Abstract
Lungs donated after cardiac death (DCD) are an underutilized resource for a dwindling donor lung transplant pool. Our study investigates the potential of a novel preservation solution, Somah, to better preserve statically stored DCD lungs, for an extended time period, when compared to low-potassium dextran solution (LPD). We hypothesize that Somah is a metabolically superior organ preservation solution for hypothermic statically stored porcine DCD lungs, possibly improving lung transplant outcomes. Porcine DCD lungs (n = 3 per group) were flushed with and submerged in cold preservation solution. The lungs were stored up to 12 h, and samples were taken from lung tissue and the preservation medium throughout. Metabolomic and redox potential were analyzed using high performance liquid chromatography, mass spectrometry, and RedoxSYS®, comparing substrate and pathway utilization in both preservation solutions. Glutathione reduction was seen in Somah but not in LPD during preservation. Carnitine, carnosine, and n-acetylcarnosine levels were elevated in the Somah medium compared with LPD throughout. Biopsies of Somah exposed lungs demonstrated similar trends after 2 h, up to 12 h. Adenosine gradually decreased in Somah medium over 12 h, but not in LPD. An inversely proportional increase in inosine was found in Somah. Higher oxidative stress levels were measured in LPD. Our study suggests suboptimal metabolic preservation in lungs stored in LPD. LPD had poor antioxidant potential, cytoprotection, and an insufficient redox potential. These findings may have immediate clinical implications for human organs; however, further investigation is needed to evaluate DCD lung preservation in Somah as a viable option for transplant.
Keywords: transplant, metabolomics, organ longevity
Lung transplantation is the only treatment for end-stage lung failure. Despite various attempts to increase graft availability, lung transplant suffers from a critical lack of suitable donor organs.1 Fewer than 20% of post-mortem donor lungs are acceptable for transplantation and despite aggressive care, ten-year survival is only 29–31%.2,3 While the majority of donor organs are donated after brain death (DBD), substantial evidence supports utilization of lungs donated after cardiac death (DCD), which would considerably increase the donor lung supply.1,4 Common barriers to both DBD and DCD availability are ischemia-induced lung inflammation, vascular leakage, and ischemia reperfusion (IR) injury occurring immediately after death and during the delay between recovery and transplant into the recipient.5,6
Over the past decades, promising prospectives have emerged in DCD lung utilization for transplant. The critical issue of warm ischemic times in a DCD setting has been addressed by continuous ex vivo lung perfusion (EVLP).7 This approach has notable potential to expand the pool of available organs, both by assessment of graft quality as well as extending preservation time.8–10 Nonetheless, in the past 15 years EVLP has not been a solution capable of diminishing the transplant gap. One important reason is that the lung evaluated in these experiments fall under Maastricht criteria III, with DCD occurring in a controlled hospital environment. Despite the impressive results demonstrated, the fraction of lungs addressed is only marginal, as the majority of possible DCD donations takes place after uncontrolled death, following Maastricht criteria I and II (Table 1). Expanding the DCD donor pool with lungs that fall under category I and II would clearly have enormous impact on the amount of available grafts. The use of Maastricht criteria III DCD lungs for transplant is generally considered as safe as DBD lung transplantation.11,12 In addition to the relatively small number of DCD grafts that can be donated when solely focusing on Maastricht criteria III lungs, the limited number of medical institutions in possession of EVLP infrastructure stands in the way of this new approach from eliminating the donor lung scarcity. EVLP could be of enormous contribution in transplant, yet its biggest addition should be considered as a technique to improve and rejuvenate marginal lungs.13–15 The study here presented therefore proposes a novel cold static storage solution to enhance current clinical procedures for DCD lungs donated for transplant.
Table 1.
Maastricht criteria for DCD lungs.
| Category | Description | Type |
|---|---|---|
| I | Dead on arrival (DoA) | Uncontrolled |
| II | Unsuccessful resuscitation | Uncontrolled |
| III | Awaiting cardiac arrest, treatment withdrawn | Controlled |
| IV | Cardiorespiratory arrest during/after diagnosis of brain death | Controlled |
A critical factor in transplant success is preserving the donor organ in optimal physiological condition between the time of collection and transplant. Current practice universally involves an ex vivo lung preservation technique based on cold static storage in a low-potassium dextran solution (LPD, Perfadex®) (Table 2). This solution provides protection against tissue edema, while the low temperature achieves a decrease in metabolic demand of the organ. Although LPD provides protection for up to 6 h of storage16 minimizing energy needs by hypothermia alone has not extended lung preservation time in clinical practice. Furthermore, IR injury occurs when blood flow is restored and oxygen is delivered to the lungs following implantation of a cold, statically stored organ. IR injury leads to apoptosis or necrosis of organ tissue triggered by mitochondrial disrupture.17,18 In order to prevent irreversible damage to the organ, it is critical to provide metabolic protection in addition to the traditional methods currently used for donor lung storage with LPD.
Table 2.
