Abstract
Notch signaling induces gene expression of the T cell lineage and discourages alternative fate outcomes. Hematopoietic deficiency in the Notch target Hes1 results in severe T cell lineage defects; however, the underlying mechanism is unknown. We found here that Hes1 constrained myeloid gene-expression programs in T cell progenitor cells, as deletion of the myeloid regulator C/EBPa restored the development of T cells from Hes1-deficient progenitor cells. Repression of Cebpa by Hes1 required its DNA-binding and Groucho-recruitment domains. Hes1-deficient multipotent progenitor cells showed a developmental bias toward myeloid and dendritic cells after Notch signaling, whereas Hes1-deficient lymphoid progenitor cells required additional cytokine signaling for diversion into the myeloid lineage. Our findings establish the importance of constraining developmental programs of the myeloid lineage early in T cell development.
Signaling via Notch receptors is essential for the generation of early T cell lineage progenitors (ETPs) in the thymus1,2. Notch signaling both upregulates T cell lineage specific gene expression and antagonizes alternative fates as progenitor cells commit to the T cell lineage3–9. ETPs retain the potential to develop into non-T cell lymphoid cells (B cell and natural killer cell), dendritic cells (DCs) and, to a degree, myeloid cells1,7,10–15, in addition to robust potential to develop into T cells; however, the intrathymic mechanisms that repress non-T cell lineage–specific programs are not well understood. Consequently, the importance of the repression of alternative fates for T cell development has not been clearly demonstrated.
Hes1 is a basic helix-loop-helix transcriptional repressor16 and an evolutionarily conserved target of Notch signaling 17,18. Germline deletion of Hes1 results in the absence of the thymus (in >90% of such mice) or a severely hypocellular thymus, in addition to defects in the pancreas, gut, bile duct and neural tube that are lethal late in embryogenesis16,19,20. The absence of a thymus in Hes1-deficient embryos may reflect defects in both hematopoietic cells and thymic stromal cells, because Hes1 is expressed in both cell types19. Hematopoietic cell–intrinsic expression of Hes1 is important for T cell development, and Hes1-deficient progenitor cells fail to generate normal numbers of T cells in competitive fetal liver (FL) or bone marrow (BM) chimeras or following direct intrathymic injection; however, the defect is not absolute19,21. It has been suggested that Hes1 facilitates T progenitor expansion, possibly via repression of Cdkn1b (which encodes the cell-cycle inhibitor p27Kip1)22,23.
Several studies suggest an antagonistic relationship between Hes1 and C/EBPa, a critical regulator of the development of myeloid cells and DCs24,25, as well as adipogenesis26. Ectopic expression of Hes1 inhibits myelopoiesis from BM progenitor cells5,27. Furthermore, during mast cell development Notch2 signaling upregulates the expression of Gata3 (which encodes the transcription factor and T cell regulator GATA-3) and Hes1, which is thought to repress the gene encoding C/EBPa (Cebpa) and prevent diversion of mast cell progenitor cells to other myeloid fates28,29. Because progenitor cells of T cells have high expression of Hes1, we considered that Hes1 may constrain C/EBPa and perhaps other myeloid factors early in T cell development19.
The attenuation of myeloid potential in hematopoietic progenitor cells begins prethymically in FL and BM and results in the generation of IL-7Ra+ lymphoid progenitor cells30,31. However, Hes1 is dispensable for the development of B cells and type 2 innate lymphoid cells, which suggests it is not essential for extrathymic lymphoid specification15,19,21,32. As progenitor cells enter the thymus and experience strong Notch signals, myeloid gene-expression programs may need to be actively repressed to allow specification to the T cell lineage to proceed. Ectopic C/EBPa expression in CD4−CD8− double-negative (DN) thymocytes experiencing Notch signaling inhibits survival and subsequent T cell development33. Consistently, ETP with higher C/EBPa expression (as identified in a reporter mouse strain) are less efficient progenitor cells of T cells than are those with lower C/EBPa expression34. Here we report that deletion of the myeloid regulator C/EBPa eliminated the requirement for Hes1 during T cell development. Thus, constraint of conflicting myeloid gene expression programs via Hes1 is a critical prerequisite for the specification and commitment of T cells.
RESULTS
Hes1 is essential for T cell lymphopoiesis
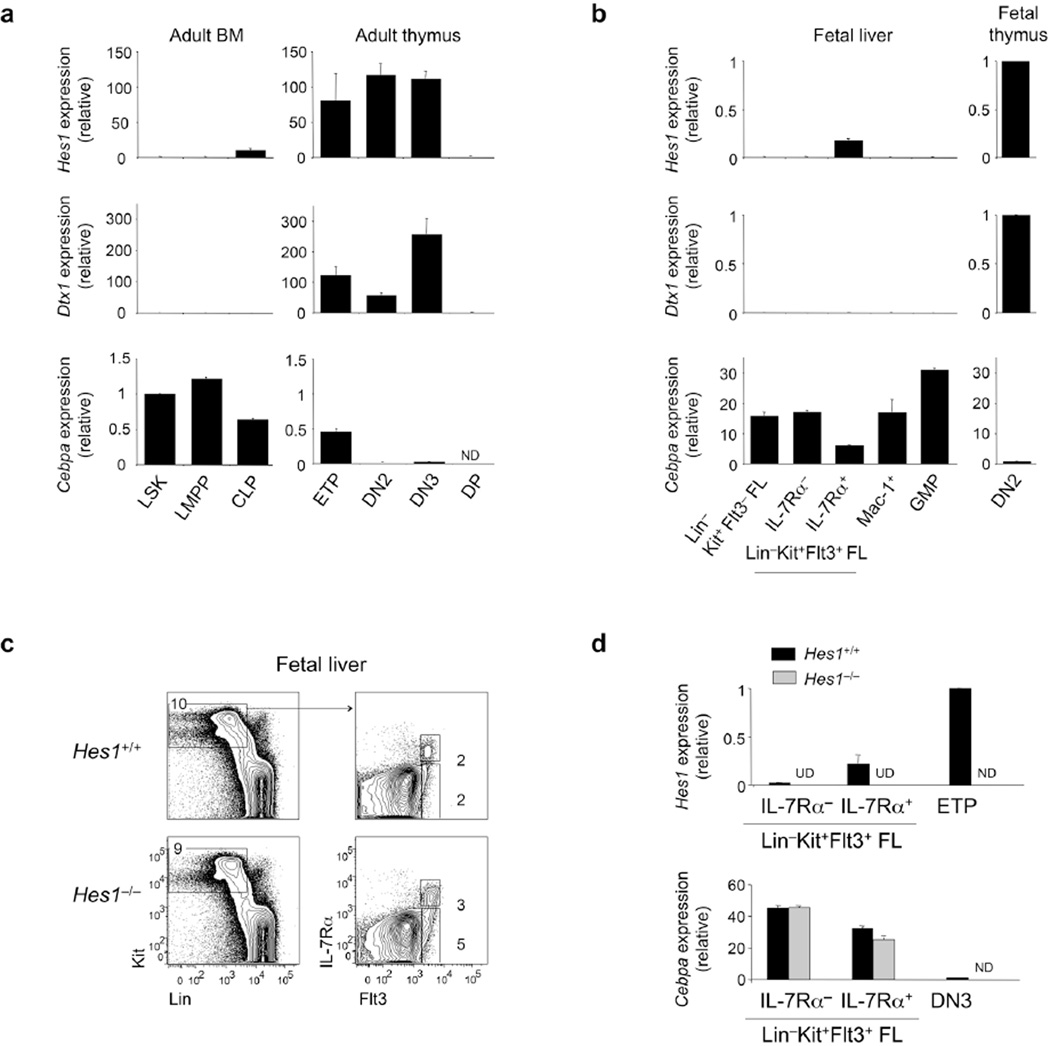
We examined Hes1 expression in BM and thymic progenitor cells of wild-type adult mice by quantitative PCR. Adult ETPs and double-negative stage 2 (DN2) and DN3 thymocytes had high expression of the Notch1 targets Hes1 and Dtx1 (which encodes the transcriptional regulator deltex-1), whereas those transcripts were low or absent in BM Lin−Sca-1+c-Kit+ (LSK) cells and lymphoid-primed multipotential progenitor cells (Fig. 1a). We did not detect expression of Hes1 or Dtx1 mRNA in CD4+CD8+ double-positive thymocytes, consistent with the termination of Notch signaling after the b-selection checkpoint35. Common lymphoid progenitor cells30 lacked Dtx1 expression but had low expression of Hes1 mRNA, perhaps because transcription factors such as E47 can induce Hes1 independently of Notch36. Expression of Cebpa followed a pattern that was reciprocal to that of Hes1. BM LSK and lymphoid-primed multipotential progenitor populations had high expression of Cebpa mRNA, which was downregulated in common lymphoid progenitor cells, consistent with the attenuated myeloid potential of those cells30. Cebpa expression was further reduced in ETPs and was almost completely extinguished in DN2 and DN3 thymocytes, in agreement with exposure to strong intrathymic Notch1 signals and correlating with upregulation of Hes1 expression. These data suggested that Hes1 may repress Cebpa in progenitor cells that have settled the thymus and are exposed to Notch1 ligands.
