Abstract
We varied the surface boundary-contour properties of binocular rivalry (BR) stimuli to measure the rivalry percept as a function of stimulus duration. Experiment 1 compared perception from BR stimuli with monocular boundary contour (MBC) and binocular boundary contour (BBC). We found global dominance is achieved with stimulus duration as short as 30 ms for the MBC rivalry stimuli, whereas it takes more than 150 ms for the BBC rivalry stimuli. This shows that global dominance can occur rapidly in the absence of a corresponding boundary contour in one half-image. Experiment 2 measured the detection of a monocular Gabor probe located centrally on a 1.5° versus 3.0° MBC rivalry stimulus. We found reliable binocular suppression is observed earlier with the 1.5° MBC stimulus, presumably because of the probe being spatially located nearer to the boundary contour. These findings, in conjunction with those in Su, He and Ooi (2011), support the notion that the representation of the dominant surface begins at the MBC and spreads toward the center of the image.
Keywords: border-to-interior strategy, boundary contour, global dominance, interocular inhibition, reaction time, surface integration
1. Introduction
Our ability to perceive single, 3-D surfaces from the 2-D retinal images is the product of binocular visual processing comprising of binocular integration and interocular inhibition. The latter process, which suppresses false matches and ecologically invalid-image representations, is assumed to play a critical role in the perception of single objects and surfaces. An important approach to understanding interocular inhibition in the laboratory is by studying binocular suppression when the two eyes are presented with dissimilar images (Blake, 1989; Fox, 1991). Figure 1a illustrates a typical binocular rivalry (BR) stimulus with similar surface boundary contour (BC) information (circular outlines framing the discs) in the two half-images but with different surface feature information (orthogonal grating orientation). With fusion, one experiences perceptual alternation between the percepts of the vertical and horizontal grating discs over time. Presumably, this reflects the visual system's momentary selection of one half-image for surface representation while suppression of the other half-image through the interocular inhibitory process. One also experiences a similar alternating percept with the BR stimulus in figure 1b, where the BC of the horizontal grating disc in the right half-image is created by a relative spatial phase-shift between the central and surrounding gratings. By using such stimuli, or their variants, researchers have been able to reveal the characteristics of interocular inhibition (e.g., Alais and Blake, 1998; Kaufman, 1974; Kovács, Papathomas, Yang, and Fehér, 1996; Lee & Blake, 2004; Nguyen, Freeman, and Alais, 2003; Ooi and He, 2005; Ooi and Loop, 1994; Papathomas, Kovács, and Conway, 2005; Shimojo and Nakayama, 1990; Smith, Levi, Harwerth, and White, 1982; Su, He, and Ooi, 2009; Suzuki and Grabowecky, 2002, 2007; Watanabe, Paik, and Blake, 2004; Wolfe, 1983).
Figure 1.
Stimuli used in Experiment 1 and the predicted percepts. (a) A binocular rivalry (BR) stimulus typically used in the laboratory. Note the disc in each half-image is clearly delineated by a boundary contour. We thus coin this stimulus a BR stimulus with binocular boundary contour (BBC). (b) A variant of a BR stimulus with BBC. Here, the boundary contour of the right half-image is produced by a 180 deg phase shift between the central and surrounding gratings. (c) The predicted percepts of the BBC rivalry stimulus (disc) from its onset to the development of global dominance. (d) A BR stimulus with monocular boundary contour (MBC), where only the left half-image has a boundary contour delineating the vertical grating disc. The right half-image has horizontal grating but no boundary contour at the corresponding retinal area. (e) The predicted percepts of the MBC rivalry stimulus (disc) from its onset to the development of global dominance.
Wolfe (1983) made an important observation regarding the influence of stimulus duration on the perception of the typical BR stimulus (similar to the stimulus in figure 1a). He found that at short stimulus durations (< 150 ms) observers reported seeing the superimposition of the dichoptic orthogonal gratings (plaid or checkerboard). Only with stimulus durations of 150 ms or longer did the observers begin to report seeing either piecemeal rivalry or the entire image of a grating that belonged to one eye (i.e., global dominance percept). This empirical finding has been taken to suggest that the interocular inhibitory process asserts its influence after a relatively long delay (> 150 ms) and that before BR's onset the two half-images are abnormally fused. Here, we consider an alternative explanation based on the broader consideration that the global dominance percept, i.e., global surface representation, of the typical BR stimulus (figure 1a) is the outcome of binocular surface representation where processes of surface integration and interocular inhibition are jointly involved (Ooi and He, 2005; Su et al., 2009). At each local binocular corresponding area the conflicting orientation signals from the two eyes compete via the interocular inhibitory process, which selects one orientation as the “winner” for perception (dominant) and suppresses the other. The local “winner” then integrates with adjacent local “winners” with similar orientation to form a larger surface patch of the same orientation. With further spatial integration, a global surface representation of the grating is formed endowing the observer with the global dominance percept. This analysis thus argues that the time required to form a global dominance percept depends on both the latency of the local interocular inhibitory process and the time required for global surface integration of like surface texture signals. Therefore, the long duration (> 150 ms) required for global perceptual dominance observed by Wolfe (1983) might also be attributed to the slowness of global surface integration with the typical BR stimulus (Su, He, and Ooi, 2011).
The boundary contours of the BR stimuli (outlines of the stimuli in figure 1a and 1b) may play a crucial role in the time required for the visual system to achieve global surface representation. Various empirical findings have led to the proposal that the visual system relies on a border-to-interior strategy to represent global surfaces (Caputo, 1998; Grossberg and Mingola, 1985; Motoyoshi, 1999; Paradiso and Nakayama, 1991; Su et al., 2011; Watanabe and Cavanagh, 1991). With this strategy, the visual system represents a texture surface from the BC of the texture image. Specifically, the visual system first codes the BC of the texture image and a sample of the surface feature adjacent to the BC (Lamme, 1995; Nothdurft, Gallant, and van Essen, 2000; Qiu and von der Heydt, 2005; Zhou, Friedman, and von der Heydt, 2000). The sample is then used to sequentially integrate with the more interiorly located, local texture signals to form the global texture surface (Su et al., 2011). The time required to achieve a global representation of the texture surface depends on the texture structure and perceptual saliency.
