This study shows in the monkey how the ability to smoothly track the current (here and now) location of a moving target is learned. We explain how practice gradually changes the coupling between two visuomotor streams (from the retina to the saccade and pursuit premotor neurons) to synchronize the movement of an effector (the eye) with the motion of a visual target in the physical world, even under constant reward conditions.
Keywords: tracking, saccade, pursuit, prediction, synchronism
Abstract
An object moving in the visual field triggers a saccade that brings its image onto the fovea. It is followed by a combination of slow eye movements and catch-up saccades that try to keep the target image on the fovea as long as possible. The accuracy of this ability to track the “here-and-now” location of a visual target contrasts with the spatiotemporally distributed nature of its encoding in the brain. We show in six experimentally naive monkeys how this performance is acquired and gradually evolves during successive daily sessions. During the early exposure, the tracking is mostly saltatory, made of relatively large saccades separated by low eye velocity episodes, demonstrating that accurate (here and now) pursuit is not spontaneous and that gaze direction lags behind its location most of the time. Over the sessions, while the pursuit velocity is enhanced, the gaze is more frequently directed toward the current target location as a consequence of a 25% reduction in the number of catch-up saccades and a 37% reduction in size (for the first saccade). This smoothing is observed at several scales: during the course of single trials, across the set of trials within a session, and over successive sessions. We explain the neurophysiological processes responsible for this combined evolution of saccades and pursuit in the absence of stringent training constraints. More generally, our study shows that the oculomotor system can be used to discover the neural mechanisms underlying the ability to synchronize a motor effector with a dynamic external event.
NEW & NOTEWORTHY
This study shows in the monkey how the ability to smoothly track the current (here and now) location of a moving target is learned. We explain how practice gradually changes the coupling between two visuomotor streams (from the retina to the saccade and pursuit premotor neurons) to synchronize the movement of an effector (the eye) with the motion of a visual target in the physical world, even under constant reward conditions.
in primates, an object moving in the visual field elicits a saccadic eye movement that brings the image of the target onto the fovea. The foveation is then maintained more or less efficiently by two types of eye movements: low-velocity movements (pursuit), and high-velocity catch-up saccades (Dodge 1903; Westheimer 1954). Sometimes, more easily in human subjects (Barnes 2008; Fuchs 1967a; Soechting et al. 2010), the visual tracking looks as if the gaze direction was locked onto the moving target, i.e., as if no delay separated the time-varying changes of target position and the time-varying changes of extraocular muscle tension. Such a spatiotemporal coincidence is remarkable when one considers that the retinal signals are transmitted to the motor neurons through multiple parallel channels connecting neuronal populations characterized by different integration times, with variable conduction speeds and substantial delays. Because of these delays, the foveal tracking cannot be driven by the current retinal signals as they correspond to past target positions.
Among the explanations proposed to account for this gaze-on-target locking (Bahill 2000; Deno et al. 1995; St-Cyr and Fender 1969), a conjecture is that the oculomotor system is driven by an internal model of the target's trajectory (Dallos and Jones 1963; Whittaker and Eaholtz 1982). Somehow, the delayed retinal signals would be backed up by extraretinal signals to derive the commands used to drive the oculomotor system (Newsome et al. 1988; Ono 2015). Several decades on, this notion of internal model remains largely used, but its neural implementation is not fully specified (Ferrera and Barborica 2010; Lisberger 2009). In fact, the major obstacle that complicates this neurophysiological characterization is the distributed nature of the representation of a visual target in the brain (e.g., Nowak and Bullier 1997; Schmolesky et al. 1998; Sparks et al. 1976). Indeed, a moving visual target involves flows of neural activity which do not remain local but propagate both “vertically” (i.e., across the successive relays interposed between the retina and the extra-ocular motor neurons) and “horizontally” (diffusion within each relay). In other words, the internal model of a moving target corresponds to entangled flows of neural activity which are spatiotemporally distributed in the brain, from the retinal ganglion cells to the oculomotor neurons (for reviews, see Krauzlis 2004; Lynch 2009; Mustari et al. 2009; and our discussion).
Yet spectacularly, when a saccade toward a target moving in the peripheral visual field is perturbed by a brief microstimulation in the deep superior colliculus (SC), the electrically-induced change in eye position is corrected in flight or after a short delay, bringing the gaze direction to the location where unperturbed saccades would have landed at about the same time (Fleuriet and Goffart 2012). Because the correction saccades did not overshoot along the motion path but landed accurately on the moving target or close to it (mostly during contralateral perturbations), it has been suggested that the interceptive saccades (i.e., the saccades made from a static gaze position toward a moving target) are driven by a command encoding at best the expected and current spatiotemporal coordinates (the here-and-now location) of the target (see also Goffart 2016; Quinet and Goffart 2015). It is not known whether the same kind of command feeds the oculomotor system during pursuit eye movements. On the basis of behavioral experiments, it has been proposed that “the saccadic and smooth pursuit systems share the same position and motion inputs” (Orban de Xivry and Lefèvre 2007, p. 15). However, it is not certain that these input signals involve the same neural channels. The idea that the “motion” signals driving pursuit eye movements involve the same neurons, the same neural channels as the “motion” signals driving saccadic eye movements, is controversial. Numerous neurophysiological observations indicate independent control of pursuit and saccadic eye movements (e.g., Büttner et al. 1994; Henn et al. 1984; Lynch 2009; May et al. 1988; Morrow and Sharpe 1995; Ohtsuka et al. 1992; our discussion). Moreover the discrepancies shown in the control of the respective trajectories (e.g., Bennett and Barnes 2006; Engel et al. 1999; Puckett and Steinman 1969) argue against the idea that “saccades and pursuit eye movements are outcomes of a single sensorimotor process” (Orban de Xivry and Lefèvre 2007).
However, the ability to generate eye movements which are locked onto the current location of a moving target suggests that the pursuit eye movements also can be driven by a command encoding the current location of the target. This performance is obviously not possible unless the upcoming target trajectory is already known. Somehow, the trajectory must have been learned. Moreover, its neural image must be retrieved during the course of the tracking to get oculomotor commands which fit with the current target location, hence our working hypothesis that the ability to accurately track the current location of a moving target requires learning. In the present study, we provide evidence supporting this hypothesis by studying the evolution of oculomotor tracking in monkeys which were never trained to track a small target displayed on a video monitor. We show how a visual target moving with a constant speed along a simple straight path is tracked and how its trajectory is gradually learned without restrictive spatiotemporal constraints (quasi-free visual tracking task). Initially, the monkeys did not exhibit an efficient smooth pursuit but a tracking which was mostly saltatory, i.e., composed of large catch-up saccades (saccades made in response to a moving target while the eye slowly moves). From this initial state where the gaze direction lagged behind the target location most of the time, the oculomotor behavior evolved with successive sessions to a mode where the gaze direction sometimes appeared locked onto the current target location. We document the smoothing of tracking eye movements at different time scales, from the single trial to successive trials in a session and across successive daily sessions.
MATERIALS AND METHODS
Subjects and surgical procedures.
Six adult (Bo and C: 4 yr old; A, G, and M: 9 yr old; Bi: 11 yr old; weights: 8–13 kg) male rhesus monkeys (Macaca mulatta) were used for this study. For the monitoring of their eye movements with the electromagnetic induction technique, a three-turn search coil was sutured with silk to the sclera under the conjunctiva of one eye under local (with oxybuprocaine) and general anesthesia (with isoflurane) and aseptic conditions. Lead wires were passed under the skin to a connector located on the top of the skull. During the same surgery, a head restraint fixture was positioned on the top front center of the skull and secured with bone cement (Palacos) and stainless screws attached to the skull. This surgical procedure and experiments were performed in accordance with the guidelines from the French Ministry of Agriculture and the European Community, as well as the Regional Ethics Committee approval (no. A13/01/13). Care and maintenance was under the auspices of a full-time veterinarian and zoo-technical staff.
Eye movement recording and visual stimulation.
During the experimental sessions, the monkeys were seated in a chair with the head restrained, facing a LCD video monitor (Samsung SyncMaster, P227f; 1,280 × 1,024 pixels, 100-Hz refresh rate, 39 × 29 cm) located at a viewing distance of 38 cm. The visual target was a Gaussian blurred white disk of 0.4° diameter displayed over a gray background. Eye movements were measured with a phase-angle detection system (CNC Engineering, 3-ft. coil frame) and voltage signals encoding separately the horizontal and vertical positions of the eye were sampled at 500 Hz. The data acquisition, the on-line control of the oculomotor performance and the triggering of stimuli were controlled by a PC using the Beethoven software package (Ryklin Software). Eye position signals were calibrated by having the animal fixate stationary targets presented at ±16° along the horizontal or vertical meridians.
Behavioral tasks.
