Abstract
OBJECTIVES: To report the long-term outcome and toxicity of locoregionally advanced nasopharyngeal carcinoma (LA NPC) treated with nimotuzumab (h-R3) plus intensity-modulated radiotherapy (IMRT) with or without chemotherapy. METHODS: From May 2008 to March 2014, 3022 newly histology-proven, nonmetastatic NPC patients were retrospectively reviewed; among them, 257 patients treated with h-R3 were enrolled in this study. The patients' age range was between 10 and 76 years. The distribution of patients by disease stage was 150 (58.4%) in stage III, 88 (34.2%) in stage IV A, and 19 (7.4%) in stage IV B. All the patients received the treatment of h-R3 plus IMRT, and from them, 239 cases were also treated with cisplatin-based chemotherapy. Acute and late radiation-related toxicities were graded according to the Acute and Late Radiation Morbidity Scoring Criteria of Radiation Therapy Oncology Group. The accumulated survival was calculated according to the Kaplan-Meier method. Log-rank test was used to compare the survival difference. Multivariate analysis was performed using Cox's proportional-hazard model. RESULTS: All 257 patients had completed combined treatment; 231 patients received h-R3 plus IMRT with induction chemotherapy (IC), while 26 patients received only h-R3 plus IMRT. With a median follow-up of 48 months (range, 13-75 months), the estimated 5-year local recurrence-free survival, regional recurrence-free survival, distant metastases-free survival, progression-free survival, and overall survival (OS) rates were 94.3%, 94.8%, 91.9%, 83.4%, and 86.2%, respectively. Univariate analysis showed that age, T stage, clinical stage, and IC were related with OS. Multivariate analysis indicated that T stage and IC were independent prognostic factors for OS. The incidence of grade 3 to 4 acute mucositis and leukocytopenia was 10.9% and 19.8%, respectively, with no cases of skin rash and infusion reaction. Xerostomia was the most common late complication, and the degree of dry mouth in most survivors was mild to moderate at the last follow-up time. CONCLUSION: h-R3 plus IMRT with or without chemotherapy showed promising outcomes in terms of locoregional control and survival without increasing the incidence of radiation-related toxicities for patients.
Introduction
Nasopharyngeal carcinoma (NPC) is a highly aggressive malignant tumor derived from nasopharyngeal epithelial cells, which is endemic in Southern China [1], [2]. Radiotherapy (RT) is the standard treatment for NPC due to the anatomical location and the high radiosensitivity [3], [4]. Based on RT combined with chemotherapy, the 5-year survival rate has been improved by 4% to 6%, complications have been reduced by 18%, and the contribution of concurrent chemoradiotherapy (CRT) has been very important [5]. CRT has become the main therapeutic option for locally advanced NPC because of its impact on overall survival (OS) increase [6]. Intensity-modulated radiation therapy (IMRT) is a novel technique for radiation therapy planning and delivery [7], [8]. IMRT allows three-dimensional (3D) dose coverage of the clinical target volume (CTV) compared to 2D-RT and 3D conformal radiotherapy (3D-CRT), which protects the surrounding normal tissue [9]. Recent phase III studies have demonstrated that IMRT is more effective than conventional 2D-RT or 3D-CRT and that it reduces morbidity [10], [11]. The dosimetric advantages of IMRT make it the first choice for radiation modality in NPC patients. The treatment of locoregionally advanced nasopharyngeal carcinoma (LA NPC) is a difficult problem for clinicians, and the efficacy needs to be further improved. There is no clear evidence for the benefits of sequential combination of targeted drugs with IMRT and chemotherapy. So the role and status of targeted drugs in the comprehensive treatment of LA NPC still need be confirmed by further research.
Epidermal growth factor receptor (EGFR) has been a hot point of study in the recent 10 years. The overexpression of EGFR has been observed in many different tumor cells, including colorectal cancer, head and neck squamous cell carcinoma, and non–small cell lung cancer [12]. Preclinical experiments have shown that overexpression of EGFR leads to increased tumor invasiveness and resistance to chemotherapy and radiotherapy, which is an independent adverse prognostic factor [13], [14], [15], [16]. Anti-EGFR drugs, including cetuximab, panitumumab, erlotinib, and gefitinib, have demonstrated good therapeutic effects [17], [18], [19]. Cetuximab combined with concurrent chemoradiotherapy in the treatment of NPC showed survival benefit with tolerable side effects [20].