Composition of organ preservation solutions investigated, mmol/L.
| Ingredient (mmol/L) | Somah | Perfadex |
|---|---|---|
| Na+ | 125 | 138 |
| K+ | 7 | 6 |
| Mg2+ | – | 0.8 |
| Cl− | – | 142 |
| KCl | 7 | 0.4 |
| MgCl2 | 0.5 | – |
| NaCO3H | 5 | – |
| MgSO4 | 0.5 | 0.8 |
| Glucose | 11 | 5 |
| Adenosine | 2 | – |
| Dextran 40 | – | 5% |
| Glutathione | 1.5 | – |
| CaCl2 | 1.3 | – |
| Phosphate | 0.44 | 0.8 |
| Insulin | 1 | – |
| l-Arginine | 5 | – |
| l-Citrulline malate | 1 | – |
| Creatine orotate | 0.5 | – |
| Creatine monohydrate | 2 | – |
| l-Carnosine | 10 | – |
| l-Carnitine | 10 | – |
| Dichloroacetate | 0.5 | – |
| Ascorbic acid | 1 | – |
| Sodium phosphate | 0.19 | – |
| pH (at 4℃) | 7.5 | 7.4 |
A novel solution, Somah (Table 2), aims to extend the ex vivo longevity of organs by supplying metabolic needs rather than minimizing energetic demand, while providing reactive oxygen species (ROS) scavenging agents and tissue protection. Studies using porcine hearts have demonstrated the potential benefit of Somah for cardiac transplantation in hypothermic and subnormothermic settings.19,20 However, in order to fully evaluate a novel solution’s mechanism and clinical potential, assessment metabolic and redox pathways are required. By investigating the metabolomics and redox aspects of this novel solution and comparison with the conventional organ storage solution, we aim to better comprehend the modus operandi of Somah and its potential benefits. In order to construct the optimal manner of pulmonary graft preservation, it is mandatory to have a broad understanding of tissue and solution interaction over time.
We evaluated the metabolomics and redox state of porcine Maastricht category I-like DCD lungs, statically stored in cold (4℃) LPD or Somah solution. Understanding the influence of different preservation solutions may provide an opportunity to expand the donor pool of lungs by safeguarding or modulating the metabolic state of DCD lungs prior to transplant.
Methods
Storage solutions
Somah was purchased from Somahlution (Jupiter, FL, USA) and Perfadex® LPD solution was purchased from XVIVO Perfusion (Gothenborg, Sweden). Solutions were freshly prepared on the day of the experiment. Preservation solutions were randomly assigned to one lung per experiment and blinded to researchers handling the organs.
Organ procurement
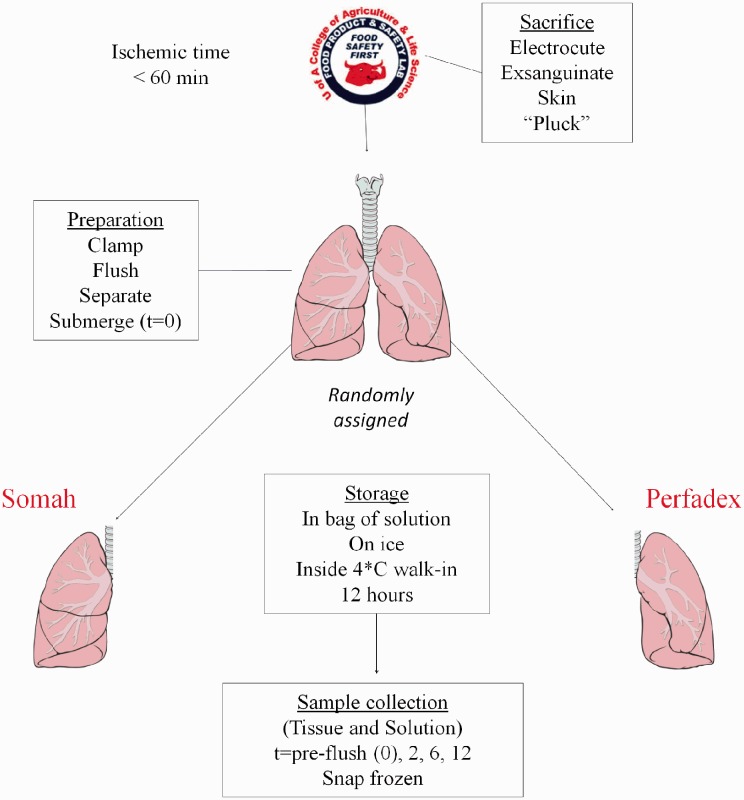
Organs were procured from Yorkshire pigs (50–60 kg) at the University of Arizona Food Product and Safety Laboratory, in accordance with United States Department of Agriculture and University of Arizona regulations. Pigs were sacrificed by electrocution and rapidly exsanguinated without anesthetics before organ harvest. Due to the traumatic nature of the injury, these circumstances mimic the Maastricht I-like criteria. The lungs were randomly assigned to either LPD or Somah and individually rinsed with 1 L of preservation solution by anterograde flushing through the pulmonary trunk, while ensuring exclusive flow through either the right or left pulmonary artery by temporarily sealing the contralateral vessel using Kelly forceps (Fig. 1). Subsequently the lungs were surgically separated and submerged in their respective cold preservation solution. As a result of splitting the lung pairs into two separate lungs, biological variability was minimized. This way, each animal used in the study is represented in both the LPD and the Somah group. Lungs that were not flushed, split, and submerged within 60 min after circulatory death of the pig were excluded from our study.
Fig. 1.
Schematic overview of experimental set-up. Pigs were shocked and rapidly exsanguinated without anesthetics before organ harvest. The lungs were randomly assigned to either LPD or Somah and individually rinsed with 1 L of preservation solution by anterograde flushing through the pulmonary trunk. Subsequently the lungs were surgically separated and submerged in their respective preservation solution. Medium samples and lung biopsies were taken from the before introducing the preservation solution to the lungs (t = 0) and at t = 2 h, t = 6 h, and at t = 12 h. Tissue biopsies and solution samples were immediately snap frozen in liquid nitrogen and stored at −80℃.
