Abstract
Although the peripheral anti-inflammatory effect of norepinephrine (NE) is well-documented, the mechanism by which this neurotransmitter functions as an anti-inflammatory/neuroprotective agent in the central nervous system is unclear. This study aimed to determine the anti-inflammatory/neuroprotective effects and underlying mechanisms of NE in inflammation-based dopaminergic neurotoxicity models. In mice, NE-depleting toxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) was injected at 6 months of lipopolysaccharide (LPS)-induced neuroinflammation. We found that NE depletion enhanced LPS-induced dopaminergic neuron loss in the substantia nigra. This piece of in vivo data prompted us to conduct a series of studies in an effort to elucidate the mechanism as to how NE affects dopamine neuron survival by using primary midbrain neuron-glia cultures. Results showed that sub-micromolar concentrations of NE dose-dependently protected dopaminergic neurons from LPS-induced neurotoxicity by inhibiting microglia activation and subsequent release of pro-inflammatory factors. However, NE-elicited neuroprotection was not totally abolished in cultures from β2-adrenergic receptor (β2-AR) deficient mice, suggesting that novel pathways other than β2-AR are involved. To this end, we found that sub-micromolar NE dose-dependently inhibited NADPH oxidase (NOX2)-generated superoxide, which contributes to the anti-inflammatory and neuroprotective effects of NE. This novel mechanism was indeed adrenergic receptors independent since both (+) and (−) optic isomers of NE displayed the same potency. We further demonstrated that NE inhibited LPS-induced NOX2 activation by blocking the translocation of its cytosolic subunit to plasma membranes. In summary, we revealed a potential physiological role of NE in maintaining brain immune homeostasis and protecting neurons via a novel mechanism.
Keywords: Extra-synaptic, Neurotransmitter, Volume Transmission, DSP-4, Neurodegeneration
Introduction
In addition to functioning as a neurotransmitter, norepinephrine (NE) has a well-documented anti-inflammatory effect in the periphery (Kin and Sanders 2006; Kohm and Sanders 2001; Severn et al. 1992; Straub et al. 2002; van der Poll et al. 1994). It is generally believed that β2-adrenergic receptor (β2-AR) on immune cells is critical in mediating NE-elicited anti-inflammatory effect through activation of cAMP/protein kinase A pathway, which leads to reduced release of pro-inflammatory factors , such as tumor necrosis factor-α(TNF-α), Interleukin-1β(IL-1β), etc. (Farmer and Pugin 2000; Flierl et al. 2009; Sanders and Straub 2002; Zamah et al. 2002). In contrast, what is known about the anti-inflammatory role of NE in the brain is limited. The effects of NE in regulating microglial inflammatory factors release or migration were previously reported (Dello Russo et al. 2004; Heneka et al. 2010; Mori et al. 2002; Troadec et al. 2001; Troadec et al. 2002). However, these immune regulatory effects were observed only in micromolar or higher concentrations of NE. It is likely that local concentrations of NE acting on microglia in the brain are less than micromolar (Gresch et al. 1995; Xie et al. 2013).
Several pieces of evidence suggest potential roles of NE in the pathogenesis and progression of neurodegenerative diseases, including Parkinson's disease (PD). Post-mortem examinations of patients with PD revealed a substantial loss of NE neurons (up to 80%) in the locus coeruleus (LC), which occurs prior to dopaminergic (DA) neuronal loss in the substantia nigra (SN) (Baloyannis et al. 2006; Braak et al. 2003; Zarow et al. 2003). NE-containing neurons in the LC region project nerve terminals to almost the entire central nervous system (CNS) (Jones and Moore 1977; Sara 2009), including the directive interaction with dopaminergic neurons in SN (Antelman and Caggiula 1993; Collingridge et al. 1979; Robertson et al. 2013). This sequential loss of brain catecholamine neurons raises a possibility that NE release in the SN region may regulate the survival of DA neurons. On the other hand, neuroinflammation has been thought to be one of the key contributors to PD development (Cunningham 2013; Gendelman 2002; Mosley et al. 2012; Phani et al. 2012). Based on these observations, we hypothesized that NE may serve as a critical brain immune regulator and dysfunction or loss of NE neurons may lead to neuroinflammation and subsequent neurodegeneration.
This study aimed to examine the anti-inflammatory/neuroprotective effects of NE during dopaminergic neurodegeneration and to understand its potential mechanisms. We utilized a lipopolysaccharide (LPS)-induced chronic neuroinflammation model of PD (Block et al. 2007; Qin et al. 2007) coupled with depletion of NE using N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4), which selectively depletes NE storage from nerve terminals (Fritschy and Grzanna 1989; Jaim-Etcheverry and Zieher 1980; Jonsson et al. 1981; Ross 1976) including SN (Fornai et al. 1996). In this study, we report that NE depletion greatly enhanced LPS-induced dopaminergic neuron loss in the SN in mice. Further in vitro studies revealed that NE protected dopaminergic neurons from inflammation-mediated neurotoxicity by directly acting on microglia and inhibiting NADPH oxidase (NOX2)-generated superoxide in a β2-AR-independent manner. This study reveals important roles of NE in the CNS, one of which is to maintain neuroimmune homeostasis and the other is to protect neurons from inflammation-mediated damage.
Materials and Methods
Animals
All experimental procedures were performed in strict accordance with the NIH guidelines. Male/female C57BL/6 and CYBB mice (B6.129S-Cybbtm1Din/J) were obtained from The Jackson Laboratory (Bar Harbor, ME). Timed-pregnant Fisher F344 rats were provided by the Charles River Laboratories (Raleigh, NC). Mice deficient in the β2-arenergic receptor (β2-AR; C.FVB-B2-AR<tm1>N10, Cat# 6277) and their background wild-type counterparts Balb/C were purchased from Taconic (Hudson, NY).
Reagents
(−)-Norepinephrine, (S)-(+)-Norepinephrine bitartrate, (L)-(−)-Norepinephrine bitartrate, 1-methyl-4-phenylpyridinium (MPP+), N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4), phentolamine, propranolol, H-89, ICI-118,551 were purchased from Sigma-Aldrich (St. Louis, MO). LPS for in vitrostudies (Escherichia coli strain O111:B4) was purchased from Calbiochem(San Diego, CA; cat# 437627) and for in vivo studies from Sigma-Aldrich (St. Louis, MO; cat#L3012). Cell culture reagents were obtained from Invitrogen(Carlsbad, CA). [3H]DA (30 Ci/mmol) was obtained from PerkinElmer LifeSciences (Boston, MA; cat# NET131250UC). The polyclonal anti-tyrosine hydroxylase (TH) antibody (cat# AB152) and monoclonal neuronal nuclei (NeuN) antibody (cat# MAB377) was purchased from CHEMICON International (Temecula, CA). The polyclonal ionized calcium binding adaptor molecule 1 (Iba-1) antibody was purchased fromWako Chemicals USA (Richmond, VA; cat# 019-19741). The rat anti-mouse CD11b antibody was purchased from AbDSerotec (Raleigh, NC, cat# MCA711G). The biotinylatedsecondaryantibodies were purchased from Vector Laboratories (Burlingame, CA).
Animal Treatment
A single systemic LPS (5 mg/kg, i.p) or vehicle (saline, 5 ml/kg, i.p) injection was administered to 3 month-old male C57BL/6 mice (Qin et al. 2007). Our previous report on the LPS-induced neurodegenerative disease models showed delayed, progressive nigral dopaminergic neurodegeneration, and motor deficits beginning at 6 months after injection (Liu et al. 2008; Qin et al. 2007). Therefore, six months later, DSP-4 (50 mg/kg, i.p.) or vehicle was injected every two weeks (4 times total) to both groups to deplete NE in the brain. DSP-4 is a neurotoxin selective for noradrenergic neurons, capable of crossing the blood-brain barrier and inducing long-term depletion of NE in brain and spinal cord (Jaim-Etcheverry and Zieher, 1980; Daw et al., 1985; Robinson et al., 1993). Two months after the first DSP-4 injection, mice were euthanized, and brains were removed and post-fixed in 4% paraformaldehyde overnight at 4°C. Brains were then placed into 30% sucrose/PBS solution at 4°C until the brains sank to the bottom of the container. Coronal sections including SN pars compacta (SNpc) were cut on a horizontal sliding microtome into 35 μm transverse free-floating sections.
Immunohistochemistry
The free-floating sections were immune-blocked with 4-10% goat serum and then incubated with polyclonal rabbit anti-TH antibody (1:2,000 dilution), or Iba-1 antibody (1: 5,000 dilution) for 48h or 24h at 4 °C, respectively. Antibody binding was visualized using a Vectastain ABC Kit (Vector Laboratories, Inc) and diaminobenzidine substrate.
Stereology
The number of TH-immunoreactive (TH-ir) neurons in the SNpc was estimated using an optical fractionator method that systematically randomizes unbiased counting frames (100 μm × 100 μm) within defined boundaries of the SN (MBF Science) (Wang et al. 2014b; Wang et al. 2012a). Section thickness was determined in a pilot study that showed initial cut thickness at 35μm shrunk to about 20 μm after the staining process. A 11 μm dissector height was used and guard zone was set at 2 μm. Counts were done with an Olympus BX50 microscope using a 60 × 1.4 NA oil immersion objective and the coefficient of error values was less than 0.1.
Confocal double-label immunofluorescence
The free-floating sections were immune-blocked with 4% goat and horse serum in 0.25% triton/PBS for 2h and then incubated with monoclonal mouse anti-NeuN antibody (1:500 dilutions) overnight at 4 °C. On the second day, the sections were washed twice (20 minutes) by 1% BSA in 0.25% triton/PBS before the incubation with polyclonal rabbit anti-TH antibody (1:2,000 dilutions) overnight at 4 °C. The double-label immunofluorescence pictures were taken under the confocal microscope by using Alexa-488 (green) conjugated anti-rabbit and Alexa-594 (red) conjugated anti-mouse secondary antibodies (1:1,000) to visualize the TH-positive and NeuN-positive neurons, respectively (Gao et al. 2011a).
Automated counting of single color images
The number of NeuN positive neurons in the immunofluorescence staining substantia nigra was measured by automated counting of single color images using ImageJ software(Fritschy and Grzanna 1989). This method was based on the protocol created by Christine Labno (University of Chicago, Integrated Light Microscopy Core). Briefly, the color images were firstly converted into grayscale, and then adjust the threshold of the image to highlight all of the structures to be counted. After using the Binary Watershed to separate the merged particles, the total number of cells was analyzed (note to adjust the size of the target particles to avoid the “noise” pixels). The threshold of the image and the size of the counted particles were kept consistent among all the images.
Primary cell cultures
Primary neuron-glia cultures were prepared as described previously (Chen et al. 2013; Liu and Hong 2003). In brief, dissociated cells from the ventral mesencephalon of embryonic day 14 ± 0.5 Fischer 334 rats, Balb/C (wild type) or β2-AR deficient mice were seeded at 5.5 × 105 cells/well (rat) or 6.5 × 105 cells/well (mice) in poly-D-lysine-coated 24-well plates, respectively. The cultures were maintained at 37°C in the incubator with 5% CO2 and 95% air in minimum essential medium. The cultures were ready for experiments seven days later, when the cultures became mature and stable of each cell component (astrocytes ~50 %, neurons ~40 % and microglia ~10 %).
Microglia depleted neuron-glia cultures were obtained by depleting microglia in neuron-glia cultures with 1.5mM of leucine methyl ester 48 h after seeding, as described previously (Qian et al. 2006).
