Introduction
KEY TEACHING POINTS
|
The association of Brugada syndrome (BrS) and atrial fibrillation (AF) is well known, but its prognostic significance has not been well defined.1 In 1992, the Brugada brothers described 8 patients with a syndrome of ST segment elevation in ECG leads V1–V3 associated with right bundle branch block appearance and the risk of ventricular fibrillation (VF) and sudden cardiac death.2 One patient was an 8-year-old girl with an additional history of paroxysmal AF. She experienced her first paroxysm of AF soon after birth, while her first episode of syncope occurred at 8 years of age. Patients with a spontaneous type 1 ECG pattern, those with inducibility of ventricular arrhythmias, or those who fulfilled criteria for an implantable cardioverter have a higher incidence of atrial arrhythmias, suggesting that atrial arrhythmias may be considered as an indicator for more severe disease.3 The recent HRS/EHRA expert consensus statement4 does not recommend genetic testing for AF because of the limited outcome data available. The most important reason was that none of the current known disease-associated genes for AF has been shown to account for >5% of the disease. However, the possibility that within the large pool of young patients with new-onset AF, a relatively large minority with latent BrS may be at risk of sudden death (as first reported by our group5) could potentially increase the diagnostic and prognostic contribution of genetic testing. Recent observations from the Brugada group5, 6, 7 have supported the results of our seminal work, posing a new challenge for all cardiologists in the management of patients with AF. In our initial experience, this association was analyzed in as many as 346 individuals with new-onset AF (median age 53 years), among whom there were 190 with lone AF and 11 with ECG Brugada pattern, all diagnosed as having lone AF (5.7%). Of these, 3 patients developed BrS, experiencing ventricular tachycardia (VT)/VF during follow-up. Similarly, Junttila et al6 reviewed cases in which the typical Brugada pattern was observed in the electrocardiogram after a range of trigger events (such as fever or propofol). Of the 9 patients in whom BrS was detected after administration of sodium-channel blockers, 1 had sudden cardiac death and another VT. Recently, the Brugada group has reported that AF was the first clinical manifestation in 35 of 611 patients with BrS.7 Of these, 11 patients were found after class IC antiarrhythmic drug therapy and 2 patients had an acute arrhythmic event. The first patient had an aborted sudden death after starting propafenone; the second patient was a 22-year-old woman who arrived at the emergency room because of a fibrillation episode and atrial flutter. A few minutes after administration of flecainide, the patient showed a type 1 ECG Brugada pattern with subsequent degeneration into VF.7
We report here a rare additional case of a pathognomonic association between AF and VF in a young patient with new-onset lone AF and latent BrS, which further emphasizes the prognostic importance of considering flecainide testing in such a selected patient population.
Case report
A 22-year-old man with lone AF presented with a 2-month history of recurrent presyncopal episodes occurring at rest and preceded by blurred vision and dizziness. In particular, he had the feeling of being just about to lose consciousness without complete loss. Symptoms were self-limiting, lasting just a few seconds, and were not related to physical activity or stress. The patient had no family history of heart disease, sudden death, or recurrent syncope, and did not take any medications before or after such episodes. At admission, baseline ECG showed no abnormalities (Figure 1A) and laboratory analysis, physical examination, and echocardiography were normal. A subcutaneously implanted ECG loop recorder did not show any atrial or ventricular tachyarrhythmias, but no episodes occurred during long-term monitoring. The patient underwent electrophysiologic testing (EPT) and during electrophysiologic study flecainide infusion (2 mg/kg over 10 minutes) resulted in type 1 ECG Brugada pattern (Figure 1B), which was followed by concomitant development of VF and AF immediately after programmed ventricular stimulation (Figure 2A). VF lasted 7 seconds, spontaneously reverting to AF (Figure 2B) and then to normal sinus rhythm with a coved BrS ECG pattern (Figure 3). After EPT, since the patient’s setting was compatible with BrS, VT/VF inducibility, and a history of recurrent presyncopal episodes, he received an implantable cardioverter-defibrillator (ICD) and after 8-month follow-up remained asymptomatic. The patient had a positive SCN5A genetic test while genetic screening of his family was underway.
