Abstract
Metallic nanoparticles have diverse applications in biomedicine, as diagnostics, image contrast agents, nanosensors and drug delivery systems. Anisotropic metallic nanoparticles possess potential applications in cell imaging and therapy+diagnostics (theranostics), but controlled synthesis and growth of these anisotropic or branched nanostructures has been challenging and usually require use of high concentrations of surfactants. Star-shaped gold nanoparticles were synthesized in high yield through a seed mediated route using HEPES as a precise shape-directing capping agent. Characterization was performed using advanced electron microscopy techniques including atomic resolution TEM, obtaining a detailed characterization of nanostructure and atomic arrangement. Spectroscopy techniques showed that particles have narrow size distribution, monodispersity and high colloidal stability, with absorbance into NIR region and high efficiency for SERS applications. Gold nanostars showed to be biocompatible and efficiently adsorbed and internalized by macrophages, as revealed by advanced FE-SEM and backscattered electron imaging techniques of complete unstained uncoated cells. Additionally, low voltage STEM and X-ray microanalysis revealed the ultra-structural location and confirmed stability of nanoparticles after endocytosis with high spatial resolution.
Introduction
Engineered nanoparticles have demonstrated their potential in diverse biomedical applications, including diagnostics, therapeutics, image contrast agents, nanosensors, drug delivery systems, among others.1–3 Properties and applications of metallic nanoparticles depend particularly on their size, shape and chemical composition. Anisotropic metallic nanoparticles, such as nano-rods, bi-pyramids and nano-stars have potential applications in cell imaging, therapy and diagnostics, but controlled synthesis and growth of these anisotropic or branched nanostructures has been challenging.4–8 Au nanoparticles are generally recognized as biocompatible materials due to their relatively low reactivity, lacking in most cases adverse and acute cytotoxic effects, making them suitable for nanomedicine applications.1,9–11 Gold nanostars (GNS) are especially attractive given their particular structural and optical properties, including localized surface plasmon resonance, surface-enhanced Raman spectroscopy (SERS) and near-infrared absorption for photothermal therapy, with potential uses in biomedicine and in cancer nanotheranostics (therapy and diagnostics).2,4,12 Interactions of nanoparticles with living systems depend on the properties of metallic nanoparticles, particularly size, shape and surface chemistry that directly mediate adsorption, uptake, intracellular trafficking and development of some cytotoxicity effects. GNS are usually synthesized with combinations of different metallic precursors, additives, reducing and capping agents, obtaining diverse grades of dispersity, superficial reactivity and control over nanostructure.4,13,14 Among all the chemicals used during steps of nucleation and growth of GNS (reductants, capping or additives) to control size and shape, only a few are recognized as biocompatible compounds: ascorbic acid, citrate, HEPES, oleic acid and Pluronic-127.4,8,15–17 In contrast, some chemicals commonly used during colloidal synthesis of gold nanoparticles have presented diverse cytotoxicological effects, particularly in the case of cetylmethylammonium (CTAB), PVP, hydrazine, sodium borohydride and Ag+ ions, among others.9,18–20 These adverse effects could limit their biological applications. To circumvent or minimize the possible cytotoxic effects, it is necessary to perform additional steps of purification with harsh conditions. Therefore, it is crucial to eliminate or replace these chemicals with inert or biocompatible compounds. During these downstream processing steps, the quality, yield and stability of nanoparticles can be compromised, resulting in nanoparticle rearrangement, surface transformation, aggregation, coalescence or poorly defined nanostructures. High interest for use and applications of gold particles in living organisms is based on its interesting and particular properties, including high biocompatibility, chemical stability, control over size, shape and surface functionalities.21–23 Although these nanoparticles have shown outstanding properties for many promising uses and applications in biomedicine, analysis of cell-nanoparticle interactions and the effects of engineered nanoparticles in living organisms is still controversial and in some cases even contradictory. Previous reports have observed that biocompatibility, cellular responses and manifestation of cytotoxic effects to gold nanoparticles depend on particular properties, such as particle size, shape, surface chemistry, charge and functionalization.4,23–28 Whereas some surface modifiers are generally recognized as biocompatible, and are commonly used to increase colloidal stability and increase circulation time (PEG, citrate, among others), others have showed evident cytotoxic effects and adverse cellular responses after exposure.26,29 Cytotoxic effects of gold nanoparticles are size-dependent, in the case of small particles (<5 nm) that can cause cell death, oxidative stress and metabolic damages, whereas bigger particles are generally not toxic, or generate minor effects in cell viability and development of inflammation responses.10,20 Besides nanoparticle size, the aspect ratio, structure and surface properties may also have an influence on adhesion and endocytosis of nanoparticles.20 Particularly for phagocytosis of nanoparticles, shape and aspect ratio are very important characteristics that promote or inhibit adsorption, internalization and mediation of cellular responses.26,27
Advanced electron microscopy techniques, like aberration-corrected S/TEM, allows a detailed characterization of nanoparticles, revealing the atomistic structure and composition of nanostructures at a sub-angstrom resolution. This information is particularly important for metallic nanoparticles with uses and applications in nanomedicine, and to understand basic principles of nanoparticle ultrastructure. Additionally, ultra-high resolution low voltage FE-SEM is employed to obtain a detailed characterization of morphology, size, nanostructuration and chemical composition of nanoparticles, and biological samples with exceptional spatial resolution. Imaging of interactions of nanostructured materials with cells is usually obtained by optical microscopy (dark field and phase contrast) and confocal microscopy. Individual nanoparticles are not distinguishable with these conventional imaging techniques, because of low contrast and since the limitation of resolution is around 200 nm.30 New generation Field Emission Scanning Electron Microscopes (FE-SEM) offers ultra-high resolution. With this advanced imaging technique, it is possible to analyze a number of interactions, adsorption and uptake of small metallic nanoparticles by cells. Resolution of 0.4–1.6 nm can be achieved by FE-SEM, with operation at low voltages (1–30 kV) and use of appropriate detectors allows imaging of individual nanoparticles with high spatial resolution. Complete unstained and uncoated cells can be examined with nanometer scale resolution, to obtain topographic and morphological information to assess the details of cellular interactions with metallic nanoparticles.30–32 Additionally, new generation of high sensitive solid-state annular backscattered electron (BSE) detectors can be used to visualize processes of adsorption and uptake of particles by living organisms, also providing rich qualitative compositional and internal structural information with resolution similar or equivalent to secondary electrons (SE) detectors (≤ 5 nm).33
In the present work, we analyzed gold nanostars obtained through a seed mediated route using 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethane-sulfonic acid (HEPES), a zwitterionic buffering compound for precise control of size, shape and structure of nanoparticles. Morphology of nanoparticles was obtained by ultra-high resolution FE-SEM and aberration corrected S/TEM to obtain detailed characterization of nanostructure and atomic arrangement. Gold nanostars were shown to be biocompatible by in vitro analysis with murine macrophages and were efficiently adsorbed and internalized by cells, as revealed by advanced FE-SEM and backscattered electron imaging of complete unstained uncoated cells. Additionally, low voltage BF/DF-STEM and X-ray microanalysis confirmed the stability of gold nanoparticles and revealed their ultra-structural location with high spatial resolution.
