Abstract
The hematopoietic stem cell (HSC) niche supports steady-state hematopoiesis and responds to changing needs during stress and disease. The nervous system is an important regulator of the niche, and its influence is established early in development when stem cells are specified. Most research has focused on direct innervation of the niche, however recent findings show there are different modes of neural control, including globally by the central nervous system (CNS) and hormone release, locally by neural crest-derived mesenchymal stem cells, and intrinsically by hematopoietic cells that express neural receptors and neurotransmitters. Dysregulation between neural and hematopoietic systems can contribute to disease, however new therapeutic opportunities may be found among neuroregulator drugs repurposed to support hematopoiesis.
Interwoven Neural and Hematopoietic Systems
Hematopoietic stem cells (HSCs) (see Glossary) can reconstitute the entire blood and immune systems, making them a curative cell therapy for many blood cancers and diseases [1]. Although HSCs have been intensely studied for decades, and are the paradigm for stem cell biology, only recently have we started to appreciate the profound contribution of the bone marrow (BM) microenvironment, or stem cell niche, to the regulation of HSC fate and function. HSCs act as a reserve for the blood system, remaining dormant for months or years, and yet can rapidly respond to stress when needed [2]. HSCs asymmetrically divide to self-renew and simultaneously generate the downstream multipotent progenitors that produce the bulk of our blood cells [3]. During aging and disease, the microenvironment may contribute to changes observed in HSCs, including lineage bias and reduced chimerism upon transplantation [4]. A better understanding of the HSC niche has the potential to improve clinical transplantation protocols and blood disease management throughout life.
The BM microenvironment has been the subject of many recent reviews (e.g., [5,6]), however this review will focus specifically on the interaction between hematopoietic and neural systems. As the blood develops together with the vasculature, and vessels extend throughout the body, nerve fibers also follow the same paths, resulting in systems that are intimately intertwined [7]. These associations continue into adulthood, where hematopoietic cells reside in perivascular niches in the BM that are innervated by the peripheral nervous system (Figure 1) [8]. Neural regulation of the immune system has been well studied [9], and now there is increasing interest in how the neural and hematopoietic systems communicate. New studies have demonstrated that critical interactions occur between hematopoietic and neural cell lineages early in embryonic development [10]. Novel discoveries show neural regulation is not only via direct innervation of the niche, but also via broad release of neurotransmitters and neurohormones [11,12]. Furthermore, neural crest lineages can give rise to rare stromal cell populations that support HSCs in the embryo and adult [13]. In this review, we will discuss several conflicting studies that have looked at the role of neural regulation during the specification and emergence of HSCs in the embryo. Overall, the nervous system has emerged as another essential layer in the complex regulation of hematopoiesis and the stem cell niche.
Figure 1. Innervation of the Bone Marrow Niche.
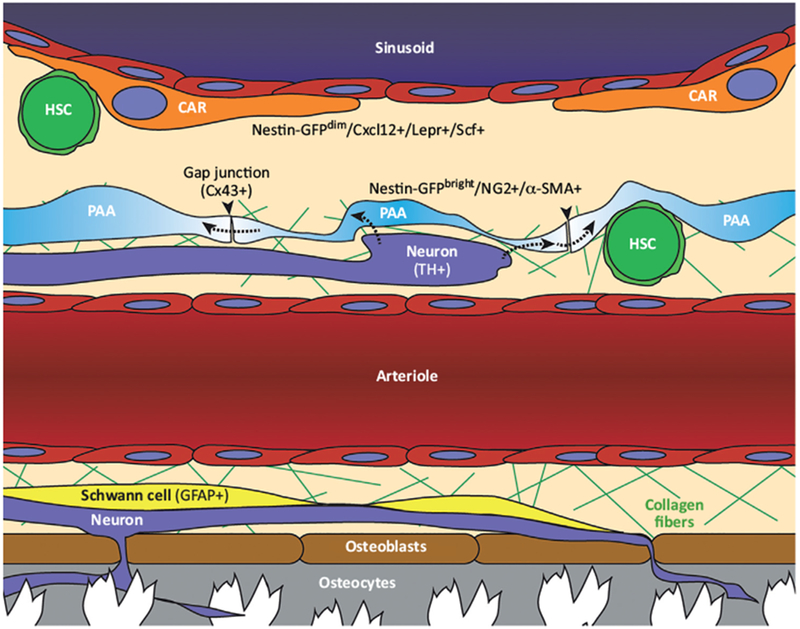
