Abstract
Reproduction in all vertebrates is controlled by the brain-pituitary-gonad (BPG) axis, which is regulated socially in males of the African cichlid fish Astatotilapia burtoni. Although social information influences GnRH1 neurons at the apex of the BPG axis, little is known about how the social environment and dominance affects the cellular and molecular composition of the testes to regulate reproductive capacity. We created an opportunity for reproductively suppressed males to ascend in status and then measured changes in gene expression and tissue morphology to discover how quickly the perception of this opportunity can influence the testes. Our results show rapid up-regulation of mRNA levels of FSH receptor and several steroid receptor subtypes in the testes during social ascent. In contrast, LH receptor was not elevated until 72 h after ascent, but this increase was coincident with elevated circulating androgens and early stages of spermatogenesis, suggesting a role in steroidogenesis. The spermatogenic potential of the testes, as measured by cellular composition, was also elevated before the overall increase in testes size. The presence of cysts at all stages of spermatogenesis, coupled with lower levels of gonadotropin and steroid receptors in subordinate males, suggests that the BPG axis and spermatogenesis are maintained at a subthreshold level in anticipation of the chance to gain a territory and become reproductively active. Our results show that the testis is stimulated extremely quickly after perception of social opportunity, presumably to allow suppressed males to rapidly achieve high reproductive success in a dynamic social environment.
Social ascent stimulates rapid changes in testicular gene transcription that occur prior to morphological changes, which allows males to quickly achieve higher reproductive success.
Interactions among conspecifics can profoundly influence reproductive capabilities, especially in highly social vertebrates. In males, position in a dominance hierarchy can dictate reproductive fitness not only by regulating the availability of breeding opportunities, but also by influencing the physiological readiness to mate (1,2,3,4). For example, through social interactions, dominant individuals within a population can suppress the activity of the brain-pituitary-gonad (BPG) axis in subordinates such that fertility, spermatogenesis, and steroid production by the testes are reduced (5,6,7). However, in species that live in dynamic social and physical environments, subordinate males often have opportunities to displace higher-ranking individuals and become reproductively active. Under these circumstances, it would be adaptive for subordinate males to quickly up-regulate their BPG axis, especially at the level of the testes, to rapidly take advantage of breeding opportunities. Aside from studies on sex-changing fishes (8,9), however, little is known about how and how quickly the perception of social opportunity initiates cellular and molecular changes in the vertebrate testis.
The testes are the final downstream effector of the BPG axis and the primary target of the pituitary gonadotropins, LH and FSH. LH and FSH receptors are rhodopsin-like receptors (Family A) within the large superfamily of seven-transmembrane G protein-coupled receptors (10). In teleosts, LH receptor (LHR) is found in both the steroid-producing Leydig (or interstitial) cells and Sertoli cells, whereas FSH receptor (FSHR) is expressed in Leydig cells, Sertoli cells, and possibly early germ cells (11,12,13). The duality of gonadotropin function is less clear in fishes than it is in mammals, because both LH and FSH are often equipotent in stimulating androgen production from the testes and they both regulate spermatogenesis at various stages (12,13). Nevertheless, changes in the gonadotropin sensitivity of the testes that are mediated by changes in LHR and FSHR expression can influence its steroidogenic and spermatogenic potential, which are essential for successful reproduction.
Testicular development and function can also be influenced by steroid hormones, and thus the expression level of steroid receptors dictates the steroid sensitivity of the testes. Androgens (e.g. testosterone, 11-ketotestosterone), for example, are known to stimulate both steroid production and mitosis and meiosis during spermatogenesis in teleost fishes and other vertebrates (14,15,16). Estradiol (E2) is also important for testicular development and male fertility (17); estrogen receptors (ERs) are found in the testes across vertebrates, and, in fact, males of many teleost fishes have levels of circulating E2 that are equivalent to or higher than that of females (18,19). Cortisol and other related stress hormones can also have profound effects on male reproduction, where at low to moderate doses they stimulate spermatogonial proliferation, but at higher levels can inhibit both spermatogenesis and steroid production (20). Variations in mRNA levels of steroid receptors can therefore provide one regulatory mechanism for structural and molecular change in the testes that has important reproductive consequences.
In males of the African cichlid fish, Astatotilapia burtoni, reproductive capacity is tightly coupled to social status (5), which makes it an excellent model to examine how the social transition from subordinate to dominant status influences the BPG axis at the level of the testes. Dominant males have bright coloration, defend a spawning territory, and display aggressive and courtship behaviors, whereas subordinate males have cryptic coloration similar to females, lack a spawning territory, display submissive behaviors, and do not court females (21). In addition to these behavioral and coloration differences, dominant and subordinate males also differ in several key measures of the BPG axis. For example, stable dominant males have larger GnRH1 somata (22,23); higher GnRH1 gene expression in the brain (24); higher LHβ, FSHβ, and GnRH receptor (GnRH-R1) mRNA levels in the pituitary (25,26); higher circulating LH, FSH (26), and androgen levels (27); higher steroid receptor mRNA levels in the brain (28); and larger testes (29) compared with stable subordinate individuals. When a subordinate male perceives an opportunity to ascend in social status and become dominant, he displays territorial and reproductive behaviors within minutes and shows evidence of rapid activation of both the brain and pituitary portions of the reproductive axis (26,30,31), but it remains unknown whether the testes are also activated on a similar timescale, or how quickly this newly ascended male becomes reproductively competent.
This is the second of two companion studies that examined how quickly the reproductive axis is up-regulated in A. burtoni as a suppressed subordinate male is given an opportunity to ascend and become dominant. Here we examine the temporal expression of cellular and molecular changes at the level of the testes, whereas the companion study examined the temporal expression of physiological traits at the pituitary gonadotropin level (26). The goal of this study was to 1) determine the temporal expression pattern of LHR, FSHR, androgen receptors (ARs), ERs, corticosteroid receptors (CRs), and aromatase CYP19a mRNA levels in the testes during social ascent; and 2) examine the cellular composition of the testes during social ascent to determine their spermatogenic potential, and to test for correlations with testes size. Our results show rapid up-regulation of the testes during status transition, suggesting that this level of the reproductive axis is also influenced by the social environment. Importantly, we also show that the socially and reproductively suppressed subordinate males maintain some activity at every level of the BPG axis and retain mature sperm in their testicular lumens that can be used for rapid spawning when given the opportunity.
Materials and Methods
Animals, social manipulation, fish measurements, and collection of blood and tissue samples were identical to those described in the companion manuscript (26), with exceptions identified here. All experimental procedures were approved by the Stanford Administrative Panel for Laboratory Animal Care.
