Abstract
Background
There is substantial need to rigorously evaluate existing and new therapies for pulmonary arterial hypertension (PAH) and other severe and relatively rare conditions affecting younger patients. However, the ability to conduct meaningful randomized clinical trials (RCTs) in such contexts often is limited by difficulties obtaining adequate patient enrollment.
Purpose
To understand the motivations of patients with PAH for participating in RCTs so as to facilitate enrollment in future trials among patients with similar diseases.
Methods
We conducted semistructured interviews of a diverse sample of patients with World Health Organization (WHO) Group I PAH. We purposefully recruited a diverse sample of participants until theoretical saturation was reached. We randomly assigned patients to review hypothetical RCTs that did or did not allow continuation of background PAH therapies and elicited their reasons for or against enrolling. Interviews were transcribed and analyzed using constant comparison techniques to code and sort data into discrete themes.
Results
The 26 PAH patients enrolled before theoretical saturation was reached identified 24 factors that would influence their RCT enrollment decisions. These factors grouped naturally into four themes: (1) personal medical benefits, (2) personal medical risks/harms, (3) nonmedical benefits, and (4) nonmedical burdens. Personal benefits were cited as commonly as altruistic motives. One third of the patients (9/26) suggested that they would defer enrollment decisions to their treating clinicians. Seventy-nine percent of patients (11/14) assigned to consider trials without background therapies expressed concerns about clinical deterioration (vs. 17% (2/12) among patients assigned to consider trials allowing background therapies).
Limitations
The sample was recruited from a single academic center. Furthermore, the use of hypothetical trials may not elicit identical decision-making processes as may be used among patients contemplating actual trial participation.
Conclusion
For PAH patients considering RCT enrollment, the potentials for personal benefit and risk are at least as important as altruistic motives. Minimizing the time demands of participating, financial remuneration, and allowing participants to continue current therapies are factors, which might enhance enrollment to trials in similar disease areas.
Introduction
The considerable difficulties in recruiting participants to randomized clinical trials (RCTs)—either in large enough numbers to meet sample size targets or sufficiently quickly to make the costs of trial conduct manageable [1]—have motivated efforts to better understand how people make decisions to participate in research. The hope is that by understanding decision-making processes, we may identify ways for investigators to design trials to improve recruitment efficiency [2]. To date, this promise has been largely unrealized, as a recent systematic review suggests marginal efficacy of most attempted interventions to improve recruitment efficiency [3].
By contrast, such efforts have proven fruitful when research on patients’ willingness to participate in trials in a given disease area has been followed up with tests of interventions deriving from those insights into the same disease area [4]. These seemingly divergent bodies of evidence can be reconciled by the hypothesis that the factors guiding patients’ willingness to participate in RCTs may be disease specific, or at least shared only among diseases sharing several common defining characteristics. If so, then what is needed are efforts to clearly define the unique characteristics of the domain under study, to study willingness to participate within this domain, and to then apply insights gained to future trials in the same domain.
The current literature on patients’ willingness to participate includes studies using quantitative [5–13] and qualitative [14–18] methods among several different types of respondents: patients eligible to participate in proposed RCTs [5–8,10,12,13,18–20], patients actually considering participation [9,11, 16,21–23], and patients having already participated in trials [14,17]. In addition to these areas of divergence, the characteristics of the disease areas studied have varied considerably, from prevalent illnesses such as congestive heart failure [9] in which multiple known effective therapies exist, to rare cancers for which no known effective therapies are available [23]. Some general themes have emerged, which guide many patients’ decisions to enroll, including the time demands of the trials [6,7,14,16,18,21,24], the risks of disease recurrence or progressions [7,23], and fears of being assigned placebo [6,7,10,15,18,21].
Largely absent from this literature have been studies exploring an increasingly common group of diseases that may be characterized by (1) low incidence and prevalence, (2) considerable associated morbidity and mortality, (3) early age of onset, (4) strong patient advocacy organizations, and (5) the existence of numerous available therapies that each carry substantive limitations in terms of their effectiveness, costs, or burdens to patients in ease of administration. Such diseases include cystic fibrosis, inflammatory bowel disease, multiple sclerosis, pulmonary arterial hypertension (PAH), and likely others. In each of these diseases, the considerable health consequences have driven the development of strong patient advocacy organizations and inspired researchers and pharmaceutical companies to identify new therapies.
However, the high morbidity has often precluded the trials of sufficiently long duration to determine these treatments’ effects on clinical end points such as survival, and the disease incidence has limited power to detect differences in such outcomes. Thus, many treatments have been developed, but evidence is largely limited to impacts on surrogate end points. Because these treatments often carry secondary costs—either financial or in their difficulty of administration—the search for new and improved therapies continues unabated, perpetuating the need for further RCTs. Yet we are aware of only one study assessing motivations for research participation among patients with such illnesses, and this study among cystic fibrosis patients assessed motivations for research in general, rather than for RCTs specifically [25].
