Abstract
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis was conducted to determine whether or not there are interstrain or site-dependent differences in the gene expression profiles of skeletal muscles in SJL/J and A/J mice as dysferlinopathy models. Upon analysis by qRT-PCR, SJL/J mice showed a trend of increased gene expression level of uncoupling protein 2 in the rectus femoris and longissimus lumborum at 30 weeks of age when dystrophic lesions became histopathologically pronounced. Heme oxygenase 1 and S100 calcium binding protein A4 were upregulated in the rectus femoris, longissimus lumborum and abdominal muscles, in which dystrophic lesions occur more commonly in SJL mice. The gene expression levels of heat shock protein 70 in most muscles of A/J mice were lower than those of BALB/c mice as control. SJL/J mice exhibited a marked lowering of decay-accelerating factor 1/CD55 gene expression level in all studied muscles except for the heart at all ages compared with that of BALB/c mice. This study showed that there were some interstrain differences in the gene expres sion profiles of skeletal muscles between SJL/J and A/J mice. Further investigation is required to reveal whether these alterations of the expression levels are the cause of dystrophic changes or occur subsequent to muscle damage.
Keywords: A/J mouse, decay-accelerating factor, dysferlin, heat shock protein, heme oxygenase-1, SJL/J mouse
Introduction
Limb-girdle muscular dystrophy type 2B (LGMD2B) and Miyoshi myopathy (MM) are both caused by recessively inherited mutations in the dysferlin gene1. LGMD2B is characterized by the progressive wasting and weakness of proximal lower limb-girdle muscles. Meanwhile, the distal muscle groups of the limbs and girdle are mostly affected in MM. Both disorders have been considered to be due to a loss of dysferlin protein at the plasma membrane in muscle fibers, which leads to abnormalities in vesicle traffic and membrane repair2,3, and are collectively called ‘dysferlinopathy’.
Two naturally occurring animal models for LGMD2B, SJL/J and A/J mice, have been identified to have mutations in the dysferlin gene associated with phenotypic features of progressive muscular dystrophy4,5. However, the type of dysferlin gene mutation differs between SJL/J and A/J mice. SJL/J mice have a splice site mutation that removes a part of the highly conserved C2E domain; the domain is known to bind to calcium, phospholipids or proteins to trigger signaling events and membrane trafficking4,6,7. On the other hand, A/J mice bear a unique ETn retrotransposon insertion near the 5’ end (intron 4) of the dysferlin gene5. Interestingly, the two strains show phenotypic differences from each other: A/J mice display a later age of onset and a slower progression of the muscle disease than SJL/J mice5. Our previous study showed that there were differences in the progress and prevalent site of skeletal muscle lesions between SJL/J and A/J mice8. In particular, the difference in sensitivity to muscular dystrophic lesions between SJL/J and A/J mice was most apparent in the lumbar (longissimus and sublumbar) muscles. These findings support the hypothesis that additional enhancers or modifiers may be involved in the progression of skeletal muscle lesions in dysferlinopathy.
To shed some light on the molecular pathogenesis of dysferlinopathy, gene expression profiling studies have been undertaken in the skeletal muscles of SJL/J mice, C57BL/10. SJL-Dysf mice and LGMD2B patients9–12. However, gene expression profiles of A/J mice or gene expression comparison studies between SJL/J and A/J mice have not yet to be reported. In this study, toward the goal of discovering ad ditional enhancers or modifiers associated with phenotypic divergence between SJL/J and A/J mice, the temporal or site-specific gene expression was analyzed by a quantitative real-time polymerase chain reaction (qRT-PCR) using Taq-Man® Gene Expression Assays.
Materials and Methods
Animals
BALB/c mice were used as control because this strain does not show abnormality in the skeletal muscles by the age of 16 months and has been used as a control in the histopathological examination of skeletal muscles in SJL/J mice13. Male BALB/c mice and male SJL/J mice were obtained from Charles River Laboratories Japan Inc. (Kanagawa, Japan). Male A/J mice were purchased from Japan SLC, Inc. (Shizuoka, Japan). All mice were brought to the test facility (Safety Research Laboratory (Kashima), Mitsubishi Tanabe Pharma Corporation, Osaka, Japan) at the age of 4, 7 or 8 weeks. These animals were housed in plastic cages in an animal room kept under controlled conditions (temperature of 23 ± 2 °C, humidity of 30 to 70%, ventilation of 12 times or more per hour, lighting for 12 hours between 06:30 to 18:30) in the test facility. The mice were given pelleted feed sterilized by 15-kGy gamma irradiation (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan) and industrial water ad libitum via an automatic water feeder.
All experimental procedures were approved by the Animal Ethics Committee of Mitsubishi Tanabe Pharma Corporation and were conducted in accordance with the Experimental Guide to the Care and Use of Laboratory Animals.
Histopathology
Three male mice from each strain were euthanized by exsanguination from the abdominal aorta under ether anesthesia at 10 and 30 weeks of age. The femoral, crural, brachial, forearm, abdominal and lumbar muscles were fixed in 10% neutral buffered formalin; skeletal muscles including bones were decalcified using 50% K-CX (Falma Co., Ltd., Tokyo, Japan) solution for decalcification, processed and embedded in paraffin. Tissue paraffin blocks were sectioned at a thickness of 2 µm, and each section was stained with hematoxylin and eosin (HE).