Figure 1.
Hes1 expression is upregulated in the thymus and is reciprocal to Cebpa expression. (a) Quantitative PCR analysis of Hes1, Dtx1 and Cebpa mRNA in adult bone marrow (BM) LSK cells, lymphoid-primed multipotential progenitor cells (LMPP), common lymphoid progenitor cells (CLP) and thymic ETP, DN2, DN3 and CD4+CD8+ double-positive (DP) progenitor cells isolated from C57/BL6 mice by cell sorting; results are relative to those of the control gene Gapdh. ND, not determined. (b) Quantitative PCR analysis of Hes1, Dtx1 and Cebpa mRNA in fetal liver (FL) Lin−Kit+Flt3− cells, MPPs (Lin−Kit+Flt3+L-7Ra−), lymphoid progenitor cells (Lin−Kit+Flt3+L-7Ra+), GMP, Mac-1+ cells and DN2 fetal thymocytes collected from C57/BL6 embryos at E12.5–E13.5 (presented as in a). (c) Flow cytometry of cells from FL of Hes1−/− mice and their Hes1+/+ littermates at E12.5–E13.5. Numbers adjacent to outlined areas indicate percent c-Kit+Lin− cells (left) or IL-7Ra+Flt3+ cells (right, top) or IL-7Ra−Flt3+ cells (right, bottom). (d) Quantitative PCR analysis of Hes1 and Cebpa mRNA in progenitor populations isolated by cell sorting as in (c) from Hes1−/− embryos (n = 2) and a Hes1+/+ littermate control embryo (n = 1) at E12.5–E13.5; Hes1+/+ fetal thymocyte progenitor cells (E13.5) (ETP or DN3) serve as a comparator population (presented as in a). UD, undetected; ND, not determined. Data are representative of three independent experiments (a–c; error bars, s.e.m. of triplicates in a and b) or are representative of one experiment (d; error bars, s.e.m.).
Hes1 and Dtx1 were expressed in fetal DN2 thymocytes but had low or absent expression in FL progenitor cells and Mac-1+ myeloid cells (Fig. 1b). We detected low expression of Hes1 mRNA in FL lymphoid progenitor cells (Lin−c-Kit+Flt3+IL-7Ra+), analogous to BM common lymphoid progenitor cells. Cebpa expression was high in FL Lin−c-Kit+Flt3− and Flt3+IL-7Ra− multipotent progenitors (MPPs) and was downregulated in Flt3+IL-7Ra+ lymphoid progenitor cells. Cebpa expression was further diminished in fetal thymocytes, indicative of a reciprocal relationship between Hes1 expression and Cebpa expression.
The thymus was either absent or extremely hypocellular in Hes1-deficient (Hes1−/−) embryos19 (Supplementary Fig. 1a), which precluded detailed analysis of fetal thymocyte populations. Germline deletion of Hes1 is lethal perinatally, but we were able to assess FL progenitor cells and found similar frequencies of Lin−c-Kit+Flt3+IL-7Ra+ lymphoid progenitor cells in Hes1−/− FL and wild-type FL at embryonic days 12.5–13.5 (E12.5–E13.5)37 (Fig. 1c). We also assessed Cebpa expression in FL cells and found it was downregulated in FL Lin−c-Kit+Flt3+IL-7Ra+ lymphoid progenitor cells from Hes1−/− mice relative to its expression in FL Lin−c-Kit+Flt3+IL-7Ra− multipotent progenitor cells from Hes1−/− mice, to a similar degree compared to control progenitor cells from their wild-type littermates (Fig. 1d). These results indicated that Hes1 was not necessary for downregulation of Cebpa expression in FL lymphoid progenitor cells before exposure to intrathymic Notch signals. Other transcription factors such as E2A that mediate lymphoid specification may fulfill this role36.
To determine the stage of T cell development that requires Hes1, we generated competitive chimeras by mixing whole FL from Hes1−/− mice or their Hes1+/+ littermates with BM from adult host-type mice (at a ratio of 1:2, FL to BM) and intravenously injecting the mixture into lethally irradiated host mice. We examined the reconstitution of hematopoietic lineages in the recipients after 8–10 weeks. The reconstitution of BM LSK cells was similar in mice that received Hes1−/− FL or Hes1+/+ control FL. However, the absence of Hes1 resulted in reduced ETP chimerism, and this defect became very pronounced by the DN2 stage15,21 (DN2 chimerism from Hes1−/− cells was <2% that of BM chimerism; Supplementary Fig. 1b). Cebpa expression was sharply downregulated by the DN2 stage of T cell development (Fig. 1a), which suggested that Hes1 may inhibit Cebpa expression during the ETP-to-DN2 transition. Furthermore, Hes1−/− Lin−c-Kit+Flt3+ progenitor cells were defective in generating CD4+CD8+ double-positive T cells when mixed with host-type Hes1+/+ BM LSK competitor cells and directly injected into the thymus of sublethally irradiated congenic hosts (Supplementary Fig. 1c), as expected21.
Notch constrains myeloid potential through Hes1
To better examine the requirement for Hes1 during T cell development, we cultured Hes1−/− FL progenitor cells with OP9 stromal cells or OP9 cells that express the Notch ligand Delta-like 4 (OP9-DL4) and stimulated the cocultures with the cytokines IL-7 (interleukin 7) and Flt3L (the ligand for the receptor tyrosine kinase Flt3). Given that MPP have robust myeloid potential, whereas lymphoid progenitor cells have attenuated that potential30,31, we investigated the differentiation potential of sorted FL Lin−c-Kit+Flt3+IL-7Ra− multipotent progenitor cells and Lin−c-Kit+Flt3+IL-7Ra+ lymphoid progenitor cells from Hes1−/− embryos and their Hes1+/+ littermates. Hes1+/+ Lin−c-Kit+Flt3+IL-7Ra− multipotent progenitor cells generated mostly myeloid cells and few B cells in OP9 cultures, whereas Lin−c-Kit+Flt3+IL-7Ra+ lymphoid progenitor cells generated predominantly B cells and few myeloid cells (Fig. 2a and Supplementary Fig. 2a). Hes1−/− FL MPP and lymphoid progenitor cells had developmental potential similar to that of their wild-type counterparts in the absence of Notch signaling (Fig. 2a and Supplementary Fig. 2a). On OP9-DL4 cells, which activate Notch signaling, the number of Thy-1+CD25+ T lineage progeny generated from either Hes1−/− multipotent progenitor cells or Hes1−/− lymphoid progenitor cells was much lower than that generated by wild-type progenitor cells (Fig. 2b and Supplementary Fig. 2b). Notch signaling effectively inhibited myeloid development from Hes1+/+ MPPs6, while Hes1−/− MPP generated high numbers of Mac-1+ progeny, many of which co-expressed Gr-1 (Fig. 2b and Supplementary Fig. 2c). In contrast to Hes1−/− MPPs, Hes1−/− lymphoid progenitor cells generated very few Mac-1+ cells, which were largely Gr-1− (Fig. 2b and Supplementary Fig. 2c). Notch-mediated inhibition of B cells remained intact in Hes1−/− MPPs and lymphoid progenitor cells (Fig. 2c). Mac-1−Thy-1− undifferentiated cells in OP9-DL4 cultures initiated with Hes1−/− FL lymphoid progenitor cells showed more annexin V staining than in cultures initiated with Hes1+/+ control lymphoid progenitor cells and decreased in number by day 4 in the former cultures (Fig. 3a). After supplementation of OP9-DL4 cultures with myeloid cytokines (SCF, Flt3L, IL-3, M-CSF, GM-CSF and G-CSF), Hes1−/− FL lymphoid progenitor cells generated more Mac-1+ cells than did wild-type FL lymphoid progenitor cells (Fig. 3b). Mac-1+ cells generated from either Hes1+/+ or Hes1−/− lymphoid progenitor cells were mainly CD11c+ (Fig. 3b). These results indicated that Hes1 was required for Notch1-mediated inhibition of the development of myeloid cells, but not B cells, from MPPs. However, in contrast to Hes1−/− MPPs, Hes1−/− lymphoid progenitor cells did not readily divert to myeloid fates in T cell–inductive conditions unless myeloid cytokines were provided.
