Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that is characterized by a defect in immune tolerance and exacerbated by both the innate and adaptive arms of the immune response. SLE-associated immune hyperactivity can be detected systemically as elevations in levels of cytokines along with their upregulated receptors expressed by hematopoietic cells. Importantly, increased levels of cytokines and their receptors can be observed in target organs, and it is clear that they have important roles in disease pathogenesis. Recent therapeutic strategies have focused on proximal cytokines, such as interferon-α, interleukin (IL)-1, IL-6, and tumor necrosis factor as a result of the efficacious use of biologic agents for intervention in rheumatoid arthritis and other autoimmune diseases. Despite the recent advances in understanding the cytokine networks involved in autoimmune diseases and more specifically in SLE, the diagnosis and prognosis of lupus remain a challenge. Lupus is heterogeneous and unpredictable; moreover, the frequency and severity of flares can be difficult to determine and treat. A better understanding of the regulation of expression of key cytokines and their receptors can likely provide important clues to the pathogenic mechanisms underlying specific forms of SLE, and pave the way toward more effective therapeutics.
Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease that can result in skin rashes, arthritis, leukopenia, nephritis, and inflammation of the nervous system. This process begins with the loss of tolerance and the presence of autoreactive lymphocytes in the periphery as the result of the combination of both environmental and genetic factors (Kumar and others 2006; Crow 2008). Multiple cell types in the adaptive and innate arms of the immune system have been documented to contribute to lupus pathogenesis, either systemically or in the end organs. One of the pathogenomic features of SLE is the elaboration of anti-DNA and related antinuclear autoantibodies. Hence, not surprisingly, T cell-dependent B cell autoantibody production lies at the heart of disease pathogenesis. Thus, cytokines that activate B and T cells and promote their interaction constitute important disease drivers.
In addition, it has become clear that cells in the innate arm of the immune system play a key role in activating autoreactive lymphocytes in SLE. Although mechanisms have yet to be fully resolved, early work demonstrated that blood plasmacytoid dendritic cells (pDC), the primary producers of interferon (IFN)-α, were decreased in the blood and recruited to inflamed tissues of SLE patients. The pDC, through IFN-α secretion, induced monocytes to become potent antigen presenting myeloid DC (mDC) (Blanco and others 2001). It was hypothesized that mDC rapidly captured apoptotic cells and nucleosomes and presented autoantigens to CD4+ T cells that became activated and underwent proliferation and clonal expansion in the secondary lymphoid organs. Subsequently, B lymphocytes stimulated by interactions with autoreactive CD4+ T cells and mDC produced autoantibodies. The autoantibodies, in turn, formed immune complexes with neutrophil products and components of nucleosomes and directly stimulated toll like receptors (TLR) on pDC, which were stimulated to secrete more IFN-α, thereby propagating the inflammatory response.
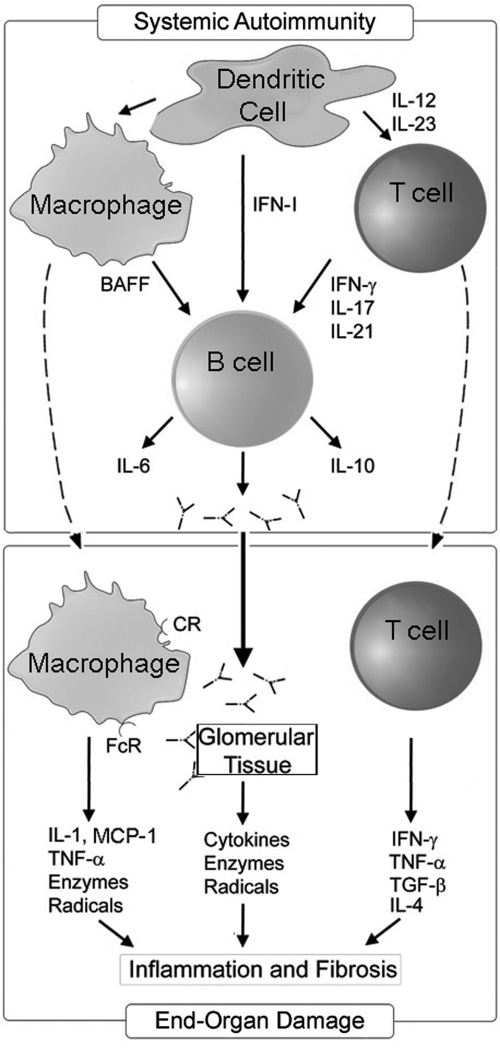
As depicted in Fig. 1, besides the systemic events, an additional series of events takes place in the end organ (eg, kidney), once autoreactive lymphocytes, myeloid cells, and autoantibodies infiltrate into the target tissues. Attracted by cytokines and chemokines produced in the inflamed target tissues, autoimmune lymphocytes respond with the elaboration of cytokines. Autoantibodies deposited in the target tissues as immune complexes further activate infiltrating myeloid cells and also the resident cells of the target tissue and perpetuate cytokine production along with release of destructive mediators such as reactive oxygen species, prostaglandins, and nitric oxide (Fu and others 2005; Fairhurst and others 2009). The resulting tissue inflammation eventually causes fibrotic tissue and the more ominous clinical manifestations of the disease. Several of the cytokines that have been implicated in SLE pathogenesis are diagramed in Fig. 1. The aim of this article is to review our current understanding of these cytokines in the context of lupus pathogenesis and the potential for intervention with anticytokine therapies.
FIG. 1.
