Introduction
When not activated, NF-κB is sequestered in the cytoplasm tightly bound to IκBs (Inhibitor of κB). These inhibitory proteins mask the nuclear localization signal (NLS) of NF-κB, preventing its nuclear translocation and subsequent activation (Baeuerle, 1998). The canonical NF-κB pathway can be activated in response to a variety of stimuli, including viruses, bacterial toxins, and cytokines. Activated IκB kinase (IKK) phosphorylates IκB leading to its ubiquitination and degradation. This exposes the NLS of NF-κB, freeing it to enter the nucleus and induce the expression of over one hundred genes (Hacker and Karin, 2006) that regulate cellular processes such as inflammation (Ghosh et al., 1998), tumor development (Karin et al., 2002) and immunity (Li and Verma, 2002). Several antiviral responses, including apoptosis (Barkett and Gilmore, 1999), autophagy (Djavaheri-Mergny et al., 2007; Espert et al., 2007), and early interferon-β (IFN-β) expression (Wang et al., 2010) are controlled by NF-κB. To evade these host defense responses or, even to enhance viral infection, many viruses have evolved strategies to modulate the NF-κB pathway (Hiscott et al., 2006).
A previous report indicated that NF-κB activation is delayed for several hours after wild-type (wt) vesicular stomatitis virus (VSV) infection, while this transcription factor is rapidly activated in L929 cells infected with the T1026R1 (R1) mutant strain of VSV (Boulares et al., 1996). The R1 virus (Stanners et al., 1977) expresses a mutant matrix (M) protein, in which there is methionine-to-arginine substitution at amino acid position 51 (Ferran and Lucas-Lenard, 1997). This position of M protein is crucial for many of the cytotoxic effects associated with VSV infection, including induction of cell rounding (Blondel et al., 1990; Lyles and McKenzie, 1997; Simon et al., 1990), regulation of apoptosis (Desforges et al., 2001; Kopecky and Lyles, 2003a, b; Kopecky et al., 2001), shut off of host transcription (Black and Lyles, 1992; Ferran and Lucas-Lenard, 1997; Paik et al., 1995), and inhibition of nuclear-cytoplasmic transport of host RNAs (Her et al., 1997; Petersen et al., 2000; von Kobbe C et al., 2000). Thus one hypothesis is that M protein may limit activation of key antiviral pathways by delaying NF-κB activation for several hours. By late times postinfection, both host transcription and translation are severely inhibited in wt-infected cells (Dunigan et al., 1986). Therefore, even if NF-κB is activated, transcription of antiviral genes would be inhibited. Garcia et al. also reported NF-κB activation at late times postinfection (8 hours) in wt-infected MEF cells (Garcia et al., 2009), however this group did not monitored NF-κB activation in cells infected with viruses encoding the M51R mutation in M. The integrity of the VSV G, L, N and P viral proteins encoded by R1 is unknown; therefore one of these proteins might also function as the inhibitor of NF-κB.
The goal of this study was to determine which VSV protein is responsible for the NF-κB inhibition phenotype in L929 cells. Our findings indicate that M protein inhibits viral-mediated activation of NF-κB when expressed during virus infection, and when expressed independently of infection, and that mutation of residue 51 to an arginine inhibits this function. We also report that the M protein is likely targeting an event upstream of IKK in the canonical pathway.
MATERIALS AND METHODS
Cells, viruses, and infections
Monolayers of mouse Fibroblast L929 cells (ATCC CCL-1) were grown in complete media containing Eagle‘s Minimum Essential Medium (MEM) supplemented with 10% Horse Serum (HS). The heat-resistant strain of the Indiana serotype of VSV (Marcus and Sekellick, 1987; Wagner and Huang, 1966) and its mutant T1026R1 (R1), isolated by Stanners et al., were grown on Vero cells as previously described (Stanners et al., 1977). Recombinant virus rHR-M containing the wt HR strain of M protein, and r1026-M encoding the HR strain of M protein containing the M51R substitution, were generously provided by Dr. Douglas Lyles (Wake Forest School of Medicine). These recombinants were isolated from infectious VSV cDNA clones and virus stocks were prepared in BHK cells as previously described (Ahmed et al., 2003). Cells were infected with each virus at a multiplicity of infection (MOI) of 5 PFU/cell unless otherwise stated. Virus was adsorbed in MEM for 1 hr at 37°C in the absence of serum, after which complete medium was added.
Plasmids
The expression vectors containing the different VSV genes regulated by the SV40 late promoter were generously provided by M. Schubert (National Institutes of Health). These constructs were generated by cloning the appropriate viral gene into the pJC119 expression vector (34) and are as follows: pSVGL containing the G gene (30), pSV-VSL1 containing the L gene (31), pKOM1 containing the M gene (4), pJS223 containing the N gene (34), and pLH7 containing the P gene (12). The empty vector pJC119 was used as a control in many of the experiments and to keep the amount of DNA per transfection constant. The pGL4.32 (luc2P/NF-κB/Hygro) plasmid (Promega) contains the firefly luciferase reporter gene cloned behind an inducible Nuclear Factor-κB (NF-κB) dependent promoter element. Plasmids were prepared using EndoFree Maxi Prep kits (Qiagen) according to the manufacturer’s instructions.
