This study is the first to quantify stress-evoked changes in the acoustic startle reflex in the upper trapezius muscle of humans, and our findings reveal a complex pattern of inhibitory and facilitatory responses consistent with observations in nonhuman primates. We further demonstrate that psychosocial stress consistently reduces the amplitude of these responses. These findings have implications for the control of motor behaviors in response to stress.
Keywords: electromyography, startle reflex, motor units
Abstract
Psychosocial stress has been shown to influence several aspects of human motor control associated with the fight-or-flight response, including augmentation of upper trapezius muscle activity. Given the established role of the reticular formation in arousal, this study investigated the contribution of reticulospinal activation to trapezius muscle activity during exposure to an acute psychosocial stressor. Twenty-five healthy adults were exposed to startling acoustic stimuli (SAS) while performing a motor task during periods of low and high psychosocial stress. Acoustic startle reflexes (ASRs) were recorded in the upper trapezius during low intensity contractions using both surface and intramuscular electromyography. Exposure to the stressor increased subjective and physiological measures of arousal (P < 0.01). The majority of participants demonstrated inhibitory ASRs, whereas a small subgroup with significantly higher trait anxiety (n = 5) demonstrated excitatory ASRs in the low stress condition. Changes in synaptic input for inhibitory ASRs were confirmed by decreases in the discharge rate of single motor units in response to the SAS. ASRs decreased in magnitude for all participants during exposure to the acute psychosocial stressor. These findings suggest that the reticular formation has predominately inhibitory effects on the human upper trapezius during an ongoing motor task and that disinhibition caused by psychosocial stress may contribute to augmentation of trapezius muscle activity. Further research is required to investigate mechanisms underlying the complex ASRs characterized by this study, particularly the phase reversal to excitatory responses observed among more anxious individuals.
NEW & NOTEWORTHY This study is the first to quantify stress-evoked changes in the acoustic startle reflex in the upper trapezius muscle of humans, and our findings reveal a complex pattern of inhibitory and facilitatory responses consistent with observations in nonhuman primates. We further demonstrate that psychosocial stress consistently reduces the amplitude of these responses. These findings have implications for the control of motor behaviors in response to stress.
psychosocial stress has been shown to influence human motor control (Christou et al. 2004; Noteboom et al. 2001; Staab et al. 2013; Stephenson and Maluf 2010). Specifically, upper trapezius muscle activity is augmented by a variety of stress-inducing tasks (Eijckelhof et al. 2013; Nilsen et al. 2007; Shahidi et al. 2013) and is considered integral to the “fight-or-flight” response; however, neurobiological mechanisms underlying these stress-induced changes in motor control are not fully understood. For example, sympathetic blockade does not eliminate the presence of stress-induced muscle activity (Nilsen et al. 2008), indicating that activation of the sympathetic nervous system is not solely responsible for elevated trapezius muscle activity in response to stress. Psychosocial stress has been shown to increase responsiveness of the corticospinal tract to transcranial magnetic stimulation (Oathes et al. 2008), specifically when stimulating over the cortical representation of the upper trapezius (Marker et al. 2013), indicating some involvement of the motor cortex and corticospinal tract. The upper trapezius is also innervated by the reticular formation (Davidson and Buford 2004, 2006), which has an established role in arousal (Holstege 1992; Jones 2003); however, reticulospinal contributions to trapezius muscle activity during periods of psychosocial stress are not known.
Responsiveness of the reticulospinal tract can be assessed noninvasively in humans through investigation of the acoustic startle reflex (ASR) (Rothwell 2006). The ASR can be elicited in response to the sudden onset of an unexpected and high amplitude acoustic stimulus (Blumenthal et al. 2005) and is characterized as a whole body patterned motor response proceeding from a rostral to caudal direction. Observed movements associated with the ASR include eye blink, cervical extension, superior and anterior movement of the shoulders, and a general shortening of body length (Yeomans and Frankland 1995). Little is known of the upper trapezius response to the ASR due to the rapid habituation of components other than the blink reflex (Aniss et al. 1998), which may be mediated by a separate pathway (Valls-Sole 2012). Recently, studies have investigated the “StartReact” phenomenon, an involuntary triggering of prepared motor responses by a startling stimulus in tasks including head and neck movement (Carlsen et al. 2011; Siegmund et al. 2001; Valls-Sole et al. 2008). In addition to early triggering of prepared motor responses, it has previously been shown that habituation of motor responses to an acoustic stimulus can be greatly reduced by increasing the state of readiness to perform a motor task using the StartReact paradigm (Carlsen et al. 2011; Valls-Sole et al. 2008).
This investigation utilized attenuated habituation of the ASR in cervical muscles during a StartReact paradigm to investigate changes in reticulospinal input to the upper trapezius muscle during periods of low and high psychosocial stress. Based on known involvement of the reticular formation in heightened arousal, we hypothesized that the magnitude of excitatory ASRs in the upper trapezius would increase during exposure to an acute psychosocial stressor, indicating enhanced excitatory effects of the reticular formation in response to stress. This finding would support the contribution of the reticular formation in stress-induced increases in upper trapezius muscle activity.
METHODS
Participants.
Healthy participants were recruited using electronic advertisements at a university medical campus and the surrounding community. All participants provided written informed consent in accordance with procedures approved by the Colorado Multiple Institutional Review Board, including additional protections for a partial waiver of consent required for the stress manipulation. Participants were screened for the absence of neck pain by a licensed physical therapist. Exclusion criteria included objective signs of structural or neurologic impairment (e.g., radiculopathy), self-reported history of traumatic injury or surgery affecting the neck or shoulder region within 12 wk of enrollment, and history of any major cardiovascular, neurological, or psychiatric medical condition.
Electromyography.
Surface electromyography (sEMG) was recorded from the nondominant upper trapezius using 8-mm diameter bipolar Ag-AgCl surface electrodes. Electrodes were positioned with a 15-mm interelectrode distance, centered 20 mm lateral to the midpoint between the seventh cervical vertebrae and the posterior lateral border of the acromion (Farina et al. 2002). A reference electrode was placed over a bony portion of the ipsilateral clavicle. sEMG data were amplified (×1,000), band-pass filtered (13-1,000 Hz LabLinc V; Coulbourn Instruments, Whitehall, PA), and sampled at 2,000 Hz (Power1401; Cambridge Electronic Design, Cambridge, UK). sEMG was also collected using identical methods from the nondominant sternocleidomastoid (SCM) muscle, with electrodes positioned one-third of the distance between the sternal notch and the mastoid process (Falla et al. 2002).