Results and Discussion
Anisotropic or branched star-shaped nanoparticles, commonly referred to as nano-stars or nano-urchins possess interesting tunable properties that can be exploited in different nanomedicine applications, including drug delivery systems, thermal-ablation, as nano-sensors or image contrast agents. As nanoparticle properties are directly related to their size and shape, it is a fundamental criterion to ensure high control and precision during the synthesis to obtain anisotropic nanoparticles with the desired properties.
Gold nanostars were synthesized by seed-mediated route, using biocompatible capping agents to control and stabilize nanoparticle morphology during the reactions.5,34,35 First step was to obtain small gold nanoparticles that served as seeds for the growth of branches to finally obtain nanoparticles with the desired star-shape. Gold seeds were obtained by chemical reaction method incorporating citrate as capping agent; colloidal nanoparticles with a narrow size distribution (14±2.5 nm in diameter) and monodisperse were efficiently obtained. HR-TEM characterization showed that gold seeds possessed a well-defined icosahedral structure (Fig. S1. Supplementary Electronic Information). Reactive surface lattices of these small icosahedral gold nanoparticles served as templates during a growth step, using Au3+ ions as precursors in a buffer solution containing 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethane-sulfonic acid (HEPES) as a precise shape-directing agent, and hydroxylamine as catalyst and soft reductant, allowing to efficiently synthesize GNS in aqueous media. HEPES is a zwitterionic organic compound with a piperazine moiety that possesses high affinity for gold and favors formation of anisotropic structures.5,34,35 During the growth of branches, hydroxylamine catalyzed the reduction of Au3+ ions on surface of gold seeds. In addition, the low temperature ice-bath favored the growing of branches over highly symmetric fcc structures of gold seeds, and helped to reduce energy differences between structures, to specifically obtain the multibranched particles with narrow size and distribution.34
This optimized method produced GNS with high yield (more than 98 %), with high control of surface branching and nanostructuration. Low-magnification FE-SEM images of nanoparticles showed GNS that maintained high levels of monodispersion and controlled size (Fig.1-A). High magnification FE-SEM imaging confirmed an efficient regular formation of branches in all faces of icosahedral gold cores, that showed narrow size and shape distributions (Fig.1-B). Anisotropic gold particles with star-shape can be obtained through seedless chemical routes. However, in most cases these nanoparticles possess poor long term stability, short aspect ratio of branches, polydispersity and suffer aggregation, or a limited formation of number of branches per particle.4,34
Figure 1. FE-SEM imaging of gold nanostars.
(A) SE Imaging at low magnification to observe monodispersity and size distribution of particles. (B) SE Imaging at high magnification revealing details of morphology and structuration of god nanostars. Sample mounted on ultra-flat silicon wafer was tilted to −32 degrees to get a detailed characterization of nanoparticle morphology.
Methods for chemical synthesis of star-shaped gold particles are divided into one-pot and seed-mediated methods. Specific combinations of reductants, capping agents, metallic ions precursors, and additives produce nanoparticles with different size, dispersity and number of branches4. Monodispersed nanoparticles with a diameter below 100 nm and capped with biocompatible compounds are preferable for biological applications. FE-SEM imaging showed that the nanoparticles produced possess these characteristics. Analysis of biocompatibility, capacity of adsorption and internalization into mammalian cells, and intracellular stability of these complex nanostructures are necessary to fully expand applications of GNS as biosensors, imaging contrast agents, and as drug delivery systems, among other uses into nanomedicine and translational research.