Generation and maintenance of stable cells lines
L929 cells were transfected with pGL4.32 using LipofectAMINE 2000 (Life Technologies) according to manufacturer’s protocol. Transfected cells were selected by growing cells in complete media supplemented with 300 µg/mL of Hygromycin B (Life Technologies). Stably transfected cells were maintained in the presence of Hygromycin B. Cells were passed into media containing 150 µg/ml Hygromycin B and were refed with media containing 300 µg/ml Hygromycin B 24 hrs after passage.
Chemical Treatments
Cells were treated with 30ng/ml TNF-α for 40 min. Bay 11–7082 (Bay, Biomol) was added to media at a 10 µM final concentration during adsorption and infection.
Nucleofector transfections
Transfection of L929 cells was done using the Nucleofector 2b device and Kit V (Lonza Group Ltd., VCA-1003), according to the manufacturer's protocol. Briefly, 106 cells were resuspended in 100 µl Nucleofector Solution V, mixed with 3 µg plasmid DNA in the provided cuvette, and nucleofected using program X-05 of the nucleofector device. 600 µl of prewarmed complete medium was added to the cuvette. Cells were gently transferred into a 100 mm plate containing 10 ml of prewarmed complete media and incubated at 37°C, 5% CO2, in a humidified atmosphere.
Immunofluorescence
Cells were grown in 6-well tissue culture dishes on sterile No. 1 coverslips and infected with VSV at an MOI of 10. At the indicated intervals postinfection, cells were washed with phosphate-buffered saline (PBS), fixed with 3.7% paraformaldehyde for 30 min, and permeabilized for 10 min at room temperature with 0.1% Triton X-100 in PBS. After washing, cells were blocked with 3% normal goat serum in PBS for 30 minutes. Cells were incubated with an NF-κB primary antibody (sc-372, Santa Cruz) for 30 minutes at room temperature. Coverslips were washed extensively with PBS and incubated with a fluorescein-conjugated goat anti-rabbit secondary antibody (sc-2012, Santa Cruz) for 30 minutes at room temperature. After several washes with PBS, coverslips were mounted on microscope slides with Vectashield mounting medium (Vector Laboratories, Inc.). The cells were visualized by confocal microscopy.
TransAM Assay
Cells were washed with ice-cold PBS with phosphatase inhibitors at the indicated times postinfection and nuclear extracts were prepared using the Nuclear Extract Kit (Active Motif) according to the vendor’s protocol. NF-κB activity was measured using equal amounts of nuclear protein extracts by the TransAM™ NF-κB p65 kit (Active Motif), an ELISA-based kit to detect and quantify NF-κB p65 subunit activation. The assay was performed according to the manufacturer's protocol. Results for the chemiluminescent TransAM kit were analyzed using a Lumicount Microplate (Packard Instrument), while results for the colorimetric TransAM kit were analyzed using the SpectraMax 190 plate reader (Molecular Devices).
Luciferase Assay
L929 cells stably transfected with pGL4.32 were passed approximately 48 hours prior to infection. Cells were infected at an MOI of 25 and collected at the indicated time postinfection. Cells were washed with ice cold PBS and harvested in 1× Reporter Lysis Buffer (Promega). After a single freeze-thaw cycle, lysates were centrifuged at 12,000g for 15 seconds and the supernatant was collected. The resultant lysates were incubated with the Luciferase Assay Reagent (Promega) and luciferase activities were measured in relative light units (RLU) using a Varioskan Flash Multimode Reader (ThermoScientific). Each sample was done in triplicate and the RLU values were averaged and normalized to the protein concentration of the respective luciferase lysate.
Immunoblot analysis
Whole cell extracts from mock-infected or virus-infected L929 cells (~1×106) were collected at the indicated time postinfection in Cytobuster (Novagen) containing protease inhibitors cocktail III (Calbiochem). Equal amounts of protein (5–50 mg) were fractionated by 10% SDS–PAGE (10% Precise Protein Gels, Pierce) and transferred to nitrocellulose membranes (Pierce) overnight at 30 volts. The membranes were incubated with the one of the following primary antibodies in TNE buffer plus 5% nonfat dry milk and 0.1% Tween-20: -VSV-G (Sigma) or -actin (sc-1616). Bound primary antibodies were detected by incubation with a HRP-conjugated secondary antibody (goat anti-rabbit sc-2030). Signal was detected by enhanced chemiluminescence (WestDura kit, Pierce) and results were visualized and quantitated using a cooled CCD camera system (Kodak ImageStation, 440). To determine the relative intensities of the VSV G band (shown below the panel), the intensity of the G band was normalized to the respective actin band.