Intramuscular EMG recordings of single motor unit (SMU) activity were collected using fine wire intramuscular electrodes custom made from two Formvar-insulated stainless steel wires (50-μm diameter; California Fine Wire, Grover Beach, CA) with the cross-sectional area exposed for recording. Wire electrodes were placed with a 30-gauge needle ∼1 cm anterior to the midpoint between surface electrodes. The needle was removed after electrode placement and small adjustments in wire position were made to enhance signal quality as necessary. A reference electrode was placed adjacent to the reference electrode for the sEMG. The intramuscular EMG signal was amplified (×1,000), band-pass filtered (20–8000 Hz; LabLinc V; Coulbourn Instruments), and sampled at 20,000 Hz (Power1401; Cambridge Electronic Design).
Motor tasks and startling acoustic stimulus.
Startling acoustic stimuli (SAS) were given in the context of a simple reaction time (RT) task to exploit the attenuation of response habituation seen during StartReact paradigms (Carlsen et al. 2011). The RT task required participants to perform a lateral pinch grip on a force transducer (HDM-915; Lode, Groningen, The Netherlands) with the dominant hand in response to a nonstartling auditory cue (76-dB tone, 50-ms duration) (model V85-05; LabLinc V, Coulbourn Instruments) presented binaurally via headphones (nonstartle trials). The SAS consisted of a 124-dB, 50-ms burst of white noise with near instantaneous rise time (model V85-05; LabLinc V, Coulbourn Instruments). The SAS replaced the RT task auditory cue in 30% of trials (startle trials) in a pseudorandom order such that the SAS was never presented as the first auditory cue in each block or during any two consecutive trials. Participants were instructed to perform the RT task as fast as possible in response to both the auditory cue and SAS. Continuous white noise (60 dB) was presented through the headphones worn during testing to minimize any effects of environmental noise. The amplitude of all auditory cues was confirmed before each experimental session (Lutron SL-4001). RTs for both startle and nonstartle trials were calculated.
To investigate the effect of the SAS on SMU behavior, participants performed a low intensity contraction with the nondominant upper trapezius by shrugging up against a force transducer positioned over the acromion (1112 N range; 7.6 mV/N; P310, Cooper Instruments, Warrenton, VA). Real-time feedback of force production and a target contraction force was displayed on a monitor positioned ∼1 m in front of the participant at eye level. The target level for the contraction was set individually for each participant at a level corresponding to the recruitment and steady discharge of one to two distinct motor units in the intramuscular EMG recording, and was held constant throughout the experimental protocol. In addition to the investigation of SMU behavior, the presence of a low intensity contraction in the upper trapezius was utilized for several other purposes: the one previous study investigating the ASR in the upper trapezius (Aniss et al. 1998) showed more consistent responses during muscle contraction; both excitatory and inhibitory responses can be observed in the presence of ongoing muscle activity, and sustained low intensity contractions simulate functionally relevant postural activity in the trapezius.
Each RT trial began with a 3-s tone (76 dB) during which participants were instructed to slowly shrug up to the target force and maintain a steady contraction throughout the rest of the trial. The auditory cue or SAS was presented 3–8 s (random interval with uniform distribution) after this initial tone. Two seconds after the auditory cue or SAS, trials ended with a 3-s tone (76 dB) during which participants were instructed to slowly relax their shoulder. The timing of all auditory cues and motor tasks for an individual trial is illustrated in Fig. 1A.
Fig. 1.
Sequence of experimental tasks and stimuli is illustrated for an individual trial (A) and the experimental protocol (B). The experimental protocol was explained during an initial familiarization. Following equipment set-up, habituation comprised 6 startling acoustic stimuli (SAS) with no other task requirements. Task practice comprised 8–10 individual trials to practice the force target matching and pinch reaction time (RT) tasks with no SAS. Low stress and high stress conditions each included 4 blocks of 10 individual trials, with the SAS replacing the 50-ms pinch task auditory cue in 30% of trials. Vertical arrows indicate collection time points for arousal measures (note that time is not shown to scale). STAI-S, Spielberger State-Trait Anxiety Index, State Score; MAP, mean arterial pressure; HR, heart rate; B, baseline; L, low stress; H, high stress.
Psychosocial stressor.
The experimental stress protocol manipulated psychosocial stress using a numeric memorization task combined with social evaluative threat (Dickerson and Kemeny 2004). Participants were initially informed that the purpose of the study was to examine the effects of mental concentration on muscle activity and therefore remained naïve to the stress manipulation until the end of the session. During both low and high stress conditions, series of five, two-digit numbers were presented on a video monitor for the participant to memorize and were removed after 10–15 s. Participants were asked to repeat the numbers in sequence after completing 10 trials of the RT task. Four blocks of 10 trials were completed in each stress condition, with a different sequence of numbers presented at the beginning of each block. The difficulty of the memorization task did not differ between stress conditions to control for the effects of cognitive demand, and participants were instructed to perform the task as accurately as possible in both conditions. Participants were told that they were “only practicing” the task during the low stress condition. They were told that their performance was not being monitored and positive feedback was provided by a familiar investigator, regardless of actual performance. The high stress condition was administered by an unfamiliar, authoritative investigator who provided no positive feedback. Participants were told that they would be videotaped and paid based on their performance. Examiners during both stress conditions were the same for all participants. Immediately after completion of the high stress condition, all participants were fully debriefed on the details and purpose of the stress manipulation. They were assured that no video recordings were made and they would receive full monetary compensation regardless of performance.
Perceived anxiety was assessed by the state portion of the Spielberger State-Trait Anxiety Inventory (STAI-S) (Spielberger 1983), and physiological arousal was assessed by mean arterial pressure (MAP) and heart rate (HR) collected with an automated oscillometric cuff (Coulbourn V series module) placed around the left arm. STAI-S was collected twice during baseline procedures and after each stress condition. MAP and HR were collected at the same two time points during baseline and after each block of RT trials in both stress conditions (see Fig. 1B).
Experimental protocol.
