Abstract
Unhealthy alcohol use is common among HIV-positive patients, yet effective evidence-based treatments are rarely provided in clinical settings providing HIV care. Further, given patient variability in response to initial treatments, stepped care approaches may be beneficial. We describe the rationale, aims and study design for the current Starting Treatment for Ethanol in Primary care Trials (STEP Trials); three parallel randomized controlled effectiveness trials being conducted in five Infectious Disease Clinics. Participants meeting criteria for: 1) at-risk drinking, 2) moderate alcohol use with liver disease (MALD), or 3) alcohol use disorder (AUD) are randomized to integrated stepped care versus treatment as usual. For those with at-risk drinking or MALD, integrated stepped care starts with a one session brief intervention and follow-up 2-week telephone booster. Based on pre-specified nonresponse criteria, participants may be “stepped up” at week 4 to receive four sessions of motivational enhancement therapy (MET) and “stepped up” again at week 12 for addiction physician management (APM) and consideration of alcohol pharmacotherapy. For those with AUD, integrated stepped care begins with APM. Non-responders may be “stepped up” at week 4 to receive MET and again at week 12 for a higher level of care (e.g. intensive outpatient program). The primary outcome is alcohol consumption assessed at 24 weeks, and secondary outcome is the VACS Index, a validated measure of HIV morbidity and mortality risk. Results from the STEP Trials should inform future research and the implementation of interventions to address unhealthy alcohol use among HIV-positive individuals.
Keywords: Multicenter study, Randomized controlled trial, Algorithms, HIV, Alcohol
1. Introduction
Unhealthy alcohol use, the spectrum of drinking that spans from at-risk drinking to alcohol use disorder [1], is common among HIV-positive patients. Depending on the sample, up to 70% of HIV-positive individuals may consume alcohol and over one third may have unhealthy alcohol use [2]. In addition, they may be more sensitive to the effects of alcohol than individuals without HIV [3, 4]. Furthermore, unhealthy alcohol use among HIV-positive individuals may lead to a range of adverse individual and public health-related consequences with effects along the HIV care continuum. For example, unhealthy alcohol use may be associated with HIV disease progression [5], decreased quality of HIV care and retention in care [6, 7], and increased risk and exacerbation of comorbid conditions, such as liver disease [8]. In addition, alcohol use, especially unhealthy alcohol use, contributes to increased sexual risk behaviors [9] and medication non-adherence [10, 11], resulting in increased HIV disease transmission.
Despite the existence of effective treatments for unhealthy alcohol use, including counseling and pharmacotherapy, HIV-positive individuals inconsistently discuss their alcohol use with HIV providers [12]. Patients may be unaware of the medical harms associated with their alcohol use and HIV providers may be underprepared to provide treatment [13–17]. Accordingly, many patients do not receive appropriate treatment. As is recommended by the Institute of Medicine [18] and supported by the Affordable Care Act, models of care which integrate screening and treatment for unhealthy alcohol use into primary care may be effective at identifying patients with unhealthy alcohol use and providing or linking to appropriate treatment. While efforts have been made to implement routine screening and treatment for unhealthy alcohol use into primary settings [19], this is not yet consistently delivered through HIV Clinics [20–22]. Given that HIV providers may not have the time and/or the appropriate skills to address unhealthy alcohol use with their patients, evaluating alternative models of integrated care that rely on multi-disciplinary teams are necessary.
Due to variability in patient responses to treatments for unhealthy alcohol use, approaches that are flexible and responsive to patient needs are indicated. Previously applied to other common chronic medical conditions (e.g. hypertension, depression, and pain) [23–25], stepped care models offer one potential solution. Such models allow patients to receive increasing intensity of treatment when unable to reach treatment goals. As substance use treatment resources are often limited in HIV clinical settings, we sought to test the impact of interventions that “self-corrected” or ramped up in those patients who needed additional services rather than providing all combined services up front. In stepped care strategies, patients initially receive low intensity interventions and their treatments are intensified, or “stepped up” based on predefined failure criteria and their response to a treatment. Stepped care models may be particularly applicable for treating patients with unhealthy alcohol use as they are individualized, incorporate evidence-based practices and place the least constraints on patients, which may be particularly relevant as many may not be seeking treatment [26].
Therefore, we are conducting the Starting Treatment for Ethanol in Primary care Trials (STEP Trials) to examine the effectiveness of integrated stepped care compared to treatment as usual for unhealthy alcohol use on alcohol consumption and other health outcomes among HIV-positive patients [27]. These three distinct randomized controlled effectiveness trials seek to enroll HIV-positive patients with unhealthy alcohol use across five Veterans Health Administration (VA) Infectious Disease Clinics. While distinct based on their target population (at-risk drinking, moderate alcohol use with liver disease [MALD] and alcohol use disorder), the three trials share the same design and are being executed contemporaneously. The goals of this paper are to, describe the rationale, aims, and study design of the STEP Trials.
2. Methods
2.1. Rationale for three distinct trials
There is a spectrum, from mild to severe, of unhealthy alcohol use [1]. Treatments are tailored to address the severity of harms associated with the level of alcohol consumption. Brief psychosocial counseling interventions are typically the primary treatment for individuals who meet criteria for milder unhealthy alcohol use (e.g. at-risk drinking) while more extensive psychosocial interventions and medications are indicated for those with more severe unhealthy alcohol use (e.g. alcohol use disorder). Similarly, alcohol consumption targets that are considered successful responses to treatment vary by diagnosis within the unhealthy alcohol use spectrum. These two factors posed a challenge to the design and execution of a single trial wherein all participants with unhealthy alcohol use assigned to the active intervention receive the same sequence of stepped care regimens with the identical a priori failure criteria for stepping up. This necessitated the conduct of three distinct trials that shared overall design and execution. To address the first challenge, we created three mutually exclusive groups of HIV-positive individuals with unhealthy alcohol use based on established diagnostic criteria and guideline recommendations (Table 1). The first met criteria for at-risk drinking according to drinking limits outlined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) [28]. The second met criteria for an alcohol use disorder (Diagnostic and Statistical Manual (DSM)-IV TR criteria for alcohol abuse or dependence) [29]. The third group consumed alcohol, had evidence of liver disease, but did not meet criteria for at-risk drinking or an alcohol use disorder. To address the challenge of varied drinking targets, we identified group specific failure criteria for stepping up treatment.
Table 1.
Unhealthy alcohol use: eligibility criteria.
| Drinking category | Description |
|---|---|
| At-risk drinking | Alcohol consumption that is 14 or more drinks per week or 4 or more drinks per occasion in men younger than or equal to 65 years old or 7 or more drinks per week or 3 or more drinks per occasion in women or men older than 65 years old [28] |
| Alcohol use disorder | Meet DSM-IV TR criteria for alcohol abuse or dependence (as DSM—5 was released after initiation of the trial) [29] |
| Moderate alcohol use with liver disease (MALD) | Report of any alcohol consumption in the past 30 days and are either hepatitis C virus (HCV) coinfected, confirmed by HCV antibody and a detectable HCV RNA viral load, or have evidence of liver fibrosis, defined as a FIB-4 score > 1.45 [8,75,76] and do not meet criteria for at-risk drinking or alcohol use disorder. |
2.2. Overall design
Study participants include HIV-positive patients with unhealthy alcohol use receiving care through one of five VA Infectious Disease Clinics in Washington, District of Columbia; Atlanta, Georgia; the Brooklyn/Manhattan, New York; and Dallas and Houston, Texas. Patients who provide written informed consent undergo assessment to confirm eligibility and if eligible, are then randomized to receive six months of integrated stepped care or treatment as usual. Depending on the patients' level of alcohol use and treatment response, those assigned to integrated stepped care are scheduled to receive a manual-guided Brief Negotiated Interview (BNI) and telephone booster (delivered by an on-site social worker); manual-guided motivational enhancement therapy (MET) over four sessions (delivered by an on-site psychologist); and/or addiction physician management (APM) (delivered by an on-site addiction psychiatrist) with an emphasis on consideration of prescribing pharmacotherapy to decrease alcohol use. Assessments are conducted at baseline, week 4, week 12, week 24 and month 12 by research coordinators. The primary outcome of each of these trials focuses on alcohol consumption. The secondary outcome is change in VACS Index score (see description below) [30–32]. Exploratory aims include antiretroviral therapy medication adherence, psychosocial outcomes, and multi-substance use.