Most sympathetic (TH+) nerves reach arterioles and their surrounding pericytes. These pericytes (PAA cells/Nestin-GFPbright/NG2+/α-SMA+) ensheath arterioles and nerves. Pericytes are linked by connexin-43 (Cx43+) gap junctions (arrowheads) that have been proposed as conduits for neuronal signals that spread through an interconnected syncytial network (dashed arrows). Schwann cells (GFAP+) are also associated with arterioles. The arteriolar niche is rich with fine collagen fibers. Neurons extend into the bone where they contact osteoblasts and osteocytes. There is minimal innervation of sinusoidal vessels that are associated with reticular mesenchymal stromal cells (CAR cells/Nestin GFPdim/Cxcl12+/Lepr+/Scf+). CAR, CXCL12-abundant reticular; GFAP, glial fibrillary acidic protein; HSC, hematopoietic stem cell; PAA, periarterial adventitial; TH, tyrosine hydroxylase.
Revisiting the Anatomy of BM Niche Innervation
The earliest analyses of nerves in the BM were performed using photomicrographs and showed there was a typical pattern in all studied mammals: the BM is innervated by myelinated and nonmyelinated nerve fibers that generally parallel the vascular architecture [8,14]. Most efferent nerve terminals in the BM approach arterial smooth muscle, however the rest reach into regions known to support hematopoiesis (mostly arterioles and very rarely sinus walls or hematopoietic parenchyma) [8]. There is no clear evidence for nerve terminals abutting hematopoietic cells in the BM, as there is for lymphocytes in the spleen [15].
One group confirmed early photomicrograph data using electron microscopy and went further by proposing the existence of a ‘neuro-reticular complex’: a functional regulatory unit made up of nerve terminals and adjacent ‘periarterial adventitial (PAA) cells’ (Figure 2) [16]. They observed that PAA cells in the BM are attached to each other by gap junctions, have no apparent basement membrane, and are instead surrounded by filaments of collagen fiber. They described nerve terminals that were closely associated with the cytoplasmic extensions of PAA cells. They proposed the fascinating model that PAA cells may act as a syncytium that would be coupled in its response, even if only a few cells are innervated. This would work similarly to the rapid transmission of an impulse through a network of cardiomyocytes [17]. In this case, neural regulation of the HSC niche could be synchronized broadly, and only defined by the proximity of HSCs to the wider network of PAA cells. This model may extend to other innervated BM niche cells that have very long cytoplasmic processes, such as CXCL12-abundant reticular (CAR) cells [18] and osteocytes [19]. Indeed, this is supported by the finding that both mouse osteolineages [20] and human BM stromal cells [21] are linked by connexin-43 gap junctions, and the latter even forms a syncytium that is necessary for calcium conduction. This model of an interconnected network of syncytial BM niche cells, only innervated at specific points, challenges the assumption that proximity of a HSC to a nerve is predictive or necessary for regulation by the peripheral nervous system.
Figure 2. Electron Microscopy Studies Show Pericyte-like PAA Cells Wrapping Arterioles.

(A) Transmission electron microscopy of arterioles (a), capillaries, and periarterial adventitial (PAA)cells in the bone marrow of amouse femur. Transverse section of an arteriole near a sinus (S) and central vein (CV). Arrowheads indicate the thin cytoplasmic processes of PAA cells surrounding the arteriole and separating it from hematopoietic parenchyma. One nerve (N) contains 5 myelinated axons and 17 unmyelinated axons and is bounded by the layers of perineurium. (B) High-power view of a zonula adherens-type junction between PAA cells [dashed red box in (A)]. (C) Three-dimensional configuration of PAA cells as observed by scanning electron microscopy. Transverse fracture across the long axis of an arteriole near the central vein and sinus. The thin, veil-like cytoplasmic processes of the PAA cells (arrows) surround the smooth muscle cells of the arteriole. Between them, fine filaments of collagen fibers (arrowheads) are present. The PAA cells also cover a nerve in a sheath-like configuration. Images are adapted, with permission from, Yamazaki and Allen [16].
