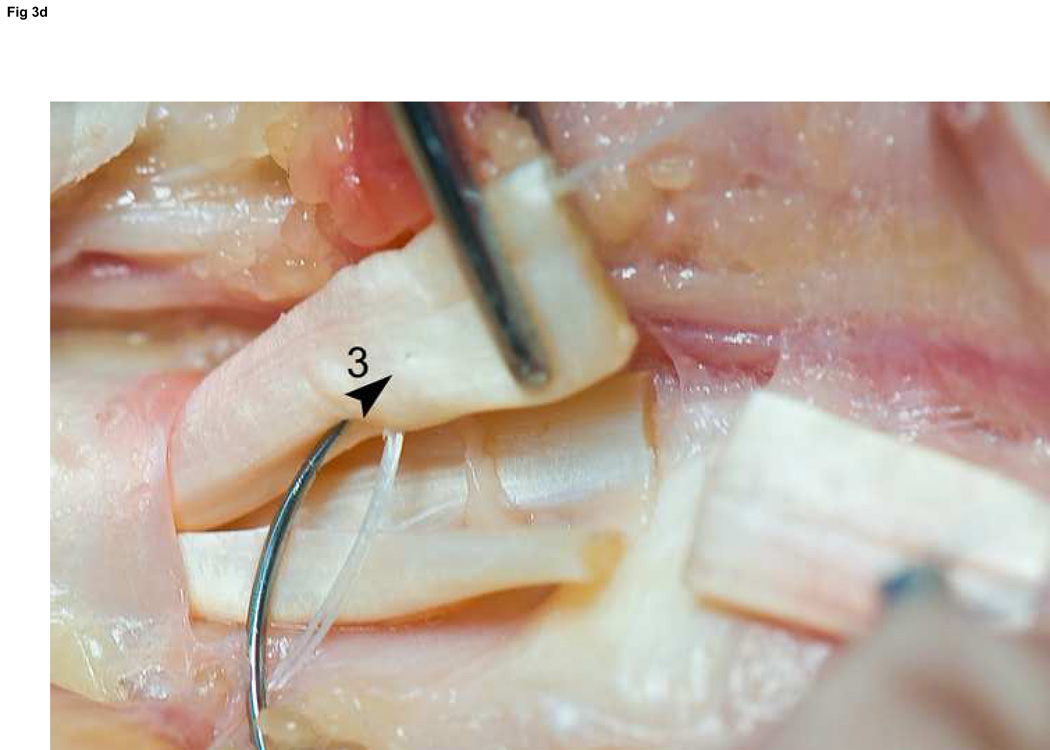
We use Winters’ core-suture technique5 with looped 4-0 pseudomonofilament nylon suture (Supramid Extra; S. Jackson Inc., Alexandria, VA), modified to achieve a locking loop as described by Pennington6 and an 8-strand repair (Figure 3).7 The suture is placed with 1.2 cm of purchase from the lacerated end of the tendon,8, 9 and a single knot placed inside the repair site.10
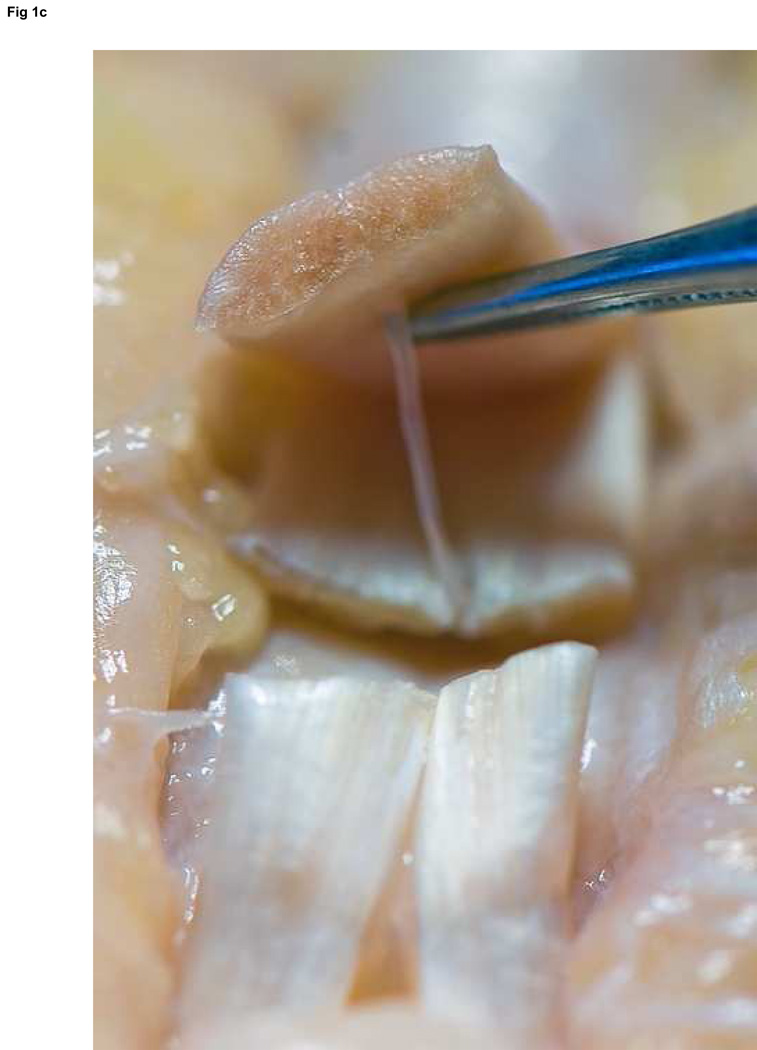
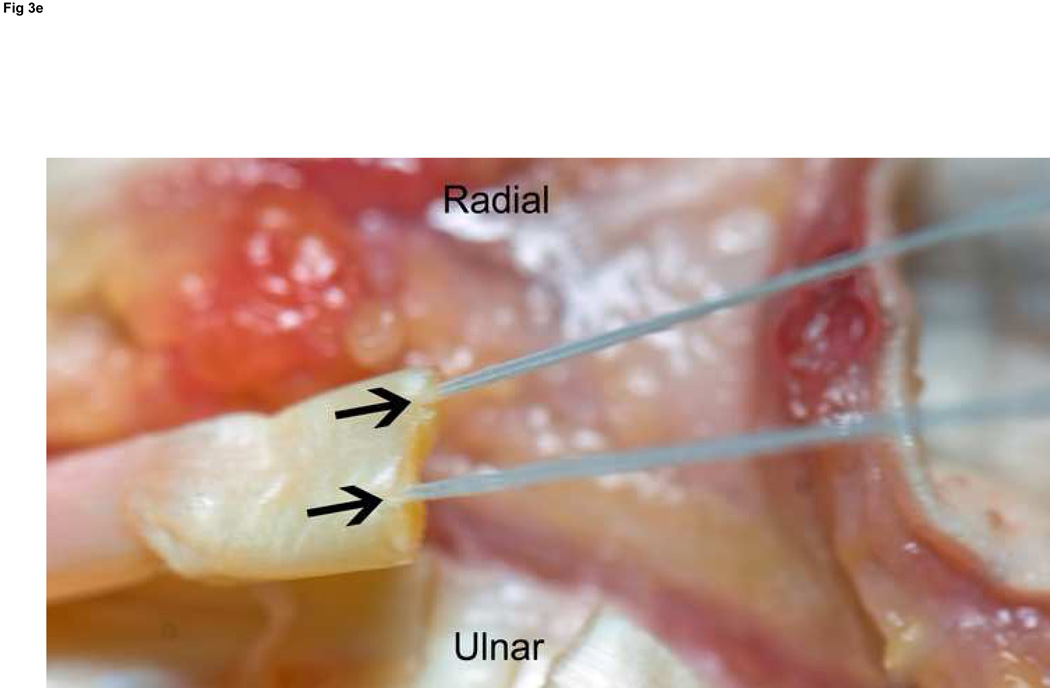