The complete experimental protocol is shown in Fig. 1B. Participants first completed a familiarization session, in which all details of the experiment (except stress manipulation) were explained. A brief physical screen was performed to rule out the presence of neck pain or related disorders, and participants completed a questionnaire containing demographic information and the trait portion of the STAI (STAI-T). Setup of sEMG and intramuscular electrodes was then performed, and participants transitioned to a custom designed experimental chair where they sat with both arms supported. Signal quality was verified for all channels, and a target contraction level was determined based on SMU recordings from the intramuscular EMG as described above. Participants were then exposed to six SAS, separated by 15–20 s, to familiarize them to the stimulus and to account for any initial habituation or orienting responses (Valls-Sole et al. 2008). Finally, participants practiced 8–10 nonstartle trials of the target matching and pinch tasks to become familiar with the motor tasks and minimize potential learning effects during the stress conditions. Participants then completed four blocks of 10 trials during both stress conditions, resulting in a total of 28 nonstartle and 12 startle trials per stress condition. SAS trials were presented in a pseudorandomized order across four blocks of trials, which was held constant across low and high stress conditions. The two stress conditions were separated by a 10-min rest period, and the low stress condition was always presented first to avoid carry-over of stress responses. After debriefing, intramuscular electrodes were removed and participants performed two to four maximum voluntary contractions (MVCs) with the nondominant upper trapezius by shrugging up against resistance to provide a reference contraction for normalization of EMG data.
Control session.
A subset of participants agreed to return for a control session to investigate the effects of time on the startle response. This session followed the same experimental protocol as that shown in Fig. 1B, except participants performed a second low stress condition instead of the high stress condition. No intramuscular EMG was collected during the control session. The target contraction level for the left upper trapezius was set to the same amplitude (%MVC) as the experimental session for each participant. The order of SAS presentations was also the same as during the experimental session.
sEMG data processing.
sEMG data were preprocessed using custom Spike2 software (Cambridge Electronic Design). All trials were visually inspected for the presence of cardiac artifacts, which were removed using a filtered template subtraction technique (Marker and Maluf 2014). Trials where a cardiac artifact was present in the 200 ms after the SAS were discarded (3% of trials). DC offset was removed and trials were exported for processing with custom LabVIEW software (National Instruments, Austin, TX).
A cumulative sum (CUSUM) analysis (Ellaway 1978) was performed on the sEMG from startle trials within each stress condition to characterize the ASR in the upper trapezius. This technique allows the characterization of inhibitory and excitatory components of a multiphasic response. The analysis was performed according to recommendations from Brinkworth and Turker (2003). The 200-ms period before and after the SAS (400 ms total) was rectified and averaged across trials within each stress condition. The CUSUM calculation was then performed according to the following equation:
where t is the time of the current data point; tp- is the preSAS analysis time; is the pre-SAS EMG mean; and bw is the inverse of the sampling rate in ms (corrects for sampling rate).
Turning points (i.e., slope equal to zero) in the resulting CUSUM signal represent time points when the averaged trial signal crosses the mean of the pre-SAS period and significant events (responses) are identified as vertical deviations between turning points greater than those identified in the pre-SAS period, representing periods above or below the pre-SAS mean sEMG for greater durations than those seen in the pre-SAS period. To reduce the occurrence of nonmeaningful turning points in the CUSUM signal, it was passed through an additional zero-phase shift low pass filter (100 Hz). This filter is lower than published recommendations (200 Hz) but was necessitated by the low number of SAS trials used in creating trial averages, and results must be interpreted with the knowledge that response onset times may be slightly shifted by the selected filter setting (Brinkworth and Turker 2003). Response onset was identified as the initial turning point of a significant event, duration as the time between the turning points of a significant event, and amplitude as the vertical distance between turning points of a significant event. Response amplitudes were converted to response strength by expressing them as a percentage of response duration (representing maximum inhibition, see Brinkworth and Turker 2003). Only responses with an onset <150 ms were identified. Figure 2 demonstrates the CUSUM procedure and response characterization with representative data. Finally, the root-mean-squared average of the sEMG signal in the 3-s period before each cue or SAS was averaged within each stress condition to quantify the amplitude of background muscle activity.
Fig. 2.
Representative data illustrating the cumulative sum (CUSUM) processing technique. An individual response to a SAS in the left upper trapezius is shown before (A) and after (B) rectification. All rectified SAS responses in a given condition (gray lines) were averaged (black line, C). The averaged response after CUSUM processing is shown in D. A response was identified when the vertical distance between 2 turning points (sign change in slope) was greater than those identified in the prestimulus period. The response onset (On) was defined as the first turning point. The response amplitude (Amp) was defined as the vertical distance between 2 consecutive turning points. The response duration (Dur) was defined as the horizontal distance between 2 consecutive turning points. The strength (Str) of a response was calculated as its amplitude divided by its duration multiplied by 100, representing the percentage of maximum excitation or inhibition. This technique allows for identification of multiple excitatory or inhibitory responses. In this example, the first response (On1, Amp1, Dur1, and Str1) is inhibitory, and the second response (On2, Amp2, Dur2, and Str2) is excitatory. Vertical dashed lines represent the onset of the startling acoustic atimulus (SAS).
Intramuscular EMG data processing.
Template matching software in Spike2 was used to discriminate SMUs in the intramuscular EMG signal in a 1-s window centered around the SAS. Discriminated SMUs were then converted to discharge rates (DRs) and exported for further analysis using MatLab software (MathWorks, Natick, MA). Large variability in SMU recruitment during the task led to few SMUs with an adequate number of SAS trials to compare activity of the same SMU across stress conditions. DR data were therefore combined for all SMUs identified within each stress condition by normalizing to the mean DR from the 150-ms pre-SAS period in a given trial and time locking to the average ASR response onset for each condition within an individual (due to large interindividual variability in response onsets). Normalized and time-locked DRs were then averaged in 10-ms bins to investigate the change in SMU DR during the startle response.
Data analysis.
All time points for STAI-S, MAP, and HR measurements were averaged within each experimental condition and compared with a repeated-measures (RM) ANOVA with one factor of Condition (baseline, low stress, high stress). RTs were compared with a 2 × 2 RM ANOVA, with factors of Trial (startle or nonstartle) and Condition (low stress or high stress). Post hoc analyses were performed with paired t-tests and Bonferroni corrected for multiple comparisons. Mean pre-SAS sEMG values were compared between low stress and high stress conditions using a paired t-test. Additionally, the root-mean-squared amplitude of sEMG within a 100-ms window immediately before and after auditory cues presented in SAS and non-SAS trials was compared with a 2 × 2 RM ANOVA, with factors of Trial (startle or nonstartle) and Time (prestimulus or poststimulus). SMU mean DRs were calculated in the 500 ms pre-SAS period and compared between low stress and high stress conditions with an unpaired t-test. Significance was set as P < 0.05.