Mixed-glia cultures were prepared from whole brains of postnatal day1 rat or mouse as reported before (Gao et al. 2002). Briefly, disassociated cells were seeded into 24-well (1×105/well) or 96-well (5×104/well) culture plates and maintained in 1 ml/well or 0.2 ml/well of DMEM/F-12 medium. The medium was changed every 3 days. On 11–12 days after plating, the cultures were mature and stable with different cell components (astrocytes ~80 %, GFAP immunopositive cells; microglia ~20 % OX-42 immunopositive cells) ready for drug treatment or superoxide assay.
Cell lines
The transfected monkey kidney COS7-PHOX cell line, stably expressing with NOX2, was a gift from Dr. Mary Dinauer (Indiana University, IN) (Suh et al. 2006). Briefly, COS7-PHOX cell line were maintained at 37 °C in DMEM (Dulbecco's modified Eagle's medium; Sigma) supplemented with 10% fetal bovine serum, 50 U/ml penicillin, 50 μg/ml streptomycin, 0.8 mg/mL geneticin (Gibco), 4 μg/ml puromycin (Inviogen), and 0.35 mg/ml hygromycin B (Invitrogen) in a humidified incubator with 5% CO2 and 95% air. The cells were split or harvested every 3-5 days.
The rat microglia HAPI cell line was a gift from Dr. J. R. Connor (Pennsylvania State University, Hershey, PA) (Cheepsunthorn et al. 2001) and maintained as described previously (Qian et al. 2008b).
[3H]DA uptake assay
[3H]dopamine (DA) uptake assays were performed as described previously (Liu et al. 2000). Briefly, cells were incubated for 21 minutes at 37°C with 1 μM [3H]DA (PerkinElmer Life Sciences) in Krebs-Ringer buffer. Cells were washed with ice-cold Krebs-Ringer buffer three times, and then were collected in 1 N NaOH. Radioactivity was determined by liquid scintillation counting. Nonspecific dopamine (DA) uptake observed in the presence of mazindol (10 μM) was subtracted.
Immunocytochemistry
Immunostaining was performed as previously described (Wang et al. 2014b)with antibody specific for TH (1: 2,000) or Iba-1(1: 5,000). Images were recorded with a CCD camera and the MetaMorph software (Molecular Devices). TH-ir neuron or Iba-1ir microglia number were counted according to published protocol (Qian et al. 2011) and was carried out by at least two investigators without knowledge of the treatment groups. For each experiment, three to six wells per treatment condition were used, and results from five to six independent experiments were obtained.
Nitric oxide and TNF-α assays
The production of nitric oxide (NO) was determined by measuring accumulated levels of nitrite in the supernatant with Griess reagent, and the release of tumor necrosis factor-α (TNF-α) was measured with a TNF-α ELISA kit from R&D Systems (Minneapolis, MN) following manufacture's protocol.
Measurement of superoxide
The production of superoxide was assessed by measuring the SOD-inhibitable reduction of the tetrazolium salt WST-1(Qin et al. 2002; Wang et al. 2012b). Briefly, primary mixed-glia cultures or COS7-PHOX cell line were washed twice with HBSS balanced salt solution and then pre-treated with indicated concentrations of NE dissolved in HBSS for 15 minutes. Immediately after the addition of LPS or PMA, 50 μl of HBSS with and without SOD (50 U/ml) was added to each well along with 50 μl of WST-1 (1 mM) in HBSS. The absorbance at 450 nm was read with a SpectraMax Plus microplate spectrophotometer (Molecular DevicesSunnyvale). The amount of SOD-inhibitable superoxide was calculated and expressed as percentage of vehicle-treated control cultures.
Membrane fractionation and Western blot analysis
HAPI microglia were lysed in hypotonic lysis buffer (1 mMTris, 1 mMKCl, 1 mM EGTA, 1 mM EDTA, 0.1 mM DTT, 1 mM PMSF, and 10 μg/ml cocktail protease inhibitor), and then subjected to Dounce homogenization (20-25 stokes, tight pestle A). The lysates were centrifuged at 1, 600 × g for 15 minutes; the supernatant was centrifuged at 100, 000 × g for 30 minutes. The pellets solubilized in 1% Nonidet P-40 hypotonic lysis buffer were used as membranous fraction. For Western blot analysis, equal amounts of protein were separated by 4-12% Bis-Tris Nu-PAGE gel and transferred to polyvinylidenedifluoride membranes. The membranes were blocked with 5% non-fat milk and incubated with rabbit antibody against p47phox (1:1000, Millipore, cat# 07-500), gp91phox (1: 1000, BD Transduction Laboratories, cat# 611414), or GAPDH (1: 1000, Abcam, cat# ab8245) overnight at 4°C. The next day, membranes were incubated with HRP-linked secondary anti-rabbit or mouse IgG (1:3,000) for 2 hours at room temperature. ECL reagents (Amersham Biosciences) were used as a detection system.
Xanthine/xanthine oxidase reaction
To determine whether NE acts as a superoxide scavenger, the superoxide-generating xanthine/xanthine oxidase system was used as described previously (Wang et al. 2012b). Assays were performed in the presence of indicated concentrations of NE, 0.01 U xanthine oxidase, 50 μM xanthine, 250 μM WST-1 in 50 mM potassium phosphate buffer (pH 7.6) in a 96-well plate (100 μL/well final volume). Xanthine was added to initiate the reaction, and absorbance at 450 nm was continuously monitored for 5 minutes using a Synergy HT multi-detection microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Statistical analysis
All group data are expressed as mean ± SEM. Group means were compared using one- or two-way ANOVA with treatment as the independent variable. When ANOVA showed a significant difference, pair wise comparisons between group means were examined by post hoc analysis by Tukey's multiple comparisons test. Statistical analysis was performed using GraphPad Prism version 6.00 for Windows with two sided α of 0.05.
Results
NE depletion enhanced the loss of nigral dopaminergic neurons in LPS-injected mice
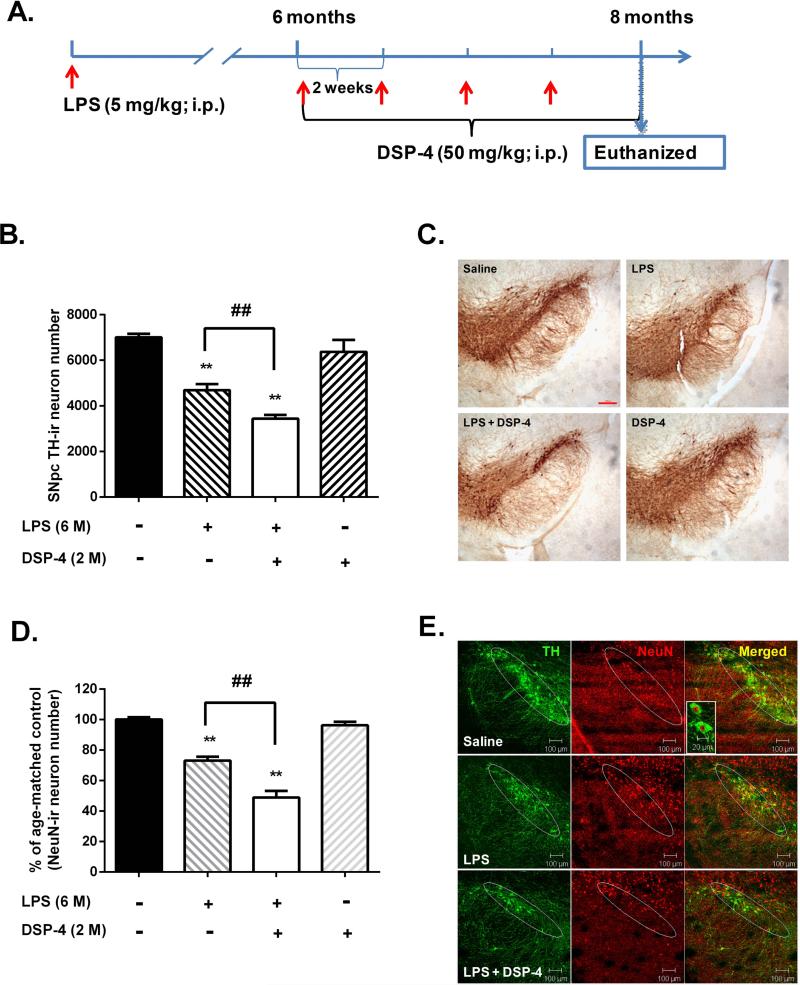
To demonstrate the neuroprotective effect of endogenous NE, we studied the consequence of NE depletion induced by DSP-4 on LPS-induced nigral dopaminergic neurodegeneration. . In this study, 6months after a single systemic LPS (5 mg/kg; i.p.) injection, mice received another 4 injections of DSP-4 at biweekly intervals (Fig. 1A). Analysis of brain monoamine content by HPLC showed that NE decreased by 60% in midbrain whereas the 5-HT or dopamine level was not influenced in DSP-4 injected mice.Comparable to our previous report (Qin et al. 2007), immunohistochemical analysis revealed a 30% loss of nigral dopaminergic neuron in mice 8 months after a single LPS injection as compared to saline-injected controls. Interestingly, in agreement with our hypothesis, depletion of NE by DSP-4 following 6months of LPS injection enhanced the LPS-induced loss of nigral dopaminergic neurons to 50% (Fig. 1B). Observations of the TH staining revealed that toxins-induced neuronal changes were not limited to the cell bodies of neurons, but the neuritis in the SN pars reticulata region(Fig. 1C).Double-labeling with anti-TH and NeuN antibodies revealed a similar decrease of NeuN-immunoreactive neurons compared with TH staining in the SNpc of LPS- and LPS + DSP-4-treated mice (Fig. 1D-E), indicating that LPS induced loss of TH-immunoreactive neurons was not due to the failure of neurons to express TH but represented the death of neurons.This finding suggests that the release of NE in the SN region may exert a protective effect on DA neurons from LPS-elicited neuroinflammatory damage.
Figure 1. NE depletion produces a greater loss of nigral dopaminergic neurons in LPS-injected mice.
(A) Schematic drawing of the treatment regimen for LPS and DSP-4. Six months after an initial single systemic injection of LPS, mice received 4 repeated injections of DSP-4 at two-week intervals. Two-week after the last injection of DSP-4, mice were sacrificed for immunohistological staining of nigral sections. (B) Quantitative measurement of TH-ir cells of the SN by stereological analysis. Counts were expressed as the total TH-ir cell numbers per SN and were of Mean ± SEM from 3 animals for each group. LPS injection alone caused 30% dopaminergic neuron loss (4691 ± 152 TH-ir neurons; n=3) compared with saline (7012 ± 88 TH-ir neurons; n=3). And depletion of NE by DSP-4 enhanced the neuronal loss into 50% (3439 ± 94 TH-ir neurons; n=3) (one-way ANOVA; F (3, 8) = 153.5, p < 0.0001; post hoc analysis by Tukey's multiple comparisons test). **p< 0.01 compared with saline control group; ##p < 0.01 compared with LPS- treated group. (C) Immunohistological staining showed that depletion of brain NE with DSP-4 produced a greater loss of both the number of cell bodies and neuritis of dopaminergic neurons (TH-ir cells) in LPS-treated mice. Scale bar, 200 μm. (D) The quantitative measurements of Neu-Nir neuronal loss were performed by auto-counting using ImageJ software on the pictures of fluorescence staining. The results are expressed as the percentage of time-matched saline controls and are Mean ± SEM from 3 mice. (E) Representative pictures of double immunofluroscence staining showed that the loss of Neu-Nir neurons (red) were comparable to the decrease of THir neurons (green) in LPS or LPS + DSP-4 groups compare to saline controls.