Sequencing of LHR and FSHR
At the start of the study, the sequences for LHR and FSHR in A. burtoni were unknown. To identify a partial cDNA sequence for these transcripts, a combination of PCR and RACE PCR (rapid amplification of cDNA ends; CLONTECH Laboratories, Inc.,) were used. cDNA for PCR was prepared by first isolating RNA from the testes of a dominant male (RNeasy mini kit; QIAGEN, Chatsworth, CA), followed by first strand cDNA synthesis for preparation of 5′- and 3′-RACE-ready cDNA according to the manufacturer’s instructions (SMART-RACE protocol, CLONTECH, Palo Alto, CA). PCR primers were designed based on the LHR and FSHR sequences of tilapia Oreochromis niloticus (LHR, GenBank accession no. AB041763; FSHR, GenBank accession no. AB041762). A fragment from each gene was amplified on a thermal cycler using a touchdown protocol: 1 min at 95 C; 14 cycles of 1 min at 95 C, 30 sec at 1 C decreasing temperature increments from 65–51 C, 1 min at 72 C; and a 5-min final extension at 72 C. The reaction products were then visualized and purified by running on a 0.8% agarose Sybr green CloneWell gel (Invitrogen, Carlsbad, CA), and the bands were collected and sequenced (Sequetech, Mountain View, CA). BLAST analysis confirmed each sequence had high similarity to its respective transcript in numerous other perciform fishes (E values ≤e−100), with highest similarity to the closely related tilapia O. niloticus. These sequences were then used to generate A. burtoni-specific primers for RACE reactions to isolate the 3′- and 5′-ends of the cDNA, and to design primers for quantitative RT-PCR (qRT-PCR). To verify sequence identity, phylogenetic and molecular evolutionary analyses were conducted on the translated amino acid sequence using MEGA 4.1 (32). The percentage of replicate trees in which A. burtoni clustered together with O. niloticus in the bootstrap test (1000 replicates; Neighbor-joining method) was 100% for FSHR and 100% for LHR.
Quantitative RT-PCR
Testes were rapidly removed, weighed to calculate the gonadosomatic index [GSI = (gonad mass/body mass) × 100], and an approximately 5-mg section in the center of the larger right testicular lobe was weighed and then flash frozen and stored at −80 C for later qRT-PCR. The remainder of the testes was fixed in 4% buffered formalin for histological processing (see below).
For qRT-PCR samples, tissue was homogenized, RNA was extracted following standard kit protocols (RNeasy Mini kit, QIAGEN), 0.5 μg of total RNA was reverse transcribed to cDNA (iScript cDNA synthesis kit; Bio-Rad Laboratories, Inc., Hercules, CA), and cDNA was diluted 1:10 before use as a template for qRT-PCRs as described in Ref. 26.
qRT-PCR was used to measure mRNA levels of LHR, FSHR, two ARs (ARα, ARβ), three ERs (ERα, ERβa, ERβb), aromatase CYP19a, and four corticosteroid receptors (CRs) [glucocorticoid receptors: GR1a, GR1b, GR2, and mineralocorticoid receptor (MR)] from the testes, with methods identical to those described in the companion manuscript (26). Primers for LHR and FSHR were designed with PrimerQuest (Integrated DNA Technologies, Coralville, IA) from the sequences for each cDNA obtained from RACE reactions described above and synthesized commercially as follows: LHR forward 5′-CAA TGG GAC AAA GCT CAA CAC GCT-3′; and reverse, 5′-AGT TGG ACC TGT GGC TCC TTC AAA-3′ (94-bp product) (GenBank accession no. HQ147567); FSHR forward, 5′-CAG CAG CTA TGG CAA AGT GAG CAT-3′; and reverse, 5′-AAG GCT TGC GAA AGG TGA GGT AGA-3′ (155-bp product) (GenBank accession no. HQ147568). Primers for steroid receptors, aromatase, and the reference genes, 18s rRNA and glyceraldehyde 3-phosphodehydrogenase (G3PDH), were also commercially synthesized and identical to those used in previous studies (19,25,28,33,34,35).
Testes size and cellular composition differ between stable subordinate and stable dominant male A. burtoni (e.g. dominant males have GSI values twice that of subordinates, but somatic to germ cell ratios about 2.5-fold less). To account for changes in mRNA expression related to testes growth and different cellular representation between social states, the mRNA levels in the testes were normalized according to methods used in other fishes to account for seasonal changes in testes composition (36,37). Relative mRNA expression in the testes was calculated according to the following equation: testes target gene mRNA levels = (PCR target gene quantity/PCR geomean reference gene quantity) × (amount of extracted RNA/tissue mass used for RNA extraction) × GSI. Measuring the concentration of total RNA extracted per testis fragment, the mass of the testis fragment before RNA isolation, and the total gonad mass normalized to body mass allows an estimation of the total amount of RNA per organ for comparisons among stable and transitioning animals that have different cellular compositions.
Testicular histology and quantification of cell composition
Testes were fixed for 24 h, after which a portion was removed (∼5 mm in length) from the center of the larger right testicular lobe (adjacent to the portion removed for qRT-PCR analysis) for histological processing. Samples were rinsed in distilled water, dehydrated in an alcohol series, cleared in xylene, embedded in paraffin, serially sectioned at 4 μm in the transverse plane, and stained with hematoxylin and eosin using standard histological techniques (Stanford School of Medicine, Digestive Disease Center).
Photomicrographs were then taken (20 × objective) of three randomly chosen sections from each individual for analysis of testicular cell composition. Randomly generated points (n = 50) were overlaid on each photograph (CPCe software) (38), and the cell type underneath each cross-hair point was identified. An initial experiment showed that a minimum of 50 points was necessary to adequately determine percentage of cell types (10, 25, 50, 100, 200 points compared; P > 0.05 for 50, 100, and 200). Cell types were identified based on previous descriptions in this and other related teleost species (15,29,39,40), and the following cells were quantified: interstitial or Leydig cells, type A spermatogonia, type B spermatogonia, spermatocytes (combined primary and secondary), spermatids, and spermatozoa (mature sperm). Each cell type was then expressed as a percentage of the total and averaged across the three sections to obtain a mean for each individual.
Because the point-count measurements described above only provide information on the relative percentage of the testes composed of each cell type, and not the amount of sperm available for release, we also quantified the density of mature spermatozoa within testicular lumens of stable subordinate and stable dominant males. Sperm density (number of sperm per μm2) was measured from three randomly selected testicular lumens in each of three cross sections per individual. Sperm heads were counted from photomicrographs with a cell-count plugin designed for NIH ImageJ, and sperm densities were averaged across the three lumens per section, and then across the three cross sections to obtain a mean for each individual.