We therefore designed the present study to understand willingness to participate within this domain of diseases, using PAH as a paradigmatic example. PAH is a rare, progressive illness characterized by increased pulmonary vascular resistance, right ventricular failure, and death [26]. The prevalence of PAH is 15 cases/million and the incidence is 2.4 cases/million per year [27]. Despite the rareness of the disease, the past 10 years have witnessed more than 20 placebo-controlled, RCTs of new drugs for PAH, leading to seven Food and Drug Administration (FDA)–approved pharmacotherapies for PAH on the basis of improvements in surrogate end points. For context, much research on willingness to participate in trials comes from more common diseases such as congestive heart failure, with prevalence estimates of 8–79 per 1000 per year, and incidence estimates of 1–28 per 1000 per year. The combination of disease epidemiology and RCT activity has resulted in most PAH patients having participated in RCTs, whereas only roughly 9.5% of congestive heart failure patients have [28,29].
Another distinction is that only one drug for PAH has ever been shown in an RCT (of only 81 patients) to improve survival [30], and this drug—continuous intravenous epoprostenol—carries a significant burden for patients due to its requirements for daily mixing, continuous infusion through a permanent intravenous catheter, and a battery-operated pump [31]. Further RCTs of new PAH therapies are therefore needed, particularly of drugs that are less burdensome, and with designs that enable clearer detection of improvements, if present, in true patient-centered outcomes. Because a number of companies are invested in developing such therapies, PAH patients are in high demand for RCT participation, with many being recruited to numerous trials at once [32]. However, the low prevalence of the disease and difficulties encountered previously with enrolling PAH patients in RCTs [33–35] have combined to create such a premium on PAH participants that working groups have been convened to discuss which among several possible RCTs to prioritize [36]. Better understanding of why patients enroll, therefore, carries great potential to improve the value of future research in this and related areas [37,38].
Methods
Patients
We recruited patients from the Pulmonary Vascular Disease Program at the University of Pennsylvania from 15 July 2009 to 20 August 2009. Two investigators (R.C. and J.A.) screened the electronic records of all patients scheduled to visit any of the programs’ physicians. Eligible patients had a diagnosis of World Health Organization (WHO) Group I pulmonary hypertension (PAH) with a current functional status equivalent to New York Heart Association Classes I–III [39]. Patients with Class IV symptoms were excluded as these patients are typically excluded from actual PAH RCTs [40–42]. This study was approved by the Institutional Review Board of the University of Pennsylvania and was assigned the project approval number 810120.
To ensure a diverse representation of views, we used purposeful recruitment in an effort to sample participants who differed in age, race, gender, socioeconomic status, prior RCT experience, and etiology of PAH. Such sampling is advocated in qualitative research to facilitate representation of all viewpoints and decision-making processes in the resulting conceptual framework [43]. We did not specify a number of patients to be interviewed in advance, but rather continued enrollment until theoretical saturation was reached, that is, until additional interviews yielded no new reasons for or against enrolling in PAH RCTs [44].
Interviews
One of the two investigators (R.C. or J.A.) who were trained in the techniques of semistructured interviewing conducted interviews among patients who consented to participate. Interviews were audiotaped and transcribed verbatim by professional medical transcriptionists. The first four interviews were conducted by the two investigators jointly to promote similarity of approach in subsequent interviews.
Each interview began with a description of a hypothetical RCT testing a novel agent to treat PAH. We predicted that a variable of particular importance to patients would be whether or not trials allowed ‘background therapies’—that is, whether patients could continue using previously prescribed endothelin receptor antagonists, phosphodiesterase inhibitors, or prostacyclin analogs during the study. Thus, we randomly assigned patients following consent to receive a description of one of two types of hypothetical RCTs: one in which patients would be removed from all specific PAH medications and one in which the new agent or placebo would be taken in addition to background therapies. Both hypothetical trials were modeled on recent real RCTs in PAH in terms of duration (16 weeks), clinic visits required (8), outcome measures (6-min walk distance, biomarkers, and echocardiographic parameters), double blinding, and other design elements.
After describing the assigned RCT, interviewers asked patients to share their thoughts regarding and reactions to participating in such a trial if it were real. Subsequent questions followed from participants’ responses, with investigators pursuing themes as they arose and seeking clarification or elaboration when appropriate. Investigators also used standard prompt questions developed at the study’s outset (Table 1) to encourage participants to more fully describe the factors that would encourage or discourage participation. Most interviews lasted 10–20 min, but were allowed to continue uninterrupted until patients could no longer identify additional influences on their enrollment decisions.
Table 1.