2.3. Study context, coordinating center and institutional review
The STEP Trials (U01-AA020795) represent the intervention component of the NIAAA-funded Consortium to improve OutcoMes in HIV/AIDS, Alcohol, Aging and multi-Substance use (COMpAAAS) (U24-AA020794), that also includes an observational component (the Veterans Aging Cohort Study - VACS) which contains all data available through the VA electronic medical record (U01-AA020790) and an operations research (U01-AA020799) component (the Operations Research Collaboration for Alcohol Abuse and AIDS – ORCAAA). COMpAAAS is a member of the NIAAA's Consortiums for HIV/AIDS and Alcohol Research Translation (CHAART). The coordinating center for the STEP Trials is located at Yale University, New Haven, CT and VA Connecticut Healthcare System, West Haven, CT. In addition, the Yale Center for Analytic Sciences serves as data and study monitor. The coordinating center sites and each of the participating sites are approved by their Institutional Review Board to conduct the study. The study is registered at www.clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT01410123).
2.4. Eligibility
To meet criteria for participation in one of the STEP Trials, individuals must be 1) HIV-positive; 2) a patient at one of the five participating VA Infectious Disease Clinics; 3) English speaking and able to provide written informed consent; and 4) meet criteria for unhealthy alcohol use (see Table 1). Patients are excluded if they meet any of the following criteria: 1) are acutely suicidal or with a psychiatric condition (e.g., psychosis, dementia, delusional) that affects their ability to provide informed consent or are participating in counseling interventions; 2) are currently enrolled in formal treatment for unhealthy alcohol use, excluding self or mutual-help groups (e.g., Alcoholics Anonymous); 3) have any medical condition(s) that would preclude completing the study or cause harm during the course of the study; and 4) are a pregnant or nursing woman, or women who do not agree to use a reliable form of birth control. Since abstinence is recommended during pregnancy and specialty care might be required to achieve this goal, this final criterion was put in place to avoid randomizing pregnant women to treatment as usual.
2.5. Recruitment and randomization
Individuals are recruited for participation through a range of mechanisms: 1)with approval from the clinic director and staff, routine or research coordinator-delivered screening with the Alcohol Use Disorders Identification Test (AUDIT-C) [33], a validated three item tool which assesses alcohol consumption and is widely used throughout the VA [19]; 2) provider-referral; 3) patient self-referral; and/or 4) a list of potentially eligible patients generated using COMpAAAS data, based on medical diagnoses and laboratory data. Individuals with an AUDIT-C score greater than zero, meet with the local research coordinator to provide written informed consent and then complete a baseline interview to confirm eligibility, and if eligible, proceed with randomization.
Randomization occurs independently for each trial in a 1:1 ratio of integrated stepped care to treatment as usual and is stratified based on site and VACS Index. Randomization schemes were implemented in TrialDB [34], a customizable web-based clinical trials management system used for the randomization as well as storage and management of clinical data. The randomization sequence was concealed in TrialDB. After reviewing eligibility criteria, study personnel entered baseline participant data into TrialDB and the treatment assignment was allocated by the system. Randomized patients are reimbursed with a $25 gift card for completing baseline assessments and an additional $50 gift card upon completing any follow-up assessments. Documentation of written informed consent was included in the electronic medical record and served as a mechanism to inform providers of their patients' participation in the study.
2.6. Data collection protocol
Assessments are collected by trained research coordinators at baseline, week 4, week 12,week 24 and month 12 (Table 2) for all of the trials' participants. The primary outcomes, assessed at week 24, are defined as drinks per week over the prior 28 days in the trials of at-risk drinking and alcohol use disorder and abstinence over the prior 28 days in the MALD trial. These are measured using the Alcohol Time-line Followback [35], a validated and widely used calendar-based method of self-reported alcohol consumption that uses prompts to facilitate recall and determine alcohol use during the past 30 days. These outcomes will be similarly evaluated at 12 months to assess durability of the intervention. The secondary outcome, also assessed at 24 weeks, is change in biological markers as measured by the VACS Index score. The VACS Index score is a validated composite biomarker that integrates routinely available laboratory-based test results to predict morbidity and mortality risk based on measures of organ system injury [30, 31, 36, 37]. It has been evaluated in both HIV-positive and negative patients. Specifically, the VACS Index score includes age, HIV biomarkers (CD4 cell count, HIV RNA viral load), hemoglobin, FIB-4 (a non-invasive measure of liver fibrosis calculated based on aspartate aminotransferase, alanine aminotransferase and platelets), estimated glomerular filtration rate (determined with serum creatinine), and presence of hepatitis C virus (HCV) infection. It is responsive to changes in antiretroviral therapy treatment [38] and varying levels of alcohol and illicit opioid use [32, 39], with higher scores being associated with greater substance use. Labs drawn in the context of routine clinical care within the VA system in the appropriate windows and that are available in the electronic medical record will be included in analyses to minimize missing data. These labs will complement labs ordered as part of the study and include available labs regardless of where they were drawn (i.e. Emergency Department, inpatient, or outpatient). The outcomes for the exploratory analyses include antiretroviral therapy medication adherence, depressive symptoms, unprotected sex after alcohol use, and the effects of treatment on multi-substance use. Antiretroviral therapy medication adherence is ascertained using VA pharmacy fill/refill data, that is available through the electronic medical record. The medication possession ratio, calculated as the number of days supply divided by the refill interval [40], has been validated against important outcomes for those with HIV infection, including treatment initiation [41], HIV viral suppression [42], and mortality [43]. Depressive symptoms are evaluated using the Patient Health Questionnaire depressive scale (PHQ-9) [44], a validated 9 item instrument where each criteria of the DSM-IV are scored from 0 to 3, where 0 indicates that symptoms are not at all present and 3 indicates presence of symptoms nearly every day. Higher scores indicate higher levels of depressive symptoms (e.g., scores of 10 to 14 indicate moderate depression, 15 to 19 indicate moderately severe depression, and 20 to 27 indicate severe depression). To determine unprotected sex after alcohol use, number of episodes of unprotected sex after alcohol use with partners whose HIV status is either unknown, positive or negative is ascertained [45, 46]. Multi-substance use is measured using the Addiction Severity Index Lite-CF [47], that captures presence and severity of drug and alcohol-related problems, in addition to psychiatric symptoms, and medical, employment, legal and social problems over the past 30 days. Presence of multi-substance use will be defined as concurrent use of other substances in addition to alcohol.
Table 2.
