Abstract
Thirty-three previously untreated patients with high risk CLL were treated before meeting standard criteria with alemtuzumab and rituximab. GM-CSF was added to the regimen to determine if it would improve treatment efficacy without increasing toxicity. High risk was defined as at least one of the following: 17p13-; 11q22.3-; unmutated IGHV (or use of VH3-21) together with elevated expression of ZAP-70 and/or CD38. Treatment was subcutaneous GM-CSF 250μg Monday-Wednesday-Friday for 6 weeks from day 1, subcutaneous alemtuzumab 3mg-10mg-30mg from day 3 and then 30 mg Monday-Wednesday-Friday for 4 weeks, and intravenous rituximab (375 mg/m2/week) for 4 weeks from day 8. Patients received standard supportive care and were monitored weekly for CMV reactivation. Using standard criteria, 31 (94%) patients responded to treatment with 9 (27%) complete responses (one with persistent cytopenia) and 9 (27%) nodular partial responses. Median progression free survival was 13.0 months and time to next treatment was 33.5 months. No patient died during treatment, seven (21%) had grade 3-4 toxicities attributable to treatment, and 10 (30%) had CMV viremia. Addition of GM-CSF to therapy with alemtuzumab and rituximab decreased treatment efficacy and increased the rate of CMV reactivation compared to a historical control.
Keywords: Chronic lymphocytic leukemia, high risk, early treatment, alemtuzumab, rituximab, GM-CSF
Introduction
Patients with high-risk chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) can be identified using biological parameters of the malignant CLL cells including specific chromosomal abnormalities[1], somatic hypermutation of the variable region of the immunoglobulin heavy chain genes (IGHV mutation)[2,3], and the expression of CD38[3] and ZAP-70[4]. Those patients in whom interphase fluorescent in-situ hybridization (FISH) detects17p13 deletions (17p-) or 11q22.3 deletions (11q-) have a significantly poorer prognosis[1]. Use of unmutated IGHV (< 2% difference from germ line sequence)[2,3] and use of VH3-21 irrespective of mutation status[5] is associated with a poorer prognosis. Patients with unmutated IGHV are reported to have a significantly worse prognosis if CLL cells express CD38 or ZAP-70[6,7] . Defining high-risk earlier stage CLL in asymptomatic patients is now feasible and the challenge is to develop highly effective low toxicity therapy for this population.
The current standard of care is to treat patients with CLL only when they have progressive disease because conventional treatment is not curative and previous randomized studies using only chemotherapy showed no survival advantage for early treatment[8-10]. Fit patients with progressive CLL are now usually treated with chemoimmunotherapy (purine analogue, cyclophosphamide, and rituximab), which achieves high response rates, prolonged response duration, and increased overall survival[11-13]. However, these therapies can cause significant toxicity and long-term complications, and are considerably less effective in patients with p53 dysfunction[13,14]. Thus, early treatment of selected CLL patients with a lower burden of disease and high risk of disease progression using effective low toxicity therapy could decrease exposure to DNA damaging agents and the risk of clonal evolution to more aggressive disease.
We have previously reported a phase II trial using a short course of alemtuzumab and rituximab for early treatment of high risk CLL patients[15]. This regimen was selected because alemtuzumab and rituximab have discrete targets and different mechanisms of action[16,17], and addition of rituximab to alemtuzumab appears to improve responses compared to historical results for patients with relapsed/refractory CLL[18,19]. Early treatment of patients with high risk CLL resulted in a 90% response rate, 37% complete response (CR) rate, median duration of response of 14.4 months and estimated median time to next treatment of 4.4 years[15]. Therapy was well tolerated and although cytomegalovirus (CMV) reactivation occurred in 3 (10%) patients, only 1 patient had clinical disease[15]. However, because of the limited duration of response, additional efforts are required to improve the efficacy of this therapy.
Phagocytosis by fixed macrophages and antibody dependent cellular cytotoxicity (ADCC) mediated by NK cells, monocytes, and neutrophils are important mechanisms of action of both rituximab and alemtuzumab and complement dependent cytotoxicity (CDC) is important for the activity of alemtuzumab[16,17,20,21]. Both alemtuzumab and rituximab decrease the circulating neutrophil count and alemtuzumab can decrease circulating NK cells and monocytes[15] while its effect on tissue macrophages is not known. Thus, both alemtuzumab and rituximab could inhibit ADCC by decreasing effector cells.