We assume the visual system represents the BR stimulus using a similar border-to-interior strategy that involves surface integration in addition to interocular inhibition (Ooi and He, 2005, 2006; Su, He, and Ooi, 2009, 2010, 2011; van Bogaert, Ooi, and He, 2008; Xu, He, and Ooi, 2010). Please refer to figure 1c for the predicted percepts of the BR stimuli in figures 1a and 1b. Note the surface texture feature (oriented grating) adjacent to the BC of each paired half-image is different (orthogonal). The dissimilar texture features between the paired half-images instigate the interocular inhibitory process, whereby one feature orientation is momentarily selected for representation while the other is suppressed. Both the relative strengths of the BC and texture features between the two eyes can affect the dominance selection process. According to the border-to-interior strategy, from the initial representation of the surface texture adjacent to the BC of each half-image, the visual system spreads the surface representation wave inward. On its inward path from each BC, the spreading wave interacts with the local “winners” emerging from local binocular competition between the dichoptic orthogonal gratings. The spreading wave integrates with the local “winners” if they have the same feature (e.g., orientation). If they have different features, the spreading wave is halted until the same feature wins again in the local binocular competition. Because the spreading waves from the two half-images have different surface features (orientation) but similar saliency, they compete for global dominance when they meet (figure 1c). Consequently, a relatively longer time is required for global dominance. This theoretical analysis based on the border-to-interior strategy thus raises the possibility that a delay in local interocular inhibition may not be the sole factor causing the long duration to perceive global dominance with the typical BR stimulus (Su et al., 2011). To test it, we investigated the perception of the monocular boundary contour (MBC) rivalry stimulus shown in figure 1d (Ooi and He, 2005, 2006).
When one free fuses the two half-images of the MBC rivalry stimulus in figure 1d, one perceives a vertical grating disc surrounded by horizontal grating. The vertical grating disc percept is relatively stable despite its corresponding area being stimulated by horizontal grating (Frisby and Mayhew, 1978; Ooi and He, 2005). The strong predominance of the MBC disc is mainly attributed to the visual system's preference to select the MBC formed between the vertical and horizontal gratings over the image with no boundary contour for surface representation (Ooi and He, 2005). To do so according to the border-to-interior hypothesis (and reiterating the above for emphasis), the visual system begins by representing the MBC and a sample of the texture adjacent to it in the same eye. Then bit-by-bit the process continues with the immediate texture area adjacent to the sample being integrated, until the entire monocular disc image is represented (see predicted percepts in figure 1e; also refer to the movie demonstration). Importantly, in contrast to the typical BR stimuli (figure 1a and 1b), the spreading wave of surface representation does not encounter another with a different grating texture from the other eye (half-image), particularly when the MBC disc has a small diameter. This is because the local homogeneous grating texture in the other half-image (without the BC) is not selected for surface representation and is suppressed. Thus, without resistance from a competing wave, the time required to represent and perceive the MBC disc is predicted to be shorter, compared to the time required to perceive global dominance with the typical BR stimuli with binocular boundary contour (BBC) (figure 1a and 1b). On the other hand, if a delayed onset of local interocular inhibition is the main factor causing the long stimulus duration to obtain global dominance, both the MBC and typical BR stimuli should produce a similar relationship between the percentage of seeing global dominance and the stimulus duration. Our first experiment provides support for the former prediction.
Our second experiment sought further evidence for the border-to-interior hypothesis by measuring the detection of a monocular Gabor probe superimposed on the center of the MBC rivalry stimulus (figure 1d; also see examples of stimuli with probes in figure 4). With binocular suppression, the chance of detecting the probe is higher in the eye viewing the MBC disc than in the eye viewing the homogeneous grating (Su et al., 2009, 2010). Thus, consistent with the observations from the first experiment, we should be able to reveal binocular suppression in the central region of the disc at very short stimulus duration (< 150 ms). Furthermore, according to the border-to-interior hypothesis, because global surface representation starts from the MBC, we expect to find the time to achieve dominance in the central disc area to increase with the diameter of the MBC disc. This predicts that interocular suppression will be observed at an earlier time with an MBC rivalry stimulus with a small disc diameter, than one with a large disc diameter. These predictions are confirmed by our experiment.
Figure 4.
Results of Experiment 2. The top panel above the graphs illustrates examples of stimuli tested in the dominance and suppression conditions, for the 1.5° (left) and 3.0° (right) MBC rivalry stimuli. The monocular Gabor probe is presented on the MBC disc half-image in the dominance condition, while it is presented on the homogeneous grating half-image in the suppression condition. Graphs (a) and (b) plot the sensitivity index () for detecting the Gabor probe in the dominance and suppression conditions as a function of SOA, respectively, for the 1.5° and 3.0° MBC rivalry stimuli. For each SOA, for the dominance condition is higher for the 1.5° MBC rivalry stimulus. Furthermore, significant binocular suppression is observed as early as 30 ms with the 1.5° MBC rivalry stimulus but not until 80 ms with the 3.0° MBC rivalry stimulus. Graphs (c) and (d) plot the reaction time to detect the Gabor probe. For the 1.5° MBC rivalry stimulus, RT is longer for detecting the probe in the suppressed than dominance condition and is evident as early as 80 ms. Such RT difference between the suppression and dominance conditions reveals the effect of binocular suppression. In contrast, for the 3.0° MBC rivalry stimulus, the RT difference becomes significant only at 120 ms SOA.