In this study, we describe the tracking and its evolution during and over several successive daily sessions in naive monkeys which were never trained to track a small visual target moving on the display of a video monitor. These sessions started several weeks after the monkeys were trained to make saccades to static targets located at various eccentricities, ranging from 0 to ±8° horizontally at vertical eccentricity of ±16° (see methods in Quinet and Goffart 2015). Thus the recovery from the eye coil surgery and the habituation of the head restrained condition could not account for the oculomotor behavior described here. Three monkeys (A, G and M) were used to study the generation of saccades toward a transient moving target (Quinet and Goffart 2015). Each experimental session (lasting 1–2 h) was composed of trials whose number depended upon the monkey's motivation (the monkeys were water-deprived in their home cage). Each trial started with a warning tone which preceded the appearance of a target at the center of visual display (straight ahead). After the monkey directed its gaze toward its location within a surrounding spatial window (4–6° radius centered on the target) and for a variable fixation interval (750, 1,000, 1,250 or 1,500 ms), the central target disappeared, while at the same time an identical peripheral target appeared 16° upward or downward on the vertical meridian, and smoothly moved in a direction orthogonal to the step, i.e., horizontally. The target motion started immediately after the extinction of the central target, except in the first tested monkey (monkey G), where a 200-ms gap interval separated the offset of the central target from the onset of the moving target. This gap was removed in the other five monkeys because it favored the generation of short-latency saccades. Consequently, most saccade landing positions were distributed near the starting position of the moving target. The target moved with a constant speed (20°/s) for 800 ms. The possible combinations of target position (16° upward or downward) and motion (leftward or rightward) were pseudorandomized to prevent the generation of anticipatory saccade and pursuit-like responses. The monkeys' task was to make a saccade toward the moving target and to track it until the location where it disappeared (16° to the right or the left, in the upper or lower visual fields) at the end of the trial. We used very relaxed fixation constraints for controlling the accuracy of eye movements. Indeed, during the early training sessions with static targets, for the monkey to be rewarded with a large drop of sweet juice or water at the end of the trial, the gaze had to be directed within a window of 5–7° radius around the target. During the tracking tasks, the gaze had to track the moving target within an ellipsoidal spatial window around the moving target (range: 10–12° horizontally and 6–10° vertically). Otherwise, the trial was aborted and a new trial started. The monkeys performed this task during 10 (monkeys A, Bi, M and G) or 12 (monkeys Bo and C) successive sessions.
Data analysis.
All data were digitized on-line and analyzed off-line using a software program that detected the onset and offset of the horizontal and vertical saccade components on the basis of a velocity threshold (30°/s). The results of this automatic detection were checked by inspecting each trial individually and were adjusted manually when necessary. To make sure that the first (interceptive) saccades were not anticipatory, those with a latency <80 ms were excluded from analysis (0.30, 0.09, 0.00, 0.00, 0.04 and 2.99% of trials in monkeys A, Bo, Bi, C, M and G, respectively). The total number of trials was 5,195, 3,253, 3,208, 3,891, 7,206, and 4,015 in monkeys A, Bo, Bi, C, M and G, respectively. Several other parameters were calculated to quantify the evolution of the oculomotor tracking behavior. The pursuit velocity gain corresponds to the ratio of eye to target velocities. The pursuit displacement gain (as a percentage of target displacement) corresponds to the horizontal eye displacement accumulated during all of the intersaccadic (pursuit) intervals. The tracking displacement gain corresponds to the ratio of eye to target displacements from the end of the interceptive saccade to the end of the trial. The amplitude, the precision and the accuracy of saccades (interceptive and catch-up) were also analyzed. By convention, positive values correspond to upward and rightward eye movements; negative values to downward and leftward eye movements. Figure 1 illustrates the different events and episodes which were used as landmarks for measuring the horizontal component of tracking eye movements. After the interceptive saccade (first saccade labeled IS), consecutive pursuit episodes (intervals P1, P2, P3) were separated from each other by catch-up saccades (CS1, CS2). In this task, we never observed slow eye movements that preceded the onset of interceptive saccades, likely because the target was fairly peripheral and the direction of its motion unpredictable (left or right).
Fig. 1.
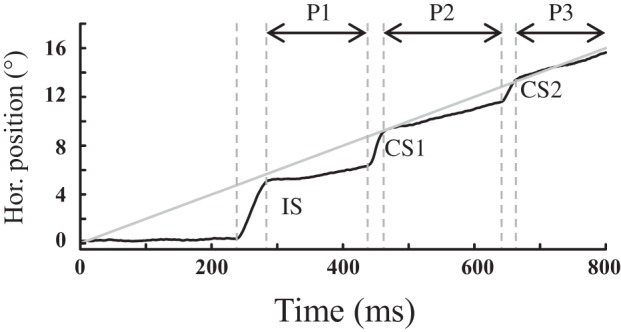
Definition of the different periods of oculomotor tracking after the onset of the motion of the target in the peripheral visual field. After the interceptive saccade (IS), a succession of catch-up saccades (CS1, CS2, etc.) are made to foveate the moving target (gray trace). During the intersaccadic intervals (P1, P2, P3, etc.), the eye pursues the target with a velocity which more or less matches with the target speed.
RESULTS
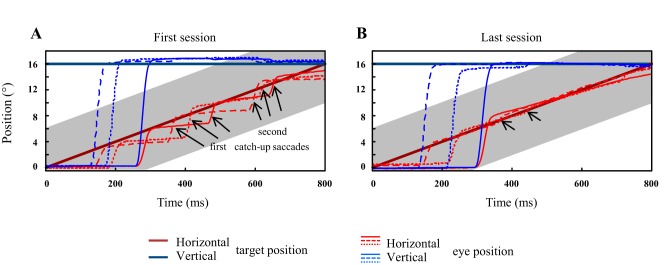
Figure 2 illustrates qualitatively with three example trials how the tracking eye movements changed between the first and last sessions in monkey A. During the first session (Fig. 2A), after the saccade to the moving target in the upper right visual field, the eye followed it with a velocity that was not sufficient to maintain its image on the fovea. A first catch-up saccade was made to refoveate the target. The velocity of the following eye movement still did not match the target speed, requiring a second catch-up saccade (see arrows). After 10 training sessions (Fig. 2B), the monkey was able to smoothly track the target immediately and continuously after the interceptive saccade; the catch-up saccades either disappeared or their amplitude was largely diminished. This saccade-free tracking was rarely, if ever, observed during the first session. In the following, we show how the oculomotor tracking changed during the course of one trial, during the course of the first and last sessions and across the successive sessions.
Fig. 2.
Typical oculomotor behavior of a monkey tracking a moving visual target. The eye position (horizontal: red; vertical: blue) is plotted as a function of time after the target motion onset for three trials recorded during the first (A) and last training sessions (B). The time course of target position is illustrated by the thick lines (horizontal: red; vertical: blue). The selected trials were recorded in monkey A when it tracked a target moving in the upper right quadrant. The shaded zone illustrates the width of the window within which the gaze had to remain to avoid the interruption of the trial. The arrows indicate the catch-up saccades.
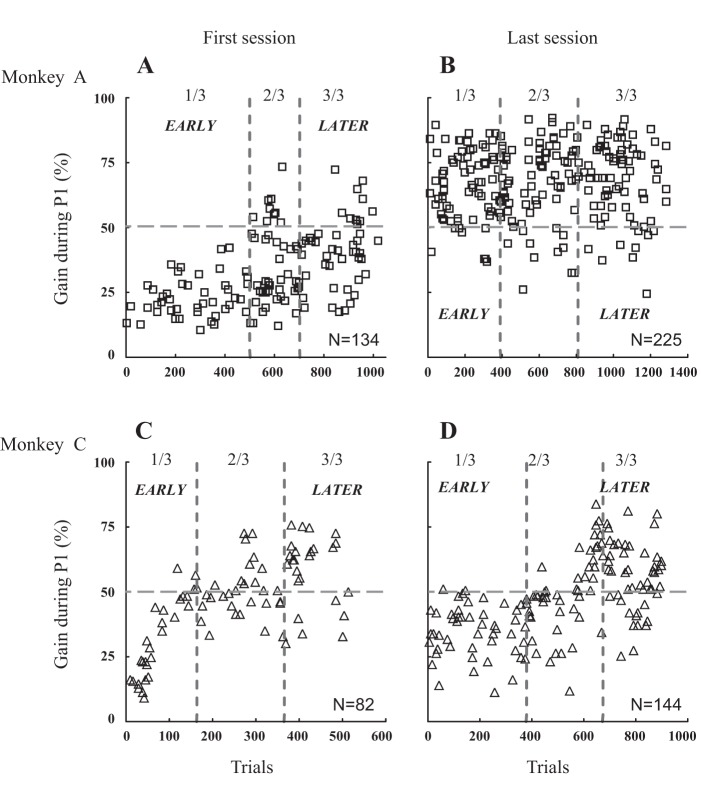
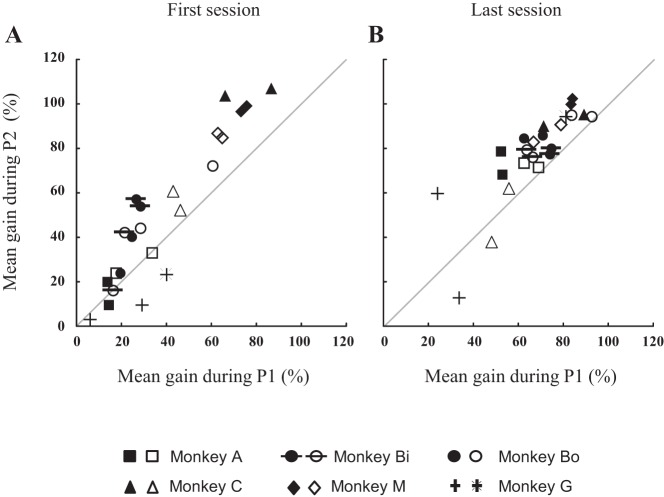
Figure 3 describes the slow (pursuit) eye movement which followed the interceptive saccade during the course of the first and last sessions when the monkeys (A and C) tracked the target moving in the upper right visual quadrant. During the first session (Fig. 3, A and C), the eye velocity rarely exceeded 10°/s, i.e., one-half value of target speed (50% gain) when we consider the early trials (first one-third of the total number of trials). During the end of the session (later trials, last one-third of the total number of trials), the velocity exceeded 10°/s in a larger proportion of trials (22% and 70% in monkeys A and C, respectively). This enhancement of velocity between the early and later parts of the session was not observed after 10 days of practice in monkey A (Fig. 3B; early: 93%, later: 88%) but still in monkey C (Fig. 3D; early: 4%, later: 73%). In the latter animal, the improvement between the first and the last sessions was not as clear cut as in the other animals (both for leftward and rightward target motions). Regardless of whether the session was the first or the last session and whether the postsaccadic eye velocity was measured early or later during the session, the graphs in Fig. 3 also show that the gain values, and by inference the pursuit velocities, were highly variable across the trials.