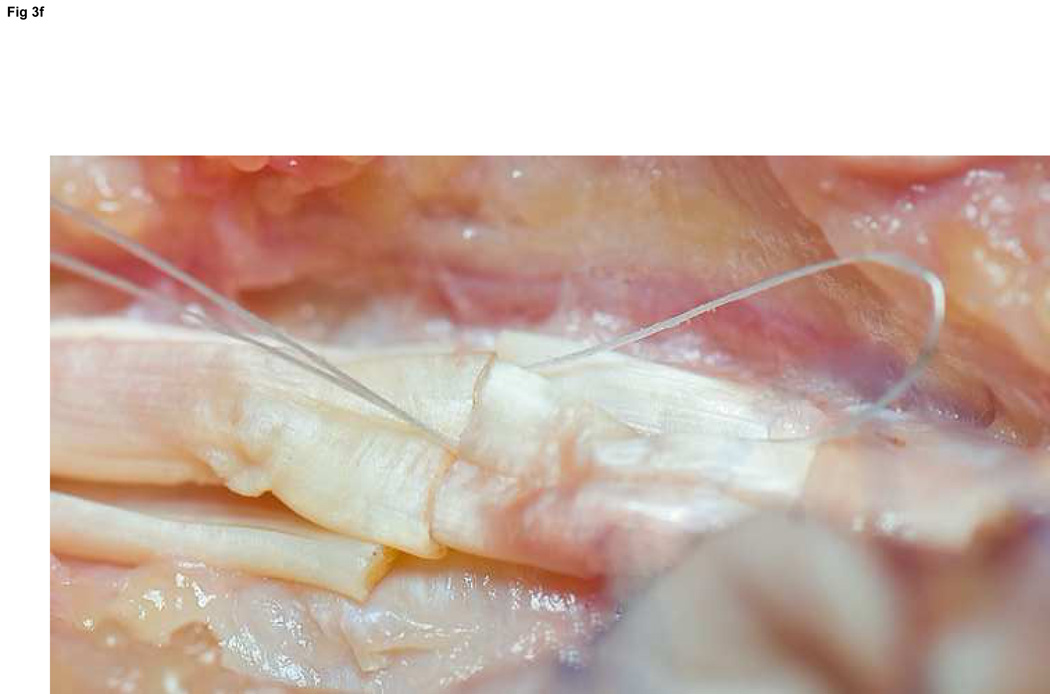
Figure 3. FDP repair.
A: Diagram depicting final repair and suture configuration. B: 4-0 looped Supramid suture. C: The initial pass is placed with a purchase length of 1.2 cm (arrowhead ‘1’); placement of transverse pass closer to lacerated tendon end to create a locking loop (arrowhead ‘2’). D: The third pass enters further from the lacerated end than the transverse pass to create a second locking loop (arrowhead ‘3’). The same passes are performed on the opposite tendon end to complete the first sequence of passes (4 strands now cross the repair site). E: Note that the suture limbs are exiting the radial and ulnar bundles of the tendon from their radial aspects (arrows); the second sequence of passes will utilize the ulnar half of the bundles to complete the 8-strand repair. F: Once the first sequence of passes is completed for the other tendon end, the ends are gently approximated to avoid ‘locking’ the ends apart prior to knot-tying.