Real-Time PCR analysis
Cells (~4 × 106 cells) were mock treated or infected for 5 hours and total RNA was isolated using the RNaqueous-4-PCR kit according to the manufacturer’s instructions (Ambion). Total RNA (1 µg) was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription kit with RNAse Inhibitor kit (ABI). The commercially available mouse IFN-β TaqMan expression assay (Mm00439546_s1) was used for Real-Time PCR analysis of IFN-β mRNA production. All PCR reactions were set up in 96-well optical plates using equal aliquots of cDNA sample, 900 nM primers, 250 nM TaqMan probe, and 1X TaqMan Universal PCR Master Mix in a total volume of 50 µl. Using the ABI 7500 Real-Time PCR System (Applied Biosystems), PCR cycling conditions were set as follows: 94 °C for 5 min, 40 cycles at 94 °C for 15 s, and 60 °C for 1 min. Samples were run in triplicate to allow for statistical analysis with IFN-β mRNA and HPRT control reactions run side by side on the reaction plate. The HPRT endogenous control Taqman Gene Expression Assay (Mm00446968_m1) was used for relative quantification because expression of this gene was shown not to vary during VSV infection (Stojdl et al., 2003). All quantitations (threshold cycle [CT] values) were normalized to that of HRPT to generate ΔCT (ΔCT IFN = CT IFN − CT HPRT), and the difference between the ΔCT value of the sample and that of the mock sample was calculated as ΔΔCT (ΔΔCT IFN = ΔCT IFN − ΔCT mock). The relative level of IFN mRNA gene expression was expressed as fold change over to mock (fold change or 2−ΔΔCT).
Statistical Analysis
Statistical analysis throughout this paper was performed using the Student’s t test and an asterisk indicates significant reduction (P< 0.05). Results were expressed as means and error bars indicate the ± standard error of the means (SEM).
Results and Discussion
NF-κB activation is delayed in cells infected with viruses encoding a functional M protein
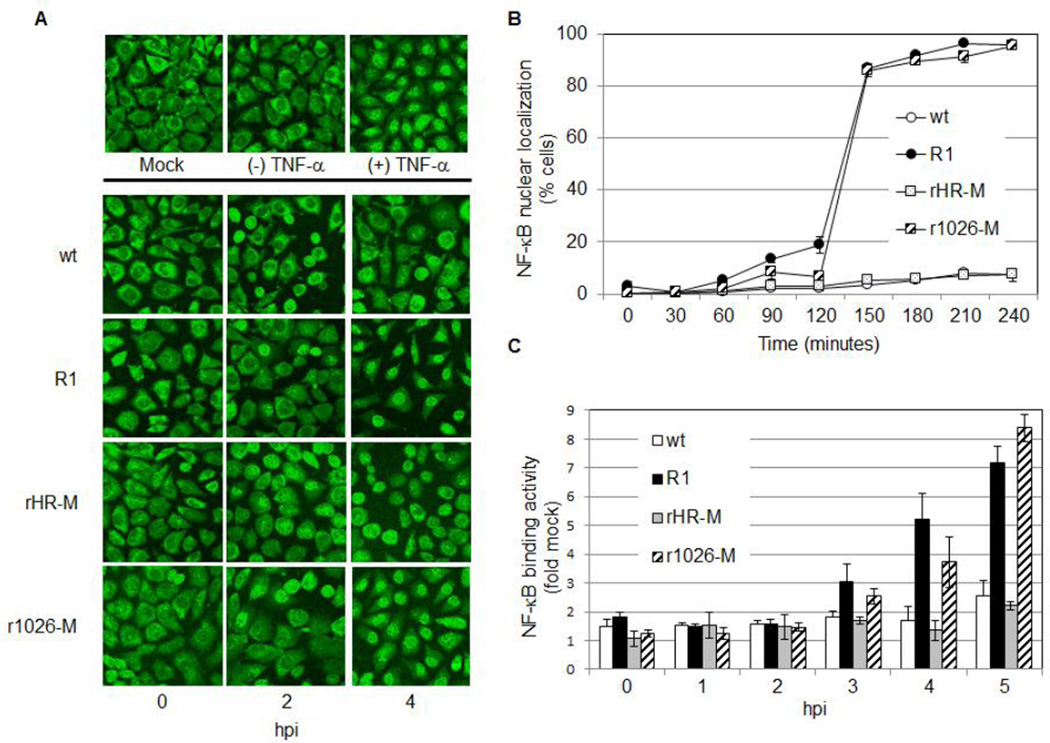
Nuclear translocation of p65 is a hallmark of NF-κB activation. Immunofluorescence was used to examine localization of this NF-κB subunit in L929 cells infected with one of the following viruses: (1) wt VSV, heat resistant (HR) Indiana strain, (2) R1, a mutated HR Indiana strain, (3) rHR-M, a recombinant virus that contains the HR strain of M protein or (4) r1026-M, a recombinant virus that is identical to rHR-M except the M protein encodes the M51R mutation. As a control, cells were untreated or treated with TNF-α, a known activator of NF-κB (Hohmann et al., 1990). As expected, NF-κB was localized to the cytoplasm in mock-infected cells or cells not treated with TNF-α. Treatment with TNF-α resulted in nuclear translocation of NF-κB (Fig. 1A). When examining virus-infected cells, little nuclear staining was observed during the 4 hour time course in cells infected with wt or rHR-M (Fig. 1A and B). In contrast, NF-κB was detected in the nucleus of 20% of R1-infected cells at 2 hours post-infection (hpi), increasing to 87% by 2.5 hpi. Similarly, NF-κB was localized to the nucleus in 85% of cells infected with r1026-M by 2.5 hpi (Fig. 1A and B). Previous studies have shown that these viruses express similar levels of the M protein in infected cells (Ahmed et al., 2003; Lodish and Porter, 1981); therefore these differences in NF-κB activity are not due to different expression levels of the M protein. These findings indicate that a functional M protein regulates a pathway that leads to nuclear localization of NF-κB, and that mutation of the methionine at position 51 to an arginine abrogates this function. To expand upon these findings the NF-κB DNA binding activity in nuclear protein extracts was examined using an ELISA-based TransAM kit (Active Motif, Fig. 1C). NF-κB DNA binding was not detected in wt or rHR-M-infected cells during the time course tested. DNA binding was detected by 3 hpi in cells infected with R1 or r1026-M (increasing to 3 and 2.5 fold of mock respectively), and this activity continued to increase after 4 and 5 hpi (Fig. 1C). These results are consistent with our immunofluorescence data.