2. Methods
2.1 Apparatus
Stimuli generated with MATLAB and Psychophysics Toolbox (Brainard, 1997; Pelli, 1997) on a Macintosh, were presented on a flat-screen CRT (1280 × 1024 pixels @ 100 Hz). A mirror haploscopic system attached to a chin-and-head rest aided fusion from a viewing distance of 75 cm. Experiment 1 was conducted with a Macintosh G4 and a 19-inch Misutbishi flat CRT, while Experiment 2 was conducted with a Macintosh MacPro and a 21-inch Samsung flat CRT.
2.2 Observers
One author and five naïve observers participated in Experiment 1. Four other naïve observers, who did not participate in Experiment 1, participated in Experiment 2. The naïve observers provided their informed consent before the experiments. All observers had normal or corrected-to-normal visual acuity and stereopsis (≤ 40 sec arc). For Experiment 2, we also ensured that the observers did not have excessive eye dominance. This was to prevent their rivalry percepts (dominance and suppression phases) from being confounded by excessive eye dominance. For example, excessive eye dominance would cause a premature termination of the dominance phase when the MBC half-image was seen by the observer's non-dominant eye, and thus interfere with our experimental procedure of presenting a probe with longer SOAs. We measured eye dominance using the method of imbalance reported elsewhere (Ooi and He, 2001; Xu, He & Ooi, 2008). For experiment 1, observers merely informed us of their dominant eye (for our record) because we did not need to ensure that the dominance/suppression phase was perceived for an adequate duration.
2.3 Experiment 1: Perception of MBC and BBC rivalry stimuli as a function of stimulus duration
2.3.1 Stimuli and Procedures
Each stimulus (figure 1a, 1b or 1d) was surrounded by a 4.1° × 4.1° square-frame fusion lock (width = 0.4°). The fusion lock was filled with black (4.0 cd/m2) and white (154.8 cd/m2) texture pattern. The vertical and horizontal sinusoidal gratings (4cpd, 79.4 cd/m2) of each stimulus had 95% contrast. In the MBC condition (figure 1d), one half-image had a vertical grating disc surrounded by horizontal grating while the other half-image had only horizontal grating. The outermost dimension (size) of each square half-image was 3.0° × 3.0°. We used two variants of the typical BR stimulus with BBC. The stimulus in the typical BR condition of figure 1b was similar to that in the MBC condition, except that a disc was created in the horizontal grating half-image at a region corresponding to the disc in the fellow half-image. This horizontal grating disc was created by a 180 deg phase shift between the designated disc area and the surrounding horizontal grating. In the typical BR condition of figure 1a, the grating disc in each half-image was surrounded by a homogeneous gray field (79.4 cd/m2) (instead of horizontal grating). For each condition, the diameter of the grating disc in each half-image was fixed at one of four predetermined values (1.0°, 1.3°, 1.6° or 1.9°). The half-image with the vertical grating disc was always presented to the dominant eye.
To begin a trial, the observer aligned his/her eyes on the nonius fixation target (0.91° × 0.91°, 153 cd/m2). He/she then pressed the spacebar to remove the nonius fixation target. Fifty ms later, the test stimulus was presented for 30, 50, 100 or 150 ms. A 3° × 3° mask (250 ms, 45° oblique plaid, 1.33cpd, 79.4 cd/m2, 12% contrast) followed to terminate the trial. The observer pressed one of four keys that corresponded to the percept of the disc area: global dominance (vertical or horizontal grating), piecemeal, plaid or unsure. The criterion for selecting dominance was strictly when the entire disc was filled with either vertical or horizontal grating. If any region within the disc > 0.5° (across 2 cycles) had a plaid pattern, the percept qualified as plaid. If the disc had grating and plaid patterns (< 0.5°), the observer reported “piecemeal”. The unsure response (rare) was made when the percept was uncertain.
For each condition, 16 combinations of variables were tested (4 durations × 4 disc diameters). Each combination was tested 20 times. Two experimental sessions, each comprising of 12 blocks (3 stimulus conditions × 4 disc diameters), were run. Each block consisted of 40 semi-randomized trials (4 durations × 10 repeats). The order of the test blocks was fully counterbalanced across observers.
2.4 Experiment 2: Performance in detecting a monocular Gabor probe on MBC rivalry stimuli with small (1.5°) versus large (3.0°) disc diameter
2.4.1 Stimuli
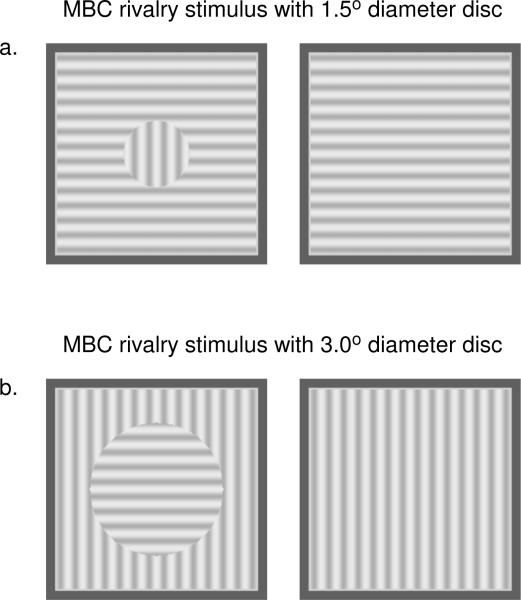
A 0.45° × 0.45° white nonius fixation target (73.1 cd/m2) and a 5° × 5° black square fusion lock frame (5 cd/m2) were used to achieve proper eye alignment before the trial. During the trial, the nonius fixation cross was removed, while the black frame remained to surround the MBC rivalry stimulus (4.5° × 4.5°). The MBC disc (1.5° or 3.0°) in one half-image was created by having a 90° grating orientation difference between the central circular area and the surrounding area of the half-image (either vertical grating disc vs. horizontal grating surround or horizontal grating disc vs. vertical grating surround). The grating orientation of the other half-image adopted the orientation of the surround grating of the fellow half-image. To counterbalance the effect of stimulus orientation, we designed two pairs of MBC rivalry stimuli for each disc diameter condition (1.5° or 3.0°), in which one pair had a vertical grating disc and the other pair had a horizontal grating disc. Thus, we tested four stimulus combinations: 1.5° MBC with vertical grating disc, 1.5° MBC with horizontal grating disc, 3.0° MBC with vertical grating disc and 3.0° MBC with horizontal grating disc rivalry stimuli. Figure 2a shows an example of a 1.5° MBC with vertical grating disc stimulus and figure 2b shows a 3° MBC with horizontal grating disc stimulus. All stimuli had 3 cpd sinusoidal grating (39.8% Michelson contrast) and were presented against a gray background of the same mean luminance (63.1 cd/m2). A 4.5° × 4.5° black-and-white random-dot mask (dot size: ~ 0.1° × 0.1°; 92.1% contrast, 250 ms) was employed at the end of the stimulus presentation. Please refer to Su et al. (2010) for further descriptions of the stimuli.