PERIPHERAL SUTURE TECHNIQUE
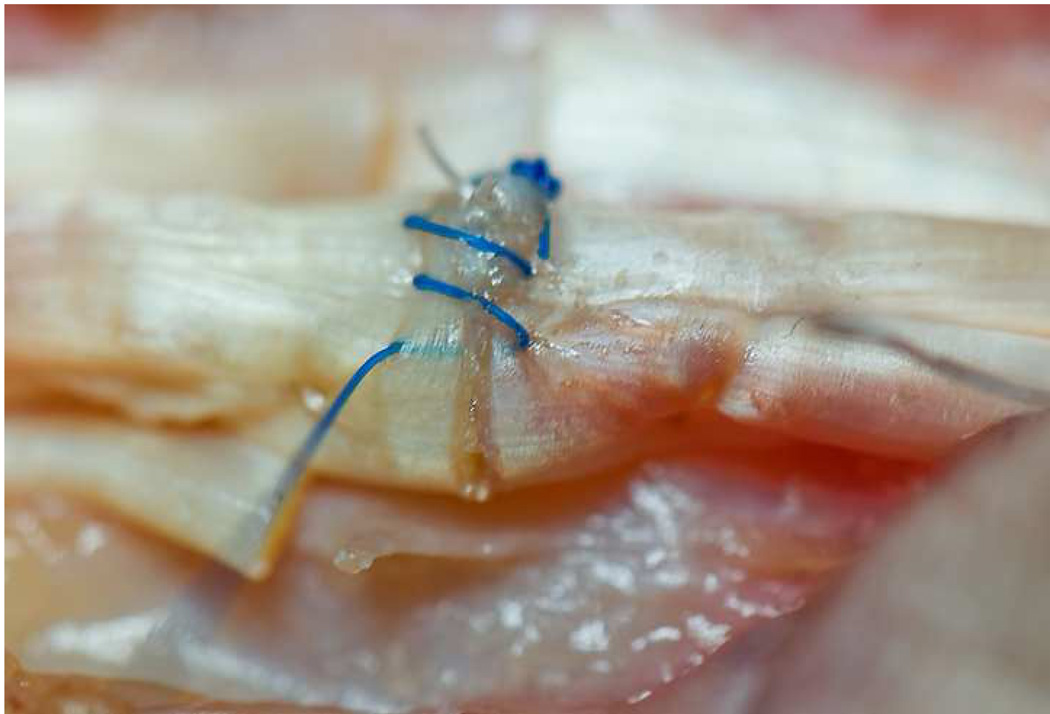
We use a running, non-locked technique with 6-0 polypropylene suture (Prolene: Ethicon Inc., Somerville, NJ) for the peripheral suture (Figure 4). The suture is placed 2 mm from the lacerated edges of the apposed tendon ends, and at least 2 mm deep into the substance of the tendon (rather than only within the epitenon).11, 12 The peripheral running suture is useful for augmentation of repair site strength, as well as ‘tidying up’ of the repair site.
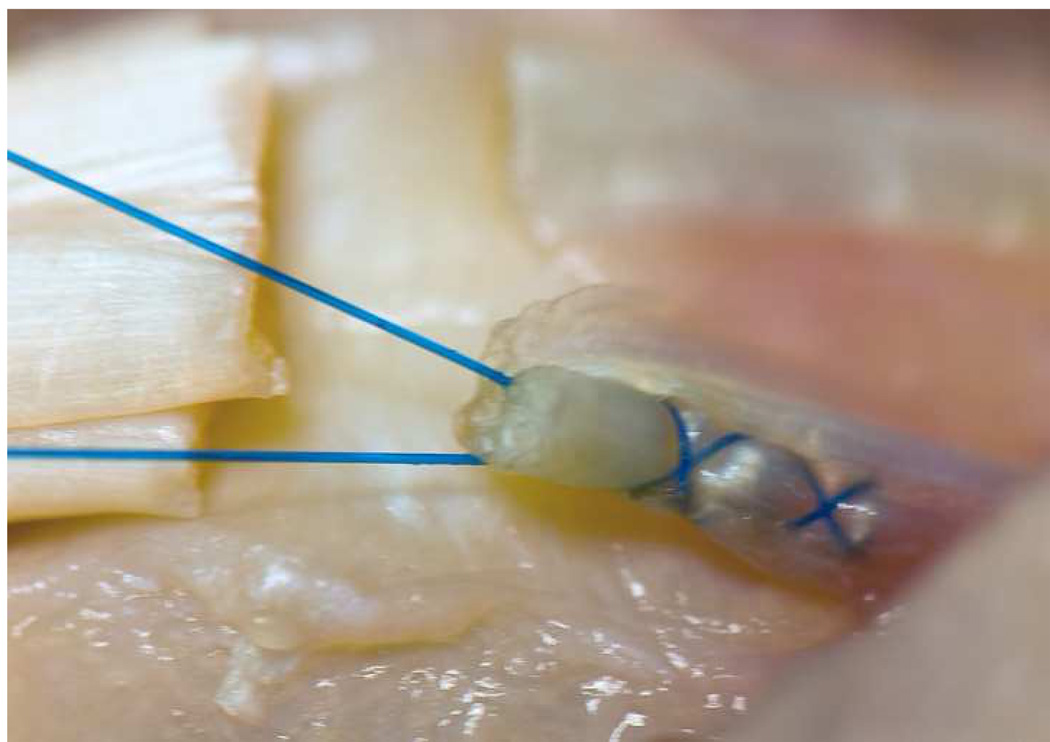
Figure 4. Peripheral suture.
6-0 Prolene is used in a running fashion, 2 mm deep and 2 mm from the lacerated end to improve the strength of the repair and tidy the repair site. The single core suture knot can be seen in the background.
Rehabilitation and Postoperative Care
An early passive mobilization rehabilitation is preferred, with the aim to generate low tensile force only.7 The postoperative splint maintains the wrist in 20 degrees of flexion, the metacarpophalangeal (MCP) joints in 50 degrees of flexion, and the interphalangeal joints fully extended.
Within 5 days postoperatively a tenodesis wrist splint is fashioned to allow 30 degrees of wrist extension (to combine with full digital flexion) and full wrist flexion ( to combine with complete finger extension against a dorsal block). Edema control using self-adhesive wrap (Coban; 3M, St Paul, MN) is initiated. Place-and-hold” exercises are generally initiated during the first week post-operatively. With the wrist extended, composite digital flexion is achieved passively and then held actively. These exercises progress with greater excursion as postoperative pain and edema subside.