Molecular markers confirmed the early anatomical findings that sympathetic nerves, positive for tyrosine hydroxylase (TH), accompany vessels in the BM [22]. Two types of BM pericytes, marked by the Nestin-GFP transgenic reporter, are found localized to either arterioles (Nestin-GFPbright) or sinusoids (Nestin-GFPdim) [23], which is consistent with the model that there are distinct anatomical niches in the BM (Table 1) [24]. BM sinusoids and their associated mesenchymal stromal cells (i.e., Lepr+/Scf-GFP+/CAR cells) are poorly innervated compared with arterioles [8,18,25]. Conversely, Nestin-GFPbright cells that are also positive for the pericyte marker NG2 (a.k.a. CSPG4), and are primarily associated with arterioles near the endosteum, are directly innervated by TH+ sympathetic nerves [23,26,27]. Functional data has shown that the sympathetic nervous system (SNS) suppresses the proliferation of these Nestin-GFP+ cells [26]. Nonmyelinating Schwann cells, marked by glial fibrillary acidic protein (GFAP), surround these TH+ nerves and run the length of arterioles [23,24,28]. Based on their location near nerves and arterioles, and their thin ‘veil-like’ morphology, it is possible that PAA cells previously described by electron microscopy (Figure 2) [16], are in fact the more recently described Nestin-GFPbright pericytes [23].
Table 1.
Bone Marrow Niche Cell Types and Associated Markers, Categorized by Sinusoidal or Arteriolar Vessel Localization
| Cell type | Sinusoidal niche | Arteriolar niche |
|---|---|---|
| Mesenchymal stromal cells and pericytes | CXCL12-abundant reticular (CAR) cells [18] | PAA cells [16] |
| Nestin-GFP+ dim [23] | Nestin-GFP+ bright [23] | |
| LepR+ [25,27] | NG2+ [23,24,27] | |
| Scf+ [25,27] | Alpha-SMA+ [24] | |
| Endothelial cells | CD31+ [23,24] | CD31+ [23,24] |
| VE-cadherin [23,24] | VE-cadherin [23,24] | |
| LDL uptake [23,24] | Sca-1+ [23,24] | |
| Neurons | Rare [8] | TH+ [22,23,26,27] |
| Schwann cells | Rare [8] | GFAP+ [23,24,28] |
The significance of HSC position relative to these innervated pericytes is controversial. One group found that HSCs (CD150+ CD48- CD41- Lineage- cells) were significantly closer to arterioles (and their associated nerves, GFAP+ Schwann cells, and NG2+ pericytes), but not sinusoids or a test set of randomly distributed dots [23]. Many HSCs (CD150+ CD48- CD41- Lineage- cells) have been found to be associated with GFAP+ Schwann cells, which are proposed to be an important BM niche cell type that ensheathes sympathetic nerves and promotes HSC quiescence by secretion of TGF-β protein [28]. Using analysis of two-dimensional BM sections, these studies placed HSCs in close proximity to the innervated arteriolar niche, with 15% of HSCs in direct contact with arterioles [23], and 23% in contact with GFAP+ Schwann cells [28]. Another group developed a different labeling and computational approach for HSC analysis that accounted for the entire three-dimensional space of the BM, and found that HSCs (α-catulin-GFP+ c-kit+ cells) were not significantly closer to arterioles or nerves [29]. However, this group did acknowledge that GFAP+ Schwann cells could be a source of a diffusible regulatory signal, such as TGF-β, that could act from a distance [28,29]. These conflicting positional data must be resolved and considered in the context of functional BM niche innervation data, as discussed below..