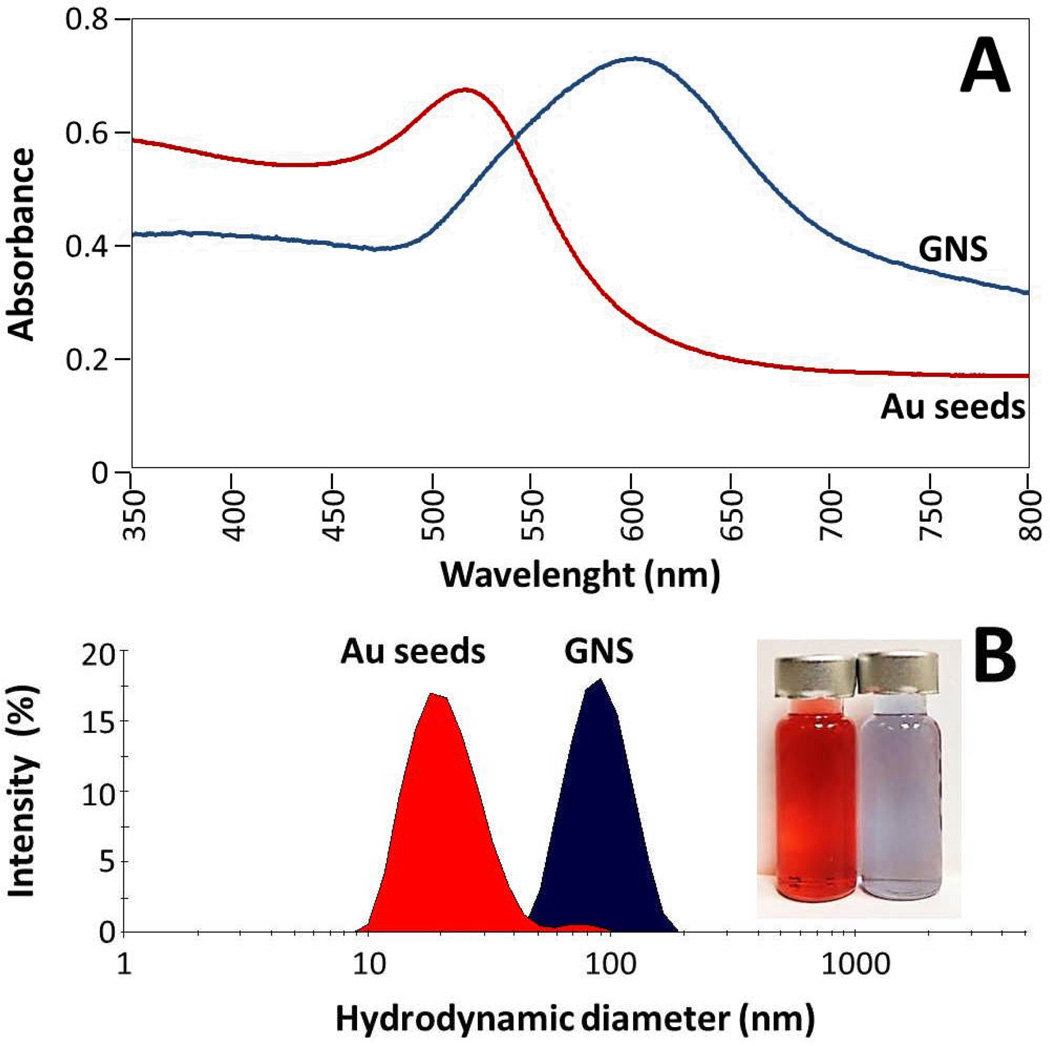
UV-Vis spectroscopy showed that GNS exhibit a maximum absorbance peak centered at near infrared (NIR) region at 612 nm (Fig.2-A). This optical behavior is attributed to the branches of GNS that cause longitudinal oscillation of absorbed electrons, and a strong increase of the electromagnetic field at peaks of nanoparticles.15,34,36,37 In comparison, gold seeds showed an absorbance peak at 518 nm, in the typical UV-Vis range for small spherical gold nanoparticles. GNS are of great interest for development of nanotechnology-based theranostic devices, taking advantage of surface plasmon resonance properties that allow them to absorb irradiated light in the NIR region. This phenomena cause resonance and collective oscillations of free electrons efficiently converting electromagnetic energy into heat.1,2,37 Plasmon resonance of nanoparticles can increase intracellular temperature to 42–47°C, that eventually can induce cell death through overexpression of apoptosis or necrosis related proteins.2,4,38,39
Figure 2. Characterization of optical properties.
(A) UV-Vis spectroscopy of gold seeds (red line, λmax=518 nm) and gold nanostars (blue line, λmax=612 nm). (B) Photon correlation spectroscopy (Dynamic Light Scattering) showing size distribution by intensity (%) of Au seeds and gold nanostars, inserted image shows vials containing nanoparticles (red: gold seeds, blue: gold nanostars).
High surface/area ratio of anisotropic particles makes them ideal substrates as nanocarriers, and by their localized surface plasmon properties, a drastically increase of Raman scattering of specific reporter compounds adsorbed on nanoparticle surface can be efficiently detected and can be combined with imaging techniques to serve as contrast agents.4,36,39,40 To probe this concept, we performed Raman spectroscopy of gold nanostars using rhodamine 6G as standard molecule. Spectra of gold nanoparticles coated with the fluorophore showed a significant increase of specific peaks assigned to rhodamine 6G (at 614, 774, 1129, 1183, 1310, 1363, 1509 and 1572 cm−1) in comparison with pure fluorophore on the same conditions (Fig. S2. Supplementary Electronic Information). These spectroscopic data confirmed high SERS efficiency of gold nanostars, and advocate their potential utility as nanosensors and contrast agents in imaging techniques.
Photon correlation spectroscopy (Dynamic Light Scattering, DLS) was employed to measure the size distribution and zeta-potential of nanoparticles in solution. Fig. 2-B shows size distribution by intensity of nanoparticles synthesized. Gold seeds had a single population (red curve, Fig. 2-B) corresponding to 98 % of the total intensity distribution with a mean hydrodynamic diameter of 20.4 nm, and a polydispersity index (PDI) of 0.214. Gold nanostars in solution had a mean hydrodynamic diameter of 72.6 nm and PDI of 0.251 corresponding to 93 % of particles measured (blue curve, Fig. 2-B). DLS data confirmed the narrow size distribution of GNS and gold seeds observed by electron microscopy. GNS showed remarkable colloidal stability and negative surface charge, with average zeta (ζ) potential value of −26.13 ± 0.49 mV in 30 mM HEPES buffer (pH 7.0), indicating high colloidal stability. In comparison, gold nanoparticles that are coated with surfactant CTAB have a positive ζ-potential (39.2 to 46.6 mV), whereas PEGylated nanoparticles also possess a negative surface charge (−23 to −9.7 mV).25 Stability of nanoformulations is important for all potential applications and to ensure that their properties remain constant. After months of storage at 4°C, GNS samples kept similar blue color and UV-Vis absorbance spectra. Besides the role of controlling size and shape, HEPES coating also contributed to long term stability of as-synthesized colloidal nanoparticles.