Fig. 3.
Evolution of pursuit velocity after the interceptive saccade across trials and during the first (A and C) and last sessions (B and D). The pursuit velocity gain was measured during the first intersaccadic interval, i.e., after the interceptive saccade when the target moved in the upper right field during the first and last session recorded in monkey A (A and B) and monkey C (C and D). Dashed lines segregate the data set into three equal parts. The early part corresponds to the first one-third of the total number of trials; the late trials, the last one-third. A: during the first session, the gains of monkey A ranged from 11 to 43% (mean ± SD = 23 ± 8%) during the early trials and from 18 to 73% (42 ± 13%) during the later trials. C: in monkey C, they ranged from 9 to 59% (31 ± 15%) during the early trials and from 30 to 76% (57 ± 14%) during the later trials. B and D: during the last session, the gains did not consistently change between the early and later trials (from 67 ± 13% to 69 ± 15% in monkey A and from 35 ± 10% to 56 ± 13% in monkey C).
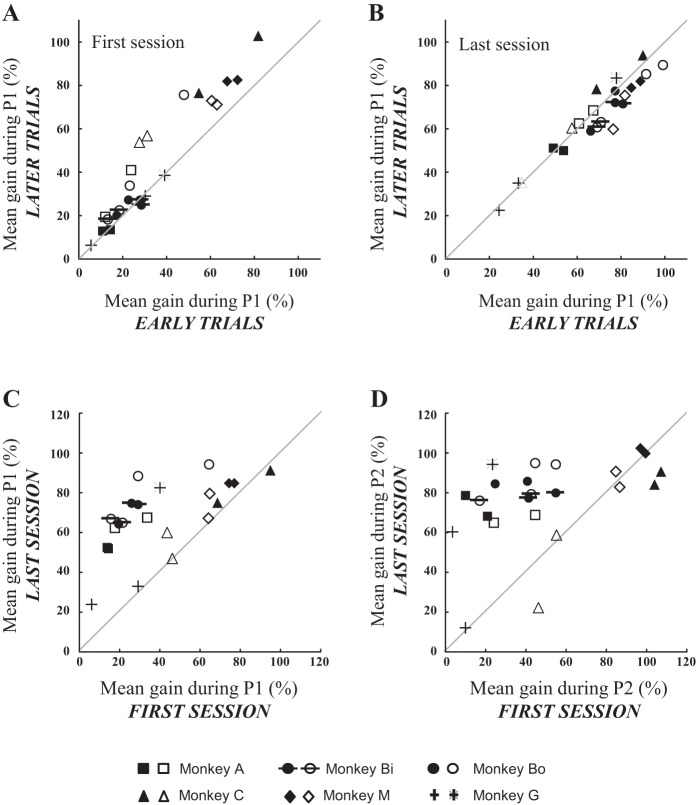
The increases in pursuit velocity after the interceptive saccade between the early and later trials and between the first and last sessions are quantitatively documented in Fig. 4 for all monkeys and all quadrants. The average pursuit gains during the interval P1 (the first intersaccadic interval) significantly increased from 34 ± 22% (range: 5–82%) to 44 ± 28% (range: 6–103%) between the early and the later trials of the first session (Fig. 4A, 28% average increase, average gain values above the diagonal line, Wilcoxon nonparametric test, P < 0.001). By contrast, they overall were not significantly changed during the last session (Fig. 4B, P value = 0.68). Concerning the increases between the first and last sessions (Fig. 4C), the average horizontal eye velocity during the first intersaccadic interval increased from 40 ± 25% (range: 6–95%) to 68 ± 18% (24–95%) between the first and the last sessions, and the paired comparison revealed a statistically significant increase (P < 0.001, 71% average increase). Figure 4D shows that the velocity enhancement was not restricted to the first intersaccadic interval (interval P1, see Fig. 1). Indeed, the eye velocity after the first catch-up saccade (during the interval P2) also increased from 49 ± 32% (3–107%) to 76 ± 22% (12–102%) between the first and last sessions, and the paired comparison revealed that this enhancement (55% on average) was statistically significant (P < 0.001).
Fig. 4.
Change of pursuit velocity after the interceptive saccade during and between the first and the last training sessions. A and B: the graphs compare the average pursuit gains between the early (first one-third of trials) and the later (last one-third) trials, for the first (A) and the last (B) training sessions. The gains were measured during the first intersaccadic interval (P1, see Fig. 1). C and D: the average gains during the first (C) and second (D) intersaccadic intervals (P1 and P2, respectively) were calculated for the total number of trials. Solid (or crosses) and open (or stars) symbols correspond to the leftward and rightward target motion, respectively. Different symbols correspond to different monkeys.
In summary, after several sessions of practice, the velocity of pursuit eye movements was higher than during the first session. Within each session, an enhancement could be observed but not systematically. Instead, the increase consisted of more frequent trials characterized by a higher pursuit gain. Before describing some of the factors that accounted for this variability, we describe how the pursuit velocity changed across successive sessions.
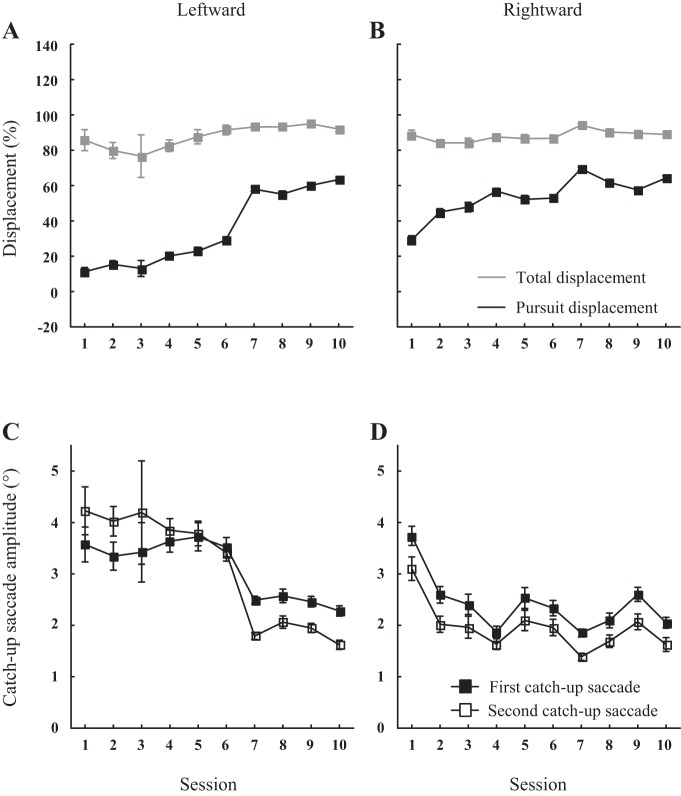
The kinetics of the changes in pursuit displacement depended on the monkey and the direction (leftward or rightward) of the target motion. Figure 5 describes for one monkey (A) how the eye displacement cumulated during the pursuit intervals (black symbols) increased, while the total eye displacement (sum of saccadic and pursuit displacements from the end of the interceptive saccade to the end of the trial; gray symbols) remained quite unchanged across the successive sessions. The difference between the leftward and rightward motions likely results from the fact that different populations of visual, visuomotor and motor neurons are involved in leftward and rightward eye movements. Depending on the monkey, the evolution could be gradual, exhibit step-like changes, short periods of stagnation and even regressions (not shown).
Fig. 5.
Evolution of visual tracking behavior over successive sessions. A and B: the evolution of the total (gray lines) and pursuit (black lines) eye displacements is illustrated for monkey A. The kinetics is documented separately for leftward (A) and rightward (B) target motion direction because they involve different neuronal populations. Error bars correspond to confidence intervals. C and D: for the same monkey, the evolution of the amplitude of the first (■) and second (□) catch-up saccades is also illustrated. The kinetics are also documented separately for leftward (C) and rightward (D). Again, error bars correspond to confidence intervals.