Sub-micromolar concentrations of NE protect dopaminergic neurons against LPS-induced neurotoxicity in midbrain neuron-glia cultures
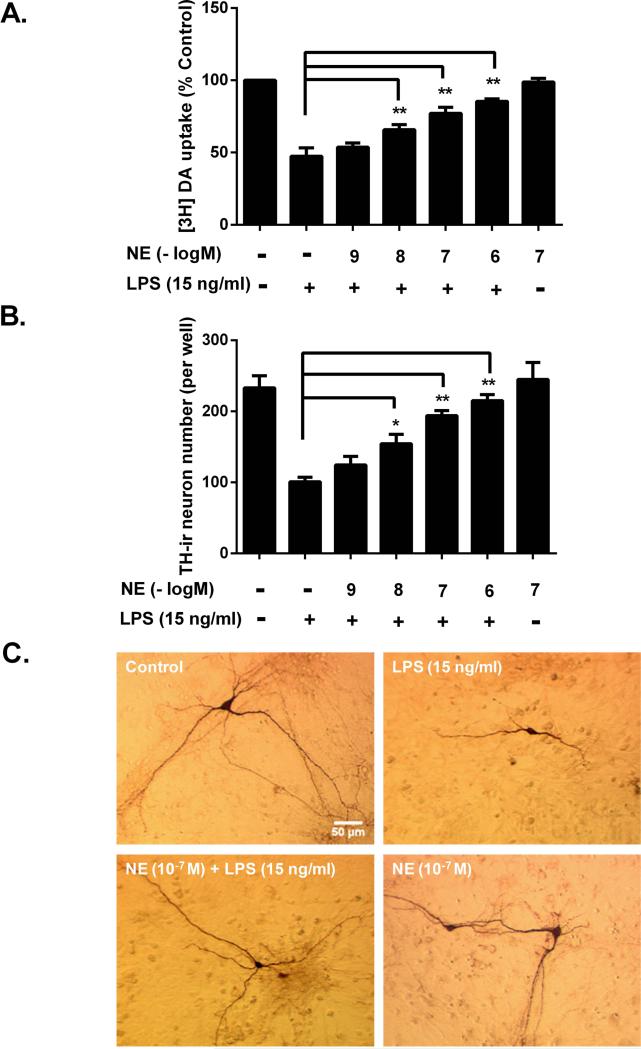
To elucidate the mechanism for the neuroprotective effects of NE in mice as shown in Fig.1, neuron-glia cultures, which do not contain NE-containing neurons, were pretreated with exogenous NE (10−9-10−6 M) for 30 minutes and subsequently stimulated with LPS (15 ng/ml). Seven days later, damage of dopaminergic neuron function was assessed by evaluating DA uptake capacity and loss of dopaminergic neurons was accessed by cell counting. The [3H]DA uptake result showed that NE pretreatment restored the culture DA uptake capacity in a dose-dependent manner, indicating that NE significantly attenuated LPS-induced dopaminergic neurotoxicity (Fig. 2A). This conclusion was further corroborated with the results from cell count, which revealed that pretreatment of NE dose-dependently mitigated the LPS-induced decrease in the number of TH-ir neurons (Fig. 2B). In addition to restoring the numbers of dopaminergic neurons, NE also rescued the dopaminergic neurite retraction and degradation associated with inflammation-mediated dopaminergic neurotoxicity (Fig. 2C). Taken together, these two measurements clearly demonstrated the neuroprotective effects of NE on LPS-induced DA neurotoxicity. Since 10−7 and 10−6 M NE showed comparable potency of neuroprotection, 10−7 M NE was primarily used for subsequent studies.
Figure 2. Sub-micromolar concentrations of NE protects cultured dopaminergic neurons from inflammation-driven neurodegeneration.
Rat mesencephalic neuron-glia cultures were pretreated with vehicle control or indicated concentrations of NE for 30 minutes before the addition of LPS (15 ng/ml). Seven days later, inflammation-elicited damage of dopaminergic neurons were assessed by quantifying functional changes with [3H]DA uptake assay and loss of TH-immunoreactive neurons. (A) NE pretreatment restored [3H]DA uptake capacity in a dose-dependent manner (53.6 ± 1.4, 65.8 ± 1.6, 77.2 ± 1.8, 88.5 ± 0.8 % of control at 10−9, 10−8, 10−7 and 10−6 M NE, respectively; n = 5) compared with LPS alone group (47.4 ± 1.7% of control; n = 12) (one-way ANOVA; F (6, 42) = 276.5, p < 0.0001; post hoc analysis by Tukey's multiple comparisons test). (B) Cell count showed pretreatment of NE dose-dependently mitigated LPS-induced TH-ir neurons number decrease (125 ± 5, 154 ± 6, 194 ± 3, 215 ± 4 per well at 10−9, 10−8, 10−7 and 10−6 M NE, respectively; n = 5) compared with LPS alone group (101 ± 3 per well; n = 5) (one-way ANOVA; F (6, 28) = 79.08, p<0.0001; post hoc analysis by Tukey's multiple comparisons test). (C) Immunostaining showed clear reduced neurite numbers and shortened processes of dopaminergic neurons in LPS-treated group. Results in (A) and (B) are means ± SEM from 5 independent experiments in triplicate.*p< 0.05, **p< 0.01 compare with LPS-treated cultures. Scale bar, 50 μm.
Microglia are essential for sub-micromolar NE-mediated dopaminergic neuroprotection
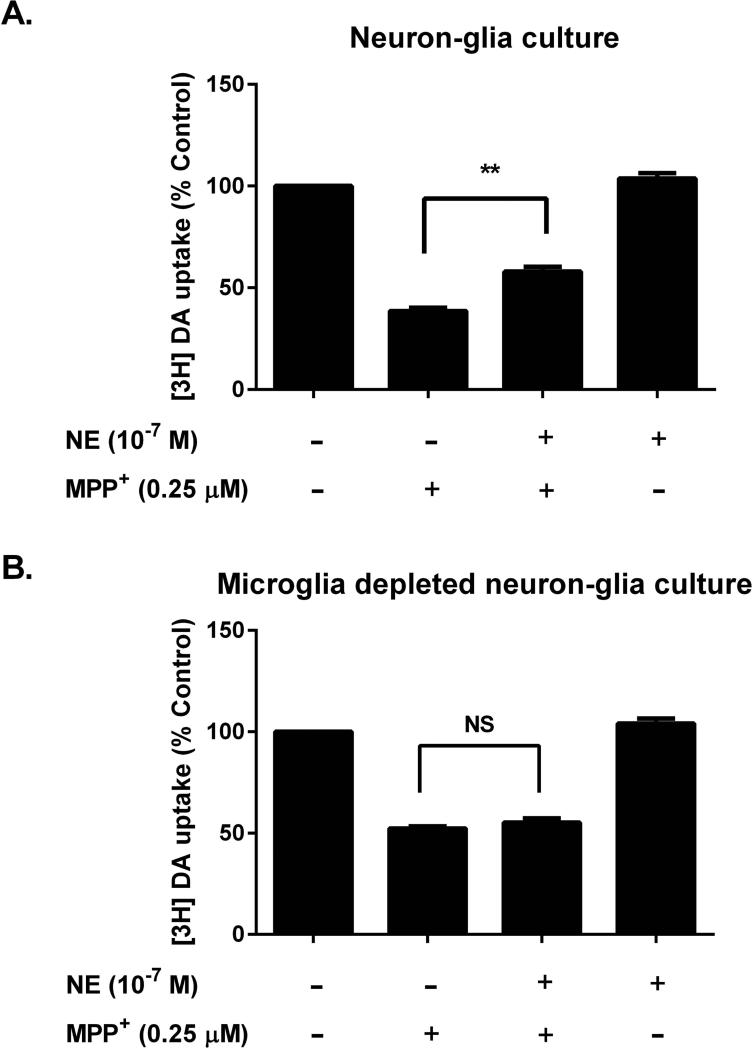
To determine whether the neuroprotective effect of NE is dependent on the presence of microglia, we compared the neuroprotective potency of NE in two different kinds of culture: 1) normal neuron-glia cultures, which contain mainly neurons, astroglia and microglia; and 2) microglia-depleted neuron-glia cultures(Qian et al. 2006). Since LPS is not able to directly damage neurons without the presence of microglia, we used MPP+, an active metabolite of MPTP, as a toxin to produce dopaminergic neurotoxicity in this study. Unlike LPS, MPP+ is known to cause dopaminergic neuron damage by dual mechanisms: 1) direct neurotoxicity through the inhibition of mitochondrial complex I; and 2) generation of reactive microgliosis through the release of cytotoxic factors from damaged neurons (Block and Hong 2005).Treatment with NE (10−7 M) reversed the MPP+-induced [3H]DA uptake decrease by 20%in neuron-glia culture (Fig. 3A), but showed no protective effect in microglia-depleted neuron-glia cultures (Fig. 3B). This finding suggests that the partial NE-mediated dopaminergic neuroprotection required the presence of microglia, most likely through the inhibition of reactive microgliosis.
Figure 3. Microglia are essential for sub-micromolar NE-mediated dopaminergic neuroprotection.
Mesencephalic neuron-glia (which contains mainly neurons, astroglia and about 10% microglia) and microglia-depleted neuron-glia cultures (microglia-depleted N-G, which contains neuron, astroglia and less than 0.01% of microglia) were pretreated with either vehicle or NE (10−7 M) for 30 min prior to the addition of MPP+ (0.25 μM). Seven days later, [3H]DA uptake assays were performed for the measurement of dopaminergic neuron function. (A) In neuron-glia culture, NE pretreatment restored MPP+-induced [3H]DA uptake decrease by 20% (57.9 ± 2.4 % of control; n = 3) compared with MPP+ alone group (38.5 ± 1.7 % of control; n = 3) (one-way ANOVA, F (3, 8) = 271.4, p < 0.0001; post hoc analysis by Tukey's multiple comparisons test). (B) In microglia- depleted neuron-glia cultures, NE pretreatment had no protective effect (one-way ANOVA, F (3, 8) = 269.3, p < 0.0001; post hoc analysis by Tukey's multiple comparisons test, p > 0.05). Results were expressed as a percentage of the vehicle treated control cultures and were the means ± SEM from three independent experiments in triplicate. **p< 0.01 compare with the MPP+-treated group.