Granulocyte monocyte colony stimulating factor (GM-CSF) promotes proliferation and maturation of neutrophils and monocytes, and increases differentiation of monocytes to macrophages and dendritic cells[22] . GM-CSF has also been reported to increase rituximab ADCC of CLL cells by monocytes in vitro[23]. A phase II study using GM-CSF and rituximab to treat patients with relapsed follicular B cell lymphoma reported higher overall response and CR rates without undue toxicity compared to the historical experience[24]. In addition, limited experience of treatment of patients with CLL with GM-CSF and rituximab suggest that this therapy is well tolerated and did not have inferior efficacy compared to rituximab monotherapy[25]. The current published data thus suggest that addition of GM-CSF to monoclonal antibody (mAb) therapy could be safe and effective.
We hypothesized that treatment with GM-CSF could increase effector cell activity resulting in improved efficacy of therapy with alemtuzumab and rituximab in patients with CLL. As the initial step in testing this hypothesis, we conducted a phase II trial of early treatment of high-risk CLL patients with GM-CSF, alemtuzumab, and rituximab. This study showed that addition of GM-CSF decreased treatment efficacy and resulted in higher rates of CMV reactivation compared to a historical experience with alemtuzumab and rituximab alone.
Methods and Materials
Patient Selection
The study was approved by the Mayo Clinic Institutional Review Board and registered with ClinicalTrials.gov (NCT00562328), and all patients provided written informed consent. Sequential patients were evaluated in the Division of Hematology at Mayo Clinic Rochester and Department of Hematology/Oncology at Mayo Clinic Arizona from January 2008 to February 2010. Eligibility required a diagnosis of CLL by flow cytometry on peripheral blood[26], previously untreated early to intermediate clinical stage disease (Rai 0–II) that did not fulfill criteria for treatment as defined by the NCI-Working Group criteria of 1996 (NCI-WG96)[27], and molecular markers predictive of a high risk of disease progression. High-risk disease was defined as at least one of the following: 1. 17p13- by FISH analysis; 2. 11q22- by FISH analysis; 3. unmutated IGHV (<2% sequence variation from germline) or use of the VH3-21 gene segment irrespective of mutation status together with expression of ZAP-70 (≥20% cells positive on flow cytometry) and/or CD38 (≥30% cells positive on flow cytometry)[15]. All patients required an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 and adequate organ function (serum creatinine ≤ 1.5 x upper limit of normal (UNL), total bilirubin ≤ 3.0 x UNL, and serum AST ≤3.0 x UNL). Exclusion criteria included any evidence of active autoimmune disease or active primary malignancy requiring treatment or limiting expected survival to <2 years.
Therapy
Patients were treated for 6 weeks. GM-CSF 250 μg/day was given subcutaneously three times a week (Monday-Wednesday-Friday) starting on day 1 of treatment and continued for a total of 6 weeks. Patients received subcutaneous alemtuzumab with dose escalation (3 mg, 10 mg, 30 mg) on days 3-5 of the first week of therapy as tolerated and then received 30 mg/d Monday-Wednesday-Friday for the next 4 weeks. After the first week of therapy, alemtuzumab could be self administered at home at the patient's discretion.
Rituximab (375 mg/m2 intravenously) was given on day 8 and then weekly for a total of 4 doses. The first dose of alemtuzumab and all doses of rituximab were premedicated with acetaminophen and diphenhydramine. Patients received allopurinol (300 mg/day) days 2-15 of therapy. All patients received prophylaxis for pneumocystis pneumonia and reactivation of herpes simplex and varicella zoster during treatment and then for an additional 6 months. Patients were monitored for CMV reactivation using PCR for viral DNA weekly during treatment and then monthly for 6 months and treated whenever CMV viremia was detected.
Response Evaluation
Patients were evaluated by physical examination and blood testing weekly during treatment, then monthly for 6 months, and then at 9 and 12 months after completing therapy. Adverse events were graded using the Common Terminology Criteria for Adverse Events (CTCAE) v3.0 http://ctep.cancer.gov/. Response to treatment was measured 2 months after completion of therapy using standard NCI-WG96 criteria[27]. In patients who achieved a complete clinical remission by NCI-WG96 criteria, the bone marrow (BM) biopsy was evaluated for residual CLL by immunohistochemical (IHC) studies (streptavidinbiotin peroxidase complex method) with antibodies directed against CD3, CD5, CD23 and PAX5 using standard techniques. Minimal residual disease (MRD) was evaluated in peripheral blood specimens by high-sensitivity flow cytometry weekly during treatment and then every month after treatment for 6 months and at 9 and 12 months after completion of therapy in patients in sustained response.
