Abstract
The engraftment of hematopoietic stem cells (HSCs) after drug resistance gene transfer and drug selection may recapitulate stress response hematopoiesis, but the processes remain elusive. Homing, trafficking, and localization of transduced cells and the impact of insertion site on focal expansion have not been well characterized. With the goal of optimizing and understanding these processes under conditions of low multiplicity of infection (MOI) lentiviral gene transfer, we used drug resistance gene O6-methylguanine-DNA methyltransferase (MGMT)-P140K and in vivo selection to enrich for transduced and transgene-expressing HSCs. To systemically monitor homing, trafficking, and expansion after transplantation and drug selection over time, we linked MGMT-P140K to the firefly luciferase gene in lentiviral self-inactivating vectors. Periodic bioluminescence imaging (BLI) of transplanted recipients was followed for up to 9 months after both primary and secondary transplantation. Initial dispersion and widespread early homing and engraftment were transient, followed by detection of persistent and discrete foci at stable tissue sites after in vivo drug selection. From these studies, we concluded that drug resistance gene transfer followed by early or late drug selection can result in stable gene expression and cell expansion in persistent foci of transduced bone marrow cells that often remain in fixed sites for extended periods of time.
Introduction
Lentiviral vectors are a preferred approach to introduce therapeutic genes into hematopoietic stem cells (HSC) because they transduce quiescent adult stem cells and have reduced risk for insertional mutagenesis.1,2,3 Still, with these systems, little is known about engraftment and repopulation dynamics of lentiviral transduced bone marrow cells, particularly long-lived stem cells. This is compounded by the observation of relatively low rates of transduced stem cells identified in vivo.4 Recently, lentiviral vector systems have been implemented in clinical trials,5,6 but a lot of studies typically used a relatively high multiplicity of infection (MOI) to raise clinical efficacy, and gene expression which increases the theoretical risk for insertional mutagenesis. To overcome the potential risks of insertion, which include gene disruption as well as insertional mutagenesis, conditions promoting low MOI lentiviral transduction would be safer for gene transfer, particularly for HSCs. Low MOI transduction, however, also reduces the efficiency of gene expression in the HSC compartment. For this reason, gene transfer that enables in vivo drug selection has been developed to enrich for an in vivo population of gene-modified hematopoietic cells.
In vivo selection is an effective strategy for enriching and expanding transgene-expressing stem cells and progenitor cells.7,8,9,10 The O6-methylguanine-DNA-methyltransferase O6-methylguanine-DNA methyltransferase (MGMT)-P140K, contains a proline-to-lysine mutation that renders the protein resistant to the clinical inhibitor, O6-benzylguanine (BG). MGMT is a major factor in resistance to alkylating agents, such as 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) or temozolomide (TMZ). As predicted, BG inactivates cellular MGMT and increases alkylating agent toxicity resulting in marrow suppression, which has limited its clinical use in anticancer treatments. Conversely, MGMT-P140K transduced bone marrow cells are much more resistant to BG and alkylating agent combinations. We and others have shown that MGMT-P140K expression in transduced human CD34+ cells protects them from BG plus BCNU treatment in vitro and in vivo and in nonmyeloablative autologous and allogeneic HSC transplantation settings in mice, dogs, and primates, results in significant levels of selection and enrichment.9,11,12,13,14 Despite the observation that these transduced cells engraft and expand after drug selection, knowledge of these processes is fairly rudimentary. Studies of HSC engraftment and in vivo selection have commonly required frequent collections of peripheral blood and sacrifice of the research animals to collect hematopoietic tissues. This precludes analysis of the time-dependent process of drug selection, which includes the periods of repeated drug treatment, stem cell expansion, repopulation, and engraftment.
To assess these processes, we have developed a lentiviral vector containing the luciferase gene to monitor engraftment after transduced HSCs were transplanted. We coupled the luciferase gene to the MGMT-P140K drug resistance gene so that cells transduced at low MOI could be monitored by bioluminescence imaging (BLI) and enriched by drug treatment in vivo. Monitoring these processes with in vivo imaging provided new data on reconstitution dynamics, stem and progenitor cell expansion and transgene expression under selection pressure.
Of the various imaging technologies available, including magnetic resonance imaging, positron emission tomography, x-ray computed tomography, and fluorescence imaging, etc.15,16 the most cost-effective monitoring over extended periods of time for small animal studies is BLI.17,18,19 Similar to fluorescence imaging, BLI is noninvasive and can be used repeatedly for long-term monitoring in vivo. However, compared to GFP imaging, BLI has much greater sensitivity due to low background signal, and more importantly, better tissue penetration because the emitted wavelength extends beyond 600 nm.20 BLI has been used to track purified human HSC and progenitor cells (CD34+ and CD34+CD38−) and mononuclear cells homing and engraftment.21,22 BLI has also been used to track regulatory T-cell trafficking and survival after allogeneic transplantation.23 However, BLI of reconstituting lentiviral transduced HSCs after in vivo drug selection has not been done. We hypothesized that low MOI lentiviral transduction of hematopoietic cells would result in long-term engraftment after MGMT-P140K-mediated selection, and that BLI would be an effective method to monitor this process and uncover physiologic processes of stem cell expansion and migration after drug selection over time, and that it would provide data very different from the “mean effect” analysis of gene transfer in peripheral blood cells.
Results
LV-mnd-P2AL transduction resulted in robust MGMT-P140K expression and provided protection against BG+BCNU treatment in vitro
To transfer the MGMT-P140K and luciferase minigenes into murine hematopoietic cells, we generated a bicistronic lentiviral vector with an MND promoter from myeloproliferative sarcoma virus, the MGMT-P140K gene placed proximal and the firefly luciferase gene placed distally to the 2A sequence in plasmid vector lentiviruses (PLV)-mnd-P2AL (Figure 1a). The titers of LVs of various preparations ranged from 1.97 × 105 to 2.04 × 106 infectious particles/ml, and they were used without further concentration. To confirm the consistency of transgene expression, we transduced 293T and K562 cells at MOI of 1. Every 2 weeks, we analyzed cells for transgene expression by flow cytometry. Over a 21-week period, MGMT-P140K positive cells remained at an average of 95 ± 4% and 69 ± 8% for 293T and K562, respectively (Figure 1b). Stable transgene expression was also observed in murine NIH-3T3 cells, at 90% under the same MOI condition (data not shown).
Figure 1.
Lentiviral vector construct and transgene expression in cell lines. (a) The construct of bicistronic lentiviral vector plasmid (pLV-mnd-P2AL) contained MGMT-P140K gene and firefly luciferase gene linked by foot and mouth disease virus (FMDV) 2A cleavage site and was controlled by an MND promoter. (b) In vitro transduction experiments by transducing 293T and K562 cells with lentiviruses (LV-mnd-P2AL) made with above bicistronic lentiviral vector plasmid at multiplicity of infection (MOI) of 1 showed robust MGMT-P140K expression over 21 weeks. (c) C3H murine bone marrow cells were transduced with LV-mnd-P2AL and treated with O6-benzylguanine (BG) and various concentrations of 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), and analysis of surviving colonies in colony-forming unit (CFU) showed strong protection in lentiviral transduced primary bone marrow cells against BCNU treatment. BM, bone marrow; MGMT, O6-methylguanine-DNA methyltransferase.
To test whether the expressed MGMT-P140K provided drug protection, we transduced primary bone marrow cells from 10-week-old C3H/HeNCrlBR mice with LV-mnd-P2AL at MOI of 1. After 48 hours of transduction, the cells were washed free of virus and treated with 20 µmol/l BG and 0–60 µmol/l BCNU for 2 hours and plated in methylcellulose. Colony counts from the cultures showed greater survival after BCNU exposure for bone marrow cells transduced with LV-mnd-P2AL compared to untransduced bone marrow cells (Figure 1c). PCR analysis for the MGMT transgene from colony-forming unit (CFU) progenitor colonies of initial transduction and from CFU progenitor colonies receiving BG and 0 µmol/l BCNU showed 50% transgene positivity whereas 100% colonies analyzed from cells that received 10 to 40 µmol/l BCNU were positive for the transgene (PCR data not shown).