STEP Trials: summary of study assessments and schedule.
| Assessment | Baseline | Week 4 |
Week 12 |
Week 24 |
Month 12 |
|---|---|---|---|---|---|
| Demographics (age, gender, race, ethnicity) | X | ||||
| AUDIT-C [33] | X | ||||
| Mini-SCID for alcohol [77] | X | ||||
| Alcohol Timeline Followback [35] | X | X | X | X | X |
| Current alcohol levels with breathalyzer | X | X | X | X | X |
| Biomarker of chronic alcohol use with phosphatidylethanol (PEth) [48–50] | X | X | |||
| VACS Index components [30–32,36–38], including CD4 count, HIV RNA viral load, hemoglobin, platelet count, creatinine, alkaline phosphatase, aspartate phosphatase, hepatitis C antibody and RNA viral load |
X | X | X | X | |
| Urine pregnancy test (women only) | X | Xa | Xa | Xa | Xa |
| Addiction Severity Index Lite-CF to assess drug and alcohol problems, psychiatric symptoms, and medical, employment, legal and social problems over past 30 days [47] |
X | X | X | ||
| Treatment Services Review [52] | X | X | X | X | X |
| Family alcohol history | X | ||||
| Readiness to change drinking, with readiness ruler | X | ||||
| Smoking assessment with Fagerstrom [54,55] and readiness to quit smoking item | X | X | X | X | X |
| Patient Health Questionnaire (PHQ-9) for depressive symptoms [44] | X | X | X | X | |
| HIV history | X | ||||
| Antiretroviral therapy medication adherence, using pharmacy fill/refill data and the medication/possession ratio [40] | |||||
| Risk assessment [45,46,78] | X | X | X | X | X |
| Patient Satisfaction Questionnaire [56] | X | X |
Only if clinically indicated.
In addition to self-reported measures of alcohol consumption, current alcohol levels are measured by breathalyzer testing and chronic measures of alcohol exposure are measured by serum phosphatidylethanol (PEth). Obtained by a finger stick, PEth is a phospholipid formed on red blood cells only after alcohol exposure and reflects alcohol exposure during the prior 2 to 3 weeks. It is a reliable marker and has been demonstrated to be superior to other biomarkers produced from ethanol metabolism [48–50] and will be used as a secondary measure of alcohol exposure. PEth values > 8 ng/mL are consistent with alcohol consumption in the past three weeks.
In combination with measures of healthcare utilization obtained from the electronic medical record [51], the Treatment Services Review [52] will catalogue the amount and type of treatment services received by participants through routinely available services both within and outside the VA. As over 20% of patients receiving care through the VA may receive treatment for acute health issues outside VA [53], systematic data collection of such services is necessary for a comprehensive understanding of treatment received. Additionally, the Treatment Services Review will capture participation in mutual help groups that would not be available through the electronic medical record (e.g. attendance at Alcoholics Anonymous meetings). Given their association with treatment responses, we include items on family alcohol history, readiness to change drinking and smoking status, as measured by the Fagerstrom [54, 55] and an item on readiness to quit smoking. In addition to demographics, comorbid conditions will be extracted from the electronic medical record using International Classification of Diseases (ICD-9) codes. Participant satisfaction is ascertained with a Patient Satisfaction Questionnaire, adapted from previous studies [56].
2.7. Intervention overview
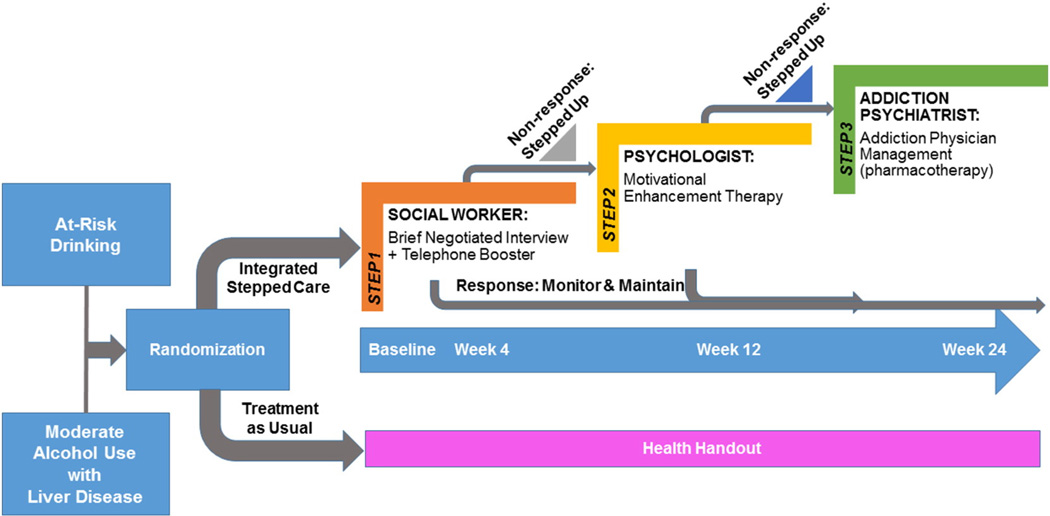
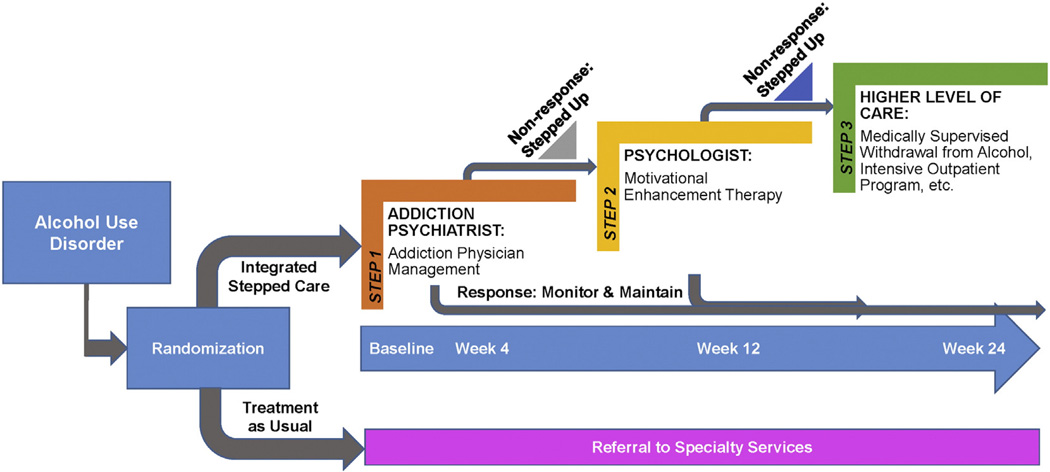
Consistent with stepped care, the integrated stepped care intervention begins with lower intensity services that are stepped up, if necessary, at predefined time points based on a priori criteria (Figs. 1 and 2). Depending on the treatment response, there are three potential intervention “steps” (Table 3). The interval between these steps was designed to allow for time to deliver the intervention and allow for a response to treatment to emerge. Further, we sought to promote standardization in the time periods applied for determining whether participants met criteria to be stepped up. In the at-risk drinking and MALD trials, Step 1 consists of a brief intervention and telephone booster delivered by a social worker; Step 2 is MET delivered by a psychologist over four sessions; and Step 3 is APM delivered by an addiction psychiatrist, with a focus on pharmacotherapy to decrease alcohol use. Similarly, in the alcohol use disorder trial, Step 1 includes APM delivered by an addiction psychiatrist, with a focus on pharmacotherapy to decrease alcohol use, Step 2 includes MET delivered by a psychologist over four sessions; and Step 3 includes a higher level of care depending on locally available services. The interventions are designed to be integrated into the VA HIV Clinics such that all services are delivered in the HIV Clinics (i.e. co-located) when possible. In addition, the interventions are based upon principles of stepped models of care as participants receive additional services if they do not meet pre-specified goals, as outlined below. Experiences on delivering integrated stepped care for unhealthy alcohol use among HIV-positive patients in the context of the STEP Trials from the perspectives of the interventionists have recently been described [27]. Participants could be referred for additional services as deemed appropriate by their providers and/or interventionists. The interventionists were advised to document visit notes consistent with their usual practice within the electronic medical record and order medications (i.e. naltrexone) through the VA.