Atomic resolution TEM significantly improves spatial resolution, sensitivity and quality in the acquired images.15,41 High resolution TEM imaging of GNS confirmed size distribution and monodispersity (70–80 nm in diameter). This advanced electron microscopy technique helped to reveal with high detail the structural organization of GNS at atomic level, revealing the atomic arrangement and the nanostructuration of nanoparticles. Fig.3 shows characterization of gold nanostars by atomic resolution TEM. Special attention was assigned to the characterization of details of crystalline nanostructure of nanostar branches. Micrographs revealed that the nanostars peaks grew as a single crystalline structure from gold seeds surface with no perceivable defects, making them exceptional active surfaces with optical and catalytic functionalities for application in electronics and biodiagnosis. Fig. 3-B revealed a GNS peak with atomic resolution, detailed analysis of nanostructure surface of particle confirmed the lattice spacing of 2.35 Å that is characteristic for gold, whereas FFT (fast Fourier transform) pattern of the selected area indicated that it corresponded to fcc gold oriented over 〈011〉 face (Fig. 3-C–D). Imaging at the edges of nanoparticle peaks showed that branches grew along 〈111〉 face, forming a stable and very well organized single crystalline structure (Fig.3-E). Braches of GNS grew lengthwise regularly through steps of two atoms along the 〈110〉 direction, as indicated by the arrows in Fig. 3-E. This very well nanostructured growing of peaks from the active surfaces of icosahedral colloidal gold seeds, produced anisotropic nanoparticles with narrow size, and the branches obtained during the growth are energetically stable. GNS are coated with an organic continuous layer formed by 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethane-sulfonic acid (HEPES), and the atomic resolution TEM confirmed the presence of an organic layer as indicated by the arrows in Fig. 3-E. This zwitterionic compound has as a central core piperazine ring that interacts with gold surface forming a π-like stacking with an interspace distance of 1.57 ± 1 Å. Similar stacking of carbon-based organic materials has been observed for FeCo nanoparticles encapsulated with graphite.34,42 It is important to highlight that the physicochemical properties of biocompatible buffering HEPES, that cover all the surfaces of the nanoparticle, made GNS highly soluble and stable in aqueous media.
Figure 3. Characterization of gold nanostars by a atomic resolution TEM.
(A) BF-TEM. (B) Detail of atomic structure of a gold nanostar branch. (C) Interplanar distance. (D) FFT patterns of area selected at (C). (E) Detail showing direction of growing on branches and presence or organic coating.
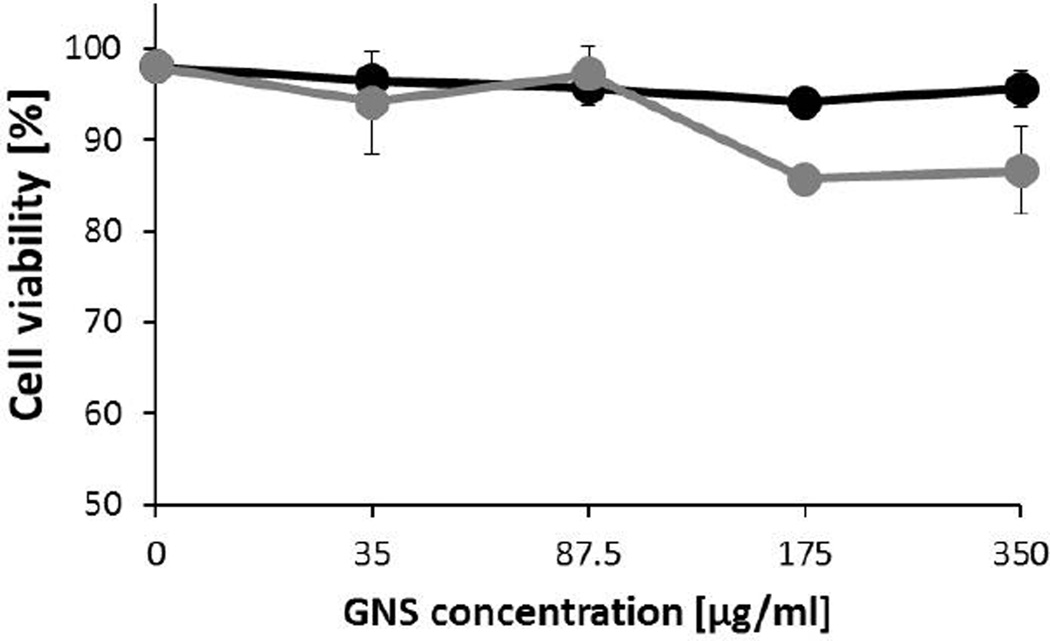
Cytotoxicity of GNS was evaluated using in vitro assays with murine macrophages after 3 and 24 h of interaction, using trypan blue exclusion and flow cytometry (propidium iodide staining) to determine cell viability and plasma membrane integrity, respectively. Treatments with a broad concentration range of gold nanoparticles (0–350 µg/ml) showed that after 3 h no significant reduction in cell viability or evident signs of toxicity; viable cells remained at 94±1 % at the highest concentration used (black line, Fig. 4). After 24 h of exposition, GNS showed a slight cytotoxic effect on macrophages, which depended on the final concentration of particles used. At the highest concentrations of 175 and 350 µg/ml, cell viability was reduced to 86±0.5 and 87±4 %, respectively (gray line, Fig.4). Plasma membrane integrity of macrophages exposed to GNS was measured by propidium iodide staining. From histograms obtained, cell viability with different treatments was calculated (Fig. S3. Supplementary Electronics Information). These data showed that GNS did not compromise cell membrane integrity even at high concentrations or 24 h of exposure; cell viability remained at 96±2.5%. Assays indicated that interaction of star-shaped particles did not significatively affect cell viability or cell membrane integrity. No signs of acute cytotoxicity in time or concentration-dependent nature was observed, indicating their high degree of biocompatibility. GNS are coated with HEPES, a common buffering agent used at a concentration of 10–25 mM in many cell culture media. Some authors have observed that the decrease in cell viability can be attributed to selective binding or clearance of essential components from cell culture media (proteins, co-factors, hormones, etc.).23,44
Figure 4. Cell viability assay.