Obviously, since the total eye displacement barely changed across the successive sessions, the evolution of tracking was not restricted to the pursuit component: changes in the generation of catch-up saccades were also observed. First, across all monkeys and quadrants, their number significantly diminished from 1.6 ± 0.5 (range: 0.9–2.5) to 1.2 ± 0.6 saccade (range: 0.4–2.9) between the first and the last sessions. Second, Fig. 5, C and D, shows how the amplitude of catch-up saccades also diminished over the sessions with a kinetics which mirrored the changes of pursuit displacements. This mirror evolution is the corollary of the increase of pursuit displacement during the intersaccadic intervals, while the total eye displacement remained scarcely changed over the training sessions (Fig. 5, A and B). Intriguingly, the amplitude of the first and second catch-up saccades exhibited similar kinetics of evolution between the same sessions: the step visible in Fig. 5A between sessions 6 and 7 is also observed in Fig. 5C. An exception was observed in the leftward tracking of monkey G. In this monkey, the amplitude of the first catch-up saccades did not evolve as quickly as the amplitude of the second ones. In the other monkeys, the reduction of saccade amplitude likely resulted from the facts that the gaze direction was closer to the target later during the course of the trial.
In summary, like the pursuit component, the generation of catch-up saccades evolved during the course of trials. The tracking tended to become smoother as a result of a reduction of both the occurrence and the amplitude of catch-up saccades, while at the same time the pursuit velocity increased. The mirror evolution of the saccadic and pursuit components suggests that the learning was not restricted to the oculomotor system controlling the generation of pursuit eye movements, but concerned both saccade-related and pursuit-related systems.
This deduction is supported by the fact that the reduction of amplitude between the first and second catch-up saccades was associated with an increase in the pursuit velocity between the first and second intersaccadic intervals. To appreciate this change of pursuit velocity, Fig. 6 shows the enhancement of pursuit velocity between the intervals P1 and P2 for all monkeys and quadrants. The average pursuit gain was significantly higher after the first catch-up saccade than before, for both the first (Fig. 6A; average difference = 12 ± 14%, P < 0.001) and the last sessions (Fig. 6B; difference = 11 ± 11%, P < 0.001).
Fig. 6.
Evolution of pursuit velocity during the course of the trial. The average pursuit velocity gains were measured during the first (P1) and second (P2) intersaccadic intervals (see Fig. 1). This figure shows the gain change between the first and the second intersaccadic intervals for the first (A) and the last (B) sessions. Solid (or crosses) and open (or stars) symbols correspond to the leftward and rightward target motion, respectively. Different symbols correspond to different monkeys.
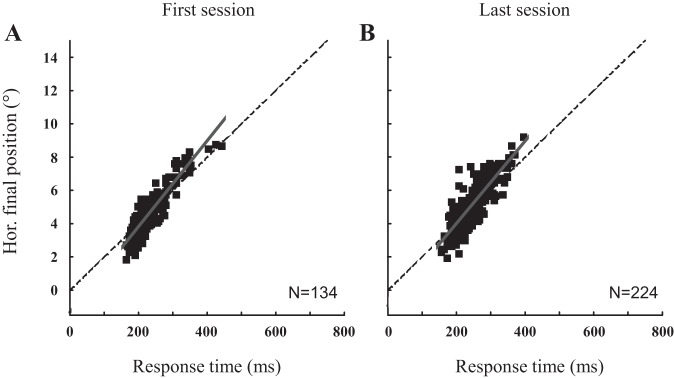
Because the interceptive saccades always precede the pursuit component, we now examine whether they can account for the enhancement of pursuit velocity. First, Fig. 7 describes for one monkey (A) the landing positions when the target moved in the upper right visual quadrant. The horizontal final position is plotted as a function of the response time (i.e., the time interval from the target motion onset to the saccade end) for the first and last sessions. The horizontal position of saccade endpoints increases linearly with the response time. The later the time of saccade landing, the more eccentric the horizontal position of its endpoint. During the first session, the slopes of the relations are close to the target speed (ranges: 15.6–31.3°/s, 17.1–35.3°/s, 16.9–29.9°/s and 20.4–28.1°/s for the upper right, upper left, lower left and lower right quadrant, respectively). They did not significantly change between the first and the last sessions (average difference = 0.8 ± 7.6°/s, Wilcoxon nonparametric P value = 0.29, all quadrants and all monkeys considered). In other words, the comparison of the slopes between the first and the last sessions did not reveal any change in the accuracy of interceptive saccades that could account for the changes in pursuit velocity.
Fig. 7.
Landing positions of interceptive saccades. For one monkey (monkey A), the horizontal final eye positions are plotted as a function of the response time (saccade landing time) of interceptive saccades made in response to a target moving in the upper right visual field for the first (A) and the last (B) session. The target position is illustrated by the dashed line. The gray line segment corresponds to the regression line fitting the data. The slope values are 25.8°/s and 24.7°/s for the first and last session, respectively.
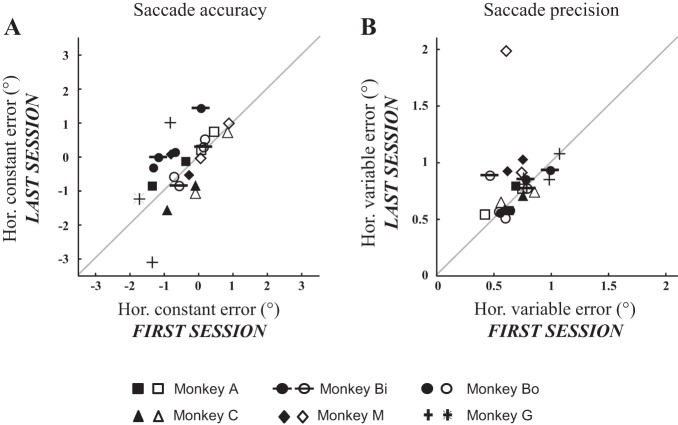
To test further the presence of any change in the horizontal amplitude of interceptive saccades between the first and last sessions, we also compared their accuracy and precision (Fig. 8). For each monkey and each quadrant, the accuracy was estimated by calculating the average value of the differences between the horizontal eye and target positions at saccade end (horizontal constant error), whereas the precision was estimated by calculating the standard deviation of these differences (horizontal variable error). The constant errors ranged from −1.7 to 0.9° (mean ± SD = −0.4 ± 0.7°) during the first session and from −3.1 to 1.5° (−0.2 ± 1.0°) during the last session. The paired comparison did not reveal any significant difference between these sessions (average difference = 0.2 ± 0.8°, P value = 0.2). Concerning the variable errors, they ranged from 0.4 to 1.1° (0.7 ± 0.2°) during the first session and from 0.5 to 2.0° (0.8 ± 0.3°) during the last session. Again, the paired comparison did not show any significant difference of precision between the first and last sessions (average difference = 0.1 ± 0.3°, P value = 0.08). Finally, we could not find any statistically significant correlation between the changes (%values) in pursuit velocity (Fig. 4C) and the changes (%values) in saccade accuracy (nonparametric correlation coefficient R = 0.31, P value = 0.14) and precision (R = 0.12, P value = 0.58). In summary, the changes in pursuit velocity that were detected after the interceptive saccade between the first and last sessions were not associated with any significant change in the amplitude of interceptive saccades.
Fig. 8.
Accuracy and precision of interceptive saccades. The average constant (A) and variable (B) errors are compared between the first and the last training sessions (see text for details). Solid (or crosses) and open (or stars) symbols correspond to the leftward and rightward target motion, respectively. Different symbols correspond to different monkeys.