Nimotuzumab (h-R3) is also a monoclonal antibody against EGFR, which is highly humanized and has a longer half-life and a higher effective dose concentration compared with other anti-EGFR drugs [21], [22]. At the same time, there are few serious complications and improved quality of life of patients associated with the use of this drug [23]. A phase II trial confirmed that h-R3 combined with RT has a good synergistic effect, with significantly improved efficacy of NPC patients; the objective rate was 100%, the complete remission rate was 90.63%, 3-year OS rate was 84.29%, and the reported adverse reactions were mild [24]. Comparative phase III clinical study of h-R3 plus RT with concurrent chemotherapy (CDDP/RT) was reported in the 2016 ASCO meeting; these recent results showed that h-R3/RT has similar efficacy than concurrent chemoradiotherapy, while the toxicities were milder in R3 group, so it could significantly improve the quality of life of patients [25]. Because of the low completion rate and unsatisfied efficacy of concurrent chemoradiotherapy in this study, it is necessary to wait for further studies and long-term follow-up results. At present, the value of h-R3 in the treatment of NPC is being investigated by some retrospective studies with small size samples and the short follow-up period, while the long-term efficacy of h-R3 combined with IMRT in the treatment of NPC has been little reported. So we evaluated the long-term survival outcomes of h-R3 combined with IMRT in patients with LA NPC and analyzed the prognostic factors.
Patients and Methods
Patients and Pretreatment
The patients enrolled into this study were hospitalized from May 2008 to April 2014 in the Department of Radiation Oncology, Zhejiang Cancer Hospital. This retrospective study was approved by the medical ethics committee of Zhejiang Cancer Hospital. The eligible patients should met the following criteria: 1) histologically proven LA NPC; 2) Eastern Cooperative Oncology Group performance status ≤1; 3) completion of radical IMRT; 4) received nimotuzumab; and 5) without previous anticancer treatment.
They had a pretreatment evaluation including complete history, physical examination, hematology and biochemistry profiles, chest radiographs, ultrasonography of the abdomen, bone scan, magnetic resonance imaging of nasopharynx, and nasopharyngoscope. All patients were staged according to 2010 AJCC staging system. Tumor histology was classified according to the World Health Organization classification.
A total of 3022 newly diagnosed LA NPC patients were registered at Zhejiang Cancer Hospital. Among them, a total of 257 NPC patients treated with h-R3 plus RT were enrolled into this study. All patients received definitive IMRT with or without chemotherapy.
RT
All patients were immobilized in the supine position with thermoplastic masks. Computed tomography scans with intravenous contrast (2.5-mm slices from the head to 2 cm below the sternoclavicular joints) were performed for planning. Target volumes were delineated according to the recommendations of the International Commission on Radiation Units and Measurements CTV delineation protocol for head and neck malignancies [26], [27]. Gross tumor volume (GTV) referred to the extent of the tumor found in clinical and imaging examinations. The extent of the primary tumor, including metastatic retropharyngeal lymph nodes, was defined as GTVnx, and the metastatic lymph nodes of the neck as GTVnd.
The CTV was defined individually according to the GTV, and the potential regions at risk surrounding the nasopharyngeal cavity. The CTV for GTVnx included CTVnx for the high-risk CTV and CTV1 when invasion was present. The CTVnx was defined as GTVnx plus a 7-mm margin that encompassed the nasopharyngeal mucosa plus 5-mm submucosal volume. For CTV1, the anatomic regions that were potentially involved included the entire nasopharyngeal cavity, the anterior one- to two-thirds of the clivus (when invasion is present, the whole clivus should be covered), the skull base, the pterygoid plates, the parapharyngeal space, the inferior sphenoid sinus (the entire sphenoid sinus should be covered for stage T3 and T4 NPC), the posterior one-quarter to one-third of the nasal cavity, and the maxillary sinus. High-risk nodes included level Ib nodes in patients with metastatic lymph nodes in level IIa, and any lymph nodes in drainage pathways containing metastatic lymph nodes. Low-risk areas for prophylactic neck irradiation areas were referred to as CTV2. These low-risk areas included levels IV and Vb without metastatic cervical lymph nodes.
The PTV was constructed automatically based on each volume with an additional 3-mm margin in three dimensions to account for setup variability. All of the PTVs, including PGTVnx, PTVnx, PTV1, and PTV2, were not delineated outside of the skin surface. Critical normal structures including the brainstem, spinal cord, parotid glands, optic nerves, chiasm, lens, eyeballs, temporal lobes, temporomandibular joints, mandible, and hypophysis were contoured and set as OARs during optimization.
All patients underwent radical IMRT with simultaneous integrated boost technique using 6 MV photons. The prescribed radiation dose was 69 or 72 Gy to PGTVnx, 66 to 69 Gy to PGTVnd, 63 to 66 Gy to PTVnx, 60 to 63 Gy to PTV1, and 51 to 54 Gy to PTV2, delivered in 30 or 33 fractions. Radiation was delivered once daily, five fractions per week, over 6 to 6.5 weeks for IMRT planning. The dose to OAR was limited on the basis of the RTOG 0225 protocol.