Trypan blue exclusion of macrophages after 3 h of incubation (black line), and 24 h of incubation (gray line). Cell culture media was dosed with concentrated gold nanoparticles to achieve the concentrations indicated, percentage of viable cells were obtained from triplicates and normalized to control (without nanoparticles).
Results obtained on cytotoxic responses of macrophages are consistent with recent reports that have showed no major cytotoxic effects at similar concentrations of 60 nm standard spherical gold nanoparticles, after 24–48 h exposure.26 In contrast, other cell lines like HeLa and human fibroblast cells treated with Au nanoparticles have showed signs of cytotoxicity, like a significant reduction in cell viability caused by an increase in reactive oxygen species (ROS), and over-expression of inflammation and stress-related genes, that finally lead to cell death.4 Gold particles coated with cationic surfactant CTAB can induce cell death, these cytotoxic effects are mainly related to desorption of surfactant and not by the direct reactivity of gold nanoparticles.19,21 Analysis of microglia and neuronal cells that are exposed to gold nanoparticles of different shapes (spheres, rods and urchins) concluded that spherical particles stabilized with CTAB developed slight cytotoxic effects after 24 h, whereas anisotropic particles (rods and multibranched) resulted in an innocuous outcome.25 Replacement of CTAB with BSA on gold nanostars resulted in a low cytotoxic response of macrophages45, confirming the importance of biocompatible coating compound usage to reduce or avoid adverse cellular responses.
Ultra-high resolution FE-SEM analytical techniques were employed to analyze adsorption-uptake of GNS by macrophages and to image morphological changes during their interaction. FE-SEM characterization included simultaneous imaging by SE (secondary electrons) and BSE (Backscattered electrons) detectors, these advanced analytical techniques were coupled with X-ray microanalysis at low voltage doses. Fig. 5 shows SE and BSE images of complete macrophages, micrographs revealed cell surface and topography with high details. Considering that all the images were obtained without use of any metal coating (Au or Pt) or heavy-metal staining (uranyl acetate or osmium tetroxide), specially to avoid interference during X-ray microanalysis. Control cells (baseline without nanoparticles) appeared rounded (Fig. 5-A–B). After interaction with moderate concentrations of gold nanoparticles (175 µg/ml), cells displayed membrane projections and signs of granularity (Fig. 5-C–D). After 3h of interaction, GNS were efficiently adsorbed on cell membrane and efficiently internalized. Gold nanoparticles appear as white dots (SE imaging) or black dots (BSE imaging), depending on the detector used in FE-SEM imaging. The high spatial resolution showed the precise location and allowed the distinction of single metallic nanoparticles. In comparison, treatments with high concentration of particles (350 µg/ml) evidenced signs of macrophage activation, with drastic changes in plasma membrane morphology and a high degree of granularity45 (Fig. 5-E–F).
Figure 5. Ultra-high resolution FE-SEM imaging of complete macrophages.
(A) SE Imaging of complete control cell. (B) BSE Imaging of complete control cell. (C) SE imaging of macrophage treated with moderate concentration of GNS (175 ug/ml) after 3h. (D) BSE imaging of macrophage treated with moderate concentration of GNS (175 ug/ml) after 3h. (E) SE imaging of macrophage treated high concentration of GNS (350 ug/ml) after 3h. (F) BSE imaging of macrophage treated high concentration of GNS (350 ug/ml) after 3h.
High magnification FE-SEM imaging displayed a detailed visualization of GNS passing through the cell membrane (Fig. 6). Acceleration voltage of 30 kV used during FE-SEM imaging allowed incoming electrons to penetrate into biological material, to obtain high spatial resolution and good contrast to locate GNS effectively. The charging effects commonly observed in biological samples were completely reduced or avoided by deposition of cells on a conductive surface.30 BSE imaging of cells dosed with GNS provided rich information of structural morphology and organization. Additionally, whereas biological materials possess low electron scattering, gold nanoparticles have high contrast on images acquired with high sensitive solid-state annular backscattered electron detectors (LABE and YAG), facilitating their location. Fig.6-B–C shows BSE of GNS internalized by macrophage. Given that the high contrast obtained by the scattering events that occur at deeper depths in the cell, and as the number of electrons scattered is directly proportional to the atomic number (Z-contrast), the gold nanoparticles presented a high contrast in comparison with the cell membrane. X-ray microanalysis (mapping Au-LA) of the same area confirmed identity, chemical composition and location of high-contrast inorganic particles adsorbed and internalized by macrophage cell (Fig.6-D).
Figure 6. FE-SEM Imaging of cells treated with gold nanostars.
(A) SE Imaging. (B) BSE Imaging (LABE detector). (C) BSE Imaging (YAG detector). (D) EDX mapping of Au-LA.