Figure 2.
Hes1-deficient multipotent progenitor cells, but not lymphoid progenitor cells, divert to myeloid fates in T cell–inductive conditions. Flow cytometry of FL progenitor cells (Lin−c-Kit+Flt3+ IL-7Ra− or IL-7Ra+) isolated by cell sorting from Hes1−/− embryos and their Hes1+/+ littermates at E12.5–E13.5 and cultured for 6 d on OP9 stromal cells (a) or OP9-DL4 stromal cells (b,c) (250 cells per well, in triplicate). Numbers adjacent to outlined areas indicate percent Mac-1+CD19− cells (a,c, top left) or Mac-1−CD19+ cells (a,c, bottom right), or percent Mac-1+CD25− cells (b, top left) or Mac-1−CD25+ cells (b, bottom right). Right (b), Thy-1+CD25+ cells (top) and Mac-1+Gr-1+ cells (bottom). *P < 0.05 and **P < 0.01 (Student’s t-test) Data are from one experiment representative of three experiments with similar results (average and s.e.m. in b, right).
Figure 3.
Hes1-deficient lymphoid progenitor cells undergo apoptosis in T cell–inductive conditions lacking myeloid cytokines. (a) Flow cytometry of FL multipotent progenitor cells (Lin−c-Kit+Flt3+IL-7Ra−) or lymphoid progenitor cells (Lin−c-Kit+Flt3+IL-7Ra+) isolated by cell sorting from Hes1−/− embryos and their Hes1+/+ littermates at E12.5–E13.5 and cultured for 4 d on OP9-DL4 stroma (500 cells per well in triplicate). Right, frequency of annexin V–positive (AnnV+) cells (left) or Mac-1−Thy-1− cells (right) among DAPI− cells. *P < 0.05 and **P < 0.01 (Student’s t-test). (b) Flow cytometry of FL multipotent progenitor cells (Lin−c-Kit+Flt3+IL-7Ra−) or lymphoid progenitor cells (Lin−c-Kit+Flt3+IL-7Ra+) isolated by cell sorting from embryos as in a and cultured for 6 d on OP9-DL1 stroma (250 cells per well in triplicate) with IL-7, Flt3L and supplemented with myeloid cytokines (IL-3, SCF, additional Flt3L, M-CSF, GM-CSF and G-CSF). Right, Mac-1+CD11c+ cells analyzed per well. *P < 0.01 (Student’s t-test). (c) Flow cytometry (left) and quantitative PCR analysis of Hes1, Gata3 and Cebpa mRNA (right) of FL multipotent progenitor cells (Lin−c-Kit+Flt3+IL-7Ra−) or lymphoid progenitor cells (Lin−c-Kit+Flt3+IL-7Ra+) isolated by cell sorting from embryos as in a and cultured for 6 d on OP9-DL4 stromal cells, followed by sorting of the Mac-1−Thy-1−CD25− population for quantitative PCR analysis. *P < 0.001 (Student’s t-test). Numbers in quadrants or adjacent to outlined areas indicate percent cells in each throughout. Data are representative of two experiments with similar results (a,b,c; error bars, s.e.m.).
We further separated FL lymphoid progenitor cells on the basis of their expression of PIR, which marks prethymic T cell lineage–committed progenitor cells and recent thymic immigrants37. PIR− and PIR+ progenitor cells were present at a similar frequency in Hes1−/− and Hes1+/+ mice37 (Supplementary Fig. 3a). Both PIR− and PIR+ Lin− c-Kit+Flt3+IL-7Ra+ progenitor cells from Hes1−/− mice failed to generate normal numbers of CD25+ T cells and did not divert to myeloid fates in OP9-DL4 cultures with IL-7 and Flt3L cytokines37 (Supplementary Fig. 3b).
To investigate whether myeloid gene expression was affected by the absence of Hes1, we cultured Hes1−/− total Lin−c-Kit+Flt3+ FL progenitor cells on OP9-DL4 stromal cells and isolated Mac-1−CD25− cells by cell sorting after 6 d for gene-expression analysis. The expression of the myeloid-lineage genes Cebpa and Csf1r was higher in cultures initiated with Hes1−/− progenitor cells than in cultures initiated with Hes1+/+ control FL progenitor cells (Supplementary Fig. 4). Those data were consistent with expanded myeloid output from this population; however, expression of Sfpi1 (which encodes the transcription factor PU.1) was similar in cultures initiated with Hes1−/− progenitor cells and those initiated with Hes1+/+ control progenitor cells. We also examined expression of Cdkn1b, which encodes the cyclin-dependent kinase inhibitor p27Kip and is suggested to be a Hes1 target22,23; however, Cdkn1b expression was not upregulated in Mac1−CD25− cells from OP9-DL4 cultures initiated with Hes1−/− FL progenitor cells relative to its expression in those initiated with Hes1+/+ FL progenitor cells (Supplementary Fig. 4). We also analyzed gene expression in Mac-1−Thy-1−CD25− cells derived from IL-7Ra− and IL-7Ra+ Lin−c-Kit+Flt3+ FL progenitor cells from Hes1+/+ and Hes1−/− mice. In OP9-DL4 cultures, Cebpa expression was higher and Gata3 expression was lower in Mac-1−Thy-1−CD25− cells differentiated from Hes1−/− Lin−c-Kit+Flt3+IL-7Ra− MPPs than in those differentiated from their Hes1+/+ counterparts (Fig. 3c), consistent with expanded myeloid output from Hes1−/− MPP (Fig. 2b). In similar conditions, we did not observe a difference between cells derived from Hes1−/− IL-7Ra+Lin−c-Kit+Flt3+ cells and those derived from their Hes1+/+ counterparts in their Cebpa expression. These results suggested that multipotent and lymphoid progenitor cells responded differently to T cell–inductive Notch signals in the absence of Hes1: Hes1−/− MPP exposed to Notch signals diverted to myeloid fates, whereas Hes1−/− lymphoid progenitor cells exposed to Notch ligands were unable to efficiently adopt either the T cell fate or myeloid cell fate and exhibited increased apoptosis.
Hes1 represses Cebpa
We studied how ectopic expression of Hes1 in hematopoietic progenitor cells resulted in downregulation of Cebpa. We treated C57BL/6 mice with the chemotherapeutic agent 5-fluorouracil to enrich for hematopoietic stem cells, collected BM from those mice, retrovirally transduced the BM with vector encoding human Hes1 or empty vector and injected the BM intravenously into irradiated host mice. In this system, expression of green fluorescent protein (GFP) is used to track transduced cells. We used cell sorting to isolate GFP+ BM LSK progenitor cells from mice reconstituted with BM transduced with the Hes1-encoding vector or empty vector and analyzed gene expression by quantitative PCR. Ectopic expression of Hes1 resulted in downregulation of Cebpa in BM LSK progenitor cells5,27,28 (Fig. 4a). To determine whether Hes1 affects the expression of C/EBPa protein, we retrovirally transduced the 32D mouse myeloid cell line with Hes1-encoding vector or empty vector and isolated transduced cells after 48 h by cell sorting according to GFP expression. Immunoblot analysis indicated that cells transduced with the Hes1-encoding vector had less C/EBPa than did those transduced with empty vector (Fig. 4b).