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Genes that have been shown to change in the skeletal muscles of SJL/J mice, dystrophin-deficient mice or muscular dystrophy patients or to be linked to these gene expression changes were mainly selected for qRT-PCR analysis.
Lipid metabolism-associated genes: SJL/J mice have a mutation in the Tbc1d1 gene that results in a truncated protein lacking the TBC Rab–GTPase-activating protein domain14 and showed increased fatty acid uptake/oxidation and reduced glucose uptake in isolated skeletal muscle. Uncoupling protein-3 and −2 (Ucp3, Ucp2) gene expressions as candidate genes in the regulation of lipids as metabolic fuels in skeletal muscle15 were measured with that of peroxisome proliferative activated receptor-γ coactivator 1α (PGC-1α) regulating the expression of many genes involved with fatty acid oxidation16.
Skeletal muscle atrophy-associated genes: Myostatin as an endogenous negative regulator of muscle growth17 and follistatin as an endogenous antagonist of myostatin18, which have attracted attention as therapeutic targets of muscular disorders19, were quantified.
Ubiquitin-proteasome system-associated genes, ubiquitin-like protein gene and ER stress-associated genes: Normal dysferlin was degraded by an endoplasmic reticulum (ER)-associated degradation system (ERAD) composed of ubiquitin/proteasome. However, mutant dysferlin spontaneously aggregated in the ER and induced eukaryotic translation initiation factor 2 alpha phosphorylation and LC3 conversion, a key step for autophagosome formation, and, finally, ER stress cell death20. Because SJL/J mice produce incomplete dysferlin protein, ERAD and ER stress-associated genes were included in the examination of gene expression.
Heat shock protein genes: An in vitro study using mouse skeletal muscle myotubes indicated that an increased level of heat shock proteins (HSPs) may provide protection against the muscle damage that occurs by a pathological increase in intracellular calcium or uncoupling of the mitochondrial respiratory chain21. Some HSPs were selected as targets for evaluation of the protective system in the skeletal muscles of SJL/J and A/J mice.
ER-associated degradation of glycoprotein-associated genes: It is known that major histocompatibility complex (MHC) class I expression is markedly upregulated in myopathic muscles of dysferlin-deficient SJL/J mice22. The assembly and folding of MHC class I molecules are associated with calnexin, calreticulin and ERp5723. Transcriptional induction of the ER degradation enhancer mannosidase alpha-like 1 (Edem1) is required for degradation of misfolded glycoprotein substrates including MHC class I24. To confirm whether or not excess MHC class I causes the upregulation of glycoprotein ERAD, these gene expression profiles of SJL/J and A/J mice were determined.
Histone deacetylase genes and nitric oxide synthase genes: Histone deacetylase inhibitors and nitro oxide donors delay the progression of muscular dystrophy in dystrophindeficient mice25,26. Because histone deacetylase and nitro oxide may be related to the development of dystrophic lesions in SJL/J and A/J mice, these gene expressions in SJL/J and A/J mice were compared with those of BALB/c mice.
Oxidative stress-associated genes: Absence of dystro phin appears to render muscle specifically more susceptible to oxidative stress27. Heme oxygenase 1 (Hmox1) is known to be induced by oxidative stress or other stress stimuli28. Thioredoxin reductase 1 (Txnrd1) is a key enzyme in the thioredoxin system as an anti-oxidation system29. To explore the relationship between oxidative stress and muscle damage in dysferlin-deficient mice, these gene expression levels were quantified in SJL/J and A/J mice.
Complement control factor genes: SJL/J mice were found to exhibit downregulation of the complement inhibitor, decay-accelerating factor 1 (Daf1)/CD55 antigen, in skeletal muscle only, and the absence of Daf1/CD55 increased susceptibility to complement attack in cultured human myotubes30. Daf1 and Daf2 were selected for quantification by qRT-PCR on the basis of these findings.
Three mice each at 10 and 30 weeks of age were prepared for qRT-PCR analysis separately from histopathological examination. The femoral (rectus femoris) and lumbar (longissimus lumborum) muscles were removed from mice, quickly cut into slices less than 0.5-cm thick and incubated overnight in RNAlater RNA Stabilization Reagent (Qiagen, Valencia, CA, USA) at 2–8 °C. The rectus femoris and longissimus lumborum, in which there was shown to be a remarkable difference of severity of muscle lesions between SJL/J and A/J mice at the age of 35 weeks old in our previous report8, were used as examined sites in the first qRT-PCR analysis. The tissue samples in the reagent were transferred to a freezer at −20 °C and stored until use. For qRT-PCR analysis, total RNA was isolated from each isolated muscle with an RNeasy® Fibrous Tissue Mini Kit (Qiagen) according to the instructions of the manufacturer. For each sample, pooled total RNA from 3 mice was reverse-transcribed using a High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA, USA). The gene-specific primers and probes used for qRT-PCR analysis were available as TaqMan® Gene Expression Assays (Applied Biosystems; Table 1). The qRT-PCR reactions were performed on a 7500 Fast Real-Time PCR System (Applied Biosystems) in 20 µL of the reaction mixture containing 1x TaqMan Fast Universal PCR Master Mix, No AmpErase® UNG (Applied Biosystems), 1x Gene Expression Assay mix and 5 µL of diluted cDNA sample as a template. MicroAmp® Fast 96-Well Reaction Plates covered by optical adhesive covers (Applied Biosystems) were used. Amplification was conducted according to the following thermal profile: 1 cycle at 95 °C for 20 sec, and 40 cycles at 95 °C for 3 sec and 60 °C for 30 sec. Initial raw data analysis was performed using the Sequence Detection Software version 1.3.1. Relative mRNA levels were calculated by the comparative threshold cycle (Ct) method31,32, as described in Applied Biosystems User Bulletin Number 2 (P/N 4303859).