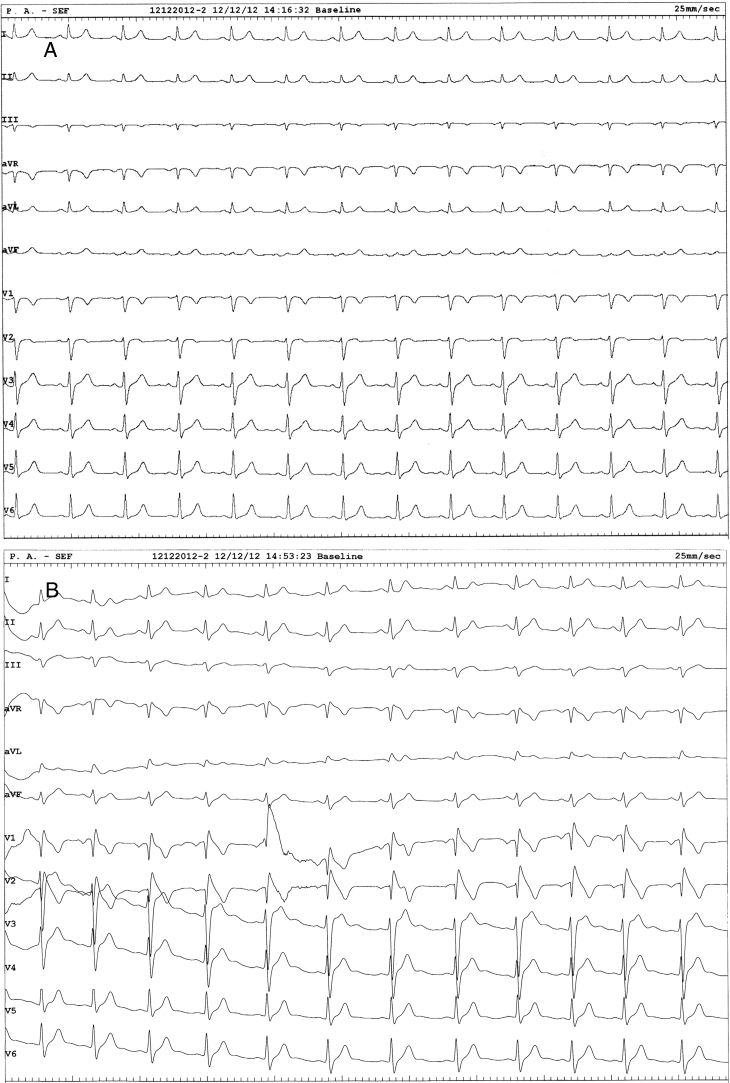
Figure 1.
A: Baseline 12-lead ECG shows no abnormality. B: After flecainide testing, a coved ST-segment elevation in leads V1–V2 compatible with a type I Brugada syndrome ECG pattern was evident.
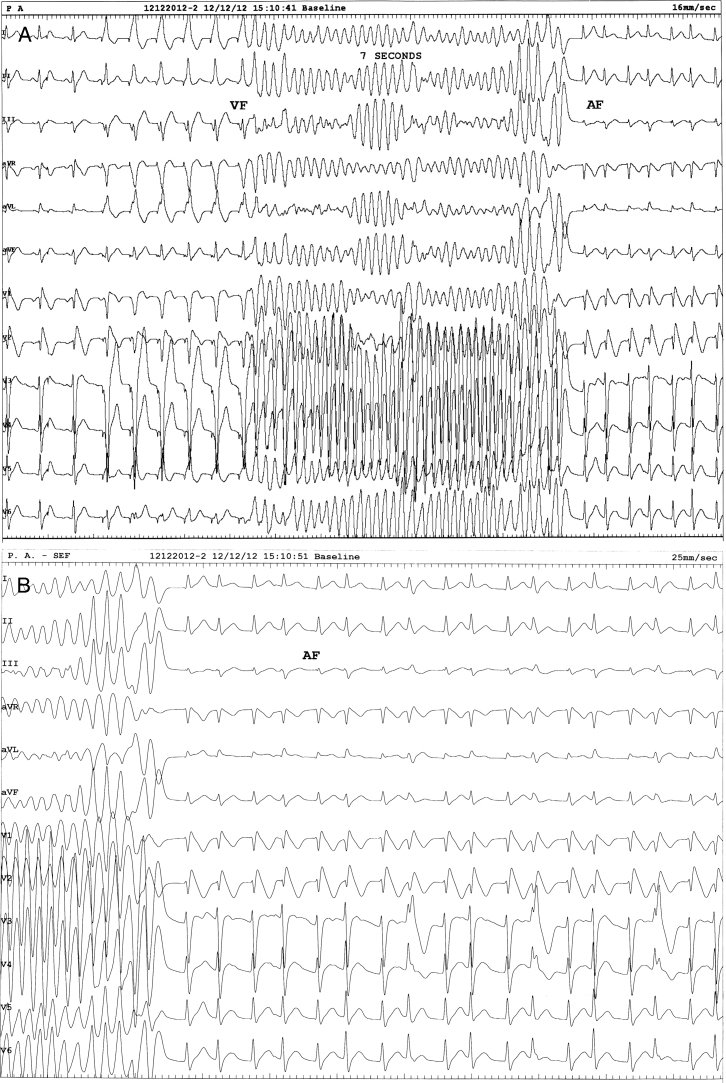
Figure 2.