Figure 4.
Hes1 inhibits myeloid development and Cebpa expression. (a) Quantitative PCR analysis of Hes1 and Cebpa mRNA in BM LSK cells from mice reconstituted 8 weeks earlier with 5-fluorouracil-conditioned BM that was transduced with empty vector (EV) or retroviral vector encoding human Hes1 (hHes1) before transfer; results are presented relative to those of 18S RNA. (b) Immunoblot analysis of C/EBPa (isoforms p42 and p30) and a-actin (loading control) in 32D cells 48 h after transduction as in a, followed by isolation of GFP+ cells by cell sorting before lysis. (c) Quantitative PCR analysis of Hes1, Sfpi1 and Cdkn1b mRNA in Hes1+/+ FL (E15.5) progenitor cells (Lin−c-Kit+Flt3+) 48 h after transduction of empty vector or with vector encoding wild-type human Hes1 (hHes1(WT)) or human Hes1 lacking the DNA-binding domain (hHes1(DDNA)) or the WRPW domain (hHes1(DGroucho)); results are presented relative to those of Gapdh. (d,e) Flow cytometry of Hes1−/− FL (E15.5) cells (Lin−c-Kit+Flt3+) transduced as in c and cultured for 8 d on OP9-DL4 cells (gated on GFP+ cells). Numbers adjacent to outlined areas indicate percent Mac-1+CD25− cells (top left) or Mac-1−CD25+ cells (bottom right). (f) ChIP analysis of the binding of Hes1 to sites 1.2 kilobases (–1.2 kb) and 165 base pairs (–165 bp) upstream of the transcriptional start site of the mouse Cebpa locus (top) and to the Hes1 promoter (prom; positive control) and Nanog promoter (negative control) in DN3 thymocytes isolated from Rag1-deficient mice. (g) Luciferase activity in 293T cells cotransfected with a luciferase reporter construct containing the Cebpa promoter sequence including one Hes1-binding site (Cebpa prom) or a mutated Hes1-binding site (Cebpa prom(DHes1)); results are normalized to empty vector and are presented relative to the activity of renilla luciferase. NS, not significant; *P < 0.001, Student’s t-test. Data are representative of two experiments (a), one experiment (b), two experiments (c), two experiments (d), two experiments (e) or three experiments (f), three experiments (g; error bars (a,c,g), s.e.m.) or (error bars (d),s.d. of triplicate wells).
Hes1 inhibits gene expression via direct DNA binding and recruitment of Groucho corepressors via its carboxy-terminal Trp-Arg-Pro-Trp (WRPW) domain16. However, Hes1 can also inhibit gene expression by interacting with positively acting basic helix-loop-helix transcription factors and decreasing their affinity for activating E-box sites16,38, a repressive mechanism that presumably does not require DNA binding by Hes1. To discriminate between those mechanisms, we transduced wild-type Lin−c-Kit+Flt3+ FL progenitor cells with retrovirus encoding a mutant human Hes1 construct lacking the DNA-binding domain or one lacking the WRPW domain (P. Zweidler-McKay, personal communication and ref. 39). We isolated transduced GFP+ cells by cell sorting 48 h later and analyzed gene expression by quantitative PCR. Wild-type Hes1 repressed Cebpa expression in Lin−c-Kit+Flt3+ FL progenitor cells, whereas neither Hes1 mutant was able to do so (Fig. 4c). Hes1 overexpression did not affect the expression of Sfpi1 or Cdkn1b (Fig. 4c), consistent with our loss-of-function data (Supplementary Fig. 4) and published data40. We also assessed the ability of the mutant Hes1 constructs to support the development of T cells and inhibit the development of myeloid cells. Expression of wild-type Hes1 in Hes1−/− progenitor cells restored T cell development and suppressed myelopoiesis in OP9-DL4 cultures, but expression of the Hes1 mutants did not (Fig. 3d,e). Transduction of retrovirus encoding the mutant Hes1 lacking the DNA-binding domain neither rescued T cell development nor inhibited myelopoiesis in OP9-DL4 cultures (Fig. 4d). Transduction of retrovirus encoding the mutant Hes1 lacking the WRPW domain led to some T cell development in OP9-DL4 cultures; however, this was much lower than that resulting from transduction of retrovirus encoding wild-type Hes1 (Fig. 4e). These results suggested that DNA binding and interaction with Groucho proteins were important for Hes1-mediated inhibition of Cebpa expression and Hes1 function.
Analysis of the mouse Cebpa promoter revealed several putative Hes1-binding (N-box) sites (Fig. 4f), which suggested a role for Hes1 in directly regulating this gene. A Hes1-binding site 165 base pairs upstream of the Cebpa transcriptional start site is bound by Hes1 in 32D cells27. Chromatin immunoprecipitation (ChIP) showed enrichment for Hes1 binding at the site 165 upstream but not at another site 1.2 kilobases upstream of the transcriptional start site in DN3 thymocytes (Fig. 4f). Hes1 also bound its own promoter in DN3 thymocytes, consistent with Hes1 autoregulation41, but did not bind a negative control site (Fig. 4f). Moreover, Hes1 repressed the activity of a luciferase reporter containing Cebpa promoter (Fig. 4g). Mutation of the Hes1-binding site in the reporter abolished that effect (Fig. 4g). These data suggested that Hes1 may directly bind the Cebpa promoter and repress its transcription in progenitors of T cells through interaction with Groucho corepressors.
Deletion of C/EBPa restores T cell lymphopoiesis in the absence of Hes1
To determine whether the primary role of Hes1 in T cell development is to repress Cebpa expression or related myeloid gene-expression programs, we investigated whether deletion of C/EBPa would restore the development of T cells from Hes1-deficient progenitor cells. We generated Hes1+/− mice with loxP-flanked Cebpa alleles (Cebpafl/fl)42 and then intercrossed those mice to obtain Hes1−/−Cebpafl/fl embryos. To delete C/EBPa, we transduced FL Lin−c-Kit+Flt3+ multipotent progenitor cells from Hes1−/−Cebpafl/fl or Hes1+/−Cebpafl/fl embryos (at E15.5) with retroviral vector encoding Cre recombinase and GFP (MSCV-Cre-GFP; called 'Cre-GFP' here) and cultured both the resulting Cre-GFP+ (transduced) and Cre-GFP− (untransduced) fractions on OP9-DL4 stromal cells. The development of myeloid cells from Cre-GFP+ Hes1−/−Cebpafl/fl and Cre-GFP+ Hes1+/−Cebpafl/fl progenitor cells was completely abrogated24,42 (Fig. 5a). Both Cre-GFP+ and Cre-GFP− Hes1+/−Cebpafl/fl progenitor cells robustly produced T cell populations on OP9-DL4 cells, because C/EBPa is not required for T cell development24. In similar cultures, only Cre-GFP+ Hes1−/−Cebpafl/fl progenitor cells gave rise to robust populations of CD25+ T lineage cells, while Cre-GFP− Hes1−/−Cebpafl/fl cells generated mostly Mac-1+ myeloid cells and very few T cells. These data indicated that deletion of C/EBPa restored the T cell potential of Hes1−/− progenitor cells. Further, they established that the effects of C/EBPa deletion were cell autonomous.
Figure 5.