Experimental procedure
Samples (5 mL) were obtained from the preservation solution before submerging the lungs (t = 0 h) and then at t = 2 h, t = 6 h, and t = 12 h post submersion (Fig. 1). Similarly, tissue biopsies (∼1 g each) were obtained from the apex of the inferior lobe of the lungs at the same time points. Tissue biopsies and solution samples were immediately snap frozen in liquid nitrogen and stored at −80℃ until further processing for analyses.
Metabolic analysis
Metabolomic and statistical analyses were conducted at Metabolon, Inc. (Durham, NC, USA) as described previously.21 Briefly, tissue samples were homogenized (Covaris), then all samples were subjected to methanol extraction then split into aliquots for analysis by ultrahigh performance liquid chromatography/mass spectrometry (UHPLC/MS) in the positive (two methods, one optimized for hydrophilic, the other hydrophobic compounds), negative or polar ion mode. Metabolites were identified by automated comparison of ion features to a reference library of chemical standard followed by visual inspection for quality control.22 For QA/QC, a pooled client matrix (or for plasma, an internal matrix) as well as several internal standards were assessed to determine instrument variability, with RSD = 3% (lung or medium) for internal standards and RSDs = 7% (lung) and 9% (medium) for endogenous biochemicals. For statistical analyses and data display, any missing values were assumed to be below the limits of detection; these values were imputed with the compound minimum (minimum value imputation). To determine statistical significance, a repeated-measures two-way ANOVA was performed in ArrayStudio (Omicsoft) or “R” on log-transformed data to compare data between experimental groups; P < 0.05 was considered significant. An estimate of the false discovery rate (Q-value) was calculated to take into account the multiple comparisons that normally occur in metabolomic-based studies, with Q < 0.05 used as an indication of high confidence in a result.
Redox potential analysis
Redox analysis was performed using RedoxSYS Diagnostics System (Aytu BioScience, Englewood, CO). The balance of all oxidants and reductants in each sample is measured as static oxidation reduction potential (sORP), which is an indicator for the oxidative stress a sample is subjected to. The driving current over the sample is measured in millivolts (mV). Lower values indicate less oxidative stress. Capacity oxidation reduction potential (cORP), also known as total antioxidant capacity (TAC), is measured in microcoulombs (µC) and expresses the antioxidant reserve in samples. Higher values are indicative of a higher ROS scavenging capacity.23
Results
Glutathione metabolism
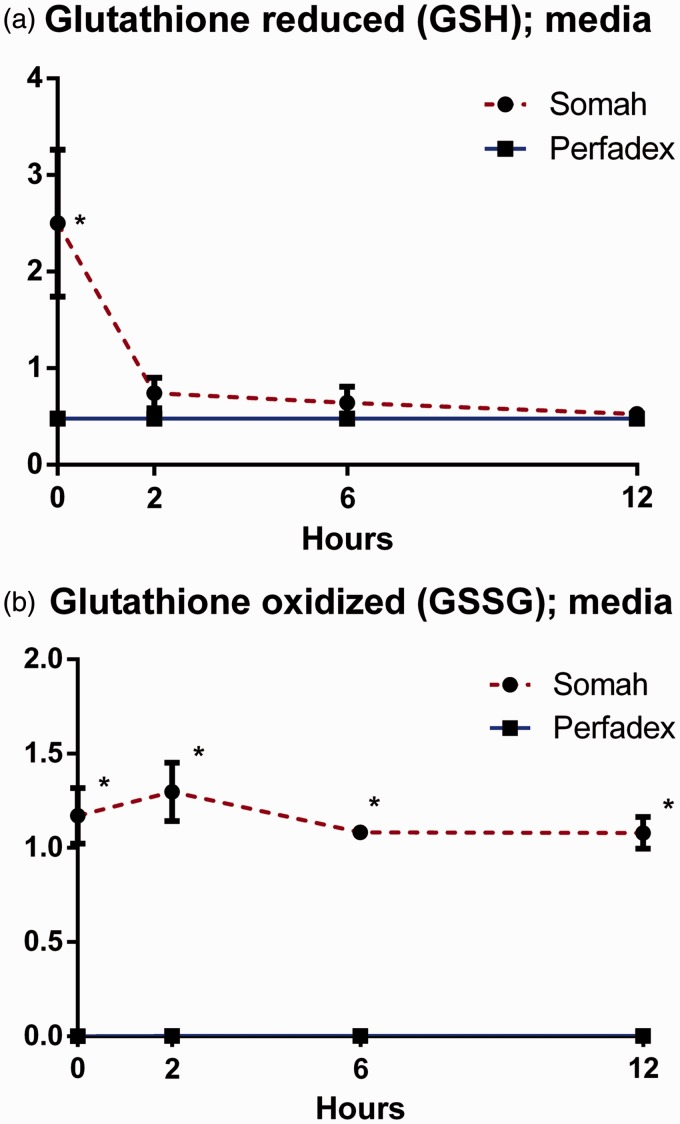
Somah demonstrated higher basal (t = 0) concentration of reduced glutathione (GSH) in the medium than LPD (P < 0.05; Fig. 2a). GSH was rapidly depleted from the Somah medium, leading to comparable concentrations of GSH in both mediums after 2 h. Oxidized glutathione (GSSG) remained stably elevated in Somah medium over LPD during the entire experiment (P < 0.05; Fig. 2b).
Fig. 2.
(a) Reduced glutathione (GSH) in Somah and LPD medium over time. (b) Oxidized glutathione (GSH) in Somah and LPD medium over time. n = 3 for each group, plotted values are mean ± SEM, *P < 0.05.