BLI tracking of murine bone marrow cell homing and early engraftment
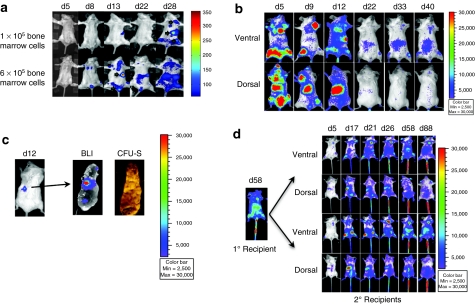
We selected white Balb/C female mice as donors and recipients for in vivo imaging because of their low photon absorption and scattering.17 We collected Balb/c bone marrow cells and transduced them with LV-mnd-P2AL at low MOI of 0.5–1. Transgene transduction was measured in CFU assay, and transgene expression was measured by flow cytometry of the whole cell cultures for MGMT-P140K before infusion. After transduction, we observed an average of 40 ± 10% transgene positivity in progenitor cell populations in CFU by PCR whereas there was only an average of 3 ± 1% transgene expressing cells in the cultured bulk bone marrow cells by flow cytometry. The difference is larger than might have been anticipated and is addressed in the Discussion section. To visualize homing, migration, and long-term engraftment of transduced stem and progenitor cells, we infused two different doses of transduced bone marrow cells (1 × 105 and 6 × 105) into groups of lethally irradiated syngeneic Balb/C mice (n = 4). Three to five days after transplantation, weak BLI signal was detected in recipients. Recipient mice that received 6 × 105 transduced cells showed a greater degree of engraftment and expansion than mice that received 1 × 105 transduced cells, suggesting a cell-dose dependent effect. Visible BLI foci first appeared at day 8 in the recipients received 6 × 105 transduced cells, and engraftment in the spleen was observed between days 12 and 14 in both groups. The BLI signal became stronger in both groups by day 28 (Figure 2a). Thereafter, the BLI signal weakened and disappeared. These initial imaging studies of engraftment were made using a laboratory prototype imaging system made by Dr David Wilson in the Department of Biomedical Engineering at Case Western Reserve University (Cleveland, OH). In the subsequent experiments, we transplanted 1 × 106 transduced cells per recipient and used the Xenogen IVIS 200 Imaging system (Caliper Life Sciences, Alameda, CA) for BLI imaging.
Figure 2.
Bone marrow cell engraftment appeared in a cell dose-dependent manner and became less active with time. (a) 1 × 105 and 6 × 105 lentivirus (LV)-mnd-P2AL transduced Balb/C bone marrow cells showed a dose-dependent engraftment pattern after transplantation. Green arrows indicated the locations of the foci. Images in this figure were taken with a Case BME prototype imaging system. Images were pseudocolored and analyzed with Matlab software. The remaining images throughout this paper were taken and analyzed with Xenogen IVIS 200 and its software. (b) 1 × 106 transduced bone marrow cells showed early rapid engraftment and expansion at day 5, but transgene expression started to fade after 3–4 weeks without any drug selection. (c) Lethally irradiated recipient received 5 × 105 transduced bone marrow cells and imaged on day 12. After BLI imaging, spleen was removed and imaged for bioluminescence, and then spleen was fixed and photographed for CFU-S. (d) Serial transplantation of lentiviral transduced bone marrow cells. At day 58 after primary transplantation, 3 × 106 bone marrow cells from primary recipient was transplanted into four secondary recipients. Identical engraftment and expansion pattern was observed in secondary recipients as seen in the primary recipients. None of the secondary recipients received any drug treatment.
In the next experiment, we transplanted 1 × 106 transduced murine bone marrow cells into each lethally irradiated recipient and observed initial engraftment as early as day 4–5 with a pattern that varied from animal to animal. The most common pattern included robust initial engraftment sites within the long bones and vertebrae. BLI signal was the strongest between days 5 and 13 but decreased with time, indicating a short burst of initial expansion from progenitor cells expressing transgene early after transplantation (Figure 2b). Thereafter, between days 8 and 14, we began to observe luciferase expression in the spleen, suggesting the formation of CFU-S spleen progenitor colonies (Figure 2c). When we counted luciferase-expressing colonies among total CFU-S colonies in animals harvested at days 12–14, 9% of the CFU-S progenitor colonies were luciferase positive by visual inspection using the BLI camera (n = 2 mice).
Although there was significant linear correlation between transduced cell numbers and BLI signal (Supplementary Figure S1a), to translate this relationship in vivo is more complex, given the attenuation of photons through different mouse tissues. Nonetheless, we were able to distinguish distinct foci in vivo with whole body BLI (Figure 2c and Supplementary Figure S1b). Under periodic observation of luciferase expression from these animals for up to 146 days, we noted that by week 4 after transplantation, most foci had faded away, and by week 8, most previously observed foci disappeared without drug selection (data not shown).
BLI monitoring of transgene expression in long-term HSCs during serial transplantation
Our results with transduction and BLI signal of CFU-S colonies indicated successful transgene expression of hematopoietic progenitor cells. However, due to low transgene expression from lentiviral transduced murine hematopoietic cells at MOI of 0.5–1 and the low BLI signal after 4–5 weeks, it was necessary to determine whether long-term engrafting cells had been transduced. To test this, we performed serial transplantation. Using the same MOI of 1 for bone marrow cells transduction, we transplanted 2 × 106 total bone marrow cells into primary recipients. In serial transplantation experiment, the primary recipient did not receive any drug treatment. After 8 weeks, we collected bone marrow cells from a primary recipient and measured transgene-expressing cells by flow cytometry. We observed only 1.9% MGMT-P140K expressing cells by flow cytometry, whereas, unexpectedly, about 72% of the marrow CFU progenitor cell colonies contained the transgene by PCR. This is further discussed in the Discussion section. We transplanted 3 × 106 bone marrow cells from the primary recipient into each of four lethally irradiated secondary recipients. Two of the mice died of transplant complications. We monitored two surviving mice over 12 weeks for luciferase expression (Figure 2d). Persistent expression of luciferase was observed in long bones, vertebrae, and spleen over 12 weeks in these secondary recipients, indicating an efficient lentiviral transduction in long-term repopulating HSCs. In addition, there was no evidence that expression of luciferase or MGMT-P140K altered the capacity for long-term engraftment.