Examples of guiding questions
| Questions |
|---|
| If you were asked to participate in this study, what would your initial thoughts and reactions be? |
| What factors would be important in your decision to participate or not? |
| What factors might motivate you to participate? |
| What factors might motivate you not to participate? |
| What concerns would you have about participating in the study? |
| Suppose you were asked to encourage someone else to participate in this study. What would you tell him/her in order to encourage him/her to participate? |
| Suppose you were asked to encourage someone else not to participate in this study. What would you tell him/her in order to discourage him/her from participating? |
Qualitative data coding
Using the transcribed interviews, we employed thematic data analysis and constant comparison techniques to code and sort data into discrete themes that emerged from the interviews [45,46]. Two investigators reviewed transcript text and assigned codes to emerging themes. Codes allowed for cataloging of key concepts while preserving the context in which they arose [47]. The constant comparative method entailed comparing new text segments with text already assigned to a code to decide whether the new segment reflected the same concept. This method allowed for refinement of existing codes while identifying new factors related to patients’ willingness to participate in the RCTs. While one author (RC) was the primary coder of data elements, a subgroup of investigators (JA, DBT, SMK, and SDH) contributed to the iterative development, identification, and cataloging of themes as a means to increase intercoder reliability [48]. Coding accuracy was further assessed by allowing the same subgroup to review samples of transcripts and provide independent codings for comparison.
Results
Among 45 patients screened for enrollment, two were ineligible because they did not have Group I pulmonary hypertension and five missed their clinic appointments. Of the 38 eligible patients, 31 (82%) agreed to participate (Figure 1). Theoretical saturation was reached after the 26th patient. The median age of these 26 participants was 56 years, 85% (22/26) were women, 65% (17/26) were non-Hispanic white, and 50% (13/26) had idiopathic PAH (Table 2). Table 3 lists patients’ motivations for and concerns regarding participating in RCTs of novel PAH agents, and summarizes the frequencies with which patients in the two groups identified each. The themes that emerged are illustrated by the representative statements in the following.
Figure 1.
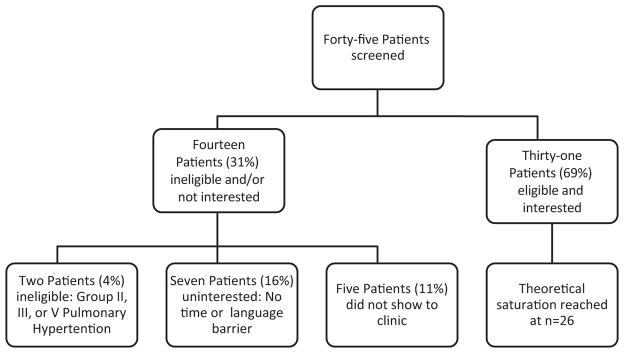
Flowchart for participant identification.
Table 2.
Characteristics of patients who participated in interviews
| Stop meds | Continue meds | Total | |
|---|---|---|---|
| Number | 14 (54%) | 12 (46%) | 26 |
| Type of PAH | |||
| Idiopathic | 5 (36%) | 8 (66%) | 13 (50%) |
| CTD associated | 5 (36%) | 1 (8%) | 6 (23%) |
| Familial | 1 (7%) | 0 (0%) | 1 (4%) |
| Congenital | 0 (0%) | 1 (8%) | 1 (4%) |
| Portopulmonary | 1 (7%) | 0 (0%) | 1 (4%) |
| Other/unknown | 2 (14%) | 2 (17%) | 4 (15%) |
| Age (years, median; [25th percentile, 75th percentile]) | 55; [40, 66] | 57; [41.5, 59] | 56; [41, 61] |
| Female | 11 (79%) | 11 (92%) | 22 (85%) |
| Race/ethnicity | |||
| Caucasian | 12 (86%) | 7 (58%) | 17 (65%) |
| African American | 1 (7%) | 4 (33%) | 5 (19%) |
| Hispanic | 1 (7%) | 1 (8%) | 4 (15%) |
| Education (years, median; [25th percentile, 75th percentile]) | 12; [12, 14] | 12; [12, 16] | 12; [12, 16] |
| Household income | |||
| <$20,000 | 2 | 4 | 6 |
| $20–40,000 | 2 | 3 | 5 |
| $40–60,000 | 2 | 2 | 4 |
| $60–80,000 | 1 | 1 | 2 |
| $80–100,000 | 2 | 0 | 2 |
| >$100,000 | 5 | 2 | 7 |
| Patients with a PAH family member | 1 (7%) | 2 (17%) | 3 (12%) |
| Years since diagnosis (median; [25th percentile, 75th percentile]) | 4.5; [3, 10] | 8.5; [5, 12] | 5.5; [4, 11] |
| Ever hospitalized for PAH | 6 (43%) | 3 (25%) | 9 (35%) |
| Agreed to participate in a previous PAH study | 7 (50%) | 5 (42%) | 12 (46%) |
| Agreed to participate in a study involving pills | 4 (29%) | 1 (8%) | 5 (19%) |
CTD: connective tissue disease.
Table 3.