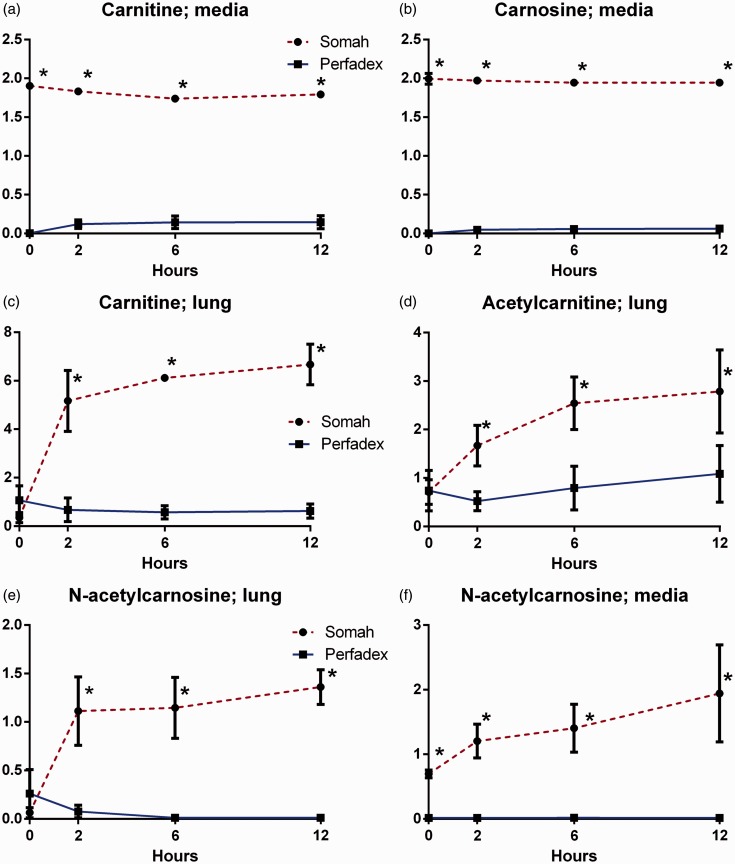
Carnitine and carnosine metabolism
Both carnitine and carnosine levels in Somah medium were significantly higher during all time points (P < 0.05; Fig. 3a, b). In the tissue biopsies, significantly increased levels of carnitine were found in the Somah group at t = 2 and were persistent during the entire experiment (P < 0.05; Fig. 3c). In addition to this finding, acetylcarnitine, a derivative from carnitine, and n-acetylcarnosine, an antioxidant intermediate in the histidine pathway, followed a similar trend in the biopsies. Though basal levels were not significantly different between groups, biopsies of lungs in Somah group contained statistically significant higher acetylcarnitine and n-acetylcarnosine values (P < 0.05; Fig. 3d, e). The Somah group exhibited higher values of n-acetylcarnosine in the medium throughout (P < 0.05; Fig. 3f).
Fig. 3.
(a) Carnitine in Somah and LPD medium over time. (b) Carnosine in Somah and LPD medium over time. (c) Carnitine in Somah and LPD lung biopsies over time. (d) Acetylcarnitine in Somah and LPD lung biopsies over time. (e) n-acetylcarnosine in Somah and LPD lung biopsies over time. (f) n-acetylcarnosine in Somah and LPD medium over time. n = 3 for each group, plotted values are mean ± SEM, *P < 0.05.
Adenosine metabolism
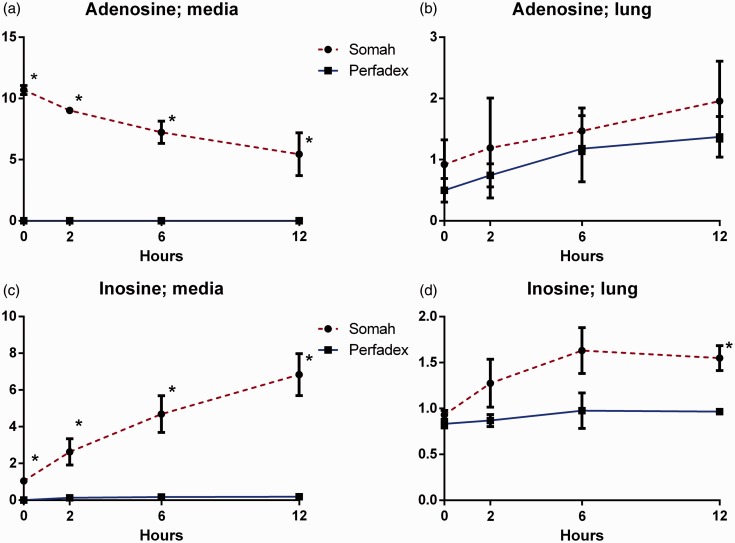
Adenosine-enriched Somah solution demonstrated significantly higher adenosine concentration in the medium throughout the experiment, with adenosine gradually depleting during the entire 12 h (P < 0.05; Fig. 4a). Tissue samples showed no differences in adenosine between groups (Fig. 4b).
Fig. 4.
(a) Adenosine in Somah and LPD medium over time. (b) Adenosine in Somah and LPD lung biopsies over time. (c) Inosine in Somah and LPD medium over time. (d) Inosine in Somah and LPD lung biopsies over time. n = 3 for each group, plotted values are mean ± SEM, *P < 0.05.
Along with adenosine catabolization, inosine in Somah medium increased gradually, a trend not found in LPD (P < 0.05; Fig. 4c). Inosine levels in Somah-preserved lung tissue were elevated at the beginning of the experiment and increased over time; the difference between groups did not reach statistical significance until t = 12 (P < 0.05; Fig. 4d).