Particle size and shape influence particular cellular mechanisms and rate of nanomaterials internalization, and also have a significant impact on biodistribution. For nanoparticles of ~80 nm in diameter like GNS analyzed in this work, cellular internalization occurs mainly through clathrin-independent and caveolin-independent endocytosis in macrophages.44 Before or during the process of cell-nanoparticle interaction, GNS added can adsorb biomolecules during the incubation time (3 h or 24 h) from cell culture media, forming a protein corona of 10–13 nm thickness, as revealed by SE and BF/DF Duo-STEM imaging. Surface modification also increased hydrodynamic diameter of particles to 117 nm and causing a shift of ζ-potential to −8.44 ± 0.35 mV (Fig. S4. Supplementary Electronic Information). The organic layer promotes adsorption and uptake of gold nanoparticles by mammalian cells, and additionally can cause sedimentation increasing probability of interaction of nanoparticles with cells growing in confluent monolayers.43 Previous reports have observed that positively charged nanoparticles exhibit greater uptake efficiency, but also caused cytotoxic effects by the presence of cationic surfactants that compromise cell membrane.25,26
We used low voltage BF/DF-STEM coupled with X-ray microanalysis to confirm adsorption, internalization and intracellular location of star-shaped gold particles. STEM imaging of resin embedded thin sections revealed that GNS were internalized into the cytoplasm, close to the cell membrane and finally located into endosome structures after 3 h of interaction. Ultrastructural analysis indicated no signs of particle invasion into the nucleus. The gold nanostars were only located close to the plasma cell membrane or contained inside lysosomal vacuoles (Fig.7). Processes of adsorption, uptake, and biodistribution depend mainly on the size of the nanoparticles. For biomedical applications, particles have to be in the range of 10–100 nm in diameter to minimize accumulation and nonspecific interactions, as some reports have indicated that small particles (<5 nm) can enter into cell nucleus and cause cell damage.20,46,47 In agreement with our observations of GNS, a previous report indicated that NIST Au nanoparticles reference material of 60 nm were located in intracellular vacuoles after uptake through endocytosis with no signs of toxicity nor pro-inflammatory responses in macrophages.26 The chemical composition of internalized nanoparticles was confirmed by X-ray microanalysis by mapping the presence of Au-LA and also DF-STEM imaging to detect high-contrast gold nanoparticles. These analytical techniques were used to clearly distinguish metallic nanoparticles from high contrast protein aggregates, subcellular structures and heavy metals (osmium and uranyl acetate) used during the processing of cells for STEM.
Figure 7. Ultrastructural location of GNS.
(A) DF-STEM Imaging. (B) BF-STEM Imaging of area selected in (A). (C) X-Ray microanalysis. (D) EDX mapping of Au-LA.
Conclusions
Gold nanoparticles with star-shape were efficiently produced through seed-mediated route, advanced electron microscopy techniques revealed the morphology, nanostructure and atomic arrangement. Aberration-corrected S/TEM revealed that branches grew along the faces of icosahedral gold seeds used as templates, forming a stable and very well organized single crystalline structure. Spectroscopy techniques indicated a narrow size distribution, monodispersity, strong SERS properties and high colloidal stability. In vitro assays indicated that interaction of star-shaped particles with macrophages did not affect significantly cell viability or cell membrane integrity, even at high concentrations (350 µg/ml) after 24 h of exposition. Imaging of cells with ultra-high resolution FE-SEM showed that macrophages efficiently internalize GNS. These advanced analytical techniques revealed with high spatial resolution details during adsorption-uptake of nanoparticles on complete unstained uncoated cells, and showed cell morphology changes indicating minimal macrophage activation. All these characteristics of gold nanostars included their high degree of biocompatibility and structural stability; whereas concentration-dependent bio-assays confirmed that GNS can be used in nanomedicine applications, expanding uses of functional nanomaterials into translational and biomedical applications.
Experimental section
Synthesis of gold nano-stars
Synthesis of anisotropic nanoparticles was obtained through a seed-mediated route.5,34,35 Citrate capped gold seeds were prepared by adding 15 µl of sodium citrate stock solution (0.5 M) to 10 ml of ddH2O containing 0.25 mM of HAuCl4. After 3 min, 50 µl of cold NaBH4 (0.1 M) aqueous solution were added, after 1 min the mixture acquired red wine color, stirring was stopped and then stored for 2h at room temperature protected from light. Synthesis of gold nanostars was achieved using 12.5 ml of HEPES (2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid) buffer (30 mM, pH 7.0) cooled in ice-bath and with gently stirring, then 50–100 µl of citrate capped gold seeds and NH2OH to a final concentration of 0.4 mM were added. Growth of gold nanostars occurred by adding 2.5 ml of 1.0 mM HAuCl4 aqueous solution, and the end of reaction the solution acquired a purple-blue color. Stirring was immediately stopped and samples were stored at 4°C protected from light. All chemicals were purchased from Sigma-Aldrich.
Spectroscopic characterization of gold nanoparticles
UV-Vis spectra were obtained on Cary 100 UV-Vis spectrophotometer (Agilent Technologies) from 350–800 nm, using 30 mM HEPES buffer as blank. Size distribution and zeta-potential were obtained by Dynamic Light Scattering (ZetasizerNanoZS, Malvern), with He-Ne laser 633nm at 5mW, backscattered detector angle at 173° and controlled temperature (25°C).
Advanced electron microscopy characterization
Nanoparticle samples were characterized by ultra-high resolution field emission scanning electron microscopy (FE-SEM HITACHI In-Lens S-5500) coupled with BF/DF Duo-STEM detector and EDX spectroscopy (Bruker). HR-TEM images obtained with JEOL 2010-F at 200kV and Atomic resolution TEM was performed on JEOL ARM-200F operated at 200 kV.