This flow cytometry method evaluated 500,000 events with a single tube, 6-color panel (CD45, CD19, CD20, CD5, kappa, lambda) on a FACS Canto flow cytometer (BD Biosciences, San Jose, CA) as previously described[28,29].
Statistical Analysis
This study used a modified Fleming two-stage design[30] with a primary endpoint of CR rate. The secondary end points were to determine: 1. Overall response rate and toxicity of treatment; 2. Overall and progression-free survival, time to next treatment, and duration of response; and 3. The quality of the response to treatment using IHC staining of BM and sensitive flow cytometry for MRD in peripheral blood. Based the results of our previous study of early treatment of high risk CLL patients with alemtuzumab and rituximab[15], the largest CR rate where the addition of GM-CSF to the alemtuzumab and rituximab regimen would be considered ineffective was defined as 35%, and the smallest complete response rate that would warrant subsequent studies was 60%. The study thus required a minimum of 16 and a maximum of 30 evaluable patients. Duration of response was defined as the time from noting a response (CR, nodular partial response (nPR), or partial response (PR)) to the earliest time that progression was documented. Survival time is defined as the time from registration to death due to any cause. Time to disease progression is defined as the time from registration to the earliest date of documentation of disease progression. Patients dying without documentation of disease progression were considered to have had tumor progression at the time of their death unless there was proof of sustained response. Time to subsequent therapy was defined as the time from the end of active treatment to the time subsequent therapy was initiated. The distribution of all time-to-event endpoints were estimated using the method of Kaplan-Meier[31]. Baseline information of patients enrolled in this trial was compared to that of patients enrolled in MC038G using two sample t-tests (for continuous data) or chi-squared tests (for categorical data).
Results
Thirty-three patients were enrolled and are described in Table 1. All patients met eligibility criteria, completed the treatment regimen protocol, and were evaluated for response and toxicity. The median follow up for surviving patients (n = 31) was 28 months (range 10 - 43).
Table 1.
Patient Characteristics
| Age (years, median & range) | 60 (42 – 77) |
| Sex | Male 23 (70%) |
| Clinical stage (Rai) | |
| 0 | 2 (6%) |
| I | 27 (82%) |
| II | 4 (12%) |
| Lymphocytes (109/L) median (range) | 27.1 (5.5 – 178.0) |
| Hemoglobin (g/dL) median (range) | 13.9 (11.3 – 15.7) |
| Platelets (109/L) median (range) | 163 (102 – 318) |
| FISH (Hierarchical) | |
| 17p- | 4 (12%) |
| 11q- | 11 (33%) |
| 12+ | 7 (21%) |
| none | 5 (15%) |
| 13q- | 5 (15%) |
| Other (6q-) | 1 (3%) |
| IGHV Somatic Hypermutation | |
| Unmutated | 30 (91%) |
| Mutated | 3 (9%) |
| ZAP-70 | |
| Positive | 25 (76%) |
| Negative | 7 (21%) |
| Unknown | 1 (3%) |
| CD38 | |
| Positive | 15 (45.5%) |
| Negative | 18 (54.5%) |
| High risk group classification | |
| 17p- | 4 (12%) |
| 11q- | 11 (33%) |
| IGHV UM & ZAP-70/CD38+ | 17 (51.5%) |
| IGHV VH3-21 & ZAP-70/CD38+ | 1 (3%) |
| Beta2 Microglobulin (mg/dL) median (range) | 2.9 (1.8 – 8.4) |
| Time from diagnosis to treatment (months) | 6.3 (0.6 – 52.2) |
| ECOG performance score | |
| 0 | 31 (93.9%) |
| 1 | 2 (6.1%) |
Toxicity
There were no deaths during treatment and seven (21%) patients had severe (grade 3-4) adverse events at least possibly related to treatment: neutropenia (n = 3), infections (n=4), dyspnea (n=1), hypersensitivity reaction (n=1), and skin reactions (n=2). The severe infections were febrile neutropenia (n=1), pneumonia (n=1) and opportunistic infections (n=2) which included one patient with CMV reactivation detailed below. In one of these patients, infection resulted in a short delay in completing treatment.
CMV viremia was detected by PCR in the peripheral blood in 10 (30%) patients with a median time to detection of 4 weeks from starting treatment (range 3-8) and a median of one positive test (range 1 – 3) per patient. Among the nine patients who started valganciclovir therapy (900 mg every 12 hours by mouth) within 24 hours of the detection of viremia, there were no significant clinical consequences of CMV reactivation. However, one patient had a multiple day delay in filling her prescription for valganciclovir because of logistical problems and developed symptomatic CMV reactivation requiring hospitalization and ganciclovir therapy. There was only one grade 2 injection site reaction.