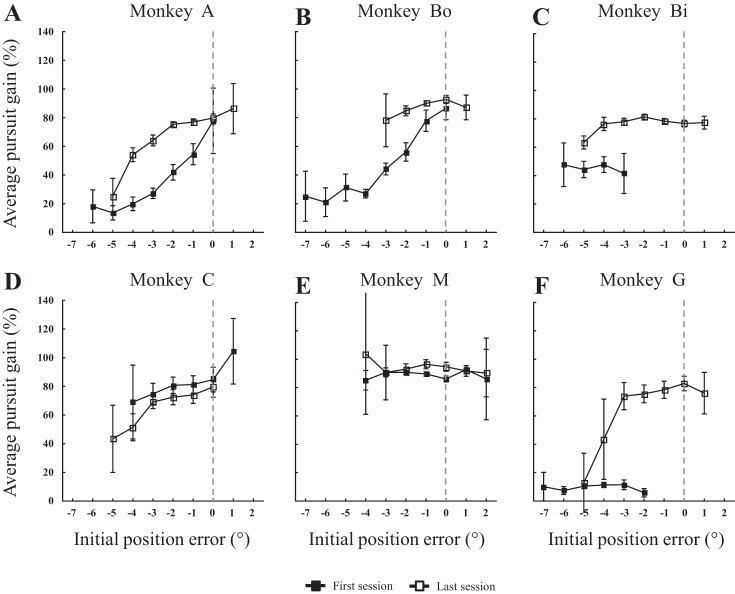
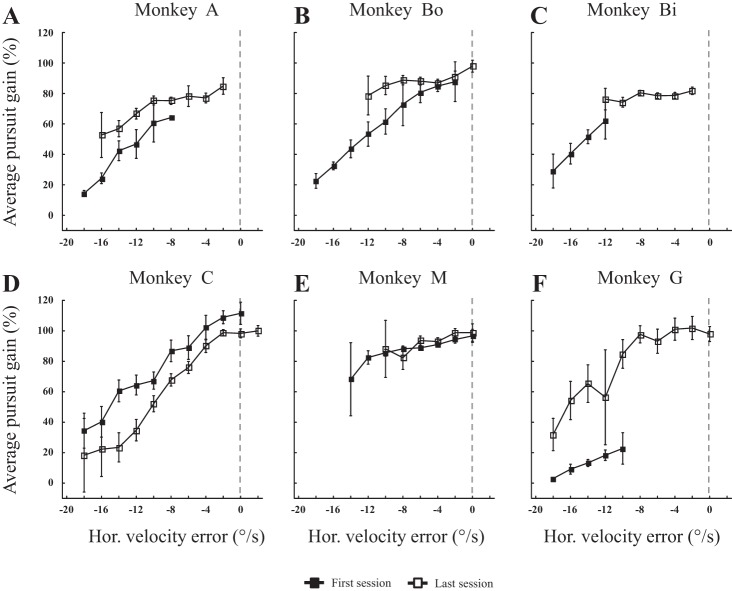
Regardless of the accuracy and precision of saccades, two types of visual signals have been proposed to enhance the pursuit velocity (Morris and Lisberger 1987; Pola and Wyatt 1980; Robinson 1965; Segraves and Goldberg 1994): 1) “position error” signals, i.e., signals related to the eccentricity of the target relative to the fovea (with the fovea estimated by the retinal zone used during fixation of a stationary target) at the onset of the catch-up saccade; and 2) “velocity error” signals related to the slip of the target image on the retina. Due to the difference between the target and eye velocities, the slip is null when the image of the moving target is maintained stable on the retina. Concerning the first type of signals, we found that they influenced the postsaccadic pursuit, regardless of whether the catch-up saccade was the first or the second one. Figure 9 documents this result for the pursuit velocity that immediately followed the first catch-up saccade: the more eccentric the target, the lower the gain of the pursuit. In four monkeys (A, Bo, Bi and G), the pursuit velocity was enhanced between the first and last sessions (Fig. 9, A, B, C and F). In two monkeys, this dependence barely changed (Fig. 9, D and E). The distribution of errors values exhibited a shift toward less negative values (negative because the gaze direction lagged behind the target).
Fig. 9.
Influence of horizontal target eccentricity at the onset of catch-up saccade on postsaccadic pursuit. A–F: for our six monkeys (monkeys A, Bo, Bi, C, M, and G, respectively), the average pursuit gain during the second intersaccadic intervals are plotted as a function of horizontal target eccentricity (called initial position error) at the onset of the first catch-up saccades. The relation is documented for the first (■) and last (□) sessions. Zero value of target eccentricity means that the horizontal eye and target position match at the onset of the catch-up saccade (dashed line). Negative values correspond to the fact that the horizontal eye position lags behind the target; positive values, when it leads ahead. The means were not calculated when any category contained less than four values.
Concerning the target-related retinal slip signals, they depend on the velocity of pursuit eye movements, such that the higher the velocity, the smaller the slip. Figure 6 (see above) showed that the gain during the second intersaccadic interval was higher than during the first one, indicating a reduction of the slip after the first catch-up saccade. Figure 10 illustrates the relation between the presaccadic horizontal velocity error and the postsaccadic pursuit gain for our six monkeys. A relation was found between these two parameters for both the first and the last sessions (solid and open squares, respectively). Indeed, the higher the retinal slip, the smaller the gain of the pursuit component after the first catch-up saccade. For matched values of retinal slip, the average pursuit gain was higher during the last session, except in monkey C where the trend was opposite, and in monkey M where it was barely noticeable. Moreover, small values of retinal slip were more frequent during the last session than during the first one. In summary, the increase of pursuit velocity between the first and the last sessions merely resulted from the fact that the target was brought to the fovea (with saccades) and more frequently maintained in the central visual field (positive feedback loop).
Fig. 10.
Influence of target retinal slip before the first catch-up saccade on the postsaccadic pursuit. A–F: for our six monkeys (monkeys A, Bo, Bi, C, M, and G, respectively), the average pursuit velocity gain during the second intersaccadic interval is plotted as a function of retinal slip (called horizontal velocity error) before the first catch-up saccade. The relation is documented for the first (■) and last (□) sessions. Zero value of target retinal slip means that the mean eye velocity during the first intersaccadic interval is equal to the target speed (dashed line). Conversely, a target-related retinal slip equal to −20°/s corresponds to the case where the eye does not move. The mean values were not calculated when less than four values were obtained for a given category.
DISCUSSION
This work describes the oculomotor behavior of monkeys tracking a visual target moving along a straight horizontal path with a constant speed and how this tracking develops over successive sessions of practice. After the interceptive saccade, gaze lagged behind the target most of the time because the velocity of pursuit eye movements rarely, if ever, matched the target speed (Figs. 2, 3 and 4) during the first session. After 10–12 days of practice, eye velocity increased on average by 70% (Figs. 2 and 4). The enhancement also occurred during the course of the trials (Fig. 6) and sessions (Figs. 3 and 4). During the intersaccadic intervals, as the pursuit velocity increased, the direction of gaze came closer to the target, a proximity which favored the enhancement of pursuit velocity (Figs. 9 and 10). In addition to the change of the slow component of visual tracking, practice also modified the generation of catch-up saccades, reducing both their occurrence and their amplitude (Fig. 5). Together, these modifications made the tracking less intermittent. The evolutions of the saccade and pursuit components exhibited a similar time course (Fig. 5), indicating that the adaptation involved the saccade and pursuit oculomotor systems in parallel. We could not find evidence for a relation between the changes in pursuit velocity and changes in the accuracy or the precision of interceptive saccades (Figs. 7 and 8). After discussing how these findings are consistent with previous studies, we explain the possible neurophysiological process underlying the link between the pursuit and saccade premotor systems. Then, we explain how the shift toward a smooth tracking is inevitable.
Learning the trajectory of a moving target.
Fuchs (1967b) reported that monkeys needed practice to be able to track a target for a sustained period of time. In response to a target moving at 30°/s, the first observed oculomotor responses were closely spaced saccades. Our study thoroughly documents this anecdotal report and describes how tracking a moving target becomes smoother with successive days of practice. The initially saltatory aspect of oculomotor tracking indicates that the signals related to the slip of the target on the fovea are not sufficient to pursue the target, even though they were immediately preceded by a relatively large interceptive saccade.
The enhancements of pursuit velocity do not result from increased levels of attention or motivation for the following reasons. First, the use of a large fixation window actually prevented our monkeys from being discouraged by trial abortions as a consequence of not fixating continuously and accurately the target during its motion in the early sessions. During each trial and from the beginning to the end of each session, the monkeys were free to track the target with saccadic or pursuit eye movements until it disappeared. Second, we could not find any evidence suggesting that the monkeys were more frequently attentive or more motivated during the late sessions than during the early sessions. Indeed, the latency of the vertical component of interceptive saccades was not different between the first and last sessions (average difference of median latencies = −2 ± 129 ms, P value = 0.93). Moreover, the peak velocity of the vertical component of interceptive saccades was significantly reduced on the last compared with the first sessions (average difference of median peak velocities = −51 ± 95 ms, P < 0.05). This velocity decrease would suggest instead a reduced (not an enhanced) level of “attention” or “motivation” during the last session. Finally, augmented “attention” (or “motivation”) is not a physiologically satisfactory argument for explaining why the size and the number of catch-up saccades diminished while the pursuit velocity increased. We will explain below how the foveation of the target provides a simpler explanation for the enhancements of pursuit velocity. It is also unlikely that the intermittent tracking was due to the fact that our monkeys were tested with the head restrained, preventing them to track the target with the head. Such a suggestion indeed implies that the head would have moved smoothly immediately after the interceptive saccade with horizontal amplitudes proportional to the target displacements. To our knowledge, such smooth tracking of the head in response of a target moving within the oculomotor range is not supported by any empirical evidence.
Instead of these general parameters (attention, motivation and head restraint), we propose that the evolution of tracking (from intermittent to continuous) over successive days merely results from the fact that, with practice, the target trajectory gradually becomes less uncertain, more “predictable.” This explanation is supported by the numerous studies which showed a low pursuit gain when an unpredictable target was being tracked (e.g., Bahill et al. 1980; Barnes and Collins 2015; Deno et al. 1995; Miall et al. 1986; Michael and Melvill-Jones 1966; Mrotek et al. 2006; St-Cyr and Fender 1969). Thus the repetitive practice of pursuing a moving target favors the learning of its trajectory such that, progressively, gaze can track the current location of the target. Interestingly, several weeks after the end of our training protocol and with no practice, the monkey A (only this monkey was tested) initially tracked the target in a saltatory manner when its tracking was reexamined under the same conditions. This anecdotal observation should be further explored because it offers the opportunity to identify the neural circuit subtending visuomotor working memory and to study the time course of its decay. The details that are learned to support the tracking is not merely the change in gaze direction (a path) completed within a given time interval. The instantaneous rate of change in position also matters (Bourrelly et al. 2015), as well as the presence of a visual target (Quinet et al. 2013). As shown elsewhere (Bennett and Barnes 2003; Churchland et al. 2003), a transient blanking of the target affects the tracking performance, as if gaze moved with a short “horizon of certainty” (or “confidence”). We do not know whether different trajectories can be learned in parallel, depending upon the shape of the target.