Table 1. TaqMan® Gene Expression Assays (Gene-specific Primers and Probes) used in qRT-PCR for the Rectus Femoris and Longissimus Lumborum from Mice at 10 and 30 Weeks of Age.
The program calculates ∆Ct and ∆∆Ct with the following formula: ∆∆Ct = ∆Ct sample [Ct endogenous control gene (from BALB/c mice at 30 weeks of age, SJL/J or A/J mice) − Ct target gene (from BALB/c mice at 30 weeks of age, SJL/J or A/J mice)] −∆Ct control [Ct endogenous control gene (from BALB/c mice at 10 weeks of age) − Ct target gene (from BALB/c mice at 10 weeks of age)]. The relative gene expression was calculated using the expression 2–(∆∆Ct). Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) gene was selected as an endogenous control gene.
Quadruplicate measurements per gene were conducted and data are presented as the mean and standard deviation. Changes in gene expression are reported as fold changes relative to those of controls (the Ct values in BALB/c mice at the age of 10 weeks). We defined that the gene expression was regulated or downregulated in the present study if the relative gene expression was more than twice or less than one-half that of the control, respectively. Data were not statistically analyzed because qRT-PCR analysis was performed using pooled samples of equal quantities of total RNA obtained from three mice in each strain.
Additional qRT-PCR analysis
To clarify the site-specificity and temporal changes of gene expression, some genes that were upregulated more than twice or downregulated less than one-half that of the control in both rectus femoris and longissimus lumborum, in one of SJL/J and A/J mice on the first qRT-PCR analysis, and other genes associated with these genes (Table 2), were measured in eight sites of striated muscles (rectus femoris, gastrocnemius, triceps brachii, flexor carpi ulnaris, longissimus lumborum, abdominal muscle, diaphragm and heart) from three mice at the ages of 5, 15 and 30 weeks old by means of the above method. The eight striated muscles used for additional qRT-PCR analysis are separated into three groups based on the severity of muscle pathological lesions in SJL/J mice at an age of 35 weeks old8. The rectus femoris, longissimus lumborum and abdominal muscle are included in the severe lesion group, which exhibits slight to moderate degeneration/necrosis with moderate central nuclei in muscle fibers. The triceps brachii are referred to as the slight lesion group, which shows minimal degeneration/necrosis with moderate central nuclei in muscle fibers. Other muscles are classified in the minimal lesion group, with only minimal degeneration/necrosis with slight central nuclei or without central nuclei in muscle fibers.
Table 2. TaqMan® Gene Expression Assays (Gene-specific Primers and Probes) used in qRT-PCR for the Skeletal Muscles from Mice at 5, 15 and 30 Weeks of Age.
Lipid metabolism-associated genes: As previously explained, SJL/J mice have a mutation in the Tbc1d1 gene. Checks were performed to ensure that the Tbc1d1 gene had not been expressed at any week of age or at any muscle site.
Apoptosis-associated gene: It was reported that Ucp2 increases the sensitivity of adult rat cardiomyocytes to hypoxia-reoxygenation by way of ATP depletion and acidosis, which in turn causes accumulation of proapoptotic protein Bcl-2 and 19-kDa interacting protein 3 (Bnip3)33. In the first qRT-PCR analysis, the gene expression levels of Ucp2 in the rectus femoris and longissimus lumborum of SJL/J mice at the age of 30 weeks old were higher than those of the control. Therefore, Bnip3 gene expression level was measured to explore the mechanism of degeneration/necrosis in skeletal muscle fibers.
Calcium binding protein gene: In muscular lesions in SJL/J mice, most infiltrating cells are F4/80 antigen-positive macrophages8. Recently, it was demonstrated that S100 calcium binding protein A4 (S100A4), a member of the S100 family of Ca2+ binding proteins, mediates macrophage recruitment and chemotaxis in vivo34. Therefore, to find an association between the severity of muscle lesions and S100A4 gene expression levels, qRT-PCR analysis was conducted using the eight striated muscles separated into three groups based on the severity of muscle lesions in SJL/J mice at an age of 35 weeks old8.
Immunologically relevant gene: It is known that MHC class I (H2-K1) expression is markedly upregulated in myopathic muscles of dysferlin-deficient SJL/J mice22. Therefore, to ascertain whether or not H2-K1 gene expression was systemically upregulated, H2-K1 gene expression level was examined by qRT-PCR analysis.
Telomere-associated genes: Telomere shortening was found in tibialis anterior and diaphragm muscles from mdx mice in comparison with age-matched wild-type mice35. In addition, it was documented that telomeric repeat binding factor-1 (Terf1) and poly (ADP-ribose) polymerase-1 (Parp1), which control telomere elongation, were overexpressed in the muscles of Duchenne muscular dystrophy36. It was ascertained whether or not two telomere elongation control factors were overexpressed in the skeletal muscles of two dysferlinopathy model mice by qRT-PCR.