The role of cytokines in systemic and end-organ autoimmune-initiated interactions in lupus. Increased IFN-α produced by pDC initiates a cascade of events that result in the activation of silent autoreactive lymphocytes in the periphery (upper panel). Although autoreactive B cells and T cells are central players driving the systemic component of lupus (upper panel), myeloid cells, and the cytokines, they elaborate constitute key determinants in shaping the autoimmune response. Autoreactive B cells interact with IFN-α-induced myeloid dendritic cells (not shown) and Th1 cells to elaborate both cytokines and autoreactive antibodies. The antibodies can form immune complexes with products released from apoptotic cells and directly stimulate pDC to propagate the immune response. Antibodies and immune complexes deposited in target organs can induce the production of chemokines, cytokines, and cytotoxic mediators from the target tissue (lower panel). Infiltrating Th cells support the local inflammatory response by producing cytokines and chemokines that further accelerate inflammation. Although there is evidence for Th2 cytokines promoting some elements of chronic nephritis, the overwhelming contribution of T cells in systemic lupus erythematosus appears to be Th1-skewed. Macrophages also contribute a number of key mediators that induce apoptosis and necrosis of target tissues, ultimately resulting in fibrosis and end-stage disease. IFN, interferon; pDC, plasmacytoid dendritic cells; IL, interleukin; TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β; BAFF, B cell activating factor.
Cytokines Elaborated by the Innate Immune System
Interferon-α
The type I IFN family consists of 12 subtypes of IFN-α encoded by 13 genes and a single gene for each of the other family members that include IFN-β, IFN-ω, IFN-κ, and IFN-ɛ/τ. All type I IFN family members bind to the same IFN-α/β receptor. Initial observations suggested that IFN-α levels were elevated in the serum of some SLE patients and that increased serum IFN-α levels correlated with SLE Disease Activity Index (SLEDAI) scores, organ involvement, anti-dsDNA as a measure of immune activation, and complement activation (Hooks and others 1979; Ytterberg and Schnitzer 1982; Bengtsson and others 2000; Lovgren and others 2004; Kirou and others 2005). Clinical parameters correlating with elevated serum IFN-α in SLE included fever, rash, and lymphopenia. Moreover, the observation that high serum IFN-α could be observed in some first-degree relatives of SLE patients over that seen in unrelated donors suggested that the high IFN-α trait is inherited in a complex polygenic fashion (Niewold and others 2007; Kariuki and Niewold 2010). Gene expression profiling studies have demonstrated increased expression of type I IFN-regulated genes (the IFN signature) both in peripheral blood mononuclear cells and within the target organs of SLE patients (Baechler and others 2003; Bennett and others 2003; Crow and others 2003; Peterson and others 2004; Yao and others 2009).
Some genetic polymorphisms in SLE risk genes have been shown to be linked to either increased production of IFN-α (IRF5, PHRF1) or potentially increased sensitivity to IFN-α (STAT4, TYK2, and IRAK1), whereas others appear to be protective by disrupting key signaling pathways associated with proinflammatory cytokines, such as TNFAIP3 (Sigurdsson and others 2005; Harley and others, 2008; Niewold and others 2008; Jacob and others 2009; Kariuki and others 2009; Vereecke and others 2009). Studies combining genetics with serologic and cytokine subphenotypes suggest that genetic variants in genes such as LRRC20 (leucine-rich repeat containing 20), PPM1H (protein phosphatase 1 H), LPAR1 (lysophosphatidic acid receptor 1), ANKS1A (sterile alpha motif domain 1A), and VSIG2 (V-set and immunoglobulin domain containing 2) may be associated with elevated serum IFN-α, disease presentation, and severity in lupus (Kariuki and others 2010).
A mechanistic role for IFN-α in disease pathogenesis was indicated by the observation that some patients treated with IFN-α developed autoantibodies (Ronnblom and Alm 2001a, 2001b). This role for IFN-α was further supported by the observation that the onset and severity of disease was reduced in some IFN-α/β receptor-deficient murine lupus models (Braun and others 2003; Santiago-Raber and others 2003). The ability of IFN-α to promote B cell differentiation and autoantibody production combined with the observation that immune complexes can directly stimulate pDC in the absence of exogenous stimuli support a potential role for dysregulated IFN-α production in SLE pathogenesis. By contrast to the large number of studies supporting a role for IFN-α in lupus, a protective role for type I IFN has been reported in a study employing the lupus-prone MRL/lpr murine model deficient for the type I IFN receptor (Hron and Peng 2004).
Initial reports with 2 monoclonal antibodies (mAbs) targeting IFN-α were encouraging, demonstrating a reduction in the IFN signature with both MEDI-545 (sifalimumab) and rhuMab IFN-α (rontalizumab) treatment (Yao and others 2009). In the MEDI-545 trial in which 62 SLE patients were given a single injection of MEDI-545 or placebo, the IFN signature was diminished in both blood and skin biopsies of a select subgroup of patients studied. Moreover, anti-IFN-α mAb therapy was also associated with a downregulation of mRNA for other proinflammatory cytokines, including interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), granulocyte-monocyte colony-stimulating factor, IL-10, and B cell activating factor (BAFF) in some patients. Another anti-IFN-α mAb, NNC 0152-0000-0001, is in early clinical trials and an IFN-α vaccine, IFN-α Kinoid, is in safety testing after successful preclinical testing in NZB/W mice (Zagury and others 2009). Although the reduced IFN signature holds promise, it has been suggested that long-term treatment with these agents could impact the immune response to viruses as well as have other unexpected side effects.
Tumor necrosis factor
Although TNF is primarily produced by macrophages and T cells, it can also be produced by other leukocytes and endothelial cells (Croft 2009). TNF binds to 2 receptors of molecular sizes 55 kDa, named the type I TNF receptor (TNFR), and 75 kDa, named the type II TNFR. Both TNFRs are found on almost every cell type; however, the membrane homotrimer expressed by mononuclear phagocytes binds only the type II TNFR. At low plasma concentrations TNF stimulates endothelial cells to express adhesion molecules and secrete chemokines that function to recruit inflammatory leukocytes. TNF also stimulates macrophages to produce IL-1 and IL-6. At moderate plasma concentrations, TNF produces a number of systemic effects acting on hepatocytes to induce acute phase proteins, the brain to produce fever, and increases circulating leukocyte numbers, whereas high concentrations of TNF produce septic shock and sustained TNF causes cachexia (Aggarwal 2003).