Fig. 1.
Nuclear Localization and DNA-binding activity of NF-κB occurs rapidly in cells infected with viruses containing the M51R mutation in M. (A) Cells were TNF-α treated, mock infected, or infected at an MOI of 10 for the indicated time. The p65 subunit of NF-κB was then visualized by confocal microscopy. The experiment was repeated three times; representative images are shown (B) The percent of total cells containing nuclear NF-κB was determined. At least 200 cells per sample were counted and these data represent the mean of two independent experiments. (C) Cells were infected at an MOI of 5, nuclear extracts prepared, and the DNA-binding activity of NF-κB determined using the TransAM NF-κB p65 Chemi Kit (Active Motif). Binding activity was expressed as fold mock and was calculated by dividing measured DNA binding activity of NF-κB in an infected sample by the activity found in mock-infected cells. Data represent the mean values from at least three independent experiments, performed in duplicate.
Our results show that viruses encoding a functional M protein (wt or rHR-M) delay NF-κB activation, while activation occurs earlier in cells infected with M-defective viruses (R1 or r1026-M). These findings suggest that the wt M protein prevents NF-κB activation, and that the M51R mutation abrogates this function.
The M protein, in the context of a viral infection, blocks virus-mediated activation of NF-κB activation
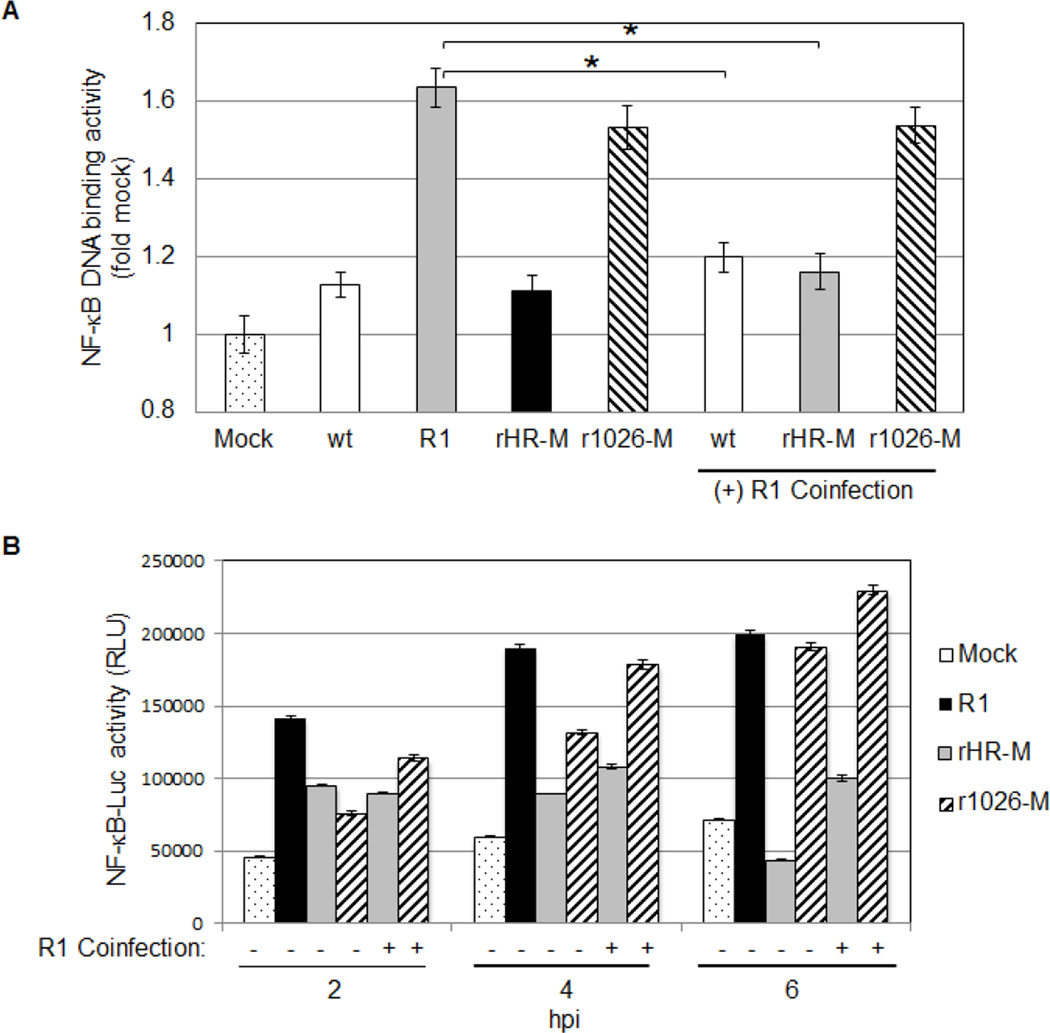
Fig. 1 suggests that NF-κB activation is delayed in cells infected with viruses containing a wt M protein. Two possible explanations exist: (1) NF-κB activation is delayed in wt-infected cells because wt virus fails to activate NF-κB, or (2) the wt M protein is able to inhibit NF-κB after it has been activated. We employed coinfection assays to examine these possibilities, using DNA binding as a measure of NF-κB activation. Similar to Fig. 1, minimal NF-κB DNA binding activity was detected in nuclei isolated from wt or rHR-M-infected cells, while NF-κB was activated in cells infected with R1 or r1026-M (Fig. 2A). Reduced DNA binding activity was detected in cells coinfected with R1 and wt or R1 and rHR-M, compared to cells infected with R1 alone. In contrast, r1026-M was not able to limit R1-mediated activation of NF-κB in coinfected cells. Therefore the functional M protein encoded by wt or rHR-M was able to block viral activation of NF-κB; however the M51R mutant M protein in r1026-M could not. These findings indicate that the M protein is essential for inhibition of activated NF-κB and that the M51R mutation inhibits this function. While the trends in NF-κB activation in Fig. 2A and Fig. 1 are similar, the fold change varied. A more sensitive chemiluminescent TransAM kit was used in Fig. 1, however for technical reasons a less-sensitive colorimetric TransAM kit was used for the rest of the study.