Target Treatment
h-R3 was administered concomitantly with induction chemotherapy and/or RT at a dose of 100 mg or 200 mg weekly; it was diluted in 250 ml saline to obtain a 100-mg or 200-mg solution and intravenously infused over 1 hour. All patients received weekly dose of nimotuzumab.
Chemotherapy
Patients received one to four cycles of platinum-based induction chemotherapy (IC). The most common induction regimens included TPF (docetaxel 60 mg/m2/day on day 1, cisplatin 25 mg/m2/day on days 1-3, and 5-fluorouracil 500 mg/m2/day on days 1-3), TP (docetaxel 60 mg/m2/day on day 1, cisplatin 25 mg/m2/day on days 1-3), GP regimen (gemcitabine 1000 mg/m2/day on days 1 and 8, cisplatin 25 mg/m2/day on days 1-3), and FP (cisplatin 25 mg/m2/day on days 1-3, and 5-fluorouracil 500 mg/m2/day on days 1-3).
NPC patients underwent ≥1 cycle concurrent chemotherapy with cisplatin (80 mg/m2) for 3 days. One hundred seventeen patients received two to three courses of adjuvant chemotherapy with FP regimen 3 weeks after RT.
Patient Evaluation and Follow-Up
The assessment of tumor response was performed thrice: after the completion of induction chemotherapy, at the end of IMRT, and 3 months after radiation, and was based on MRI and nasopharyngeal fiberscope according to Response Evaluation Criteria for Solid Tumors criteria. Systemic chemotherapy adverse effects were graded using the National Cancer Institute Common Toxicity Criteria (NCI CTCAE, version 3.0), whereas RT-induced toxicities were scored according to the Acute and Late Radiation Morbidity Scoring Criteria of the Radiation Therapy Oncology Group (RTOG).
All the subjects underwent weekly examinations for treatment response and toxicities during radiation therapy. Patients were followed up every 3 months in the first 2 years, every 6 months from the third to the fifth year, and then annually. The examinations in each follow-up included careful examination of the nasopharynx and neck nodes by an experienced doctor, MRI scan of the nasopharynx, nasopharyngeal fiberscope, chest computed tomography radiograph, and ultrasound of abdomen were performed 3 months after the completion of RT and every 6-12 months thereafter. Additional examinations were performed when it is indicated to evaluate local relapse or distant metastasis.
Statistical Analysis
Survival curves were generated using the Kaplan-Meier method. The curves were compared using log-rank tests. Multivariate analysis was performed using Cox regression models to identify significant prognostic factors. Hazard ratios and 95% confidence intervals were calculated for each prognostic factor. IBM SPSS Statistics version 19.0 was used for all data analysis. A P < .05 was considered statistically significant. Survival time was calculated from the date of diagnosis to the most recent follow-up or to either the date of relapse (event-free, local recurrence-free, or distant metastasis-free) or death (OS). After recurrence or metastasis, patients were given salvage therapy as determined by their physicians.
Results
Patient Characteristics and Completion of Treatment
The flowchart of patients is shown in Figure 1. Between May 2008 and April 2014, the clinical data of 257 newly diagnosed LA NPC patients, who were initially treated with h-R3 plus IMRT with or without chemotherapy in the Department of Radiation Oncology, Zhejiang Cancer Hospital (Hangzhou, People's Republic of China), were collected and retrospectively reviewed. Basic characteristics of patients are summarized in Table 1.
Figure 1.
Flowchart of patients.
Table 1.
Baseline Characteristic of 257 LA NPC Patients
| Characteristics | N (%) |
|---|---|
| Age (years) | |
| Range | 10-76 |
| Median | 47 |
| <60 years | 200 (77.8) |
| ≥60 years | 57 (22.2) |
| Sex | |
| Man | 192 (74.7) |
| Female | 65 (25.3) |
| WHO histological classification | |
| Type I | 7 (2.7) |
| Type II | 247 (96.1) |
| Type III | 3 (1.2) |
| T stage⁎ | |
| 1 | 7 (2.7) |
| 2 | 37 (14.4) |
| 3 | 122 (47.5) |
| 4 | 91 (35.4) |
| N stage⁎ | |
| 0 | 24 (9.3) |
| 1 | 73 (28.4) |
| 2 | 140 (54.5) |
| 3 | 20 (7.8) |
| Clinical stage⁎ | |
| III | 145 (56.4) |
| IV | 112 (43.6) |
| Chemotherapy | |
| Yes | 239 (93.0) |
| No | 18 (7.0) |
| Induction chemotherapy | |
| Yes | 231 (89.9) |
| No | 26 (10.1) |
| Concurrent chemotherapy | |
| Yes | 214 (83.3) |
| No | 43 (16.7) |
| Adjuvant chemotherapy | |
| Yes | 113 (44.0) |
| No | 144 (56.0) |
WHO, World Health Organization.
The 7th AJCC/UICC staging system.