Basic aspects of pursuit learning.
Other studies have documented the learning properties of the pursuit system in monkeys which had been already trained to pursue a moving target. For example, it has been shown that the repeated exposure to a change in target speed 100 ms after the motion onset gradually changed the pursuit acceleration. For instance, Kahlon and Lisberger (1996) showed that the target motion-related signals which feed the oculomotor system during the initiation of pursuit are complemented by slightly delayed extraretinal signals built upon the experience of previous trials. Our study extends this influence of learning and practice to a larger time scale, since we show changes which happen not only after a moving target is foveated, but also over successive days. Kahlon and Lisberger (1996) also found that the changes in eye acceleration depended upon the target motion direction. Learning a change in speed when the target moved in one direction did not alter the response when the target moved in the opposite direction. Later, Chou and Lisberger (2002) demonstrated that the learning did not depend upon the orientation of the eye in the orbit: it happened even for motions initiated from untrained starting positions. These last two results are consistent with the different kinetics of evolution for leftward and rightward tracking eye movements reported here (Fig. 5, A and B: pursuit-related component; C and D: saccade-related component), regardless of whether the target moved in the upper or lower visual field.
However, in these other studies, the monkeys were required to track the moving target within a very small window (∼2°), whereas, in our protocol, the tolerance window surrounding the target was very large (10–12° horizontally and 6–10° vertically) and permitted our monkeys to freely track the target. The trial was not aborted when the direction of gaze was far from the target. The monkeys could track the target with a succession of saccades, and a reward was given at the end of each trial, regardless of the kind of tracking. This free tracking task allowed us to demonstrate that changes in pursuit eye movements can happen without experiencing unrewarded outcomes (or punishment). Thus conditional reward and supervised control of gaze direction are unnatural and unnecessary conditions for modifying the oculomotor behavior. They are unnatural because no “electronic fixation window” guides the oculomotor behavior in the natural environment. It is the brain processing of foveal and parafoveal signals which does it, naturally (Basso et al. 2000; Krauzlis et al. 2000; Lisberger and Westbrook 1985; Newsome et al. 1985). The learning could merely result from sustained changes in the functional connectivity induced by the repeated dynamic activation of the same chain of neuronal populations (Hebb 1961). Thus the predictability would correspond to a recruitment of neurons, where some neurons that were initially silent become active. The repeated foveation of the target permitted by saccades likely helped to induce this sustained activation (see below).
Linking the saccade-related and pursuit-related systems.
Saccades and pursuit eye movements are not outcomes of a single visuo-oculomotor process, as proposed by Orban de Xivry and Lefèvre (2007). Lesion and clinical studies indicate that the saccadic and pursuit eye movements are responses controlled by separate systems which involve different neuronal streams. For example, in the monkey, the lesion of the paramedian pontine reticular formation (place of saccade-related premotor neurons) abolishes almost completely the possibility to produce saccades (their amplitudes do not exceed 3°), but spares the generation of pursuit eye movements up to 30° (Henn et al. 1984). In the human subject, clinical studies show that, depending upon their location, cerebellar lesions can lead to saccadic disorders without impairment in pursuit (Büttner et al. 1994) or to pursuit deficits without saccadic alterations (Ohtsuka et al. 1992) (see also Engel et al. 2004). Finally, anatomical and electrophysiological studies in monkey show that several areas in the cerebral cortex are involved in the generation of pursuit and saccadic eye movements (frontal eye field, lateral intraparietal cortex, supplementary eye field, etc.). However, each of these areas is subdivided in adjacent subregions which do not overlap and which are, respectively, involved in saccades or pursuit (Keating 1993; Lynch 2009; May et al. 1988; Morrow and Sharpe 1995; Mustari et al. 2009; Suzuki et al. 1990, 1999, 2003). The parallel organization of the neural streams controlling saccadic and pursuit eye movements do not exclude interactions between them. Indeed, modulations in the firing rate can be found in some neural populations of the brain stem (nucleus raphe interpositus: Missal and Keller 2002; SC: Basso et al. 2000; Krauzlis 2003), and the cerebellum [caudal fastigial nucleus (cFN): Fuchs et al. 1994; posterior vermis: Sato and Noda 1992; Suzuki et al. 1981; Suzuki and Keller 1988] during both saccadic and pursuit eye movements. Our study offers the opportunity to further explore the flexibility of this coupling. Indeed, the similar kinetics which characterizes the changes in the saccadic and pursuit components of tracking (Fig. 5) suggests a coupling between the two visuomotor streams (Keller and Heinen 1991; Krauzlis 2004; Lynch 2009). The changes in pursuit and saccade could result from changes in their coupling and premotor interactions. But they could also result from a restriction of visuomotor interactions to the central visual field, i.e., at a functional site located upstream from the two preoculomotor systems.
Before addressing this point in more detail, we want to discuss the fact that, during the first sessions, the tracking was interrupted by saccades. This observation leads us to wonder why there was an initial preference for saccades rather than an increase in the pursuit velocity. Many studies indeed show the capability of monkeys to smoothly track a target moving with a velocity that varies sinusoidally (e.g., Eckmiller and Mackeben 1980; Fuchs 1967b; Kettner et al. 1996; Leung and Kettner 1997). During the accelerating phases of sinusoidal motions, the target speed increases and the monkeys are quite able to increase the velocity of their pursuit. Such increase was not observed in our monkeys; catch-up saccades were made instead. This kind of correction could result from the fact that both the target and pursuit velocities were constant. It has indeed been suggested that pursuit eye movements are primarily driven by signals related to velocity difference between the target and the eye motions, and that saccades are triggered by signals related to position error (Morris and Lisberger 1987; Pola and Wyatt 1980; Segraves and Goldberg 1994, but see also Heywood and Churcher 1971; Newsome et al. 1988). The saccades reduce the distance between the gaze and target directions because of their high velocity and their facilitative influence on pursuit eye movements. This facilitation is visual and motor. On the one hand, saccades bring the target image within the central field, where the visual motion signals are more effective for driving slow eye movements compared with signals from the peripheral field (Lisberger and Westbrook 1985; Newsome et al. 1985). On the other hand, the slow eye movements match the target speed better when they are preceded by a saccade (Lisberger 1998).
This leads us to the neurophysiological explanation of how the changes in the saccade and pursuit components of tracking result from changes in a process located upstream from their dedicated premotor systems. We propose that change in tracking performance results from an expansion of the “mass” of neural activity linked to the representation of the central field. The saccades indeed orient the fovea toward locations situated next to the moving target. As a result, neurons which are sensitive to the visual stimulation of the fovea become excited by the presence of the target. Their repeated excitation by the successive saccades likely contributed to delay their adaptation, and the variability of saccade endpoints enlarged the population of active neurons. Such an enlargement would correspond to an expansion of the central visual field, which is also known to involve neurons in the rostral SC. Therefore, we propose that the increase in pursuit velocity and the reduction of saccade size resulted from an enlargement of the population of active neurons in the rostral portion of the SC, possibly under the influence of the cFN. The rostral SC is indeed involved in both foveal and parafoveal pursuit (Basso et al. 2000; Krauzlis et al. 2000) and fixational saccades (Hafed and Krauzlis 2012; Krauzlis et al. 1997). The facts that larger target size not only improves the gain of pursuit eye movements (Helmick 1951; Pola and Wyatt 1985), but also increase the spatial spread of activity in the rostral SC (Goffart et al. 2012; Hafed et al. 2008), are consistent with this hypothesis. Concerning the cFN, their unilateral pharmacological inactivation is known to impair the generation of pursuit (Robinson et al. 1997) and saccadic eye movements (Buzunov et al. 2013; Goffart et al. 2004), as well as the direction of gaze relative to a fixated target, whether the latter is static (Goffart and Pélisson 1998; Guerrasio et al. 2010; Quinet and Goffart 2005) or moving (Robinson et al. 1997). This gaze “positional” disorder (called fixation offset) likely involves the fastigiocollicular connection (May et al. 1990; Quinet and Goffart 2015). Indeed, similar fixation offsets are observed in the head-restrained monkey during unilateral inactivation of rostral SC (Goffart et al. 2012; Hafed et al. 2008). Pursuit-related, saccade-and-pursuit-related and target-velocity-related neurons have also been reported in cFN (Büttner et al. 1991; Fuchs et al. 1994), as well as in the posterior vermis (Sato and Noda 1992; Suzuki et al. 1981; Suzuki and Keller 1988) from which the cFN receive inhibitory afferents and whose lesion impairs the generation of saccades and pursuit (Takagi et al. 1998, 2000). However, further research is required to identify the perturbed streams which are responsible for the pursuit disorders observed during the perturbation of these cerebellar regions.
About the notion of internal model.