Sub-micromolar NE attenuates LPS-induced microglial activation and pro-inflammatory factor release
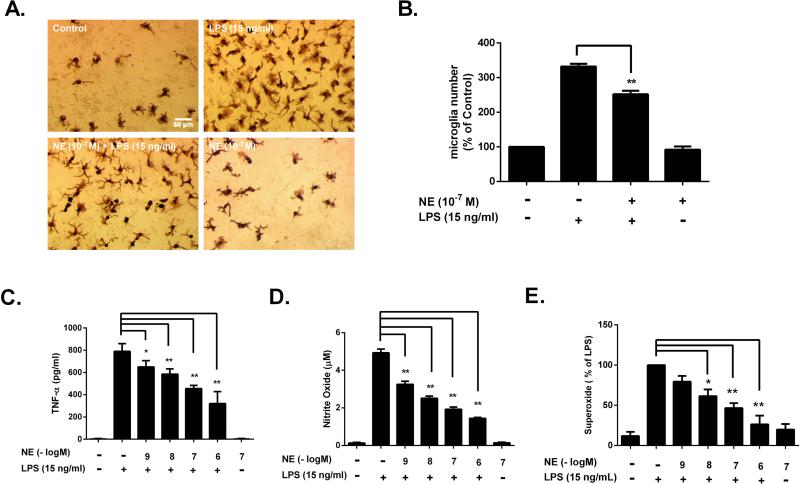
Chronic microglial activation and the release of cytotoxic pro-inflammatory factors are essential for inflammation-mediated dopaminergic neurotoxicity (Block et al. 2007; Qin et al. 2004). To determine whether NE inhibits microglial activation, neuron-glia cultures were pretreated with NE (10−7 M) for 30 minutes followed by addition of LPS. Microglial morphology and pro-inflammatory factors were assessed at different time points following LPS treatment. Immunocytochemistry of Iba-1, a microglia marker whose expression is up-regulated during microglial activation (Ito et al. 1998), showed that 24 hours after NE exposure, microglia reverted from hypertrophied cells (i.e., fewer projections, enlarged soma) in response to LPS activation to a ramified morphology (i.e., numerous projections, small soma) that indicates a quiescent state (Fig. 4A). Further, cellular quantification showed that NE pretreatment significantly suppressed the up-regulated expression of Iba-1 and thus reduced the number of Iba-1-positive cell after LPS stimulation (Fig. 4B). It is important to note that the decrease in Iba-1positive cells was not due to the microglial cytotoxicity caused by NE. Instead, it was because of the decrease of Iba-1immunoreactivity in some microglia, which were below the sensitivity of immunostaining. This conclusion was supported by estimating the number of viable microglia by MTT assay, which indicated that sub-micromolar concentrations of NE did not produce cytotoxicity (data not shown).
Figure 4. Sub-micromolar NE attenuates LPS-induced microglial activation and release of pro-inflammatory factors.
Rat primary mesencephalic neuron-glia cultures were pretreated with NE for 30 minutes before LPS stimulation. (A) Microglia morphology was assessed using an antibody against Iba-1 at 24 hours after LPS treatment. (B) The number of Iba-1-immunoreactive cells of each well was counted under the microscope. NE pretreatment reduced the number of Iba-1-positive cell in NE/LPS group (251.7 ± 10.3 % of control; n = 5) compared to LPS alone group (332.2 ± 7.6 % of control; n = 5) (one-way ANOVA, F (3, 16) = 221.5, p <0.0001; post hoc analysis by Tukey's multiple comparisons test). Data was shown as the percentage of control and expressed as the means ± SEM from 5 independent experiments in triplicate. (C) The concentrations of TNF-αin the supernatant of neuron-glia culture were determined after 3h of LPS treatment. The result showed that NE pretreatment reduced TNF-α production in a dose-dependent manner (650.0 ± 25.1, 585.4 ± 21.0, 454.3 ± 13.2, 320.8 ± 48.1 pg/ml at 10−9, 10−8, 10−7 and 10−6 M NE, respectively versus LPS alone 790.0 ± 22.1 pg/ml; n = 5) (one-way ANOVA, F (6, 43) = 318.1, p < 0.0001; post hoc analysis by Tukey's multiple comparisons test). (D) The concentrations of NO in the supernatant of neuron-glia culture were determined after 24h of LPS treatment. The result showed that NE pretreatment reduced NO in a dose-dependent manner (3.25 ± 0.16, 2.51 ± 0.12, 1.93 ± 0.11, 1.44 ± 0.06 μM at 10−9, 10−8, 10−7 and 10−6 M, respectively versus LPS alone group 4.93 ± 0.20 μM; n = 5) (one-way ANOVA, F(6, 43) = 234.7, p < 0.0001; post hoc analysis by Tukey's multiple comparisons test).(E) The production of superoxide was measured by SOD-inhibitable reduction of WST-1in rat primary mix-glia culture prepared from postnatal day-1 pups. The result showed that NE pretreatment inhibited superoxide production in a dose-dependent manner (79.4 ± 7.1, 61.4 ± 8.2, 46.7 ± 6.0, 26.4 ± 10.8 % of LPS-treated group at 10−9, 10−8, 10−7 and 10−6 M, respectively versus LPS alone group) (one-way ANOVA, F (6, 27) = 23.98, p < 0.0001; post hoc analysis by Tukey's multiple comparisons test). Results were expressed as the means ± SEM from 5 independent experiments in triplicate. *p< 0.05, **p< 0.01 compare with the LPS-treated cultures.
In addition to morphological observations, the inhibitory effects of NE on the release of pro-inflammatory factors from microglia were measured. The release of the pro-inflammatory factors superoxide (15 minutes), TNF-α (3h) and NO (24h) were determined in supernatant at their individual peak release time (Qin et al. 2007). Pre-treatment of NE markedly reduced the production of TNF-α and inhibited the release of both nitric oxide (measured as nitrite) (Fig. 4D) and superoxide (Fig. 4E) in a dose-dependent manner. We have previously reported that the release of proinflammatory factors from microglia was the driving force causing LPS-induced neurodegeneration. Thus, we conclude that NE reduced inflammation-driven dopaminergic neurotoxicity by inhibiting the release of pro-inflammatory factors during microglia activation.
β2-AR is not the sole mechanism mediating the anti-inflammatory and neuroprotective effects of sub-micromolar NE
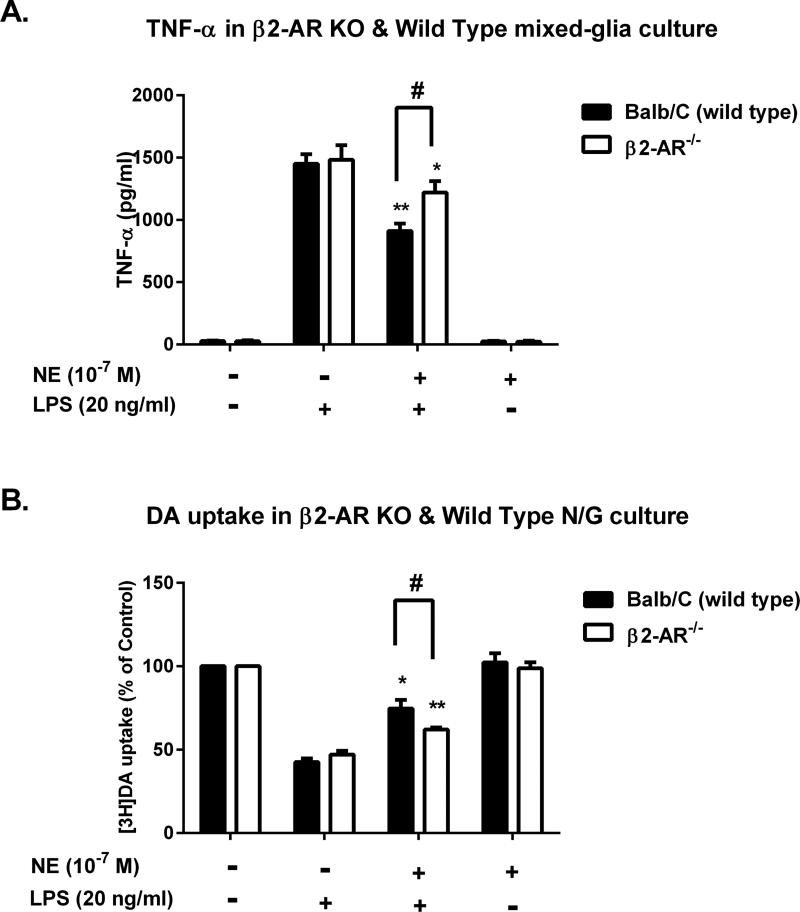
Expression of mRNA of different adrenergic receptor subtypes α1, α2, β1, and β2 was found in rat microglia(Mori et al. 2002). In micromolar concentrations, β2 receptor are reported to be the major site mediating the anti-inflammatory effect of NE in the brain (Mori et al. 2002). To examine the involvement ofβ2 receptorin the anti-inflammatory effects of sub-micromolar NE, mixed-glia cultures were prepared from wild type (WT) and β2-AR−/− mice. We use the mix-glia culture instead of neuron-glia mainly for the purpose to determine the TNF-α production of microglia in response to LPS stimulation to avoid any influence from neurons. The cultures were pre-treated with 10−7 M NE or vehicle control before LPS stimulation. To obtain similar levels of LPS-induced neurotoxicity between rat and mouse cultures, we have adjusted the LPS concentration to 20 ng/ml in mouse primary cultures. Surprisingly, we found that compared to WT, the NE-elicited inhibitory effect of LPS-induced TNF-α release was only partially reversed in the β2-AR−/− culture (40% in WT vs. 20% in β2-AR−/−), suggesting that another signaling pathway may mediate the NE-elicited reduction in LPS-induced TNF-α (Fig. 5A). Consistently, measurement of [3H]DA uptake capacity in midbrain neuron-glia cultures also confirmed that the neuroprotective effect of NE was only partially affected by β2-AR deletion. LPS caused more than a 50% reduction in DA uptake capacity in both WT and β2-AR−/− cultures, which was reversed by NE to 80% in WT, but only 65% in β2-AR−/− cultures (Fig. 5B). Also different from the previous report, which showed increased production of intracellular cAMP level in microglia in micromolar concentrations of both NE and β2 agonist, we found that sub-micromolar NE failed to induce cAMP production (Supplementary Fig. 1). Taken together, the data indicates that the signaling pathways mediating the anti-inflammatory/neuroprotective effects of sub-micromolar NE are different from that of high concentrations of NE (>10−6 M), suggesting that a β2-AR-independent pathway was involved in mediating the anti-inflammatory/neuroprotective effects of sub-micromolar NE.
Figure 5. β2-AR is partially involved in the anti-inflammatory and neuroprotective effects of sub-micromolar NE.
Mixed-glia and mesencephalic neuron-glia cultures prepared from β2-adrenergic receptor (β2-AR)-deficient mice or Balb/C mice (wild type, WT) were pretreated with NE (10−7 M) for 30 minutes prior to LPS challenge. (A) Three hours later, the levels of TNF-α in the mixed-glia supernatant were determined using commercial ELISA kit. The inhibitory effect of NE was reduced in the β2-AR−/− culture (NE/LPS 1219.4 ± 52.64 versus LPS 1482.44 ± 67.03 pg/ml; n = 3) compared to WT (NE/LPS 911.09 ± 34.66 versus LPS 1450.98 ± 44.39 pg/ml; n = 3), but still had 20% significant change (two-way ANOVA, F (3, 16) = 8.577, p = 0.0013; post hoc analysis by Tukey's multiple comparison test). Results were expressed as means ± SEM from 3 independent experiments in triplicate. (B) The effects of NE on [3H]DA uptake capacity were measured after 7 days following LPS treatment in neuron-glia cultures and the results were expressed as a percentage of the control cultures. The result showed that the protective effect in the β2-AR−/− culture was 15% (NE/LPS 62.0 ± 0.8 % versus LPS 47.0 ± 1.3 % of control; n = 3) whereas it was 38% in the WT culture (NE/LPS 80.8 ± 8.9 % versus LPS 42.6 ± 1.3 % of control; n = 3) (two-way ANOVA, F (3, 16) = 7.712, p = 0.0021; post hoc analysis by Tukey's multiple comparison test). Results were expressed as the means ± SEM from 3 independent experiments in triplicate. *p< 0.05, **p< 0.01 compared with the corresponding LPS alone cultures, #p < 0.05 compared to WT.