In murine lupus models, TNF can have protective effects or accelerate disease depending on the mouse strain and degree of disease activity (Aringer and Smolen 2008). High serum levels of TNF and the soluble TNFRs have been reported in SLE patients with active disease (Studnicka-Benke and others 1996) and elevated TNF has been documented in the target organ in renal disease (Malide and others 1995). Of note, TNF induced the differentiation of the potent IFN-α-secreting immature pDC to become mature pDC, which caused the downregulation of IFN-α production and the induction of antigen presenting cell function (Palucka and others 2005; Bave and others 2001). This might explain why a deficiency in TNF related to treatment with anti-TNF inhibitors can trigger a syndrome that shares a number of features with SLE (Katz and Zandman-Goddard 2010). These patients present with cutaneous lupus, hypocomplementemia, a decrease in antihistone antibodies, and an increased frequency of higher anti-dsDNA titers. Nevertheless, studies have been carried out to determine the impact of anti-TNF therapies on lupus arthritis. Treatment of lupus patients with the chimeric anti-TNF mAb, infliximab, for up to 10 weeks improved the symptoms associated with arthritis and nephritis in a majority of patients for extended periods. However, this therapy caused lupus nephritis patients to experience an increase in anti-dsDNA antibody levels and longer treatment regimens were associated with severe adverse events (Aringer and others 2009). These studies demonstrate the potential for TNF to act as a double-edged sword in complex autoimmune diseases.
Interleukin-1
The major functions of IL-1α and IL-1β, like TNF, are to act as mediators of the host inflammatory reaction to infections (Dinarello 2011). The principle source of IL-1β is the activated macrophage. IL-1β is secreted in response to bacterial stimuli that serve as TLR ligands such as lipopolysaccharide. IL-1 can be secreted by many cell types, including neutrophils, lymphocytes, epithetial cells, and endothelial cells. Most of the IL-1 detected in the periphery is IL-1β. IL-1 has 2 membrane receptors with the type I receptor being expressed on almost all cell types. The type II receptor is expressed on B lymphocytes and is inducible on other cell types. The type II receptor might act as a decoy receptor to competitively inhibit the type I IL-1 receptor (IL-1R). IL-1 has many similar actions to those of TNF. At low concentrations IL-1 promotes local inflammation and upregulates adhesion molecule expression on endothelial cells and induces production of a number of mediators, including prostaglandins, nitric oxide, and cyclooxygenase 2. IL-1 can also costimulate B and T lymphocyte responses. At higher concentrations, IL-1 exerts endocrine effects, inducing fever and acute-phase proteins. Although IL-1 and TNF share cell signaling pathways, IL-1 does not induce apoptosis or cause pathophysiologic alterations that occur with septic shock.
Of the IL-1 family members, the IL-1R antagonist (IL-1Ra) and a related family member, IL-18, have been the most studied in autoimmune diseases. Whereas low serum IL-1Ra levels and high serum IL-18 levels tend to correlate with nephritis in SLE, high serum IL-1 levels tend to correlate with disease activity (Sturfelt and others 1997; Tucci and others 2008; Brugos and others 2010). Two open-label trials with anakinra, a recombinant form of IL-1Ra, suggested limited but positive effects; however, it is unlikely that further trials will be conducted with IL-1 inhibitors for SLE (Moosig and others 2004; Ostendorf and others 2005; Ronnblom and Elkon 2010).
Interleukin-6
IL-6 is produced by many cell types. Like TNF and IL-1, IL-6 also has pleiotropic effects on a variety of tissues, and this includes the induction of acute phase proteins and fever. IL-6 is primarily produced by macrophages, but can also be secreted in lower amounts by many other cell types, including lymphocytes, mesangial cells, and endothelial cells. The IL-6R is also expressed by many cell types, including leukocytes, megakaryocytes, and hepatocytes. The IL-6R is composed of 1 cytokine-binding component that can be soluble or membrane bound and the common gp130 signal transduction component, which is shared with other family members, including IL-11, oncostatin, and leukemia inhibitory factor (Taga and Kishimoto 1997; Jones 2005; Nishimoto and Kishimoto 2006). In the periphery, IL-6 promotes macrophage activation, B lymphocyte activation, and immunoglobulin secretion by plasma cells as well as T cell effector functions. IL-6 and transforming growth factor-β (TGF-β) with or without IL-1, IL-18, and TNF-α promote Th17 cell differentiation through the activation of the transcription factors STAT3 and RORγt. Conversely, IL-6 suppresses the transcription factor forkhead box P3 (FOXP3), which results in decreased numbers of T regulatory cells. Th17 cells have been implicated as pathogenic effector cells in a variety of autoimmune disorders (see below).
IL-6 is elevated in the serum and more so in the urine of some lupus patients and studies in murine lupus models support a role for IL-6 in nephritis (Iwano and others 1993; Peterson and others 1996; Tsai and others 2000; Liang and others 2006; Cash and others 2010). IL-6 is also observed in kidney biopsies of patients with renal disease (Malide and others 1995). A specific antibody to the IL-6R, tocilizumab, has shown efficacy in the treatment of rheumatoid arthritis (Smolen and others 2008). A trial with tocilizumab for lupus patients demonstrated decreased disease activity, but patients also experienced several adverse reactions, indicating that tocilizumab will be an unlikely choice for future lupus trials (Illei and others 2010).
Interleukin-10
IL-10 functions primarily as a negative feedback inhibitor for activated macrophages and dendritic cells, suppressing expression of IL-12, HLA-DR, and costimulatory molecules such as CD40, CD80, and CD86. IL-10 also directly suppresses T cell effector function, but IL-10 appears to promote B cell activation. IL-10 is produced mainly by activated macrophages and it can also be produced in lower amounts by other cell types, including T cells and keratinocytes. IL-10 binds to a type II cytokine receptor that forms a heterodimer. IL-10 receptors are expressed mostly on immune cells with the highest expression on monocytes and macrophages (Sabat 2010).
In lupus, the serum levels of IL-10 are elevated and tend to correlate with disease activity (Park and others 1998). Work in murine models support a therapeutic role for IL-10 antagonists in lupus (Llorente and others 1995) A small trial with a single injection of the murine anti-IL-10 mAb, B-N10, was conducted in lupus patients with active disease. Although there was an overall improvement in disease activity, all patients developed antimurine antibodies (Llorente and others 2000). No follow-up studies have been reported.