Triplicate measurements for each gene were conducted and data are presented as the mean and standard deviation. Changes in gene expression are described as fold changes relative to that of controls (the Ct values in BALB/c mice at the age of 5 weeks).
Results
Histopathology
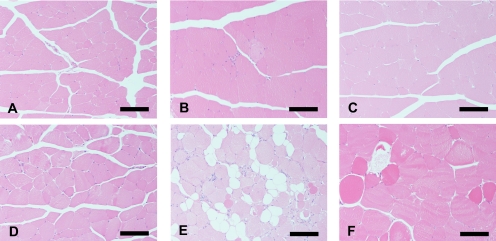
The results of histopathological examination corresponded to our published data8. Fig. 1 shows typical histopathological findings in the femoral muscles of BALB/c, SJL/J and A/J mice at 10 and 30 weeks of age.
Fig. 1.
Histopathology of the rectus femoris in BALB/c, SJL/J and A/J mice. At 10 weeks of age (upper figures), no significant changes are observed in the skeletal muscle fibers of BALB/c (A) and A/J mice (C), and a few muscle fibers show minimal degeneration with mono-nuclear cell infiltration in SJL/J mice (B). At 30 weeks of age (lower figures), BALB/c mice do not exhibit histopathological changes in any skeletal muscles (D). The histopathological lesions of skeletal muscles in SJL/J mice progress in severity with age and are characterized by the following findings: degenerative/necrotic muscle fibers, centronuclear muscle fibers, fatty infiltration and variation in size of muscle fibers (E). The muscle fibers in A/J mice show only degenerative/necrotic features and variation in size (F). HE staining. Bar: 100 µm.
In brief, at 10 weeks of age, no significant changes were observed in the skeletal muscle fibers of BALB/c and A/J mice, and some skeletal muscles (particularly femoral, brachial, abdominal and lumbar muscles) showed minimal degeneration and/or necrosis of muscle fibers in SJL/J mice. At 30 weeks of age, BALB/c mice did not exhibit histopathological changes in any skeletal muscles. However, the histopathological lesions of skeletal muscles in SJL/J mice progressed in severity and were increasingly frequent with age, and SJL/J mice revealed macrophage infiltration around degeneration and/or necrosis of muscle fibers. In contrast, histological lesions of these skeletal muscles in A/J mice showed a slow progression with age.
qRT-PCR
Tables 3 and 4 show the results of qRT-PCR analysis.
Table 3. Relative Expression Levels of mRNAs in The Rectus Femoris of BALB/c, SJL/J and A/J Mice.
Table 4. Relative Expression Levels of MRNAs in The Longissimus Lumborum of BALB/c, SJL/J and A/J Mice.
The first qRT-PCR analysis revealed genes that were upregulated more than twice or downregulated less than one-half that of the control in both rectus femoris and longissimus lumborum in one of SJL/J and A/J mice.
Ucp2 as a lipid metabolism-associated gene was up-regulated in the rectus femoris and longissimus lumborum of SJL/J mice and downregulated in the longissimus lumborum of A/J mice at 30 weeks of age.
The gene expression level of heat shock protein 70 (Hsp70) in the rectus femoris and longissimus lumborum of BALB/c mice at 30 weeks of age was more than 30 times higher than that of the control. The gene expression level of heat shock protein 40 (Hsp40), which is known to be a co-chaperone regulating Hsp70, in the rectus femoris and longissimus lumborum of BALB/c mice at 30 weeks of age was also more than four times higher than that of the control. Hsp70 was upregulated in the rectus femoris and longissimus lumborum of SJL/J mice at 10 and 30 weeks of age more than 10 times higher than that of the control. Likewise, Hsp40 was upregulated in the rectus femoris and longissimus lumborum of SJL/J mice at 10 weeks of age more than three times higher than that of the control. However, the gene expression levels of Hsp70 and Hsp40 in SJL/J mice were lower than those of BALB/c mice at 30 weeks of age. Hsp70 and Hsp40 were downregulated in the longissimus lumborum or rectus femoris of A/J mice at 30 weeks of age compared with those of the control.
Edem1 mRNA in the rectus femoris and longissimus lumborum, and ERp57 mRNA in the longissimus lumborum showed high expression levels in SJL/J mice at 30 weeks of age compared with those of the control.
The expression level of Hmox1 mRNA in the rectus femoris and longissimus lumborum of SJL/J mice at 30 weeks of age was more than two times higher than that of the control.
The gene expression level of Daf1/CD55 in the rectus femoris and longissimus lumborum of SJL/J mice at 10 and 30 weeks of age was lower than that of the control.
Additional qRT-PCR
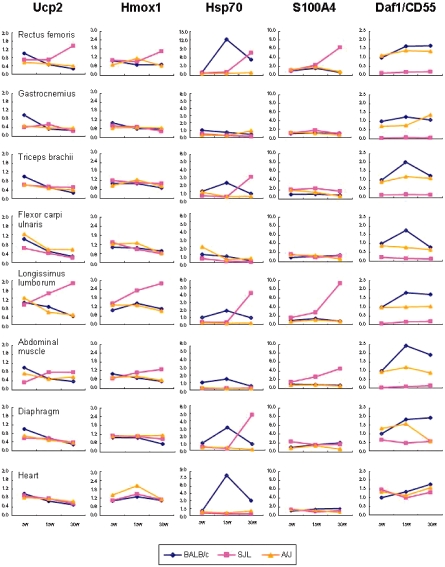
Tables 5 to 7 show the results of an additional qRT- PCR analysis. Fig. 2 indicates the changes of principal genes in the tested muscles.