Visual inspection of individual ASR responses revealed both inhibitory and excitatory response patterns in the upper trapezius, therefore participants were categorized into subgroups based on similar ASR response patterns in the low stress condition. Further analyses were performed with nonparametric statistics due to smaller sample sizes within subgroups. Response onsets, durations, and strengths were compared between stress conditions using Wilcoxon signed-rank tests within subgroups. Differences between subgroups in demographic characteristics were investigated using exact k-sample permutation tests (Curran-Everett 2012). Significance for these analyses was set as P < 0.05 and nonsignificant trends were identified as P < 0.1. All statistical analyses were performed using R software (R Development Core Team, Vienna, Austria).
RESULTS
Experimental session.
Twenty-five participants were recruited for the study [mean age = 30 (range 24–49) yr, 18 women, all right-hand dominant]. Mean (SD) STAI-T was 30 (6.6) out of a possible 80 points, with higher scores indicating higher trait anxiety (Spielberger 1983).
Results for STAI-S, MAP, and HR analyses are shown in Fig. 3. The main effect of Condition was significant for STAI-S (F2,23 = 28, P < 0.01), MAP (F2,23 = 78, P < 0.01), and HR (F2,23 = 26, P < 0.01). The high stress condition was significantly greater than the low stress and baseline conditions for all three measures (P < 0.01 for all). The low stress condition was significantly greater than the baseline condition for all three measures (STAI-S P = 0.04; MAP and HR P < 0.01). Participants performed slightly but significantly worse on the mental concentration task in the high stress (13/20 numbers correctly memorized) compared with the low stress (15/20 numbers correctly memorized) condition (t = −4.39, P < 0.05).
Fig. 3.
Changes in perceived anxiety [Spielberger State-Trait Anxiety Index State Score (STAI-S); A] and indexes of physiological arousal, including mean arterial pressure (MAP; B) and heart rate (HR; C) during the experimental protocol. All time points (see Fig. 1) collected for each measure are shown. Time points within each condition were averaged before statistical analyses that compared mean scores for the baseline (B1 and B2), low stress (L1, L2, L3, and L4), and high stress (H1, H2, H3, and H4) conditions.
The target force for upper trapezius contraction was set at a mean (SD) of 7.4 (9.5) %MVC force (range = 0.3–22%MVC), a range similar to that observed for low intensity postural contractions. Pre-SAS mean sEMG did not change between low stress and high stress conditions performed at the same target force [8.3 (6.1) %MVC vs. 8.6 (6.8) %MVC, respectively, t = 0.20, P = 0.84]. RTs demonstrated a main effect of Trial (F1,24 = 110, P < 0.01) but not Condition (F1,24 = 0.71, P = 0.41). RTs were significantly faster during startle trials [236 (84) ms] compared with nonstartle trials [340 (74) ms, t = −11.5, P < 0.01].
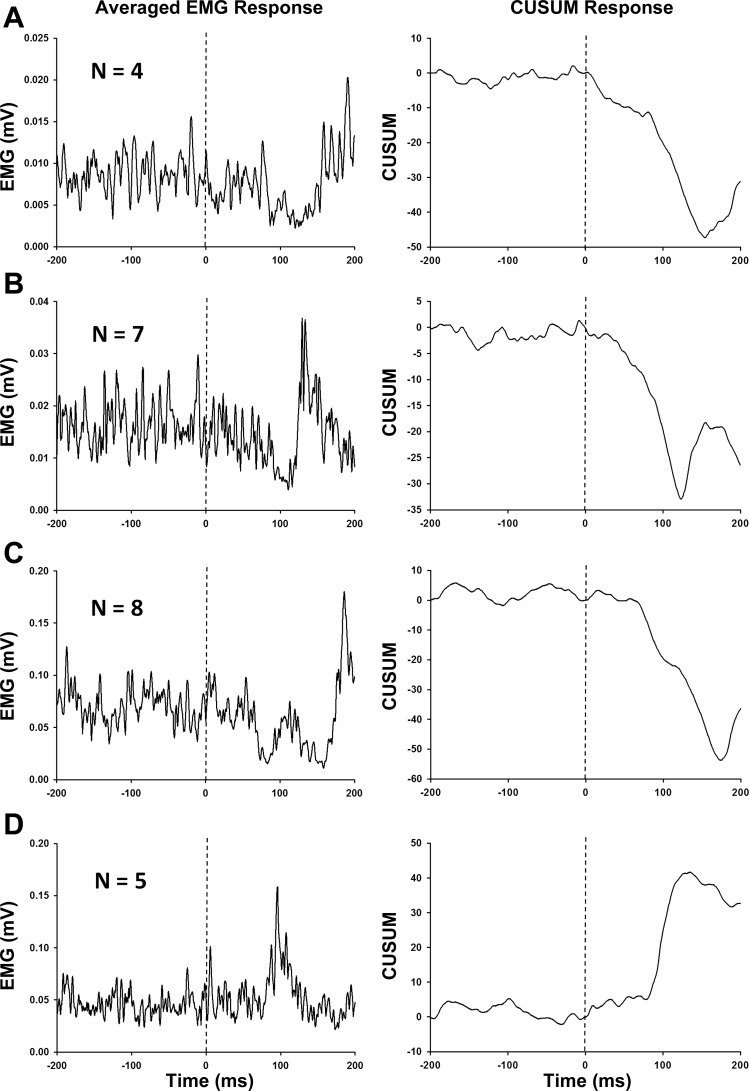
Four characteristic response patterns were observed in the ASR during the low stress condition; representative examples of each response pattern are shown in Fig. 4. One participant showed no identifiable ASR and was not included in subsequent analyses. Observable startle response patterns included 1) a single period of inhibition (Fig. 4A); 2) inhibition followed by excitation (Fig. 4B); 3) two periods of inhibition (Fig. 4C); and 4) a single period of excitation (Fig. 4D). Subgroup classification was based on the initial phase of the ASR, resulting in an inhibitory ASR group (n = 19) and an excitatory ASR group (n = 5). No participants demonstrated a change from inhibition to excitation or vice versa between the low and high stress conditions. One participant in the inhibitory ASR group demonstrated no identifiable ASR in the high stress condition, and the response strength was set to zero for this case. The two subgroups did not differ in age (Z = −0.7, P = 0.53); however, the group with excitatory ASR responses showed significantly higher STAI-T compared with those with inhibitory ASR responses [36 (8) vs. 28 (4), Z = 2.8, P < 0.01].
Fig. 4.