Sub-micromolar NE inhibits NOX2-generated superoxide in an adrenergic receptor-independent manner
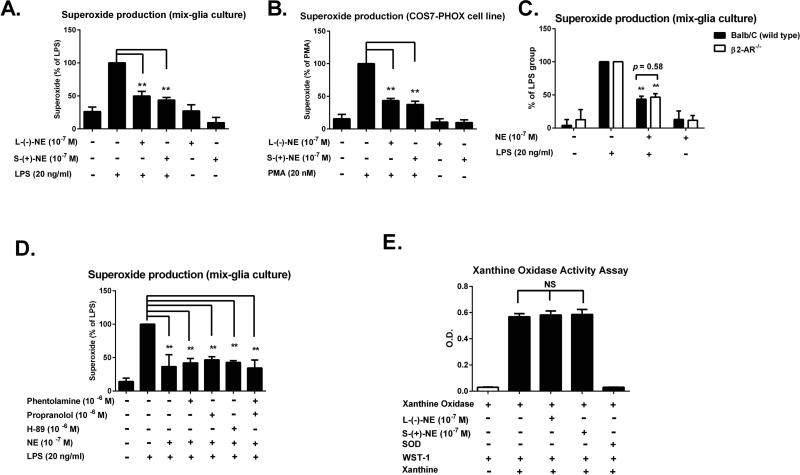
The partial involvement of the β2-AR in the anti-inflammatory effects of NE in microglia prompted us to explore other pathways that may contribute to the modulation of neuroinflammation by NE. We chose to focus on the superoxide-producing enzyme NOX2 because of its reported critical role in microglia-mediated inflammation and neurotoxicity (Qin et al. 2013; Qin et al. 2004). Our previous work indicated that microglial NOX2 was the main source of LPS-mediated superoxide production (Wang et al. 2004).We discovered that pretreatment with NE significantly inhibited the release of LPS-induced superoxide in a dose-dependent manner (Fig. 4E). We further evaluated the potential role of adrenergic receptors in modulating NE-dependent superoxide inhibition by comparing two NE optic isomers, which display more than 100-fold difference in adrenergic receptors binding affinity: (−)-NE, an active isomer; (+)-NE, an inactive isomer (Bylund and Snyder 1976; Deupree and Kennedy 1979). In LPS-treated mixed-glia cultures, we found that (+)-NE and (−)-NE were equi-potent in inhibiting LPS-induced superoxide production (Fig. 6A). This adrenergic receptor-independent inhibitory action of NE on superoxide production was further demonstrated in COS7 cells, which expressed very low levels of adrenergic receptor subtypes (Regan et al. 1988; Schwinn et al. 1990; Strader et al. 1987). Both NE isomers displayed similar potency in inhibiting phorbolmyristate acetate (PMA)-induced superoxide in COS7 cells transfected with all the essential subunits of NOX2 (Mizrahi et al. 2006)(Fig. 6B). Moreover, we found that NE exerted a similar potency of superoxide inhibitory effect in mixed-glia cultures prepared from both wild type control and β2-AR−/− mice (Fig. 6C). Furthermore, we ruled out the possibility that other types of adrenergic receptors besides the β2-AR were involved in mediating NE-elicited superoxide production. Pretreatment with non-selective α1 (phentolamine) or β1 (propranolol)-AR antagonists either alone or in combination failed to influence the NE elicited inhibition of superoxide production in LPS-stimulated mix-glia cultures (Fig. 6D). Since PKA is a common pathway mediating most of the adrenergic receptor signaling, we also eliminated this possibility by showing that a PKA inhibitor H-89 did not affect the inhibitory effect of NE on superoxide production (Fig. 6D).
Figure 6. Sub-micromolar NE inhibits NOX2-generated superoxide in a β2-AR-independent manner.
(A-B) Mixed-glia cultures prepared from rat postnatal day-1 pup, or COS7-PHOX cells transfected with all subunits of NOX2 were pre-treated with NE, including (−)- or (+)-isomers, for 15 minutes before LPS or phorbolmyristate acetate (PMA) stimulation, respectively. The production of extracellular superoxide was detected by SOD-inhibitable reduction of WST-1. (A) In LPS-treated mixed-glia cultures, (+)-NE and (−)-NE were equi-potent in inhibiting LPS-induced superoxide production ((−)-NE 49.8 ± 7.2 versus (+)-NE 43.7 ± 4.0 % of LPS alone; n = 5) (one-way ANOVA, F (5, 24) = 22.03, p< 0.0001;post hoc analysis by Tukey's multiple comparison test). Results were expressed as the percentage of LPS alone-treated group and were the means ± SEM from 5 independent experiments in triplicate. **p< 0.01 compare with the LPS-treated cultures. (B) In phorbolmyristate acetate (PMA)-treatedCOS7-NOX2 cells, (+)-NE and (−)-NE also showed the comparable potency in inhibiting superoxide production ((−)-NE 43.5 ± 3.3 versus (+)-NE 37.4 ± 5.2 % of LPS alone; n = 5) (one-way ANOVA, F (5, 24) = 55.07, p< 0.0001; post hoc analysis by Tukey's multiple comparison test). Results were expressed as the percentage of PMA alone-treated group and were the means ± SEM from 5 independent experiments in triplicate. **p< 0.01 compare with the PMA-treated cultures. (C) Mixed-glia cultures prepared from β2-AR−/− and WT mice were pretreated with NE for 15 minutes before the addition of LPS. The effects of NE on superoxide production were determined. The similar superoxide inhibition was found in wild type (43.6 ± 1.9 % of LPS alone; n = 5) and β2-AR−/−mice (46.6 ± 2.4 % of LPS alone; n = 5)(two-way ANOVA, F (3, 32) = 0.6678, p = 0.5780; post hoc analysis by Tukey's multiple comparison test). Results were expressed as the percentage of LPS alone-treated group and were the means ± SEM from 5 independent experiments in triplicate. **p< 0.01 compare with the LPS-treated cultures. (D) Mixed-glia cultures prepared from rat postnatal day-1 pup were incubated with the non-selective α-AR blocker phentolamine or β-AR blocker propranolol alone or in combination for 15 minutes before the pretreatment of NE. Fifteen minutes after NE addition, the production of LPS-induced extracellular superoxide was measured. A similar experiment was performed with a protein kinase A (PKA) inhibitor H-89. Blockade of adrenergic receptors or inhibition of PKA didn't affect the inhibitory effect of NE on LPS-induced superoxide production (one-way ANOVA, F (6, 14) = 8.652, p = 0.0005;post hoc analysis by Tukey's multiple comparison test). Results were expressed as the percentage of LPS alone-treated group and were the means ± SEM from 3 independent experiments in triplicate. **p< 0.01 compare with the LPS-treated cultures. (E) NE isomers failed to inhibit xanthine/xanthine oxidase-generated superoxide.(−)- or (+)-NE, xanthine oxidase (10 mU), and WST-1 (250 μM) were mixed in potassium phosphate buffer (PBS, 50 mM, pH 7.6). Xanthine (50 μM, final concentration) was added to initiate the reaction (final volume, 1 ml). Absorbance at 450 nm was continuously monitored. Results are expressed as the means of O.D. value ± SEM from 5 independent experiments in triplicate. Either (−)-NE or (+)-NE failed to scavenge the produced free radical compared to positive control SOD.
Finally, we also addressed the question whether the suppression of LPS-induced superoxide production could be due to the scavenging effect of NE. NE was reported to display free radical scavenging activity in minimolar (10−4 −10−3M) concentrations (Liu and Mori 1993). In our study, stoichiometrically, we believe that the sub-micromolar concentrations of NE were not sufficient to clear micromolar concentrations of superoxide by scavenging properties. To obtain further support, we evaluated the effects of NE isomers on superoxide production using a xanthine-xanthine oxidase systemtorule out the possibility that the inhibitory effects of NE on superoxidewere attributed to its radical scavenging properties. The increased superoxide levels induced by the xanthine-xanthine oxidase system was largely inhibited by SOD, but not by both NE isomers (Fig. 6E), indicating that the NE-inhibited superoxideproduction was not due to its superoxidescavenging capacity. Taken together, these results revealed that NOX2/superoxide is a novel pathway in mediating NE-elicited inhibition of microglial activation, which is independent of adrenergic receptors.
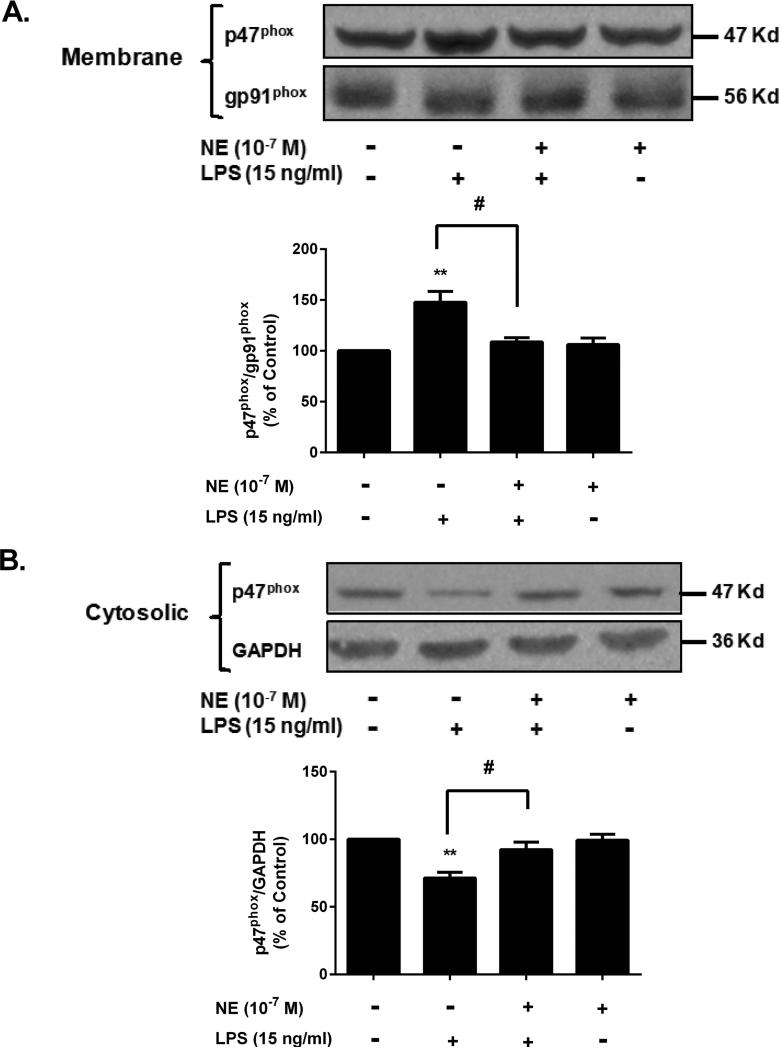
NE prevents LPS-induced membrane translocation of the cytosolic subunit p47phox
To elucidate the detailed molecular mechanism by which NE suppresses superoxide production by NOX2, we examined the effects of NE on the membrane translocation of cytosolic subunit p47phox, which is required for NOX2 activation (Koshkin et al. 1996). For this purpose, rat microglial cell line (HAPI cells) was used. The reasons why we used cell lines instead of enriched microglia were: 1) primary enriched microglia are known to be short-lived and become activated in the condition without the presence of either neurons or astrocytes; 2) the high purity and long-lived nature of HAPI cell line. Western blot analysis showed that LPS significantly reduced the immunoreactivity of p47phox in the cytosol fraction (Fig. 7A), but increased the amount in the membrane (Fig. 7B), indicating an increase in the translocation of cytosolic subunits of NOX2. This increase in membrane translocation was prevented by the pretreatment with NE. We had attempted to show a possible direct interaction of NE to NOX2 by a binding assay using [3H] NE, but unfortunately, we were not able to draw a definite conclusion due to a high non-specific binding. Nonetheless, the collective findings suggest that a NE-induced decrease in the translocation of cytosolic p47phox to the cell membrane is the mechanism by which NE suppresses superoxide production by NOX2.