Statistical analyses
Data sets that were normally distributed (Shapiro-Wilk test) with equal variances (Levene median test) were analyzed with one-way ANOVA with post hoc Student Newman Keul’s tests for multiple comparisons, or with Student’s t tests, whereas data that did not meet the assumptions of parametric statistics were compared with Kruskal-Wallis (KW) tests with post hoc Dunn’s tests, or with Mann-Whitney rank sum tests. Correlations were assessed with either Pearson product moment tests (parametric) or Spearman rank tests (nonparametric). For consistency, however, all data are plotted as mean ± ses with appropriate statistical test values reported in the text. Statistical comparisons were performed with SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA.).
Results
LH and FSH receptors
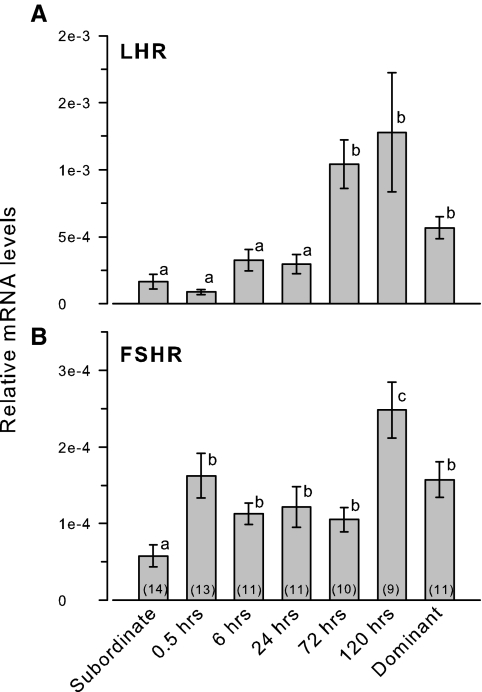
Testicular FSHR mRNA levels were rapidly elevated by 30 min after social ascent, remained relatively stable through 72 h, and were then 2-fold higher at 120 h before returning to a lower level in stable dominant males (KW, H = 22.88; df = 6; P < 0.001) (Fig. 1). In contrast, LHR mRNA levels did not change from low subordinate levels during the first 24 h of social transition but increased by 3-fold at 72–120 h (KW, H = 36.65; df = 6; P < 0.001).
Figure 1.
Relative mRNA levels of LHR and FSHR in the testes of male A. burtoni. A, LHR mRNA levels were elevated by 72 h after perception of social opportunity. B, FSHR mRNA levels were rapidly elevated at 30 min and were further increased by 120 h after ascent. Data are plotted as relative mRNA levels (mean ± se) referenced to the geometric mean of two housekeeping genes (18s and G3PDH) and corrected for testes size and cellular composition. Bars with different letters represent significant differences (P < 0.05), and sample sizes are indicated within each bar.
LHR mRNA levels in the testes were not correlated with circulating levels of LH (r = 0.20; P = 0.121) or FSH (r = 0.08; P = 0.526). FSHR mRNA levels, however, were positively correlated with circulating FSH levels (r = 0.29; P = 0.019), but not with LH levels (r = 0.21; P = 0.111).
Androgen receptors
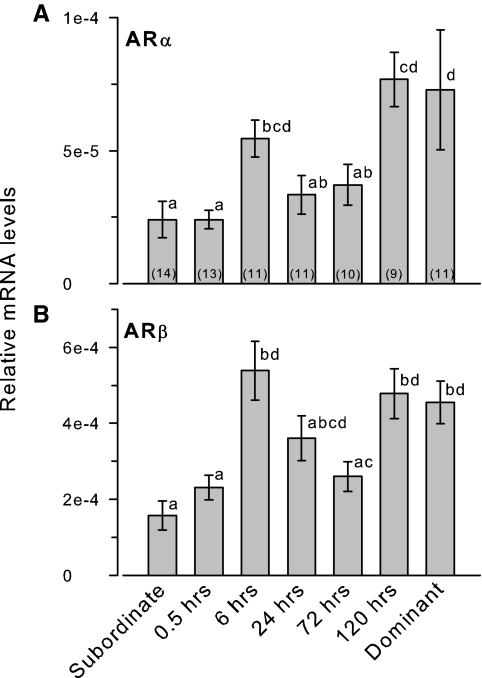
ARα and ARβ mRNA levels were both higher in stable dominant compared with stable subordinate males (Fig. 2). mRNA levels of both AR subtypes were elevated by 6 h after social ascent, were lower during the 24- to 72-h time points, and then were elevated again at 120 h after social ascent to reach stable dominant male levels (ARα: KW, H = 29.26; df = 6; P < 0.001; ARβ: KW, H = 29.68; df = 6; P < 0.001).
Figure 2.
Relative mRNA levels of AR subtypes in the testes of male A. burtoni. A, ARα mRNA levels were elevated by 6 h after perception of social opportunity. B, ARβ mRNA levels were also elevated by 6 h after social ascent. mRNA levels of both ARs were also higher in stable dominant compared with stable subordinate males. Data are plotted as relative mRNA levels (mean ± se) referenced to the geometric mean of two housekeeping genes (18s and G3PDH) and corrected for testes size and cellular composition. Bars with different letters represent significant differences (P < 0.05), and sample sizes are indicated within each bar.
ERs and aromatase
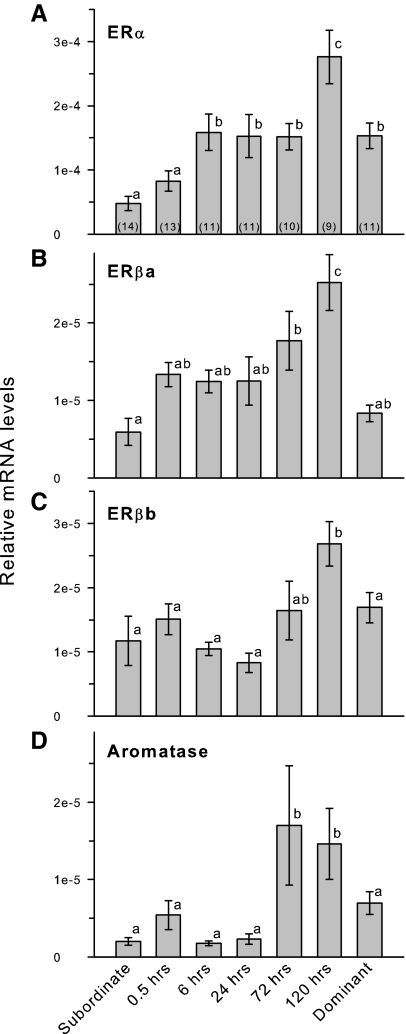
Testicular mRNA levels of ERα, but not ERβa, ERβb, or aromatase, were higher in stable dominant compared with stable subordinate males (Fig. 3). ERα mRNA levels were elevated by 6 h after social ascent, remained at this level through 72 h, and then increased further at 120 h before returning to the lower stable dominant male levels (KW, H = 32.58; df = 6; P < 0.001). ERβa mRNA levels showed a gradual increase during social transition, but were not elevated above stable subordinate levels until 72–120 h after ascent (KW, H = 29.10; df = 6; P < 0.001). ERβb mRNA levels did not differ from stable subordinate levels until 120 h after ascent and were then reduced in stable dominant animals to a level that did not differ from subordinates (KW, H = 20.99; df = 6; P = 0.002). Aromatase mRNA levels remained low for the first 24 h after social ascent but were then elevated by 72–120 h, although the variance was quite high during these time points (KW, H = 33.84; df = 6; P < 0.001).