Fig. 2.
Viruses containing a wt M protein block virus-induced activation of NF-κB. (A) Nuclear extracts were isolated from cells infected with a single viral strain, or coinfected with R1 (MOI of 5 for each virus, 5 hpi). The NF-κB DNA binding activity was measured using the TransAM NF-κB p65 Kit (colorimetric, Active Motif). Each sample was standardized to mock infected samples. Data represent the mean of five independent experiments, performed in duplicate. (B) Luciferase transcriptional reporter activity in stably transfected cells following viral infection (MOI of 25 for each virus). A representative experiment of three is shown.
A luciferase reporter assay was developed as another measure of NF-κB activation. Since detection of luciferase also relies on events downstream of NF-κB, such as expression of the luciferase protein, this assay also monitors the effect of the M protein on transcription and translation. A stably transfected L929 cell line containing the pGL4.32 plasmid, which contains the Luc2P gene under the control of five κB response elements, was established. Stably transfected cells were infected for 2, 4, or 6 hours and luciferase activity was measured. NF-κB dependent-luciferase expression increased over the times tested in cells infected with R1 or r1026-M, while luciferase expression decreased over time in cells infected with rHR-M. At 6 hours of coinfection, rHR-M limited R1-mediated luciferase expression; however r1026-M was not able to do so (Fig. 2B). These results suggest that viruses containing a functional M protein can limit virus-mediated expression from a NF-κB-dependent promoter; however viruses encoding the M51R mutation in the M protein are not able to limit this expression.
The wt M protein alone blocks virus-mediated activation of NF-κB
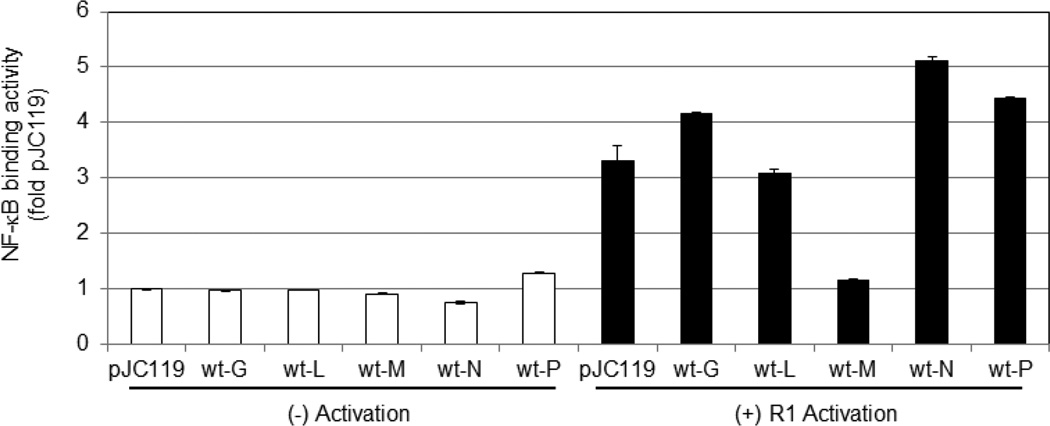
To determine if another VSV protein regulates NF-κB, L929 cells were nucleofected with an expression vector encoding one of the VSV genes. Expression of these viral proteins in transfected cells was previously confirmed (Ferran and Lucas-Lenard, 1997). At 40–48 hours post-transfection cells were mock or R1-infected, and NF-κB binding activity was measured. As shown in Fig. 3, little NF-κB was activated in nucleofected cells that were not infected with R1, confirming that nucleofection alone did not activate NF-κB. Compared to mock-infected cells nucleofected with the empty vector pJC119, R1-infection activated 3 fold more NF-κB in cells nucleofected with the same plasmid. R1 infection did activate NF-κB in cells expressing the VSV G, L, N, or P protein, suggesting that these viral proteins do not prevent virus-mediated NF-κB activation. Expression of the wt M protein significantly blocked the ability of R1 to activate NF-κB; therefore the wt M protein inhibits NF-κB when expressed alone and in the context of a viral infection.