BAFF and APRIL
Lupus serum contains elevated levels of BAFF, APRIL, and BAFF/APRIL heterotrimers (Davidson 2010). BAFF, which stands for B cell activating factor [also known as B-lymphocyte stimulator (BLyS, TALL1) and TNF (ligand) superfamily member 13b or TNFSF13B], and APRIL (a proliferation inducing ligand; TNFSF13) are both produced by monocytes and macrophages after encountering pro-inflammatory cytokines such as the IFNs (Mackay and Schneider 2009). BAFF trimers bind to 3 TNF superfamily members: (a) CD267 or transmembrane activator and CAML interactor (TACI) or TNFR superfamily member 13B (TNFRSF13B); (b) CD268 or BAFF receptor (BR3, BAFF-R, and TNFRSF13C), and (c) CD269 or B-cell maturation antigen (BCMA; TNFRSF17). BAFF and APRIL trimers bind to TACI and BCMA, but only BAFF binds to the BAFF-R. In general, BAFF and APRIL promote B cell survival during different stages of B cell differentiation. B cell survival, enhanced germinal center reactions, and antibody class-switch recombination are supported by BAFF-R engagement. TACI is involved in T-cell-independent B cell immunoglobulin responses, and it also plays a role in class-switch recombination during antibody production. BCMA is important later during B cell differentiation into plasma cells, and it is thus involved in sustaining humoral antibody responses (Khan 2009; Mackay and Schneider 2009).
Autoreactive B cells are more dependent on BAFF relative to naïve mature B cells (Chan 2011). BAFF, APRIL, and BAFF/APRIL heterotrimers are elevated in the serum of SLE patients and levels correlate with disease activity (Zhang and others 2001; Roschke and others 2002). Moreover, overexpression of BAFF in mice results in a lupus-like disorder that does not rely on T cells (Groom and others 2007). While 4 biologic agents have been developed to targets in this pathway, only a fully humanized mAb that binds to BAFF, known as belimumab, has completed testing in phase III trials (Thanou-Stavraki and Sawalha 2011). Belimumab has recently received FDA approval for the treatment of SLE. Of note, the criteria for phase II clinical trials were measured by a new composite score called the SLE Responder Index (SRI), which required that patients on belimumab demonstrated an improved score on the Safety of Estrogens in Lupus Erythematosus: National Assessment (SELENA) version of the SLEDAI and no decrease in British Isles Lupus Assessment Group (BILAG) scores as well as other criteria. While the primary endpoint for the phase II study was not met, the results led to the development of the new index employed for the phase III trials. Improved responses to belimumab were observed in patients with positive antinuclear antibodies or antidouble-stranded DNA antibodies or both, and there was a marked reduction in naïve B cells, activated B cells, and CD20+ plasmablasts in the treated subjects. Results for the 2 phase III trials, BLISS-52 and BLISS-76, along with the earlier trials, have recently been summarized in a review (Thanou-Stavraki and Sawalha 2011). It is noteworthy that belimumab is the first biologic to meet its primary endpoint in a phase III clinical trial for lupus patients and gain FDA approval.
Cytokines Elaborated by the Adaptive Immune System
Interferon-γ
IFN-γ is increased in the serum of some SLE patients and IFN-γ levels have been shown to correlate with disease activity (al-Janadi and others 1993; Akahoshi and others 1999). IFN-γ is produced as a homodimer and its receptor is a type II cytokine receptor composed of 1 cytokine-binding receptor and 1 signal transduction (STAT1 activating) protein. IFN-γ is produced by NK cells during the initiation of an immune response and by Th-1 cells and CD8+ T cells during the adaptive phase of an immune response. IFN-γ production by T cells is enhanced by IL-12 and IL-18. IFN-γ activates macrophage microbicidal functions, upregulates class I and class II MHC molecules on antigen presenting cells, modulates T cell differentiation, and is involved in B cell isotype switch to opsonizing and complement-fixing antibody subclasses. IFN-γ can be detected in the kidneys of renal SLE patients and might be involved in the disease pathogenesis (Uhm and others 2003) This observation is supported by murine studies in which disease was advanced by IFN-γ administration to (NZB×NZW)F1 lupus prone mice and ameliorated by IFN-γ receptor deficiency (Jacob and others 1987; Haas and others 1998; Schwarting and others 1998). Of note, SLE patients have more IFN-γ-producing effector cells in the periphery as compared to healthy controls, which correlates with increased BAFF production by myeloid cells (Harigai and others 2008). A fully humanized anti-IFN-γ mAb, AMG 811, is in phase 1b trials; however, because of the risk of infection this therapy would likely only be utilized in a small subset of patients, as discussed elsewhere (Ronnblom and Elkon 2010).
Interleukin-17
Elevated serum levels of IL-17A and increased numbers of T cells secreting IL-17 (Ouyang and others 2008; Yang and others 2009) have been detected in SLE patients and in murine lupus models. The IL-17 family consists of IL-17A–IL-17F and each cytokine can be composed of either homodimers or heterodimers, with IL-17A being the most intensely studied form. IL-17 binds to 3 of 4 family member receptor subunits, IL-17RA, IL-17RC, and CBAD, which appear to form a complex that results in signal transduction much like gp130. IL-17RA is ubiquitously expressed with the highest levels being found on hematopoietic tissues. Of interest, fibroblasts, epithelial cells, and endothelial cells are the most responsive to IL-17. These cytokines enhance the production of TNF, IL-1, granulocyte colony-stimulating factor, chemokines, and other molecules. IL-17A is a potent neutrophil chemoattractant and it is the primary form of IL-17 secreted by Th17 cells. While several cytokines, including IL-6, IL-1, IL-21, and TGF-β, can drive Th17 development, the cytokine IL-23, which is produced by myeloid lineage cells, has been shown to be required for the expansion and terminal differentiation of Th17 cells (Ouyang and others 2008; Korn and others 2009). In lupus, as in a number of other autoimmune diseases, Th17 cells traffic to inflamed tissues, such as the kidney and promote inflammation by enhancing cytokine production, which can in turn activate B cell antibody production, activate dendritic cells, and stimulate resident cells in the target tissues (Yang and others 2009; Zhang and others 2009). Both CD3+CD4+Th17 cells and increased numbers of double-negative cells (CD3+CD4−CD8−) derived from CD3+CD8+ T cells have been reported to contribute to IL-17A production in lupus. Along with IL-17, CD4+ Th17 cells that express RORα and RORγt can also produce the cytokines IL-17F, IL-21, and IL-22, which can propagate the inflammatory response. At least 3 mAbs targeting components of the IL-17 pathway are under study for various autoimmune diseases, including rheumatoid arthritis, inflammatory bowel disease, and psoriasis (Ronnblom and Elkon 2010). One is a fully humanized neutralizing anti-IL-17 mAb (AIN457) in phase II trials as is the anti-IL-17 mAb LY2439821. Ustekinumab, a mAb to the p40 subunit that blocks binding of both IL-12 and IL-23 to the IL-17 receptor, is in clinical trials for psoriasis and related diseases. The efficacy of these therapies for lupus remains to be determined.