Averaged surface EMG responses and corresponding cumulative sum (CUSUM) plots for representative participants illustrating the 4 major response patterns of the upper trapezius muscle to startling acoustic stimuli (SAS): a single period of inhibition (A), inhibition followed by excitation (B), 2 periods of inhibition (C), and a single period of excitation (D). Participants were categorized based on the direction of the initial phase of the acoustic startle reflex (ASR) observed during the low stress condition. Vertical dashed lines represent onset of the SAS.
To confirm that ASRs were generated specifically in response to the SAS, startle and nonstartle trials were compared. In the inhibitory response group, a significant Trial × Time interaction (F1,18 = 13.3, P < 0.01) indicated that sEMG decreased pre- to poststimulus for SAS trials [8.7 (7.0) %MVC to 7.8 (6.3) %MVC] but not for non-SAS trials [8.9 (7.0) %MVC to 8.7 (7.0) %MVC]. The pre- to poststimulus change in sEMG in the excitatory response group similarly confirmed the presence of ASR responses in SAS trials [6.9 (3.4) %MVC to 8.2 (4.5) %MVC] but not non-SAS trials [7.2 (3.3) %MVC to 7.0 (3.3) %MVC], although the interaction was not significant (F1,4 = 3.2, P = 0.15), likely due to the small sample size.
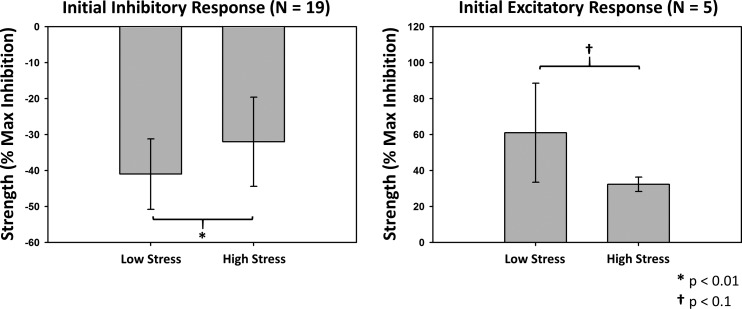
ASR response onsets and durations for both subgroups during each stress condition are shown in Table 1. Neither onset (Inhibitory, W = 74, P = 0.64; Excitatory, W = 2, P = 0.19) nor duration (Inhibitory, W = 75, P = 0.67; Excitatory, W = 5, P = 0.62) were significantly different between conditions. Changes in ASR response strength between stress conditions for both groups are shown in Fig. 5. The inhibitory ASR group demonstrated a significant reduction in the strength of inhibition in the high compared with low stress condition [−32 (12) % vs. −40 (9) %maximum inhibition, respectively, W = 166, P < 0.01]. The excitatory ASR group showed a trend for reduced strength of excitation in the high compared with low stress condition [61 (27) % vs. 32.3 (4) % maximum inhibition, respectively, W = 0, P = 0.06].
Table 1.
Acoustic startle response characteristics during low and high stress conditions
| Low Stress |
High Stress |
|||
|---|---|---|---|---|
| Group | Onset, ms | Duration, ms | Onset, ms | Duration, ms |
| Inhibition (n = 19) | 57.0 (14) | 57.0 (21) | 54.8 (21) | 52.4 (22) |
| Excitation (n = 5) | 60.2 (15) | 37.6 (15) | 49.2 (9) | 30.9 (13) |
Values are means (SD).
Fig. 5.
Strength of the initial phase of the acoustic startle reflex (ASR) during low stress and high stress conditions. ASR response strength was calculated using amplitude and duration estimates from the cumulative sum (CUSUM) analysis. The strength of the initial phase of inhibitory responses significantly decreased from low to high stress, with a similar trend for reduced strength of the initial phase of excitatory responses.
A total of 37 SMUs in 17 participants were discriminated from startle trials in the low stress condition, and 33 SMUs in 16 participants were discriminated from startle trials in the high stress condition. Mean (SD) DR of SMUs during the 500 ms pre-SAS period was 11.2 (2.3) Hz in the low stress condition and 11.5 (1.9) Hz in the high stress condition and was not significantly different between conditions (t = 0.85, P = 0.40). Due to the limited number of SMUs identified during SAS trials in the excitatory response group (6 SMUs in low stress; 5 SMUs in high stress, n = 3), results for these participants could not be combined for analysis. Therefore, further analyses were performed only for the inhibitory response group (31 SMUs from 14 participants in the low stress condition; 28 SMUs from 13 participants in the high stress condition). Representative examples of SMU recordings from the inhibitory ASR group, and the averaged DR profiles are shown in Fig. 6. A sustained decrease in DR is seen during inhibitory ASRs in both low and high stress conditions.
Fig. 6.
Representative data for an inhibitory surface EMG response (A) and corresponding single motor unit (SMU) activity (B) in response to the startling acoustic stimulus (SAS). Vertical dashed lines represent onset of the SAS. SMU analyses were performed only for inhibitory ASRs due to the low number of identifiable SMUs in the excitatory ASR group. Discharge rates (DR) were normalized to the average DR from a 150-ms prestimulus period, and the average response onset for each participant was set as time 0 to account for variability in response latencies. DRs from all SMUs were then averaged in 10-ms bins within each stress condition. Periods of decreased DR were noted in both the low (C) and high (D) stress conditions.
Control session.
Twelve participants returned for the control session. No significant main effect for Condition was seen in STAI-S (F2,10 = 1.7, P = 0.24), whereas a trend was observed in HR (F2,10 = 4, P = 0.05) with a small increase observed in the low stress condition (HR = 72 beats/min) compared with both other conditions (HR = 69 beats/min). A significant main effect for MAP (F2,10 = 5, P = 0.04) was seen, with post hoc analyses revealing an increase from the baseline condition to the high stress condition (t = 3.1, P = 0.01). Results for RTs and pre-SAS sEMG means were similar to the experimental session. RTs were significantly shorter in startle trials [179.5 (38) s] compared with nonstartle trials [272.2 (52.6) s, F1,11 = 153, P < 0.01]. Pre-SAS sEMG was not significantly different between the first [7.2 (4.8) %MVC] and second [7.8 (6.2) %MVC] low stress condition (t = 0.62, P = 0.55).