Figure 2.
Two examples of MBC stimuli used in Experiment 2. (a) A 1.5° MBC rivalry stimulus with vertical grating disc. (b) A 3.0° MBC rivalry stimulus with horizontal grating disc. Not shown at the counterparts of these stimuli, namely, a 1.5° MBC rivalry stimulus with horizontal grating disc and a 3.0° MBC rivalry stimulus with vertical grating disc. All four stimulus conditions were tested.
During a trial, the monocular Gabor probe (20 ms) was presented either in the center of the MBC grating disc (dominance condition), or in the center of the homogeneous grating half-image (suppression condition). (See the examples in the upper panel of figure 4.) The definition of the probe was given by the formula,
In the above formula, L(x, y) represents the luminance at a specified location (x, y). Lm is the mean luminance (63.1 cd/m2); c is the grating contrast (39.8%); f is the spatial frequency (3 cpd); a is the peak increment contrast ratio of the probe (observer-specific, see section 2.4.2 below); σ is the standard deviation of the Gaussian kernel (0.24°). The probe was always presented to the observer's dominant eye.
2.4.2 Procedures of determining the Gabor probe strength
The increment contrast ratio (a) of the Gabor probe used in the proper experiment was determined separately for each observer to control for individual differences in sensitivity. To do so, we measured the increment contrast threshold (at 79.4% correct) of a monocular Gabor probe while the observer experienced global perceptual dominance, or suppression, with the MBC rivalry stimulus. The probe was always presented to the observer's dominant eye. The parameters of the MBC rivalry stimulus were the same as those in the proper experiment; however, only the MBC rivalry stimuli with the 1.5° discs were used for the contrast threshold measurements.
We used a 2-temporal AFC design to determine the contrast threshold of the Gabor probe on the MBC rivalry stimulus. To begin a trial, the observer steadied himself/herself on a head-and-chin rest and maintained eye alignment on the nonius fixation and fusion lock. He/she then pressed the spacebar on the computer keyboard to present the MBC rivalry stimulus, which was diplayed 250 ms after the removal of the nonius fixation target. Five hundred ms (interval-1) or 1000 ms (interval-2) after the onset of the MBC stimulus, a monocular Gabor probe (20 ms) was presented on one half-image (pedestal) of the MBC stimulus. Two brief tones, each presented at 500 and 1000 ms after the onset of the MBC stimulus, were used to aid the observers in discriminating between the two separate intervals. After the second interval, the MBC stimulus was displayed for another 500 ms. A 250 ms random-dot mask followed to terminate the trial. The observer's task was to press one of two keys to indicate whether the probe was seen on the first or second interval. No feedback regarding the response accuracy was given to the observer.
Once a trial was completed, the observer would press the space bar to initiate the next trial. The probe contrast in the subsequent trial was determined by the QUEST procedure. To ensure that the observer only responded to trials with total (global) dominance or suppression, he/she was instructed to abort trials where either parts, or all, of the MBC disc was suppressed, or where piecemeal rivalry was experienced. This was done by pressing the right-arrow key.
Pairs of dominance and suppression thresholds were measured for detecting both horizontal and vertical probes over three days. We then took the average threshold over the three days. From this, we determined the contrast of the vertical and horizontal probes to be used in the proper RT experiment using the following criteria: (i) if the observer had an average dominance threshold of at least 0.2 log unit lower than his/her average suppression threshold, the probe contrast in the proper test would be set to 0.4 log unit above the average dominance threshold; (ii) if the average dominance threshold was less than 0.2 log unit lower than his/her average suppression threshold, the probe contrast would be set to 0.1 log unit above the average suppression threshold. We set these criteria based on the assumption that the typical observer has a depth of suppression of about 0.3 log unit during BR. Doing so ensures that the probe contrast was suprathreshold. With these criteria, two observers used probes that were 0.4 log unit above their dominance thresholds, and one observer used probes that were 0.1 log unit above the suppression thresholds, in the proper experiment. The last observer had an orientation specific asymmetry; thus, her vertical probe was 0.4 log unit above the dominance threshold while her horizontal probe was 0.1 log unit above the suppression threshold.
2.4.3 Procedures of measuring probe detection performance and reaction time
The Gabor probe, whose increment contrast level was determined above, was presented on either half-image of the MBC rivalry stimulus (see examples on the top panel of figure 4). For each test trial, the probe duration was 20 ms and its onset relative to the onset of the MBC rivalry stimulus (SOA, stimulus onset asynchrony) was 30, 50, 80, or 120 ms. We measured both the hit and false alarm rates, as well as the response time to detect the probe in the dominance and suppression conditions. To check for the reliability of the observer's responses, we also included catch trials in which the MBC rivalry stimulus was presented without the probe. The catch trials and test trials were intermingled within a block of 180 trials {[4 SOAs × 2 test conditions (dominance and suppression) × 2 probe orientations (horizontal and vertical) × 10 repeats] + 20 catch trials}. The trials were semi-randomized with the provision that no more than three consecutive trials had exactly the same combination of test condition and probe orientation. Additionally, four warm-up trials were provided at the beginning of each block of trials. The computer randomly chose the stimuli used for the warm-up trials. In all, each observer was tested over 12 blocks (2 disc sizes × 6 repeats) of trials. The order in which the blocks were tested was pairwise (1.5°/3.0°, etc.).