Figure 3.
Relative mRNA levels of ER subtypes and aromatase in the testes of male A. burtoni. A, ERα mRNA levels were elevated by 6 h after ascent and were doubled at 120 h before being lowered to stable dominant male levels. B, ERβa mRNA levels did not differ between stable subordinate and stable dominant animals but were elevated during social ascent. C, ERβb mRNA levels were only higher in ascending males at 120 h after social ascent. D, Aromatase CYP19a mRNA levels were elevated only at 72–120 h after ascent and were then reduced in stable dominant males to levels that did not differ from stable subordinate animals. Data are plotted as relative mRNA levels (mean ± se) referenced to the geometric mean of two housekeeping genes (18s and G3PDH) and corrected for testes size and cellular composition. Bars with different letters represent significant differences (P < 0.05), and sample sizes are indicated within each bar.
Corticosteroid receptors
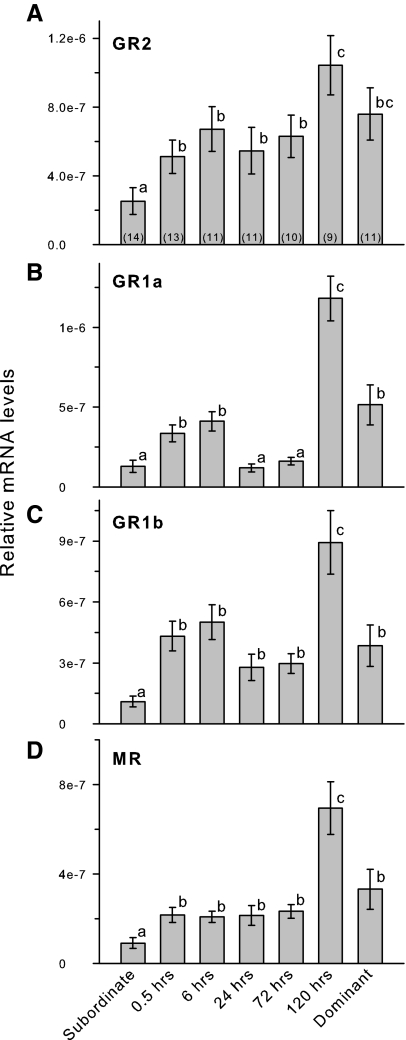
Testicular mRNA levels of all four corticosteroid receptors were higher in stable dominant compared with stable subordinate males, and expression levels for all CRs were greatest at 120 h (Fig. 4). During social transition, GR2 mRNA levels showed a general increase over time but were not elevated above stable subordinate levels until 120 h after social ascent (KW, H = 21.08; P = 0.002). GR1a mRNA levels were elevated by 6 h after social ascent, were then reduced from 24–72 h, and then finally showed a 5-fold increase at 120 h (KW, H = 45.08; df = 6; P < 0.001). GR1b mRNA levels were elevated by 30 min after ascent, remained at this level through 72 h, and then increased approximately 2.5-fold at 120 h (KW, H = 33.04; df = 6; P < 0.001). MR mRNA levels were low during social transition until they increased by about 3-fold at 120 h after ascent (KW, H = 30.58; df = 6; P < 0.001).
Figure 4.
Relative mRNA levels of corticosteroid receptor subtypes in the testes of male A. burtoni. A, GR2 mRNA levels were elevated by 30 min after social ascent, remained unchanged through 72 h, and then were further elevated at 120 h. B, GR1a mRNA levels were also elevated by 30 min, but were then lower at 24–72 h before showing a 5-fold increase at 120 h after social ascent. C, GR1b mRNA levels were elevated by 30 min and then showed a further increase at 120 h after social ascent. D, MR mRNA levels were elevated by 30 min after ascent, remained at this level through 72 h, and then showed a 3-fold increase at 120 h. All CRs were higher in stable dominant compared with stable subordinate males. Data are plotted as relative mRNA levels (mean ± se) referenced to the geometric mean of two housekeeping genes (18s and G3PDH) and corrected for testes size and cellular composition. Bars with different letters represent significant differences (P < 0.05), and sample sizes are indicated within each bar.
Temporal changes in testicular cell composition
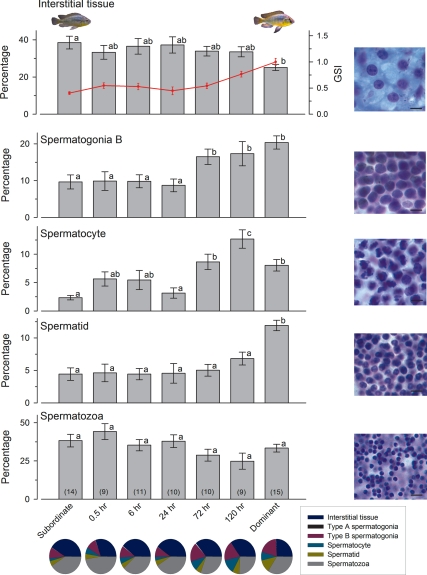
GSI was elevated above stable subordinate male levels by 120 h after social ascent (Fig. 5) (KW, H = 43.19; df = 6; P < 0.001). The percentage of the testes composed of interstitial tissue was greater in stable subordinates compared with stable dominants, but percentages remained relatively constant during social transition (KW, H = 15.03; df = 6; P = 0.020). Type A spermatogonia were relatively rare compared with all other cell types (<1%) and did not differ among stable subordinate, ascending, and stable dominant animals (KW, H = 2.83; df = 6; P = 0.830). The percentage of type B spermatogonia remained near 10% until 72 h after ascent where it increased to more than 15% to reach stable dominant male values (ANOVA, F(6, 72) = 5.36; P < 0.001). Spermatocytes were also elevated by 72 h, were highest at 120 h, and then fell back down to the 72 h values in stable dominant males (KW, H = 32.13; df = 6; P < 0.001). The percentage of spermatids remained constant (∼5%) throughout the social transition but were higher in stable dominant males (ANOVA, F(6, 72) = 8.59; P < 0.001). The percentage of the testes composed of mature spermatozoa did not differ among stable subordinate, ascending, and stable dominant animals (ANOVA, F(6, 72) = 2.16; P = 0.060) (Fig. 5). The pie charts in Fig. 5 illustrate the transition from stable subordinate males with testes primarily composed of interstitial tissue and mature sperm within testicular lumens, to stable dominant males with greater percentages of multiple cell types that is indicative of active spermatogenesis.