BLI showed effective selection of transduced bone marrow cells with BG and TMZ in both lethally irradiated and nonmyeloablated recipients
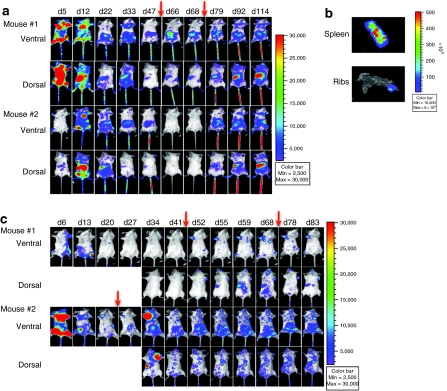
Monitoring limited numbers of stem cells for transduced gene expression after low MOI transduction can be challenging due to low level of transgene expression. In vivo selection has been shown to be an effective way to enrich for MGMT-transduced HSC. We used BLI to observe the impact of the drug selection process on hematopoietic engraftment and expansion, and the distribution of transduced cells in real time. Recipients (n = 3) received 750 cGy total body irradiation followed by infusion of 1 × 106 lentiviral transduced bone marrow cells. Two sample mice were shown in Figure 3a. After initial engraftment and early expansion at day 5, and hematopoietic-organ-oriented foci (marrow and spleen) of expansion at day 12, transgene expression decreased gradually for 7 weeks after cell infusion (Figure 3a). After transgene expression started to decrease, we administered the first 3-day injection of BG and TMZ starting on day 48 and the second 3-day drug treatment starting on day 75 after cell infusion. The first treatment had a modest effect on transgene expressing cells in mouse #1 and no effect on mouse #2 in terms of BLI intensity (Figure 3a). In mouse #1, a diffuse signal from the abdominal region increased, and there was a slight increase of BLI signal in spleen (Figure 3a). The second drug treatment led to substantial transgene expression in the spleens, spine, abdomen, and lower legs of mouse #1 and #2. This expression persisted for >2.5 months after second drug treatment, suggesting that transduction and selection of long-term repopulating cells had occurred. In addition, the foci were persistent in discrete locations and migration of these sites did not appear to take place. At week 17, spleens and ribs were removed and imaged ex vivo, the BLI signal was confirmed and was focal (Figure 3b). In both mice, the spleens appeared normal in size, and histological examination with hematoxylin and eosin stain showed no evidence of abnormal clonal expansion or disruption of the normal splenic architecture (data not shown).
Figure 3.
BG+TMZ treatment enhanced transgene expression in lethally irradiated and nonmyeloablated recipients. (a) Two lethally irradiated mice received 1 × 106 lentivirus (LV)-mnd-P2AL transduced bone marrow cells and BG+TMZ treatments for 3 days beginning on day 48 and day 75 (indicated with red arrows). Both ventral and dorsal views are shown. (b) Spleen and chest rib were collected at the termination of the experiment, placed in a 100-mm dish, and imaged to confirm bioluminescence signal in both locations. (c) In two nonmyeloablated recipients, sample mouse #1 received 5 × 105 transduced bone marrow cells and 5 × 105 untransduced supporting cells and was treated with BG+TMZ for 3 days beginning on days 44 and 69, and sample mouse #2 received 5 × 105 transduced bone marrow cells and 5 × 105 untransduced supporting cells and three rounds of BG+TMZ treatments for 3 days beginning on days 21, 44, and 69 (drug treatments indicated with red arrows). Both ventral and dorsal views are presented in the figure. BG, O6-benzylguanine; TMZ, temozolomide.
We also compared the engraftment of lentiviral transduced bone marrow cells in both lethally irradiated recipients and nonmyeloablated recipients before and after drug treatments (for treatment schedules, see Materials and Methods section) (n = 15). The imaging data showed a similar early engraftment after both methods of preconditioning (Figure 3a,c). After nonmyeloablated and lethally irradiated preconditioning of the recipients, the BLI signal subsided within 3–4 weeks and did not return throughout the remaining observation period when the recipients did not receive any drug treatment (Supplementary Figure S2). To determine whether transgene-expressing cells could be enriched and induced to expand and whether these progeny would express the MGMT transgene, we treated mouse #2 in Figure 3 with BG and TMZ for 3-day treatment starting on day 21. The transgene expression increased dramatically 2 weeks later in the bone marrow of the upper right limb and in the spleen (Figure 3c). To determine whether rescue of expressing cells could occur later after transplant, when transgene expression was not detected with BLI, we gave mouse #1 the first BG and TMZ treatment starting on day 44, at the same time that we gave mouse #2 the second BG and TMZ treatment. In mouse #1, we observed persistent foci 8 days after drug treatment. Transgene expression in mouse #2 increased slightly after the second round of drug treatment and persistent foci were easy to identify. These persistent foci were visible for an additional 10 weeks. Following a third cycle of BG and TMZ treatment at day 69, we observed further enhancement of transgene expression in the foci from the chest, vertebrae, and lower limbs of mouse #2 (Figure 3c).
The detection of luciferase expression is highly sensitive for relative small numbers of cells and can accurately present transgene expression in the whole animal. For instance, in a mouse with clearly visible BLI foci on day 32, peripheral blood analysis by flow cytometry revealed very low levels of (~2%) MGMT-P140K positive cells (Supplementary Figure S3).
Semiquantitative analysis of BLI confirmed enhanced engraftment of lentiviral transduced bone marrow cells
To semiquantify the BLI signal in the mouse, we used the Xenogen Living Image 2.5 software to select regions of interest (ROI). We selected bones and hematopoietic and lymphoid organs for further analysis. As noted from the qualitative observations, total body BLI signals were strong during the early phase of homing and engraftment. Photon emission then decreased in most mice 3–4 weeks after transplantation. However, after receiving BG and TMZ treatments, in both lethally irradiated and nonmyeloablated recipients, imaging data showed a three- to fourfold increase in BLI signal compared to untreated recipients (Figure 4a). Imaging detection in ROI of hematopoiesis, such as in marrows and spleen, indicated cell expansion manifested by a significant increase in transgene expression after drug selection that persisted for 90–115 days (Figure 4b–f). ROI analysis also showed more robust transgene expression at late time points in spleen and vertebrae of lethally irradiated recipient mice compared to nonmyeloablated mice (Figure 4e,f). This suggested that long-term engraftment and cell expansion were enhanced in lethally irradiated recipients, and that in both settings, persistent expression after drug selection was observed.
Figure 4.
Semiquantitative analysis of bioluminescence imaging (BLI) signal showed increased engraftment and transgene expression after BG+TMZ treatment. Four groups of animals are compared longitudinally for evidence of BLI positive cells based on preconditioning status. The groups are: (i) untransduced bone marrow cells in nonmyeloablated recipients (n = 2), (ii) transduced bone marrow cells (BMC) in nonmyeloablated recipients without BG+TMZ treatments (n = 1), (iii) transduced BMC in nonmyeloablated recipients given BG+TMZ treatments (n = 2), and (iv) transduced BMC in lethally irradiated recipients given BG+TMZ treatments (n = 2). Image intensity was compared over time for the total body, as well as regions and organ sites. (a) Whole body comparison, (b) comparison in bone marrows of all of the limbs, (c) chest, (d) abdomen, (e) spleen, (f) vertebral bodies. Cyan arrows (↓) in c indicate the start of the 3-day drug treatments in nonmyeloablated recipients and green arrows (↓) in d indicate the start of the 3-day drug treatments in lethally irradiated recipients. BG, O6-benzylguanine; TMZ, temozolomide.
In vivo drug selection resulted in persistent BLI foci
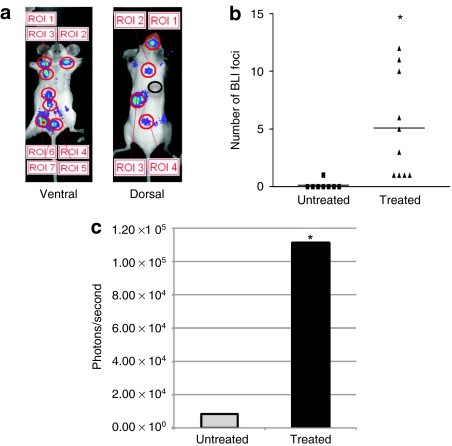
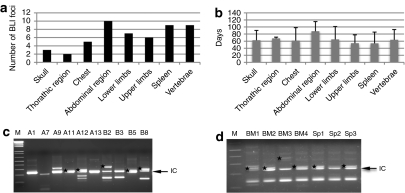
To analyze BLI foci more carefully, we increased the minimum imaging detection setting to 2,500 p/second/cm2/sr to eliminate background noise and used a pseudocolor image to identify and semiquantify the number of persistent foci. Identified persistent foci were analyzed using a fixed size ROI for their intensity (Figure 5a). Eight weeks after bone marrow transplantation, only rare foci were noted. However, after two or three rounds of BG and TMZ treatment in either irradiated or nonmyeloablated recipients, there was sharp increase in the numbers and signal intensity of persistent foci (Figure 5b,c) (treated mice: n = 10; untreated mice: n = 8). Locations of those persistent foci were estimated based on BLI images, and the most common sites were spleen (9 out of 10 animals), vertebrae (5 out of 10), abdominal regions (4 out of 10), skull (3 out of 10), thoracic regions (2 out of 10), chest (5 out of 10), and both upper and lower limbs (6 out of 10). The organs containing the most foci were in the abdominal region (most likely mesentery), spleen, and vertebrae (Figure 6a). Interestingly, many of those sites had appeared in BLI during early engraftment. The duration of transgene expression among those persistent foci varied across sites and animals. In many instances, these foci persisted in intensity and location over extended periods of time (Figure 6b). Most notable, some of those BLI foci appeared then disappeared after transplantation, and only reappeared at the same locations after drug selection.