Patients received between 3 and 17 weeks of nimotuzumab's treatment, with a weekly dose interval. Forty-seven patients received 3 to 5 doses of nimotuzumab; 171 patients received 6 to 8 doses, and 39 patients received more than 9 doses of nimotuzumab. The number of radiation fields IMRT delivered to the patients was 7 to 9.
Disease Response
At the end of treatment, complete remission (CR) and partial remission (PR) for lesions of the nasopharynx in 257 LA NPC patients accounted for 86.4% (222/257) and 14.6% (33/257), respectively. For 233 patients with neck metastatic lymph nodes, CR and PR rates of cervical lymph nodes were 90.1% (210/233) and 9.9% (23/233), respectively.
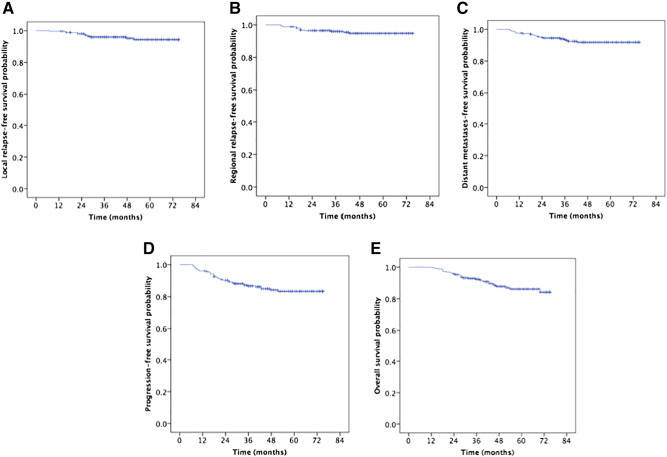
Rates of Local Control and Survival
The median follow-up time was 48 months (range, 13-75). The estimated 5-year local recurrence-free survival (LRFS), regional recurrence-free survival (RRFS), distant metastases-free survival (DMFS), progression-free survival (PFS), and OS rates were 94.3%, 94.8%, 91.9%, 83.4%, and 86.2%, respectively (Figure 2). The 5-year OS rates were 100%, 89.1%, 90.8%, and 77.1% for patients with stage T1, T2, T3, and T4, respectively (P = .002, Figure 3A). The 5-year OS rate of patients treated with IC was higher than that without IC (87.7% vs 71.6%, P = .020, Figure 3B). And the patients 60 years and older obtained poorer OS rates than those <60 years (78.6% vs 88.4%, P = .019, Figure 3C). The 5-year OS and PFS rates were 92.3%, 78.4% and 89.2%, 75.1% for patients with stage III and IV, respectively (P < .001, Figure 3D; P = .005 Figure 4A). The 5-year PFS rates were 87.5%, 88.3%, 83.2%, and 58.3% for patients with stage N0, N1, N2, and N3, respectively (P = .003, Figure 4B). The 5-year PFS rates for patients with adjuvant chemotherapy (AC) versus without AC and CR patients versus non-CR patients were 89.3% vs 77.0% (P = .023, Figure 4C) and 88.0% vs 66.6%, (P = .003, Figure 4D), respectively.
Figure 2.
Kaplan-Meier estimates of the survival in patients with nasopharyngeal carcinoma. (A) LRFS; (B) RRFS; (C) DMFS; (D) PFS; (E) OS.
Figure 3.
Kaplan-Meier estimates of the OS in nasopharyngeal carcinoma patients for different variable. (A) Overall survival for T stages; (B) overall survival for with IC versus without IC; (C) overall survival for <60 years versus ≥60 years; and (D) overall survival for clinical stage.
Figure 4.
Kaplan-Meier estimates of the PFS in nasopharyngeal carcinoma patients for univariate. (A) PFS for clinical stages; (B) PFS for N stages; (C) PFS of patients with or without AC; and (D) PFS for tumor response.
For all patients, the effect of h-R3 used by fraction dose and total dose on 5-year OS and PFS was analyzed as follows: 5-year OS rate of 86.7% for 100 mg vs 85.9% for 200 mg (P = .803), 86% for <1200 mg vs 86.7% for ≥1200 mg (P = .998); 5-year PFS of 87% for 100 mg vs 82.8% for 200 mg (P = .716), 81.3% for <1200 mg vs 87.4% for ≥1200 mg (P = .293). There were no significant statistical differences. In the subgroup analysis, higher total dose of h-R3 had a tendency to improve 5-year PFS rate in female patients and non-CR (85.1% for <1200 mg vs 100% for ≥1200 mg in female patients, P = .056, Figure 5A; 56.4% for <1200 mg vs 83.1% for ≥1200 mg in patients with non-CR, P = .094, Figure 5B), although no statistical significance of difference was found.
Figure 5.
Kaplan-Meier estimates of PFS in nasopharyngeal carcinoma patients by subgroup analysis. (A) PFS for total dose of h-R3 in female patients; (B) PFS for total dose of h-R3 in patients with non-CR.