Deletion of C/EBPa restores T cell development from Hes1-deficient progenitor cells in vitro. (a) Flow cytometry of FL (E15.5) multipotent progenitor cells (Lin−c-Kit+Flt3+) isolated by cell sorting from Hes1−/−Cebpafl/fl or Hes1+/−Cebpafl/fl (control) embryos and transduced with retroviral vector encoding Cre-GFP and cultured for 7 d on OP9-DL4 cells. Numbers adjacent to outlined areas indicate percent Cre-GFP+CD45+ (transduced) cells (left, top) or Cre-GFP−CD45+ (untransduced) cells (left, bottom), or percent Mac-1+CD25− cells (right; top left) or Mac-1− CD25+ cells (right, bottom right). (b) Flow cytometry of Hes1−/−Cebpafl/fl FL (E15.5) progenitor cells (Lin−c-Kit+Flt3+) transduced with empty retroviral vector (left) or retroviral vector encoding Cre-GFP (right) and sorted for GFP expression, then cultured for 7 d on OP9-DL4 cells (numbers adjacent to outlined areas as in a, right). Below, quantification of CD25+ T cells, normalized to GFP+ cells plated. *P < 0.05 (Student’s t-test). (c) Limiting-dilution analysis of the frequency of T cell progenitors among Hes1−/−Cebpafl/fl or Hes1+/+Cebpafl/fl FL (E15.5) progenitor cells (Lin−c-Kit+Flt3+) transduced and sorted as in b, then cultured for 6 d on OP9-DL4 cells and analyzed by flow cytometry. Data are representative of three experiments (a), two experiments (b; average and s.e.m.) or one experiment (c).
To quantify the effect of the deletion of C/EBPa in Hes1−/− progenitor cells, we transduced Hes1−/−Cebpafl/fl Lin−c-Kit+Flt3+ FL progenitor cells with Cre-GFP or with an empty retroviral vector encoding GFP (referred to here as empty vector), cultured them on OP9-DL4 stromal cells and examined the cultures by flow cytometry after 7 d. Significantly more CD25+ T cells were generated by Hes1−/−Cebpafl/fl progenitor cells transduced with Cre-GFP than by those transduced with empty vector (Fig. 5b). We also assessed Cre-GFP+ progenitor cells in OP9-DL4 cultures by limiting-dilution analysis. Although the frequency of T cell progenitors among transduced cells was lower than that among untransduced cells (data not shown), the frequency of T cell progenitors was similar among Cre-GFP+ Hes1−/−Cebpafl/fl cells (1/43) and Cre-GFP+ Hes1+/+Cebpafl/fl cells (1/56), and both of those frequencies were significantly higher than that of Hes1−/−Cebpafl/fl cells transduced with empty vector (1/170) (Fig. 5c). Thus, deletion of C/EBPa substantially improved the generation of T cells from Hes1−/− progenitor cells. Collectively, these data were consistent with the idea that T cell development requires Hes1-mediated repression of Cebpa and perhaps related myeloid genes.
Next we determined whether deletion of C/EBPa would restore the development of T cells from Hes1−/− thymus-settling progenitor cells in vivo. We bred Hes1+/−Cebpafl/fl mice with Vav1-Cre mice, which results in specific expression of Cre recombinase in hematopoietic cells43. We bred the resulting Hes1+/−Cebpafl/+Vav1-Cre mice with Hes1+/−Cebpafl/fl mice to obtain Hes1−/−Cebpafl/flVav1-Cre embryos. We mixed whole FL from Hes1−/− Cebpafl/flVav1-Cre embryos (at E15.5) or their littermates (Hes1+/−Cebpafl/flVav1-Cre or Hes1−/−Cebpafl/+Vav1-Cre) with CD45.1+ wild-type adult BM and transplanted the mixture into lethally irradiated CD45.1+ recipient mice. T cell development in mice reconstituted with Hes1−/−Cebpafl/flVav1-Cre progenitor cells was restored to wild-type levels (Fig. 6a–d and Supplementary Fig. 5). As an additional control, we did not detect Hes1 mRNA in DN3 thymocytes derived from Hes1−/−Cebpafl/flVav1-Cre cells (Fig. 6b). The controls used in these experiments were either Hes1+/−Cebpafl/flVav1-Cre cells or Hes1+/−Cebpafl/+Vav1-Cre cells (Fig. 6d), and we saw normal development of T cells from cells of both those genotypes in vivo (data not shown).
Figure 6.
Deletion of C/EBPa restores T cell development from Hes1-deficient progenitor cells in vivo. (a) Flow cytometry of cells from irradiated CD45.1+ mice 10–12 weeks after intravenous injection of a mixture of FL from CD45.2+ Hes1−/−Cebpafl/flVav1-Cre, Hes1−/−Cebpafl/+Vav1-Cre or Hes1+/−Cebpafl/flVav1-Cre embryos (at E15.5) and BM from CD45.1+ mice. Numbers adjacent to outlined areas indicate percent CD45.2+ (FL donor) cells. (b) Quantitative PCR analysis of Hes1 mRNA and Tcf7 mRNA (which encodes TCF-1) in CD45.2+ FL-derived DN3 thymocytes sorted from mice reconstituted with Hes1−/−Cebpafl/flVav1-Cre FL, or Hes1+/+ DN3 cells (positive control), to verify absence of Hes1 mRNA. (c) Quantification of CD45.2+ donor–derived thymocytes in chimeras generated as in a (n = 3 with Hes1−/−Cebpafl/flVav1-Cre or Hes1+/−Cebpafl/flVav1-Cre FL and 1 with Hes1−/− Cebpafl/+Vav1-Cre FL). (d) Frequency of CD45.2+ donor chimerism in various populations (horizontal axis) of chimeras generated as in a (n = 6 with Hes1−/−Cebpafl/flVav1-Cre FL, 5 with Hes1+/− Cebpafl/+Vav1-Cre FL and 2 with Hes1−/−Cebpafl/+Vav1-Cre FL); BM chimerism was inferred from CD19+ splenic B cell chimerism; mice reconstituted with Hes1+/− Cebpafl/+Vav1-Cre or Hes1+/−Cebpafl/flVav1-Cre cells had equivalent T cell development, so those groups were pooled. (e) Flow cytometry of FL multipotent progenitor cells (Lin−c-Kit+Flt3+IL-7Ra−) or lymphoid progenitor cells (Lin−c-Kit+Flt3+IL-7Ra+) obtained from Hes1−/−Cebpafl/flVav1-Cre or Hes1+/− Cebpafl/flVav1-Cre embryos (E13.5) and cultured for 6 d on OP9-DL1 cells. Numbers adjacent to outlined areas indicate percent Mac-1−CD25+ cells. Right, quantification of Thy-1+CD25+ cells among those at left. Data are from one experiment representative of two experiments with similar results (a,b,c,e; average and s.e.m.) or represent two independent experiments (d) (d; average and s.e.m.).
Because Hes1−/− MPPs and lymphoid progenitor cells had different developmental potential in OP9-DL4 cultures, we assessed the effect of deletion of C/EBPa in each Hes1−/− progenitor cell type in vitro. We cultured Lin− c-Kit+Flt3+IL-7Ra− multipotent progenitor cells or Lin−c-Kit+Flt3+IL-7Ra+ lymphoid progenitor cells from Hes1−/− Cebpafl/flVav1-Cre embryos or their Hes1+/−Cebpafl/+Vav1-Cre littermates with OP9-DL4 stromal cells and examined T cell development by flow cytometry. The development of T cells from either Hes1−/− MPPs or lymphoid progenitor cells was restored to wild-type levels by deletion of C/EBPa (Fig. 6e). These results suggested that failure to downregulate myeloid lineage gene expression prohibited T cell development, even in the absence of overt myeloid diversion. These findings demonstrated that deletion of the myeloid factor C/EBPa eliminated the requirement for Hes1 in T cell development in vivo.
DISCUSSION
To investigate the mechanism and importance of alternative lineage gene repression during early T cell development, we focused on the canonical Notch target and basic helix-loop-helix transcriptional repressor Hes1. Hes1-deficient progenitor cells have a profound, but incomplete, developmental defect in the T cell lineage attributed to inadequate population expansion of early progenitor cells19,21; however, the precise cause of the defect has remained unknown. Because Hes1 robustly inhibits Cebpa expression and myeloid potential5,27,28, we investigated whether Hes1 constrains myeloid-lineage gene-expression programs in early T cell development. We found that deletion of C/EBPa in Hes1-deficient progenitor cells restored in vivo T cell development to wild-type levels. Hence, our results establish that the main, and perhaps only, role of Hes1 in T cell development is to inhibit myeloid gene-expression programs.