Intermittent oculomotor tracking composed of catch-up saccades is found in young subjects. The pursuit gain is lower in children than in adults (Accardo et al. 1995), and it increases with age (Salman et al. 2006). Accardo and colleagues (1995) proposed that the low gain resulted from an immature pursuit system, itself resulting from an incomplete maturation of a “prediction mechanism.” This explanation does not seem to concern our study because our monkeys were adult animals. Moreover, no strong difference was observed during horizontal (sinusoidal) pursuit between young and mature monkeys (Takeichi et al. 2003). However, we do agree with the association between low pursuit gain values and the fact that the target moved in a manner which was initially unforeseeable to the monkey (see above). Thus the evolution of the visual tracking could be viewed as a reduction in a signal encoding the error between the time-varying changes in target position and the expectations of the monkey. The notion of internal model has indeed been proposed to help think about how animals solve problems and produce accurate actions (Kawato 1999; Mischiati et al. 2015; Wolpert et al. 1995). Employed in robotics, this notion was also incorporated in studies of arm movement control and motor learning (Bo et al. 2008; Wolpert et al. 1998) and in oculomotor studies more recently (Ethier et al. 2008; Lisberger 2009; Shibata et al. 2005). When human subjects track with no delay a periodically moving target (Barnes 2008; Kettner et al. 1996; Leung and Kettner 1997), “it is as if they create an internal model of the target movement and then track the output of this model, rather than the actual visual target” (Bahill et al. 1980). The dynamics of the oculomotor response (output) matches that of the target (input). Thus the output is not considered anymore as the outcome of complex and parallel processes causing the generation of a motor response; it has become the driving input, shaped by teaching signals. Hence, with practice, the behavior would evolve to mimic its environmental conditions, even under constant rewarding conditions (our study). The preceding paragraph illustrates the tremendous complexity which is hidden behind the notion of internal model, i.e., neural processes that connect several regions of the cerebral cortex, the brain stem and the cerebellum, within a medium which is radically different (it is neither homogeneous nor isotropic) from the arithmetical fabric with which the motion and the position of a visual target are formalized.
Conclusion.
Contrary to the belief that pursuit eye movements “stabilize the image of the moving object on the fovea once saccades have placed it there” (Thier and Ilg 2005), our study shows that such a performance requires training. We show how practice gradually changes the coupling between two visuomotor streams (from the retina to the saccade and pursuit premotor nuclei) by reducing the lag and synchronizing the motor performance with the current visual conditions, even under constant reward conditions. Future neurophysiological studies will unravel how processes spatiotemporally distributed in the brain cooperate to establish those sensorimotor interactions which take place locally in “space” and “time,” neither in the past nor in the future, but here and now.
GRANTS
This work was fully supported by the Centre National de la Recherche Scientifique and the European Research Council (Grant POSITION), and partly by the Agence Nationale de la Recherche (Grant VISAFIX). This work was also made possible thanks to support to J. Quinet from the Fondation de France (Berthe Fouassier 0039352).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.B., J.Q., and L.G. performed experiments; C.B. and L.G. analyzed data; C.B. and L.G. interpreted results of experiments; C.B. prepared figures; C.B., J.Q., P.C., and L.G. drafted manuscript; C.B., J.Q., P.C., and L.G. edited and revised manuscript; C.B., J.Q., P.C., and L.G. approved final version of manuscript; L.G. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Marc Martin, Luc Renaud and Dr. Ivan Balansard for veterinarian care and surgical assistance, Xavier Degiovanni and Joël Baurberg for technical assistance, and finally Drs. Ivo Vanzetta and Frédéric Chavane for lending their monkeys (M and Bi and G, respectively).
REFERENCES
- Accardo AP, Pensiero S, Pozzo SDA, Perissutti P. Characteristics of horizontal smooth pursuit eye movements to sinusoidal stimulation in children of primary school age. Vision Res 35: 539–548, 1995. [DOI] [PubMed] [Google Scholar]
- Bahill AT. Modeling the smooth-pursuit eye-movement system. Automedica 19: 1–17, 2000. [Google Scholar]
- Bahill AT, Iandolo MJ, Troost BT. Smooth pursuit eye movements in response to unpredictable target waveforms. Vision Res 20: 923–931, 1980. [DOI] [PubMed] [Google Scholar]
- Barnes GR. Cognitive processes involved in smooth pursuit eye movements. Brain Cogn 68: 309–326, 2008. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Collins S. Influence of predictability on control of extra-retinal components of smooth pursuit during prolonged 2D tracking. Exp Brain Res 233: 885–897, 2015. [DOI] [PubMed] [Google Scholar]
- Basso MA, Krauzlis RJ, Wurtz RH. Activation and inactivation of rostral superior colliculus neurons during smooth-pursuit eye movements in monkeys. J Neurophysiol 84: 892–908, 2000. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Barnes GR. Human ocular pursuit during the transient disappearance of a visual target. J Neurophysiol 90: 2504–2520, 2003. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Barnes GR. Combined smooth and saccadic ocular pursuit during the transient occlusion of a moving visual object. Exp Brain Res 168: 313–321, 2006. [DOI] [PubMed] [Google Scholar]
- Bo J, Block HJ, Clark JE, Bastian AJ. A cerebellar deficit in sensorimotor prediction explains movement timing variability. J Neurophysiol 100: 2825–2832, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourrelly C, Quinet J, Goffart L. Evolution of the oculomotor tracking with an accelerating or decelerating target. J Vis 15: 1016, 2015. [Google Scholar]
- Büttner U, Fuchs AF, Markert-Schwab G, Buckmaster P. Fastigial nucleus activity in the alert monkey during slow eye and head movements. J Neurophysiol 65: 1360–1371, 1991. [DOI] [PubMed] [Google Scholar]
- Büttner U, Straube A, Spuler A. Saccadic dysmetria and “intact” smooth pursuit eye movements after bilateral deep cerebellar nuclei lesions. J Neurol Neurosurg Psychiatry 57: 832–834, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzunov E, Mueller A, Straube A, Robinson FR. When during horizontal saccades in monkey does cerebellar output affect movement? Brain Res 1503: 33–42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou IH, Lisberger SG. Spatial generalization of learning in smooth pursuit eye movements: implications for the coordinate frame and sites of learning. J Neurosci 22: 4728–4739, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Chou IH, Lisberger SG. Evidence for object permanence in the smooth-pursuit eye movements of monkeys. J Neurophysiol 90: 2205–2218, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos PJ, Jones RW. Learning behavior of the eye fixation control system. IEEE Trans Automat Contr 8: 218–227, 1963. [Google Scholar]
- Deno DC, Crandall WF, Sherman K, Keller EL. Characterization of prediction in the primate visual smooth pursuit system. Biosystems 34: 107–128, 1995. [DOI] [PubMed] [Google Scholar]
- Dodge R. Five types of eye movements in the horizontal meridian plane of the field of regard. Am J Physiol 8: 307–329, 1903. [Google Scholar]
- Eckmiller R, Mackeben M. Pre-motor single unit activity in the monkey brain stem correlated with eye velocity during pursuit. Brain Res 184: 210–214, 1980. [DOI] [PubMed] [Google Scholar]
- Engel KC, Anderson JH, Soechting JF. Oculomotor tracking in two dimensions. J Neurophysiol 81: 1597–1602, 1999. [DOI] [PubMed] [Google Scholar]
- Engel KC, Anderson JH, Gomez CM, Soechting JF. Deficits in ocular and manual tracking due to episodic ataxia type 2. Mov Disord 19: 778–787, 2004. [DOI] [PubMed] [Google Scholar]
- Ethier V, Zee DS, Shadmehr R. Changes in control of saccades during gain adaptation. J Neurosci 28: 13929–13937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Barborica A. Internally generated error signals in monkey frontal eye field during an inferred motion task. J Neurosci 30: 11612–11623, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuriet J, Goffart L. Saccadic interception of a moving visual target after a spatiotemporal perturbation. J Neurosci 32: 452–461, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF. Saccadic and smooth pursuit eye movements in the monkey. J Physiol 191: 609–631, 1967a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF. Periodic eye tracking in the monkey. J Physiol 193: 161–171, 1967b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Robinson FR, Straube A. Participation of the caudal fastigial nucleus in smooth-pursuit eye movements. I. Neuronal activity. J Neurophysiol 72: 2714–2728, 1994. [DOI] [PubMed] [Google Scholar]
- Goffart L. Saccadic eye movements: basic neural processes. Neurosci Biobehav Psychol. In press, 2016.