Figure 5.
Persistent bioluminescence imaging (BLI) foci resulted from in vivo drug selection. Predefined “regions of interest” (ROI) analysis was used to measure persistent BLI foci from both ventral and dorsal sides of recipient mice. Measurements from 18 recipients of both lethally and nonmyeloablated preconditioning were pooled together to measure persistent foci, stratified by drug treatment. (a) An example of persistent foci selected with predefined ROI. The black circle in the dorsal image is an example of background used in the comparative measurement of foci intensity. (b) The number of persistent foci identified averaged 0.125 per mouse in untreated mice compared to 5.1 per mouse in treated recipients, P < 0.01. (c) The mean ± SD of BLI intensity were 8,389.43 ± 6,353.17 photons/second (data range: 5,826.11–24,027.67) in untreated mice and 11,1019.15 ± 18,7641.07 photons/second (~13-fold increase, data range: 24,033.33–10,45000) in chemotherapy treated mice, P < 0.01.
Figure 6.
Distribution of persistent foci in recipient animals and insertion analysis of colony-forming unit (CFU) colonies of transduced bone marrow cells in vitro and after in vivo drug selection. Detailed analysis of persistent bioluminescence imaging (BLI) foci was performed from 10 recipients after drug treatment. (a) Tissue localization of persistent foci was enumerated based on 2D BLI images. (b) The duration of visual detection of these persistent tissue foci (n = 51 foci analyzed). (c) Linear amplification-mediated PCR (LAM-PCR) of transgene positive CFU colonies (n = 19) collected from LV-mnd-P2AL transduced bone marrow cells before transplantation (without prior drug treatment). (d) LAM-PCR of transgene positive CFU colonies collected from bone marrow (lanes BM1–BM4) and spleen (lanes Sp1–Sp3) of one of the recipient mice after in vivo selection (BM: n = 4 and spleen: n = 10). Black arrows in c and d and labeled IC indicate the band identified by amplification of the proviral internal control sequence that simply indicates presence of the provirus. The amplicons sequenced and listed in Table 1 are marked with black stars in c and d. (M: 1 kb plus ladder)
We also analyzed the integration sites of CFU progenitor colonies from transduced cells before transplantation and from recipients after in vivo drug selection by linear amplification-mediated PCR (LAM-PCR) and direct sequencing to better understand those BLI foci. Initial analysis of CFU from lentiviral transduced cells before transplantation showed a variety of clones with a low number of insertion sites (1–3 insertions per colony) (Figure 6c). After in vivo selection, CFU progenitor colonies from both bone marrow and spleen obtained from mouse #2 in Figure 3a had a similar pattern of integration by LAM-PCR (Figure 6d), indicating that a small number of transduced hematopoietic progenitors contributed to both bone marrow and spleen after drug treatment. Further sequencing was performed directly on excised DNA bands from LAM-PCR, and BLAT search showed identical insertion sites from different CFU progenitor colonies recovered from both bone marrow and spleen of the same animal, indicating progenies of a common progenitor in both bone marrow and spleen (Table 1).
Table 1. Insertion site analysis of LAM-PCR from CFU colonies of hematopoietic stem and progenitor cells before transplantation and after in vivo selection.
Discussion
This study was performed to assess long-term patterns of engraftment and expansion of lentiviral transduced murine hematopoietic cells after in vivo selection. BLI allowed observation of low-level early engraftment of genetically modified stem and progenitor cells before in vivo selection as well as sequential drug selection-mediated enrichment of transduced cells over time. The most important contribution to our knowledge of the engraftment of genetically altered stem cells is the observation of patterns and locations of transgene expression by long-term engrafting cells and the discovery of discrete persistent foci at various locations in the recipients after in vivo selection.
After lentiviral gene transfer of murine hematopoietic cells, we observed a discrepancy between the transduction efficiency in progenitor cell population by CFU assay and transgene expression in bulk whole bone marrow cells. This is similar to the results in our earlier retroviral study in primary murine cells.24 It has also been observed in lentiviral transduction of murine Sca+Kit+Lin− cells and a foamy viral transduction study.25,26 In these lentiviral and retroviral studies, the differences between transduction and gene expression were likely in part due to transduction mosaicism and heterogeneous gene expression in CFU colonies.26 Another possible reason for the discrepancy, as suggested in a study of foamy virus transduction and selection, rests in the different cell populations that were analyzed. CFU colonies include more rapidly dividing progenitor cells whereas the majority of bulk cells in whole bone marrow were nondividing hematopoietic cells. Lentiviral transduction rates in these two populations were different. These differences may have been exacerbated by our low MOI transduction conditions. It is of interest that the drug selection allowed selection under these conditions, because the nonexpressing transduced cells were selected against, in favor of MGMT-expressing progenitors. To determine whether MGMT expression studies by flow underestimated the proportion of cells expressing the transgene, we inserted GFP alone with MGMT-P140K into the same lentiviral backbone, conducted transduction studies in 293T and K562 cell lines and murine primary bone marrow cells, and observed similar expression pattern (Supplementary Figure S4). Due to the low MOI lentiviral transduction, we suspect that a large portion of transduced progenitor cells did not express the transgene in primary murine hematopoietic cells in vivo. Because none of the PCR showed evidence of a rearranged transgene, the next most likely explanation is lentiviral gene silencing, although we have no direct evidence.
Although other BLI studies have tracked purified human CD34 cells and HSCs transplanted from luciferase transgenic mice,27,28 our imaging study provides visual evidence of the dynamic complexity of engraftment, transgene expression, stem cell persistence, and in vivo selection of low MOI lentiviral transduced hematopoietic bone marrow cells. The emergence late after transplantation of gene expressing clones after in vivo selection has not been previously observed. During the early engraftment process before drug treatment, even though transplanted cells home to bone marrow and splenic spaces within 24 hours, detectable bioluminescence signal only appears 5 days later, which is consistent with the proliferation of clusters of stem, progenitor, and mature cells for a short period of time post-transplantation.29,30,31 Yoshimoto et al. used GFP+ HSC transplantation to study niche sites occupied by stem cells soon after transplantation in bone marrow.32 They were able to detect individual cells resident within the endosteal niche by fluorescent stereomicroscope in ribs and vertebrae and noted that these stem cells proliferate in a “stepping-stone”-like manner. The shortcoming of these observations include that they are not able to track these niche occupying cells weeks to months later. We have also observed engraftment foci in ribs and vertebrae and cell expansion by increased transgene expression with selection over extended periods of time, indicating that these long-term engrafting cells become resident at these niche sites for extended periods of time. Unlike previous studies, however, we were able to observe the sequential processes after transplantation of HSC homing, CFU-S formation, and conversion to an unobserved quiescent state resulting in undetectable transgene expression 3–4 weeks after transplantation. Recrudescence of expression took place after drug selection, and transgene expression is more likely due to cell expansion because drug treatment does not have direct effect on gene expression. Although we have no way of identifying these cells months later without selection, the appearance of gene-expressing cells after selection suggests that they are also present in untreated mice.