Of the returning participants, one had no identifiable startle response in the experimental or control session. Nine participants from the inhibitory ASR group returned, although one had no identifiable startle response in the control session. Two participants from the excitatory ASR group returned. The excitatory group in the control session still demonstrated increased STAI-T compared with the inhibitory group [38 (10) vs. 26 (3) out of 80 points] and continued to exhibit excitatory responses. In the inhibitory ASR group, the eight participants with identifiable ASRs continued to demonstrate inhibitory ASRs, although no significant change in response strength was seen from the first low stress to the second low stress condition [−37 (11) % and −39 (13) % maximum inhibition, respectively, W = 9, P = 0.25]. No statistical analysis was performed on the returning excitation group due to low sample size, but a decrease in response strength from the first low stress to the second low stress condition was seen (36 and 20% maximum inhibition, respectively).
DISCUSSION
This investigation is the first to utilize the reduced attenuation of motor responses to acoustic stimuli seen during the StartReact paradigm to characterize stress-induced changes in the upper trapezius ASR. The upper trapezius showed predominantly inhibitory responses to the SAS, although excitatory responses were observed in some individuals with elevated trait anxiety. Contrary to expectation, the magnitude of both inhibitory and excitatory responses decreased in the presence of an acute psychosocial stressor, indicating a generalized reduction in reticulospinal effects on trapezius motor activity under stress.
Motor RT.
The StartReact paradigm is typically used to investigate preparation of a motor response (Carlsen et al. 2012), which attenuates habitation of the ASR in cervical muscles (Carlsen et al. 2011; Valls-Sole et al. 2008). Although results from the control experiment indicate that habituation of the ASR was effectively suppressed in the present investigation, changes in motor preparation (i.e., pinch grip RT) were unchanged in response to the acute psychosocial stressor. RTs were significantly shortened in SAS trials compared with non-SAS trials but were still longer than those seen in previous studies of the StartReact effect (Carlsen et al. 2012). Both these findings may be explained by the complexity of the task in the present experiment. Participants were required to perform isometric contractions of the trapezius muscle with both visual (target matching) and auditory (contract and relax) cues, combined with ongoing mental concentration and a motor RT task in response to an auditory cue. A recent study investigating the effects of dual-task performance on the StartReact effect showed similar results to those observed in the present study, with longer RTs during dual-task performance (Maslovat et al. 2015). These investigators also reported decreased sensitivity of RT changes in response to a SAS when one task was cognitive and the other motor. Thus it seems likely that the complex task in the current investigation increased motor RTs and potentially reduced their responsiveness to the psychosocial stressor.
Characterization of the upper trapezius ASR.
The authors are aware of only one previous investigation of ASRs in the upper trapezius muscle (Aniss et al. 1998). Results from the current study are consistent with previous observations of both inhibitory and excitatory components in the ASR, with onset latencies averaging ∼55 ms for the upper trapezius in both studies. This response latency is longer than that produced in the trapezius by transcranial electrical and magnetic stimulation (Alexander et al. 2007; Gandevia and Applegate 1988). This could be attributed to reduced conduction velocity within the reticulospinal tract (Rothwell 2006) and/or mediation of the ASR through spinal inhibitory interneurons (Davidson and Buford 2004). Specificity of the startle reflex was verified in the present study by poststimulus changes in muscle activity observed only for trials in which the pinch task was preceded by a startling acoustic stimulus. Results from this analysis further indicate that differences in the ASR cannot be attributed to changes in motor preparation associated with the pinch task, as no changes in trapezius muscle activity were observed following the presentation of non-SAS.
We categorized participants based on two observed response patterns: those with an initial period of inhibition during the ASR either with or without a subsequent period of inhibition or excitation, and those with an initial period of excitation during the ASR. The mechanisms underlying these complex response patterns remain unclear. The reticulospinal tract is capable of coordinating complex tasks in primates (Lemon et al. 2012; Rothwell 2006). Specifically, the trapezius muscle appears to be highly responsive to activation of the reticular formation in nonhuman primates (Davidson and Buford 2006), exhibiting both excitatory and inhibitory responses (Davidson and Buford 2004).
The ASR is typically thought to facilitate motor activity during a “fight-or-flight” response (Grillon 2008); however, the upper trapezius muscle was inhibited by SAS in the majority of our participants, likely due to activation of spinal inhibitory interneurons by the reticular formation (Davidson and Buford 2004). It is important to note that these inhibitory responses were observed during a precisely controlled isometric contraction of the trapezius muscle. Thus the inhibitory influence of the reticular formation may assist in the cessation of an ongoing motor task to help prepare the body for “fight-or-flight,” or may contribute to the freeze component of a “freeze, flight, fight, or fright” conceptualization of the stress response (Bracha et al. 2004). Subgroup analyses revealed that individuals with higher trait anxiety show an initial excitatory rather than inhibitory response to SAS. This finding is consistent with a previous study showing larger excitatory eye blink startle reflexes in women with higher trait anxiety (Poli and Angrilli 2015), although the present finding involved an apparent reversal in direction of the initial phase of the ASR response. Together, these findings suggest greater net excitation of the trapezius motor neuron pool resulting from reticulospinal activation among more anxious individuals.
Decreases in the DR of SMUs observed at the onset of inhibitory responses (Fig. 6) support that the initial inhibitory phase of the ASR is a result of altered synaptic input to spinal motor neurons and not recording artifact or EMG cross talk, which have been reported in upper trapezius reflexes previously (Vangsgaard et al. 2013). Although our sample size was insufficient to confirm increases in the DR of SMUs during excitatory ASRs, an increase in sEMG was evident from the CUSUM analysis for a small subgroup of participants. Together, these observations support the ability of the reticular formation to both suppress and facilitate upper trapezius muscle activity, consistent with previous findings from studies of nonhuman primates (Davidson and Buford 2004, 2006). Together with a previous investigation (Aniss et al. 1998), our findings indicate that the trapezius ASR is more complex than traditionally described in terms of a generalized protective response (Yeomans and Frankland 1995), as the magnitude and direction of the response depend on both participant characteristics and the task being performed. Additional studies are warranted to elucidate mechanisms underlying the complex pattern of ASR responses to reticulospinal activation.
Psychosocial stress reduces strength of the upper trapezius ASR.