Figure 7. Sub-micromolar NE prevents LPS-induced membrane translocation of the cytosolic subunit p47phox.
HAPI microglia cells were pretreated with NE for 30 minutes and followed by LPS stimulation. Fifteen minutes later, cells were harvested, and the fractions of membrane and cytosol were isolated for Western blot analysis of p47phoxlevels. GAPDH and gp91phoxwere used for internal cytosolic and membrane controls, respectively. The band density including cytosolic and membrane fractions were quantified. (A) The results showed that NE pretreatment inhibited the LPS-induced p47phox increase in cell membrane (NE/LPS 108.8 ± 4.1 % of control vs. LPS alone 147.7 ± 10.7 % of control; n = 3) (one-way ANOVA, F (3, 8) = 10.70, p = 0.0036; post hoc analysis by Tukey's multiple comparison test). (B) The band showed the decreased level of p47phox in cytosolic. The quantified data described 20% decrease of p47phox after LPS stimulation, but not NE pre-treated group (NE/LPS 92.3 ± 5.6 % of control vs. LPS 71.3 ± 4.2 % of control; n = 3)(one-way ANOVA, F (3, 8) = 10.43, p = 0.0039; post hoc analysis by Tukey's multiple comparison test). The results were expressed as a percentage of the control and were mean ± SEM from three independent experiments performed in triplicate.**p< 0.01 compared with control group, #p< 0.05 compared with LPS alone group.
NOX2 mediates β2-AR-independent anti-inflammatory/neuroprotective effects of sub-micromolarNE
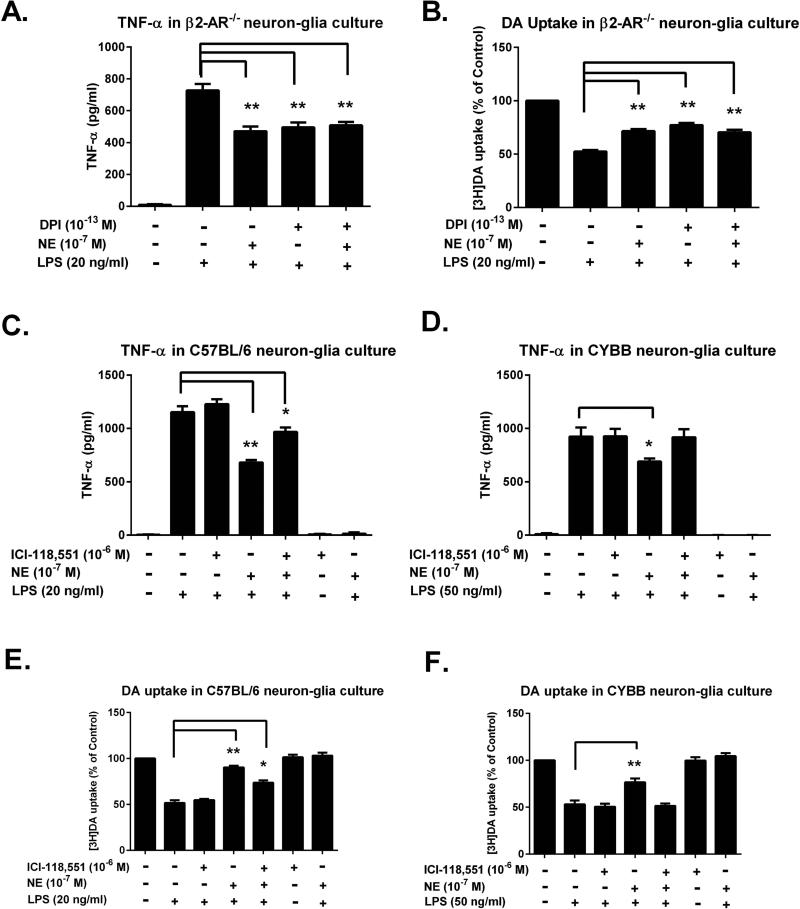
In Fig. 5, we found that β2-AR partially involved in the anti-inflammatory and neuroprotective effects of sub-micromolar NE. To determine whether NOX2 is the putative β2-AR-independent target for NE-elicited anti-inflammatory/neuroprotective effect, NOX2 inhibitor diphenyliodonium (DPI) was added to neuron-glia cultures prepared from β2-AR−/− mice. An ultra-low concentration (10−13 M) of DPI was used since our previous report showed that DPI at this concentration was capable of inhibiting cultured microglial NOX2 with high specificity(Wang et al. 2014a). The inhibitory effects of DPI on LPS-induced TNF-α production in β2-AR−/− culture were comparable to those of NE and no further reduction was observed when DPI and NE were combined (Fig. 8A). Further [3H]DA uptake measurement confirmed that the neuroprotective effect of DPI on LPS-induced neurotoxicity were comparable to those of NE and no additive protection was observed when DPI and NE were combined (Fig. 8B). These results imply that DPI and NE may exert their effects through a common mechanism.
Figure 8. NOX2 mediates β2-AR-independent anti-inflammatory/neuroprotective effects of sub-micromolar NE.
(A-B) β2-AR genetic ablated neuron-glia cultures were pre-treated with 10−13 M DPI for 30 minutes. Cultures were then treated with NE for 30 minutes prior to LPS insult. (A) Levels of TNF-α in the supernatant were measured 3 hours after LPS treatment. In β2-AR−/− culture, the inhibitory effects of DPI (495.14 ± 31.68 pg/ml; n = 5) on LPS-induced TNF-α production were comparable to those of NE (471.07 ± 29.28 pg/ml; n = 5) and no further reduction was observed when DPI and NE were combined (one-way ANOVA, F (4, 20) = 87.62, p< 0.0001; post hoc analysis by Tukey's multiple comparisons test). The results were expressed as means ± SEM from 5 independent experiments in triplicate. (B) The protective effects of NE on dopaminergic neurons were determined by [3H]DA uptake assay after 7 days of LPS insult and the results were expressed as percentage of controls (means ± SEM) from 5 independent experiments in triplicate. The neuroprotective effect of DPI (70.40 ± 2.43 % of control; n = 5) on LPS-induced neurotoxicity were comparable to those of NE (77.22 ± 1.91 % of control; n = 5) (one-way ANOVA, F (4, 20) = 97.76, p< 0.0001) and no additive protection was observed when DPI and NE were combined (post hoc analysis by Tukey's multiple comparisons test, p> 0.05). (C-D) Neuron-glia cultures prepared from gp91−/− (CYBB) and C57BL/6 mice were pre-treated with a β2-AR antagonist (ICI-118,551) for 30 minutes followed by 30 minutes NE treatment. Then the cultures were stimulated by LPS of 20 ng/ml for C57BL/6 and 50 ng/ml for gp91−/−. TNF-α production in the supernatant were detected at 3 hours after LPS addition. The results were expressed as means ± SEM from 3 independent experiments in triplicate. With β2-AR blockade in C57BL/6 culture, NE still reduced TNF-α production (966.7 ± 40.9 pg/ml) compared to LPS alone (1151.9 ± 55.6 pg/ml) (one-way ANOVA, F (6, 14) = 283.0, p< 0.0001, post hoc analysis by Tukey's multiple comparison test), while NE failed to reduce TNF-α production in CYBB culture after β2-AR blockade (one-way ANOVA, F (6, 14) = 80.12, post hoc analysis by Tukey's multiple comparison test, p> 0.05). (E-F) The DA uptake assay was performed at 7 days after treatment. The results were expressed as percentage of control alone group (means ± SEM) from 3 independent experiments in triplicate. In C57BL/6 neuron-glia culture, NE pretreatment restored DA uptake (73.54 ± 2.68% of control) compared to LPS alone (51.56 ± 2.93% of control) even with β2-AR blockade (one-way ANOVA, F (6, 14) = 86.31, p< 0.0001; post hoc analysis by Tukey's multiple comparison test); Whereas in CYBB neuron-glia culture, NE failed to restore DA uptake with β2-AR blockade (one-way ANOVA, F (6, 14) = 59.18, p<0.0001; post hoc analysis by Tukey's multiple comparison test, p> 0.05).*p< 0.05, **p< 0.01, compare with the LPS alone-treated cells.
To further confirm the contribution of NOX2 in sub-micromolar NE-mediated anti-inflammatory and neuroprotective effect neuron-glia cultures prepared from NOX2-deficient mice (CYBB, gp91phox−/−, the catalytic subunit of NOX2) were compared with its wild type C57BL/6. Firstly, the cultures were pre-treated with the β2-AR specific antagonist ICI-118,551for 30 minutes and then treated with NE 10−7 M for another 30 minutes before the addition of LPS. Since LPS added to microglia in the CYBB neuron-glia culture is less effective than when added to C57BL/6 cultures, a higher concentration (50 ng/ml) of LPS was used in gp91phox−/− neuron-glia culture, while a lower concentration (20 ng/ml) was used in C57BL/6 neuron-glia cultures. The TNF-α production results showed that blockade of the β2-AR by the antagonist partially reversed the inhibitory effect of NE in C57BL/6 neuron-glia culture (Fig. 8C), whereas a total reversal of the inhibitory effect of NE occurred in CYBB neuron-glia culture (Fig. 8D). Similar results were obtained in[3H]DA uptake studies, i.e., the β2-AR antagonist partially decreased the neuroprotective effect of NE in the C57BL/6 neuron-glia culture (Fig. 8E). In contrast, in the presence of the β2-AR antagonist, the neuroprotective effect of NE in CYBB neuron-glia culture was totally prevented (Fig. 8F). Altogether, these data suggest thatβ2-AR-independent anti-inflammatory effects of NE were indeed mediated through microglial NOX2.
Discussion
In this study, we have provided strong evidence to indicate a protective effect of the neurotransmitter NE on the survival of nigral dopaminergic neurons. We found that the depletion of endogenous NE exacerbated inflammation-driven nigral dopaminergic neurodegeneration in LPS-injected mice. These in vivo findings led to the discovery of a novel mechanism underlying the anti-inflammatory/neuroprotective actions of NE. We found that sub-micromolar concentrations of exogenous NE protected dopaminergic neurons from inflammatory mediator-induced toxicity in primary neuron-glia cultures. This study showed for the first time that this NE-induced anti-inflammatory and neuroprotective effect was mediated by not only the activation of the previously reported β2-AR-cAMP-protein kinase pathway (Farmer and Pugin 2000; Sanders and Straub 2002; van der Poll et al. 1994; Zamah et al. 2002), but also by the microglial superoxide-generating enzyme NOX2. Moreover, the inhibition of superoxide production was achieved at a much lower concentrations of NE (10−8 to 10−6 M) than that required for activation of the β2-AR signaling pathway (>10−6 M) (Alexander et al. 1975; Bylund and Snyder 1976; Deupree and Kennedy 1979). We provided evidence that the sub-micromolar NE-induced inhibition of NOX2-generated superoxide, as well as the subsequent reduction in pro-inflammatory factors release, are independent of the β2-AR. Taken together, our findings strongly suggest that the endogenous neurotransmitter NE may play a critical role in not only maintaining the homeostasis of immune functions, but also protecting neurons from oxidative stress-induced damage through modulation of the NOX2/superoxide signaling pathway. In view of the well-documented evidence in PD patients suggesting that the NE-containing neurons in the LC region are degenerated years before the loss of nigral dopaminergic neurons (Baloyannis et al. 2006; Zarow et al. 2003), our findings might suggest that the removal of the NE-mediated protection of nigral dopaminergic neurons precedes disease progression and may provide novel insight for understanding the pathogenesis of the sequential progressive neuronal loss in PD.