The engraftment and expansion of transduced hematopoietic bone marrow cells after in vivo selection was highly influenced by drug treatment. The observation that, after in vivo selection, these visual foci appeared and were stable for over 6 months has not been previously reported. Although this pattern of engraftment after selection was specific for MGMT-P140K transduced repopulating cells augmented by the drug selection procedure, it is likely that the patterns of engraftment, expansion, and persistent proliferation may be common to all dormant repopulating stem cells called stochastically over time into proliferation due to inflammation, cytopenia, or other stress. Of course, some clusters may be macrophage, lymphoid, or dendritic cells that persist in marrow, splenic, hepatic, or lymphoid environments. However, those clusters seem to contain stem and progenitor cells able to expand upon drug selection pressure and to undergo secondary transplantation and engraftment. These highly generalized observations represent some of the first to assess the dynamic of foci of repopulating hematopoietic cells, some or many of which may be clonal, over extended periods of time. Other types of stress—such as lipopolysaccharides, other chemotherapy, infection, growth factors, or chemokines may be expected to also result in recruitment of small foci of tissue resident stem cells, it would be difficult to identify without a visualization strategy.
The sites of persistent foci of transduced stem cells appeared to be randomly distributed among various marrow sites and the spleen. Because expansion of the observed foci occurred almost exclusively after drug selection, drug cytotoxicity followed by hematopoietic expansion appears to be the predominant instigator of clonal expansion. In any stem cell transduction and transplant study, there needs to be an assessment of clonal expansion due to insertional mutagenesis. In these studies, there was no evidence that the lentiviral transduction led to clonal expansion in the absence of drug selection. Furthermore, no alteration in hematopoiesis in terms of either splenic morphology, blood counts, or marrow cellularity was noted in any animal. This was consistent with other studies of MGMT-P140K-mediated drug selection that have documented expansion of the MGMT-P140K-expressing cells but not overexpansion of individual clones.13,33,34 Thus, MGMT-P140K remains the most effective method of protecting human CD34+, murine, and canine HSCs against chemotherapeutic drugs in vivo, resulting in multi-log enrichment and selection over time.11,35,36
The sensitivity of BLI was astonishing throughout our studies. In many cohorts, there was a very low level of MGMT-P140K transgene-expressing cells in peripheral blood samples and bone marrows that were detectable by flow cytometry. However, BLI signals were clearly visible within the animals, providing clues to possible stem cell niches, including bone marrow spaces of the extremities, vertebral bodies, ribs, and even lymph nodes, as found in other studies.37,38 In addition, BLI imaging showed that in vivo selection resulted in cell proliferation and increased transgene expression with persistent focal expansion of engrafted cells. To further explore these sites, we will use the recently developed Case Cryoimaging system that performs automated cryosections with block-face imaging and reconstitution of images of the entire mouse.39 In initial studies, we were able to analyze a limited number of transgene positive samples collected from the abdominal region. These sites contained transgene positive cells originating from progenitors with only two to three different insertions. Of note, these insertions were not the same as those recovered from the peripheral blood cells of the same animal. Although these results are very preliminary, the foci could represent a composite of HSCs that were quiescent and only started cycling after transplantation and drug selection, but were not contributing sufficient cells to be identified in the blood, which may explain the low transgene expression in peripheral blood samples. A more complete cryoimaging sample collection from MGMT-P140K–GFP-Luc transduced and drug-selected mice is underway to identify the cells at specific foci.
These studies identify a pattern of late emergence of hematopoietic progenitor and stem cells at various marrow and splenic sites in a sub-population of transduced and drug-selected HSC-derived cells in the mouse. It would seem likely that this pattern of focal engraftment and expansion particularly the pattern that emerges after drug selection would also be present in HSC gene therapy clinical trials. Furthermore, this pattern suggests an inhomogeneity in the HSC engraftment and expansion process after gene transduction. Since blood sampling may not reflect this pattern, other approaches to assess the full scale of engraftment of cells that remain tissue resident foci are needed.
Materials and Methods
Vector and LV production. The luciferase lentiviral plasmid, pCSO-rre-cppt-MCU3-LUC, was kindly provided by Dr Donald B. Kohn (Children's Hospital Los Angeles, Los Angeles, CA), containing firefly luciferase gene under the control of MND promoter, which increases transgene expression in hematopoietic cells, in a self-inactivating lentiviral backbone.22,40 MGMT-P140K gene was excised from pLV-mnd-P140K plasmid with ClaI and MluI. 2A sequence from foot and mouth disease virus was used as a linker for MGMT-P140K and luciferase genes because it offers 1:1 of expression.41 2A sequence was inserted between MGMT-P140K and luciferase by designing overlapping MGMT-2A primer (5′-CTGCTGGCCGAAA CGCACCGGTGAAACAGCTTTTGAGCTTTGACCTGCTCAAGTTGGCAGG-3′) and 2A-luc primer (5′-TCAAGTTGGCA GGGGACGTCGAGTCCAACCCTGGGCCTATGGAAGACGCCAAAAACATAA-3′). MluI and ScaI sites were used to digest the MGMT-2A-luc PCR fragment, and then the fragment was ligated to P140K fragment cut out from pLV-mnd-P140K. Then MGMT-P140K-2A-luc long fragment was cloned into pCSO-rre-cppt-MCU3-LUC with ClaI and ScaI to generate plasmid pLV-mnd-P140K-2A-luc (pLV-mnd-P2AL). After cloning, plasmid was digested and sequenced to ensure the correct orientation and in frame. LV, named LV-mnd-P2AL, was generated by triple transient transfection in 293T cells as described previously.42 In brief, component plasmids included pMD.G (VSVG envelop plasmid), pCMVΔR8.91 (packaging plasmid), and pLV-mnd-P140K-2A-luc were added to 293T cells at a ratio of 1:3:3 with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in Opti-MEM (Gibco, Paisley, UK), and 12 hours later, Opti-MEM was replaced with fresh Dulbecco's modified Eagle's medium (Mediatech, Manassas, VA). After another 12-hour incubation, lentiviral supernatant was collected and filtered with Millipore Steriflip Filter (Millipore, Billerica, MA). The titer of lentiviral vectors was measured in K562 cells by measuring MGMT-P140K expression by flow cytometry with an anti-MGMT monoclonal antibody (Kamiya Biomedical, Seattle, WA).
Mice. Female Balb/C mice (Charles River, Wilmington, MA) were used as donors and recipients in all imaging studies. Mice were housed in pathogen-free Animal Resource Center according to approved animal protocol. In nonmyeloablative preconditioning, 2 days before transplantation, recipient mice received 30 mg/kg of BG and 7.5 mg/kg of BCNU; in lethal irradiation preconditioning, recipient mice received lethal dose of 750 cGy with a Cs137 source within 1–5 hours before transplantation.