The magnitude of the ASR response strength decreased in both the inhibitory and excitatory response groups in the presence of an acute psychosocial stressor (Fig. 5). Although the magnitude of the response decreased, it was completely eliminated in only one participant and SMU data showed similar changes in DR in both stress conditions. The observed reduction in ASR response strength is contrary to the hypothesized stress-induced increase in excitatory reticulospinal input to the trapezius. The more often studied eye blink startle reflex has been shown to increase during periods of threat, fear, and anxiety (Bublatzky et al. 2013; Davis et al. 2010; Dichter et al. 2002). However, the eye blink reflex may be mediated by a separate pathway than startle reflexes in other body regions (Valls-Sole 2012) and thus may show different changes in response to the same environmental threat. The decrease in ASR response strength could be explained by habituation to the SAS; however, results from the control session indicate that the decrease in inhibition during the acute stressor was not due to habituation. Excitatory responses in the control session decreased over time for two participants, suggesting possible habituation of excitatory ASR responses to psychosocial stress among more anxious individuals; however, the sample size for this group was too small to make any statistical inferences.
When considering functional implications of a stress-induced reduction in the magnitude of the trapezius startle reflex, it is important to note that the psychosocial stressor in this study induced anxiety, or apprehension, caused by unpredictable and symbolic threats (Grillon 2008). Although the ASR may serve to facilitate motor responses to a sudden and immediate threat, when this threat is partially expected (as during anxiety), it may be more beneficial to facilitate voluntary (i.e., cortically driven) action that is more specific to a given threat in the environment. Functionally, the observed reduction in reticulospinal input with heightened anxiety may serve a similar purpose as reductions in muscle spindle activity observed with sympathetic activation (Matsuo et al. 1995; Roatta et al. 2002). Specifically, a reduction in peripheral and subcortical inputs during periods of increased anxiety may allow the body to make faster voluntary responses, via corticospinal mechanisms, to a partially anticipated threat. This idea is supported by previously observed increases in corticospinal responsiveness in response to acute psychosocial stressors (Marker et al. 2013; Oathes et al. 2008). It is also possible that the withdrawal of reticulospinal inhibitory effects (i.e., disinhibition) may contribute to stress-induced increases in trapezius muscle activity (Eijckelhof et al. 2013; Shahidi et al. 2013), which is particularly relevant for clinical pain populations who also have impairments of cortical inhibition (Marker et al. 2013; Schwenkreis et al. 2010).
Limitations.
The major limitation of this study is the small number of startle trials available to quantify the ASR. Although the number of trials utilized for the CUSUM calculations was less than those previously recommended (Brinkworth and Turker 2003), it was necessary to limit the number of trials as physiological arousal in response to an acute stressor cannot be maintained indefinitely. Accordingly, a reduction in HR had already become evident near the end of the high stress condition (Fig. 3C). The SAS was also presented at a slightly higher frequency (30% of trials) than previously recommended for StartReact paradigms (25% of trials) (Carlsen et al. 2011) but was similar to other studies investigating the StartReact during head movements (Siegmund et al. 2001). Therefore, the number of SAS trials in the current study was optimized for constraints of the stress protocol, and reliable responses were obtained across sessions. Sample sizes for subgroup analyses of inhibitory and excitatory ASR responses were also imbalanced and small. However, the statistical analysis used to compare groups makes no normality assumptions and is well suited for handling small, unbalanced groups (Curran-Everett 2012). The initial characterization of excitatory ASR responses, particularly regarding their presence and habituation among individuals with higher trait anxiety, should be confirmed in a larger sample.
Another limitation of this study is the lack of direct confirmation that a startle response occurred in each SAS trial. Previous studies have confirmed the presence of an ASR based on reflex responses in the SCM sEMG (Carlsen et al. 2011); however, this was not possible in the present study due to highly variable levels of background muscle activity in the SCM. Several indirect confirmations support the proposal that the analyzed responses in the upper trapezius were true ASRs, reflecting modulation of reticulospinal input to the muscle. First, the upper trapezius responses in this investigation were similar in onset and form to upper trapezius ASRs in the one other published investigation of this response (Aniss et al. 1998). No significant sEMG changes were observed during nonstartle trials, supporting that the responses were a direct consequence of the SAS in the startle trials. RTs were significantly shorter in startle compared with nonstartle trials, similar to previous studies showing that motor RTs are reduced during a startle response (Maslovat et al. 2015). Finally, we utilized a protocol of SAS presentation based on StartReact paradigms previously shown to consistently elicit ASRs in cervical muscles (Carlsen et al. 2011; Siegmund et al. 2001).
We cannot rule out potential influences from systems other than the reticulospinal system, however. For example, declines in performance on the cognitive task in the high stress condition may reflect a shift in cognitive attention that could have affected trapezius motor responses, although this seems unlikely given the similarity of background EMG and RT between stress conditions. Alternatively, there is some evidence that SAS can induce changes in the responsiveness of corticomotor and autonomic pathways (Valls-Sole 2012). Future studies using direct stimulation or lesioning of specific brain regions are needed to elucidate underlying neural mechanisms of stress induced changes in cervical motor activity.
Conclusions.
This study is the first to quantify stress-induced changes in the ASR in the upper trapezius muscle of humans, and our findings reveal a complex pattern of inhibitory and facilitatory responses consistent with observations in nonhuman primates. We further demonstrate that exposure to an acute psychosocial stressor consistently attenuates the strength of the ASR response in all participants. These findings have implications for the control of motor behaviors in the presence of stress and indicate a need for future studies to identify task determinants, physiological mechanisms, and the functional significance of complexities in the human ASR.
GRANTS
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01-AR-056704 (to K. S. Maluf), National Science Foundation Grant DGE-0742434 (to R. J. Marker), and a Foundation for Physical Therapy Promotion of Doctoral Studies II Scholarship (to R. J. Marker).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.J.M. and K.S.M. performed experiments; R.J.M. analyzed data; R.J.M., S.C., and K.S.M. interpreted results of experiments; R.J.M. prepared figures; R.J.M. drafted manuscript; R.J.M., S.C., and K.S.M. edited and revised manuscript; R.J.M., S.C., and K.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. John Buford for helpful comments on an initial draft of this manuscript.