Fig. 1.
The STEP Trials: protocol overview at-risk drinking and moderate alcohol and liver disease groups.
Fig. 2.
The STEP Trials: protocol overview for alcohol use disorder group.
Table 3.
STEP Trials: overview of intervention components.
| Drinking category | Step | Treatment service | Integrated stepped care | Treatment as usual* |
|---|---|---|---|---|
| At-risk | 1 | BNI | Yes | No |
| 1 | Telephone booster | Yes | No | |
| 2 | MET | Yes | No | |
| 3 | APM with pharmacotherapy | Yes | No | |
| Moderate alcohol use with liver disease | 1 | BNI | Yes | No |
| 1 | Telephone Booster | Yes | No | |
| 2 | MET | Yes | No | |
| 3 | APM with pharmacotherapy | Yes | No | |
| Alcohol use disorder | 1 | APM with pharmacotherapy | Yes | No |
| 2 | MET | Yes | No | |
| 3 | Referral for higher level of care | Yes | No |
Notes: asterisk (*) indicates that services may be available at the medical center but are not coordinated onsite.
2.7.1. Step 1 for at-risk drinking and MALD trials
Participants in the at-risk drinking and MALD trials who are randomized to integrated stepped care, receive a brief psychosocial intervention, the Brief Negotiated Interview (BNI) delivered by an onsite social worker. This manual-guided brief intervention is based upon principles of motivational interviewing and stages of change model of behavior change and has demonstrated efficacy in decreasing alcohol in patients with at-risk drinking [57, 58]. For the current trials, the content of the BNI manual was modified to include HIV and HCV specific content. The main goals of the session, designed to be 15 to 20 min in duration, are to 1) decrease participant ambivalence to reduce alcohol use by reviewing the participant's perceptions regarding pros and cons of alcohol use and 2) negotiate strategies for change based on the participant's readiness to change. The specific components include: 1) raising the subject of alcohol; 2) providing feedback, including reviewing the participant's alcohol consumption, making a connection to the participant's medical condition using the STEP Trials Feedback Form (Fig. 3), and reviewing the NIAAA guidelines for lower risk drinking (e.g."We know that our adherence to medication decreases even with one or two drinks. Drinking at any level may affect your liver.”); 3) enhancing motivation, by having the participant identify their level of motivation using a readiness to change ruler and developing discrepancy; and 4) negotiating and advising, during which a drinking goal is negotiated, participants are given specific advice, and a drinking agreement is completed, summarizing and arranging follow-up. Participants are also referred to web-based resources for self-help. The information provided in the Feedback Form for “other drug use” was determined based on the Addiction Severity Index-Lite CF and categorized as “high,” “medium,” or “low” risk based on drug type and frequency of use. Information provided in other domains of the Feedback Form was based on research assessments as well as laboratories as indicated. Detailed guides for interpreting the Feedback Form were created for both interventionists and participants.
Fig. 3.
STEP Trials Feedback Form.
Consistent with Project Treat [59], the BNI is followed by a 15–20 min telephone call at 2 weeks, which follows a similar structure as the BNI and aims to review participant progress and challenges towards meeting the goals outlined in the drinking agreement. We anticipated that any impact of this intervention would be reliably observed by week 4 and recognize that treatment effects likely decay over time [60].
2.7.2. Step 1 for alcohol use disorder trial
Participants in the alcohol use disorder trial who are randomized to integrated stepped care, will receive APM. Consistent with prior work with integrated addiction treatment into HIV Clinics [21, 22], APM includes: 1) assessment of the impact of alcohol use on medical, psychiatric, social, employment and legal functioning; 2) education of the participant of the harms associated with alcohol use with the STEP Trials Feedback Form (Fig. 3); 3) offering a prescription of pharmacotherapy to decrease cravings for alcohol use, prioritizing Food and Drug Administration (FDA)-approved medications for this indication (i.e. naltrexone, acamprosate, disulfiram) and those with strong efficacy data, unless contraindicated; 4) encouragement of the participant to attain or maintain their drinking target and adhere to medications (if indicated); 5) encouragement of lifestyle changes, avoidance of triggers and attendance at mutual-help groups; 6) identification and response to medical complications of alcohol use; and 7) referral of participants to other treatment services as appropriate. These sessions occur weekly for two weeks, then every other week for four weeks, and then monthly up to a total of eight sessions over the 24-week treatment period. The week 4 assessment to determine treatment response is consistent with data demonstrating that return to heavy drinking will occur among approximately 40% by week 4 [61].
2.7.3. Step 2 for at-risking drinking, MALD and alcohol use disorder trials
Participants randomized to integrated stepped care are evaluated for their response to Step 1 at week 4. Based on their alcohol use during the prior 14 days as determined by the TLFB method, participants will be stepped up to Step 2 of the intervention if they do not meet pre-specified goals (Table 4) [62]. In all trials, Step 2 consists of MET delivered over 4 sessions [63] with sessions every other week over the course of 6 weeks, by onsite psychologists. The MET manual is based on the ELM Brief Intervention Study Treatment Manual [64] and includes adaptations for HIV-positive and HCV-positive patients. Consistent with the BNI, this intervention is grounded in motivational interviewing and the stages of change model for behavior change. It is designed such that psychologists use reflective listening techniques to help patients identify internal sources of motivation to decrease their alcohol use; skill building techniques are also used as indicated for those who exhibit high levels of motivation. Psychologists review an updated STEP Trials Feedback Form (Fig. 3), including data from the week 4 assessments, with participants to deliver individually-tailored feedback about the way in which alcohol use may be impacting the participant's health. In addition, participants are again referred to web-based self-help resources.
Table 4.
Criteria for being stepped up to additional treatment based on Timeline Followback.
| Unhealthy alcohol use category |
Criteria |
|---|---|
| At-risk drinking |
|
| Alcohol use disorder |
|
| Moderate alcohol use with liver disease |
|
Notes: asterisk (*) indicates part of the criteria at week 12 only.
2.7.4. Step 3 for at-risk drinking, MALD and alcohol use disorder trials
Participants who are stepped up to receive MET are evaluated for their response to Step 2 at week 12. Consistent with the procedures for Step 2, based on their alcohol use during the prior 14 days as determined by the TLFB method, participants will be stepped up to Step 3 of the intervention if they do not meet pre-specified goals (Table 4). For those in the at-risk drinking and MALD trials, Step 3 provides APM (see Section 2.7.2), for a total of up to five sessions. For those in the alcohol use disorder trial, Step 3 includes referral to a higher level of care based on locally available resources (e.g., medically supervised withdrawal from alcohol, intensive outpatient program).