Our results suggest that C/EBPa must be downregulated for specification to the T cell lineage to proceed, consistent with the observed decrease in Cebpa mRNA as thymocytes progressed from ETP to DN2. Notably, the consequences of Hes1 deficiency differed in MPP and lymphoid progenitor populations. Hes1-deficient MPPs displayed a developmental bias toward myeloid cells and dendritic cell after Notch signaling, even in the absence of exogenous myeloid cytokines, whereas Hes1-deficient lymphoid progenitor cells underwent apoptosis in the same conditions. Additionally, our results indicated that the threshold for myeloid diversion of lymphoid progenitor cells was higher than that for diversion of multipotent progenitor cells after Notch signaling in vitro, because in our studies it required the provision of myeloid cytokines. Our results are consistent with other published work showing that ectopic expression of myeloid transcription factors in DN thymocytes conferred a survival disadvantage in T cell–inductive conditions33,44. These data may also explain why diversion of Hes1-deficient T cell progenitor cells to myeloid fates is not apparent in the thymus of FL chimeras15,21. Notably, the deletion of C/EBPa restored the development of both Hes1-deficient multipotent progenitor cells and Hes1-deficient lymphoid progenitor cells into T cells, which indicated that failure to constrain myeloid-lineage gene expression inhibits T cell development even in the absence of overt diversion into the myeloid lineage.
Our work and that of others27 suggests that Hes1 directly represses Cebpa, because the Hes1 DNA-binding domain is necessary for inhibition of C/EBPa and Hes1 binds to the Cebpa promoter in DN3 thymocytes. Furthermore, Hes1 may rely on Groucho corepressors for its function45, as alteration of the carboxy-terminal WRPW Groucho interaction domain greatly decreased Hes1 function. Although our data suggest that Hes1 might repress Cebpa directly in progenitors of T cells, Hes1 may also act on other effectors in the same pathway.
Hes1-deficient cells are resistant to induction of T cell acute lymphoblastic leukemia via introduction of intracellular Notch, and continuous Hes1 signals are required to maintain Notch-induced T cell acute lymphoblastic leukemia 21. Moreover, Hes1 may contribute to human myeloid malignancies via C/EBPa suppression46. At this time, it is unknown whether similar mechanisms downstream of Hes1 operate during the processes of T cell development and the induction or maintenance of T cell acute lymphoblastic leukemia.
Our observations help explain the sequential loss of non-T cell potential during early T cell development. We propose that the main function of Hes1 during T cell development is to constrain myeloid gene-expression programs. Hes1 was not required for the inhibition of B cell fate, a process that is probably mediated by other Notch effectors (such as TCF-1 and GATA-3)8,9. The transcriptional regulator Bcl11b may constrain myeloid potential after the ETP stage, as it is expressed in DN2 thymocytes and inhibits Cebpa expression47,48. Consistent with the idea that biochemically and temporally distinct mechanisms act together to inhibit non-T cell lineage programs, analysis of sequential T cell progenitor populations by ChIP and deep sequencing has revealed that diverse histone modifications mark different genes encoding hematopoietic regulatory molecules across development49. In FL-derived DN1 cells cultured with Notch ligands49 and in wild-type ETP, DN2 and DN3 thymocytes (our unpublished data), the Cebpa transcriptional start site was bivalently marked with the activating histone marks H3K4me3 or H3K(9,14)Ac, as well as the repressive mark H3K27me3, which suggests that repression had initiated in at least some cells. Hes1 is no longer expressed after the DN3 stage, which indicates that additional mechanisms are probably needed to silence myeloid genes in committed progenitors of T cells and mature T cells. Because progenitor cells lacking Cebpa and Hes1 generated normal numbers of T cells, they may be a useful tool with which to delineate stage-specific mechanisms by which regulators of the myeloid lineage are initially constrained and finally silenced during T cell development.
Our data support a model in which Notch signals upregulate expression of the transcriptional repressor Hes1 shortly after entry into the thymus, coincident with the generation of ETPs. Hes1 directly inhibits expression of Cebpa, which encodes a critical myeloid regulator, and perhaps other myeloid-lineage genes, which remain accessible in early thymus-settling progenitor cells15. Thus, our findings establish the importance of constraining developmental programs of the myeloid lineage early in intrathymic T cell development, a task accomplished by inclusion of Hes1 in the gene-regulatory network that establishes T cell identity.
ONLINE METHODS
Mice
Female C57BL/6 (CD45.2+) and B6.Ly5.2 (CD45.1+) mice (National Cancer Institute animal facility) were used at 5–8 weeks of age. Hes1−/− mice were provided by L. Raetzman (used with the permission of R. Kageyama). Hes1−/− mice were crossed with C57BL/6 mice. Cebpafl/fl mice were from Jackson Laboratories. To obtain FL, embryos were generated from timed matings. Detection of the vaginal plug was designated E0.5. All animal experiments were done according to protocols approved by the Office of Regulatory Affairs of the Perelman School of Medicine at the University of Pennsylvania (Philadelphia, Pennsylvania) in accordance with guidelines set forth by the US National Institutes of Health.
Cell preparations
BM cells were obtained from mouse femurs and red blood cells were removed through the use of ammonium chloride–potassium bicarbonate lysis buffer. Prior to cell sorting, BM underwent depletion with antibody to CD19 (anti-CD19) (1D3; BioXCell) and anti-Gr-1 (8C5; BioXCell) by removal of antibody-bound cells with magnetic beads conjugated to antibody to rat immunoglobulin G (IgG) (310107; Qiagen). Fetal liver cells were treated with ACK lysis buffer to lyse red blood cells. Prior to sorting of ETP, thymocyte cell suspensions were depleted using anti-CD4 (GK1.5; BioXCell) and anti CD8a (53-6.72; BioXCell) followed by removal of antibody-bound cells with magnetic beads conjugated to goat antibody to rat IgG (310107; Qiagen).
Flow cytometry and cell sorting
Bone marrow, thymocyte and FL cell suspensions were stained with optimized dilutions of directly conjugated fluorescent antibodies. In most experiments, the lineage (Lin) antibody 'cocktail' used included anti-B220 (RA3-6B3; eBioscience) and anti-CD19 (1D3; eBioscience) for the exclusion of B lineage cells; anti-CD11b (anti-Mac-1; M1/70; BD Pharmingen) and anti-Gr-1 (8C5; BioLegend) for the exclusion of myeloid cells; anti-CD11c (N418; eBioscience) for the exclusion of dendritic cells, anti-Ter119 (TER119; eBioscience) for the exclusion of erythroid cells; anti-NK1.1 (PK136; BD Pharmingen) for the exclusion of natural killer cells; and anti-CD3e (2C11; eBioscience), anti-CD8a (53-6.72; eBioscience ), anti-CD8b (H35-17.2; eBioscience), anti-TCRb (H57; eBioscience) and anti-TCRgd (GL-3; eBioscience ) for exclusion of the T cell lineage. For FL experiments, anti-CD11b (anti-Mac-1) was not included in the lineage 'cocktail'. Additional antibodies used include: anti-CD45.1 (A20; eBioscience ), anti-CD45.2 (104; eBioscience), anti-c-Kit (2B8; eBioscience), anti-Sca-1 (D7; eBioscience), anti-Flt3 (), anti-Thy-1 (53-2.1; BD Pharmingen), anti-CD25 (PC61.5; eBioscience). All of the antibodies noted above were directly conjugated to fluorescein isothiocyanate, phycoerythrin, allophycocyanin or biotin. Biotin-conjugated antibodies were visualized with either peridinine chlorophyll protein–cyanine 5.5–streptavidin (BD Biosciences) or streptavidin–Pacific blue (Molecular Probes). Cells were analyzed on a two-laser FACSCanto or a four-laser LSR II (Becton Dickinson). DAPI (4,6-diamidino-2-phenylindole) was used for exclusion of dead cells. Cells were sorted on a FACSAria (BD). Data were analyzed with FlowJo software, version 8.8.6 (TreeStar). Progenitor populations were gated as published15. FL multipotent progenitor cells were sorted as Lin−c-Kit+Flt3+IL-7Ra− cells. FL lymphoid progenitor cells were sorted as Lin−c-Kit+Flt3+IL-7Ra+ cells. The BM LSK population was sorted as Lin−Sca-1+c-Kit+ cells. BM lymphoid-primed multipotential progenitor cells were sorted as Lin−Sca-1+c-Kit+Flt3+ cells. Thymocyte populations were defined and sorted as ETPs (Lin−c-Kit+CD25− or Lin−c-Kit+CD25−), DN2 cells (Lin−c-Kit+CD25+ or Lin−c-Kit+CD25+), DN3 cells (Lin−c-Kit−CD25+ or Lin−c-Kit+CD25+) or double-positive cells (CD4+CD8+, CD4+ SP: CD4+CD8−,CD8+ SP: CD4− CD8+).).