Major themes that emerged during interviews
| Patient-identified themes | All patients (n = 26) | Stop meds (n = 14) | Continue meds (n = 12) |
|---|---|---|---|
| Medical benefit | |||
| Hope for a cure | 14 (54%) | 6 (43%) | 8 (66%) |
| Possible lack of benefit given comorbidities | 5 (19%) | 1 (7%) | 4 (33%) |
| Benefit of more tests/physician surveillance/ability to rapidly detect deterioration | 4 (15%) | 4 (29%) | 0 (0%) |
| Benefit of coming off or changing meds | 3 (12%) | 2 (14%) | 1 (8%) |
| As a last option/failure of current meds | 3 (12%) | 2 (14%) | 1 (8%) |
| Provision of free care to the underinsured | 2 (8%) | 2 (14%) | 0 (0%) |
| Doubts that new meds would work for them | 1 (4%) | 1 (7%) | 0 (0%) |
| Medical risk/harm | |||
| Side effects of experimental drug | 17 (65%) | 7 (50%) | 10 (83%) |
| Concerns of clinical deterioration | 13 (50%) | 11 (79%) | 2 (17%) |
| Concerns about coming off meds | 13 (50%) | 12 (86%) | 1 (8%) |
| Concerns about placebo | 10 (38%) | 8 (57%) | 2 (17%) |
| More likely to enroll if continue meds | 5 (19%) | 4 (29%) | 1 (8%) |
| Concerns about drug interactions | 4 (15%) | 1 (7%) | 3 (25%) |
| Would only participate if recently diagnosed or if not as ill or old | 4 (15%) | 3 (21%) | 1 (8%) |
| Concerns about new drug/being guinea pig | 4 (15%) | 2 (14%) | 2 (17%) |
| Concerns about additional tests | 1 (4%) | 0 (0%) | 1 (8%) |
| Nonmedical benefit | |||
| Altruism | 14 (54%) | 7 (50%) | 7 (58%) |
| Deference to doctor’s opinion | 9 (35%) | 6 (43%) | 3 (25%) |
| Financial compensation/reimbursement | 6 (23%) | 3 (21%) | 3 (25%) |
| Opportunity to meet other PAH patients | 1 (4%) | 0 (0%) | 1 (8%) |
| Disclosure of results | 1 (4%) | 1 (7%) | 0 (0%) |
| Nonmedical burden | |||
| Time demands/inconvenience | 17 (65%) | 8 (57%) | 9 (75%) |
| Duration of trial | 6 (23%) | 4 (29%) | 2 (17%) |
| Disliking of having to take medicines | 4 (15%) | 0 (0%) | 4 (33%) |
Medical benefits
More than half of all patients interviewed expressed hope that participation in RCTs of novel therapies for PAH would result in personal benefit with improved health status. As one patient stated, ‘If it is something that they think is going to help, I am willing to try … I am only 61 and I don’t want to die anytime soon so I will try anything to help me’.
Four patients who were presented with the RCT prohibiting background therapies also mentioned the benefit of increased physician surveillance, including additional clinic visits, and the assumption that study-related procedures would provide physicians further opportunities to recognize deteriorations in their health. One patient reported, ‘[I] would be getting some regular blood work, chest x-rays, echocardiograms or whatever. That’s another selling point because I’d be getting care’. The same patient also noted the potential financial benefits of what she perceived to be free care, saying, ‘You’re talking echocardiograms. Some insurance companies’ co-pays are high’.
Medical risks or harms
Of the patients interviewed, 65% (17/26) expressed concerns about the side effects of experimental drugs, and several noted that this was among the most important considerations guiding whether they would participate. One patient reported, ‘One of my biggest concerns is the safety… I would want somebody to clearly communicate to me what they expect the medication side effects might be. [T]he possible side effects from the medications, those are things that would pull me [into the study] or take me away from it’.
All but one patient evaluating the RCT without background therapy were concerned about foregoing their usual treatments. Some of these patients specifically noted a greater willingness to enroll in a study that allowed receipt of current medications. One patient stated, ‘You were going pretty good until you said I would have to stop taking my [endothelin receptor antagonist] and take the experimental drug, not knowing whether or not I had the placebo or the real thing. That is a matter of concern to me’. Another patient explained, ‘Well, it’s kind of my impression that pulmonary hypertension is a pretty serious illness but it is an illness that can be controlled. So that is what makes me concerned about not taking my medication is getting to a point where I can’t go to work or walk up a hill or things like that’. Significantly, more patients who addressed the no-background-therapy trial expressed concerns about deteriorating clinically during the study than among those who addressed the RCT in which background therapies could be continued (79% (11/14) vs. 17% (2/12)).
Nonmedical benefits
In addition to the factors related to individual patients’ own health that seemed to influence their willingness to enroll, multiple ‘nonmedical’ themes also emerged. For example, altruistic motives were identified by 54% (14/26) of patients. As one patient said, ‘I want to do anything that will help research for this disease. I want to help people that are being diagnosed now and that are younger [than me]’. As another patient put it, ‘Someone’s got to step up!’ Another commented, ‘[There’s] a certain altruistic component to this, that it’s not just for me, and realizing that PAH needs a lot of study. So the fact that you could be part of something important I think is a component that grabs people. You’ve been given this particular illness so it’s the way to turn around and instead of say “oh poor me” it’s “how can I use this to help?”’