Interleukin-21
Several cytokine genes have also been directly associated with SLE susceptibility, including IL-10, IFNG, and IL-21, and the receptor for IL-21, IL-21R (Sawalha and others 2008; Gateva and others 2009; Webb and others 2009; Kim and others 2010). Produced by T cells, IL-21 binds a heterodimeric receptor expressed on mononuclear cells. The expression of IL-21 and its receptor can be regulated by IFN-α (Strengell and others 2004; Rochman and others 2009). Although IL-21 can have proapoptotic effects on naïve B cells, it is a potent inducer of B cell proliferation and differentiation into plasma cells. In this regard, IL-21 is secreted by specialized T follicular helper cells in B cell follicles. IL-21 is also produced by peripheral T cells to induce Th17 cells (Rochman and others 2009). As with the other cytokines, elevated serum levels of IL-21 have been observed in lupus patients and an allelic variant of IL-21 and its receptor have been identified as conferring SLE susceptibility (Sawalha and others 2008; Wong and others 2010). These results suggest that IL-21 could be an excellent target for therapeutic intervention in a subset of SLE patients. Indeed, limited evidence from murine lupus studies supports the pathogenic role of IL-21 in lupus (Herber and others 2007; Bubier and others 2009; Linterman and others 2009).
Th2 cytokines
Th2 cytokines include IL-4, IL-5, IL-9, IL-13, and IL-25 (also known as IL-17E). IL-4 has been the most extensively studied of the Th2 cytokines in autoimmune disease because of its powerful role in suppressing both Th1 and macrophage responses. The IL-4R is a type I cytokine receptor and is composed of the cytokine-binding α-chain and the associated common γ-chain, which generates downstream signal transduction. IL-4 activates the transcription factors STAT6 and GATA3, which generate classical Th2 cells. Limited evidence indicates that Th2 cytokines, notably IL-4, can potentially play a role in murine lupus pathogenesis (Shimizu and others 2005; Shiroiwa and others 2007; Charles and others 2010), whereas other reports demonstrate no systemic role for IL-4 in murine lupus (Kono and others 2000; Hasegawa and others 2002). Indeed, Th2-regulated JAK/STAT activation appears to underlie the pathogenesis of glomerulosclerotic lesions in murine lupus nephritis (Singh and others 2003). A few studies have also reported that Th2 cytokines may be elevated in a subset of SLE patients (Houssiau and others 1995; Richaud-Patin and others 1995; Nagy and others 2000; Munoz-Valle and others 2003). In contrast to the other cytokines discussed above, Th2 blockade has not emerged as an attractive therapeutic modality in lupus.
Conclusions
These studies support a pivitol role for cytokines in lupus pathogenesis. It is clear that the actions of individual cytokines (eg, IL-10) can be distinct and even at odds with their ability to drive autoimmune inflammation in combination with other mediators. To date only 1 biologic, belimumab, has received FDA approval for the treatment of SLE. The studies with belimumab bring up the urgent need for better measurements of disease activity, higher-quality biomarkers, and improved trial design in order to determine the efficacy and safety of new anticytokine therapies. Novel biomarkers combined with new genetic information employed to subset patient groups for specific treatment regimens could vastly improve the outcomes of clinical trials for new therapies.
Author Disclosure Statement
No competing financial interests exist.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Akahoshi M. Nakashima H. Tanaka Y. Kohsaka T. Nagano S. Ohgami E. Arinobu Y. Yamaoka K. Niiro H. Shinozaki M. Hirakata H. Horiuchi T. Otsuka T. Niho Y. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis Rheum. 1999;42:1644–1648. doi: 10.1002/1529-0131(199908)42:8<1644::AID-ANR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- al-Janadi M. al-Balla S. al-Dalaan A. Raziuddin S. Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J Clin Immunol. 1993;13:58–67. doi: 10.1007/BF00920636. [DOI] [PubMed] [Google Scholar]
- Aringer M. Houssiau F. Gordon C. Graninger WB. Voll RE. Rath E. Steiner G. Smolen JS. Adverse events and efficacy of TNF-alpha blockade with infliximab in patients with systemic lupus erythematosus: long-term follow-up of 13 patients. Rheumatology (Oxford) 2009;48:1451–1454. doi: 10.1093/rheumatology/kep270. [DOI] [PubMed] [Google Scholar]
- Aringer M. Smolen JS. The role of tumor necrosis factor-alpha in systemic lupus erythematosus. Arthritis Res Ther. 2008;10:202. doi: 10.1186/ar2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baechler EC. Batliwalla FM. Karypis G. Gaffney PM. Ortmann WA. Espe KJ. Shark KB. Grande WJ. Hughes KM. Kapur V. Gregersen PK. Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bave U. Vallin H. Alm GV. Ronnblom L. Activation of natural interferon-alpha producing cells by apoptotic U937 cells combined with lupus IgG and its regulation by cytokines. J Autoimmun. 2001;17:71–80. doi: 10.1006/jaut.2001.0519. [DOI] [PubMed] [Google Scholar]