- Goffart L, Chen LL, Sparks DL. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. J Neurophysiol 92: 3351–3367, 2004. [DOI] [PubMed] [Google Scholar]
- Goffart L, Hafed ZM, Krauzlis RJ. Visual fixation as equilibrium: evidence from superior colliculus inactivation. J Neurosci 32: 10627–10636, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffart L, Pélisson D. Orienting gaze shifts during muscimol inactivation of caudal fastigial nucleus in the cat. I. Gaze dysmetria. J Neurophysiol 79: 1942–1958, 1998. [DOI] [PubMed] [Google Scholar]
- Guerrasio L, Quinet J, Büttner U, Goffart L. Fastigial oculomotor region and the control of foveation during fixation. J Neurophysiol 103: 1988–2001, 2010. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. Superior colliculus inactivation causes stable offsets in eye position during tracking. J Neurosci 28: 8124–8837, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Krauzlis RJ. Similarity of superior colliculus involvement in microsaccade and saccade generation. J Neurophysiol 107: 1904–1916, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: Science Editions, 1961. [Google Scholar]
- Helmick JS. Pursuit learning as affected by size of target and speed of rotation. J Exp Psychol 41: 126–138, 1951. [DOI] [PubMed] [Google Scholar]
- Henn V, Lang W, Hepp K, Reisine H. Experimental gaze palsies in monkeys and their relation to human pathology. Brain 107: 619–636, 1984. [DOI] [PubMed] [Google Scholar]
- Heywood S, Churcher J. Eye movements and the afterimage. I. Tracking the afterimage. Vision Res 11: 1163–1168, 1971. [DOI] [PubMed] [Google Scholar]
- Kahlon M, Lisberger SG. Coordinate system for learning in the smooth pursuit eye movements of monkeys. J Neurosci 16: 7270–7283, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol 9: 718–727, 1999. [DOI] [PubMed] [Google Scholar]
- Keating EG. Lesions of the frontal eye field impair pursuit eye movements, but preserve the predictions driving them. Behav Brain Res 53: 91–104, 1993. [DOI] [PubMed] [Google Scholar]
- Keller EL, Heinen SJ. Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neurosci Res 11: 79–107, 1991. [DOI] [PubMed] [Google Scholar]
- Kettner RE, Leung HC, Peterson BW. Predictive smooth pursuit of complex two-dimensional trajectories in monkey: component interactions. Exp Brain Res 108: 221–235, 1996. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Neuronal activity in the rostral superior colliculus related to the initiation of pursuit and saccadic eye movements. J Neurosci 23: 4333–4344, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol 91: 591–603, 2004. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Shared motor error for multiple eye movements. Science 276: 1693–1695, 1997. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol 84: 876–891, 2000. [DOI] [PubMed] [Google Scholar]
- Leung HC, Kettner R. Predictive smooth pursuit of complex two-dimensional trajectories demonstrated by perturbation responses in monkeys. Vision Res 37: 1347–1354, 1997. [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Post-saccadic enhancement of the initiation of smooth pursuit eye movements in monkeys. J Neurophysiol 79: 1918–1930, 1998. [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience 162: 763–776, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci 5: 1662–1673, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JC. Oculomotor control: anatomical pathways. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford, UK: Academic, 2009, p. 17–23. [Google Scholar]
- May JG, Keller EL, Suzuki DA. Smooth-pursuit eye movement deficits with chemical lesions in the dorsolateral pontine nucleus of the monkey. J Neurophysiol 59: 952–977, 1988. [DOI] [PubMed] [Google Scholar]
- May PJ, Hartwich-Young R, Nelson J, Sparks DL, Porter JD. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience 36: 305–324, 1990. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Manual tracking of visual targets by trained monkeys. Behav Brain Res 20: 185–201, 1986. [DOI] [PubMed] [Google Scholar]
- Michael JA, Melvill-Jones G. Dependence of visual tracking capability upon stimulus predictability. Vision Res 6: 707–716, 1966. [DOI] [PubMed] [Google Scholar]
- Mischiati M, Lin HT, Herold P, Imler E, Olberg R, Leonardo A. Internal models direct dragonfly interception steering. Nature 517: 333–338, 2015. [DOI] [PubMed] [Google Scholar]
- Missal M, Keller EL. Common inhibitory mechanism for saccades and smooth pursuit eye movements. J Neurophysiol 88: 1880–1892, 2002. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Lisberger SG. Different responses to small visual errors during initiation and maintenance of smooth-pursuit eye movements in monkeys. J Neurophysiol 6: 1351–1369, 1987. [DOI] [PubMed] [Google Scholar]
- Morrow MJ, Sharpe JA. Deficits of smooth-pursuit eye movement after unilateral frontal lobe lesions. Ann Neurol 37: 443–451, 1995. [DOI] [PubMed] [Google Scholar]
- Mrotek LA, Flanders M, Soechting JF. Oculomotor responses to gradual changes in target direction. Exp Brain Res 172: 175–192, 2006. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Ono S, Das VE. Signal processing and distribution in cortical-brainstem pathways for smooth pursuit eye movements. Ann N Y Acad Sci 1164: 147–154, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Dursteler MR, Mikami H. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci 5: 825–840, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurosci 60: 604–620, 1988. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Bullier J. The timing of information transfer in the visual system. In: Cerebral Cortex. Extrastriate Cortex in Primates, edited by Rockland KS, Kaas JH, Peters A. New York: Plenum, 1997, vol. 12, p. 205–241. [Google Scholar]
- Ohtsuka K, Igarashi Y, Chiba S. Cerebellar peduncle lesion without saccadic abnormalities. Int J Ophthalmol 204: 44–48, 1992. [DOI] [PubMed] [Google Scholar]
- Ono S. The neuronal basis of on-line visual control in smooth pursuit eye movements. Vision Res 110: 257–264, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Lefèvre P. Saccades and pursuit: two outcomes of a single sensorimotor process. J Physiol 584: 11–23, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pola J, Wyatt HJ. Target position and velocity: the stimuli for smooth pursuit eye movements. Vision Res 20: 523–534, 1980. [DOI] [PubMed] [Google Scholar]
- Pola J, Wyatt HJ. Active and passive smooth eye movements: effects of stimulus size and location. Vision Res 25: 1063–1076, 1985. [DOI] [PubMed] [Google Scholar]
- Puckett JD, Steinman RM. Tracking eye movements with and without saccadic correction. Vision Res 9: 695–703, 1969. [DOI] [PubMed] [Google Scholar]
- Quinet J, Bourrelly C, Goffart L. Building an internal model of expected target position for the control of visual tracking. Soc Neurosci Abstr 363.02, 2013. [Google Scholar]
- Quinet J, Goffart L. Saccade dysmetria in head unrestrained gaze shifts after muscimol inactivation of the caudal fastigial nucleus in the monkey. J Neurophysiol 93: 2343–2349, 2005. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Does the brain extrapolate the position of a transient moving target? J Neurosci 35: 11780–11790, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. The mechanics of human smooth pursuit eye movement. J Physiol 180: 569–591, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Participation of caudal fastigial nucleus in smooth pursuit eye movements. II. Effects of muscimol inactivation. J Neurophysiol 78: 848–859, 1997. [DOI] [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, Lillakas L, Dennis M, Steinbach MJ. Smooth pursuit eye movements in children. Exp Brain Res 169: 139–143, 2006. [DOI] [PubMed] [Google Scholar]
- Sato H, Noda H. Posterior vermal Purkinje cells in macaques responding during saccades, smooth pursuit, chair rotation and/or optokinetic stimulation. Neurosci Res 12: 583–595, 1992. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol 79: 3272–3278, 1998. [DOI] [PubMed] [Google Scholar]
- Segraves MA, Goldberg ME. Effect of stimulus position and velocity upon the maintenance of smooth pursuit eye velocity. Vision Res 18: 2477–2482, 1994. [DOI] [PubMed] [Google Scholar]
- Shibata T, Tabata H, Schaal S, Kawato M. A model of smooth pursuit in primates based on learning the target dynamics. Neural Netw 18: 213–224, 2005. [DOI] [PubMed] [Google Scholar]
- Soechting JF, Rao HM, Juveli JZ. Incorporating prediction in models for two-dimensional smooth pursuit. PLoS One 5: e12574, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL, Holland R, Guthrie BL. Size and distribution of movement fields in the monkey superior colliculus. Brain Res 113: 21–34, 1976. [DOI] [PubMed] [Google Scholar]
- St-Cyr GJ, Fender DH. Nonlinearities of the human oculomotor system: time delays. Vision Res 9: 1491–1503, 1969. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, Keller EL. The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. II. Target velocity-related Purkinje cell activity. J Neurophysiol 59: 19–40, 1988. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, May JG, Keller EL, Yee RD. Visual motion response properties of neurons in dorsolateral pontine nucleus of alert monkey. J Neurophysiol 63: 37–59, 1990. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, Noda H, Kase M. Visual and pursuit eye movement-related activity in posterior vermis of monkey cerebellum. J Neurophysiol 46: 1120–1139, 1981. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, Yamada T, Hoedema R, Yee RD. Smooth-pursuit eye-movement deficits with chemical lesions in macaque nucleus reticularis tegmenti pontis. J Neurophysiol 82: 1178–1186, 1999. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, Yamada T, Yee RD. Smooth-pursuit eye-movement-related neuronal activity in macaque nucleus reticularis tegmenti pontis. J Neurophysiol 89: 2146–2158, 2003. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80: 1911–1931, 1998. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol 83: 2047–2062, 2000. [DOI] [PubMed] [Google Scholar]
- Takeichi N, Fukushima J, Kurkin S, Yamanobe T, Shinmei Y, Fukushima K. Directional asymmetry in smooth ocular tracking in the presence of visual background in young and adult primates. Exp Brain Res 149: 380–390, 2003. [DOI] [PubMed] [Google Scholar]
- Thier P, Ilg UJ. The neural basis of smooth-pursuit eye movements. Curr Opin Neurobiol 15: 645–652, 2005. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Eye movement responses to a horizontally moving visual stimulus. AMA Arch Ophthalmol 52: 932–941, 1954. [DOI] [PubMed] [Google Scholar]
- Whittaker SG, Eaholtz G. Learning patterns of eye motion for foveal pursuit. Invest Ophthalmol Vis Sci 23: 393–397, 1982. [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science 269: 1880–1882, 1995. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall R, Kawato M. Internal models in the cerebellum. Trends Cogn Sci 2: 338–347, 1998. [DOI] [PubMed] [Google Scholar]