Table 5. Expression Levels of MRNAs in BALB/c Mice at 15 and 30 Weeks of Age Relative to the Control (BALB/c Mice at 5 Weeks of Age).
Table 6. Expression Levels of MRNAs in SJL/J Mice at 5, 15 and 30 Weeks of Age Relative to the Control (BALB/c Mice at 5 Weeks of Age).
Table 7. Expression Levels of MRNAs in A/J Mice at 5, 15 and 30 Weeks of Age Relative to the Control (BALB/c Mice at 5 Weeks of Age).
Fig. 2.
The changes of principal genes in each muscle site of BALB/c, SJL/J and A/J mice. The presented genes (Ucp2, Hmox1, Hsp70, S100A4 and Daf1/CD55) are those for which the changes are suspected of involvement in muscular lesions observed in SJL/J and A/J mice. The severity of muscular lesions is as follows: lumbar muscle (longissimus lumborum) > femoral muscle (rectus femoris) > abdominal muscle > brachial muscle (triceps brachii) > crural muscle (gastrocnemius) > forearm muscle (flexor carpi ulnaris) > diaphragm. Ucp2 gene expression in the rectus femoris and longissimus lumborum of SJL/J mice shows a tendency to be upregulated with age. Hmox1 gene expression in the rectus femoris and longissimus lumborum of SJL/J mice is upregulated at 30 weeks of age. Hsp70 gene expression levels in most muscles of A/J mice are lower at all ages. S100A4 gene expression in the rectus femoris, longissimus lumborum and abdominal muscles of SJL/J mice is upregulated at 30 weeks of age. Daf1/CD55 gene expression in all studied muscles except for the heart of SJL/J mice shows a marked downregulation at all ages.
Lipid metabolism-associated genes: Ucp2 gene expression showed a tendency to be upregulated with age in the rectus femoris and longissimus lumborum of SJL/J mice, but to be downregulated with age in other muscles of SJL/J mice and all muscles of BALB/c and A/J mice. Tbc1d1 gene expression level in SJL/J mice was lowered regardless of age and site.
Heat shock protein genes: The gene expression levels of Hsp70 and Hsp40 in BALB/c mice peaked at 15 weeks of age. Hsp70 gene in the rectus femoris and heart of BALB/c mice exhibited a persistently higher expression level than that of the control. On the other hand, SJL/J mice showed upregulation of Hsp70 gene expression in the rectus femoris, triceps brachii, longissimus lumborum and diaphragm at 30 weeks of age. The gene expression levels of Hsp70 and Hsp40 in most muscles of A/J mice were lower than those of the control.
ER-associated degradation of glycoprotein-associated genes: The rectus femoris and longissimus lumborum of SJL/J mice showed a tendency toward an increase in the gene expression levels of Edem1 and ERp57 at 30 weeks of age.
Oxidative stress-associated genes: Hmox1 gene expression level was increased with age in the longissimus lumborum of SJL/J mice, and there was a trend for an increase in its level in the rectus femoris of SJL/J mice at 30 weeks of age.
Complement control factor genes: Daf1/CD55 gene expression level in BALB/c mice showed a trend to increase by 15 weeks of age. In contrast, SJL/J mice exhibited a marked lowering of Daf1/CD55 gene expression level in the limb, lumbar and abdominal muscles at all ages. There was no abnormality in the gene expression levels of Daf1/CD55 in all muscles of A/J mice and in the heart of SJL/J mice in comparison to that of the control.
Apoptosis-associated genes: Bnip3 mRNA level was sporadically increased in BALB/c mice and was temporally increased in the heart in A/J mice with age.
Calcium binding protein genes: The rectus femoris, longissimus lumborum and abdominal muscles of SJL/J mice showed an increase in S100A4 gene expression level with age.
Immunologically relevant genes: H2-K1 gene was sig nificantly expressed in SJL/J and A/J mice regardless of age and site.
Telomere-associated genes: Terf1 and Parp1 gene expression levels in SJL/J and A/J mice showed no alteration in any site or age compared with those of the control.
Discussion
This study showed that there were interstrain and site- dependent differences in the gene expression profiles of skeletal muscles in dysferlinopathy model mice, SJL/J and A/J mice.
Upon analysis by qRT-PCR, Tbc1d1 gene expression level in SJL/J mice was lowered regardless of age by a mutation in the Tbc1d1 gene responsible for increased fatty acid uptake/oxidation and decreased glucose uptake14. In quantitative analysis of the gene expression levels of PGC1α, Ucp2 and Ucp3 as lipid metabolism-associated genes, mRNA expression level of Ucp2 showed a trend to be upregulated in the rectus femoris and longissimus lumborum of SJL/J mice at 30 weeks of age in contrast to those of BALB/c and A/J mice. Forced expression of Ucp2 in pancreatic islets was found to result in decreased ATP content, and the islet cells of UCP2 knockout mice showed increased ATP level37. Overexpression of UCP2 in primary cardiomyocytes led to a significant decline in ATP level and enhanced sensitivity to hypoxia-reoxygenation33. Ucp2-mediated energy loss may be related to muscle degeneration/necrosis in SJL/J mice. Tbc1d gene-deficient cells exhibited inhibited trafficking of glucose transporter GLUT4 from intracellular vesicles to plasma membrane38, which is suggested to show a decrease in intracellular glucose level and a subsequent enhancement of fatty acid oxidation. As a result, the skeletal muscles in SJL/J mice are likely to have uncoupling.