Most of the earlier studies documenting the anti-inflammatory function of NE focused on peripheral organs. It is generally believed that the β2-AR associated with macrophage or microglia is the major site of action for mediating the NE-induced anti-inflammatory effects (Farmer and Pugin 2000; Kohm and Sanders 2001; Sanders 1998; Zamah et al. 2002). In contrast, considerably less is known about the mechanism by which NE regulates brain immune function. In vitro reports showed that NE regulates microglia (Heneka et al. 2010) and inhibits microglial inflammatory factors release through stimulation of the microglial β2-AR (Dello Russo et al. 2004; Mori et al. 2002). However, in these studies, micromolar or higher concentrations of NE were used. These high concentrations of NE are needed for binding to the β2-AR of microglia, since the reported Ki value of NE for β2-AR binding is approximately 20 μM (Alexander et al. 1975; Bylund and Snyder 1976). Indeed, micromolar concentrations of NE can be released from nerve terminals to act on the post-synaptic receptors, but it is questionable whether NE reaches such high levels outside of a synaptic junction where microglial β2-AR stimulation would occur. We hypothesized that the NE that escapes from the re-uptake by nerve terminals or enzymatic breakdown will diffuse out of the synaptic junction to act on the surrounding microglia and should be much less than micromolar concentrations, likely in the range of 10−9~10 −7 M (Gresch et al. 1995; Xie et al. 2013). Due to these low concentrations that are 10 to 100-fold lower than its Ki value of binding affinity to adrenergic receptors (Alexander et al. 1975; Bylund 2007; Bylund and Snyder 1976; Deupree and Kennedy 1979), we speculated that a novel signaling pathway other than that activated by adrenergic receptors might mediate the anti-inflammatory function of NE. Earlier reports using microdialysis support our hypothesis by showing that the extracellular concentration of NE in brain is in the range of sub-micromolar amounts (Abercrombie and Zigmond 1989; Gresch et al. 1995; Jing et al. 2007). Our study, using sub-micromolar concentrations of NE, demonstrated that NE induces anti-inflammatory effects in a β2-AR-independent manner by inhibiting microglial NOX2-mediated superoxide production, and thus reveals a novel mechanism for the anti-inflammatory effect of NE in the brain.
The concept of extra-synaptic transmission (also known as volume transmission) has been suggested for several neurotransmitters (Castaneda-Hernandez and Bach-y-Rita 2003; Fuxe et al. 2012; Mechawar et al. 2002; Vargova and Sykova 2008). Among them, GABA has been the most extensively studied (Taylor 1997). It was reported that GABA interacts with receptors outside of synaptic junctions. One potential source of the extra-synaptic GABA may be neuronal dendrites, which are able to release small amounts of GABA. Alternatively, GABA released to synaptic junctions may diffuse to extra-synaptic spaces after escaping reuptake by nerve terminals or enzymatic degradation. It is believed that extra-synaptic GABA may play a critical role in regulating long-term neuronal transmission, which is different from the acute conventional synaptic transmission. The sub-micromolar NE-elicited anti-inflammatory effects reported in the present study may represent another example of extra-synaptic transmission. More importantly, our study extends this concept by demonstrating long-term effects of extra-synaptic NE on non-neuronal cells such as microglia, which is mediated partly through a novel β2-AR-independent microglial NOX2/superoxide signaling pathway. This conclusion was supported by several lines of evidence: 1) NE inhibited LPS-induced superoxide production in microglia from β2-AR null mice and not affected by non-selective α- and β-adrenergic antagonists; 2) The optic isomers of NE, (+)-NE and (−)-NE, displayed the same potency in inhibiting NOX2-generated superoxide; 3) Preliminary data from our laboratory showed that biological metabolites of NE, such as nor-metanephrine and 3, 4-dihydroxymandelic acid, which lack affinity in binding to the β2-AR, inhibited activation of NOX2 to the same potency as NE (Jiang et al, preliminary observations). Taken together, our data indicate that the NE-induced inhibition of microglial NOX2, which is mediated independently of the β2-AR, may constitute a major pathway signaling the extra-synaptic transmission of NE. In addition to microglia, neurons also express this superoxide-producing enzyme, but in much smaller quantities. Although we cannot totally exclude the possibility that the inhibition of neuronal NOX2 might also contribute to the NE-elicited neuroprotection, inhibition of microglial NOX2 plays a major role since depletion of microglia almost abolished the neuroprotective effects of NE. We speculate that under physiological conditions, the extra-synaptic concentration of NE is too low to influence the β2-AR singling pathway. However, we cannot rule out the possibilities that during either a disease process or pharmacological manipulations, such as inhibition of NE uptake or degradation, the extra-synaptic concentration of NE may reach to a level that is capable of modulating β2-AR function.
This study also addresses the mechanism by which NE inhibits LPS-induced superoxide production, as well as the link between superoxide inhibition and the NE-induced anti-inflammatory/neuroprotection effects. We first ruled out the possibility that the inhibitory effect on LPS-induced superoxide by NE is due to its free radical scavenging effect by showing that NE failed to decrease the xanthine/xanthine oxidase-produced superoxide (Fig. 6E). Instead, the data showed that NE inhibited the activation of NOX2-generated superoxide by reducing the LPS-induced translocation of cytosolic subunits p47phox to the plasma membrane (Fig. 7). These findings suggest that microglial NOX2 is a potential target for the action of NE. Inhibition of superoxide production plays a key role in signaling the subsequent events, as shown by the reduction of LPS-induced release of TNF-α (Fig. 8A) and protection of dopaminergic neurons from LPS-induced damage (Fig. 8B). It is interesting to note that in neuron-glial cultures prepared from β2-AR−/− mice, inhibition of NOX2 by either DPI or NE showed comparable anti-inflammatory, i.e., inhibition of TNF-α release, and neuroprotective effects, but no additive effect when DPI and NE were added together, suggesting that microglial NOX2 may be a common target that is mediating the actions of both DPI and NE. Finally, combined use of a β2-AR blocker and cultures prepared from NOX-deficient mice (CYBB) further confirmed that NE acts on NOX2 in mediating its β2-AR-independent anti-inflammatory and neuroprotective effects (Fig. 8 C-F).
In summary, strong evidence suggests that microglial NOX2/superoxide is a critical signaling pathway capable of mediating the anti-inflammatory/neuroprotective effects of sub-micromolar NE. Furthermore, this study supports the notion that in addition to its function as a conventional neurotransmitter mediating acute neurotransmission function, NE may also play a neuromodulator role in maintaining long-term immune homeostasis in the brain by inhibiting NOX2/superoxide in an adrenergic receptor-independent manner. This novel extra-synaptic function of NE warrants further investigation to expand our understanding of the pathophysiological processes involved in the development of neurodegenerative diseases.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health. We thank Anthony Lockhart for assistance with animal colony management and maintenance. We also thank Dr. Honglei Chen (Epidemiology Branch, NIEHS/NIH), Dr. Ronald E. Cannon (Laboratory of Toxicology and Pharmacology, NIEHS/NIH), and Dr. Sabrina D Robertson (Laboratory of Neurobiology, NIEHS/NIH) for reviewing this manuscript and Dr. Kissling, Grace (Biostatistics Branch) for the assistance of statistical analysis.
Footnotes
Disclosures
The authors declare no competing financial interests.
Reference
- Abercrombie ED, Zigmond MJ. Partial injury to central noradrenergic neurons: reduction of tissue norepinephrine content is greater than reduction of extracellular norepinephrine measured by microdialysis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1989;9:4062–7. doi: 10.1523/JNEUROSCI.09-11-04062.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RW, Williams LT, Lefkowitz RJ. Identification of cardiac beta-adrenergic receptors by (minus) [3H]alprenolol binding. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:1564–8. doi: 10.1073/pnas.72.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR. Briley M, Marien M, editors. Norepinephrine-dopamine interactions: one step beyond. Noradrenergic Mechanisms in Parkinson's Disease. 1993:127–139. [Google Scholar]
- Baloyannis SJ, Costa V, Baloyannis IS. Morphological alterations of the synapses in the locus coeruleus in Parkinson's disease. Journal of the neurological sciences. 2006;248:35–41. doi: 10.1016/j.jns.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Progress in neurobiology. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature reviews Neuroscience. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Bylund DB. Characterization of Adrenoceptors. Current Protocols in Pharmacology. 2007:1.5. 1–1.5. 18. [Google Scholar]
- Bylund DB, Snyder SH. Beta adrenergic receptor binding in membrane preparations from mammalian brain. Molecular pharmacology. 1976;12:568–80. [PubMed] [Google Scholar]
- Castaneda-Hernandez GC, Bach-y-Rita P. Volume transmission and pain perception. TheScientificWorldJournal. 2003;3:677–83. doi: 10.1100/tsw.2003.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- Chen SH, Oyarzabal EA, Hong JS. Preparation of rodent primary cultures for neuron-glia, mixed glia, enriched microglia, and reconstituted cultures with microglia. Methods in molecular biology. 2013;1041:231–40. doi: 10.1007/978-1-62703-520-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, James TA, MacLeod NK. Neurochemical and electrophysiological evidence for a projection from the locus coeruleus to the substantia nigra [proceedings]. The Journal of physiology. 1979;290:44P. [PubMed] [Google Scholar]
- Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- Daw NW, Videen TO, Parkinson D, Rader RK. DSP-4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine) depletes noradrenaline in kitten visual cortex without altering the effects of monocular deprivation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1985;5:1925–33. doi: 10.1523/JNEUROSCI.05-07-01925.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Russo C, Boullerne AI, Gavrilyuk V, Feinstein DL. Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1beta production. Journal of neuroinflammation. 2004;1:9. doi: 10.1186/1742-2094-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deupree JD, Kennedy RH. Stereospecific (--)-[3H]norepinephrine binding to bovine hypothalamus. Possible identification of the catecholamine uptake site in synaptic vesicles. Biochimica et biophysica acta. 1979;582:470–85. doi: 10.1016/0304-4165(79)90138-7. [DOI] [PubMed] [Google Scholar]