To begin a trial, the observer aligned his/her eyes with the nonius fixation target and pressed the spacebar of the computer keyboard. This led to the removal of the nonius fixation (while the surrounding square fusion lock remained) and 250 ms later, the presentation of the MBC rivalry stimulus. Depending on the type of trial (test or catch), the monocular probe could be added at the appropriate SOA. The probe was always presented to the observer's dominant eye. The observer's task was to respond as quickly as possible by pressing the right arrow key of the keyboard if he/she saw the Gabor probe. Once the response was made, the trial terminated with the presentation of a mask (250 ms). If no response was made (because the probe was either not detected or absent), the MBC rivalry stimulus would be removed after 1.5 sec and the trial terminated with the presentation of the mask. If no probe was detected, the observer should not press any key. Unlike the threshold measurements to determine the probe contrast (section 2.4.2 above), observers need not abort a trial if the MBC disc was not seen in global dominance. However, the observer was instructed to abort the trial if the contour of the MBC disc either disappeared or was perceived as broken. This never occurred with either the 1.5° or 3.0° MBC rivalry stimulus.
In addition, several precautionary measures were implemented. Audio feedbacks with different tones were given to convey two possible types of false alarms: (i) responding to a catch trial, and (ii) responding less than 100 ms upon the probe onset (anticipatory response). Trials with anticipatory responses (< 0.1%) would be repeated. A third audio feedback accompanied the “regular” test trials where probe detection were made. In this way, the observer could monitor the reliability of his/her responses. For each block of trials, the observer was allowed to make a maximum of four false alarms (20%) when responding to the catch trials. If a fifth false alarm was made, the test program would abort and the observer would have to repeat the entire block of trials. Furthermore, a one-minute rest period was inserted after every 40 trials to reduce the possibility of observer fatigue during the 180-trial block.
The data from the 12 blocks of trials from each observer were pooled for analysis of reaction time data. To increase data reliability, responses whose reaction times deviated from the mean by larger than three standard deviations (in each condition) were excluded from analysis. This rarely occurred (< 0.1%).
We estimated , the sensitivity index for detecting the Gabor probe, from the hit rate (h) and the false alarm rate (f) according to the formula, . We combined the values of the two probe orientations, horizontal and vertical, in the final data presentation because they are not statistically different.
3. Results
3.1 Experiment 1: Perception of MBC and BBC rivalry stimuli as a function of stimulus duration
The observer viewed a briefly presented MBC or BBC rivalry stimulus (figure 1a, 1b or 1d). He/she then reported the perceived grating pattern (global dominance, piecemeal, or plaid), or that he/she was unsure of the percept (average occurrence rate<1%), by pressing one of four keys. The full-stacked area plots in figure 3 show the average percentages of seeing the four percepts as a function of stimulus duration for the three conditions (figure 3a–c) and disc diameters. We found that the global dominance percept is experienced as early as 30 ms [t-tests against zero, t(5) ≥ 2.874, p < 0.05] for all four MBC stimuli with different disc sizes/diameters (figure 3c). Notably, since the MBC rivalry stimuli have conflicting texture features that activate the interocular inhibitory mechanism, this finding indicates the onset of interocular inhibition has a short latency. The percentage of seeing global dominance increases with stimulus duration in a manner that is opposite to the trend of seeing piecemeal and plaid patterns [F(1.558, 7.789) = 15.503, p = 0.003, Greenhouse-Geisser correction, 2-way ANOVA with repeated measures]. Furthermore, the percentage of perceiving global dominance is significantly higher with the MBC rivalry stimuli with small disc sizes compared to those with large disc sizes [F(3, 15) = 5.467, p < 0.01]. This supports the prediction of the border-to-interior surface representation hypothesis that it takes longer time for the spreading wave from the border to cover the entire surface area (figure 1e).
Figure 3.
Results of Experiment 1. Percentages of seeing the four percepts as a function of stimulus duration for the BBC rivalry stimuli (rows a and b) and MBC rivalry stimulus (row c). The four columns of graphs plot the data from the four disc sizes (diameters) tested. The percentage of perceiving global dominance is evident at 30 ms, increases with stimulus duration, and is significantly earlier and higher with the MBC rivalry stimulus. In contrast, with the BBC rivalry stimuli, the percentage of perceiving global dominance is not significantly different from zero even at 150 ms.
In contrast, for the typical BR (BBC) stimuli (figure 3a and 3b), the percentages of seeing global dominance are not significantly different from zero even for the longest stimulus duration tested (150 ms) [t-tests, t(5) ≤ 1.536, p ≥ 0.185]. This confirms the previous observations by Wolfe (1983). This finding, when compared to that of the MBC rivalry stimulus (figure 3c), does not indicate that the interocular inhibitory mechanism has a long latency. Instead, it is more likely related to the extra time required for global surface representation of BR stimuli with BBC (figure 1c).
3.2 Experiment 2: Performance in detecting a monocular Gabor probe on MBC rivalry stimuli with small (1.5°) versus large (3.0°) disc diameter
Figure 4a and 4b, respectively, show the average for detecting the monocular Gabor probes on the 1.5° and 3.0° diameter MBC rivalry stimuli. As predicted, the difference between the dominance (circles) and suppression (squares) conditions, i.e., the suppression effect, is affected by the diameter of the MBC disc. With the small disc (1.5°), the overall suppression effect is strong [F(1, 14) = 13.852, p = 0.002, ANOVA with repeated measures] and increases with SOA [F(1, 14) = 6.783, p = 0.021]. However, with the large MBC disc (3.0°), the overall suppression effect is only marginally significant [F(1, 14) = 4.365, p = 0.055] although it also increases significantly with SOA [F(1, 14) = 33.913, p < 0.001].