Resolving the Role of the Nervous System in Regulation of the HSC Niche
After decades of anatomical descriptions showing innervation of the BM niche, the functional role of the SNS in regulating hematopoiesis has been more difficult to discern. Many studies have explored SNS function in the BM niche by using surgical, chemical, or genetic denervation models. Surgical denervation has produced conflicting results, although direct comparison between studies is difficult because of different conditions and genetic backgrounds. For example, in one study BM cellularity was decreased, with fewer BM progenitors, but more circulating progenitors [30]. In another study, there was no change in BM cellularity or BM progenitor numbers [31]. In a third study, there was no change in BM cellularity, but there was a decrease in bona fide BM HSCs [28]. This latter study is one of the few to suggest the SNS has a role in regulating steady-state HSC numbers.
Bone tissue itself is an important functional component of the HSC niche and is regulated by the SNS. Early BM photomicrographs detected innervation of osteoblasts [32], and later molecular analysis confirmed sympathetic nerves reach osteoblasts and osteocytes that express functional β2-adrenergic target receptors [33,34]. The SNS suppresses bone-forming osteoblasts and activates bone-resorbing osteoclasts [35], promoting mobilization of HSCs from the niche by lowering levels of the attractive chemokine CXCL12 [36], and increasing release of proteolytic enzymes [37], respectively. Bone turnover also increases extracellular calcium levels and HSCs express a calcium-sensing receptor that is required for localization to the endosteal niche [38]. Treatment with granulocyte-colony stimulating factor (G-CSF), used clinically to promote hematopoietic stem and progenitor cell (HSPC) mobilization, produced striking changes in the osteocyte network, and surgical denervation showed these changes were dependent on SNS signaling to the bone [34].
Chemical sympathectomy using the neurotoxin 6-hydroxydopamine (6-OHDA) has produced slightly different results than surgical denervation. Most studies found there was no change in steady-state BM progenitor or HSC numbers, however there was impaired trafficking and mobilization of HSCs [30,31,36,39,40]. Furthermore, chemical denervation, or neuropathy resulting from chemotherapy (e.g., cisplatin), further delayed the recovery of transplant recipients, and reduced survival after challenge with 5-fluorouracil (5FU) [40]. Interestingly, it was also found that the SNS may regulate the size of the niche, as SNS signal disruption (by 6-OHDA denervation or β-blockade) increased the number of Nestin-GFP+ cells and endothelial cells, and impaired recovery of these niche cells after challenge [40]. However, in another study surgical denervation did not produce any measurable change in niche endothelial cells, mesenchymal stem cells (MSCs), or osteoblasts [28]. How the SNS regulates the supportive cells of the niche will need to be explored in more depth. A very compelling study of patients with spinal cord injury found that progenitors taken from decentralized BM had reduced longterm colony formation potential, providing evidence in humans that there is a role for SNS regulation of the BM niche [41]. In summary, most studies support the conclusion that the SNS has an important role in HSC trafficking and regeneration of the BM niche after challenge, however a role for the SNS in steady-state HSC maintenance has not been well-defined [30,31,36,39,40,42,43].
Telling Time in the Niche: Circadian Rhythms and HSC Trafficking
In one of the earliest functional experiments to demonstrate neural regulation of hematopoiesis, one group performed chemical sympathectomy on BM transplant recipient mice using 6-OHDA [43]. Consistent with a role for the SNS in regulating hematopoietic trafficking, this resulted in a significant increase in the number of peripheral blood leukocytes, which could be mimicked by the α1-adrenoceptor antagonist prazosin. They also made the additional striking observation that when mice were kept under continuous 24-hour lighting, there was even greater dysregulation of blood leukocyte numbers, with or without 6-OHDA sympathectomy. This finding was important because the rhythmicity of the circadian clock in mammals is regulated by periodic exposure to light and dark cycles [44]. It was later discovered that under normal physiological conditions, HSC trafficking follows a rhythmic pattern that is influenced by the circadian clock (Box 1) [39].
Box 1. The Clock Is Ticking: Circadian Rhythms and HSC Trafficking.