REFERENCES
- Alexander C, Miley R, Stynes S, Harrison PJ. Differential control of the scapulothoracic muscles in humans. J Physiol 580: 777–786, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniss AM, Sachdev PS, Chee K. Effect of voluntary muscle contraction on the startle response to auditory stimuli. Electromyogr Clin Neurophysiol 38: 285–293, 1998. [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology 42: 1–15, 2005. [DOI] [PubMed] [Google Scholar]
- Bracha HS, Ralston TC, Matsukawa JM, Williams AE, Bracha AS. Does “fight or flight” need updating? Psychosomatics 45: 448–449, 2004. [DOI] [PubMed] [Google Scholar]
- Brinkworth RS, Turker KS. A method for quantifying reflex responses from intra-muscular and surface electromyogram. J Neurosci Methods 122: 179–193, 2003. [DOI] [PubMed] [Google Scholar]
- Bublatzky F, Guerra PM, Pastor MC, Schupp HT, Vila J. Additive effects of threat-of-shock and picture valence on startle reflex modulation. PLoS One 8: e54003, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Franks IM. Preparation for voluntary movement in healthy and clinical populations: evidence from startle. Clin Neurophysiol 123: 21–33, 2012. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev 35: 366–376, 2011. [DOI] [PubMed] [Google Scholar]
- Christou EA, Jakobi JM, Critchlow A, Fleshner M, Enoka RM. The 1- to 2-Hz oscillations in muscle force are exacerbated by stress, especially in older adults. J Appl Physiol 97: 225–235, 2004. [DOI] [PubMed] [Google Scholar]
- Curran-Everett D. Explorations in statistics: permutation methods. Adv Physiol Educ 36: 181–187, 2012. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173: 25–39, 2006. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol 92: 83–95, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35: 105–135, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Baucom BR. Startle modulation before, during and after exposure to emotional stimuli. Int J Psychophysiol 43: 191–196, 2002. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 130: 355–391, 2004. [DOI] [PubMed] [Google Scholar]
- Eijckelhof BH, Huysmans MA, Bruno Garza JL, Blatter BM, van Dieen JH, Dennerlein JT, van der Beek AJ. The effects of workplace stressors on muscle activity in the neck-shoulder and forearm muscles during computer work: a systematic review and meta-analysis. Eur J Appl Physiol 113: 2897–2912, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45: 302–304, 1978. [DOI] [PubMed] [Google Scholar]
- Falla D, Dall'Alba P, Rainoldi A, Merletti R, Jull G. Location of innervation zones of sternocleidomastoid and scalene muscles–a basis for clinical and research electromyography applications. Clin Neurophysiol 113: 57–63, 2002. [DOI] [PubMed] [Google Scholar]
- Farina D, Madeleine P, Graven-Nielsen T, Merletti R, Arendt-Nielsen L. Standardising surface electromyogram recordings for assessment of activity and fatigue in the human upper trapezius muscle. Eur J Appl Physiol 86: 469–478, 2002. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Applegate C. Activation of neck muscles from the human motor cortex. Brain 111: 801–813, 1988. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology (Berl) 199: 421–437, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G. The emotional motor system. Eur J Morphol 30: 67–79, 1992. [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Front Biosci 8: s438–451, 2003. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Landau W, Tutssel D, Lawrence DG. Lawrence and Kuypers (1968a,b) revisited: copies of the original filmed material from their classic papers in Brain. Brain 135: 2290–2295, 2012. [DOI] [PubMed] [Google Scholar]
- Marker RJ, Maluf KS. Effects of electrocardiography contamination and comparison of ECG removal methods on upper trapezius electromyography recordings. J Electromyogr Kinesiol 24: 902–909, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker RJ, Stephenson JL, Kluger BM, Curran-Everett D, Maluf KS. Modulation of intracortical inhibition in response to acute psychosocial stress is impaired among individuals with chronic neck pain. J Psychosom Res 76: 249–256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslovat D, Drummond NM, Carter MJ, Carlsen AN. Reduced motor preparation during dual-task performance: evidence from startle. Exp Brain Res 33: 2673–2683. 2015. [DOI] [PubMed] [Google Scholar]
- Matsuo R, Ikehara A, Nokubi T, Morimoto T. Inhibitory effect of sympathetic stimulation on activities of masseter muscle spindles and the jaw jerk reflex in rats. J Physiol 483: 239–250, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen KB, Sand T, Borchgrevink P, Leistad RB, Ro M, Westgaard RH. A unilateral sympathetic blockade does not affect stress-related pain and muscle activity in patients with chronic musculoskeletal pain. Scand J Rheumatol 37: 53–61, 2008. [DOI] [PubMed] [Google Scholar]
- Nilsen KB, Sand T, Stovner LJ, Leistad RB, Westgaard RH. Autonomic and muscular responses and recovery to one-hour laboratory mental stress in healthy subjects. BMC Musculosket Disord 8: 81, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noteboom JT, Fleshner M, Enoka RM. Activation of the arousal response can impair performance on a simple motor task. J Appl Physiol 91: 821–831, 2001. [DOI] [PubMed] [Google Scholar]
- Oathes DJ, Bruce JM, Nitschke JB. Worry facilitates corticospinal motor response to transcranial magnetic stimulation. Depress Anxiety 25: 969–976, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli E, Angrilli A. Greater general startle reflex is associated with greater anxiety levels: a correlational study on 111 young women. Front Behav Neurosci 9: 10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roatta S, Windhorst U, Ljubisavljevic M, Johansson H, Passatore M. Sympathetic modulation of muscle spindle afferent sensitivity to stretch in rabbit jaw closing muscles. J Physiol 540: 237–248, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC. The startle reflex, voluntary movement, and the reticulospinal tract. Suppl Clin Neurophysiol 58: 223–231, 2006. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Scherens A, Ronnau AK, Hoffken O, Tegenthoff M, Maier C. Cortical disinhibition occurs in chronic neuropathic, but not in chronic nociceptive pain. BMC Neurosci 11: 73, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi B, Haight A, Maluf K. Differential effects of mental concentration and acute psychosocial stress on cervical muscle activity and posture. J Electromyogr Kinesiol 3: 1082–1089, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund GP, Inglis JT, Sanderson DJ. Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol 535: 289–300, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, 1983. [Google Scholar]
- Staab JP, Balaban CD, Furman JM. Threat assessment and locomotion: clinical applications of an integrated model of anxiety and postural control. Semin Neurol 33: 297–306, 2013. [DOI] [PubMed] [Google Scholar]
- Stephenson JL, Maluf KS. Discharge behaviors of trapezius motor units during exposure to low and high levels of acute psychosocial stress. J Clin Neurophysiol 27: 52–61, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J. Assessment of excitability in brainstem circuits mediating the blink reflex and the startle reaction. Clin Neurophysiol 123: 13–20, 2012. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp Brain Res 187: 497–507, 2008. [DOI] [PubMed] [Google Scholar]
- Vangsgaard S, Norgaard LT, Madeleine P, Taylor JL. Crossed responses found in human trapezius muscles are not H-reflexes. Muscle Nerve 49: 362–369, 2013. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Brain Res Rev 21: 301–314, 1995. [DOI] [PubMed] [Google Scholar]