Similar to the results of the first experiment with the MBC rivalry stimuli above, binocular suppression is observed at 30 ms SOA for the small MBC disc stimulus [F(1, 3) = 21.961, p = 0.018]. The suppression effect for the large MBC disc stimulus, however, is not significant even by 50 ms SOA [F(1, 3) = 2.483, p = 0.213], and only reaches significance at 80 ms SOA [F(1, 3) = 14.550, p = 0.032]. This indicates at least 50 ms is required for the central region of the 3.0° MBC disc to become dominant (i.e., for completion of global surface representation). These findings confirm the prediction of the border-to-interior hypothesis that the larger the MBC disc the longer is the time needed for the spreading wave to travel from the boundary contour to reach the center of the disc (figure 1e).
We should emphasize that the finding of similar detection performance in the dominance and suppression conditions at 30 ms SOA with the 3° MBC rivalry stimulus simply reveals there is no consistent dominance or suppression in the central region. It does not, however, necessarily indicate the lack of local interocular inhibition. This is because our psychophysical measurement does not distinguish between an equal amount of local interocular inhibition between the two eyes or the absence of local interocular inhibition.
The average reaction times (RT) reveal a similar effect of MBC disc size on binocular suppression (figure 4c and 4d). For the small MBC rivalry stimulus (1.5°), RT is significantly longer for detecting the probe on the suppressed half-image (squares) than on the dominant half-image (circles) and is evident as early as 80 ms SOA [F(1, 3) = 15.808, p = 0.028]. Such RT difference between the suppression and dominance conditions reveals the effect of binocular suppression (O'Shea and Crassini, 1981). In contrast, for the large MBC rivalry stimulus (3°), the RT difference at 80 ms SOA is smaller and fails to reach the statistically significant level of 0.05 [F(1, 3) = 8.121, p = 0.065]. The RT difference becomes significant only at 120 ms SOA [F(1, 3) = 23.432, p = 0.017].
The difference in RT between the dominance and suppression conditions increases significantly with SOA for the small (1.5°) MBC rivalry stimulus [F(1, 14) = 9.076, p = 0.009, interaction effect]. But the effect of SOA is only moderate for the large (3.0°) MBC rivalry stimulus [F(1, 14) = 2.751, p = 0.119, interaction effect]. In addition, figure 4c and 4d reveal the overall RT is longer with the large MBC rivalry stimulus than with the small MBC rivalry stimulus, for both the dominance [F(1, 14) = 15.604, p = 0.001, 2-way ANOVA with repeated measures] and suppression conditions [F(1, 14) = 15.113, p = 0.002, 2-way ANOVA with repeated measures].
Finally, we describe here a related phenomenon that we observed in this and previous studies (e.g., Su et al., 2010). This pertains to an observation we encountered when measuring the contrast threshold for detecting the monocular Gabor probe in the suppressed eye (e.g., when determining the appropriate probe contrast for the current experiment in section 2.4.2). We noticed that when the monocular probe in the suppressed eye was detected, one sometimes observed the suppressed half-image surrounding the probe also became dominant. Sometimes the dominant grating pattern (surface) expanded from the probe region much like a spreading wave. This phenomenon reminds us of the traveling wave in BR reported by Wilson, Blake, and Lee (2001), and also of the common observation where a transient increase in contrast energy (“contour strength”) breaks BR suppression (von Helmholtz, 1962; Hering, 1942; Levelt, 1965). Since the monocular Gabor probe used in our experiment had a weak BC strength, the dominance switch it triggered can mainly be attributed to the local saliency of its surface feature. This observation thus reinforces the proposal that both the BC strength and surface feature saliency determine binocular surface representation in BR (Ooi and He, 2005; Su et al., 2009, 2010; Xu et al., 2010).
4. Discussion
Our study confirms Wolfe's (1983) observations that longer stimulus duration (>150 ms) is required to obtain global dominance in the typical BR stimuli with BBC (figure 1a and 1b). However, we found that when the BR stimulus has an MBC (figure 1c), global dominance is perceived as early as 30 ms after stimulus onset. This finding indicates that the interocular inhibitory mechanism does not require a long processing period (delay) to produce effective binocular suppression. Furthermore, by measuring performance in detecting a monocular Gabor probe at the center of the MBC rivalry stimulus, we were able to observe reliable binocular suppression as short as 30 ms after stimulus onset when the MBC disc is small (1.5° in diameter). In contrast, there is a significant delay in observable binocular suppression when the MBC disc is large (3.0° in diameter). Although this finding can also be explained by the general assumption that it takes a longer time to achieve global dominance with a larger binocular rivalry stimulus, it is more in line with the specific hypothesis that the visual system employs a border-to-interior strategy to represent the MBC disc, wherein the representation of the MBC disc begins at the MBC (border) and spreads inward (figure 1e). Accordingly, because it takes time for the surface-spreading wave to travel from the border, the representation of an MBC rivalry stimulus with a larger disc diameter (3.0°) takes longer.