Intriguingly, HSC trafficking between the niche and peripheral blood follows a daily light and dark cycle. Photic cues travel along the retinal-hypothalamic tract to reach the suprachiasmatic nucleus(SCN)that is the central clock located in the anterior hypothalamus [44]. The asynchronous expression of certain ‘core’ or ‘molecular clock’ genes, such as Arntl (a.k.a. Bmal1) and Clock, within the SCN and peripheral tissues regulates the expression of other target genes, such as Per1, thereby generating feedback loops [79,80]. The rhythmic release of HSCs in mice has been shown to peak 5 hours after induction of light (5ZT, Zeitgeber time) and trough at 17 hours (17ZT) when mice are kept under normal 12:12 hour light-dark conditions (12LD). Altering circadian timing by exposing animals to continuous light or to a 12-hour shift (i.e., ‘jet lag’) disrupts the rhythmicity of HSC release, providing further evidence that HSC trafficking is entrained by light [39]. This pattern is asynchronous to the cycling levels of CXCL12 ligand, with low levels of CXCL12 in the niche corresponding to high numbers of circulating HSCs [39]. In fact, it is not only CXCL12 levels in the niche that change with light cycles, but CXCR4 levels on HSCs also fluctuate so they are synchronized with CXCL12 to optimize mobilization [81]. Furthermore, treatment with mobilizing agents such as G-CSF [82,83], or the CXCR4 antagonist AMD3100 [84], elicited significantly higher HSC recovery at 5ZT than at 13ZT [81]. This suggested that synchronizing blood apheresis in the clinic with circadian timing could enhance HSC recovery from transplant donors [81]. Another interesting observation that came out of these studies was that peak HSC mobilization corresponds to the beginning of the rest period for a given species, which is early evening for humans and early morning for mice. A fascinating recent study extended these findings by using a humanized mouse model generated by intrahepatic injection of human fetal liver CD34+ HSPCs into irradiated neonatal NOD-SCID IL-2Rγ−/− (NSG) mice [85]. This group found that xenotransplanted human HSPCs had inverted daily oscillations compared with the endogenous HSPCs in the same recipient mouse. Mechanistically, this resulted from the opposite effects of stress-kinase on reactive oxygen species levels, which increased in mouse cells and decreased in human cells, followed by differential downstream regulation of hypoxia-inducible factor 1α (HIF-1α) expression. The knowledge that daily cyclic HSC mobilization is not only regulated by the genetic clock, but also by cellular stress and metabolism, could lead to a better understanding of tissue repair and regeneration.
Although an association had been found between HSC migration patterns and circadian rhythms, a mechanism to directly connect photic cues to BM niche regulation had not been determined. Elegant experiments demonstrated that the SNS regulates the rhythmic release of norepinephrine from nerve terminals that innervate the BM niche. This in turn modulates Cxcl12 expression levels by acting on the β3-adrenergic receptors expressed on stromal cells [39,45]. HSC retention in the niche is regulated by the CXCL12-CXCR4 signaling axis, with CXCL12 ligand expressed on niche cells and CXCR4 receptor expressed on HSCs [18,46–48]. Both physiological and G-CSF-enforced mobilization of progenitor cells depends on the SNS-mediated downregulation of Cxcl12 levels [36,39]. β3-Adrenergic receptor activation was shown to alter Cxcl12 expression levels by mediating degradation of the transcription factor Sp1, an upstream activator of Cxcl12 [39]. In Bmal1−/− mice that have impaired circadian rhythms, the number of circulating progenitors and Cxcl12 levels did not follow a daily cycle, indicating these oscillations were entrained by light [39]. Rhythmic fluctuations in circulating progenitor numbers and Cxcl12 mRNA levels within the BM could be similarly disrupted by surgical denervation or 6-OHDA sympathectomy [39]. Together, these studies demonstrate there is a mechanism that connects photic cues to the HSC niche via adrenergic signals delivered by SNS nerves. Furthermore, these adrenergic signals have the effect of changing the adhesive properties of the niche by changing CXCL12 levels on stromal cells, and can therefore regulate the mobilization of HSCs.