In vitro transgene expression and drug selection. 5 × 105 293T or K562 cells were plated in 6-well plate in triplicates, and lentiviral vector LV-mnd-P2AL supernatant was added to each well at MOI of 1 with 8 µg/ml polybrene (Sigma-Aldrich, Milwaukee, WI) for 48 hours. Transduced 293T and K562 cells were maintained in Dulbecco's modified Eagle's medium and Iscove's MEM respectively, and passed every 3–4 days for over 4 months. Every 2–3 weeks, cells were measured for MGMT-P140K expression by flow cytometry. For in vitro assessments of gene transfer, murine bone marrow cells were collected and transduced with lentiviral vector LV-mnd-P2AL at MOI of 1.5 supplemented with 20 ng/ml mIL-3 (R&D Systems, Minneapolis, Minnesota), 50 ng/ml mIL-6 (R&D Systems), 50 ng/ml rSCF (R&D Systems), and 20% fetal bovine serum (Invitrogen) with 8 µg/ml polybrene for 48 hours. Then cells were treated with 20 µmol/l BG for 1 hour and followed by treating with 0–60 µmol/l of BCNU for 2 hours. After drug treatments, cells were plated in murine methylcellulose for CFU analysis. CFU colonies were counted and tested for the present of transgene by PCR.
Lentiviral gene transfer of murine bone marrow cells. Bone marrow cells from 6- to 8-week-old female Balb/C mice were collected by flushing femurs, tibia, and hip bones with α-MEM (Mediatech) containing 10% Heparin (Baxter Healthcare, Deerfield, IL). Cells were washed in fresh α-MEM and counted with a hemocytometer after mixed with 4% (vol/vol) acetic acid. Based on viral titer and MOI of 0.5–1, appropriate amount of whole bone marrow cells or lineage-depleted cells were incubated in lentiviral supernatant with the same supplements described above. Lineage-depleted cells were isolated by using anti-phycoerythrin microbeads (Miltenyi Biotec, Auburn, CA) to separate Lin+ cells stained with phycoerythrin-conjugated CD3, CD4, Ter119, B220, and CD11b (BD Pharmingen, San Diego, CA). After transduction, cells were washed and resuspended in fresh α-MEM before transplantation. Lin− cells were mixed back to Lin+ bone marrow cells for transplantation. Mice received transduced whole bone marrow cells or transduced Lin− cells were pooled together for BLI foci analysis and grouped based on preconditioning and drug treatments. 5 × 105 total bone marrow cells were used to measured MGMT-P140K expression by flow cytometry, and 1–2 × 105 cells were plated in CFU. Transduction efficiency was measured by testing CFU progenitor colonies for the presence of transgene by PCR.
Transplantation and drug treatments. Transduced bone marrow cells were washed and resuspended in α-MEM. 1 × 105 to 1 × 106 bone marrow cells were transplanted into preconditioned syngeneic mice by tail-vein injection, and about 1 × 105 bone marrow cells were saved for in vitro analysis. After preconditioning of recipients, animals were provided with water supplied with Bacitricin/Neomycin (Sigma-Aldrich, St Louis, MO). Every 3–4 weeks after transplantation, recipient mice received 3-consecutive day treatments of 30 mg/kg of BG and 60 mg/kg of TMZ. BG was dissolved in 40% polyethylene glycol and diluted in 60% pH 8.0 phosphate-buffered saline, and TMZ was dissolved in 10% dimethyl sulfoxide and 90% phosphate-buffered saline. TMZ was administered 1 hour after BG treatment. In serial transplantation experiment, at day 58 post-transplantation, one primary recipient mouse was sacrificed, and 3 × 106 recovered whole bone marrow cells were transplanted into lethally irradiated secondary recipients.
CFU-S assay. Six-to-eight-week old recipient Balb/C mice were lethally irradiated (750 cGy) 1–3 hours before cell infusion. Whole bone marrow cells from donor mice were transduced with LV-mnd-P2AL at MOI of 1 two days before cell infusion. 1 × 105 or 5 × 105 transduced bone marrow cells were transplanted into irradiated recipients via tail-vein injection. At 12 days or 14 days later, recipient mice were imaged with IVIS 200 system (Caliper Life Sciences), and spleens were removed and imaged again. After imaging, spleens were fixed in Bouin's solution for 15 minutes and photographed for visible colonies.
BLI of bone marrow cells in vivo. BLI of in vivo selection studies was performed on Xenogen IVIS 200 system (Caliper Life Sciences) at Case Western Reserve University Small Animal Imaging Center. A diluted anesthesia mix (ketamine HCl, xylazine HCl, and acepromazine), prepared by Case Animal Resource Center, was injected 5–7 minutes intraperitoneally before another intraperitoneal injection of 125 mg/kg of -luciferin (Biosynth AG, Staad, Switzerland) into each mouse, and the animals were taped down on black photographic paper. The imaging parameters were maintained throughout the experiments. The exposure time for each image was 5 minutes, and first image was taken 7 minutes after injecting -luciferin. Two consecutive ventral images were followed by one dorsal image. Only the second ventral image and the dorsal image from each animal were used for analysis because the peak bioluminescence signal emitted from most organs was reached 15–20 minutes after the injection of -luciferin based on preliminary studies that identified the signal intensity as a function of time (data not shown). For confirmation of the exact location of foci, some mice were sacrificed after BLI imaging, and their internal organs were imaged again to confirm the bioluminescence signal.
BLI imaging analysis. Xenogen Living Image 2.5 software was used to quantify BLI signals. ROI, including the whole body, four limbs (bones of extremities), chest, the whole abdominal region, spleen, and vertebrates, were created for quantification. Identified persistent BLI foci were also selected as ROIs and compared for BLI intensity. ROIs for foci were selected as circle with a diameter of 1 cm. Only visible focus that appeared at least 8 weeks after bone marrow transplantation, had twice the intensity as the background signal taken from each animal, and lasted >3 weeks at a specific location was defined as a persistent focus. Number of foci and signals were quantified and analyzed using paired t-test (GraphPad Software, La Jolla, CA). Some locations of the foci were estimated from BLI images due to the 2D nature of BLI and compared for the occurring frequency and duration.
LAM-PCR and sequencing. CFU colonies were collected and washed once with 1-ml phosphate-buffered saline, and digested with 25 µl of protease K solution at 50 °C overnight. Samples were heat-inactivated at 95 °C for 10 minutes and spun down. LAM-PCR was performed according to published protocol. Linear amplification was done with 5′-biotinylated primer, LV-LTR1 (5′-(biotin)-GAGCTCTCTGGCTAACTAGG-3′). Dynabeads M-280 Strepavidin magnetic beads (Invitrogen) were used in the following steps to wash and pull-down PCR products. Random priming was performed to convert linear product to double stranded DNA fragments with Klenow polymerase and hexanucleotide mix (Roche, Indianapolis, IN) at 37 °C for 1 hour. Enzyme digestion was done with Tsp509I (New England Biolabs, Ipswich, MA) at 65 °C for 1 hour. Digested DNA fragments were ligated to a linker cassette (5′-GACCCGGGAGATCTGAATTCAGTGGCACAGCAGTTAGG-3′) with a complementary overhang. Then two nested PCR with performed with two sets of primers. First nested PCR used LV-LTR2 (5′-GACCCGGGAGATCTGAATTC-3′) and LC-1 (5′-GACCCGGGAGATCTGAATTC-3′), and the second nested PCR used LV-LTR3 (5′-AGTAGTGTGTGCCCGTCTGT-3′) and LC2 (5′-GATCTGAATTCAGTGGCACAG-3′). The products of the second nested PCR were run on 2% agarose gels. PCR bands were then excised and purified with QIAquick gel extraction kit (Qiagen, Valencia, CA) and sent for sequencing. Sequencing results were searched with BLAT or BLAST for matching mouse genome.