Programmed ventricular stimulation resulted in concomitant development of ventricular fibrillation (VF) and atrial fibrillation (AF). VF lasted 7 seconds and spontaneously reverted to sustained AF. The paper speeds are as follows: A: 16 mm/s; B: 25 mm/s.
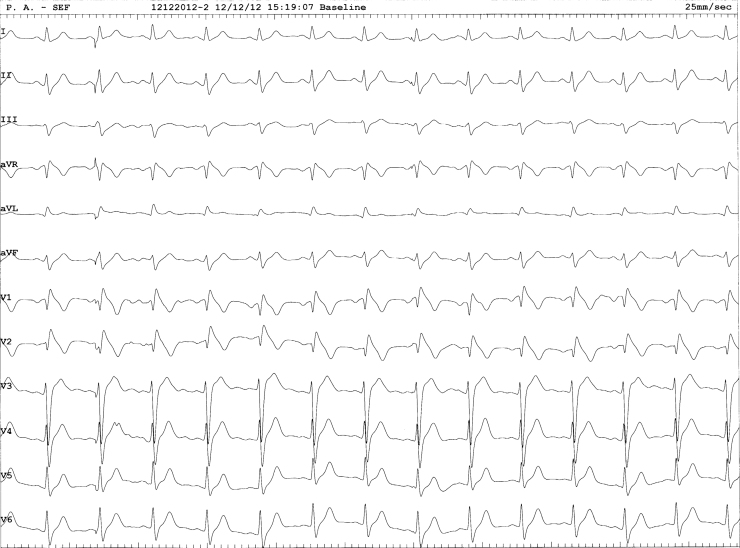
Figure 3.
Twelve-lead ECG after electrophysiologic testing showing a stable sinus rhythm with coved Brugada syndrome pattern.
Discussion
The actual prevalence of BrS is unknown. It is estimated to affect around 5 in 10 000 individuals, but this figure is an underestimate owing to the existence of concealed electrocardiographic forms,8, 9 which can occasionally be unmasked by fever or sodium-channel blocking drugs, as in our case. Aborted sudden cardiac death is the first manifestation of BrS in 6%–21% of patients,10, 11, 12, 13 and in 17%–31% of BrS patients the diagnosis is made following etiologic study of syncope, which is generally secondary to self-limited episodes of polymorphic VT,8, 9, 10, 11 as in this patient. The case presented here, a young male patient with lone AF who exhibited coved-type ECG and VF under administration of flecainide, calls our attention to life-threatening risk because a portion of such patients would manifest BrS and administration of class I antiarrhythmic drugs could yield devastating results. Indeed, this case emphasizes that some patients with new-onset lone paroxysmal AF having latent undiagnosed BrS can indeed be at risk of sudden death, because intravenous flecainide or class I antiarrhythmic drugs are commonly used in AF patients without structural heart disease to acutely treat episodes of paroxysmal AF, as in our case. Of note, in our patient, immediately after flecainide testing VF and AF developed concomitantly, in all probability as a result of global atrial and ventricular vulnerability. According to the latest consensus statement,14 this patient would have been considered as a Class IIb recommendation for ICD implantation (inducible VF, no spontaneous VT/VF or syncope). Although he experienced “presyncopal” episodes without complete loss of consciousness like frank syncope, EPT demonstrated flecainide-induced type I BrS ECG pattern and inducibility of transient, spontaneously reverting VF before AF. Therefore, after EPT and both flecainide-induced coved BrS ECG pattern and inducibility of VF, we considered the prognostic significance of the spontaneous presyncopal episodes to be equivalent to that of frank syncope and then we decided to implant an ICD as a therapeutic approach similar to that of BrS patients with arrhythmic cardiac arrest. These findings, taken together, emphasize the prognostic significance of recurrent presyncopal episodes in young patients with lone AF potentially having a latent BrS. Identification of such selected patients is crucial, since they should be managed with ICD implantation and not with the use of sodium-channel blockers, which are now widely used acutely or chronically to treat or prevent AF recurrences. These findings also suggest revisiting current guidelines on AF to include flecainide/ajmaline testing in selected patients with new-onset AF in daily clinical practice to avoid fatalities.15