The current findings complement those of our recent study (Su et al., 2011), which provided evidence for the border-to-interior surface representation hypothesis using a different approach. We measured the spreading speed of the surface representation wave of a MBC rivalry stimulus. To facilitate our measurements, we employed an MBC surface in the shape of a rectangle with horizontal grating texture. Our observers viewed the MBC rivalry stimulus, presented for various durations (30–500 ms), and reported the perceived grating texture pattern within the MBC rectangle. With sufficiently long stimulus duration, observers perceived an MBC rectangle completely filled with horizontal grating. But with very short stimulus duration, observers perceived a wide area of plaid pattern (superimposition of horizontal and vertical grating textures from the two eyes) at the center of the MBC rectangle, flanked by horizontal grating texture on its left and right sides. This percept supports the prediction of the border-to-interior hypothesis, where the surface representation of the horizontal grating texture spreads from the left and right borders of the MBC. Most importantly, the proportion of the represented horizontal grating area expanded inward while the plaid area shrank as the stimulus duration increased. This indicates an inward spreading of surface representation with time. Based on the perceived proportion of the horizontal grating texture within the rectangular MBC as a function of the stimulus duration, we were able to estimate the presumed neural spreading speeds of the surface representation wave from the right and left borders according to the cortical magnification factors (V1, V2, V3v and V4v). We found the cortical speed is more or less constant over the cortical distance traveled by the surface representation wave. Furthermore, we measured the spreading speed of surface representation with a monocular stimulus that does not instigate local interocular competition (i.e., one eye views a blank field and the other eye views the half-image with the rectangular MBC stimulus). We obtained similar results, except the estimated neural spreading speed is faster probably due to the absence of local interocular inhibition.
Finally, the current finding that effective interocular inhibition occurs after a relatively short duration of binocular processing augments the notion that binocular integration and interocular inhibition co-exist to construct binocular surface perception (e.g., Blake, Westendorf, and Overton, 1980; Kaufman, 1974; Su et al., 2009; Treisman, 1962; Wolfe, 1983, 1986, 1988). In fact, this adds to our findings on the co-existence theory (Su et al., 2009). For instance, in that study the observer viewed one half-image with a vertical grating disc on a larger phase-shifted vertical grating surround and the other half-image with a homogeneous vertical grating (i.e., an MBC phase-shift stimulus). We were able to reveal this stimulus causes the observer to simultaneously experience both global stereo depth and binocular suppression. Specifically, the grating disc was seen as separated in depth from the surrounding grating, indicating the involvement of the binocular integration process in computing binocular disparity from the relative phase shift of the dichoptic gratings. Also, the observer's contrast threshold for detecting a monocular Gabor probe at the center of the MBC grating disc was lower than that on the homogeneous grating in the fellow eye, indicating some degree of binocular suppression of the homogeneous grating half-image. Our current study adds to the Su et al. (2009) study by revealing that the interocular inhibitory mechanism functions quite early and is thus likely to work in conjunction with the binocular integration process at a relatively early temporal phase of binocular information processing.
4.1 Conclusions
It is proposed that processes of interocular inhibition (resolving conflict between locally dissimilar features) and global surface integration determine the global dominance percept of a BR stimulus. Supporting this, we found that the surface boundary contour properties of the BR stimuli can affect the stimulus duration required to achieve global dominance. Specifically, global dominance is perceived as early as 30 ms when the BR stimulus has an MBC, whereas it takes 150 ms or longer to perceive global dominance when the BR stimulus has a BBC. In addition, we found that binocular suppression (thus, the representation of the dominant surface) is observed earlier at a location closer to the MBC. This, in conjunction with our findings in Su et al (2011), confirms the notion that the global surface integration process begins at the boundary contour.
Supplementary Material
Acknowledgement
This study was supported by a grant from the NIH (R01 EY 015804) to TLO and ZJH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alais D, Blake R. Interactions between global motion and local binocular rivalry. Vision Research. 1998;38:637–644. doi: 10.1016/s0042-6989(97)00190-9. [DOI] [PubMed] [Google Scholar]
- Blake R. A neural theory of binocular rivalry. Psychological Review. 1989;96:145–167. doi: 10.1037/0033-295x.96.1.145. [DOI] [PubMed] [Google Scholar]
- Blake R, Westendorf DH, Overton R. What is suppressed during binocular rivalry? Perception. 1980;9:223–231. doi: 10.1068/p090223. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Caputo G. Texture brightness filling-in. Vision Research. 1998;38:841–851. doi: 10.1016/s0042-6989(97)00237-x. [DOI] [PubMed] [Google Scholar]
- Fox R. Binocular rivalry. In: Regan DM, editor. Vision and Visual Dysfunction Vol. 9, Binocular Vision. Macmillan; London: 1991. pp. 93–110. [Google Scholar]
- Frisby JP, Mayhew JEW. The relationship between apparent depth and disparity in rivalrous-texture stereogram. Perception. 1978;7:661–678. doi: 10.1068/p070661. [DOI] [PubMed] [Google Scholar]
- Grossberg S, Mingolla E. Neural dynamics of form perception: Boundary completion, illusory figures, and neon color spreading. Psychological Review. 1985;92:173–211. [PubMed] [Google Scholar]
- Hering E. In: Spatial Sense and Movements of the Eye. Radde C, editor. American Academy of Optometry; Baltimore, MD: 1942. [Google Scholar]
- Kaufman L. Sight and Mind. Oxford University Press; New York: 1974. [Google Scholar]