Although the scripts read to patients made no mention of compensation and/or reimbursement for research participation, roughly one quarter of patients identified financial considerations as relevant to their decisions. A college-aged patient stated, ‘If there was compensation I would probably do it. Most definitely if you are going to cover my money to pay for my gas to get here. It can be a conservative amount but as long as I am getting back. Compensation would be a huge factor’. Other patients identified compensation as a relevant issue, but downplayed its importance. One remarked, ‘[Compensation] would help but if I would not be compensated that would not prevent me from doing it because I still feel that’s my contribution to help and I would never sign on to something because you’re giving me X number of dollars. That’s not why I would be doing this’. Another patient commented, ‘I personally would [enroll] if expenses were covered. Like, you shouldn’t have to pay to park if you’re coming in to do this kind of thing. But I would think you wouldn’t want to pay them because then you would have people participating for the wrong reasons’.
Nonmedical burdens
Patients also identified several nonmedical factors such as time demands and other inconveniences that may dissuade enrollment. Of the 26 patients interviewed, 17 (65%) said that the time demands of the trials—eight visits during 16 weeks—may discourage their enrollment. Travel to and from study appointments on alternating weeks was described by several patients as ‘difficult’, ‘inconvenient’, ‘a hassle’, and ‘a bit too much’. One patient offered, ‘I might consider doing it depending on the leniency of when I could come in or what hours can I come into the office. I would be more drawn to a study that brought me in fewer times out of the week’. Although most patients placed priority on the convenience of trial participation, patients differed in how burdensome they viewed the same trials. As one patient commented, ‘What you described would be doable. But if … I would be missing work then that would be an issue’.
Some patients specified limits regarding how far they would travel to participate in such a study. One patient commented, ‘There would be a limit. I would say probably not more than 20 minutes or so’. Another stated, ‘If I thought a study was valuable, I would probably go up to an hour’.
Discussion
To our knowledge, this is the first study to examine the motivations for and concerns regarding participation in RCTs of novel therapies among patients with a disease that is relatively rare, commonly fatal, and characterized by early age of onset, strong patient advocacy communities, and multiple available therapies that have uncertain effectiveness and unfavorable cost or administration characteristics. Investigators conducting research in diseases fitting these descriptions, including PAH, cystic fibrosis, inflammatory bowel disease, and multiple sclerosis, also face tensions between being unable to conduct active-controlled trials due to the larger sample sizes required and the difficulties of running placebo-controlled trials due to views that withholding available therapies carries greater than minimal risks [49].
By contrast, most studies of willingness to participate in RCTs have addressed patients with common illnesses with multiple inexpensive therapies, such as diabetes, heart failure, or systemic hypertension [6,9,14]. These diseases are each characterized by more indolent progression, greater affordability and clearer efficacy of treatments, and greater prevalence, enriching the pool of eligible RCT participants and thereby making active-controlled trials more feasible. The present study, therefore, complements and extends this literature by examining how patients with previously unstudied disease types choose to participate in clinical trials.
Our central finding is that many of the same motives and concerns found among patients with more common illnesses also pervade the thoughts of patients with these rarer, more life-threatening illnesses. However, the relative importance of these factors may differ.
Specifically, we found that while PAH patients recognize the need for more research, many are concerned about the potential deterioration of their health if they were to enroll in RCTs. This fear was particularly common among patients asked to consider enrollment in trials that precluded ongoing use of current PAH therapies. By contrast, many of these same patients were comforted by the perceived increase in disease surveillance they would receive while in the study. Similar to findings from a quantitative study in essential hypertension [6], this qualitative study in PAH suggests that patients would prefer to enroll in add-on drug trials, but might consider placebo-controlled trials without background therapies if emphasis was placed on frequent monitoring of their health.
Unfortunately, the procedures required to instill this sense of comfort may carry drawbacks, as many patients are concerned with the time demands of trials. Patients were concerned particularly about the frequency of required clinic visits, the duration of the study, and the amount of time they spend traveling to and from clinic visits. The observation that some patients (15% (4/26)) prefer more visits due to the potential for closer health surveillance contrasts with the desires of a majority (65% (17/26)) of patients to limit the number of clinic visits during the study so as to minimize the time demands. It may be difficult to meet the needs of both groups of patients. Nonetheless, knowledge that this tension exists may help investigators design studies to include a minimal number of visits that is unlikely to be perceived as overly burdensome, while also offering optional visits for those who desire closer supervision. However, such designs would require prospective plans to account for surveillance and other biases that may ensue if patients in different groups have different follow-up schedules. Alternatively, the option of home visits instead of clinic visits could also enhance the appeal of RCTs for patients, though studies offering home visits may be time and resource intensive.