References
- 1.Francis J., Antzelevitch C. Atrial fibrillation and Brugada syndrome. J Am Coll Cardiol. 2008;51:1149–1153. doi: 10.1016/j.jacc.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 3.Bordachar P., Reuter S., Garrigue S., Caï X., Hocini M., Jaïs P., Haïssaguerre M., Clementy J. Incidence, clinical implications and prognosis of atrial arrhythmias in Brugada syndrome. Eur Heart J. 2004;25:879–884. doi: 10.1016/j.ehj.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman M.J., Priori S.G., Willems S. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Pappone C., Radinovic A., Manguso F. New-onset atrial fibrillation as first clinical manifestation of latent Brugada syndrome: prevalence and clinical significance. Eur Heart J. 2009;30:2985–2992. doi: 10.1093/eurheartj/ehp326. [DOI] [PubMed] [Google Scholar]
- 6.Junttila M.J., Gonzalez M., Lizotte E., Benito B., Vernooy K., Sarkozy A., Huikuri H.V., Brugada P., Brugada J., Brugada R. Induced Brugada-type electrocardiogram, a sign for imminent malignant arrhythmias. Circulation. 2008;117:1890–1893. doi: 10.1161/CIRCULATIONAHA.107.746495. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Mañero M., Namdar M., Sarkozy A., Casado-Arroyo R., Ricciardi D., de Asmundis C., Chierchia G.B., Wauters K., Rao J.Y., Bayrak F., Van Malderen S., Brugada Prevalence, clinical characteristics and management of atrial fibrillation in patients with Brugada syndrome. Am J Cardiol. 2013;111:362–367. doi: 10.1016/j.amjcard.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher M.M., Forleo G.B., Behr E.R., Magliano G., De Luca L., Morgia V., De Liberato F., Romeo F. Prevalence and significance of Brugada-type ECG in 12,012 apparently healthy European subjects. Int J Cardiol. 2008;130:44–48. doi: 10.1016/j.ijcard.2007.07.159. [DOI] [PubMed] [Google Scholar]
- 9.Patel S.S., Anees S., Ferrick K.J. Prevalence of a Brugada pattern electrocardiogram in an urban population in the United States. Pacing Clin Electrophysiol. 2009;32:704–708. doi: 10.1111/j.1540-8159.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- 10.Probst V., Veltmann C., Eckardt L. Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 11.Brugada J., Brugada R., Antzelevitch C., Towbin J., Nademanee K., Brugada P. Long-term follow-up of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Circulation. 2002;105:73–78. doi: 10.1161/hc0102.101354. [DOI] [PubMed] [Google Scholar]
- 12.Priori S.G., Napolitano C., Gasparini M. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105:1342–1347. doi: 10.1161/hc1102.105288. [DOI] [PubMed] [Google Scholar]
- 13.Eckardt L., Probst V., Smits J.P. Long-term prognosis of individuals with right precordial ST-segment—elevation Brugada syndrome. Circulation. 2005;111:257–263. doi: 10.1161/01.CIR.0000153267.21278.8D. [DOI] [PubMed] [Google Scholar]
- 14.Priori S.G., Wilde A.A., Horie M. HSR/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Anguita M., Worner F., Domenech P. New evidence, new controversies: a critical review of the European Society of Cardiology 2010 clinical practice guidelines on atrial fibrillation. Rev Esp Cardiol. 2012;65:7–13. doi: 10.1016/j.recesp.2011.10.003. [DOI] [PubMed] [Google Scholar]