Most upregulation of Edem1, ERp57, Hmox1 and S100A4 was observed in the rectus femoris, longissimus lumborum or abdominal muscles, in which dystrophic lesions occur more commonly in SJL mice. These upregulations approximately coincide with the occurrence of dystrophic changes in these sites.
Edem1 is needed for degradation of misfolded glycoprotein substrates including MHC class I24. ERp57 contributes to the formation of native disulfide bonds in nascent MHC class I heavy chains24. SJL/J mice show marked upregulation of MHC class I expression in myopathic muscles22. In fact, the expression level of MHC class I (H2-K1) gene in the rectus femoris and longissimus lumborum of SJL/J mice was significantly higher regardless of age and site compared with those of A/J and BALB/c mice in our study. Therefore, gene changes of Edem1 and ERp57 in SJL/J mice may be associated with a very marked upregulation of H2-K1 expression in the rectus femoris and longissimus lumborum.
Hmox1 provides the first line of defense against oxidative stress because it rapidly responds to oxidants39. However, Txnrd1, which is a part of the anti-oxidation system as well as Hmox1, was not upregulated in any muscles of SJL/J mice. Recently, calcium-dependent upregulation of Hsp70 and Hmox-1 in skeletal muscle cells or hepatocytes was reported40,41. Because dysferlin null muscle fibers are defective in Ca2+-dependent resealing of sarcolemma disruptions42, these muscle fibers may cause persistent calcium influx into the cytoplasm after membrane injury. The gene expression levels of Hmox1 were correlated with the severity of histopathological lesions in femoral (rectus femoris), lumbar (longissimus lumborum) and abdominal muscles; calcium influx into the cytoplasm following muscle injury may induce Hmox1 gene expression. However, Hsp70 was also upregulated in the diaphragm of SJL/J mice, in where there were few histopathological changes at all examined times. In addition, change of Hsp70 gene expression level was not observed in the abdominal muscles of SJL/J mice, in which histopathological changes were found at 30 weeks of age. The above muscles and hearts without histopathological abnormalities in BALB/c mice exhibited upregulation of this gene expression from 15 weeks of age. It was reported that significant increases in Hsp70 were observed at 12 weeks postpartum in normal rats43. The physiological gene expression mechanism of Hsp70 may develop somewhat later in these muscles excluding the heart of SJL/J mice. Therefore, an unknown factor other than persistent calcium influx may also cause Hmox1 induction in these muscles of SJL/J mice.
In contrast, the gene expression levels of Hsp70 in most muscles of A/J mice were lower than that of the control. Loss of fer-1, dysferlin homolog, in C. elegans causes down-regulation of hsp-7044. It is possible that the downregulation of Hsp70 gene expression in the skeletal muscles of A/J mice is caused by the functional loss of dysferlin.
In muscle lesions in SJL/J mice, most infiltrating cells are F4/80 antigen-positive macrophages8. Recently, it was demonstrated that S100A4, a member of the S100 family of Ca2+-binding proteins, mediates macrophage recruitment and chemotaxis in vivo34. S100A4 upregulation in the rectus femoris and longissimus lumborum of SJL/J mice may be linked to muscle pathological characteristics in SJL/J mice.
SJL/J mice exhibited a marked lowering of Daf1/CD55 gene expression level in all studied muscles except for the heart at all ages compared with that of BALB/c mice. In contrast, there was no predominant difference in the Daf1/ CD55 gene expression levels of A/J mice compared with that of BALB/c mice. It was reported that the gene expression of Daf1/CD55 as a complement inhibitor was down-regulated in the skeletal muscles of LGMD2B patients or SJL/J mice30. Moreover, the serum concentration of the fifth component of complement (C5) in SJL/J mice is known to be significantly greater than that of other strains45. On the other hand, A/J mice are genetically deficient in C546. These results show the possibility that the difference in sensitivity to complement-dependent cytotoxicity causes the differ ence in phenotype between the two dysferlin-deficient mice. However, the downregulation of Daf1/CD55 alone cannot explain site-specificity of muscle lesions.
In LGMD2B patients or SJL mice, it is shown that MHC class I is overexpressed on muscle fibers22,47. Meanwhile, the results of study using SJL.129P2(B6)-B2mtm1Unc mice developed by targeted gene mutation of β-2-microglobulin, which is required for proper assembly of MHC class I proteins on the cell surface, revealed that MHC class I is not required for the appearance of spontaneous myopathy in SJL/J mice22. Despite prolonged overexpression of MHC class I gene, there were few histopathological changes in the heart and diaphragm of SJL/J mice or A/J mice in our previous study8. Accordingly, it was suggested that upregulation of MHC class I in SJL/J and A/J mice was not directly associated with the progression of dystrophic lesions.
This study showed that there were some interstrain dif ferences in the gene expression profiles of skeletal muscles between SJL/J and A/J mice (both dysferlinopathy model mice). The genes, the changes of which correlate with the severity of muscular lesion, were Ucp2, Hmox1 and S100A4 in SJL/J mice. SJL/J mice showed a marked downregulation of Daf1/CD55 gene expression in all studied muscles except for the heart at all ages. The downregulation of Hsp70 gene expression was observed in the examined skeletal muscles of A/J mice. Further investigation is required to reveal whether alterations of their expression levels are the cause of dystrophic changes or occur subsequent to muscle damage.