- Bengtsson AA. Sturfelt G. Truedsson L. Blomberg J. Alm G. Vallin H. Ronnblom L. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9:664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- Bennett L. Palucka AK. Arce E. Cantrell V. Borvak J. Banchereau J. Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco P. Palucka AK. Gill M. Pascual V. Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- Braun D. Geraldes P. Demengeot J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- Brugos B. Kiss E. Dul C. Gubisch W. Szegedi G. Sipka S. Zeher M. Measurement of interleukin-1 receptor antagonist in patients with systemic lupus erythematosus could predict renal manifestation of the disease. Hum Immunol. 2010;71:874–877. doi: 10.1016/j.humimm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Bubier JA. Sproule TJ. Foreman O. Spolski R. Shaffer DJ. Morse HC., 3rd Leonard WJ. Roopenian DC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash H. Relle M. Menke J. Brochhausen C. Jones SA. Topley N. Galle PR. Schwarting A. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol. 2010;37:60–70. doi: 10.3899/jrheum.090194. [DOI] [PubMed] [Google Scholar]
- Chan AC. B cell immunotherapy in autoimmunity -2010 update. Mol Immunol. 2011;48:1344–1347. doi: 10.1016/j.molimm.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Charles N. Hardwick D. Daugas E. Illei GG. Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MK. Collaboration, genetic associations, and lupus erythematosus. N Engl J Med. 2008;358:956–961. doi: 10.1056/NEJMe0800096. [DOI] [PubMed] [Google Scholar]
- Crow MK. Kirou KA. Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36:481–490. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- Davidson A. Targeting BAFF in autoimmunity. Curr Opin Immunol. 2010;22:732–739. doi: 10.1016/j.coi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst AM. Xie C. Fu Y. Wang A. Boudreaux C. Zhou XJ. Cibotti R. Coyle A. Connolly JE. Wakeland EK. Mohan C. Type I interferons produced by resident renal cells may promote end-organ disease in autoantibody-mediated glomerulonephritis. J Immunol. 2009;183:6831–6838. doi: 10.4049/jimmunol.0900742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. Xie C. Yan M. Li Q. Joh JW. Lu C. Mohan C. The lipopolysaccharide-triggered mesangial transcriptome: Evaluating the role of interferon regulatory factor-1. Kidney Int. 2005;67:1350–1361. doi: 10.1111/j.1523-1755.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- Gateva V. Sandling JK. Hom G. Taylor KE. Chung SA. Sun X. Ortmann W. Kosoy R. Ferreira RC. Nordmark G. Gunnarsson I. Svenungsson E. Padyukov L. Sturfelt G. Jonsen A. Bengtsson AA. Rantapaa-Dahlqvist S. Baechler EC. Brown EE. Alarcon GS. Edberg JC. Ramsey-Goldman R. McGwin G., Jr. Reveille JD. Vila LM. Kimberly RP. Manzi S. Petri MA. Lee A. Gregersen PK. Seldin MF. Ronnblom L. Criswell LA. Syvanen AC. Behrens TW. Graham RR. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41:1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom JR. Fletcher CA. Walters SN. Grey ST. Watt SV. Sweet MJ. Smyth MJ. Mackay CR. Mackay F. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C. Ryffel B. Le Hir M. IFN-gamma receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB x NZW)F1 mice. J Immunol. 1998;160:3713–3718. [PubMed] [Google Scholar]
- Harigai M. Kawamoto M. Hara M. Kubota T. Kamatani N. Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181:2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- Harley JB. Alarcon-Riquelme ME. Criswell LA. Jacob CO. Kimberly RP. Moser KL. Tsao BP. Vyse TJ. Langefeld CD. Nath SK. Guthridge JM. Cobb BL. Mirel DB. Marion MC. Williams AH. Divers J. Wang W. Frank SG. Namjou B. Gabriel SB. Lee AT. Gregersen PK. Behrens TW. Taylor KE. Fernando M. Zidovetzki R. Gaffney PM. Edberg JC. Rioux JD. Ojwang JO. James JA. Merrill JT. Gilkeson GS. Seldin MF. Yin H. Baechler EC. Li QZ. Wakeland EK. Bruner GR. Kaufman KM. Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K. Hayashi T. Maeda K. Promotion of lupus in NZB x NZWF1 mice by plasmids encoding interferon (IFN)-gamma but not by those encoding interleukin (IL)-4. J Comp Pathol. 2002;127:1–6. doi: 10.1053/jcpa.2002.0556. [DOI] [PubMed] [Google Scholar]
- Herber D. Brown TP. Liang S. Young DA. Collins M. Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- Hooks JJ. Moutsopoulos HM. Geis SA. Stahl NI. Decker JL. Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- Houssiau FA. Lefebvre C. Vanden Berghe M. Lambert M. Devogelaer JP. Renauld JC. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–395. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- Hron JD. Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173:2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- Illei GG. Shirota Y. Yarboro CH. Daruwalla J. Tackey E. Takada K. Fleisher T. Balow JE. Lipsky PE. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M. Dohi K. Hirata E. Kurumatani N. Horii Y. Shiiki H. Fukatsu A. Matsuda T. Hirano T. Kishimoto T. Urinary levels of IL-6 in patients with active lupus nephritis. Clin Nephrol. 1993;40:16–21. others. [PubMed] [Google Scholar]
- Jacob CO. van der Meide PH. McDevitt HO. In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J Exp Med. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob CO. Zhu J. Armstrong DL. Yan M. Han J. Zhou XJ. Thomas JA. Reiff A. Myones BL. Ojwang JO. Kaufman KM. Klein-Gitelman M. McCurdy D. Wagner-Weiner L. Silverman E. Ziegler J. Kelly JA. Merrill JT. Harley JB. Ramsey-Goldman R. Vila LM. Bae SC. Vyse TJ. Gilkeson GS. Gaffney PM. Moser KL. Langefeld CD. Zidovetzki R. Mohan C. Identification of IRAK1 as a risk gene with critical role in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2009;106:6256–6261. doi: 10.1073/pnas.0901181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- Kariuki SN. Franek BS. Kumar AA. Arrington J. Mikolaitis RA. Utset TO. Jolly M. Crow MK. Skol AD. Niewold TB. Trait-stratified genome-wide association study identifies novel and diverse genetic associations with serologic and cytokine phenotypes in systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R151. doi: 10.1186/ar3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki SN. Kirou KA. MacDermott EJ. Barillas-Arias L. Crow MK. Niewold TB. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J Immunol. 2009;182:34–38. doi: 10.4049/jimmunol.182.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki SN. Niewold TB. Genetic regulation of serum cytokines in systemic lupus erythematosus. Transl Res. 2010;155:109–117. doi: 10.1016/j.trsl.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz U. Zandman-Goddard G. Drug-induced lupus: an update. Autoimmun Rev. 2010;10:46–50. doi: 10.1016/j.autrev.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183:3561–3567. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- Kim K. Cho SK. Sestak A. Namjou B. Kang C. Bae SC. Interferon-gamma gene polymorphisms associated with susceptibility to systemic lupus erythematosus. Ann Rheum Dis. 2010;69:1247–1250. doi: 10.1136/ard.2009.117572. [DOI] [PubMed] [Google Scholar]
- Kirou KA. Lee C. George S. Louca K. Peterson MG. Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- Kono DH. Balomenos D. Park MS. Theofilopoulos AN. Development of lupus in BXSB mice is independent of IL-4. J Immunol. 2000;164:38–42. doi: 10.4049/jimmunol.164.1.38. [DOI] [PubMed] [Google Scholar]
- Korn T. Bettelli E. Oukka M. Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kumar KR. Li L. Yan M. Bhaskarabhatla M. Mobley AB. Nguyen C. Mooney JM. Schatzle JD. Wakeland EK. Mohan C. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312:1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- Liang B. Gardner DB. Griswold DE. Bugelski PJ. Song XY. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119:296–305. doi: 10.1111/j.1365-2567.2006.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA. Rigby RJ. Wong RK. Yu D. Brink R. Cannons JL. Schwartzberg PL. Cook MC. Walters GD. Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente L. Richaud-Patin Y. Garcia-Padilla C. Claret E. Jakez-Ocampo J. Cardiel MH. Alcocer-Varela J. Grangeot-Keros L. Alarcon-Segovia D. Wijdenes J. Galanaud P. Emilie D. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Llorente L. Zou W. Levy Y. Richaud-Patin Y. Wijdenes J. Alcocer-Varela J. Morel-Fourrier B. Brouet JC. Alarcon-Segovia D. Galanaud P. Emilie D. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovgren T. Eloranta ML. Bave U. Alm GV. Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- Mackay F. Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- Malide D. Russo P. Bendayan M. Presence of tumor necrosis factor alpha and interleukin-6 in renal mesangial cells of lupus nephritis patients. Hum Pathol. 1995;26:558–564. doi: 10.1016/0046-8177(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Moosig F. Zeuner R. Renk C. Schroder JO. IL-1RA in refractory systemic lupus erythematosus. Lupus. 2004;13:605–606. doi: 10.1191/0961203304lu1047cr. [DOI] [PubMed] [Google Scholar]
- Munoz-Valle JF. Vazquez-Del Mercado M. Garcia-Iglesias T. Orozco-Barocio G. Bernard-Medina G. Martinez-Bonilla G. Bastidas-Ramirez BE. Navarro AD. Bueno M. Martinez-Lopez E. Best-Aguilera CR. Kamachi M. Armendariz-Borunda J. T(H)1/T(H)2 cytokine profile, metalloprotease-9 activity and hormonal status in pregnant rheumatoid arthritis and systemic lupus erythematosus patients. Clin Exp Immunol. 2003;131:377–384. doi: 10.1046/j.1365-2249.2003.02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G. Pallinger E. Antal-Szalmas P. Aleksza M. Marschalko M. Brozik M. Falus A. Gergely P. Measurement of intracellular interferon-gamma and interleukin-4 in whole blood T lymphocytes from patients with systemic lupus erythematosus. Immunol Lett. 2000;74:207–210. doi: 10.1016/s0165-2478(00)00265-0. [DOI] [PubMed] [Google Scholar]
- Niewold TB. Hua J. Lehman TJ. Harley JB. Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB. Kelly JA. Flesch MH. Espinoza LR. Harley JB. Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N. Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- Ostendorf B. Iking-Konert C. Kurz K. Jung G. Sander O. Schneider M. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann Rheum Dis. 2005;64:630–633. doi: 10.1136/ard.2004.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W. Kolls JK. Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka AK. Blanck JP. Bennett L. Pascual V. Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A. 2005;102:3372–3377. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YB. Lee SK. Kim DS. Lee J. Lee CH. Song CH. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:283–288. [PubMed] [Google Scholar]
- Peterson E. Robertson AD. Emlen W. Serum and urinary interleukin-6 in systemic lupus erythematosus. Lupus. 1996;5:571–575. doi: 10.1177/096120339600500603. [DOI] [PubMed] [Google Scholar]
- Peterson KS. Huang JF. Zhu J. D'Agati V. Liu X. Miller N. Erlander MG. Jackson MR. Winchester RJ. Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J Clin Invest. 2004;113:1722–1733. doi: 10.1172/JCI19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richaud-Patin Y. Alcocer-Varela J. Llorente L. High levels of TH2 cytokine gene expression in systemic lupus erythematosus. Rev Invest Clin. 1995;47:267–272. [PubMed] [Google Scholar]
- Rochman Y. Spolski R. Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnblom L. Alm GV. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol. 2001a;22:427–431. doi: 10.1016/s1471-4906(01)01955-x. [DOI] [PubMed] [Google Scholar]
- Ronnblom L. Alm GV. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J Exp Med. 2001b;194:F59–F63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnblom L. Elkon KB. Cytokines as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:339–347. doi: 10.1038/nrrheum.2010.64. [DOI] [PubMed] [Google Scholar]