Altogether, 35 patients developed treatment failure by the last follow-up: local relapse only was found in 9 patients, regional relapse occurred in 8 patients and locoregional relapse in 2 patients; and 16 patients experienced distant failure. Patterns of treatment failure in NPC patients were listed in Table 2.
Table 2.
Patterns of Treatment Failure in Nasopharyngeal Carcinoma Patients
| Sites | Number of Patients (n = 35) |
|---|---|
| Local relapse only | 9 |
| Regional relapse only | 8 |
| Local and regional failure | 2 |
| Regional and distant failure | 2 |
| Distant metastasis only | 14 |
| Lung metastasis only | 3 |
| Bone metastasis only | 3 |
| Liver metastasis only | 2 |
| Lung, liver, bone, and other | 6 |
Identification of Prognostic Factors
We evaluated several potential prognostic factors including patient age, gender, clinical stage, adjusted tumor (T) and lymph node (N) stage, IC, concurrent chemotherapy (CC), AC, dose of nimotuzumab, and tumor response at the end of treatment. Univariate analysis revealed that age, T stage, clinical stage, and IC were significant prognostic factors for OS, while N stage, clinical stage, AC, and tumor response at the end of treatment were significant prognostic factor for PFS (Table 3). Multivariate analysis indicated that stage T4 and without IC were poorer prognostic factors for OS; stage N2-3 for PFS, RRFS, and DMFS; non-CR for PFS, LRFS, and RRFS; and IV for DMFS (Table 4).
Table 3.
Univariate Analysis of Prognostic Factors on OS and PFS in LA NPC Patients
| Characteristic | N | 5-Year OS (%) | P | 5-Year PFS (%) | P |
|---|---|---|---|---|---|
| Gender | .729 | .130 | |||
| Male | 192 | 85.5 | 80.7 | ||
| Female | 65 | 88.2 | 90.5 | ||
| Age (years) | .019 | .201 | |||
| <60 | 200 | 88.4 | 82.0 | ||
| ≥ 60 | 57 | 78.6 | 88.5 | ||
| T stage* | .002 | .269 | |||
| T1 | 7 | 100.0 | 87.5 | ||
| T2 | 37 | 89.1 | 76.3 | ||
| T3 | 122 | 90.8 | 87.7 | ||
| T4 | 91 | 77.1 | 80.4 | ||
| N stage⁎ | .780 | .003 | |||
| N0 | 24 | 91.5 | 87.5 | ||
| N1 | 73 | 88.4 | 88.3 | ||
| N2 | 140 | 84.9 | 83.2 | ||
| N3 | 20 | 80.2 | 58.3 | ||
| Clinical stage⁎ | <.001 | .005 | |||
| III | 145 | 92.3 | 89.2 | ||
| IV | 112 | 78.4 | 75.1 | ||
| IC | .020 | .390 | |||
| No | 26 | 71.6 | 92.1 | ||
| Yes | 231 | 87.7 | 82.6 | ||
| CC | .218 | .906 | |||
| No | 43 | 81.0 | 79.1 | ||
| Yes | 214 | 87.4 | 83.9 | ||
| AC | .570 | .023 | |||
| No | 144 | 86.3 | 89.3 | ||
| Yes | 113 | 86.8 | 77.0 | ||
| Fractional dose of h-R3 | .803 | .716 | |||
| 100 mg | 23 | 86.7 | 87.0 | ||
| 200 mg | 234 | 85.9 | 82.8 | ||
| Total dose of h-R3 | .998 | .293 | |||
| <1200 mg | 169 | 86.0 | 81.3 | ||
| ≥1200 mg | 88 | 86.7 | 87.4 | ||
| Tumor response | .496 | .003 | |||
| CR | 204 | 85.6 | 88.0 | ||
| Non-CR | 53 | 79.7 | 66.6 |
The 7th AJCC/UICC staging system.
Table 4.
Multivariate Analysis of Prognostic Factors in LA NPC Patients
| Characteristic | HR | 95% CI | P Value | |
|---|---|---|---|---|
| OS | T1–3 vs T4 | 0.266 | 0.126-0.560 | <.001 |
| Without vs with IC | 2.910 | 1.183-3.156 | .020 | |
| PFS | N0-1 vs N2-3 | 0.313 | 0.143-0.683 | .004 |
| CR vs non-CR | 0.449 | 0.234-0.861 | .016 | |
| LRFS | CR vs non-CR | 0.287 | 0.093-0.892 | .031 |
| RRFS | N0-1 vs N2-3 | 0.274 | 0.074-1.014 | .053 |
| CR vs non-CR | 0.294 | 0.095-0.912 | .034 | |
| DMFS | III vs IV | 0.158 | 0.052-0.478 | .001 |
| N0-1 vs N2-3 | 0.079 | 0.010-0.590 | .013 |
HR, hazard ratio; CI, confidence interval.