We also found that a majority of patients with PAH viewed RCTs as ways to improve their own health. Indeed, for many, the perceived potential for personal benefit—through increased surveillance or exposure to a potentially superior drug—was a prominent influence on patients’ expressed willingness to enroll. This well-described phenomenon, termed the therapeutic misconception (i.e. research participants misconceive research as therapy) [50] raises several concerns about the extent to which patients understand the distinctions between research and clinical care. Thus, physicians—particularly those who simultaneously serve as clinicians and investigators in PAH trials—should work to ensure that their patients understand these distinctions prior to enrolling in RCTs [51]. It has been suggested that offering financial compensation for research participation could help combat the therapeutic misconception because patients are not ordinarily paid for transportation costs or undergoing tests as parts of their health care [52]. Though plausible, we are not aware of evidence to support or refute this suggestion.
Finally, we found that 54% (14/26) of interviewed PAH patients cited altruistic motives for research participation. PAH and the aforementioned similar diseases are characterized by prominent patient advocacy communities whose abilities to unite patients with less common diseases have only grown with the Internet and chat rooms. We had hypothesized that within these diseases, patients would have been further motivated to participate in research for altruistic reasons. However, as in studies in other disease areas [6], we instead found that personal motives often figure more prominently than do altruistic ones.
This study has limitations. First, the recruitment of patients from a single academic program in the United States could limit the generalizability of the results. Second, most patients in our sample were women. However, PAH more commonly afflicts women [39], suggesting that our findings may generalize to the PAH population at large. Third, this study involved treated patients, so the results may not extend to newly diagnosed patients who have yet to begin therapy. Fourth, the percentages of patients citing different factors (Table 3) should not be interpreted as reflecting population-level proportions, but rather serve only to help readers understand the relative frequencies with which factors were cited among our sample. Although our strategy of purposive sampling of patients was appropriate for our goal of establishing a conceptual framework for why patients with PAH or other rare devastating diseases enroll in trials, this strategy precludes the ability to estimate population-level proportions.
Fifth, the use of hypothetical trials, though common in studies of willingness to participate, may not elicit identical decision-making processes as would be found if patients were contemplating actual trial participation. Thus, future research should aim to quantify the impacts of various features of RCT design on the actual enrollment decisions patients with PAH make.
Finally, we intentionally assigned approximately half of patients to respond to placebo-controlled trials that prohibited concomitant use of other PAH-specific therapies to add evidence to an ongoing debate about the appropriateness of this trial design in the modern treatment era [49,51,53]. In the time since we designed this study, the proportion of new RCTs in PAH that include such restrictions on background therapy seems to have declined. The heightened concerns our patients expressed regarding the risks of such trials suggest that such changes may enhance the enrollment.
In summary, this study suggests that several elements of a trial—including the duration and frequency of required clinic visits, restrictions on concomitant drug use, the views of physicians requesting enrollment, and perhaps the use of financial incentives or remuneration for study-related expenses—may influence PAH patients’ enrollment in RCTs. Future research is needed to explore the extent to which modifying these factors may augment participation in actual RCTs, to identify interventions to mitigate the prevalent misconceptions of research as care, and to confirm hypotheses that the research participation decision-making processes among patients with PAH may resemble those made by patients with other similar diseases.
Acknowledgments
Funding
This study was supported by an American Thoracic Society/Pfizer Award in Pulmonary Hypertension and by a University of Pennsylvania Center for Clinical Epidemiology and Biostatistics Summer Research Award.
Footnotes
Reprints and permission: http://www.sagepub.co.uk/journalsPermissions.nav
References