Fig. 3.
The VSV M protein blocks virus-mediated activation of NF-κB. An expression vector encoding the indicated VSV gene was Nucleofected into cells. After approximately 48 hours post-nucleofection cells were mock or R1-infected (MOI of 5 for 5 hours), and the DNA-binding activity in nuclear extracts was determined using the TransAM Kit (colorimetric). The data was calculated by dividing DNA binding activity of NF-κB in a sample by the activity found in cells nucleofected with the empty vector pJC119 and mock infected. Three independent experiments were performed. The mean of a representative experiment done in quadruplicate is shown.
Inhibition of IKK limits NF-κB activation and IFN-β mRNA expression in cells infected with R1 or r1026-M
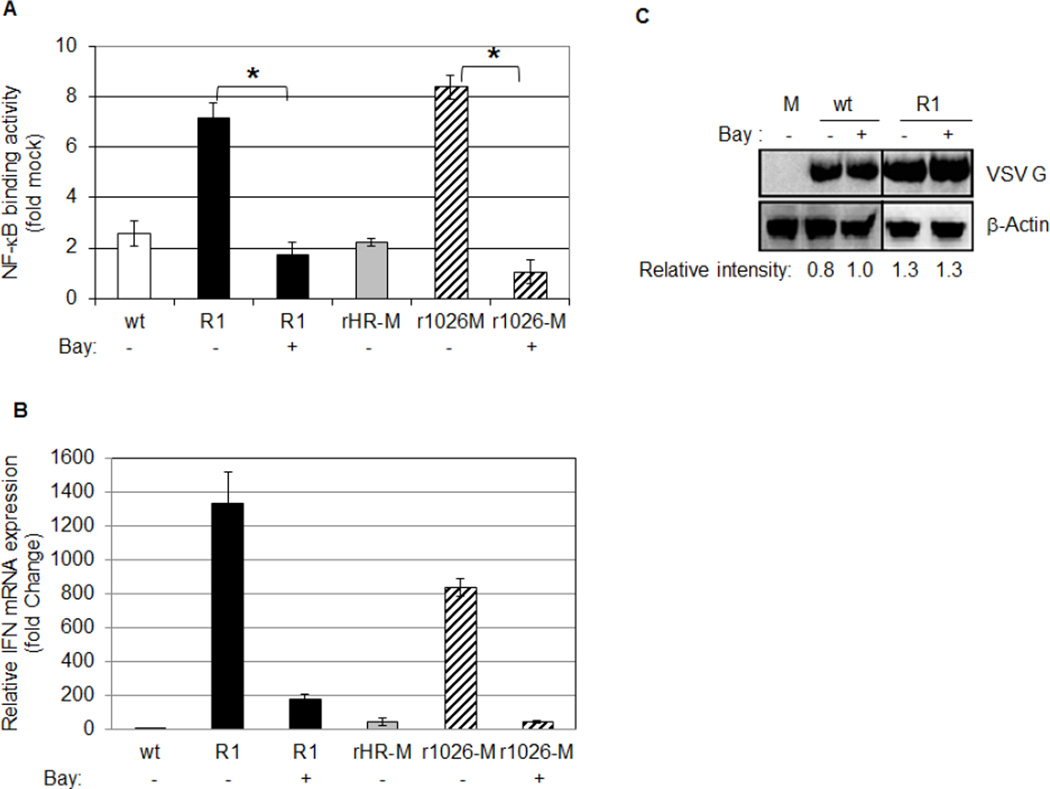
A previous study found a correlation between NF-κB activation and IκB-α degradation in R1-infected cells (Boulares et al., 1996). To further investigate the pathway VSV targets to limit NF-κB activation, Bay 11–7082 (Bay), a pharmacological inhibitor of the IκB kinase (IKK) that phosphorylates IκB-α (Pierce et al., 1997), was added to cells during adsorption and infection. In comparison to untreated cells, Bay treatment significantly decreased NF-κB activation in cells infected with R1 (7.1 fold down to 1.7 fold) or r1026-M (8.4 fold down to 1 fold). These results suggest that IKK is necessary for activation of NF-κB following R1 or r1026-M infection (Fig. 4A) and indicate that the direct target of the M protein may be involved in the IKK activation pathway.
Fig. 4.