Safety and Toxicity
The most common treatment-related acute adverse events included hematologic and nonhematologic toxicity. During the period of IC, hematologic toxicity was reported as grade 3 and worse in severity in 51 (19.8%) patients. Of these patients, neutropenic fever occurred in 10 cases. It was tolerated without delaying the chemotherapy and without interrupting radiotherapy by GMSF treatment. The gastrointestinal toxicities were mild or moderate, and patients recovered rapidly with or without symptomatic medication. Grade 3 to 4 hematologic toxicities and radiotherapy-related oral mucositis during the period of CCRT were reported in 19 (7.4%) and 28 (10.9%) patients. Among those 28 patients, 5 patients stopped using h-R3 because of severe oral mucositis. At the same time, there were 2 cases of liver damage, which recovered quickly and did not interrupt radiotherapy plan after treatment for liver protection. Grade 3 dermatitis was observed in 5 patients within the RT field. No acneiform eruptions were found among these subjects.
The most commonly observed late complication was xerostomia. However, the degree typically decreased over time. The degree of dry mouth in most patients was mild to moderate at the time of the last follow-up. There were only 22 (8.6%) patients who complained of serious xerostomia. Finally, 79 patients developed either unilateral or bilateral hearing impairment, and 15 were found to have temporal lobe damage, which was diagnosed during follow-up based on magnetic resonance imaging. Likewise, second primary tumors occurred in 9 cases: 2 cases of thyroid cancer and of prostate cancer, and 1 case each of breast cancer, kidney cancer, liver cancer, gastric cancer, and lung cancer. These patients could be treated by surgery.
Discussion
With the further research of the molecular mechanism of tumorigenesis and tumor development, molecular targeted therapy in patients with NPC will become the research hot point. Ninety-four percent of patients with NPC were detected for overexpression of EGFR [14]. Cetuximab, as the anti-EGFR monoclonal antibody most commonly used, has a good curative effect in the treatment of nasopharyngeal carcinoma, with 2-year PFS of 86.5% to 89.3% and 3-year OS of 90.9% [20], but severe oral mucositis and itchy acneiform rash limited its application in NPC. To minimize cetuximab-related toxicities, novel EGFR-targeted agent was developed.
h-R3, is a humanized immunoglobulin G1 isotype monoclonal antibody with unique safety profile and low skin toxicity. It has been approved for the treatment of non-NPC HNSCC [12], [28]. The advantage of the drug is that the affinity constant is quite lower than that of cetuximab, allowing for high tumor uptake and low normal tissues uptake [29]. h-R3 requires bivalent binding for stable attachment, which renders the agent to selectively bind to tumors with moderate to high EGFR levels. When EGFR expression is low as on the normal tissues, cetuximab still had high ability of binding because of its higher affinity constant [29]. Our experiment confirmed that nimotuzumab has sensitization of radiotherapy on NPC cell line CNE-2 in vitro and can reduce cancer cell proliferation, induce cell apoptosis, and change cell cycle distribution [30]. All of these indicated that h-R3 plus RT could be selected in the design of the clinical trial of NPC.
To date, there are only four studies of small sample size about addition of h-R3 to RT or CCRT for NPC patients. In a retrospective paired study by Li et al. [31], the OS and PFS rates for h-R3/RT treatment group were lower than those for cisplatin/RT treatment group, but in stage II or older than 60 years subgroup, there were no significant differences for the OS and PFS. Zhai et al. reported that addition of h-R3 to IMRT showed promising locoregional control and survival outcomes for LA NPC patients [32]. Huang et al. [33] and Liu et al. [34] found that concurrent administration of h-R3 and CCRT obtained encouraging survival outcomes in LA NPC patients, with tolerable treatment-related toxicity results. For the former two studies, due to the severe acute sequel of CCRT, h-R3, as a preferred option replacing cisplatin, increased the quality of life in selected NPC patients, with similar treatment outcomes. But in last two studies, h-R3 added into the intensive scheme of NAC followed by CCRT may improve the survival of LA NPC patients. So the role of h-R3 in the combined treatment of LA NPC still remained unclear.
This study investigated the efficacy and safety of h-R3 plus IMRT with or without chemotherapy for LA NPC patients. Our study showed promising clinical outcomes, with 5-year LRFS of 94.3%, 5-year RRFS of 94.8%, 5-year DMFS of 91.9%, 5-year PFS of 83.4%, and 5-year OS of 86.2%. Univariate analysis revealed that age, T stage, clinical stage, and IC were significant prognostic factors for OS, while N stage, clinical stage, AC, and tumor response at the end of treatment were significant prognostic factor for PFS. Multivariate analysis indicated that stage T4 and without IC were poorer prognostic factors for OS; stage N2-3 for PFS, RRFS, and DMFS; non-CR for PFS, LRFS, and RRFS; and IV for DMFS. However, 19.8% of patients experienced grade ≥ 3 hematologic toxicity, and 10.9% had grade ≥ 3 radiotherapy-related oral mucositis. Only 5 patients had grade 3 dermatitis within the RT field. No acneiform eruptions were found among these subjects.