Cell culture and in vitro interaction with gold nanoparticles
J774A.1 murine macrophage cells were grown in T-25 flasks with complete DMEM media (supplemented with 10% FBS, NEAA, glutamine and 1% penicillin-streptomycin) (GIBCO, Carlsbad, CA, USA) at 37°C in a humidified atmosphere of 5% CO2. Cells were cultured to achieve 80–90% confluency before dosing with different quantities (100–1000 µl) of 2.5 mM solution of gold nanostars. After 3 h or 24 h of incubation, exhausted media was removed, cell monolayers were washed twice with sterile PBS and scraped carefully before been resuspended in 2 ml of sterile PBS. Cell viability was evaluated by Trypan blue exclusion method.
FE-SEM imaging of cells
For SEM microscopy analysis, cells were centrifuged 10 min at 1000 rpm, PBS buffer was removed and cells were fixed with 1 ml of fixative buffer (phosphate buffered 4% formaldehyde, 1% glutaraldehyde, pH 7.2). After 2 h incubation at room temperature samples were stored at 4°C. Samples were dehydrated using a series of ethanol solutions (25, 50, 75, 95 and 100%), loaded onto a 5 mm2 ultra-flat silicon wafer, and stored in a desiccation chamber under vacuum. FE-SEM was carried out with an S-5500 In-Lens ultra-high resolution FE-SEM (HITACHI) coupled with LABE (Low Angle Backscattered Electron), YAG-BSE (Yttrium-Aluminum-Garnet Backscattered Electron) and EDX detector (Bruker) operated with an accelerating voltage of 5–30 kV.
Ultra-structural localization of nanoparticles
Fixed cells were rinsed twice with PBS buffer, post-fixed using 1% OsO4 in PBS for 1 h. Subsequently cell pellets were gradually dehydrated using a series of ethanol solutions (25, 50, 75, 95 and 100%). Samples were infiltrated with 50% LX112 resin (Ladd Research) in propylene oxide for 1 h and consequently infiltrated with 100% LX112 resin and polymerized at 60°C for 48 h. Ultrathin sections (90–100 nm) were cut using 45° diamond knife on a Leica Ultracut ultramicrotome and transferred to 300 mesh copper grids (2PSI). Thin sections were then post-stained with 1% uranyl acetate aqueous solution for 30 min. Cell sections were imaged with BF/DF-Duo STEM Detector and EDX mapping modes in S-5500 In-Lens FE-SEM HITACHI operated with an accelerating voltage of 30 kV.
Supplementary Material
Acknowledgements
This work was supported by Welch Foundation (AX-1615), NSF (DMR-1103730), NSF-PREM (DMR-0934218) and CONACyT Fellowship (147947 and 173179). Cell culture facilities of Department of Biology UTSA. Technical assistance of Dr. Ekaterina Vinogradova. Facilities of Kleberg Advanced Microscopy Center (KAMiC), NIH RCMI Nanotechnology and Human Health Core (RCMI grant 5G12RR013646-12) and NIH RCMI Biophotonics Core (RCMI grant G12MD007591) at UTSA.
References
- 1.Cai W, Gao T, Hong H, Sun J. Nanotech. Sci. Appl. 2008;1:17–32. doi: 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kievit FM, Zhang M. Adv. Mater. 2011;23:H217–H247. doi: 10.1002/adma.201102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minelli C, Lowe SB, Stevens MM. Small. 2010;21:2336–2357. doi: 10.1002/smll.201000523. [DOI] [PubMed] [Google Scholar]
- 4.Guerrero-Martínez A, Barbosa S, Pastoriza-Santos I, Liz-Marzán LM. Curr. Op. Colloid. Interface. Sci. 2011;16:118–127. [Google Scholar]
- 5.Maiorano G, Rizzello L, Malvindi MA, Shankar SS, Martiradonna L, Falqui A, Cingolani R, Pompa PP. Nanoscale. 2011;3:2227–2232. doi: 10.1039/c1nr10107b. [DOI] [PubMed] [Google Scholar]
- 6.Jones MR, Osberg KD, Macfarlane RJ, Langille MR, Mirkin CA. Chem. Rev. 2011;111:3736–3827. doi: 10.1021/cr1004452. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Wu X, Zhang X, Liu Y, Zhou D, Sun H, Zhang H, Yang B. J. Phys. Chem. C. 2011;115:3630–3637. [Google Scholar]
- 8.Rodríguez-Lorenzo L, Krpetic Z, Barbosa S, Alvarez-Puebla RA, Liz-Marzán LM, Prior IA, Brust M. Integr. Biol. 2011;3:922–926. doi: 10.1039/c1ib00029b. [DOI] [PubMed] [Google Scholar]
- 9.Lim ZZ, Li JE, Ng CT, Yung LY, Bay BH. Acta. Pharmacol. Sin. 2011;32:983–990. doi: 10.1038/aps.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khlebtsov N, Dykman L. Chem. Soc. Rev. 2011;40:1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 11.Alkilany AM, Murphy CJ. J. Nanopart. Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zandberg WF, Bakhtiari AB, Erno Z, Hsiao D, Gates BD, Claydon T, Branda NR. Nanomedicine. 2011;8:908–915. doi: 10.1016/j.nano.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed W, Kooij ES, van Silfhout A, Poelsema B. Nanotechnology. 2010;21:125605. doi: 10.1088/0957-4484/21/12/125605. [DOI] [PubMed] [Google Scholar]
- 14.Burt J, Elechiguerra J, Reyesgasga J, Montejano-Carrizales JM, Jose-Yacaman M. J. Cryst. Growth. 2005;285:681–691. [Google Scholar]