Acknowledgments
The authors are grateful to all the staff of Tanabe R&D Service Co., Ltd. for their help with maintenance of animals.
References
- 1.Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 23: 1456–1471 2000 [DOI] [PubMed] [Google Scholar]
- 2.Han R, Bansal D, Miyake K, Muniz VP, Weiss RM, Mc-Neil PL, Campbell KP. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J Clin Invest. 117: 1805–1813 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glover L, Brown RH., JrDysferlin in membrane trafficking and patch repair. Traffic. 8: 785–794 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bittner RE, Anderson LV, Burkhardt E, Bashir R, Vafiadaki E, Ivanova S, Raffelsberger T, Maerk I, Hoger H, Jung M, Karbasiyan M, Storch M, Lassmann H, Moss JA, Davison K, Harrison R, Bushby KM, Reis A. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat Genet. 23: 141–142 1999 [DOI] [PubMed] [Google Scholar]
- 5.Ho M, Post CM, Donahue LR, Lidov HG, Bronson RT, Goolsby H, Watkins SC, Cox GA, Brown RH., JrDisruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum Mol Genet. 13: 1999–2010 2004 [DOI] [PubMed] [Google Scholar]
- 6.Vafiadaki E, Reis A, Keers S, Harrison R, Anderson LV, Raffelsberger T, Ivanova S, Hoger H, Bittner RE, Bushby K, Bashir R. Cloning of the mouse dysferlin gene and genomic characterization of the SJL-Dysf mutation. Neuroreport. 12: 625–629 2001 [DOI] [PubMed] [Google Scholar]
- 7.Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 273: 15879–15882 1998 [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi K, Izawa T, Kuwamura M, Yamate J. The distribution and characterization of skeletal muscle lesions in dysferlin-deficient SJL and A/J mice. Exp Toxicol Pathol. 62: 509–517 2010 [DOI] [PubMed] [Google Scholar]
- 9.Campanaro S, Romualdi C, Fanin M, Celegato B, Pacchioni B, Trevisan S, Laveder P, De Pittà C, Pegoraro E, Hayashi YK, Valle G, Angelini C, Lanfranchi G. Gene expres sion profiling in dysferlinopathies using a dedicated muscle microarray. Hum Mol Genet. 11: 3283–3298 2002 [DOI] [PubMed] [Google Scholar]
- 10.von der Hagen M, Laval SH, Cree LM, Haldane F, Pocock M, Wappler I, Peters H, Reitsamer HA, Hoger H, Wiedner M, Oberndorfer F, Anderson LV, Straub V, Bittner RE, Bushby KM. The differential gene expression profiles of proximal and distal muscle groups are altered in pre-pathological dysferlin-deficient mice. Neuromuscul Disord. 15: 863–877 2005 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki N, Aoki M, Hinuma Y, Takahashi T, Onodera Y. Ish igaki A, Kato M, Warita H, Tateyama M, and Itoyama Y. Expression profiling with progression of dystrophic change in dysferlin-deficient mice (SJL). Neurosci Res. 52: 47–60 2005 [DOI] [PubMed] [Google Scholar]
- 12.Turk R, Sterrenburg E, van der Wees CG, de Meijer EJ, de Menezes RX, Groh S, Campbell KP, Noguchi S, van Om-men GJ, den Dunnen JT, ‘t Hoen PA. Common pathological mechanisms in mouse models for muscular dystrophies. FASEB J. 20: 127–129 2006 [DOI] [PubMed] [Google Scholar]
- 13.Weller AH, Magliato SA, Bell KP, Rosenberg NL. Spontaneous myopathy in the SJL/J mouse: pathology and strength loss. Muscle Nerve. 20: 72–82 1997 [DOI] [PubMed] [Google Scholar]
- 14.Chadt A, Leicht K, Deshmukh A, Jiang LQ, Scherneck S, Bernhardt U, Dreja T, Vogel H, Schmolz K, Kluge R, Zierath JR, Hultschig C, Hoeben RC, Schürmann A, Joost HG, Al-Hasani H. Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nat Genet. 40: 1354–1359 2008 [DOI] [PubMed] [Google Scholar]
- 15.Samec S, Seydoux J, Dulloo AG. Skeletal muscle UCP3 and UCP2 gene expression in response to inhibition of free fatty acid flux through mitochondrial beta-oxidation. Pflugers Arch. 438: 452–457 1999 [DOI] [PubMed] [Google Scholar]
- 16.Bonen A. PGC-1alpha-induced improvements in skeletal muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab. 34: 307–314 2009 [DOI] [PubMed] [Google Scholar]
- 17.Carnac G, Vernus B, Bonnieu A. Myostatin in the pathophysiology of skeletal muscle. Curr Genomics. 8: 415–422 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev Biol. 270: 19–30 2004 [DOI] [PubMed] [Google Scholar]
- 19.Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 39: 283–296 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, Hayashi YK, Momoi T. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum Mol Genet. 16: 618–629 2007 [DOI] [PubMed] [Google Scholar]
- 21.Maglara AA, Vasilaki A, Jackson MJ, McArdle A. Damage to developing mouse skeletal muscle myotubes in culture: protective effect of heat shock proteins. J Physiol. 548: 837–846 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostek CA, Dominov JA, Miller JB. Upregulation of MHC class I expression accompanies but is not required for spontaneous myopathy in dysferlin-deficient SJL/J mice. Am J Pathol. 160: 833–839 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Baig E, Williams DB. Functions of ERp57 in the folding and assembly of major histocompatibility complex class I molecules. J Biol Chem. 281: 14622–14631 2006 [DOI] [PubMed] [Google Scholar]