Our experiences found that h-R3 plus IMRT with or without chemotherapy for LA NPC patients is safe and effective. However, due to retrospective study nature, our results should be regarded as preliminary.
Conclusion
In conclusion, our study observed that the administration of h-R3 with IMRT with or without chemotherapy in LA NPC patients was well tolerated and showed promising clinic outcomes. Further randomized, controlled, multicenter phase III clinical trials are needed to confirm the ultimate therapeutic gain.
Funding
This study was supported by grants from the Medical Science Foundation of Zhejiang Health Bureau (No. 2013KYB033, No. 2009B026, No. 2006A016, No. 2005B012, No. 2004B014) and the National Natural Science Foundation of China (No. 81502646, No. 81502647).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Contributor Information
Fangzheng Wang, Email: wangfz76@126.com.
Yangming Jiang, Email: jym_wm@126.com.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lorlet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA cancer J Clin. 2015;65(1):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Peng H, Chen L, Guo R, Zhang Y, Li WF, Mao YP, Sun Y, Zhang F, Liu LZ, Tian L. Clinical treatment considerations in the intensity-modulated radiotherapy era for patients with N0-category nasopharyngeal carcinoma and enlarged neck lymph nodes. Chin J Cancer. 2017;36:32. doi: 10.1186/s40880-017-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W, Shen G, Zhang Y, Chen G, Wu X, Li Y, Li A, Kang S, Yuan X, Hou X. Development and validation of a nomogram for predicting the survival of patients with non-metastatic nasopharyngeal carcinoma after curative treatment. Chin J Cancer. 2016;35:98. doi: 10.1186/s40880-016-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard P, Lee A, Marguet S, Ledlercq J, Ng WT, Ma J, Chan AT, Huang PY, Benhamou E, Zhu G. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–655. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 6.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol. 1998;16(4):1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 7.Intensity Modulated Radiation Therapy Collaborative Working Group Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51:880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 8.Ezzell GA, Galvin JM, Low D, Palta JR, Rosen I, Sharpe MB, Xia P, Xiao Y, Xing L, Yu CX. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: report of the IMRT Subcommittee of the AAPM Radiation Therapy Committee. Med Phys. 2003;30:2089–2115. doi: 10.1118/1.1591194. [DOI] [PubMed] [Google Scholar]
- 9.Mao YP, Yin WJ, Guo R. Dosimetric benefit to organs at risk following margin reductions in nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Chin J Cancer. 2015;34:16. doi: 10.1186/s40880-015-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J Clin Oncol. 2006;24:2618–2623. doi: 10.1200/JCO.2005.04.7225. [DOI] [PubMed] [Google Scholar]
- 11.Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, Lai M, Ho R, Cheung KY, Yu BK. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 12.Reddy BK, Lokesh V, Vidyasagar MS, Shenoy K, Babu KG, Shenoy A, Naveen T, Joseph B, Bonanthaya R, Nanjundappa Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: a randomized, open-label, phase IIb, 5-year study in Indian patients. Oral Oncol. 2014;50(5):498–505. doi: 10.1016/j.oraloncology.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94(5):1593–1611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- 14.Chua DT, Nicholls JM, Sham JS, Au GK. Prognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(1):11–20. doi: 10.1016/j.ijrobp.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Ma BB, Poon TC, KF To, Zee B, Mo FK, Chan CM, Ho S, Teo PM, Jonson PH, Chan AT. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma – a prospective study. Head Neck. 2003;25(10):864–872. doi: 10.1002/hed.10307. [DOI] [PubMed] [Google Scholar]
- 16.Leong JL, Loh KS, Putti TC, Goh BC, Tan LK. Epidermal growth factor receptor in undifferentiated carcinoma of the nasopharynx. Laryngoscope. 2004;114(1):153–157. doi: 10.1097/00005537-200401000-00029. [DOI] [PubMed] [Google Scholar]
- 17.Dorsey K, Agulnik M. Promising new molecular targeted therapies in head and neck cancer. Drugs. 2013;73(6):315–325. doi: 10.1007/s40265-013-0025-3. [DOI] [PubMed] [Google Scholar]