Blood Disorders Reveal Conflict between the HSC Niche and Nervous System
Disease models have shown how neuropathy can be intrinsically linked with dysregulation of hematopoiesis [40,49]. For example, in a MLL-AF9 acute myelogenous leukemia (AML) model, chemical denervation increased BM infiltration of transplanted leukemic cells and decreased survival of recipients [50]. This study also showed that AML infiltration in normal BM reduced TH+ nerve fiber density, suggesting leukemic cells may reinforce the disease state by inducing sympathetic neuropathy. The SNS is also involved in disease progression in myeloproliferative neoplasms (MPNs). Many patients with this disease have a V617F mutation of Janus kinase 2 (JAK2) in HSCs that leads to uncontrolled cell expansion [51–54]. Surprisingly, these JAK2(V617F) mutant HSCs produce excessive interleukin (IL)-1β that is damaging to BM neurons and Schwann cells [49]. This in turn leads to reduction of Nestin-GFP + MSCs that produce CXCL12 and support healthy HSCs. Protecting sympathetic nerve fibers or restoring β3-adrenergic signaling using small molecules, essentially rescuing a healthy microenvironment, had the effect of blocking MPN progression. These studies support the emerging concept that malignancy can corrupt the BM microenvironment to favor itself over healthy HSCs [55–60]. There is now evidence that treatments to restore the BM niche could complement conventional chemotherapy and ultimately lead to better patient outcomes [40,49,55].
Systemic Regulation of HSCs via the Nervous System
Until recently, neural regulation of the BM niche has primarily focused on direct innervation by peripheral nerves. However, recent studies have shown there are mechanisms employed by the central nervous system (CNS) to systemically regulate hematopoiesis during development and in the adult [11,12]. This is significant because it demonstrates there is global control of the entire HSC pool, regardless of local microenvironment or proximity to peripheral nerves that reach into the BM. Conceptually, this was first demonstrated by systemic hormones, such as estrogen and parathyroid hormone, that regulate HSCs directly via surface receptors or indirectly via modulation of niche cells (for a review see [61]). More recently, it was shown that glucocorticoid (GC) hormones promote HSC migration [12]. Interestingly, this signal originates in the brain and regulates HSCs in the BM via the hypothalamic-pituitary-adrenal (HPA) axis. The corticosterone nuclear hormone receptor Nr3c1 is expressed throughout the BM, but is required cell autonomously on the surface of HSCs for G-CSF-induced mobilization.
Intriguingly, human CD34+ progenitors themselves express many classes of neuroreceptors, including adrenergic, dopamine, serotonin, and GABAB receptors [62–64]. The neurotransmitters epinephrine and norepinephrine, as well as dopamine, have been shown to act directly on CD34+ progenitors via receptor activation on the cell surface [63]. HSPCs also express receptors for neurotrophic factors such as GDNF [65] and cannabinoids [66]. Another signal that is important for hematopoiesis is the monoamine neurotransmitter serotonin, or 5-hydroxytryptamine (5-HT) [67]. Serotonin expands human cord blood CD34+ HSPCs ex vivo and increases engraftment in nonobese diabetic (NOD)/severe combined immunodeficient (SCID) recipients [68]. Explants of aorta-gonad-mesonephros (AGM) cultured in the presence of serotonin or fluoxetine (a selective serotonin reuptake inhibitor), produced more colonies in colony forming unit culture and spleen assays [67]. Serotonin and fluoxetine were also identified in a zebrafish embryo screen for increased HSPC production in the dorsal aorta [69]. Together, these results suggest serotonin is not only a positive regulator of HSPCs, but may also be involved in HSPC production during development, although some of these results are controversial (Box 2).
Box 2. Serotonin and HSCs: Generating Controversy or Feelings of Well-Being?