- 24.Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell. 4: 265–271 2003 [DOI] [PubMed] [Google Scholar]
- 25.Minetti GC, Colussi C, Adami R, Serra C, Mozzetta C, Parente V, Fortuni S, Straino S, Sampaolesi M, Di Padova M, Illi B, Gallinari P, Steinkühler C, Capogrossi MC, Sartorelli V, Bottinelli R, Gaetano C, Puri PL. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. 12: 1147–1150 2006 [DOI] [PubMed] [Google Scholar]
- 26.Brunelli S, Sciorati C, D’Antona G, Innocenzi A, Covarello D, Galvez BG, Perrotta C, Monopoli A, Sanvito F, Bottinelli R, Ongini E, Cossu G, Clementi E. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci USA. 104: 264–269 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rando TA, Disatnik MH, Yu Y, Franco A. Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromuscul Disord. 8: 14–21 1998 [DOI] [PubMed] [Google Scholar]
- 28.Soares MP, Bach FH. Heme oxygenase-1: from biology to therapeutic potential. Trends Mol Med. 15: 50–58 2009 [DOI] [PubMed] [Google Scholar]
- 29.Arnér ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 267: 6102–6109 2000 [DOI] [PubMed] [Google Scholar]
- 30.Wenzel K, Zabojszcza J, Carl M, Taubert S, Lass A, Harris CL, Ho M, Schulz H, Hummel O, Hubner N, Osterziel KJ, Spuler S. Increased susceptibility to complement attack due to downregulation of decay-accelerating factor/CD55 in dysferlin-deficient muscular dystrophy. J Immunol. 175: 6219–6225 2005 [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30: e36 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodyak N, Rigor DL, Chen YS, Han Y, Bisping E, Pu WT, Kang PM. Uncoupling protein 2 modulates cell viability in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 293: H829–H835 2007 [DOI] [PubMed] [Google Scholar]
- 34.Li ZH, Dulyaninova NG, House RP, Almo SC, Bresnick AR. S100A4 regulates macrophage chemotaxis. Mol Biol Cell. 21: 2598–2610 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lund TC, Grange RW, Lowe DA. Telomere shortening in diaphragm and tibialis anterior muscles of aged mdx mice. Muscle Nerve. 36: 387–390 2007 [DOI] [PubMed] [Google Scholar]
- 36.Aguennouz M, Vita GL, Messina S, Cama A, Lanzano N, Ciranni A, Rodolico C, Di Giorgio RM, Vita G. Telomere shortening is associated to TRF1 and PARP1 overexpression in Duchenne muscular dystrophy. Neurobiol Aging. 2010; . [DOI] [PubMed] [Google Scholar]
- 37.Langin D. The role of uncoupling protein 2 in the development of type 2 diabetes. Drugs Today (Barc). 39: 287–295 2003 [DOI] [PubMed] [Google Scholar]
- 38.Roach WG, Chavez JA, Mîinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 15(403): 353–358 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Islam T, McConnell R, Gauderman WJ, Avol E, Peters JM, Gilliland FD. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am J Respir Crit Care Med. 177: 388–395 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorquera G, Juretić N, Jaimovich E, Riveros N. Membrane depolarization induces calcium-dependentupregulation of Hsp70 and Hmox-1 in skeletal muscle cells. Am J Physiol Cell Physiol. 297: C581–C590 2009 [DOI] [PubMed] [Google Scholar]
- 41.Silomon M, Bauer I, Bauer M, Nolting J, Paxian M, Rensing H. Induction of heme oxygenase-1 and heat shock protein 70 in rat hepatocytes: the role of calcium signaling. Cell Mol Biol Lett. 12: 25–38 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 423: 168–172 2003 [DOI] [PubMed] [Google Scholar]
- 43.O’Neill DE, Noble EG. Constitutive expression of inducible Hsp70 is linked to natural shifts in skeletal muscle phenotype. Acta Physiol Scand. 181: 35–41 2004 [DOI] [PubMed] [Google Scholar]
- 44.Krajacic P, Hermanowski J, Lozynska O, Khurana TS, Lamitina T. The C. elegans Dysferlin homolog fer-1 is expressed in muscle and fer-1 mutations initiate altered gene expression of muscle enriched genes. Physiol Genomics. 40: 8–14 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch DM, Kay PH. Studies on the polymorphism of the fifth component of complement in laboratory mice. Exp Clin Immunogenet. 12: 253–620 1995 [PubMed] [Google Scholar]
- 46.Rhodes JC, Wicker LS, Urba WJ. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect Immun. 29: 494–499 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Confalonieri P, Oliva L, Andreetta F, Lorenzoni R, Dassi P, Mariani E, Morandi L, Mora M, Cornelio F, Mantegazza R. Muscle inflammation and MHC class I upregulation in muscular dystrophy with lack of dysferlin: an immunopathological study. J Neuroimmunol. 142: 130–136 2003 [DOI] [PubMed] [Google Scholar]