Differential excursion of the FDS and FDP tendons is particularly important for combined repairs of the FDS and FDP tendons distal to the A2 pulley due to the intimacy of the FDS slips and the FDP tendon at that level. Several passive exercises are initiated after the first week to maximize differential excursion. These include isolated distal interphalangeal (DIP) joint, isolated proximal interphalangeal (PIP) joint flexion, flat fist (IP joint extension, MCP joint flexion), and hook fist (IP joint flexion, MCP joint extension.
At 6 weeks the exercises are converted to an active protocol, maintaining an emphasis on differential excursion of FDS and FDP tendons. Resisted exercises are not introduced until 12 weeks postoperatively.
Clinical Case
A 31 year-old right-hand dominant laborer presents to the emergency department with an isolated injury to his right ring digit within 12 hours of injury. He slipped while using a boxcutter and sustained a deep palmar transection at the distal proximal phalanx level. Clinical examination demonstrates a well-perfused digit with intact digital nerves. Function of the FDS and FDP tendons, however, cannot be demonstrated actively. The digit rests in a more extended position than the typical cascade, and does not demonstrate a tenodesis effect with wrist motion.
The patient has sustained isolated FDS and FDP tendon transections. Without repair reduced grip strength and altered hand function are expected. Early operative repair provides the most reliable means of restoring function.
Pearls and Pitfalls
Keys to success include atraumatic technique, preservation of the flexor sheath (particularly the A2 and A4 pulleys), utilization of multi-strand core and peripheral sutures, and controlled rehabilitation.