Intrathymic transfer
Female CD45.1+ host mice were irradiated with 600 rads before intrathymic injection. Intrathymic transfers were done as described15. Mice were anesthetized and a thoracic incision was made to expose the thymus. Freshly sorted FL progenitor cells were injected directly into the thymus in a total volume of 10 ml.
Cell culture
OP9 and OP9-DL4 cells were used essentially as described8. For most cultures, IL-7 was added at a final concentration of 1 ng/ml and Flt3L was added at a final concentration of 5 ng/ml. Stromal cell cultures were not supplemented with myeloid cytokines except where specifically noted. Myeloid cytokine conditions50 were as follows: 25 ng/mL SCF, 25 ng/mL Flt3L, 5 ng/mL IL-3, 5 ng/mL M-CSF, 5 ng/mL GM-CSF and 10 ng/mL G-CSF. To ensure adequate cytokine for B cell development, some cultures included IL-7 at a final concentration of 5 ng/ml. For flow cytometry, cells in 24-well plates were resuspended in a final volume of 300 ml and were analyzed on the cytometer for 30–60 s, which allowed a fraction of the well to be analyzed. Cell numbers per well reflect the number of events collected during a given collection time, to allow comparison between wells. Stromal cells were plated 2 d before initiation of culture at a density of 20,000 cells per ml in 24-well plates for bulk cultures or 96-well plates for limiting-dilution analysis. For limiting-dilution analysis, wells in which more than 1% of CD45+ cells were Mac-1+Gr-1+ or Thy-1+CD25+, respectively, were considered 'positive', and all other wells were considered 'negative.' The progenitor frequency was calculated by the method of maximum likelihood applied to Poisson distribution with the help of the L-Calc software (StemCell Technologies). For limiting-dilution analysis of transduced progenitor cells, the number of cells plated per well reflects the number of live transduced cells per well 6 h after plating.
Annexin staining
Cells were stained with annexin V according to the manufacturer instructions (BD Biosciences). After cell surfaces were stained, cells were washed in PBS and resuspended in 1× annexin binding buffer containing phycoerythrin-conjugated annexin V. After 15 min of incubation at room temperature, cells were diluted in 1× annexin V binding buffer and analyzed by flow cytometry.
Retroviral transduction
Sorted progenitor cells were cultured in stimulation 'cocktail' containing 5 ng/ml IL-3, IL-6 and Flt3L, 50 ng/ml SCF, and polybrene. Progenitors were spun with retroviral preparations at 2,300 r.p.m. for 2 h at room temperature. After 24–48 h, cells were plated onto OP9 or OP9-DL4 stromal cell layers, after being sorted as GFP+ cells where needed.
Hes1 mutant constructs
Hes1 constructs in an empty mouse stem cell virus (MSCV)-based retroviral vector were obtained from P. Zweidler-McKay. The Hes1 mutant lacking DNA binding contained substitution of alanine for three residues (Glu43, Lys44 and Arg47) in the basic region of Hes1 responsible for DNA binding. The Hes1 mutant lacking the Groucho-interaction region featured substitution of the carboxy-terminal WRPW domain to with Gly-Phe-Pro-Gly (GFPG)39. Confirmation that wild-type and mutant forms of Hes1 protein were expressed was achieved by retroviral transduction of 3T3 fibroblasts and intracellular staining for Hes1 (data not shown). Hes1 protein was detected with rabbit polyclonal antibody to Hes1 (sc-25392; Santa Cruz Biotechnology) and a goat anti-rabbit F(ab’)2 fragment of IgG conjugated to Alexa Fluor 647 (A21244; Invitrogen).
Luciferase assay
The Cebpa promoter reporter construct was made by cloning of about 1 kilobase of sequence upstream of the Cebpa transcriptional start site, which encodes one Hes1-binding element, upstream of the minimal SV40 promoter in the pGL3-promoter reporter vector (Promega). 293T cells were seeded 1 d before transfection to reach 80% confluency, then were transiently cotransfected through the use of LipoD293 reagent according to the manufacturer's protocol (Stratagene). The total amount of DNA was the same for all wells, and constructs were used in the following amounts: Promega pGL3 promoter vector, 300 ng per well; vector encoding Hes1-GFP or empty vector, 300 ng per well; and renilla luciferase, 50 ng per well). DMEM containing 10% l-glutamine and 10% penicillin-streptomycin was added 12 h after transfection, and cells were harvested 24 h after transfection and analyzed with a Dual Assay Reporter kit (Promega) and GloMax 96 Microplate luminometer (Promega). Results were analyzed by comparison of firefly luciferase activity to renilla luciferase activity and were normalized to reflect the increase over background. We verified that Hes1 expression inhibited the activity of a positive control luciferase reporter containing the Hes1 promoter sequence39 but did not repress the empty Promega PGL3prom luciferase reporter, as a negative control (data not shown).
ChIP assay
Putative Hes1-binding sites were identified in the Cebpa promoter with the evolutionarily conserved region (ECR) browser. Thymus from mice deficient in recombination-activating gene 1 were used for ChIP, as thymus of this genotype is enriched for DN3 thymocytes that express Hes1. Cells were fixed for 10 min at room temperature in 1% formaldehyde and were treated with 125 mM glycine. The crosslinked chromatin was lysed with 1% SDS lysis buffer and sheared by sonication, which produced fragments of 200–800 base pairs in length. Sheared chromatin was immunoprecipitated with 2 mg anti-Hes1 (sc-25392; Santa Cruz Biotechnology) or rabbit IgG (control antibody) (sc-3888; Santa Cruz Biotechnology). After samples were washed, bound chromatin was eluted and treated to reverse crosslinking. After treatment with RNaseA and proteinase K, DNA was purified with a PCR purification kit (Qiagen) and was analyzed by quantitative PCR. The primer sets used amplify genomic fragments containing two conserved Hes1-binding sites in the Cebpa promoter were as follows: site 165 base pairs upstream of the transcriptional start site: forward, TTGCAGCGCAGGAGTCAGT, and reverse, ATGGTGCCTGCTGGGTCTTA; site 1.2 kilobases upstream of the transcriptional start site: forward, CGGCTGTGGGTAGGAGTTTG, and reverse, GACGAAAGGCCTCAGCTCAA. Nanog negative control primers were as follows: forward, 5¢-GGCTGCCTCTCCTCGCCCT-3¢, and reverse, 5¢-GTGCACACAGCTGGGCCTGA-3¢. Hes1 promoter: forward, 5¢-CGTGTCTCTTCCTCCCATTG-3¢, and reverse, 5¢-CCAGGACCAAGGAGAGAGGT-3¢.
PCR
For real-time PCR, mRNA from sorted populations was isolated with an RNeasy kit (Qiagen) and was reverse-transcribed with Superscript II (Invitrogen). The resultant cDNA was then amplified and detected with pre-made Taqman primers and probes for Dtx1, Cebpa, Csf1r, Hes1, Sfpi1, Gata3, Tcf7 and Cdkn1b (Applied Biosystems). A StepOnePlus Real-Time PCR System (Applied Biosystems) was used for amplification and analysis. Relative transcript abundance was determined by the change-in-cycling-threshold (DDCt) method after normalization with the control gene Gapdh or 18S RNA.