- Roschke V. Sosnovtseva S. Ward CD. Hong JS. Smith R. Albert V. Stohl W. Baker KP. Ullrich S. Nardelli B. Hilbert DM. Migone TS. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J Immunol. 2002;169:4314–4321. doi: 10.4049/jimmunol.169.8.4314. [DOI] [PubMed] [Google Scholar]
- Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21:315–324. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Santiago-Raber ML. Baccala R. Haraldsson KM. Choubey D. Stewart TA. Kono DH. Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawalha AH. Kaufman KM. Kelly JA. Adler AJ. Aberle T. Kilpatrick J. Wakeland EK. Li QZ. Wandstrat AE. Karp DR. James JA. Merrill JT. Lipsky P. Harley JB. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis. 2008;67:458–461. doi: 10.1136/ard.2007.075424. [DOI] [PubMed] [Google Scholar]
- Schwarting A. Wada T. Kinoshita K. Tesch G. Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol. 1998;161:494–503. [PubMed] [Google Scholar]
- Shimizu S. Sugiyama N. Masutani K. Sadanaga A. Miyazaki Y. Inoue Y. Akahoshi M. Katafuchi R. Hirakata H. Harada M. Hamano S. Nakashima H. Yoshida H. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1) J Immunol. 2005;175:7185–7192. doi: 10.4049/jimmunol.175.11.7185. [DOI] [PubMed] [Google Scholar]
- Shiroiwa W. Tsukamoto K. Ohtsuji M. Lin Q. Ida A. Kodera S. Ohtsuji N. Nakamura K. Tsurui H. Kinoshita K. Nishimura H. Shirai T. Hirose S. IL-4Ralpha polymorphism in regulation of IL-4 synthesis by T cells: implication in susceptibility to a subset of murine lupus. Int Immunol. 2007;19:175–183. doi: 10.1093/intimm/dxl134. [DOI] [PubMed] [Google Scholar]
- Sigurdsson S. Nordmark G. Goring HH. Lindroos K. Wiman AC. Sturfelt G. Jonsen A. Rantapaa-Dahlqvist S. Moller B. Kere J. Koskenmies S. Widen E. Eloranta ML. Julkunen H. Kristjansdottir H. Steinsson K. Alm G. Ronnblom L. Syvanen AC. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RR. Saxena V. Zang S. Li L. Finkelman FD. Witte DP. Jacob CO. Differential contribution of IL-4 and STAT6 vs STAT4 to the development of lupus nephritis. J Immunol. 2003;170:4818–4825. doi: 10.4049/jimmunol.170.9.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen JS. Beaulieu A. Rubbert-Roth A. Ramos-Remus C. Rovensky J. Alecock E. Woodworth T. Alten R. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- Strengell M. Julkunen I. Matikainen S. IFN-alpha regulates IL-21 and IL-21R expression in human NK and T cells. J Leukoc Biol. 2004;76:416–422. doi: 10.1189/jlb.1003488. [DOI] [PubMed] [Google Scholar]
- Studnicka-Benke A. Steiner G. Petera P. Smolen JS. Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol. 1996;35:1067–1074. doi: 10.1093/rheumatology/35.11.1067. [DOI] [PubMed] [Google Scholar]
- Sturfelt G. Roux-Lombard P. Wollheim FA. Dayer JM. Low levels of interleukin-1 receptor antagonist coincide with kidney involvement in systemic lupus erythematosus. Br J Rheumatol. 1997;36:1283–1289. doi: 10.1093/rheumatology/36.12.1283. [DOI] [PubMed] [Google Scholar]
- Taga T. Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- Thanou-Stavraki A. Sawalha AH. An update on belimumab for the treatment of lupus. Biologics. 2011;5:33–43. doi: 10.2147/BTT.S13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CY. Wu TH. Yu CL. Lu JY. Tsai YY. Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron. 2000;85:207–214. doi: 10.1159/000045663. [DOI] [PubMed] [Google Scholar]
- Tucci M. Quatraro C. Lombardi L. Pellegrino C. Dammacco F. Silvestris F. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 2008;58:251–262. doi: 10.1002/art.23186. [DOI] [PubMed] [Google Scholar]
- Uhm WS. Na K. Song GW. Jung SS. Lee T. Park MH. Yoo DH. Cytokine balance in kidney tissue from lupus nephritis patients. Rheumatology (Oxford) 2003;42:935–938. doi: 10.1093/rheumatology/keg255. [DOI] [PubMed] [Google Scholar]
- Vereecke L. Beyaert R. van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30:383–391. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Webb R. Merrill JT. Kelly JA. Sestak A. Kaufman KM. Langefeld CD. Ziegler J. Kimberly RP. Edberg JC. Ramsey-Goldman R. Petri M. Reveille JD. Alarcon GS. Vila LM. Alarcon-Riquelme ME. James JA. Gilkeson GS. Jacob CO. Moser KL. Gaffney PM. Vyse TJ. Nath SK. Lipsky P. Harley JB. Sawalha AH. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum. 2009;60:2402–2407. doi: 10.1002/art.24658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CK. Wong PT. Tam LS. Li EK. Chen DP. Lam CW. Elevated production of B cell chemokine CXCL13 is correlated with systemic lupus erythematosus disease activity. J Clin Immunol. 2010;30:45–52. doi: 10.1007/s10875-009-9325-5. [DOI] [PubMed] [Google Scholar]
- Yang J. Chu Y. Yang X. Gao D. Zhu L. Wan L. Li M. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- Yao Y. Richman L. Higgs BW. Morehouse CA. de los Reyes M. Brohawn P. Zhang J. White B. Coyle AJ. Kiener PA. Jallal B. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- Ytterberg SR. Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 1982;25:401–406. doi: 10.1002/art.1780250407. [DOI] [PubMed] [Google Scholar]
- Zagury D. Le Buanec H. Mathian A. Larcier P. Burnett R. Amoura Z. Emilie D. Peltre G. Bensussan A. Bizzini B. Gallo RC. Koutouzov S. IFNalpha kinoid vaccine-induced neutralizing antibodies prevent clinical manifestations in a lupus flare murine model. Proc Natl Acad Sci U S A. 2009;106:5294–5299. doi: 10.1073/pnas.0900615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Roschke V. Baker KP. Wang Z. Alarcon GS. Fessler BJ. Bastian H. Kimberly RP. Zhou T. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Kyttaris VC. Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]