Figure 5.
GSI and cell composition of the testes in male A. burtoni. Interstitial tissue was higher in stable subordinate males compared with stable dominant males but remained unchanged in ascending animals. Type B spermatogonia and spermatocytes were elevated by 72 h after ascent, whereas the percentage of spermatids was only higher in stable dominant males. There was no difference in the percentage of mature spermatozoa among stable phenotypes or during ascension. Data are plotted as the percentage of testis (mean ± se) composed of each cell type, and representative photomicrographs of each cell type are shown at right (cresyl violet stain; scale bars, 10 μm). GSI is overlaid on the top graph (red symbols and line), and increased to near stable dominant male levels by 120 h after ascent. Bars with different letters represent significant differences (P < 0.05), and sample sizes are indicated in parentheses within each bar. Pie charts show the percentage of each cell type represented in the testes of stable subordinate, stable dominant, and ascending males at each time point to illustrate the progressive increase in absolute spermatogenic potential during ascent.
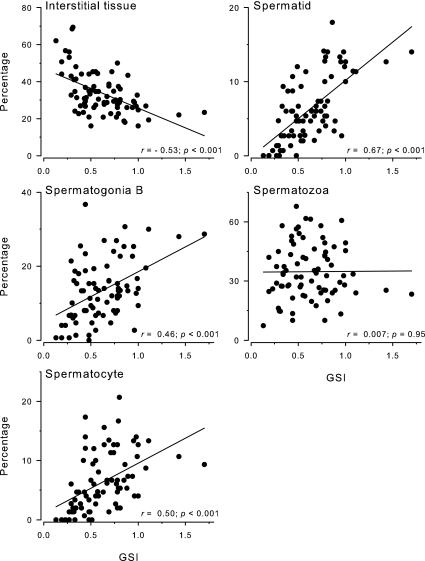
The percentage of interstitial tissue in the testes was negatively correlated with GSI, whereas the percentage of type B spermatogonia, spermatocytes, and spermatids were all positively correlated with GSI when all animals were examined together (Fig. 6). In contrast, there was no relationship between the percentage of mature spermatozoa in the testis and GSI (r = 0.007; P = 0.950).
Figure 6.
Correlations between GSI and spermatogenic cell type in the testes of male A. burtoni. GSI was negatively correlated with interstitial tissue and positively correlated with type B spermatogonia, spermatocytes, and spermatids. There was no relationship between GSI and the percentage of mature spermatozoa in the testis. Correlation coefficients (r) and P values are shown.
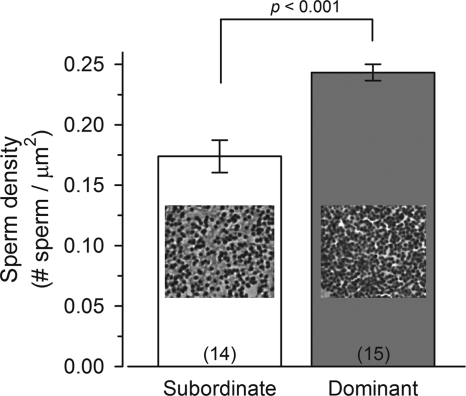
Sperm density within testicular lumens of stable subordinate and dominant males
Even though there was no difference in the percentage of the testes that contained mature sperm among males, the density of that sperm within the testicular lumens was higher in stable dominant males compared with stable subordinate males (Mann-Whitney, U = 19.0; P < 0.001) (Fig. 7).
Figure 7.
Density of mature sperm within testicular lumens of male A. burtoni. Stable dominant males had a greater density of sperm within their lumens compared with stable subordinate males that were socially suppressed for 4–5 wk. Data are plotted as mean ± se, and sample sizes are indicated within each bar. Representative photomicrographs of mature sperm within the testicular lumen are shown.
Discussion
Our results show rapid up-regulation of FSHR and several steroid receptor subtypes in the testes during social ascent in an African cichlid fish. The presence of cells at all stages of spermatogenesis, coupled with lower but measurable levels of gonadotropin and steroid receptors in stable subordinate male testes, further supports the idea that the BPG axis and spermatogenesis are not arrested in these socially suppressed individuals, but rather, is maintained at a lower level in anticipation of the chance to gain a territory and become reproductively active. This is consistent with the idea that males transitioning from subordinate to dominant status undergo a reactivation of the already functional BPG axis that is akin to puberty in mammals (41). We also show that, in addition to the previously described rapid stimulation of the brain (30) and pituitary (26), the testes are also stimulated extremely quickly, presumably as an adaptation for suppressed males to rapidly achieve higher reproductive success in a dynamic social environment where territory tenure can be relatively short lived.
LHR and FSHR
Whereas LHβ mRNA levels in the pituitary and LH levels in the bloodstream were rapidly elevated by 30 min after ascent (26), LHR mRNA levels in the testes remained relatively low during the first 24 h after ascent, but then increased nearly 3-fold at 72 h. LHRs are expressed primarily in Leydig cells of most teleosts [although a recent study has also localized LHR to Sertoli cells in the zebrafish Danio rerio (13)] and play a major role in mediating steroid production (12). In fact, the 72 h elevation in testicular LHR mRNA levels is coincident with an increase in circulating 11-ketotestosterone levels, as well as the increase in percentage of early stages of spermatogenesis (type B spermatogonia, spermatocytes) in these same animals. These early spermatogenic stages were shown to be associated with high levels of androgen-synthesizing enzymes in other fishes (36,42). Thus, the expression of LHR in the testes of A. burtoni during social ascent is consistent with a role in steroid production. However, in seasonally breeding teleosts, LH also plays a role in the final stages of spermatogenesis and spermiation (12,43); thus it is possible that changes in LHR expression during social transition play a role in germ cell development as well.