Carbohydrate metabolism
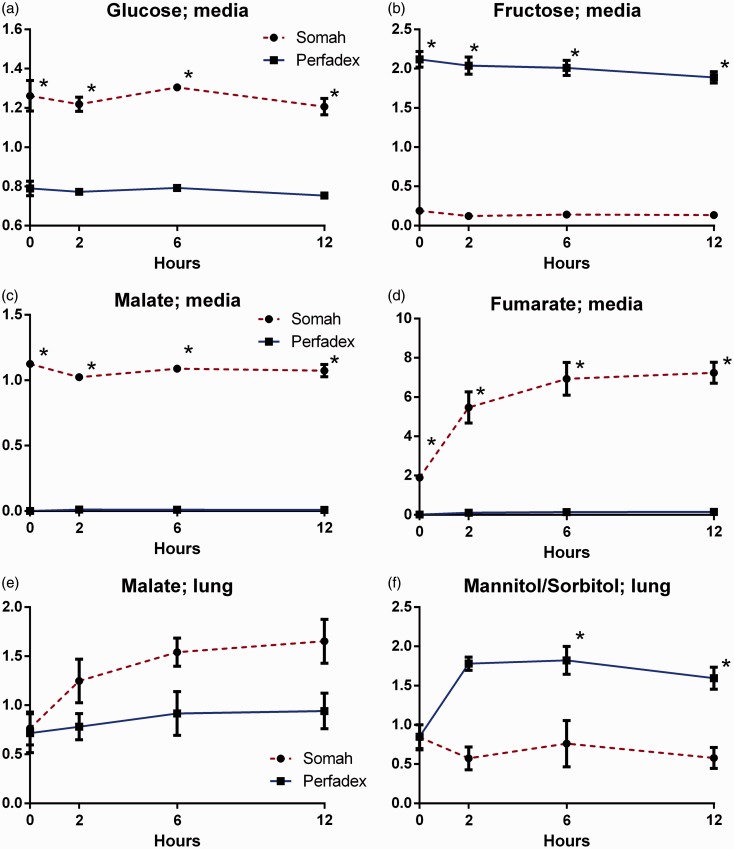
Somah contains higher basal levels of glucose, malate, and fumerate, while fructose is higher in LPD (P < 0.05; Fig. 5a–d). Citric acid (TCA) cycle intermediates showed larger increments in Somah than in LPD across all time points (P < 0.05; Table 3). In tissue samples, significantly higher mannitol/sorbitol was found when preserved in LPD after t = 2 (P < 0.05; Fig. 5e). Malate in the tissue showed a trend of greater increase in Somah than LPD, but did not reach statistical significance (Fig. 5f).
Fig. 5.
(a) Glucose in Somah and LPD medium over time. (b) Fructose in Somah and LPD medium over time. (c) Malate in Somah and LPD medium over time. (d) Fumarate in Somah and LPD medium over time. (e) Malate in Somah and LPD lung biopsies over time. (f) Mannitol/sorbitol in Somah and LPD lung biopsies over time. n = 3 for each group, plotted values are mean ± SEM, *P < 0.05.
Table 3.
TCA cycle components in preservation solutions over time; n = 3 for each sample.
Redox state
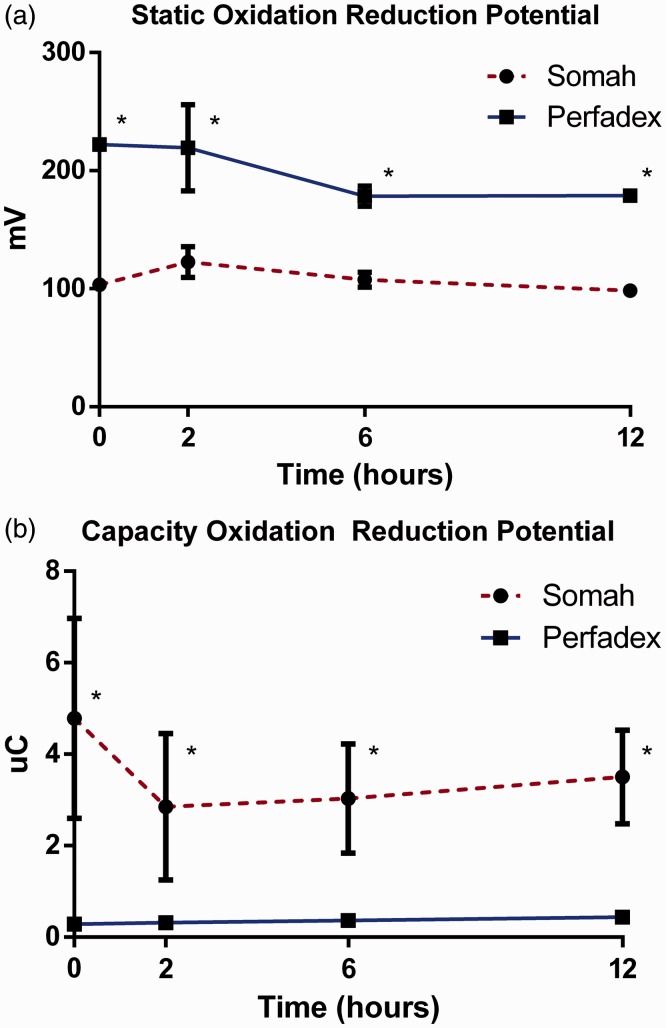
Samples of LPD medium demonstrated elevated levels of sORP compared to Somah samples during the entire preservation (P < 0.05; Fig. 6a). Levels of cORP in Somah medium were significantly higher at each time point than LPD (P < 0.05; Fig. 6b).
Fig. 6.