An inhibitor of IKK reduces NF-κB activation and IFN-β mRNA expression in cells infected with viruses containing mutant M proteins. L929 cells were untreated or treated with Bay during adsorption and infection with the indicated virus at an MOI of 5. (A) Nuclear lysates were prepared at 5 hpi and NF-κB activation was measured using the TransAM Kit (colorimetric). Each sample was standardized to mock infected samples. The data represent the mean of two independent experiments, performed in duplicate. (B) Total RNA was collected from infected cells at 5 hpi and reverse transcribed into cDNA and submitted to Real-Time PCR analysis of mouse IFN-β mRNA production. Samples were done in triplicate and were normalized to HPRT gene expression in respective samples. Data is represented as fold change relative to mock-infected cells. The mean of one representative experiment of three is shown. (C) Whole cell protein lysates were collected after 8 hours of infection and analyzed by western blot analysis. A representative experiment of three is shown. Relative intensities of the VSV G band are shown below the panel.
The promoter of the IFN-β gene is activated by NF-κB, IRF3 and AP-1. To determine if IFN-β mRNA expression correlated with NF-κB activation, total RNA was isolated at 5 hpi and analyzed by Real-Time PCR analysis. IFN-β mRNA was expressed in cells infected with R1 or r1026-M (1300 and 800 fold change respectively); however very little mRNA was detected in cells infected with viruses containing a wt M protein (see Fig. 4B). We repeatedly observed 1.5–2.5 times more IFN-β mRNA expression in R1-infected cells, compared to cells infected with r1026-M. Addition of Bay severely limited IFN-β mRNA production in cells infected with R1 or r1026-M, indicating that IKK activity and NF-κB activation are necessary for IFN-β mRNA production in infected L929 cells.
To confirm that Bay treatment did not limit VSV infection, cells were infected in the presence or absence of Bay, and the production of the G protein was analyzed via Western blot analysis (Fig. 4C). Bay treatment had little to no effect on the amount of the G protein in wt or R1-infected cells. Results from trypan blue exclusion assays (Strober, 2001) indicated that Bay treatment had no effect on viability of mock or R1-infected L929 cells during the times tested (data not shown).
By blocking activation of NF-κB, the M protein may limit induction of NF-κB target genes and therefore limit many crucial NF-κB-dependent antiviral pathways including apoptosis, autophagy, and the IFN-β response. Further experiments are necessary to identify the step in the NF-κB pathway that the M protein disrupts; however our results suggest that M is regulating the conical pathway. We also propose that VSV regulates NF-κB in a cell specific manner. Our preliminary findings indicate that NF-κB is activated in wt-infected HeLa cells at late times postinfection, and activation occurs even later in cells infected with R1 (data not shown). This cell type specific regulation of NF-κB is currently under investigation.
Highlights.
VSV M protein is sufficient to block activation of NF-κB.
M residue 51 is essential for this function
IκB kinase (IKK) activity is necessary for NF-κB activation and interferon-β mRNA expression.
Acknowledgments
We thank Douglas Lyles for generously providing the recombinant virus strains. We thank Sanjay Maggirwar for his invaluable scientific input, and generous use of reagents and equipment. We also thank Jonelle Mattiacio, Joanna Shisler, Hyla Sweet, and Brian Ward for their support and insightful comments on the manuscript. We thank the Rochester Institute of Technology (RIT) College of Science (COS) for helping to support this work and the COS Summer Undergraduate Research Program and the RIT Honors Program for supporting many of the undergraduates who contributed to this work. This research was supported by the NIH/NIAID grant R15 AI058969 (M. F.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J Virol. 2003;77:4646–4657. doi: 10.1128/JVI.77.8.4646-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle PA. IkappaB-NF-kappaB structures: at the interface of inflammation control. Cell. 1998;95:729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- Black BL, Lyles DS. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J Virol. 1992;66:4058–4064. doi: 10.1128/jvi.66.7.4058-4064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel D, Harmison GG, Schubert M. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J Virol. 1990;64:1716–1725. doi: 10.1128/jvi.64.4.1716-1725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulares AH, Ferran MC, Lucas-Lenard J. NF-kappaB activation Is delayed in mouse L929 cells infected with interferon suppressing, but not inducing, vesicular stomatitis virus strains. Virology. 1996;218:71–80. doi: 10.1006/viro.1996.0167. [DOI] [PubMed] [Google Scholar]
- Desforges M, Charron J, Bérard S, Beausoleil S, Stojdl DF, Despars G, Laverdière B, Bell JC, Talbot PJ, Stanners CP, Poliquin L. Different host-cell shutoff strategies related to the matrix protein lead to persistence of vesicular stomatitis virus mutants on fibroblast cells. Virus Res. 2001;76:87–102. doi: 10.1016/s0168-1702(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Djavaheri-Mergny M, Amelotti M, Mathieu J, Besançon F, Bauvy C, Codogno P. Regulation of autophagy by NFkappaB transcription factor and reactives oxygen species. Autophagy. 2007;3:390–392. doi: 10.4161/auto.4248. [DOI] [PubMed] [Google Scholar]