2.7.5. Interventionists, training and monitoring
The interventionists, including the social workers, psychologists and addiction psychiatrists are front-line clinical staff. Their training includes an orientation to their role in the trials and the study manuals. In the first year of the study, all study interventionists attended a full day orientation and training that used didactics and case-based materials. In the event of new interventionists joining the trials after this initial training, similar training is provided via teleconference by members of the coordinating center. All interventionists are provided with structured encounter forms to guide each visit and manuals as indicated. In addition, the BNI and MET sessions are digitally recorded and reviewed periodically by a psychologist to ensure fidelity to the intervention using standardized measures. Further, interventionists are provided the opportunity to participate in teleconferences occurring every 1 to 2 months with the study investigators, including three clinical psychologists (social workers and psychologist teleconferences), and at least two physicians certified in Addiction Medicine (social workers, psychologist and addiction psychiatrist teleconferences) to review recent experiences with participants and discuss fidelity to the intervention. Consistent with an effectiveness trial, there were no contingencies in place to ensure patient or provider adherence to visits scheduled per protocol.
2.8. Treatment as usual
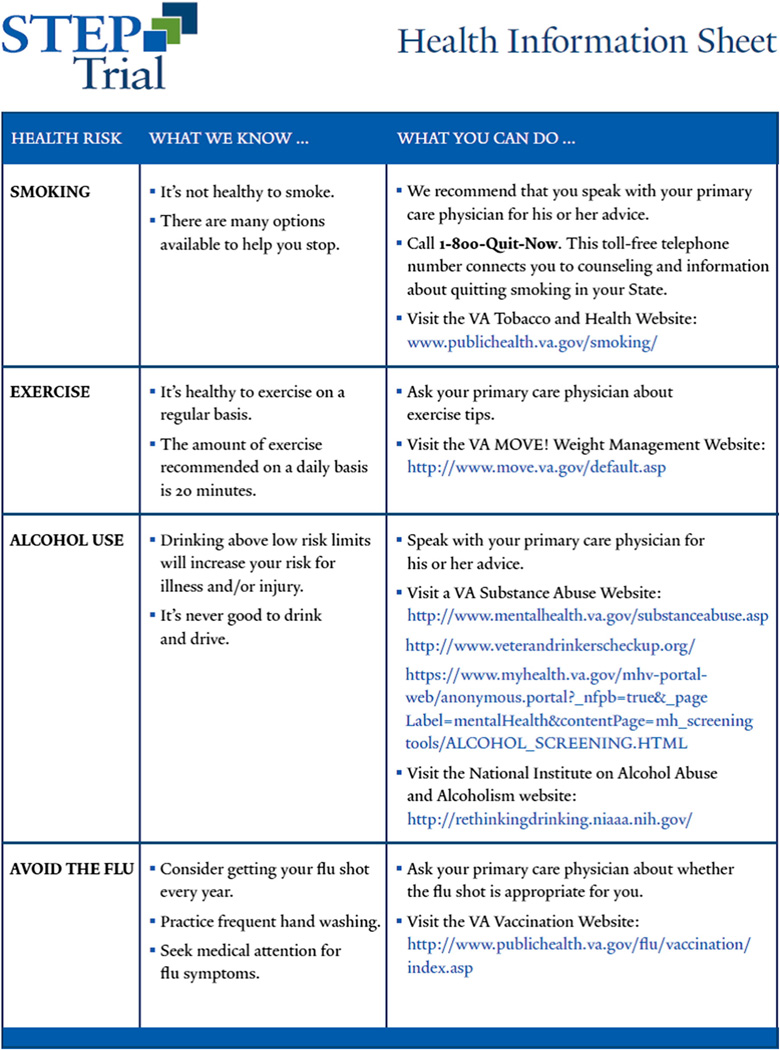
We elected to compare the integrated stepped care intervention to treatment as usual to test its effectiveness against a “real world” control and because it is a comprehensive standalone intervention that would substitute for treatment as usual [65]. Participants in the at-risk drinking and MALD trials who are randomized to treatment as usual, receive a health handout that provides advice about drinking in the context of general health advice (e.g. smoking cessation, exercise), in addition to online resources regarding alcohol use (Fig. 4). The primary care provider and/or clinic director of participants in the alcohol use disorder trial who are randomized to treatment as usual is notified so that a referral to appropriate treatment services may be made. Specifically, it was conveyed that their patient likely meets criteria for alcohol use disorder and that they may want to consider referral to addiction treatment services within their VA Medical Center. This care was in addition to standard practice occurring in the VA, including mandatory annual AUDIT-C screening and, for patients who screen positive for unhealthy alcohol use, an electronic clinical reminder prompts a brief intervention [66]. Furthermore, alcohol pharmacotherapy is available through the VA in addition to other addiction treatment services [67–69]. These services may be more robust than those available in some non-VA HIV treatment settings [16]. Participants could be referred for additional services as deemed appropriate by their providers. Thus, we chose treatment as usual for the control condition while tracking delivered services to monitor use of such services.
Fig. 4.
STEP Trials: health information sheet.
2.9. Statistical considerations
2.9.1. Sample size calculations
The primary aim of the STEP Trials is to examine the effectiveness of integrated stepped care for unhealthy alcohol use, compared to treatment as usual, on decreasing alcohol use. Each of the three trials are evaluated separately with distinct enrollment targets. In the at-risk drinking trial, assuming a standard deviation of 13 drinks per week, to detect an additional decrease of 5 drinks per week above the expected decrease of 6.7 drinks per week in those with treatment as usual [70], 108 participants in each group are needed to have 80% power at the two-sided 0.05 significance level. Given an anticipated 15% dropout rate, the target enrollment is 254. Among those with alcohol use disorder, to detect a difference in reduction of 15 drinks per week, 64 participants in each group are needed to have 80% power at the two-sided 0.05 significance level. Given an anticipated 20% dropout rate, the target enrollment is 160. Among those in the MALD trial, to detect an increase of 15% (from10% to 25%) in self-reported abstinence with 80% power at the two-side 0.05 significance level, 97 participants per group are needed. Given an anticipated 15% dropout rate, the target enrollment is 228.
2.9.2. Statistical analyses
Analyses for each of the three trials will be conducted separately. Descriptive statistics will be used to evaluate balance in baseline characteristics across the treatment groups.
2.9.2.1. Primary outcomes
A likelihood-based ignorable analysis using a mixed model will be used for primary comparisons of alcohol consumption between groups [71, 72]. This analysis will assume that missing data occurs at random. The inclusion of 4, 12, 24 week and 12 month outcome data in the model will assist in meeting this assumption. Linear mixed effects models will be used to evaluate the primary outcome (number of drinks per week) for the at-risk and alcohol use disorder trials. The generalized linear mixed model (GLMM) will be used to compare the primary outcome of abstinence in the MALD trial. More specifically, the mixed models will include fixed effects for intervention (integrated stepped care vs. treatment as usual), time (4, 12, 24 weeks, 12 months), and the interaction of intervention and time. Additional fixed effects will be included for design factors (site) as well as the baseline outcome and gender. Random effects will be included for participant and time. Linear contrasts will be used to estimate intervention group differences and 95% confidence intervals at the primary 24 week time point. The amount and pattern of missing data will be described. Logistic regression will be used to determine factors associated with dropout. Factors associated with missing data will be included in the primary outcome models. Sensitivity analyses to account for data missing not at random will be conducted as necessary.
2.9.2.2. Secondary and exploratory outcomes
We will examine the correlation between self-reported alcohol use as measured by the TLFB with PEth data and linear mixed effect repeated measures models similar to those described above will be used to compare outcomes using PEth data. Additionally, we will create models to examine the effect of the intervention on the VACS Index, depressive symptoms and antiretroviral therapy medication adherence. Generalized linear mixed models will be used to compare engagement in unprotected sex after alcohol use as well as presence of multi-substance use. We will also compare the actual receipt and patterns of alcohol treatment services overall, by intervention condition, and among specific subgroups as assessed by the electronic medical record and self-report to inform an operations research model. In addition, we will measure adherence to the interventionist visits among those eligible (i.e. proportion of participants who attend MET sessions among those stepped up to Step 2) as well as assess patient satisfaction to better understand patient-preferences for this type of intervention.