(a) Static oxidation reduction (sORP) in millivolts (mV) of Somah and LPD medium over time. (b) Capacity oxidation reduction potential (cORP) in microcoulombs (µC) of Somah and LPD medium over time. n = 3 for each group, plotted values are mean ± SEM, *P < 0.05.
Discussion
Limited ex vivo storage time has been one of the main restricting factors in the pulmonary transplant success rate. Trials exploring the potential of EVLP as a preservation standard provide exciting prospects, but are increasingly aiming to rejuvenate marginal grafts rather than increasing preservation time. Moreover, EVLP has limitations that impede widespread usage of continuous perfused preservation. The need for improved cold static storage of donor lungs therefore remains unabated. Current static lung preservation protocols often lead to the transplantation of a marginal graft and may lead to the organ being rejected. Extending ex vivo storage time with the right conditions would ensure better quality of organs transplanted. In addition to this enormous benefit, the organs’ transplantable geographic radius would increase, providing the optimal HLA-match, and ideally facilitating a global organ donor program. However, little is known about the metabolomics of the lung allograft at hypothermia with the current golden standard lung transplant preservation solution, LPD. We evaluated the metabolomics pathways of the current practice organ preservation medium and compared the effects and implications of a novel solution on cellular and mitochondrial metabolism during cold static storage of porcine DCD lungs. Our study shows that LPD is unable to scavenge ROS effectively at hypothermia. ROS is a major cause of tissue apoptosis and necrosis in IR injury. Both the lack of antioxidants and the redox status seen in the medium raise questions about the current use of LPD. Somah’s ROS scavenging ability could be an enhancing alternative. Another important cytoprotective agent that is missing in LPD is adenosine, which is supplemented in Somah’s formula. The reduction of adenosine along with the elevation in inosine, as observed in the Somah medium, are strong indicators of active adenosine catabolism during organ storage. LPD does not provide cellular protection or inflammatory modulation in a similar manner. Better characterization of the metabolomics in donor lung organ preservation solutions leads us to understanding the foundations of limited longevity when lungs are stored in cold LPD. It also gives an insight on how Somah may better protect or possibly even recondition DCD marginal lungs. This could theoretically prevent IR injury following lung transplantation and thus improve primary graft dysfunction and transplant outcomes.
Glutathione metabolism
Depletion of glutathione pathway intermediates in Somah medium after 2 h suggests tissue utilization of these substrates. This reduction might be indicative of ROS scavenging and redox potential stabilization. GSH and GSSG can be used as a marker for oxidative stress.24–26 The penetration of GSH appears to be suboptimal due to the static environment. It could be suggested that the tissue penetration would be better if the organ were continually perfused. Combining the benefits of continuous organ perfusion by EVLP and an optimal preservation solution that features higher glutathione metabolism has enormous potential in the search for increasing ex vivo graft storage time.
Carnitine and carnosine metabolism
Carnitine has an important role as a transporter of fatty acids over the mitochondrial membrane. The increase in carnitine in Somah lungs implies β-oxidation progression into the TCA-cycle and ATP production. More importantly, a deficit in carnitine during ischemic periods can cause a defect in metabolic processing and ATP production upon reperfusion.27
Furthermore, carnitine plays a role in gluconeogenesis through fatty acid oxidation as well as being a stabilizing agent in cell membranes,28,29 providing protection against cytotoxic free fatty acids.
In addition to these findings, carnosine intermediates found in Somah-preserved lungs highlight a strong ROS scavenging role. This further supports the suggested antioxidant capacity of Somah. The accumulation of acetylcarnosine in lung tissue can play a vital, beneficial role against ROS upon reperfusion of the lungs. Carnitine and carnosine levels are significantly higher after t = 2 in Somah-preserved lung tissue and in Somah solution itself, implying the benefits of increased carnitine and carnosine uptake are seen in the Somah group and not the LPD group.
Adenosine metabolism
Adenosine in preservation solutions has notable influences on multiple levels. We have previously shown that adenosine has a cytoprotective role;30,31 in lungs it is of great importance to protect cellular function during ischemic periods. Once the organ is transplanted, immediate and adequate functioning (i.e. gas exchange) is of vital importance.
Moreover, adenosine possesses anti-inflammatory properties.32,33 During ischemia, adenosine can play a pivotal role in preventing reperfusion injury.34,35 The absence of adenosine in LPD raises concerns about the protective measures on the inflammatory response as well as IR injury.
Carbohydrate metabolism
Glucose levels, although being supplemented in both solutions, are higher in the basal formulation of Somah. Significant evidence of different carbohydrate metabolism is found in the basal variation in the medium, as Somah contains higher malate and fumerate levels and LPD demonstrates increased fructose levels. The effects of this contrast may be shown in the tissue biopsies. Lungs preserved in Somah demonstrate increased TCA cycle utilization, based on the increase in malate and succinate, whereas LPD lung tissue showed a muted response, although no statistical significance was found. This lack of significance might be due to the hypothermic storage conditions, effectively decelerating all cellular processes including TCA cycle reactions. Fructose metabolism in LPD lungs is demonstrated by the increments of sorbitol over time.
These finding suggest a better bioenergetic profile for organs in Somah. This is supported by the accumulation of glycolytic metabolites such as pyruvate and lactate. Although this response is seen in both groups, the increase is greater in Somah medium. Altogether these results indicate the favorable impact of Somah on glycolytic circumstances, such as ischemic ex vivo storage.