Responses to treatment
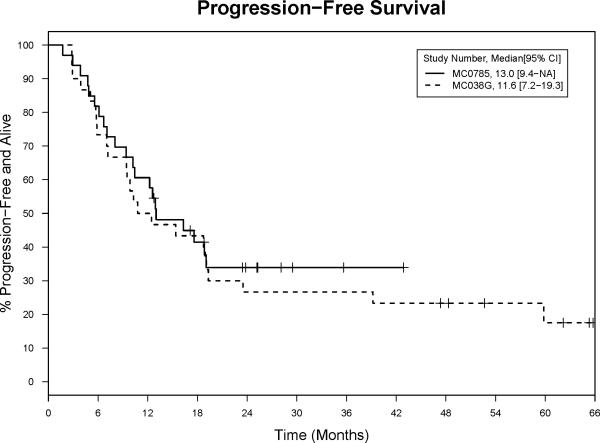
Thirty-one (94%, 95% confidence interval (CI): 80–99%) patients responded to treatment with eight (24%) CR, one complete response with persistent cytopenia (CRi) (with subsequent normalization of blood counts), nine (27%) nPR, and 13 (39%) PR. Median progression free survival (PFS) was 13.0 months (95% CI: 9.4–not yet reached (NR))(Figure 1), duration of response was 14.9 months (95% CI: 8.8–NR), time to next treatment 33.5 months (95% CI: 25.5–NR)(Figure 2), and overall survival has not been reached. Of the first 30 evaluable patients enrolled, there were 8 (27%) CR and thus according to the study design, we conclude that addition of GM-CSF to alemtuzumab and rituximab did not increase the efficacy of this regimen.
Figure 1. Progression Free Survival.
These Kaplan Meier curves plot the progression free survival for patients on the MC0785 (alemtuzumab, rituximab, and GM-CSF) and MC038G (alemtuzumab and rituximab studies).
Figure 2. Time to Retreatment.
These Kaplan Meier curves plot the percentage of patients that did not require retreatment for their CLL on the MC0785 (with GM-CSF) and MC038G (without GMCSF) studies.
There were 2 patient deaths after completion of therapy. One patient with 17p13- CLL presented with back pain 9 days after completion of therapy and was found to have a renal mass caused by EBV negative diffuse large B cell lymphoma that was fatal. The role of treatment with alemtuzumab in the development of the diffuse large B cell lymphoma cannot be determined. Alemtuzumab induced immunosuppression has been associated with the development of more aggressive lymphomas but the reported cases were EBV positive[32,33]. The absence of evidence of EBV reactivation and the temporal sequence of the complication suggest that this patients diffuse large B cell lymphoma could have been present prior to initiation of therapy with alemtuzumab. A second patient with 17p13- CLL achieved PR of 4 months duration. He then failed treatment for progressive disease with fludarabine, cyclophosphamide and rituximab (FCR) and died from his CLL 10 months later. Eleven additional patients required subsequent treatment and received FCR (n=6), pentostatin, cyclophosphamide, and rituximab (PCR)(n=1), bendamustine and rituximab (n=1), rituximab, cyclophosphamide, vincristine and prednisone (n=2), and rituximab (n=1).
Neutrophil and Monocyte Counts
GM-CSF decreased treatment induced neutropenia (Figure 3) but not alemtuzumab mediated monocytopenia (Figure 4) compared to our previous study of early treatment of high risk CLL patients with alemtuzumab and rituximab[15].
Figure 3. Neutrophil Counts.
Absolute blood neutrophil counts (median, 25th, and 75th quartiles) were plotted for patients treated with (solid squares) or without (open circles) GM-CSF. The vertical interrupted lines indicate duration of treatment. The median neutrophil counts (x 109/L) were significantly higher for patients on MC0785 compared to MC038G for measurements at three time points during therapy with GM-CSF (marked by the vertical interrupted lines) at day 8 (3.5 vs. 2.7, p<0.001), day 22 (3.9 vs. 3.1. p=0.003), and day 29 (4.1 vs. 2.9, p=0.002) (Wilcoxon Rank Sum Test).
Figure 4. Monocyte Counts.