- 1.Hunningshake DB, Darby CA, Probstfield JL. Recruitment experience in clinical trials: Literature summary and annotated bibliography. Control Clin Trials. 1987;8:6S–30S. doi: 10.1016/0197-2456(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 2.Halpern SD. Prospective preference assessment: A method to enhance the ethics and efficiency of randomized clinical trials. Control Clin Trials. 2002;23:274–88. doi: 10.1016/s0197-2456(02)00191-5. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell PH, Hamilton S, Tan A, et al. Strategies for increasing recruitment to randomised controlled trials: Systematic review. PLoS Med. 2010;7:e1000368. doi: 10.1371/journal.pmed.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donovan J, Mills N, Smith M, et al. Quality improvement report: Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Brit Med J. 2002;325:766–70. doi: 10.1136/bmj.325.7367.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Israni AK, Halpern SD, McFadden C, et al. Willingness of dialysis patients to participate in a randomized controlled trial of daily dialysis. Kidney Int. 2004;65:990–98. doi: 10.1111/j.1523-1755.2004.00460.x. [DOI] [PubMed] [Google Scholar]
- 6.Halpern SD, Karlawish JHT, Casarett D, et al. Hypertensive patients’ willingness to participate in placebo-controlled trials: Implications for recruitment efficiency. Am Heart J. 2003;146:985–92. doi: 10.1016/S0002-8703(03)00507-6. [DOI] [PubMed] [Google Scholar]
- 7.Solomon MJ, Pager CK, Young JM, et al. Patient entry into randomized clinical trials of colorectal cancer treatment: Factors influencing participation. Surgery. 2003;133(6):608–13. doi: 10.1067/msy.2003.119. [DOI] [PubMed] [Google Scholar]
- 8.Sugarman J, Kass N, Goodman S, et al. What patients say about medical research. IRB. 1998;20(4):1–7. [PubMed] [Google Scholar]
- 9.Peterson ED, Lytle BL, Biswas MS, et al. Willingness to participate in cardiac trials. Am J Geriatr Cardiol. 2004;13(1):11–15. doi: 10.1111/j.1076-7460.2004.01709.x. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins V, Farewell D, Batt L, et al. The attitudes of 1066 patients with cancer towards participation in randomized clinical trials. Brit J Cancer. 2010;103(12):1801–07. doi: 10.1038/sj.bjc.6606004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A, Wilke HJ, II, Mingay D, et al. Patient attitudes toward granting consent to participate in perioperative randomized clinical trials. J Clin Anesth. 2004;16(6):426–34. doi: 10.1016/j.jclinane.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Durant RW, Legedza AT, Marcantonio ER, et al. Willingness to participate in clinical trials among African Americans and whites previously exposed to clinical research. J Cult Divers. 2011;18:8–19. [PMC free article] [PubMed] [Google Scholar]
- 13.Davison BJ, So A, Goldenberg SL, et al. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: A Canadian perspective. BJU Int. 2008;101:982–87. doi: 10.1111/j.1464-410X.2007.07349.x. [DOI] [PubMed] [Google Scholar]
- 14.Robiner WN, Yozwiak JA, Bearman DL, et al. Barriers to clinical research participation in a diabetes randomized clinical trial. Soc Sci Med. 2009;68(6):1069–74. doi: 10.1016/j.socscimed.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson CL, Morley KC, Teeson M, et al. Issues with recruitment to randomized controlled trials in the drug and alcohol field: A literature review and Australian case study. Drug Alcohol Rev. 2008;27(2):115–22. doi: 10.1080/09595230701829561. [DOI] [PubMed] [Google Scholar]
- 16.Sharp L, Cotton SC, Alexander L, et al. TOMBOLA Group. Reasons for participation in a randomized controlled trial: Postal questionnaire surveys of women eligible for TOMBOLA (Trial of Management of Borderline and Other Low-Grade Abnormal smears) Clin Trials. 2006;3(5):431–42. doi: 10.1177/1740774506070812. [DOI] [PubMed] [Google Scholar]
- 17.Glogowska M, Roulstone S, Enderby P, et al. Who’s afraid of the randomized controlled trial? Parents’ views of an SLT research study. Int J Lang Comm Dis. 2001;36(Suppl):499–504. doi: 10.3109/13682820109177936. [DOI] [PubMed] [Google Scholar]
- 18.Agoritsas T, Deom M, Perneger TV. Study design attributes influenced patients’ willingness to participate in clinical research: A randomized vignette-based study. J Clin Epidemiol. 2011;64:107–15. doi: 10.1016/j.jclinepi.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Fallowfield LJ, Jenkins V, Brennan C, et al. Attitudes of patients to randomised clinical trials of cancer therapy. Eur J Cancer. 1998;34:1554–59. doi: 10.1016/s0959-8049(98)00193-2. [DOI] [PubMed] [Google Scholar]
- 20.Halpern SD, Metzger DS, Berlin JA, et al. Who will enroll? Predicting participation in a phase II AIDS vaccine trial. J Acq Immun Def Synd. 2001;27:281–88. doi: 10.1097/00126334-200107010-00011. [DOI] [PubMed] [Google Scholar]
- 21.Lemieux J, Goodwin PJ, Pritchard KI, et al. Identification of cancer care and protocol characteristics associated with recruitment in breast cancer clinical trials. J Clin Oncol. 2008;26(27):4458–65. doi: 10.1200/JCO.2007.15.3726. [DOI] [PubMed] [Google Scholar]
- 22.Goode PS, Fitzgerald MP, Richter HE, et al. Enhancing participation of older women in surgical trials. J Am Coll Surgeons. 2008;207(3):303–11. doi: 10.1016/j.jamcollsurg.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baggstrom MQ, Waqar SN, Sezhiyan AK, et al. Barriers to enrollment in non-small cell lung cancer therapeutic clinical trials. J Thorac Oncol. 2011;6:98–102. doi: 10.1097/JTO.0b013e3181fb50d8. [DOI] [PubMed] [Google Scholar]