SUPPLEMENTARY MATERIAL Figure S1. BLI signal correlated to cell numbers. (a) BLI imaging of transduced bone marrow cells showed linear correlation between BLI signal to cell numbers. (b) BLI image of lethally irradiated recipient received 1×105 cells at day 14, and spleen was removed and imaged, showing two strong distinct foci that matched whole body BLI. Figure S2. Loss of transgene expression in nonmyeloablative and lethally irradiated recipients without drug selection. Mice #1 and #2 received non-myeloablative pre-conditioning, and mouse #3 received lethally irradiated pre-conditioning. Mouse #1 received 1×106 untransduced bone marrow cells, mouse #2 received 5×105 transduced and 5×105 untransduced bone marrow cells, and mouse #3 received 1×106 tranduced bone marrow cells. Transgene expression in mouse #2 and #3 lost overtime without any BG and TMZ treatment. Figure S3. Transgene expression measured by BLI and flow cytometry at day 32 after transplantation. BLI images captured transgene expression in the whole animal at day 32 after transplantation. Peripheral blood was also collected after imaging, and MGMT-P140K expression was measured by flow cytometry, showing low level of transgene expression around 2%. PCR testing of peripheral blood showed presence of the transgene. (M: 1 kb plus ladder; + as PCR positive control; - as H2O control). Figure S4. Tricistronic lentiviral vector with MGMT-P140K, GFP, and luciferase, and transgene expression. (a) Construct of tricistronic lentiviral vector contains MGMT-P140K, GFP, and firefly luciferase gene, linking by two FMDV 2A sequences. (b) MGMT-P140K and GFP expression in initially transduced bulk bone marrow cells were 1.53% and 1.57%, respectively, and CFU progenitor cell colonies from initially transduced bone marrow cells showed 25.6% positivity in transgene. Efficient transgene expression in 293T and K562 cells similar to Fig 1b were observed, and efficient in vivo selection were also observed in recipients (data not shown).
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH-NCI RO1-CA073062) and also in part supported by grants NIH-P30-CA43703 and NIH R24-CA110943. We would also like to express thanks to Dr Zhenghong Lee for his valuable suggestion in the preparation of this manuscript. Y.L., P.C., J.C.R. and D.L.W. declared no competing financial interests. S.L.G. holds a patent through Case Western Reserve University for BG-resistant mutant forms of MGMT as gene transfer agents in stem cell selection. A license for lentiviral vector constructs of mutant MGMTs has been issued to Lentigen Inc. S.L.G. would receive a royalty benefit should this process be commercialized.
Supplementary Material
BLI signal correlated to cell numbers. (a) BLI imaging of transduced bone marrow cells showed linear correlation between BLI signal to cell numbers. (b) BLI image of lethally irradiated recipient received 1×105 cells at day 14, and spleen was removed and imaged, showing two strong distinct foci that matched whole body BLI.
Loss of transgene expression in nonmyeloablative and lethally irradiated recipients without drug selection. Mice #1 and #2 received non-myeloablative pre-conditioning, and mouse #3 received lethally irradiated pre-conditioning. Mouse #1 received 1×106 untransduced bone marrow cells, mouse #2 received 5×105 transduced and 5×105 untransduced bone marrow cells, and mouse #3 received 1×106 tranduced bone marrow cells. Transgene expression in mouse #2 and #3 lost overtime without any BG and TMZ treatment.
Transgene expression measured by BLI and flow cytometry at day 32 after transplantation. BLI images captured transgene expression in the whole animal at day 32 after transplantation. Peripheral blood was also collected after imaging, and MGMT-P140K expression was measured by flow cytometry, showing low level of transgene expression around 2%. PCR testing of peripheral blood showed presence of the transgene. (M: 1 kb plus ladder; + as PCR positive control; - as H2O control).
Tricistronic lentiviral vector with MGMT-P140K, GFP, and luciferase, and transgene expression. (a) Construct of tricistronic lentiviral vector contains MGMT-P140K, GFP, and firefly luciferase gene, linking by two FMDV 2A sequences. (b) MGMT-P140K and GFP expression in initially transduced bulk bone marrow cells were 1.53% and 1.57%, respectively, and CFU progenitor cell colonies from initially transduced bone marrow cells showed 25.6% positivity in transgene. Efficient transgene expression in 293T and K562 cells similar to Fig 1b were observed, and efficient in vivo selection were also observed in recipients (data not shown).
REFERENCES
- De Palma M, Montini E, Santoni de Sio FR, Benedicenti F, Gentile A, Medico E.et al. (2005Promoter trapping reveals significant differences in integration site selection between MLV and HIV vectors in primary hematopoietic cells Blood 1052307–2315. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C.et al. (2006Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration Nat Biotechnol 24687–696. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH.et al. (1996In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector Science 272263–267. [DOI] [PubMed] [Google Scholar]
- Woods NB, Ooka A., and, Karlsson S. Development of gene therapy for hematopoietic stem cells using lentiviral vectors. Leukemia. 2002;16:563–569. doi: 10.1038/sj.leu.2402447. [DOI] [PubMed] [Google Scholar]
- D'Costa J, Mansfield SG., and, Humeau LM. Lentiviral vectors in clinical trials: Current status. Curr Opin Mol Ther. 2009;11:554–564. [PubMed] [Google Scholar]
- Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allay JA, Persons DA, Galipeau J, Riberdy JM, Ashmun RA, Blakley RL.et al. (1998In vivo selection of retrovirally transduced hematopoietic stem cells Nat Med 41136–1143. [DOI] [PubMed] [Google Scholar]
- Milsom MD., and, Williams DA. Live and let die: in vivo selection of gene-modified hematopoietic stem cells via MGMT-mediated chemoprotection. DNA Repair (Amst) 2007;6:1210–1221. doi: 10.1016/j.dnarep.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge G, Beard BC., and, Kiem HP. Hematopoietic stem cell transduction and amplification in large animal models. Hum Gene Ther. 2005;16:1355–1366. doi: 10.1089/hum.2005.16.1355. [DOI] [PubMed] [Google Scholar]
- Zaboikin M, Srinivasakumar N., and, Schuening F. Gene therapy with drug resistance genes. Cancer Gene Ther. 2006;13:335–345. doi: 10.1038/sj.cgt.7700912. [DOI] [PubMed] [Google Scholar]
- Gerull S, Beard BC, Peterson LJ, Neff T., and, Kiem HP. In vivo selection and chemoprotection after drug resistance gene therapy in a nonmyeloablative allogeneic transplantation setting in dogs. Hum Gene Ther. 2007;18:451–456. doi: 10.1089/hum.2006.039. [DOI] [PubMed] [Google Scholar]
- Larochelle A, Choi U, Shou Y, Naumann N, Loktionova NA, Clevenger JR.et al. (2009In vivo selection of hematopoietic progenitor cells and temozolomide dose intensification in rhesus macaques through lentiviral transduction with a drug resistance gene J Clin Invest 1191952–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff T, Beard BC, Peterson LJ, Anandakumar P, Thompson J., and, Kiem HP. Polyclonal chemoprotection against temozolomide in a large-animal model of drug resistance gene therapy. Blood. 2005;105:997–1002. doi: 10.1182/blood-2004-08-3169. [DOI] [PubMed] [Google Scholar]
- Zielske SP, Reese JS, Lingas KT, Donze JR., and, Gerson SL. In vivo selection of MGMT(P140K) lentivirus-transduced human NOD/SCID repopulating cells without pretransplant irradiation conditioning. J Clin Invest. 2003;112:1561–1570. doi: 10.1172/JCI17922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt IJ., and, Gambhir SS. Molecular imaging applications for immunology. Clin Immunol. 2004;111:210–224. doi: 10.1016/j.clim.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Koo V, Hamilton PW., and, Williamson K. Non-invasive in vivo imaging in small animal research. Cell Oncol. 2006;28:127–139. doi: 10.1155/2006/245619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger M, Hoffmann P, Contag CH., and, Negrin RS. Evaluation of effector cell fate and function by in vivo bioluminescence imaging. Methods. 2003;31:172–179. doi: 10.1016/s1046-2023(03)00127-0. [DOI] [PubMed] [Google Scholar]