Redox potential
The increased degree of sORP found in LPD samples are indicative of elevated oxidative stress, and the lower sORP levels in Somah highlight its ROS scavenging potential. Additionally, the cORP results indicate greater antioxidant capacity in Somah throughout.23 The measurement of sORP and cORP may provide some benefits over the more standard methods of ROS assessment. The application of traditional ROS assays has been up for debate as they are known to have various limitation.36–38 By measuring the complete static and capacity ORP we aim to overcome the difficulties in ROS measurement, while demonstrating redox state, of which ROS is an integral part. Combining glutathione assays with redox potential provides a powerful evaluation of oxidative stress in the tissue.26
A limitation of our study is the absence of reperfusion after cold static storage. The investigators acknowledge the pivotal role of oxygen as a mediator of IR injury. As mentioned before, mitochondrial rupture upon reperfusion of the organ with oxygenated blood is a key mediator of tissue apoptosis. However, given the lack of understanding of what bioenergetic requirements are met during cold static storage as well as the protective means of current preservation solutions, a deeper understanding of the metabolic pathways was our focus. The present study aims to investigate differences in metabolic pathways, and experimental methodology has been developed accordingly. As a result of the set-up, however, it remains unclear if the effects are seen locally rather than throughout the entire pulmonary milieu. We also acknowledge that functional lung parameters were unable to be measured at hypothermia in this experimental setting and that further studies would be required following reperfusion of DCD lungs.
Lastly, biological variability between the donor pigs could be regarded as a limitation of our study. Our experimental set-up counteracted this issue by splitting the lungs before flushing with preservation solution, creating matched pairs. Thus, each animal was represented in both groups, with one half of the lung serving as its own control, and the variation between animals was abated.
Conclusion
There has been little progress over the decade in lung transplantation preservation solutions. Altered cellular metabolism by hypothermia does decelerate energy depletion and tissue damage, but does not provide circumstances that exceed the 6 hour ex vivo time limit before critical damage occurs.39,40 In pursuit of extending this time limit for cardiothoracic organs it is essential to minimize IR injury. The formulation of Somah solution could be a logical step towards maintenance of sufficient ATP levels, mitochondrial integrity and protection against IR injury. Although the tissue damage occurs upon reperfusion after transplant, the environment in which the graft is preserved plays a large role. Insufficient bioenergetic capacity or reserve and the loss in ATP associated with that is a major trigger for IR injury. Given the basal formulation of LPD, insufficient glycolytic capability and heightened fructose metabolism was expected. Consistent with this we observed an increase in fructose metabolites. The opposite was seen in Somah lungs, where the accumulation of TCA cycle components is representative of a favorable energetic profile upon transplant and glucose enrichment entails more glycolytic capacity, findings that were expected with Somah’s basal formulation. Furthermore, DCD lungs remain underutilized as transplantable organs after cold static storage. The warm ischemic times often seen in DCD organ harvest as well as a cytokine storm and inflammation upon cardiac arrest, further elucidate the need of preservation approaches that safeguard bioenergetic state and possess anti-inflammatory capabilities. Our study illustrates the potential to enhance metabolic and redox conditions in a way not seen in current practice with traditional LPD. Lung preservation solutions have hardly evolved over the past decades and this is underlined by insignificant extension of static ex vivo graft preservation time. Thus, in conclusion, to increase the lung donor pool, it is essential to find novel approaches that improve the metabolic state of the organ. Somah does so in a way that could introduce DCD lungs as an acceptable alternative resource pool for lung transplant with the potential of reducing primary graft dysfunction and its sequelae. Despite the highly encouraging results demonstrated in the current study, the effects of organ reperfusion after preservation remain unknown, as well as functional lung parameters. Studies investigating these factors and outcomes are needed in order to validate the suggested observations.
Acknowledgments
The authors thank Julia Fisher from the University of Arizona Statistical Consulting Laboratory for statistical guidance. The authors also thank Samuel Garcia, Crystal Carr, and all the employees of the University of Arizona Food Product and Safely lab for facilitating the procurement of porcine organs. Lastly, the authors acknowledge the support of the US-Polish Fulbright Commission during the study.
Conflict of interest
One author is an unpaid consultant for Somahlution, the manufacturer of Somah solution investigated in this study. The terms of this arrangement have been reviewed and appropriately managed by the University of Arizona in accordance with its conflict of interest policies. All other authors declare no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Valapour M, Skeans MA, Heubner BM, et al. OPTN/SRTR 2012 Annual Data Report: lung. Am J Transplant 2014; 14 Suppl. 1: 139–165. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report–2013; focus theme: age. J Heart Lung Transplant 2013; 32(10): 965–978. [DOI] [PubMed] [Google Scholar]
- 3.Yusen RD, Shearon TH, Qian Y, et al. Lung transplantation in the United States, 1999–2008. Am J Transplant 2010; 10(4 Pt 2): 1047–1068. [DOI] [PubMed] [Google Scholar]
- 4.Wigfield CH, Love RB. Donation after cardiac death lung transplantation outcomes. Curr Opin Organ Transplant 2011; 16(5): 462–468. [DOI] [PubMed] [Google Scholar]