Absolute blood monocyte counts (median, 25th, and 75th quartiles) were plotted for patients treated with (solid squares) or without (open circles) GM-CSF. The vertical interrupted lines indicate duration of treatment. The median monocyte counts (x 109/L) were not significantly different for patients on MC0785 compared to MC038G during therapy with GM-CSF at day 8 (0.5 vs. 0.3, p=0.06), day 15 (0.1 vs. 0.1, p=0.178), day 22 (0.1 vs. 0.1, p=0.86) and day 29 (0.1 vs. 0.1, p=0.75) (Wilcoxon Rank Sum Test).
Testing for Residual Disease
BM biopsies of the six patients achieving a CR/CRi with no morphological evidence of CLL were studied with IHC for residual disease. The three patients with IHC negative CR had a longer PFS (not reached at 25 months). Serial high sensitivity flow cytometry for MRD in blood was performed during treatment and then until disease progression in 29 patients. Seventeen (59%) patients achieved at least 1 negative MRD test during treatment and 12 (41%) had negative results after completion of therapy. However, only four (14%) of these patients were still MRD negative at 2 months after completion of therapy with two having evidence of residual disease on IHC examination of the BM (PFS 19 months and not reached at 19 months) and two having a IHC negative BM (PFS not reached at 25 months). The longest duration of MRD negative status was 9 months after completion of therapy.
Addition of GM-CSF to alemtuzumab and rituximab
To assess if GM-CSF improved the efficacy of alemtuzumab and rituximab for early treatment of high risk CLL, we compared the results of this trial (MC0785) to a preceding trial (MC038G) which used similar eligibility criteria[15]. As seen in Table 2, there were significantly more patients on the MC0785 trial with unmutated IGHV. In contrast the higher number of patients on MC038G with 17p13- (30% vs. 12%) did not represent a significant difference (p = 0.12). Use of GM-CSF was associated with a significantly higher rate of CMV reactivation (30% vs. 3%, p = 0.007), but only one patient in each trial had symptomatic CMV reactivation requiring hospitalization. There were no other significant differences in toxicities. Use of GM-CSF was associated with a similar overall response rate (94%, 95% CI 80-99% vs. 90%, 95% CI 73-98%) and PFS (13.0, 95% CI 9.4-NR vs. 11.6, 95% CI 7.2-19.3 months) (Figure 1) but lower CR rates (24%, 95% CI 11-42% vs. 37%, 95% CI 19-56%) and time to next treatment (33.5, 95% CI 25.5-37.6 vs. 43, 95% CI 29.4-66.3 months) (Figure 2).
Table 2.
Comparison of Patients Treated with Alemtuzumab and Rituximab (MC038G) and Alemtuzumab, Rituximab, and GM-CSF (MC0785)
| MC038G (N=30) | MC0785 (N=33) | Total (N=63) | p value | |
|---|---|---|---|---|
| Age (years, median and range) | 61 (29 – 77) | 60 (42 – 77) | 60 (29 – 77) | 0.8014 |
| Sex (male) | 20 (66.7%) | 23 (69.7%) | 43 (68.3%) | 0.7964 |
| Clinical Stage (Rai) | 0.1313 | |||
| 0 | 7 (23.3%) | 2 (6.1%) | 9 (14.3%) | |
| I | 21 (70.0%) | 27 (81.8%) | 48 (76.2%) | |
| II | 2 (6.7%) | 4 (12.1%) | 6 (9.5%) | |
| Lymphocytes (×109/L, median, range) | 25.3 (4.8-124.7) | 27.1 (5.9-178.0) | 26.0 (4.8-178.0) | 0.1447 |
| Hemoglobin (g/dL) | 14.2 (12.0-17.7) | 13.9 (11.3-15.7) | 13.9 (11.3-17.7) | 0.4872 |
| Platelets (×109/L, median, range) | 178 (100-312) | 163 (102-318) | 176 (100-318) | 0.1559 |
| Beta2 microglobulin (ng/mL) | 2.5 (1.3-8.5) N = 24 | 2.9 (1.8-8.4) | 2.7 (1.3-8.5) N = 57 | 0.1657 |
| Diagnosis to treatment (months, median, range) | 7.8 (0.7-73.1) | 6.3 (0.6-52.2) | 6.7 (0.6-73.1) | 0.9663 |
| Follow up (months, median, range) | 54.7 (13.9 – 70.8) | 28.3 (2.0 – 42.9) | 36.9 (2.0 – 70.2) | |
| FISH Hierarchical | 0.5121 | |||
| 17p- | 9 (30.0%) | 4 (12.1%) | 13 (20.6%) | |
| 11q- | 8 (26.7%) | 11 (33.3%) | 19 (30.2%) | |