2.10. Protection of participants
The STEP Trials are approved by the Human Investigation Committees/Institutional Review Boards and the coordinating centers and each of the sites. The study is HIPAA compliant and a Certificate of Confidentiality has been obtained from the National Institute on Alcohol Abuse and Alcoholism. The Data Safety and Monitoring Board (DSMB) reviews study progress every 6 months to review enrollment, baseline characteristics of enrolled participants, delivery of the intervention and adverse events.
2.11. Current status of the STEP Trials
As of July 10, 2016, a total of 303 participants have been enrolled across the three trials. This reflects 40%, 53%, and 82% of target enrollment in the at-risk drinking, MALD and alcohol use disorder trials respectively. That recruitment was unlikely to meet targets for the at-risk drinking and MALD trials were reviewed during DSMB calls after approximately 3.5 years of enrollment. After carefully reviewing options with the DSMB to change recruitment strategies, change outcomes, and conditional power with the option to halt the trial(s), the decision was made to continue recruitment and procedures in accordance with the original protocol for the at-risking drinking and alcohol use disorder trials. The DSMB indicated that relevant lessons will be learned regarding treatment of unhealthy alcohol use using an integrated stepped care approach for those in the MALD trial, but further recruitment was not warranted.
3. Discussion
By focusing on HIV-positive patients with either at-risk drinking, MALD, or alcohol use disorder, the STEP Trials will provide important data regarding the effectiveness of integrated stepped care to address a range of unhealthy alcohol use when delivered through HIV Clinics. The methods are innovative for several reasons. First, in addition to targeting at-risk drinking and alcohol use disorder, we expand the definition of unhealthy alcohol use [1] to an often overlooked population by targeting lower levels of drinking in the presence of liver disease. Liver disease is a major contributor to morbidity and mortality among HIV-positive patients [73] and hastened by alcohol use [8]; interventions to decrease patient exposure to this important hepatotoxin are important. In addition, as alcohol use impacts medication adherence in a dose-dependent fashion [10], targeting alcohol use may be important as patients are being evaluated for HCV treatment. Second, the STEP Trials provide a model for integrating treatment into HIV Clinics, rather than referring patients off-site. This is important as through screening, the STEP Trials are identifying non-treatment seeking patients with a low level of motivation to address their unhealthy alcohol use who are unlikely to adhere to an off-site referral. Third, the STEP Trials use a stepped care approach, that has not been previously applied to address unhealthy alcohol use among HIV-positive patients. Fourth, we are examining the impact of integrated stepped care on changes in the VACS Index, a novel biomarker that predicts morbidity and mortality. No studies to date have prospectively examined the impact of an intervention to address substance use on the VACS Index.
Results from the STEP Trials will have important implications. If effectiveness is demonstrated, integrated stepped care will provide a self-correcting mechanism for tailoring treatment intensity to patients' alcohol use. In addition, as we are cataloguing both receipt of the components of the intervention as well as use of other routine services, this will inform which services participants adhere to and inform development and tailoring of clinical programs. Notably, this study is occurring in five distinct sites that are part of the system that is the single largest provider of HIV care in the United States, namely the VA. The VA has been a leader in adopting models of care consistent with patient-centered medical homes (PACTs) and integrated treatment of comorbid conditions with multidisciplinary teams (i.e., Primary Care-Mental Health Integration). In addition, given the VA's focus on screening and delivering treatment for unhealthy alcohol use [19], this study has the potential to directly inform future implementation efforts to improve treatment of unhealthy alcohol use for both HIV-positive and negative patients [27]. One goal of these trials was to conduct research to inform practice in HIV clinical settings. As such, we elected to compare the stepped interventions to current practice – treatment as usual. While we considered other control conditions, we believe that a comparison of a variety of sequenced interventions (e.g. stepped care) with increasing levels of intensity delivered within HIV Clinics to treatment as usual will provide the field with the most pertinent information at this time. If effectiveness is demonstrated through these trials, further comparative effectiveness research would be warranted. Lastly, our findings comparing self-reported alcohol use to PEth may inform the measurement of alcohol exposure in future interventional and observational studies.
Our study has some limitations. Due to the characteristics of patients served by VA HIV Clinics our findings will not be generalizable to women. In addition these findings may not apply to other settings, lacking on-site multidisciplinary team members (e.g. psychologists, addiction psychiatrists). While the MALD trial will provide information on patient interest, adherence to and preliminary alcohol and health-related outcomes, it is unlikely that the MALD trial will provide data on the effectiveness of the integrated stepped care model for this population. Anecdotally, barriers to enrollment have included lack of interest in addressing this perceived safe level of alcohol use, transportation issues, and concerns that reporting active alcohol use would prohibit hepatitis C treatment. Resulting preliminary data will be timely, however, and should inform subsequent interventions to promote liver-related health, especially as new treatments for chronic HCV are provided to this patient population. Also, we do not measure use of e-cigarettes which became more widespread since we launched our study. Lastly, as this study includes research coordinators who are actively involved in determining whether participants assigned to the intervention meet criteria for being stepped up, processes for streamlining ongoing outcome evaluation in routine clinical practice still need to be refined.
The STEP Trials represent an important and novel treatment approach for addressing unhealthy alcohol use among HIV-positive patients. In addition, lessons learned from these studies can inform future implementation work to improve delivery and uptake of treatment for unhealthy alcohol use in HIV Clinics, as well as inform stepped care approaches for the treatment of other chronic medical conditions in clinical settings.
Acknowledgments
This work was generously supported by funding from the National Institute on Alcohol Abuse and Alcoholism (1U01AA020795 and 2U01AA020790). EJ Edelman was supported as a Yale-Drug Abuse, HIV, and Addiction Research Scholar (National Institute on Drug Abuse K12DA033312-02) during this time.