- 5.den Hengst WA, Gielis JF, Lin JY, et al. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol 2010; 299(5): H1283–1299. [DOI] [PubMed] [Google Scholar]
- 6.de Perrot M, Liu M, Waddell TK, et al. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med 2003; 167(4): 490–511. [DOI] [PubMed] [Google Scholar]
- 7.Steen S, Sjoberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001; 357(9259): 825–829. [DOI] [PubMed] [Google Scholar]
- 8.Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant 2008; 27(12): 1319–1325. [DOI] [PubMed] [Google Scholar]
- 9.Hsin MK, Iskender I, Nakajima D, et al. Extension of donor lung preservation with hypothermic storage after normothermic ex vivo lung perfusion. J Heart Lung Transplant 2016; 35(1): 130–136. [DOI] [PubMed] [Google Scholar]
- 10.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011; 364(15): 1431–1440. [DOI] [PubMed] [Google Scholar]
- 11.De Oliveira NC, Osaki S, Maloney JD, et al. Lung transplantation with donation after cardiac death donors: long-term follow-up in a single center. J Thorac Cardiovasc Surg 2010; 139(5): 1306–1315. [DOI] [PubMed] [Google Scholar]
- 12.Cypel M, Levvey B, Van Raemdonck D, et al. International Society for Heart and Lung Transplantation Donation After Circulatory Death Registry Report. J Heart Lung Transplant 2015; 34(10): 1278–1282. [DOI] [PubMed] [Google Scholar]
- 13.Steen S, Ingemansson R, Eriksson L, et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Annals Thorac Surg 2007; 83(6): 2191–2194. [DOI] [PubMed] [Google Scholar]
- 14.Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant 2009; 9(10): 2262–2269. [DOI] [PubMed] [Google Scholar]
- 15.Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Annals Thorac Surg 2009; 87(1): 255–260. [DOI] [PubMed] [Google Scholar]
- 16.Soares PR, Braga KA, Nepomuceno NA, et al. Comparison between Perfadex and locally manufactured low-potassium dextran solution for pulmonary preservation in an ex vivo isolated lung perfusion model. Transplant Proc 2011; 43(1): 84–88. [DOI] [PubMed] [Google Scholar]
- 17.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res 2004; 61(3): 372–385. [DOI] [PubMed] [Google Scholar]
- 18.Jassem W, Fuggle SV, Rela M, et al. The role of mitochondria in ischemia/reperfusion injury. Transplantation 2002; 73(4): 493–499. [DOI] [PubMed] [Google Scholar]
- 19.Lowalekar SK, Lu XG, Thatte HS. Further evaluation of Somah: long-term preservation, temperature effect, and prevention of ischemia-reperfusion injury in rat hearts harvested after cardiocirculatory death. Transplant Proc 2013; 45(9): 3192–3197. [DOI] [PubMed] [Google Scholar]
- 20.Thatte HS, Rousou L, Hussaini BE, et al. Development and evaluation of a novel solution, Somah, for the procurement and preservation of beating and nonbeating donor hearts for transplantation. Circulation 2009; 120(17): 1704–1713. [DOI] [PubMed] [Google Scholar]
- 21.Shin SY, Fauman EB, Petersen AK, et al. An atlas of genetic influences on human blood metabolites. Nat Genet 2014; 46(6): 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehaven CD, Evans AM, Dai H, et al. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform 2010; 2(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stagos D, Goutzourelas N, Bar-Or D, et al. Application of a new oxidation-reduction potential assessment method in strenuous exercise-induced oxidative stress. Redox Rep 2015; 20(4): 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ 2009; 16(10): 1303–1314. [DOI] [PubMed] [Google Scholar]
- 25.Zitka O, Skalickova S, Gumulec J, et al. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett 2012; 4(6): 1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aquilano K, Baldelli S, Ciriolo MR. Glutathione: new roles in redox signaling for an old antioxidant. Front Pharmacol 2014; 5: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvani M, Reda E, Arrigoni-Martelli E. Regulation by carnitine of myocardial fatty acid and carbohydrate metabolism under normal and pathological conditions. Basic Res Cardiol 2000; 95(2): 75–83. [DOI] [PubMed] [Google Scholar]
- 28.Carter AL, Abney TO, Lapp DF. Biosynthesis and metabolism of carnitine. J Child Neurol 1995; 10 Suppl. 2: S3–7. [PubMed] [Google Scholar]
- 29.Borum PR. Carnitine. Annu Rev Nutr 1983; 3: 233–259. [DOI] [PubMed] [Google Scholar]
- 30.Khalpey Z, Yuen AH, Lavitrano M, et al. Mammalian mismatches in nucleotide metabolism: implications for xenotransplantation. Mol Cell Biochem 2007; 304(1–2): 109–117. [DOI] [PubMed] [Google Scholar]
- 31.Khalpey Z, Yuen AH, Kalsi KK, et al. Loss of ecto-5'nucleotidase from porcine endothelial cells after exposure to human blood: Implications for xenotransplantation. Biochim Biophys Acta 2005; 1741(1–2): 191–198. [DOI] [PubMed] [Google Scholar]
- 32.Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol (1985) 1994; 76(1): 5–13. [DOI] [PubMed] [Google Scholar]
- 33.Antonioli L, Pacher P, Vizi ES, et al. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013; 19(6): 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 2001; 41: 775–787. [DOI] [PubMed] [Google Scholar]
- 35.Gazoni LM, Laubach VE, Mulloy DP, et al. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. J Thorac Cardiovasc Surg 2008; 135(1): 156–165. [DOI] [PubMed] [Google Scholar]
- 36.Batandier C, Fontaine E, Keriel C, et al. Determination of mitochondrial reactive oxygen species: methodological aspects. J Cell Mol Med 2002; 6(2): 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dikalov SI, Harrison DG. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal 2014; 20(2): 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winterbourn CC. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim Biophys Acta 2014; 1840(2): 730–738. [DOI] [PubMed] [Google Scholar]
- 39.Snell GI, Rabinov M, Griffiths A, et al. Pulmonary allograft ischemic time: an important predictor of survival after lung transplantation. J Heart Lung Transplant 1996; 15(2): 160–168. [PubMed] [Google Scholar]
- 40.Okada Y, Kondo T. Preservation solution for lung transplantation. Gen Thorac Cardiovasc Surg 2009; 57(12): 635–639. [DOI] [PubMed] [Google Scholar]