| 12+ | 7 (23.3%) | 7 (21.2%) | 14 (22.2%) | |
| none | 3 (10.0%) | 5 (15.2%) | 8 (12.7%) | |
| 13 | 3 (10.0%) | 5 (15.2%) | 8 (12.7%) | |
| other | 0 (0.0%) | 1 (3.0%) | 1 (1.6%) | |
| IGHV Somatic Hypermutation | 0.0001 | |||
| Mutated (≥ 2%) | 17 (56.7%) | 3 (9.1%) | 20 (31.7%) | |
| Unmutated (< 2%) | 13 (43.3%) | 30 (90.9%) | 43 (68.3%) | |
| ZAP-70 | 0.6242 | |||
| Missing | 0 (0.0%) | 1 (3.0%) | 1 (1.6%) | |
| Positive | 23 (76.7%) | 25 (75.8%) | 48 (76.2%) | |
| Negative | 7 (23.3%) | 7 (21.2%) | 14 (22.2%) | |
| CD38 | 0.6622 | |||
| Positive | 12 (40.0%) | 15 (45.5%) | 27 (42.9%) | |
| Negative | 18 (60.0%) | 18 (54.5%) | 36 (57.1%) | |
| Risk Category | 0.2844 | |||
| 17p- | 9 (30.0%) | 4 (12.1%) | 13 (20.6%) | |
| 11q- | 8 (26.7%) | 11 (33.3%) | 19 (30.2%) | |
| IGHV UM+* | 13 (43.3%) | 18 (54.5%) | 30 (47.6%) | |
| ECOG performance score | 0.5635 | |||
| 0 | 27 (90.0%) | 31 (93.9%) | 58 (92.1%) | |
| 1 | 3 (10.0%) | 2 (6.1%) | 5 (7.9%) |
This group includes all patients who were eligible for enrollment in either trial because of use of UM IGHV or use of VH3-21 irrespective of mutation and concomitant expression of either ZAP-70 and/or CD38.
Discussion
Early treatment of high risk CLL could be beneficial if the highest risk patients can be accurately identified and treated with safe and effective non-genotoxic therapy. In this study we show that addition of GM-CSF did not improve the efficacy of alemtuzumab and rituximab therapy for early treatment of high risk CLL and increased the rate of CMV reactivation.
The rationale for using GM-CSF was to enhance ADCC by increasing the efficacy of monocyte-macrophage-dendritic cells and preventing mAb induced neutropenia. Although neutrophil counts were well maintained, there was no increase in circulating monocytes counts or treatment efficacy. GM-CSF was used at the dose recommended by the manufacturers based on their experience in vaccine and immunotherapy trials. A higher GM-CSF dose could have increased the circulating monocyte level but could also have increased the side effects of malaise, myalgia and fevers.
Use of GM-CSF could have adversely altered monocyte-macrophage function in the patients treated in this study. CLL patients have recently been shown to have increased levels of myeloid-derived suppressor cells which are mediators of cancer tolerance[34] and production of monocyte-derived survival factors such as soluble CD14 that have been shown to increase CLL cell survival in vitro[35]. We have previously shown that patients enrolled in this study had an increased baseline percentage of immunosuppressive CD14+ monocytes with reduced HLA-DR expression compared to healthy controls, and higher percentages of these CD14+HLA-DRlo/neg cells predicted significantly shorter PFS[36]. These data suggest that GM-CSF could stimulate an immunosuppressive monocyte-macrophage-dendritic cell response in patients with CLL that decreases ADCC and thus impairs the response to alemtuzumab and rituximab.
The use of GM-CSF could decrease the risk of infection. However we observed a three fold increase in the rate of CMV reactivation compared to the previous trial[15]. This finding is compatible with previous reports suggesting that G-CSF could increase the risk of CMV reactivation in CLL patients treated with alemtuzumab[37]. The mechanism of increased risk of CMV activation in CLL patients treated with these growth factors is unknown. Compared to patients on the previous trial (MC038G), patients treated with GM-CSF had a lower rate of grade 3-4 neutropenia (9% vs. 17%) but there was no decrease in grade 3-4 infections (12 vs. 6%). Addition of GM-CSF to alemtuzumab and rituximab therapy does not appear to be useful in decreasing the risk of infection in this patient population.