In contrast to the LHR, mRNA levels of FSHR were rapidly elevated in the testes at 30 min after social ascent, with a further increase at 120 h. This 30-min elevation was coincident with the increases of FSH in both the pituitary and circulation and indicates a rapid increase in FSH sensitivity of the testes during social ascent. In teleosts, FSHR is expressed in both Leydig and Sertoli cells and has some affinity for both FSH and LH (12). This suggests that quick up-regulation of only the FSHR in the testes can accomplish the dual function of stimulating spermatogenesis as well as increasing steroid production via signals from both LH and FSH. In the zebrafish, FSH was 20-fold more potent at stimulating androgen production than LH, and it also up-regulated testicular mRNA levels of key steroidogenic-related genes both in vitro and in vivo (13), suggesting a role for FSH as the constitutive driving force for both steroidogenesis and spermatogenesis. In A. burtoni, however, the relative affinities of LH and FSH for their receptors, and the ability of each gonadotropin to influence steroid production and spermatogenesis, are not yet known and require future testing.
Steroid receptors
Our results show that stable dominant males have higher mRNA levels of ARα, ARβ, ERα (but not ERβa or ERβb), and all four CRs in the testes compared with stable subordinate males. These results differ from a previous study in A. burtoni that showed no difference in mRNA levels of any ARs or ERs in the testes of stable subordinate compared with stable dominant males (28). This discrepancy is likely due to the fact that we corrected the mRNA levels to account for the differences in cellular composition and size of the testes among social states (36), a method that was not used in the previous study.
Levels of the two AR subtypes, ARα and ARβ, showed similar temporal patterns during ascent. AR mRNA levels were elevated above stable subordinate male levels at 6 h after ascent, which was followed by a reduction from 24–72 h and then another increase at 120 h to reach stable dominant male levels. In other fishes, ARs are expressed in Sertoli and Leydig cells (44), as well as in germ cells of most stages of spermatogenesis, but generally reach their highest levels in spermatocytes (42,45). However, it is also possible that the elevation at 6 h is due to diurnal changes in the expression pattern of AR mRNA because this was the only time point sampled in the afternoon rather than the morning.
In A. burtoni, mRNA levels of each ER subtype differed slightly in their temporal expression patterns during social ascent, and only ERα levels differed between stable dominant and stable subordinate males. The E2-synthesizing capacity of the vertebrate testes is well known, evidenced by the presence of the aromatase enzyme that is required for conversion of testosterone to estrogens (17), and its changes in expression levels during spermatogenesis (36,42). Dominant male A. burtoni also have higher levels of circulating E2 compared with subordinates (19), but how plasma E2 levels change during social transition is not yet known. In the sea bass Dicentrarchus labrax, ERs were expressed in all germ cell stages but were highest in spermatogonia and spermatocytes (42). In that species, estrogen synthesis and sensitivity of the testes were highest during early spermatogenesis, which is consistent with the hypothesized role of E2 in stimulating mitosis and spermatogonial renewal (42,46). E2 is important for proper development and function of the male testes, and the changes in ER expression during social transition in A. burtoni are likely essential for the rapid testicular growth that occurs during this time.
In A. burtoni, mRNA levels of all four CR subtypes were elevated in the testis by 30 min and then showed highest expression levels at 120 h after ascent. Circulating levels of cortisol in male A. burtoni of different social states are variable and may be highly dependent on many parameters such as tank density, stability of the social environment, and recent social interactions (19,47,48). The majority of studies in teleosts indicate that cortisol inhibits male reproductive physiology by acting at all levels of the BPG axis, including the testes (20). In another teleost with two male reproductive morphs, the plainfin midshipman Porichthys notatus, mRNA levels of GR and MR in the testes did not differ between territorial nesting type I and sneaker type II males (49). However, the studies are not directly comparable because the midshipman study did not adjust mRNA levels for the large differences in testicular size and cell composition among morphs, as done here for A. burtoni. Nevertheless, the changes in CR mRNA levels during social ascent suggest that the sensitivity of the testes to stress hormones also varies and may contribute to the regulation of spermatogenesis and steroid production.
Nearly every testicular mRNA measure in A. burtoni showed their highest expression levels at 120 h after social ascent, before settling at the stable dominant levels. This may be due to an overcompensation by the reproductive axis upon removal of the suppressive effects. Because the default pathway for male A. burtoni is the dominant state, the reproductive axis of subordinate individuals is suppressed by both social signals and negative feedback from androgens (50). The higher expression levels at 120 h after social ascent may therefore be due to an additive effect from both removal of the suppression and a coincident increase in mRNA levels from new testicular growth.
GSI and testes cellular composition
GSI was first elevated above stable subordinate male levels at 120 h after ascent in A. burtoni, but earlier changes in cellular composition of the testes occurred before this GSI increase. GSI is a common metric used to estimate reproductive investment in fishes, but in males, it does not provide information on more subtle cellular changes that can have important functional consequences for sperm and steroid production (51). For example, in the ascending A. burtoni males, the percentages of type B spermatogonia and spermatocytes were elevated above stable subordinate levels by 72 h after ascent, a time point that is 2 d before the GSI increase and is also associated with elevated serum 11-ketotestosterone levels in these same animals (31). Importantly, although GSI levels are lower in the stable subordinate males, they still contained cysts of every spermatogenic cell type, indicating that spermatogenesis was still taking place. This finding was also recently confirmed by bromodeoxyuridine injections that showed similar active cell proliferation in stable subordinate, ascending, and stable dominant male testes (J.M. Kustan, J.M., K.P. Maruska, and R.D. Fernald, unpublished data). In addition, depletion of gonadotropins in mammals results in increased proapoptotic signals such that one role of FSH and/or LH may be to maintain spermatogenic homeostasis by inhibiting cell-death signals in germ cells (52). The low levels of gonadotropins and their receptors in stable subordinate male A. burtoni may therefore promote apoptotic pathways in the testis during social suppression, a condition that is then quickly reversed by the rapid plasma increase in FSH and LH upon social ascent, but this hypothesis requires testing.
One significant finding of our study was that the percentage of mature sperm in the testes did not differ with social status or during ascent, and there was no correlation between mature sperm and GSI. This suggests that suppressed, but previously dominant, males can retain mature sperm in their testes during the social suppression period in anticipation of a future chance to ascend in status. This adaptation ensures that a newly ascended male can quickly spawn with females to help secure territorial status and improve reproductive success, without having to wait the approximately 11–12 d required to make sperm. We also know that these suppressed males can successfully spawn with gravid females within a few hours of social ascent, a latency equivalent to that of stable dominant males with large testes (J.M. Kustan, J.M., K.P. Maruska, and R.D. Fernald, unpublished data). Even though these suppressed males have viable sperm, the density of spermatozoa within their testicular lumens is lower than that in stable dominant males, which suggests they may release some sperm during sneak fertilizations [a tactic that has been observed in laboratory tanks (our unpublished data)], or that sperm is broken down over time without being replenished in the volume seen in stable dominant male testes. A previous study in A. burtoni also showed that group-reared subordinate males that had never been dominant had lower percentages of sperm in their testes compared with dominant males (29). This suggests that the phenotype of these males that have never been dominant may be very different from that of suppressed subordinate males that have previously held a territory and spawned with females.