Conflicting models support either a global or local signaling effect for serotonin during HSPC emergence in the embryo. Serotonin is synthesized by the enzyme tryptophan hydroxylase (Tph) into two forms, Tph1 which acts predominantly in the periphery, and Tph2 that functions centrally in the CNS [86]. However, Tph1 is also expressed in human brain and pituitary [87], and Tph2 has been detected in the AGM of mouse [67] and zebrafish [88]. In addition, other proteins required for serotonin biosynthesis and serotonergic neuron formation are expressed in the AGM; for example, dopa decarboxylase [DDC; a.k.a. aromatic amino acid decarboxylase (AADC or AAAD)], and the ETS family transcription factor Fev (a.k.a. Pet1) [67]. Interestingly, the zebrafish homolog of Fev was recently shown to have a role in zebrafish HSPC development [89]. In the mouse, endogenous serotonin was detected in the AGM, and reduced serotonin levels in Tph2 mutant mouse embryos correlated with reduced HSPC numbers. Endothelial-specific deletion of Tph2 demonstrated that the hemogenic endothelium of the dorsal aorta is the main source of serotonin during HSPC specification [67]. Furthermore, AGM explants treated ex vivo with serotonin or the selective serotonin reuptake inhibitor fluoxetine have increased HSPCs numbers [67]. Together, these data all support a local effect model [67]. However, it has been argued that serotonin exerts a global effect during HSPC emergence in the zebrafish embryo. Contrary to the studies above, another group found there was no expression of zebrafish homologs tph1a, tph1b, or tph2 in the AGM, and these genes were only found in the nervous system [11]. When these genes were knocked down, HSPC production in the AGM was still reduced, suggesting that the positive effect of serotonin must not be in the periphery. It was proposed that serotonin must work via the CNS because it still had a positive effect when peripheral adrenergic and dopaminergic signaling were disrupted (i.e., with 6-OHDA and nepicastat treatment) [11]. However, that model may be interpreted differently, given that catecholamines and serotonin work via different signaling pathways. That aside, the study did show that serotonin positively regulates the HPA system, and that global HPA and GC activation are required for serotonin-dependent stimulation of HSPCs [11]. This is consistent with a systemic role for GC signaling in regulating HSPCs, as was shown in the adult mouse BM [12]. Lastly, there are many non-neural sources of serotonin in the body [90], and it will be necessary to evaluate the importance of these for HSC regulation.
Neural Crest Contribution to the HSC Niche
Determining the developmental origins of the various cell types in the highly heterogeneous BM microenvironment is challenging. However, knowledge of common origins can provide insight into how related niche cells communicate and how they may be differentiated in vitro for regenerative medicine and tissue engineering. It was recently shown that a subset of stromal support cells in the niche are MSCs derived from the neural crest [13]. The neural crest is made up of cells that delaminate from the dorsal aspect of the neural tube early in development, are highly migratory, and contribute to many different tissues throughout the body [70]. The neural crest gives rise to a diverse complement of lineages, including bone, cartilage, connective tissues, glia, neurons, and melanocytes.
Using lineage tracing, neural crest-derived MSCs have been found to contribute to and help establish the fetal BM niche [13]. These Nestin-GFP+ MSCs share a common origin with sympathetic peripheral neurons and glial cells, but are distinct from other MSCs that give rise to osteoblasts and chondrocytes. There are two populations of Nestin-GFP+ MSCs in the fetal BM that contribute to either HSC-supportive stroma (Pdgfra+) or glial Schwann cell precursors (Pdgfrα-). As further evidence of the intricate development of the BM niche and nervous system, Nestin-GFP+ MSCs use the Erb-B2 Receptor Tyrosine Kinase 3 [ERBB3 receptor; a member of the epidermal growth factor receptor (EGFR) family], to migrate along developing peripheral nerves to promote establishment of the fetal BM niche [13]. Secretion of the chemokine CXCL12 by Nestin-GFP+ MSCs is functionally required for HSC colonization of the nascent BM niche [13]. In the adult, neural crest-derived Nestin-GFP+ cells likely make up only a small population of total Nestin-GFP+ MSCs that contribute to the BM niche [26]. Together, these lineage tracing studies have shown that a subset of BM MSCs share a common origin with peripheral sympathetic nerves and Schwann cells, and that these stromal cells have a unique ability to communicate and coordinate with the nervous system [13].