References
- 1.Saitz R. Clinical practice. Unhealthy alcohol use. N. Engl. J. Med. 2005;352:596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- 2.Conigliaro J, Justice AC, Gordon AJ, Bryant K. Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: a conceptual model to guide the implementation of evidence-based interventions into practice. Med. Care. 2006;44:S1–S6. doi: 10.1097/01.mlr.0000223659.36369.cf. [DOI] [PubMed] [Google Scholar]
- 3.McGinnis K, Fiellin DA, Tate JP, Cook RL, Braithwaite RS, Bryant KJ, et al. Number of drinks to “feel a buzz” by HIV status and viral load in men. AIDS Behav. 2015 Mar;20(3):504–511. doi: 10.1007/s10461-015-1053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCance-Katz EF, Lum PJ, Beatty G, Gruber VA, Peters M, Rainey PM. Untreated HIV infection is associated with higher blood alcohol levels. J. Acquir. Immune Defic. Syndr. 2012;60:282–288. doi: 10.1097/QAI.0b013e318256625f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn JA, Samet JH. Alcohol and HIV disease progression: weighing the evidence. Curr. HIV/AIDS Rep. 2010;7:226–233. doi: 10.1007/s11904-010-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korthuis PT, Fiellin DA, McGinnis KA, Skanderson M, Justice AC, Gordon AJ, et al. Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. J. Acquir. Immune Defic. Syndr. 2012;61:171–178. doi: 10.1097/QAI.0b013e31826741aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monroe AK, Lau B, Mugavero MJ, Mathews WC, Mayer KH, Napravnik S, et al. Heavy alcohol use is associated with worse retention in HIV care. J. Acquir. Immune Defic. Syndr. 2016 Dec 1;73(4):419–425. doi: 10.1097/QAI.0000000000001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim JK, Tate JP, Fultz SL, Goulet JL, Conigliaro J, Bryant KJ, et al. Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin. Infect. Dis. 2014 May;58(10):1449–1458. doi: 10.1093/cid/ciu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook RL, McGinnis KA, Kraemer KL, Gordon AJ, Conigliaro J, Maisto SA, et al. Intoxication before intercourse and risky sexual behavior in male veterans with and without human immunodeficiency virus infection. Med. Care. 2006;44:S31–S36. doi: 10.1097/01.mlr.0000223710.35008.d9. [DOI] [PubMed] [Google Scholar]
- 10.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol. Clin. Exp. Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 11.Grodensky CA, Golin CE, Ochtera RD, Turner BJ. Systematic review: effect of alcohol intake on adherence to outpatient medication regimens for chronic diseases. J. Stud. Alcohol Drugs. 2012;73:899–910. doi: 10.15288/jsad.2012.73.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metsch LR, Pereyra M, Colfax G, Dawson-Rose C, Cardenas G, McKirnan D, et al. HIV-positive patients' discussion of alcohol use with their HIV primary care providers. Drug Alcohol Depend. 2008;95:37–44. doi: 10.1016/j.drugalcdep.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Elliott JC, Aharonovich E, O'Leary A, Johnston B, Hasin DS. Perceivedmedical risks of drinking, alcohol consumption, and hepatitis C status among heavily drinking HIV primary care patients. Alcohol. Clin. Exp. Res. 2014;38:3052–3059. doi: 10.1111/acer.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss SM, Munoz-Plaza C, Tiburcio NJ, Maisto SA, Conigliaro J, Gwadz M, et al. HIV care providers' role legitimacy as supporters of their patients' alcohol reduction. Open Infect. Dis. J. 2009;3:13–20. doi: 10.2174/1874279300903010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauss SM, Tiburcio NJ, Munoz-Plaza C, Gwadz M, Lunievicz J, Osborne A, et al. HIV care providers' implementation of routine alcohol reduction support for their patients. AIDS Patient Care STDs. 2009;23:211–218. doi: 10.1089/apc.2008.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montague BT, Kahler CW, Colby SM, McHugh RK, Squires D, Fitzgerald B, et al. Attitudes and training needs of New England HIV care and addiction treatment providers: opportunities for better integration of HIV and alcohol treatment services. Addict. Disord. Their Treat. 2015;14:16–28. doi: 10.1097/ADT.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredericksen RJ, Edwards TC, Merlin JS, Gibbons LE, Rao D, Batey DS, et al. Patient and provider priorities for self-reported domains of HIV clinical care. AIDS Care. 2015;27:1255–1264. doi: 10.1080/09540121.2015.1050983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute of Medicine. Quality Chasm Series. Washington, D.C.: 2005. Improving the Quality of Health Care for Mental and Substance- Use Conditions. [Google Scholar]
- 19.Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am. J. Manag. Care. 2006;12:597–606. [PubMed] [Google Scholar]
- 20.Walley AY, Palmisano J, Sorensen-Alawad A, Chaisson C, Raj A, Samet JH, et al. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J. Subst. Abus. Treat. 2015;59:59–66. doi: 10.1016/j.jsat.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Fiellin DA, Weiss L, Botsko M, Egan JE, Altice FL, Bazerman LB, et al. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J. Acquir. Immune Defic. Syndr. 2011;56(Suppl. 1):S33–S38. doi: 10.1097/QAI.0b013e3182097537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss L, Netherland J, Egan JE, Flanigan TP, Fiellin DA, Finkelstein R, et al. Integration of buprenorphine/naloxone treatment into HIV clinical care: lessons from the BHIVES collaborative. J. Acquir. Immune Defic. Syndr. 2011;56(Suppl. 1):S68–S75. doi: 10.1097/QAI.0b013e31820a8226. [DOI] [PubMed] [Google Scholar]
- 23.van Straten A, Hill J, Richards DA, Cuijpers P. Stepped care treatment delivery for depression: a systematic review and meta-analysis. Psychol. Med. 2015;45:231–246. doi: 10.1017/S0033291714000701. [DOI] [PubMed] [Google Scholar]
- 24.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 25.Bair MJ, Ang D, Wu J, Outcalt SD, Sargent C, Kempf C, et al. Evaluation of Stepped Care for Chronic Pain (ESCAPE) in veterans of the Iraq and Afghanistan conflicts: a randomized clinical trial. JAMA Intern. Med. 2015;175:682–689. doi: 10.1001/jamainternmed.2015.97. [DOI] [PubMed] [Google Scholar]
- 26.Sobell MB, Sobell LC. Stepped care as a heuristic approach to the treatment of alcohol problems. J. Consult. Clin. Psychol. 2000;68:573–579. [PubMed] [Google Scholar]
- 27.Edelman EJ, Hansen NB, Cutter CJ, Danton C, Fiellin LE, O'Connor PG, et al. Implementation of integrated stepped care for unhealthy alcohol use in HIV clinics. Addict. Sci. Clin. Pract. 2016;11:1. doi: 10.1186/s13722-015-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DoHaH. Helping patients who drink too much: a clinician's guide. Services; 2005. National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. fifth. Washington, D.C.: American Psychiatric Press; 2013. [Google Scholar]
- 30.Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, et al. Predictive accuracy of the Veterans Aging Cohort Study index formortality with HIV infection: a North American cross cohort analysis. J. Acquir. Immune Defic. Syndr. 2013;62:149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27:563–572. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfectedmen. Drug Alcohol Depend. 2016;161:95–103. doi: 10.1016/j.drugalcdep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 34.Nadkarni PM, Brandt C, Frawley S, Sayward FG, Einbinder R, Zelterman D, et al. Managing attribute—value clinical trials data using the ACT/DB client-server database system. J. Am. Med. Inform. Assoc. 1998;5:139–151. doi: 10.1136/jamia.1998.0050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobell LC, Sobell SM. Handbook of Psychiatric Measures. Washington, D.C.: American Psychiatric Association; 1996. Alcohol Timeline Followback (TLFB) [Google Scholar]
- 36.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin. Infect. Dis. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Justice AC, McGinnis KA, Skanderson M, Chang CC, Gibert CL, Goetz MB, et al. Towards a combined prognostic index for survival in HIV infection: the role of ‘non- HIV’ biomarkers. HIV Med. 2010;11:143–151. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bebu I, Tate J, Rimland D, Mesner O, Macalino GE, Ganesan A, et al. The VACS index predicts mortality in a young, healthy HIV population starting highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2014;65:226–230. doi: 10.1097/QAI.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGinnis K, Edelman EJ, Tate JP, Justice AC, Fiellin DA. College on Problems of Drug Dependence. San Juan, Puerto Rico: Using the VACS Index to Track Health Outcomes Associated With Abstinence Among HIV-infected Patients Receiving Opioid Agonist Treatment, Poster Presentation. [Google Scholar]