Sensitive assays for residual CLL could be useful in determining the extent of response to treatment. In this study, most patients had a negative sensitive flow cytometry assay for MRD in the blood during treatment but this did not predict the quality or duration of response to treatment. Our data thus supports the previously published finding that MRD testing in the peripheral blood during and for the first 3 months after alemtuzumab therapy is not a reliable method of determining response to treatment[38]. In contrast, patients with CR/CRi and negative IHC examinations for residual CLL cells in the BM of patients had the most durable responses to treatment. This data supports previous reports that MRD testing in the bone marrow during and within 3 months of completion of therapy is predictive of response to treatment for regimens using alemtuzumab[38]. However, the small number of patients in our study precludes any definitive conclusions about the clinical utility of these assays.
The results of this trial show that addition of GM-CSF to the combination of alemtuzumab and rituximab does not increase the efficacy of alemtuzumab and rituximab therapy. Determining the definitive role of GM-CSF would require a randomized controlled trial, but the results of this phase II study suggest that this would not be an appropriate use of resources and that alternative drugs should be tested for their ability to improve mAb cytotoxicity in patients with CLL. Our clinical data also suggests that the complex effects of GM-CSF on monocyte-macrophage-dendritic cell physiology could be detrimental to mAb therapy of CLL.
Acknowledgments
Funding
This study was supported by Genentech, Bayer, Genzyme, and the National Institutes of Health (University of Iowa/Mayo Clinic Lymphoma SPORE CA97274)
Footnotes
Potential Conflict of Interests
Clive S. Zent is the principal investigator of research projects at Mayo Clinic that are funded by Genentech, Genzyme, Novartis, GlaxoSmithKline, and Biothera.
References
- 1.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 2.Hamblin T, Davis Z, Gardiner A, Oscier D, Stevenson F. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 3.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 4.Wiestner A, Rosenwald A, Barry TS, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101:4944–4951. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 5.Tobin G, Thunberg U, Johnson A, et al. Chronic lymphocytic leukemias utilizing the VH3-21 gene display highly restricted Vlambda2-14 gene use and homologous CDR3s: implicating recognition of a common antigen epitope. Blood. 2003;101:4952–4957. doi: 10.1182/blood-2002-11-3485. [DOI] [PubMed] [Google Scholar]
- 6.Zent CS, Kay NE. Management of patients with chronic lymphocytic leukemia with a high risk of adverse outcome: the Mayo Clinic approach. Leukemia & lymphoma. 2011;52:1425–1434. doi: 10.3109/10428194.2011.568654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pepper C, Majid A, Lin TT, et al. Defining the prognosis of early stage chronic lymphocytic leukaemia patients. Br J Haematol. 2012;156:499–507. doi: 10.1111/j.1365-2141.2011.08974.x. [DOI] [PubMed] [Google Scholar]
- 8.Dighiero G, Maloum K, Desablens B, et al. Chlorambucil in indolent chronic lymphocytic leukemia. N Engl J Med. 1998;338:1506–1514. doi: 10.1056/NEJM199805213382104. [DOI] [PubMed] [Google Scholar]
- 9.Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists' Collaborative Group. J Natl Cancer Inst. 1999;91:861–868. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 10.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) updating the National Cancer Institute-Working Group (NCI-WG) 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keating MJ, O'brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 12.Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B-chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 14.Stilgenbauer S, Zenz T. Understanding and managing ultra high-risk chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:481–488. doi: 10.1182/asheducation-2010.1.481. [DOI] [PubMed] [Google Scholar]
- 15.Zent CS, Call TG, Shanafelt TD, et al. Early treatment of high risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Cancer. 2008;113:2110–2118. doi: 10.1002/cncr.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zent CS, Secreto CR, Laplant BR, et al. Direct and complement dependent cytotoxicity in CLL cells from patients with high-risk early-intermediate stage chronic lymphocytic leukemia (CLL) treated with alemtuzumab and rituximab. Leuk Res. 2008;32:1849–1856. doi: 10.1016/j.leukres.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Curr Opin Immunol. 2008;20:444–449. doi: 10.1016/j.coi.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faderl S, Thomas DA, O'Brien S, et al. Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood. 2003;101:3413–3415. doi: 10.1182/blood-2002-07-1952. [DOI] [PubMed] [Google Scholar]