Excessive scarring due to rough handling of the tissues overshadows technically sound tendon repair. Disruption of the A2 and A4 pulleys reduces the efficiency of flexor mechanics and can lead to a fixed flexion deformity of the digit. Poor choice of suture type, caliber, or configuration reduces the strength of the repair, and excessive bulk at the coaptation site increases friction within the sheath. While we prefer an 8-strand repair with looped 4-0 core suture, larger caliber sutures might provide greater tensile strength13 and thereby may provide equivalent strength with fewer strands. An increased preponderance of adhesions has not been correlated with an increased number of strands.14 It is important to approximate the tendon ends after the first sequence of passes of the suture (Figure 3f). Although some suture sliding is possible despite the locking loops, approximating the tendon ends prior to the second pass will eliminate a gap due to a ‘locked’ and gapped position of the tendon ends.
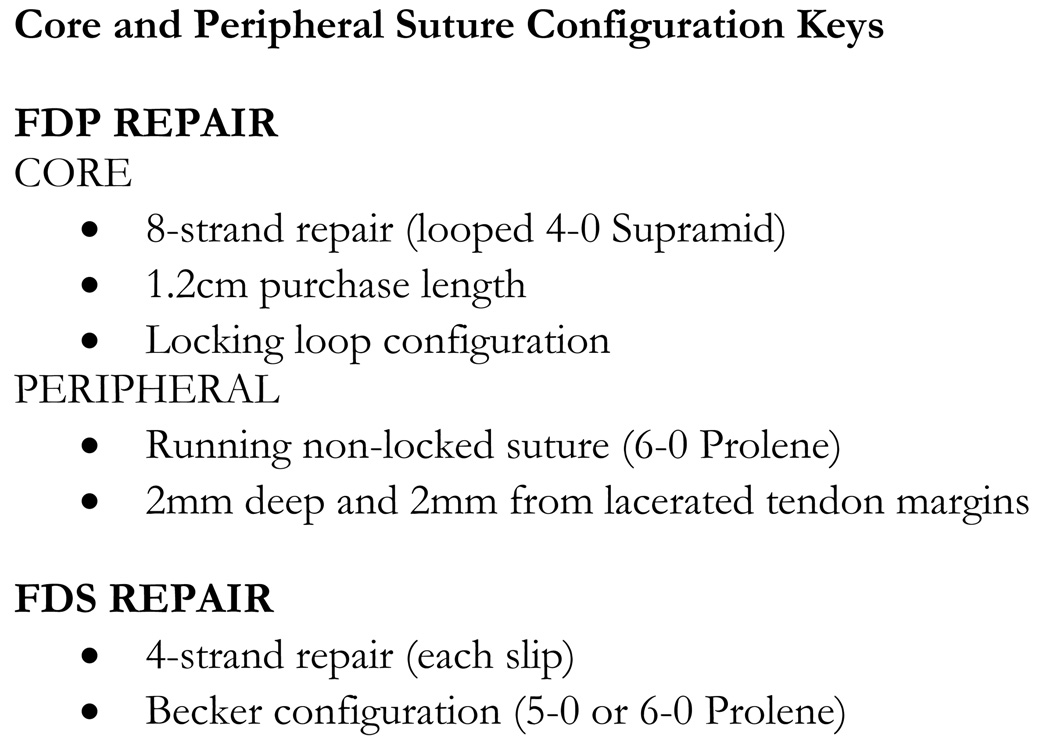
The configuration of suture placement is important (Figure 5). We utilize a core suture purchase length of 1.2 cm to maximize strength and gap resistance.8, 9 The peripheral suture is placed 2 mm deep and 2mm from the lacerated tendon margins to maximize strength11, 12 and in a running non-locked fashion to decrease excessive bulk and tidy the repair site.
Figure 5.
Key points for the core and peripheral suture configuration.