Summary of reproductive physiological changes during social ascent in A. burtoni
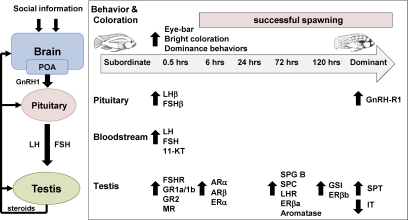
How and how quickly does the perception of social opportunity influence the reproductive axis of ascending A. burtoni males? The transformation from subordinate to dominant status occurs along a continuum, and changes at different levels occur on different time scales (e.g. behavioral, physiological, morphological). Figure 8 summarizes the data from this and the companion manuscript to show the physiological changes that occur in the pituitary and testes as a suppressed subordinate male ascends in status to become a dominant reproductively active male. Several important points are evident from these data. First, socially suppressed subordinate males are not reproductively incompetent, but rather, maintain some level of activity at every level of their reproductive axis, from the brain down to the testes. This is important because it gives males a physiological substrate on which to act, and allows for rapid changes in reproductive ability in a dynamic environment where territory tenure typically lasts only a few weeks (53). This reactivation of the BPG axis upon social ascent (e.g. after initial sexual maturity) is similar to the initiation of puberty in mammals, as well as the reoccurrence of puberty observed in seasonally breeding animals (41). Second, there is evidence for rapid (i.e. within 30 min) socially induced up-regulation of reproductive capability at every level of the reproductive axis. These rapid changes in mRNA levels suggest that both transcriptional and posttranscriptional mechanisms such as changes in mRNA stability, or small RNA-mediated regulation (e.g. microRNAs) might play a role during social ascent. Third, our data on rapid changes at the testes level raise the question: are these cellular and molecular changes mediated via the linear BPG axis or are there other pathways? The quick changes in mRNA levels in the testes raise the possibility that there may be additional and parallel signaling pathways that bypass the linear GnRH1-LH/FSH-gonadotropin receptor activation scheme, but future studies are needed to test this hypothesis. Collectively, these data highlight the fact that social ascent in male A. burtoni is a reactivation of the already functional BPG axis, and examination of the underlying regulatory mechanisms of this transition could improve our understanding of the processes that control puberty in other vertebrates, including humans.
Figure 8.
Temporal summary of physiological changes along the reproductive axis during social ascent in male A. burtoni. Arrows indicate the time point at which the first significant increase (up arrows) or decrease (down arrows) from stable subordinate male values was observed. Note that any further significant differences after this initial change in each measure are not shown. These data were compiled from the present study, the companion manuscript, and Ref. 31. Schematic simplified diagram of the BPG axis is shown at left. 11-KT, 11-ketotestosterone; IT, interstitial tissue; SPC, spermatocytes; SPG B, type B spermatogonia; SPT, spermatids.
Acknowledgments
We thank Pauline Chu (Stanford School of Comparative Medicine) for histological processing; we also thank the reviewers for their insightful comments that improved the manuscript.
Footnotes
This research was funded by National Institutes of Health Grants F32NS061431 (to K.P.M.) and NS 034950 (to R.D.F.).
Disclosure summary: The authors have nothing to disclose.
Abbreviations: AR, Androgen receptor; BPG, brain-pituitary-gonad; CR, corticosteroid receptor; E2, estradiol; ER, estrogen receptor; FSHR, FSH receptor; GR, glucocorticoid receptor; GSI, gonadosomatic index; KW, Kruskal-Wallis; LHR, LH receptor; MR, mineralocorticoid receptor; qRT-PCR, quantitative RT-PCR; RACE, rapid amplification of cDNA ends.
First Published Online November 17, 2010
References
- Sapolsky RM 2005 The influence of social hierarchy on primate health. Science 308:648–652 [DOI] [PubMed] [Google Scholar]
- Creel SN, Wildt DE, Monfort SL 2002 Behavioral and endocrine mechanisms of reproductive suppression in Serengeti dwarf mongooses. Anim Behav 43:231–245 [Google Scholar]
- Ellis L 1995 Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol Sociobiol 16:257–333 [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE 2009 Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim Behav 77:873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald RD 2009 Social regulation of reproduction: what changes and why? Hormones Brain Behav 1:683–691 [Google Scholar]
- Kruczek M, Styrna J 2009 Semen quantity and quality correlate with bank vole males’ social status. Behav Processes 82:279–285 [DOI] [PubMed] [Google Scholar]
- Pizzari T, Cornwallis CK, Froman DP 2007 Social competitiveness associated with rapid fluctuations in sperm quality in male fowl. Proc R Soc B 274:853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin J 2009 Social determination of sex in reef fishes. Semin Cell Dev Biol 20:264–270 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Nakamura M, Sunobe T, Usami T, Kobayashi T, Manabe H, Paul-Prasanth B, Suzuki N, Nagahama Y 2009 Sex change in the Gobiid fish is mediated through rapid switching of gonadotropin receptors from ovarian to testicular portion or vice versa. Endocrinology 150:1503–1511 [DOI] [PubMed] [Google Scholar]
- Gether U 2000 Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev 21:90–113 [DOI] [PubMed] [Google Scholar]
- Garcia-López A, Bogerd J, Granneman J, van Dijk W, Trant JM, Taranger GL, Schulz RW 2009 Leydig cells express follicle-stimulating hormone receptors in African catfish. Endocrinology 150:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levavi-Sivan B, Bogerd J, Mananos EL, Gomez A, Lareyre JJ 2010 Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol 165:412–437 [DOI] [PubMed] [Google Scholar]
- Garcia-López A, de Jonge H, Nóbrega RH, de Waal PP, van Dijk W, Hemrika W, Taranger GL, Bogerd J, Schulz RW 2010 Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins. Endocrinology 151:2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T, Yamauchi K, Takahashi H, Nagahama Y 1991 Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica). Proc Natl Acad Sci USA 88:5774–5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz RW, de Franca LR, Lareyre JJ, Legac F, Chiarini-Garcia H, Nobrega RH, Miura T 2010 Spermatogenesis in fish. Gen Comp Endocrinol [Erratum (2010) 167:179]165:390–411 [DOI] [PubMed] [Google Scholar]
- Walker WH 2009 Molecular mechanisms of testosterone action in spermatogenesis. Steroids 74:602–607 [DOI] [PubMed] [Google Scholar]