- 40.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med. Care. 1988;26:814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapywhen the CD4+cell count is 0.200 to 0.350 × 10 (9) cells/L. Ann. Intern. Med. 2003;139:810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 42.Kitahata MM, Reed SD, Dillingham PW, Van Rompaey SE, Young AA, Harrington RD, et al. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. Int. J. STD AIDS. 2004;15:803–810. doi: 10.1258/0956462042563666. [DOI] [PubMed] [Google Scholar]
- 43.Hogg RS, Heath K, Bangsberg D, Yip B, Press N, O'Shaughnessy MV, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciccarone DH, Kanouse DE, Collins RL, Miu A, Chen JL, Morton SC, et al. Sex without disclosure of positive HIV serostatus in a US probability sample of persons receiving medical care for HIV infection. Am. J. Public Health. 2003;93:949–954. doi: 10.2105/ajph.93.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duru OK, Collins RL, Ciccarone DH, Morton SC, Stall R, Beckman R, et al. Correlates of sex without serostatus disclosure among a national probability sample of HIV patients. AIDS Behav. 2006;10:495–507. doi: 10.1007/s10461-006-9089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the addiction severity index. J. Subst. Abus. Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 48.Viel G, Boscolo-Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int. J. Mol. Sci. 2012;13:14788–14812. doi: 10.3390/ijms131114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walther L, de Bejczy A, Lof E, Hansson T, Andersson A, Guterstam J, et al. Phosphatidylethanol is superior to carbohydrate-deficient transferrin and gammaglutamyltransferase as an alcohol marker and is a reliable estimate of alcohol consumption level. Alcohol. Clin. Exp. Res. 2015;39:2200–2208. doi: 10.1111/acer.12883. [DOI] [PubMed] [Google Scholar]
- 50.Wurst FM, Thon N, Yegles M, Schruck A, Preuss UW, Weinmann W. Ethanolmetabolites: their role in the assessment of alcohol intake. Alcohol. Clin. Exp. Res. 2015 Nov;39(11):2060–2072. doi: 10.1111/acer.12851. [DOI] [PubMed] [Google Scholar]
- 51.Harris AH, Ellerbe L, Phelps TE, Finney JW, Bowe T, Gupta S, et al. Examining the specification validity of the HEDIS quality measures for substance use disorders. J. Subst. Abus. Treat. 2015;53:16–21. doi: 10.1016/j.jsat.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 52.McLellan AT, Alterman AI, Cacciola J, Metzger D, O'Brien CP. A new measure of substance abuse treatment. Initial studies of the Treatment Services Review. J. Nerv. Ment. Dis. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern. Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.deMeneses-Gaya IC, Zuardi AW, Loureiro SR, Crippa JAD. Psychometric properties of the Fagerstrom Test for Nicotine Dependence. J. Bras. Pneumol. 2009;35:73–82. doi: 10.1590/s1806-37132009000100011. [DOI] [PubMed] [Google Scholar]
- 55.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 56.Fiellin DA, Moore BA, Sullivan LE, Becker WC, Pantalon MV, Chawarski MC, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2–5 years. Am. J. Addict. 2008;17:116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- 57.D'Onofrio G, Pantalon MV, Degutis LC, Fiellin DA, Busch SH, Chawarski MC, et al. Brief intervention for hazardous and harmful drinkers in the emergency department. Ann. Emerg. Med. 2008;51:742–750. e2. doi: 10.1016/j.annemergmed.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D'Onofrio G, Pantalon MV, Degutis LC, Fiellin DA, O'Connor PG. Development and implementation of an emergency practitioner-performed brief intervention for hazardous and harmful drinkers in the emergency department. Acad. Emerg. Med. 2005;12:249–256. doi: 10.1197/j.aem.2004.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers. A randomized controlled trial in community-based primary care practices. JAMA. 1997;277:1039–1045. [PubMed] [Google Scholar]
- 60.O'Donnell A, Anderson P, Newbury-Birch D, Schulte B, Schmidt C, Reimer J, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol. 2014;49:66–78. doi: 10.1093/alcalc/agt170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 62.Falk D, Wang XQ, Liu L, Fertig J, Mattson M, Ryan M, et al. Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol. Clin. Exp. Res. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- 63.Matching alcoholism treatments to client heterogeneity: project MATCH posttreatment drinking outcomes. J. Stud. Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- 64.Maisto SA, Conigliaro J, McNeil M, Kraemer K, Conigliaro RL, Kelley ME. Effects of two types of brief intervention and readiness to change on alcohol use in hazardous drinkers. J. Stud. Alcohol. 2001;62:605–614. doi: 10.15288/jsa.2001.62.605. [DOI] [PubMed] [Google Scholar]
- 65.Nunes EV, Ball S, Booth R, Brigham G, Calsyn DA, Carroll K, et al. Multisite effectiveness trials of treatments for substance abuse and co-occurring problems: have we chosen the best designs? J. Subst. Abus. Treat. 2010;38(Suppl. 1):S97–112. doi: 10.1016/j.jsat.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams EC, Rubinsky AD, Chavez LJ, Lapham GT, Rittmueller SE, Achtmeyer CE, et al. An early evaluation of implementation of brief intervention for unhealthy alcohol use in the US Veterans Health Administration. Addiction. 2014;109:1472–1481. doi: 10.1111/add.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iheanacho T, Issa M, Marienfeld C, Rosenheck R. Use of naltrexone for alcohol use disorders in the Veterans' Health Administration: a national study. Drug Alcohol Depend. 2013;132:122–126. doi: 10.1016/j.drugalcdep.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 68.Marienfeld C, Iheanacho T, Issa M, Rosenheck RA. Long-acting injectable depot naltrexone use in the Veterans' Health Administration: a national study. Addict. Behav. 2014;39:434–438. doi: 10.1016/j.addbeh.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Harris AH, Ellerbe L, Reeder RN, Bowe T, Gordon AJ, Hagedorn H, et al. Pharmacotherapy for alcohol dependence: perceived treatment barriers and action strategies among Veterans Health Administration service providers. Psychol. Serv. 2013;10:410–419. doi: 10.1037/a0030949. [DOI] [PubMed] [Google Scholar]
- 70.D'Onofrio G, Fiellin DA, Pantalon MV, Chawarski MC, Owens PH, Degutis LC, et al. A brief intervention reduces hazardous and harmful drinking in emergency department patients. Ann. Emerg. Med. 2012 Aug;60(2):181–192. doi: 10.1016/j.annemergmed.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dmitrienko A, Molenberghs G, Chuang-Stein C, Offen W. Analysis of Clinical Trials Using SAS: A Practical Guide. Cary, NC: SAS Institute, Inc.; 2005. [Google Scholar]
- 72.Molenberghs G, Thijs H, Jansen I, Beunckens C, Kenward MG, Mallinckrodt C, et al. Analyzing incomplete longitudinal clinical trial data. Biostatistics. 2004;5:445–464. doi: 10.1093/biostatistics/5.3.445. [DOI] [PubMed] [Google Scholar]
- 73.Joshi D, O'Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377:1198–1209. doi: 10.1016/S0140-6736(10)62001-6. [DOI] [PubMed] [Google Scholar]
- 75.Mendeni M, Foca E, Gotti D, Ladisa N, Angarano G, Albini L, et al. Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clin. Infect. Dis. 2011;52:1164–1173. doi: 10.1093/cid/cir071. [DOI] [PubMed] [Google Scholar]
- 76.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 77.Fiellin DA, Reid MC, O'Connor PG. Screening for alcohol problems in primary care: a systematic review. Arch. Intern. Med. 2000;160:1977–1989. doi: 10.1001/archinte.160.13.1977. [DOI] [PubMed] [Google Scholar]
- 78.Raj A, Saitz R, Cheng DM, Winter M, Samet JH. Associations between alcohol, heroin, and cocaine use and high risk sexual behaviors among detoxification patients. Am. J. Drug Alcohol Abuse. 2007;33:169–178. doi: 10.1080/00952990601091176. [DOI] [PubMed] [Google Scholar]