Statistical analysis
Data sets were analyzed using the Student’s t-test on Microsoft Excel, with a two-tailed distribution assuming equal sample variance.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Raetzman (University of Illinois at Urbana-Champaign) for Hes1−/− mice (used with permission from R. Kageyama (Kyoto University Institute for Virus Research); P. Zweidler-McKay (University of Texas MD Anderson Cancer Center) for mutant Hes1 constructs; A. Chi for advice on the design of luciferase reporter constructs; and N. Speck, D. Allman, S. Reiner, Q. Yang, S. Zhang, W. Bailis and D. Northrup for critical comments on the manuscript. Supported by the US National Institutes of Health (AI059621 and AI098428 to A.B., AI047833 to W.S.P., T32 GM-07229 and T32 CA-09140 to M.E.D., 1-F32-AI-080091-01A1 to J.J.B. and F30-HL-099271 to D.A.Z.).
Footnotes
Accession codes. GEO: microarray data, QQQ.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS
M.E.D., J.J.B. and A.B. designed the study and did the experiments; X.W., C.H., Y.Y.-O., D.A.Z, D.A.S. and J.H.D. did experiments; W.S.P. contributed conceptual expertise and provided input into the design of the study; and M.E.D. and A.B. wrote the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Sambandam A, et al. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat. Immunol. 2005;6:663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 2.Tan JB, Visan I, Yuan JS, Guidos CJ. Requirement for Notch1 signals at sequential early stages of intrathymic T cell development. Nat. Immunol. 2005;6:671–679. doi: 10.1038/ni1217. [DOI] [PubMed] [Google Scholar]
- 3.Radtke F, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 4.Pui JC, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 5.Kawamata S, Du C, Li K, Lavau C. Overexpression of the Notch target genes Hes in vivo induces lymphoid and myeloid alterations. Oncogene. 2002;21:3855–3863. doi: 10.1038/sj.onc.1205487. [DOI] [PubMed] [Google Scholar]
- 6.de Pooter RF, et al. Notch signaling requires GATA-2 to inhibit myelopoiesis from embryonic stem cells and primary hemopoietic progenitors. J. Immunol. 2006;176:5267–5275. doi: 10.4049/jimmunol.176.9.5267. [DOI] [PubMed] [Google Scholar]
- 7.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 8.Weber BN, et al. A critical role for TCF-1 in T-lineage specification and differentiation. Nature. 2011;476:63–68. doi: 10.1038/nature10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Ojeda ME, et al. GATA-3 promotes T-cell specification by repressing B-cell potential in pro-T cells in mice. Blood. 2013;121:1749–1759. doi: 10.1182/blood-2012-06-440065. [DOI] [PubMed] [Google Scholar]
- 10.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 11.Wada H, et al. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452:768–772. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]
- 12.Feyerabend TB, et al. Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity. 2009;30:67–79. doi: 10.1016/j.immuni.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Schlenner SM, et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Luc S, et al. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nat. Immunol. 2012;13:412–419. doi: 10.1038/ni.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Obaldia ME, Bell JJ, Bhandoola A. Early T-cell progenitors are the major granulocyte precursors in the adult mouse thymus. Blood. 2013;121:64–71. doi: 10.1182/blood-2012-08-451773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- 17.Jarriault S, et al. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura M, et al. Structure, chromosomal locus, and promoter of mouse Hes2 gene, a homologue of Drosophila hairy and Enhancer of split. Genomics. 1998;49:69–75. doi: 10.1006/geno.1998.5213. [DOI] [PubMed] [Google Scholar]
- 19.Tomita K, et al. The bHLH gene Hes1 is essential for expansion of early T cell precursors. Genes Dev. 1999;13:1203–1210. doi: 10.1101/gad.13.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi M, et al. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- 21.Wendorff AA, et al. Hes1 is a critical but context-dependent mediator of canonical Notch signaling in lymphocyte development and transformation. Immunity. 2010;33:671–684. doi: 10.1016/j.immuni.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Murata K, et al. Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1. Mol. Cell. Biol. 2005;25:4262–4271. doi: 10.1128/MCB.25.10.4262-4271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong GW, Knowles GC, Mak TW, Ferrando AA, Zuniga-Pflucker JC. HES1 opposes a PTENdependent check on survival, differentiation, and proliferation of TCRb-selected mouse thymocytes. Blood. 2012;120:1439–1448. doi: 10.1182/blood-2011-12-395319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang DE, et al. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein a-deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welner RS, et al. C/EBPs is required for development of dendritic cell progenitors. Blood. 2013;121:4073–4081. doi: 10.1182/blood-2012-10-463448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross DA, et al. Functional analysis of Hes-1 in preadipocytes. Mol. Endocrinol. 2006;20:698–705. doi: 10.1210/me.2005-0325. [DOI] [PubMed] [Google Scholar]
- 27.Klinakis A, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473:230–233. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakata-Yanagimoto M, et al. Coordinated regulation of transcription factors through Notch2 is an important mediator of mast cell fate. Proc. Natl. Acad. Sci. USA. 2008;105:7839–7844. doi: 10.1073/pnas.0801074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taghon T, Yui MA, Rothenberg EV. Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3. Nat. Immunol. 2007;8:845–855. doi: 10.1038/ni1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 31.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. doi: 10.1016/j.immuni.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBPa and PU.1 transcription factors. Immunity. 2006;25:731–744. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Wölfler A, et al. Lineage-instructive function of C/EBPa in multipotent hematopoietic cells and early thymic progenitors. Blood. 2010;116:4116–4125. doi: 10.1182/blood-2010-03-275404. [DOI] [PubMed] [Google Scholar]
- 35.Taghon T, Yui MA, Pant R, Diamond RA, Rothenberg EV. Developmental and molecular characterization of emerging b- and gd-selected pre-T cells in the adult mouse thymus. Immunity. 2006;24:53–64. doi: 10.1016/j.immuni.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Ikawa T, Kawamoto H, Goldrath AW, Murre C. E proteins and Notch signaling cooperate to promote T cell lineage specification and commitment. J. Exp. Med. 2006;203:1329–1342. doi: 10.1084/jem.20060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuda K, et al. Prethymic T-cell development defined by the expression of paired immunoglobulin-like receptors. EMBO J. 2005;24:4052–4060. doi: 10.1038/sj.emboj.7600878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992;6:2620–2634. doi: 10.1101/gad.6.12b.2620. [DOI] [PubMed] [Google Scholar]
- 39.Zhang P, Yang Y, Nolo R, Zweidler-McKay PA, Hughes DP. Regulation of NOTCH signaling by reciprocal inhibition of HES1 and Deltex 1 and its role in osteosarcoma invasiveness. Oncogene. 2010;29:2916–2926. doi: 10.1038/onc.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Real MM, Rothenberg EV. Architecture of a lymphomyeloid developmental switch controlled by PU.1, Notch and Gata3. Development. 2013;140:1207–1219. doi: 10.1242/dev.088559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takebayashi K, et al. Structure, chromosomal locus, and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES-1. Negative autoregulation through the multiple N box elements. J. Biol. Chem. 1994;269:5150–5156. [PubMed] [Google Scholar]
- 42.Zhang P, et al. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBPa. Immunity. 2004;21:853–863. doi: 10.1016/j.immuni.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 43.de Boer J, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 44.Franco CB, et al. Notch/Delta signaling constrains reengineering of pro-T cells by PU.1. Proc. Natl. Acad. Sci. USA. 2006;103:11993–11998. doi: 10.1073/pnas.0601188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paroush Z, et al. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell. 1994;79:805–815. doi: 10.1016/0092-8674(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 46.Nakahara F, et al. Hes1 immortalizes committed progenitors and plays a role in blast crisis transition in chronic myelogenous leukemia. Blood. 2010;115:2872–2881. doi: 10.1182/blood-2009-05-222836. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Leid M, Rothenberg EV. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science. 2010;329:89–93. doi: 10.1126/science.1188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikawa T, et al. An essential developmental checkpoint for production of the T cell lineage. Science. 2010;329:93–96. doi: 10.1126/science.1188995. [DOI] [PubMed] [Google Scholar]
- 49.Zhang JA, Mortazavi A, Williams BA, Wold BJ, Rothenberg EV. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell. 2012;149:467–482. doi: 10.1016/j.cell.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansson R, et al. Single-cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2010;115:2601–2609. doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.