- Farmer P, Pugin J. beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. American journal of physiology Lung cellular and molecular physiology. 2000;279:L675–82. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Nadeau BA, Sarma JV, Day DE, Lentsch AB, Huber-Lang MS, Ward PA. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PloS one. 2009;4:e4414. doi: 10.1371/journal.pone.0004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Bassi L, Torracca MT, Alessandri MG, Scalori V, Corsini GU. Region- and neurotransmitter-dependent species and strain differences in DSP-4-induced monoamine depletion in rodents. Neurodegeneration : a journal for neurodegenerative disorders, neuroprotection, and neuroregeneration. 1996;5:241–9. doi: 10.1006/neur.1996.0032. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Immunohistochemical analysis of the neurotoxic effects of DSP-4 identifies two populations of noradrenergic axon terminals. Neuroscience. 1989;30:181–97. doi: 10.1016/0306-4522(89)90364-3. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Diaz-Cabiale Z, Rivera A, Ferraro L, Tanganelli S, Tarakanov AO, Garriga P, Narvaez JA. Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronalglial networks. Frontiers in physiology. 2012;3:136. doi: 10.3389/fphys.2012.00136. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H-M, Zhang F, Zhou H, Kam W, Wilson B, Hong J-S. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson's disease. Environmental health perspectives. 2011a;119:807. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:782–90. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. 2011b;31:1081–92. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE. Neural immunity: Friend or foe? Journal of neurovirology. 2002;8:474–9. doi: 10.1080/13550280290168631. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. Journal of neurochemistry. 1995;65:111–6. doi: 10.1046/j.1471-4159.1995.65010111.x. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, Jardanhazi-Kurutz D, Walter J, Kirchhoff F, Hanisch UK. Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6058–63. doi: 10.1073/pnas.0909586107. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Zhang D, Pang H, Caudle WM, Li Y, Gao H, Liu Y, Qian L, Wilson B, Di Monte DA. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. J Immunol. 2008;181:7194–204. doi: 10.4049/jimmunol.181.10.7194. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain research Molecular brain research. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Jaim-Etcheverry G, Zieher LM. DSP-4: a novel compound with neurotoxic effects on noradrenergic neurons of adult and developing rats. Brain research. 1980;188:513–23. doi: 10.1016/0006-8993(80)90049-9. [DOI] [PubMed] [Google Scholar]
- Jing FC, Chen H, Li CL. Rapid determination of dopamine and its metabolites during in vivo cerebral microdialysis by routine high performance liquid chromatography with electrochemical detection. Biomedical and environmental sciences : BES. 2007;20:317–20. [PubMed] [Google Scholar]
- Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain research. 1977;127:25–53. [PubMed] [Google Scholar]
- Jonsson G, Hallman H, Ponzio F, Ross S. DSP4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine)--a useful denervation tool for central and peripheral noradrenaline neurons. European journal of pharmacology. 1981;72:173–88. doi: 10.1016/0014-2999(81)90272-7. [DOI] [PubMed] [Google Scholar]
- Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. Journal of leukocyte biology. 2006;79:1093–104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacological reviews. 2001;53:487–525. [PubMed] [Google Scholar]
- Koshkin V, Lotan O, Pick E. The cytosolic component p47(phox) is not a sine qua non participant in the activation of NADPH oxidase but is required for optimal superoxide production. The Journal of biological chemistry. 1996;271:30326–9. doi: 10.1074/jbc.271.48.30326. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. The Journal of pharmacology and experimental therapeutics. 2000;293:607–17. [PubMed] [Google Scholar]
- Liu B, Hong JS. Primary rat mesencephalic neuron-glia, neuron-enriched, microglia-enriched, and astroglia-enriched cultures. Methods in molecular medicine. 2003;79:387–95. doi: 10.1385/1-59259-358-5:387. [DOI] [PubMed] [Google Scholar]
- Liu J, Mori A. Monoamine metabolism provides an antioxidant defense in the brain against oxidant- and free radical-induced damage. Archives of biochemistry and biophysics. 1993;302:118–27. doi: 10.1006/abbi.1993.1189. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin L, Wilson B, Wu X, Qian L, Granholm AC, Crews FT, Hong JS. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology. 2008;29:864–70. doi: 10.1016/j.neuro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechawar N, Watkins KC, Descarries L. Ultrastructural features of the acetylcholine innervation in the developing parietal cortex of rat. The Journal of comparative neurology. 2002;443:250–8. doi: 10.1002/cne.10114. [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Berdichevsky Y, Ugolev Y, Molshanski-Mor S, Nakash Y, Dahan I, Alloul N, Gorzalczany Y, Sarfstein R, Hirshberg M. Assembly of the phagocyte NADPH oxidase complex: chimeric constructs derived from the cytosolic components as tools for exploring structure-function relationships. Journal of leukocyte biology. 2006;79:881–95. doi: 10.1189/jlb.1005553. others. [DOI] [PubMed] [Google Scholar]
- Mori K, Ozaki E, Zhang B, Yang L, Yokoyama A, Takeda I, Maeda N, Sakanaka M, Tanaka J. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology. 2002;43:1026–34. doi: 10.1016/s0028-3908(02)00211-3. [DOI] [PubMed] [Google Scholar]
- Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE. Inflammation and adaptive immunity in Parkinson's disease. Cold Spring Harbor perspectives in medicine. 2012;2:a009381. doi: 10.1101/cshperspect.a009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phani S, Loike JD, Przedborski S. Neurodegeneration and inflammation in Parkinson's disease. Parkinsonism & related disorders. 2012;18(Suppl 1):S207–9. doi: 10.1016/S1353-8020(11)70064-5. [DOI] [PubMed] [Google Scholar]
- Qian L, Block ML, Wei SJ, Lin CF, Reece J, Pang H, Wilson B, Hong JS, Flood PM. Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. The Journal of pharmacology and experimental therapeutics. 2006;319:44–52. doi: 10.1124/jpet.106.106351. [DOI] [PubMed] [Google Scholar]
- Qian L, Wei SJ, Zhang D, Hu X, Xu Z, Wilson B, El-Benna J, Hong JS, Flood PM. Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J Immunol. 2008a;181:660–8. doi: 10.4049/jimmunol.181.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Wei SJ, Zhang D, Hu X, Xu Z, Wilson B, El-Benna J, Hong JS, Flood PM. Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. Journal of immunology. 2008b;181:660–8. doi: 10.4049/jimmunol.181.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Wu HM, Chen SH, Zhang D, Ali SF, Peterson L, Wilson B, Lu RB, Hong JS, Flood PM. beta2-adrenergic receptor activation prevents rodent dopaminergic neurotoxicity by inhibiting microglia via a novel signaling pathway. Journal of immunology. 2011;186:4443–54. doi: 10.4049/jimmunol.1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Cooper C, Liu B, Wilson B, Hong JS. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. Journal of neurochemistry. 2002;83:973–83. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Hong JS, Crews FT. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61:855–68. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. The Journal of biological chemistry. 2004;279:1415–21. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JW, Kobilka TS, Yang-Feng TL, Caron MG, Lefkowitz RJ, Kobilka BK. Cloning and expression of a human kidney cDNA for an alpha 2-adrenergic receptor subtype. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:6301–5. doi: 10.1073/pnas.85.17.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nature neuroscience. 2013;16:1016–23. doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GB, Fluharty SJ, Zigmond MJ, Sclabassi RJ, Berger TW. Recovery of hippocampal dentate granule cell responsiveness to entorhinal cortical input following norepinephrine depletion. Brain research. 1993;614:21–8. doi: 10.1016/0006-8993(93)91013-i. [DOI] [PubMed] [Google Scholar]
- Ross SB. Long-term effects of N-2-chlorethyl-N-ethyl-2-bromobenzylamine hydrochloride on noradrenergic neurones in the rat brain and heart. British journal of pharmacology. 1976;58:521–7. doi: 10.1111/j.1476-5381.1976.tb08619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VM. The role of norepinephrine and beta-2-adrenergic receptor stimulation in the modulation of Th1, Th2, and B lymphocyte function. Advances in experimental medicine and biology. 1998;437:269–78. doi: 10.1007/978-1-4615-5347-2_30. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain, behavior, and immunity. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nature reviews Neuroscience. 2009;10:211–23. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Schwinn DA, Lomasney JW, Lorenz W, Szklut PJ, Fremeau RT, Jr., Yang-Feng TL, Caron MG, Lefkowitz RJ, Cotecchia S. Molecular cloning and expression of the cDNA for a novel alpha 1-adrenergic receptor subtype. The Journal of biological chemistry. 1990;265:8183–9. [PubMed] [Google Scholar]
- Severn A, Rapson NT, Hunter CA, Liew FY. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. Journal of immunology. 1992;148:3441–5. [PubMed] [Google Scholar]
- Strader CD, Sigal IS, Register RB, Candelore MR, Rands E, Dixon RA. Identification of residues required for ligand binding to the beta-adrenergic receptor. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:4384–8. doi: 10.1073/pnas.84.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Gunzler C, Miller LE, Cutolo M, Scholmerich J, Schill S. Anti-inflammatory cooperativity of corticosteroids and norepinephrine in rheumatoid arthritis synovial tissue in vivo and in vitro. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:993–1000. doi: 10.1096/fj.02-0085com. [DOI] [PubMed] [Google Scholar]
- Suh CI, Stull ND, Li XJ, Tian W, Price MO, Grinstein S, Yaffe MB, Atkinson S, Dinauer MC. The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcgammaIIA receptor-induced phagocytosis. The Journal of experimental medicine. 2006;203:1915–25. doi: 10.1084/jem.20052085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CP. Mechanisms of action of gabapentin. Revue neurologique. 1997;153(Suppl 1):S39–45. [PubMed] [Google Scholar]
- Troadec JD, Marien M, Darios F, Hartmann A, Ruberg M, Colpaert F, Michel PP. Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. Journal of neurochemistry. 2001;79:200–10. doi: 10.1046/j.1471-4159.2001.00556.x. [DOI] [PubMed] [Google Scholar]
- Troadec JD, Marien M, Mourlevat S, Debeir T, Ruberg M, Colpaert F, Michel PP. Activation of the mitogen-activated protein kinase (ERK(1/2)) signaling pathway by cyclic AMP potentiates the neuroprotective effect of the neurotransmitter noradrenaline on dopaminergic neurons. Molecular pharmacology. 2002;62:1043–52. doi: 10.1124/mol.62.5.1043. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infection and immunity. 1994;62:2046–50. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargova L, Sykova E. Extracellular space diffusion and extrasynaptic transmission. Physiological research / Academia Scientiarum Bohemoslovaca. 2008;57(Suppl 3):S89–99. doi: 10.33549/physiolres.931603. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chu CH, Oyarzabal E, Jiang L, Chen SH, Wilson B, Qian L, Hong JS. Subpicomolar diphenyleneiodonium inhibits microglial NADPH oxidase with high specificity and shows great potential as a therapeutic agent for neurodegenerative diseases. Glia. 2014a;62:2034–43. doi: 10.1002/glia.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Chu CH, Qian L, Chen SH, Wilson B, Oyarzabal E, Jiang L, Ali S, Robinson B, Kim HC. Substance P Exacerbates Dopaminergic Neurodegeneration through Neurokinin-1 Receptor-Independent Activation of Microglial NADPH Oxidase. J Neurosci. 2014b;34:12490–503. doi: 10.1523/JNEUROSCI.2238-14.2014. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Shin EJ, Nguyen XK, Li Q, Bach JH, Bing G, Kim WK, Kim HC, Hong JS. Endogenous dynorphin protects against neurotoxin-elicited nigrostriatal dopaminergic neuron damage and motor deficits in mice. J Neuroinflammation. 2012a;9:124. doi: 10.1186/1742-2094-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhou H, Gao H, Chen SH, Chu CH, Wilson B, Hong JS. Naloxone inhibits immune cell function by suppressing superoxide production through a direct interaction with gp91phox subunit of NADPH oxidase. J Neuroinflammation. 2012b;9:32. doi: 10.1186/1742-2094-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Qin L, Liu B, Liu Y, Wilson B, Eling TE, Langenbach R, Taniura S, Hong J-S. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. Journal of neurochemistry. 2004;88:939–947. doi: 10.1046/j.1471-4159.2003.02242.x. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system. The Journal of biological chemistry. 2002;277:31249–56. doi: 10.1074/jbc.M202753200. [DOI] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Archives of neurology. 2003;60:337–41. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.