Complications
Complications following combined FDS and FDP repair include rupture and stiffness. The greatest protection against rupture is a strong core and peripheral suture repair, plus a cooperative patient who is engaged in the rather complex rehabilitation process. An educated patient with the guidance of the therapist can minimize adhesions and therefore stiffness. Rupture or disabling stiffness may require further operative intervention. Relative shortening of the FDP tendon by repair leads to a quadrigia effect, adversely affecting the excursion of the adjacent digits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silverman PM, Gordon L. Early motion after replantation. Hand Clin. 1996;12:97–107. [PubMed] [Google Scholar]
- 2.Lundborg G, Myrhage R, Rydevik B. The vascularization of human flexor tendons within the digital synovial sheath region--structureal and functional aspects. J Hand Surg. 1977;2A:417–427. doi: 10.1016/s0363-5023(77)80022-1. [DOI] [PubMed] [Google Scholar]
- 3.Sourmelis SG, McGrouther DA. Retrieval of the retracted flexor tendon. Journal of hand surgery (Edinburgh, Scotland) 1987;12:109–111. doi: 10.1016/0266-7681_87_90071-4. [DOI] [PubMed] [Google Scholar]
- 4.Miller L, Mass DP. A comparison of four repair techniques for Camper's chiasma flexor digitorum superficialis lacerations: tested in an in vitro model. J Hand Surg. 2000;25A:1122–1126. doi: 10.1053/jhsu.2000.20151. [DOI] [PubMed] [Google Scholar]
- 5.Winters SC, Gelberman RH, Woo SL, Chan SS, Grewal R, Seiler JG., 3rd The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: a biomechanical study in dogs. J Hand Surg. 1998;23A:97–104. doi: 10.1016/s0363-5023(98)80096-8. [DOI] [PubMed] [Google Scholar]
- 6.Pennington DG. The locking loop tendon suture. Plastic Reconstr Surg. 1979;63:648–652. doi: 10.1097/00006534-197905000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. The J Bone Joint Surg. 2001;83-A:891–899. [PubMed] [Google Scholar]
- 8.Tang JB, Zhang Y, Cao Y, Xie RG. Core suture purchase affects strength of tendon repairs. J Hand Surg. 2005;30A:1262–1266. doi: 10.1016/j.jhsa.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Zhu B, Xie RG, Tang JB. Influence of core suture purchase length on strength of four-strand tendon repairs. J Hand Surg. 2006;31A:107–112. doi: 10.1016/j.jhsa.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Momose T, Amadio PC, Zhao C, Zobitz ME, An KN. The effect of knot location, suture material, and suture size on the gliding resistance of flexor tendons. J Biomed Mater Res. 2000;53:806–811. doi: 10.1002/1097-4636(2000)53:6<806::aid-jbm23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Diao E, Hariharan JS, Soejima O, Lotz JC. Effect of peripheral suture depth on strength of tendon repairs. J Hand Surg. 1996;21A:234–239. doi: 10.1016/S0363-5023(96)80106-7. [DOI] [PubMed] [Google Scholar]
- 12.Merrell GA, Wolfe SW, Kacena WJ, Gao Y, Cholewicki J, Kacena MA. The effect of increased peripheral suture purchase on the strength of flexor tendon repairs. J Hand Surg. 2003;28A:464–468. doi: 10.1053/jhsu.2003.50074. [DOI] [PubMed] [Google Scholar]
- 13.Taras JS, Raphael JS, Marczyk SC, Bauerle WB. Evaluation of suture caliber in flexor tendon repair. J Hand Surg. 2001;26A:1100–1104. doi: 10.1053/jhsu.2001.28946. [DOI] [PubMed] [Google Scholar]
- 14.Strick MJ, Filan SL, Hile M, McKenzie C, Walsh WR, Tonkin MA. Adhesion formation after flexor tendon repair: a histologic and biomechanical comparison of 2-and 4-strand repairs in a chicken model. J Hand Surg. 2004;29A:15–21. doi: 10.1016/j.jhsa.2003.09.003. [DOI] [PubMed] [Google Scholar]