- 19.Nabhan C, Patton D, Gordon L, et al. A Pilot Trial of Rituximab and Alemtuzumab Combination Therapy in Patients with Relapsed and/or Refractory Chronic Lymphocytic Leukemia (CLL). Leukemia & lymphoma. 2004;45:2269–2273. doi: 10.1080/10428190412331286096. [DOI] [PubMed] [Google Scholar]
- 20.Golay J, Manganini M, Rambaldi A, Introna M. Effect of alemtuzumab on neoplastic B cells. Haematologica. 2004;89:1476–1483. [PubMed] [Google Scholar]
- 21.Siders WM, Shields J, Garron C, et al. Involvement of neutrophils and natural killer cells in the anti-tumor activity of alemtuzumab in xenograft tumor models. Leukemia & lymphoma. 2010;51:1293–1304. doi: 10.3109/10428191003777963. [DOI] [PubMed] [Google Scholar]
- 22.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 23.Voso M, Pantel G, Rutella S, et al. Rituximab reduces the number of peripheral blood B-cells in vitro mainly by effector cell-mediated mechanisms. Haematologica. 2002;87:918–925. [PubMed] [Google Scholar]
- 24.Cartron G, Zhao-Yang L, Baudard M, et al. Granulocyte-macrophage colony- stimulating factor potentiates rituximab in patients with relapsed follicular lymphoma: results of a phase II study. J Clin Oncol. 2008;26:2725–2731. doi: 10.1200/JCO.2007.13.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrajoli A. Incorporating the use of GM-CSF in the treatment of chronic lymphocytic leukemia. Leukemia & lymphoma. 2009;50:514–516. doi: 10.1080/10428190902763541. [DOI] [PubMed] [Google Scholar]
- 26.Morice WG, Kurtin PJ, Hodnefield JM, et al. Predictive value of blood and bone marrow flow cytometry in B-cell lymphoma classification: comparative analysis of flow cytometry and tissue biopsy in 252 patients. Mayo Clin Proc. 2008;83:776–785. doi: 10.4065/83.7.776. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 28.Goldin LR, Lanasa MC, Slager SL, et al. Common occurrence of monoclonal B- cell lymphocytosis among members of high-risk CLL families. Br J Haematol. 2010;151:152–158. doi: 10.1111/j.1365-2141.2010.08339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanafelt TD, Kay NE, Jenkins G, et al. B-cell count and survival: differentiating chronic lymphocytic leukemia from monoclonal B-cell lymphocytosis based on clinical outcome. Blood. 2009;113:4188–4196. doi: 10.1182/blood-2008-09-176149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleming TR. One sample multiple testing procedures for phase II clinical trials. Biometrics. 1982:38. [PubMed] [Google Scholar]
- 31.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 32.Abruzzo LV, Rosales CM, Medeiros LJ, et al. Epstein-Barr virus-positive B-cell lymphoproliferative disorders arising in immunodeficient patients previously treated with fludarabine for low-grade B-cell neoplasms. Am J Surg Pathol. 2002;26:630–636. doi: 10.1097/00000478-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Sohani AR, Ferry JA, Chang PS, Abramson JS. Epstein-barr virus-positive diffuse large B-cell lymphoma during therapy with alemtuzumab for T-cell prolymphocytic leukemia. J Clin Oncol. 2010;28:e69–72. doi: 10.1200/JCO.2009.24.4194. [DOI] [PubMed] [Google Scholar]
- 34.Tadmor T, Attias D, Polliack A. Myeloid-derived suppressor cells--their role in haemato-oncological malignancies and other cancers and possible implications for therapy. Br J Haematol. 2011;153:557–567. doi: 10.1111/j.1365-2141.2011.08678.x. [DOI] [PubMed] [Google Scholar]
- 35.Seiffert M, Schulz A, Ohl S, Dohner H, Stilgenbauer S, Lichter P. Soluble CD14 is a novel monocyte-derived survival factor for chronic lymphocytic leukemia cells, which is induced by CLL cells in vitro and present at abnormally high levels in vivo. Blood. 2010;116:4223–4230. doi: 10.1182/blood-2010-05-284505. [DOI] [PubMed] [Google Scholar]
- 36.Gustafson MP, Abraham RS, Lin Y, et al. Association of an increased frequency of CD14(+) HLA-DR(lo/neg) monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL). Br J Haematol. 2012;156:674–676. doi: 10.1111/j.1365-2141.2011.08902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin TS, Flinn IW, Lucas MS, et al. Filgrastim and alemtuzumab (Campath-1H) for refractory chronic lymphocytic leukemia. Leukemia. 2005;19:1207–1210. doi: 10.1038/sj.leu.2403782. [DOI] [PubMed] [Google Scholar]
- 38.Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21:956–964. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]