- Kovács I, Papathomas TV, Yang M, Fehér Á. When the brain changes its mind: Interocular grouping during interocular rivalry. Proceedings of the National Academy of Sciences (USA) 1996;93:15508–15511. doi: 10.1073/pnas.93.26.15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme VA. The neurophysiology of figure-ground segregation in primary visual cortex. Journal of Neuroscience. 1995;15:1605–1615. doi: 10.1523/JNEUROSCI.15-02-01605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Blake R. A fresh look at interocular grouping during binocular rivalry. Vision Research. 2004;44:983–991. doi: 10.1016/j.visres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Levelt W. On Binocular Rivalry. Royal Van Gorcum, Assen; The Netherlands: 1965. [Google Scholar]
- Motoyoshi I. Texture filling-in and texture segregation revealed by transient masking. Vision Research. 1999;39:1285–1291. doi: 10.1016/s0042-6989(98)00248-x. [DOI] [PubMed] [Google Scholar]
- Nguyen V, Freeman A, Alais D. Increasing depth of binocular rivalry suppression along two visual pathways. Vision Research. 2003;43:2003–2008. doi: 10.1016/s0042-6989(03)00314-6. [DOI] [PubMed] [Google Scholar]
- Nothdurft HC, Gallant JL, van Essen DC. Response profiles to texture border patterns in area V1. Visual Neuroscience. 2000;17:421–436. doi: 10.1017/s0952523800173092. [DOI] [PubMed] [Google Scholar]
- O'Shea RP, Crassini B. The sensitivity of binocular rivalry suppression to changes in orientation assessed by reaction-time and forced-choice techniques. Perception. 1981;10:283–293. doi: 10.1068/p100283. [DOI] [PubMed] [Google Scholar]
- Ooi TL, He ZJ. Sensory eye dominance. Journal of the American Optometric Association. 2001;72(3):168–178. [PubMed] [Google Scholar]
- Ooi TL, He ZJ. Surface representation and attention modulation mechanisms in binocular rivalry. In: Alais D, Blake R, editors. Binocular Rivalry. MIT Press; Cambridge, MA: 2005. pp. 117–135. [Google Scholar]
- Ooi TL, He ZJ. Binocular rivalry and surface-boundary processing. Perception. 2006;35:581–603. doi: 10.1068/p5489. [DOI] [PubMed] [Google Scholar]
- Ooi TL, Loop MS. Visual suppression and its effect upon color and luminance sensitivity. Vision Research. 1994;34:2997–3003. doi: 10.1016/0042-6989(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Papathomas TV, Kovács I, Conway T. Interocular grouping in binocular rivalry: Attributes and combinations. In: Alais D, Blake R, editors. Binocular Rivalry. MIT Press; Cambridge, MA: 2005. pp. 155–168. [Google Scholar]
- Paradiso MA, Nakayama K. Brightness perception and filling-in. Vision Research. 1991;31:1221–1236. doi: 10.1016/0042-6989(91)90047-9. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Qiu FT, von der Heydt R. Figure and ground in the visual cortex: V2 combines stereoscopic cues with Gestalt rules. Neuron. 2005;47:155–166. doi: 10.1016/j.neuron.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo S, Nakayama K. Real world occlusion constraints and binocular rivalry. Vision Research. 1990;30:69–80. doi: 10.1016/0042-6989(90)90128-8. [DOI] [PubMed] [Google Scholar]
- Smith EL, Levi DM, Harwerth RS, White JM. Color vision is altered during the suppression phase of binocular rivalry. Science. 1982;218:802–804. doi: 10.1126/science.7134975. [DOI] [PubMed] [Google Scholar]
- Su Y, He ZJ, Ooi TL. Coexistence of binocular integration and suppression determined by surface border information. Proceedings of the National Academy of Sciences (USA) 2009;106:15990–15995. doi: 10.1073/pnas.0903697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su RY, He ZJ, Ooi TL. The magnitude and dynamics of interocular suppression affected by monocular boundary contour and conflicting local features. Vision Research. 2010;50:2037–2047. doi: 10.1016/j.visres.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su RY, He ZJ, Ooi TL. Seeing grating-textured surface begins at the border. Journal of Vision. 2011;11(1):14, 1–14. doi: 10.1167/11.1.14. http://www.journalofvision.org/content/11/1/14, doi:10.1167/11.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Grabowecky M. Evidence for perceptual “trapping” and adaptation in multistable binocular rivalry. Neuron. 2002;36:143–157. doi: 10.1016/s0896-6273(02)00934-0. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Grabowecky M. Long-term speeding in perceptual switches mediated by attention-dependent plasticity in cortical visual processing. Neuron. 2007;56:741–753. doi: 10.1016/j.neuron.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Treisman A. Binocular rivalry and stereoscopic depth perception. The Quarterly Journal of Experimental Psychology. 1962;14:23–37. [Google Scholar]
- van Bogaert EA, Ooi TL, He ZJ. The monocular boundary contour mechanism in binocular surface representation and suppression. Perception. 2008;37:1197–1215. doi: 10.1068/p5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Helmholtz H. In: Treatise on Physiological Optics. Southall JPC, editor. Vol. 3. Dover; New York: 1962. [Google Scholar]
- Watanabe T, Cavanagh P. Texture and motion spreading, transparency and the aperture problem. Perception & Psychophysics. 1991;50:459–464. doi: 10.3758/bf03205062. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Paik Y, Blake R. Preserved gain control for luminance contrast during binocular rivalry suppression. Vision Research. 2004;44:3065–3071. doi: 10.1016/j.visres.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Wilson HR, Blake R, Lee S. Dynamics of travelling waves in visual perception. Nature. 2001;412:907–910. doi: 10.1038/35091066. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Influence of spatial frequency, luminance, and duration on binocular rivalry and abnormal fusion of briefly presented dichoptic stimuli. Perception. 1983;12:447–456. doi: 10.1068/p120447. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Stereopsis and binocular rivalry. Psychological Review. 1986;93:269–282. [PubMed] [Google Scholar]
- Wolfe JM. Parallel ideas about stereopsis and binocular rivalry: A reply to Blake and O'Shea. Psychological Review. 1988;95:155–158. [Google Scholar]
- Xu J, He ZJ, Ooi TL. Sensory eye dominance is retinal location specific and affects stereopsis. Journal of Vision. 2008;8(6):14. 1089, doi: 10.1167/8.6.1089. [Google Scholar]
- Xu J, He ZJ, Ooi TL. Surface boundary contour strengthens image dominance in binocular competition. Vision Research. 2010;50:155–170. doi: 10.1016/j.visres.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Friedman HS, von der Heydt R. Coding of border ownership in monkey visual cortex. Journal of Neuroscience. 2000;20:6594–6611. doi: 10.1523/JNEUROSCI.20-17-06594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.