- 24.Ross S, Grant A, Counsell C, et al. Barriers to participation in randomised controlled trials: A systematic review. J Clin Epidemiol. 1999;52:1143–56. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 25.Lowton K. Trials and tribulations: Understanding motivations for clinical research participation amongst adults with cystic fibrosis. Soc Sci Med. 2005;61(8):1854–65. doi: 10.1016/j.socscimed.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 26.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: Results from a national registry. Am J Resp Crit Care. 2006;173:1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 28.van Jaarsveld CH, Ranchor AV, Kempen GI, et al. Epidemiology of heart failure in a community-based study of subjects aged > or = 57 years: Incidence and long-term survival. Eur J Heart Fail. 2006;8(1):23–30. doi: 10.1016/j.ejheart.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5(2):167–73. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 30.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 31.Barst RJ, Rubin LJ, McGoon MD, et al. Survival in primary pulmonary hypertension with long-term continuous intravenous prostacyclin. Ann Intern Med. 1994;121:409–15. doi: 10.7326/0003-4819-121-6-199409150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Farber H, Halpern S, Bodin F, et al. Pulmonary hypertension roundtable: Of ethics, trials, and tribulations. Adv Pulm Hypertens. 2011;9:226–32. [Google Scholar]
- 33.Reichenberger F, Mainwood A, Morrell NW, et al. Intravenous epoprostenol versus high dose inhaled iloprost for long-term treatment of pulmonary hypertension. Pulm Pharmacol Ther. 2010;24(1):169–73. doi: 10.1016/j.pupt.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Denton CP, Humbert M, Rubin L, et al. Bosentan treatment for pulmonary arterial hypertension related to connective tissue disease: A subgroup analysis of the pivotal clinical trials and their open-label extensions. Ann Rheum Dis. 2006;65:1336–40. doi: 10.1136/ard.2005.048967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaughlin VV, Suissa S. Prognosis of pulmonary arterial hypertension: The power of clinical registries of rare diseases. Circulation. 2010;122(2):106–08. doi: 10.1161/CIRCULATIONAHA.110.963983. [DOI] [PubMed] [Google Scholar]
- 36.Halpern S, Badesch DB, McGoon MD, et al. Pulmonary hypertension roundtable: Ethical considerations for RCTs in PAH. Adv Pulm Hypertens. 2009;8:42–46. [Google Scholar]
- 37.Halpern SD. Prospective preference assessment: A method to enhance the ethics and efficiency of randomized clinical trials. Control Clin Trials. 2002;23:274–88. doi: 10.1016/s0197-2456(02)00191-5. [DOI] [PubMed] [Google Scholar]
- 38.Freedman B. Scientific value and validity as ethical requirements for research: A proposed explication. IRB. 1987;9(6):7–10. [PubMed] [Google Scholar]
- 39.Mandel J, Taichman D. Pulmonary vascular disease. Saunders; Philadelphia, PA: 2006. pp. 66–83. [Google Scholar]
- 40.Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: A phase II study. Eur Respir J. 2010;36(4):792–99. doi: 10.1183/09031936.00182909. [DOI] [PubMed] [Google Scholar]
- 41.Barst RJ, McGoon M, McLaughlin V, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41(12):2119–25. doi: 10.1016/s0735-1097(03)00463-7. [DOI] [PubMed] [Google Scholar]
- 42.Galie N, Humbert M, Vachiery JL, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2002;39(9):1496–502. doi: 10.1016/s0735-1097(02)01786-2. [DOI] [PubMed] [Google Scholar]
- 43.Curry LA, Nembhard IM, Bradley EH. Qualitative and mixed methods provide unique contributions to outcomes research. Circulation. 2009;119:1442–52. doi: 10.1161/CIRCULATIONAHA.107.742775. [DOI] [PubMed] [Google Scholar]
- 44.Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. J Am Med Assoc. 2000;284:357–62. doi: 10.1001/jama.284.3.357. [DOI] [PubMed] [Google Scholar]
- 45.Strauss AL, Corbin J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. SAGE; Thousand Oaks, CA: 1998. [Google Scholar]
- 46.Ryan GW, Bernard HR. Techniques to identify themes. Field Method. 2003;15(1):85–109. [Google Scholar]
- 47.Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: Developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758–72. doi: 10.1111/j.1475-6773.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hruschka DJ, Schwartz D, St John DC, et al. Reliability in coding open-ended data: Lessons learned from HIV behavioral research. Field Method. 2004;16(3):307–31. [Google Scholar]
- 49.Halpern SD, Doyle R, Kawut SM. The ethics of randomized clinical trails in pulmonary arterial hypertension. Proc Am Thorac Soc. 2008;5:631–35. doi: 10.1513/pats.200802-019SK. [DOI] [PubMed] [Google Scholar]
- 50.Applebaum PS, Roth LH, Lidz CW, et al. False hopes and best data: Consent to research and the therapeutic misconception. Hastings Center Rep. 1987;17(2):20–24. [PubMed] [Google Scholar]
- 51.Macchia A, Marchioli R, Marfisi R, et al. A meta-analysis of trials of pulmonary hypertension: A clinical condition looking for drugs and research methodology. Am Heart J. 2007;153(6):1037–47. doi: 10.1016/j.ahj.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 52.Dickert N, Grady C. What’s the price of a research subject? Approaches to payment for research participation. N Engl J Med. 1999;341:198–203. doi: 10.1056/NEJM199907153410312. [DOI] [PubMed] [Google Scholar]
- 53.Helman DL, Jr, Brown AW, Jackson JL, et al. Analyzing the short-term effect of placebo therapy in pulmonary arterial hypertension: Potential implications for the design of future clinical trials. Chest. 2007;132(3):764–72. doi: 10.1378/chest.07-0236. [DOI] [PubMed] [Google Scholar]