- 18.Prenen H, Vecchione L, Van Cutsem E. Role of targeted agents in metastatic colorectal cancer. Target Oncol. 2013;8(1):83–96. doi: 10.1007/s11523-013-0281-x. [DOI] [PubMed] [Google Scholar]
- 19.Yewale C, Baradia D, Vhora I, Patil S, Misra A. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials. 2013;34(12):8690–8707. doi: 10.1016/j.biomaterials.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 20.Feng HX, Guo SP, Li GR, Zhong WH, Chen L, Huang LR, Qin HY. Toxicity of concurrent chemoradiotherapy with cetuximab for locoregionally advanced nasopharyngeal carcinoma. Med Oncol. 2014;31(2):170–180. doi: 10.1007/s12032-014-0170-x. [DOI] [PubMed] [Google Scholar]
- 21.Talavera A, Friemann R, Gomez-Puerta S, Martinez-Fleltes C, Garrido G, Rabasa A, Lopez-Requena A, Pupo A, Johansen RF, Sanchez O. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res. 2009;69(15):5851–5859. doi: 10.1158/0008-5472.CAN-08-4518. [DOI] [PubMed] [Google Scholar]
- 22.Crombet T, Torres L, Neninger E, Catala M, Solano ME, Perera A, Torres O, Iznaga N, Torres F, Perez R. Pharmacological evaluation of humanized anti- epidermal growth factor receptor, monoclonal antibody h-R3, in patients with advanced epithelial-derived cancer. J immunother. 2003;26(2):139–148. doi: 10.1097/00002371-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Du F, Zheng Z, Shi S, Jiang Z, Qu T, Yuan X, Sun Y, Song Y, Yang L, Zhao J. S-1 and Cisplatin With or Without Nimotuzumab for Patients With Untreated Unresectable or Metastatic Gastric Cancer: A Randomized, Open-Label Phase 2 Trial. Medicine. 2015;94 doi: 10.1097/MD.0000000000000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang XD, Xu GZ, Gao L, Xu GZ, Jin J, Yang WZ, Lu TX, Wu SX, Wu RR, Hu WH. Multi-center phase II clinical trial of huanized anti-epidermal factor receptor monoclonal antibody h-R3 combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Oncol. 2007;29(3):197–201. [PubMed] [Google Scholar]
- 25.Kong L, Lin Q, Hu CS. Radiation plus concurrent nimotuzumab versus CDDP in locally advanced nasopharyngeal cancer: Results of a phase III randomised trial. J Clin Oncol. 2016;34 [suppl; abstr 6002] [Google Scholar]
- 26.International Commission on Radiation Units and Measurements . ICRU; Bethesda: 1993. Prescribing, recording, and reporting photon beam therapy. [Google Scholar]
- 27.International Commission on Radiation Units and Measurements . ICRU; Bethesda: 1999. Prescribing, recording, and reporting photon beam therapy (supplement to ICRU report 50) [Google Scholar]
- 28.Rodriguez MO, Rivero TC, del Castillo Bahi R, Muchull CR, Bilbao MA, Vinageras EN, Alert J, Galainena JJ, Rodriguez E, Graclas E. Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biol Ther. 2010;9(5):343–349. doi: 10.4161/cbt.9.5.10981. [DOI] [PubMed] [Google Scholar]
- 29.Ramakrishnan MS, Eswaraiah A, Crombet T, Piedra P, Saurez G, Iyer H, Arvind AS. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs. 2009;1(1):41–48. doi: 10.4161/mabs.1.1.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hua YH, Ma SL, Fu ZF, Hu QY, Du LB, Jiang H. Effect of nimotuzumab on the radiation sensitivity of nasopharyngeal carcinoma cell line CNE-2. Chin J Zhejiang Med. 2011;33(6):836–839. [Google Scholar]
- 31.Li HM, Li P, Qian YJ, Wu X, Xie L, Wang F, Zhang H, Liu L. A retrospective paired study: efficacy and toxicity of nimotuzumab versus cisplatin concurrent with radiotherapy in nasopharyngeal carcinoma. BMC cancer. 2016;16(1):946–955. doi: 10.1186/s12885-016-2974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhai RP, Ying HM, Kong FF, Du CR, Huang S, Zhou JJ, Hu CS. Experience with combination of nimotuzumab and intensity-modulated radiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. OncoTargets Ther. 2015;8(12):3383–3390. doi: 10.2147/OTT.S93238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang JF, Zhang FZ, Zou QZ, Zhou LY, Yang B, Chun JJ, Yu JH, Zhang HW, Yuan XP, Tai GM. Induction chemotherapy followed by concurrent chemoradiation and nimotuzumab for locoregionally advanced nasopharyngeal carcinoma: preliminary results from a phase II clinical trial. Oncotarget. 2017;8(2):2457–2465. doi: 10.18632/oncotarget.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu ZG, Zhao Y, Tang J, Zhou YJ, Yang WJ, Qiu YF, Wang H. Nimotuzumab combined with concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a retrospective analysis. Oncotarget. 2016;7(17):24429–24435. doi: 10.18632/oncotarget.8225. [DOI] [PMC free article] [PubMed] [Google Scholar]