- Shaha C 2008 Estrogens and spermatogenesis. Adv Exp Med Biol 636:42–64 [DOI] [PubMed] [Google Scholar]
- Maruska KP, Korzan WJ, Mensinger AF 2009 Individual, temporal, and population-level variations in circulating 11-ketotestosterone and 17β-estradiol concentrations in the oyster toadfish Opsanus tau. Comp Biochem Physiol A Mol Integr Physiol 152:569–578 [DOI] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD 2010 Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. BMC Neurosci 11:58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milla S, Wang N, Mandiki SN, Kestemont P 2009 Corticosteroids: Friends or foes of teleost fish reproduction? Comp Biochem Physiol A Mol Integr Physiol 153:242–251 [DOI] [PubMed] [Google Scholar]
- Fernald RD, Hirata NR 1977 Field study of Haplochromis burtoni: quantitative behavioral observations. Anim Behav 25:964–975 [Google Scholar]
- Davis MR, Fernald RD 1990 Social control of neuronal soma size. J Neurobiol 21:1180–1188 [DOI] [PubMed] [Google Scholar]
- Francis RC, Soma K, Fernald RD 1993 Social regulation of the brain-pituitary-gonadal axis. Proc Natl Acad Sci USA 90:7794–7798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SA, Nguyen T, Fernald RD 2002 Social regulation of gonadotropin-releasing hormone. J Exp Biol 205:2567–2581 [DOI] [PubMed] [Google Scholar]
- Au TM, Greenwood AK, Fernald RD 2006 Differential social regulation of two pituitary gonadotropin-releasing hormone receptors. Behav Brain Res 170:342–346 [DOI] [PubMed] [Google Scholar]
- Maruska KP, Levavi-Sivan B, Biran J, Fernald RD 2011 Plasticity of the reproductive axis caused by social status change in an African cichlid fish: I. pituitary gonadotropins. Endocrinology 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh VN, Clement TS, Fernald RD 2006 Androgen level and male social status in the African cichlid, Astatotilapia burtoni. Behav Brain Res 166:291–295 [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Kailasanath V, Fernald RD 2007 Social dominance regulates androgen and estrogen receptor gene expression. Horm Behav 51:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley NB, Fernald RD 1982 Social control of developmental rate in the African cichlid fish, Haplochromis burtoni. Z Tierpsychol 60:66–82 [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD 2005 Rapid Behavioral and Genomic Responses to Social Opportunity. PLoS Biol 3:e363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD 2010 Behavioral and physiological plasticity: Rapid changes during social ascent in an African cichlid fish. Horm Behav 58:230–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S 2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Butler PC, White RB, DeMarco U, Pearce D, Fernald RD 2003 Multiple corticosteroid receptors in a teleost fish: distinct sequences, expression patterns, and transcriptional activities. Endocrinology 144:4226–4236 [DOI] [PubMed] [Google Scholar]
- Zhao S, Fernald RD 2005 Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol 12:1047–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD 2010 Reproductive status regulates expression of sex steroid and GnRH receptors in the olfactory bulb. Behav Brain Res 213:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe M, Nakamura I, Evans J, Swanson P, Young G 2006 Changes in mRNAs encoding steroidogenic acute regulatory protein, steroidogenic enzymes and receptors for gonadotropins during spermatogenesis in rainbow trout testes. J Endocrinol 189:541–554 [DOI] [PubMed] [Google Scholar]
- Maugars G, Schmitz M 2008 Expression of gonadotropin and gonadotropin receptor genes during early sexual maturation in male Atlantic salmon parr. Mol Reprod Dev 75:403–413 [DOI] [PubMed] [Google Scholar]
- Kohler KE, Gill SM 2006 Coral point count with Excel extensions (CPC3): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269 [Google Scholar]
- Schulz RW, Menting S, Bogerd J, Françca LR, Vilela DA, Godinho HP 2005 Sertoli cell proliferation in the adult testis–evidence from two fish species belonging to different orders. Biol Reprod 73:891–898 [DOI] [PubMed] [Google Scholar]
- Hyder M 1969 Gonadal development and reproductive activity of the cichlid fish Tilapia leucosticta (Trewavas) in an equatorial lake. Nature 224:1112 [DOI] [PubMed] [Google Scholar]
- Ebling FJ 2005 The neuroendocrine timing of puberty. Reproduction 129:675–683 [DOI] [PubMed] [Google Scholar]
- Viñas J, Piferrer F 2008 Stage-specific gene expression during fish spermatogenesis as determined by laser-capture microdissection and quantitative-PCR in sea bass (Dicentrarchus labrax) gonads. Biol Reprod 79:738–747 [DOI] [PubMed] [Google Scholar]
- Yaron Z, Gur G, Melamed P, Rosenfeld H, Elizur A, Levavi-Sivan B 2003 Regulation of fish gonadotropins. Int Rev Cytol 225:131–185 [DOI] [PubMed] [Google Scholar]
- Ikeuchi T, Todo T, Kobayashi T, Nagahama Y 2001 Two subtypes of androgen and progestogen receptors in fish testes. Comp Biochem Physiol B Biochem Mol Biol 129:449–455 [DOI] [PubMed] [Google Scholar]
- Takeo J, Yamashita S 2001 Immunohistochemical localization of rainbow trout androgen receptors in the testis. Fish Sci 67:518–523 [Google Scholar]
- Miura T, Miura C 2001 Japanese eel: a model for analysis of spermatogenesis. Zool Sci 18:1055–1063 [Google Scholar]
- Fox HE, White SA, Kao MH, Fernald RD 1997 Stress and dominance in a social fish. J Neurosci 17:6463–6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Fernald RD, Hofmann HA 2008 Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc Biol Sci 275:2393–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arterbery AS, Deitcher DL, Bass AH 2010 Corticosteroid receptor expression in a teleost fish that displays alternative male reproductive tactics. Gen Comp Endocrinol 165:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Francis RC, Wingfield JC, Fernald RD 1996 Androgen regulation of hypothalamic neurons containing gonadotropin-releasing hormone in a cichlid fish: integration with social cues. Horm Behav 30:216–226 [DOI] [PubMed] [Google Scholar]
- Maruska KP, Cowie EG, Tricas TC 1996 Periodic gonadal activity and protracted mating in elasmobranch fishes. J Exp Zool 276:219–232 [Google Scholar]
- Pareek TK, Joshi AR, Sanyal A, Dighe RR 2007 Insights into male germ cell apoptosis due to depletion of gonadotropins caused by GnRH antagonists. Apoptosis 12:1085–1100 [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Benson ME, Fernald RD 1999 Social status regulates growth rate: consequences for life-history strategies. Proc Natl Acad Sci USA 96:14171–14176 [DOI] [PMC free article] [PubMed] [Google Scholar]