- 15.Mayoral A, Magen C, Jose-Yacaman M. Cryst. Growth. Des. 2011;11:4538–4543. doi: 10.1021/cg2007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayoral A, Vazquez-Duran A, Ferrer D, Montejano-Carrizales JM, Jose-Yacaman M. Crys. Eng. Comm. 2010;12:1090–1095. [Google Scholar]
- 17.Sau TK, Rogach AL. Adv. Mater. 2010;22:1781–1804. doi: 10.1002/adma.200901271. [DOI] [PubMed] [Google Scholar]
- 18.Barbosa S, Agrawal A, Rodríguez-Lorenzo L, Pastoriza-Santos I, Alvarez-Puebla RA, Kornowski A, Weller H, Liz-Marzán LM. Langmuir. 2010;26:14943–14950. doi: 10.1021/la102559e. [DOI] [PubMed] [Google Scholar]
- 19.Grabinski C, Schaeublin N, Wijaya A, D’Couto H, Baxamusa SH, Hamad-Schifferli K, Hussain SM. ACS Nano. 2011;5:2870–2879. doi: 10.1021/nn103476x. [DOI] [PubMed] [Google Scholar]
- 20.Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B. Biochim. Biophys. Acta. 2011;1810:361–373. doi: 10.1016/j.bbagen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Soenen SJ, Manshian B, Montenegro JM, Amin F, Meermann B, Thiron T, Cornelissen M, Vanhaecke F, Doak S, Parak WJ, De Smedt S, Braeckmans K. ACS Nano. 2012;6:5767–5783. doi: 10.1021/nn301714n. [DOI] [PubMed] [Google Scholar]
- 22.Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. Chem. Soc. Rev. 2012;41:2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walkey CD, Chan WC. Chem. Soc. Rev. 2012;41:2780–2799. doi: 10.1039/c1cs15233e. [DOI] [PubMed] [Google Scholar]
- 24.Albanese A, Sykes EA, Chan WC. ACS Nano. 2010;4:2490–2493. doi: 10.1021/nn100776z. [DOI] [PubMed] [Google Scholar]
- 25.Hutter E, Boridy S, Labrecque S, Lalancette-Hebert M, Kriz J, Winnik FM, Maysinger D. ACS Nano. 2010;4:2595–2606. doi: 10.1021/nn901869f. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Hitchins VM, Schrand AM, Hussain SM, Goering PL. Nanotoxicology. 2011;5:284–295. doi: 10.3109/17435390.2010.512401. [DOI] [PubMed] [Google Scholar]
- 27.Schaeublin NM, Braydich-Stolle LK, Maurer EI, Park K, MacCuspie RI, Afrooz AR, Vaia RA, Saleh NB, Hussain SM. Langmuir. 2012;28:3248–3258. doi: 10.1021/la204081m. [DOI] [PubMed] [Google Scholar]
- 28.Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 29.Sapsford KE, Tyner KM, Dair BJ, Deschamps JR, Medintz IL. Anal. Chem. 2011;83:4453–4488. doi: 10.1021/ac200853a. [DOI] [PubMed] [Google Scholar]
- 30.Hartsuiker L, Van Es P, Petersen W, Van Leeuwen TG, Terstappen LW, Otto C. J. Microsc. 2011;244:187–193. doi: 10.1111/j.1365-2818.2011.03528.x. [DOI] [PubMed] [Google Scholar]
- 31.Schatten H. Micron. 2011;42:175–185. doi: 10.1016/j.micron.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Schrand AM, Schlager JJ, Dai L, Hussain SM. Nat. Protoc. 2010;5:744–757. doi: 10.1038/nprot.2010.2. [DOI] [PubMed] [Google Scholar]
- 33.Koh AL, Shachaf CM, Elchuri S, Nolan GP, Sinclair R. Ultramicroscopy. 2008;109:111–121. doi: 10.1016/j.ultramic.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie J, Lee JY, Wang DI. Chem. Mater. 2007;19:2823–2830. [Google Scholar]
- 35.Brown KR, Natan MJ. Langmuir. 1998;14:726–728. [Google Scholar]
- 36.Hao F, Nehl CL, Hafner JH, Nordlander P. Nano. Lett. 2007;7:729–732. doi: 10.1021/nl062969c. [DOI] [PubMed] [Google Scholar]
- 37.Nehl CL, Liao H, Hafner JH. Nano. Lett. 2006;6:683–688. doi: 10.1021/nl052409y. [DOI] [PubMed] [Google Scholar]
- 38.Cherukuri P, Glazer ES, Curley SA. Adv. Drug. Deliv. Rev. 2010;62:339–345. doi: 10.1016/j.addr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao H, Nehl CL, Hafner JH. Nanomedicine. 2006;1:201–208. doi: 10.2217/17435889.1.2.201. [DOI] [PubMed] [Google Scholar]
- 40.Velázquez-Salazar JJ, Esparza R, Mejía-Rosales SJ, Estrada-Salas R, Ponce A, Deepak FL, Castro-Guerrero C, José-Yacamán M. ACS Nano. 2011;5:6272–6278. doi: 10.1021/nn202495r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Schooneveld MM, Gloter A, Stephan O, Zagonel LF, Koole R, Meijerink A, Mulder WJ, de Groot FM. Nat. Nanotechnol. 2010;5:538–544. doi: 10.1038/nnano.2010.105. [DOI] [PubMed] [Google Scholar]
- 42.Lee SJ, Cho JH, Lee C, Cho J, Kim YR, Park JK. Nanotechnology. 2011;22:375603. doi: 10.1088/0957-4484/22/37/375603. [DOI] [PubMed] [Google Scholar]
- 43.Walkey CD, Olsen JB, Guo H, Emili A, Chan WC. J. Am. Chem. Soc. 2012;134:2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 44.Petros RA, DeSimone JM. Nat. Rev. Drug. Disc. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 45.Xia W, Song HM, Wei Q, Wei A. Nanoscale. 2012;4:7143–7148. doi: 10.1039/c2nr32070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, Frangioni JV. Nat. Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dam DH, Lee JH, Sisco PN, Co DT, Zhang M, Wasielewski MR, Odom TW. ACS Nano. 2012;6:3318–3326. doi: 10.1021/nn300296p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.