- Dunigan DD, Baird S, Lucas-Lenard J. Lack of correlation between the accumulation of plus-strand leader RNA and the inhibition of protein and RNA synthesis in vesicular stomatitis virus infected mouse L cells. Virology. 1986;150:231–246. doi: 10.1016/0042-6822(86)90282-5. [DOI] [PubMed] [Google Scholar]
- Espert L, Codogno P, Biard-Piechaczyk M. Involvement of autophagy in viral infections: antiviral function and subversion by viruses. J Mol Med (Berl) 2007;85:811–823. doi: 10.1007/s00109-007-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferran MC, Lucas-Lenard JM. The vesicular stomatitis virus matrix protein inhibits transcription from the human beta interferon promoter. J Virol. 1997;71:371–377. doi: 10.1128/jvi.71.1.371-377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Gallego P, Campagna M, González-Santamaría J, Martínez G, Marcos-Villar L, Vidal A, Esteban M, Rivas C. Activation of NF-kB Pathway by Virus Infection Requires Rb Expression. PLoS ONE. 2009;4:e6422. doi: 10.1371/journal.pone.0006422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Her LS, Lund E, Dahlberg JE. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science. 1997;276:1845–1848. doi: 10.1126/science.276.5320.1845. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Nguyen TL, Arguello M, Nakhaei P, Paz S. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene. 2006;25:6844–6867. doi: 10.1038/sj.onc.1209941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann HP, Brockhaus M, Baeuerle PA, Remy R, Kolbeck R, van Loon AP. Expression of the types A and B tumor necrosis factor (TNF) receptors is independently regulated, and both receptors mediate activation of the transcription factor NF-kappa B. TNF alpha is not needed for induction of a biological effect via TNF receptors. J Biol Chem. 1990;265:22409–22417. [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Kopecky SA, Lyles DS. Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J Virol. 2003a;77:4658–4669. doi: 10.1128/JVI.77.8.4658-4669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky SA, Lyles DS. The cell-rounding activity of the vesicular stomatitis virus matrix protein is due to the induction of cell death. J Virol. 2003b;77:5524–5528. doi: 10.1128/JVI.77.9.5524-5528.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky SA, Willingham MC, Lyles DS. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J Virol. 2001;75:12169–12181. doi: 10.1128/JVI.75.24.12169-12181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Lodish HF, Porter M. Vesicular stomatitis virus mRNA and inhibition of translation of cellular mRNA--is there a P function in vesicular stomatitis virus? J Virol. 1981;38:504–517. doi: 10.1128/jvi.38.2.504-517.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles DS, McKenzie MO. Activity of vesicular stomatitis virus M protein mutants in cell rounding is correlated with the ability to inhibit host gene expression and is not correlated with virus assembly function. Virology. 1997;229:77–89. doi: 10.1006/viro.1996.8415. [DOI] [PubMed] [Google Scholar]
- Marcus PI, Sekellick MJ. Interferon induction by viruses. XV. Biological characteristics of interferon induction-suppressing particles of vesicular stomatitis virus. J Interferon Res. 1987;7:269–284. doi: 10.1089/jir.1987.7.269. [DOI] [PubMed] [Google Scholar]
- Paik SY, Banerjea AC, Harmison GG, Chen CJ, Schubert M. Inducible and conditional inhibition of human immunodeficiency virus proviral expression by vesicular stomatitis virus matrix protein. J Virol. 1995;69:3529–3537. doi: 10.1128/jvi.69.6.3529-3537.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JM, Her LS, Varvel V, Lund E, Dahlberg JE. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol Cell Biol. 2000;20:8590–8601. doi: 10.1128/mcb.20.22.8590-8601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- Simon KO, Whitaker-Dowling PA, Youngner JS, Widnell CC. Sequential disassembly of the cytoskeleton in BHK21 cells infected with vesicular stomatitis virus. Virology. 1990;177:289–297. doi: 10.1016/0042-6822(90)90482-7. [DOI] [PubMed] [Google Scholar]
- Stanners CP, Francoeur AM, Lam T. Analysis of VSV mutant with attenuated cytopathogenicity: mutation in viral function, P, for inhibition of protein synthesis. Cell. 1977;11:273–281. doi: 10.1016/0092-8674(77)90044-7. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.ima03bs21. Appendix 3, Appendix 3B. [DOI] [PubMed] [Google Scholar]
- von Kobbe C, van Deursen JM, Rodrigues JP, Sitterlin D, Bachi A, Wu X, Wilm M, Carmo-Fonseca M, Izaurralde E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol Cell. 2000;6:1243–1252. doi: 10.1016/s1097-2765(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Wagner RR, Huang AS. Inhibition of RNA and interferon synthesis in Krebs-2 cells infected with vesicular stomatitis virus. Virology. 1966;28:1–10. doi: 10.1016/0042-6822(66)90300-x. [DOI] [PubMed] [Google Scholar]
- Wang J, Basagoudanavar SH, Wang X, Hopewell E, Albrecht R, García-Sastre A, Balachandran S, Beg AA. NF-kappa B RelA subunit is crucial for early IFN-beta expression and resistance to RNA virus replication. J Immunol. 2010;185:1720–1729. doi: 10.4049/jimmunol.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]