- Luker GD., and, Luker KE. Optical imaging: current applications and future directions. J Nucl Med. 2008;49:1–4. doi: 10.2967/jnumed.107.045799. [DOI] [PubMed] [Google Scholar]
- Welsh DK., and, Kay SA. Bioluminescence imaging in living organisms. Curr Opin Biotechnol. 2005;16:73–78. doi: 10.1016/j.copbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Rice BW, Cable MD., and, Nelson MB. In vivo imaging of light-emitting probes. J Biomed Opt. 2001;6:432–440. doi: 10.1117/1.1413210. [DOI] [PubMed] [Google Scholar]
- Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P.et al. (2007Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium Stem Cells 252677–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rosol M, Ge S, Peterson D, McNamara G, Pollack H.et al. (2003Dynamic tracking of human hematopoietic stem cell engraftment using in vivo bioluminescence imaging Blood 1023478–3482. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH.et al. (2007In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation Blood 1092649–2656. [DOI] [PubMed] [Google Scholar]
- Davis BM, Reese JS, Lingas K., and, Gerson SL. Drug selection of mutant methylguanine methyltransferase from different oncoretroviral backbones results in multilineage hematopoietic transgene expression in primary and secondary recipients. J Hematother Stem Cell Res. 2003;12:375–387. doi: 10.1089/152581603322286015. [DOI] [PubMed] [Google Scholar]
- Cai S, Ernstberger A, Wang H, Bailey BJ, Hartwell JR, Sinn AL.et al. (2008In vivo selection of hematopoietic stem cells transduced at a low multiplicity-of-infection with a foamy viral MGMT(P140K) vector Exp Hematol 36283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola H, Woods NB, Sjögren M, Helgadottir H, Hamaguchi I, Jacobsen SE.et al. (2000Lentivirus gene transfer in murine hematopoietic progenitor cells is compromised by a delay in proviral integration and results in transduction mosaicism and heterogeneous gene expression in progeny cells J Virol 7411911–11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS.et al. (2004Shifting foci of hematopoiesis during reconstitution from single stem cells Proc Natl Acad Sci USA 101221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D, Gelovani J, Savoldo B, Robinson SN, Decker WK, Brouard N.et al. (2009Noninvasive bioluminescent imaging demonstrates long-term multilineage engraftment of ex vivo-expanded CD34-selected umbilical cord blood cells Stem Cells 271932–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenasy N, Stein J, Yaniv I., and, Farkas DL. The topologic and chronologic patterns of hematopoietic cell seeding in host femoral bone marrow after transplantation. Biol Blood Marrow Transplant. 2003;9:496–504. doi: 10.1016/s1083-8791(03)00150-2. [DOI] [PubMed] [Google Scholar]
- Askenasy N, Zorina T, Farkas DL., and, Shalit I. Transplanted hematopoietic cells seed in clusters in recipient bone marrow in vivo. Stem Cells. 2002;20:301–310. doi: 10.1634/stemcells.20-4-301. [DOI] [PubMed] [Google Scholar]
- Stein J, Yaniv I., and, Askenasy N. Critical early events in hematopoietic cell seeding and engraftment. Folia Histochem Cytobiol. 2005;43:191–195. [PubMed] [Google Scholar]
- Yoshimoto M, Shinohara T, Heike T, Shiota M, Kanatsu-Shinohara M., and, Nakahata T. Direct visualization of transplanted hematopoietic cell reconstitution in intact mouse organs indicates the presence of a niche. Exp Hematol. 2003;31:733–740. doi: 10.1016/s0301-472x(03)00108-5. [DOI] [PubMed] [Google Scholar]
- Ball CR, Pilz IH, Schmidt M, Fessler S, Williams DA, von Kalle C.et al. (2007Stable differentiation and clonality of murine long-term hematopoiesis after extended reduced-intensity selection for MGMT P140K transgene expression Blood 1101779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard BC, Sud R, Keyser KA, Ironside C, Neff T, Gerull S.et al. (2009Long-term polyclonal and multilineage engraftment of methylguanine methyltransferase P140K gene-modified dog hematopoietic cells in primary and secondary recipients Blood 1135094–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide LM, Javazon E., and, Spencer HT. Transduction of murine hematopoietic stem cells and in vivo selection of gene-modified cells. Methods Mol Biol. 2008;433:213–228. doi: 10.1007/978-1-59745-237-3_13. [DOI] [PubMed] [Google Scholar]
- Persons DA, Allay ER, Sawai N, Hargrove PW, Brent TP, Hanawa H.et al. (2003Successful treatment of murine beta-thalassemia using in vivo selection of genetically modified, drug-resistant hematopoietic stem cells Blood 102506–513. [DOI] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA.et al. (2007Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues Cell 131994–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D.et al. (2009Detection of functional haematopoietic stem cell niche using real-time imaging Nature 45797–101. [DOI] [PubMed] [Google Scholar]
- Wilson D, Roy D, Steyer G, Gargesha M, Stone M., and, McKinley E. Whole Mouse Cryo-Imaging. Proc Soc Photo Opt Instrum Eng. 2008;6916:69161I–69161I9. doi: 10.1117/12.772840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halene S, Wang L, Cooper RM, Bockstoce DC, Robbins PB., and, Kohn DB. Improved expression in hematopoietic and lymphoid cells in mice after transplantation of bone marrow transduced with a modified retroviral vector. Blood. 1999;94:3349–3357. [PMC free article] [PubMed] [Google Scholar]
- Donnelly ML, Hughes LE, Luke G, Mendoza H, ten Dam E, Gani D.et al. (2001The ‘cleavage' activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like' sequences J Gen Virol 82Pt 51027–1041. [DOI] [PubMed] [Google Scholar]
- Zielske SP., and, Gerson SL. Lentiviral transduction of P140K MGMT into human CD34(+) hematopoietic progenitors at low multiplicity of infection confers significant resistance to BG/BCNU and allows selection in vitro. Mol Ther. 2002;5:381–387. doi: 10.1006/mthe.2002.0571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BLI signal correlated to cell numbers. (a) BLI imaging of transduced bone marrow cells showed linear correlation between BLI signal to cell numbers. (b) BLI image of lethally irradiated recipient received 1×105 cells at day 14, and spleen was removed and imaged, showing two strong distinct foci that matched whole body BLI.
Loss of transgene expression in nonmyeloablative and lethally irradiated recipients without drug selection. Mice #1 and #2 received non-myeloablative pre-conditioning, and mouse #3 received lethally irradiated pre-conditioning. Mouse #1 received 1×106 untransduced bone marrow cells, mouse #2 received 5×105 transduced and 5×105 untransduced bone marrow cells, and mouse #3 received 1×106 tranduced bone marrow cells. Transgene expression in mouse #2 and #3 lost overtime without any BG and TMZ treatment.
Transgene expression measured by BLI and flow cytometry at day 32 after transplantation. BLI images captured transgene expression in the whole animal at day 32 after transplantation. Peripheral blood was also collected after imaging, and MGMT-P140K expression was measured by flow cytometry, showing low level of transgene expression around 2%. PCR testing of peripheral blood showed presence of the transgene. (M: 1 kb plus ladder; + as PCR positive control; - as H2O control).
Tricistronic lentiviral vector with MGMT-P140K, GFP, and luciferase, and transgene expression. (a) Construct of tricistronic lentiviral vector contains MGMT-P140K, GFP, and firefly luciferase gene, linking by two FMDV 2A sequences. (b) MGMT-P140K and GFP expression in initially transduced bulk bone marrow cells were 1.53% and 1.57%, respectively, and CFU progenitor cell colonies from initially transduced bone marrow cells showed 25.6% positivity in transgene. Efficient transgene expression in 293T and K562 cells similar to Fig 1b were observed, and efficient in vivo selection were also observed in recipients (data not shown).