Neural crest cells also interact with HSCs at the earliest stages of specification. We know that HSCs emerge directly from the hemogenic endothelium of the dorsal aorta [71–73], however we do not have a complete knowledge of the signals and cellular interactions required for HSC specification. Trunk neural crest cells in the zebrafish have been found to undergo ventromedial migration until they are in close contact with the dorsal aorta prior to HSC emergence (Figure 3A,B) [10]. In the same study, it has also been demonstrated that neural crest cell migration to the dorsal aorta is dependent on Pdgf receptor signaling. Blockade of Pdgfrα signaling prevents neural crest cells from reaching the dorsal aorta and leads to disruption of HSC specification. The specific signals produced by neural crest cells that contribute to HSC specification must still be determined.
Figure 3. Neural Crest Derivatives are Found Adjacent to the Site of HSC Emergence in the Dorsal Aorta of Mouse and Zebrafish.

(A,B) Zebrafish transgenic reporter showing neural crest (red) and endothelium (green). (A) Cells derived from the neural crest contact the ventral wall of the dorsal aorta. Transverse confocal section of midtrunk region at 24 hours postfertilization (hpf); dorsal up, ventral down; white arrowhead indicates pigment cell lineages migrating ventrolaterally. Scale bars: 50 microns. (B) Zoomed region shown in white box in (A) with yellow arrowhead indicating a neural crest cell between the dorsal aorta (da) and posterior cardinal vein (pcv). (C) E11.5 mouse embryo showing sympathoadrenal neural crest-derived cells (green) adjacent to the ventral wall of the dorsal aorta (red). Lateral confocal Z-stack image (anterior left, posterior right). no, Notochord; nt, neural tube; sg, sympathetic ganglia. Images are adapted with permission from: (A,B) Damm and Clements [10]; (C) Lumb and Schwarz [75].
Catecholamine neurotransmitters may be one of the signals from neural crest derivatives that contribute to HSC production in the dorsal aorta, however contradictory results from mouse and zebrafish have yet to be resolved [10,11,74]. In mouse studies, TH expression is present during HSC specification in the mouse AGM region at embryonic day (E) 11.5 (Figure 3C) [74]. One study found that HSCs were reduced in Gata3 null embryos, and this was not because of a cell autonomous role for Gata3 in HSCs, but instead the result of Gata3-dependent loss of TH regulation in surrounding nonhematopoietic tissues. Gata3 and TH are expressed in neural crest-derived sympathoadrenal progenitors and sympathetic ganglia that are adjacent to the AGM [75]. This study also showed that after testing for expression of several adrenergic receptors on the surface of HSCs, only the β2-adrenergic receptor was expressed, which is also true of human CD34+ cord blood and mobilized HSPCs [63,74]. Together, these results suggest that catecholamine neurotransmitters produced by SNS tissues surrounding the mammalian dorsal aorta signal directly to HSCs via β2-adrenergic receptors and play a role in their specification.
Given the above results in mouse, another group wanted to determine if the neural crest-derived cells they found adjacent to the dorsal aorta in zebrafish (Figure 3A,B) were also a source of catecholamines for HSPC specification [10]. In zebrafish, the th and dbh genes encode TH and dopamine β-hydroxylase (DBH) enzymes that produce catecholamines and mark differentiated catecholaminergic neurons. However, during early HSPC specification stages [~12–36 hours postfertilization (hpf)], th and dbh are only expressed in a few neurons in the head [10], and robust protein expression is not detected until 48 hpf [76]. This means th and dbh are not expressed in the region of the dorsal aorta during HSPC specification. Knockdown of th resulted in no detectable change of HSPC marker expression in the dorsal aorta at 36 hpf [10]. Another group also knocked down th [11], and looking at later stages (~72 hpf), found HSPC marker expression was reduced in the caudal hematopoietic tissue (CHT), the zebrafish equivalent of the mammalian fetal liver [77,78]. Similar results were obtained by treating embryos with 6-OHDA and the DBH inhibitor nepicastat that antagonizes catecholaminergic signaling [11]. To summarize these zebrafish results, the catecholamine synthesis enzymes TH and DBH are not expressed near the dorsal aorta during HSPC specification stages, and TH is not required for HSPC specification. However, after HSPC emergence from the dorsal aorta, there appears to be a role for catecholamines in expansion of HSPCs in the CHT. Although there are differences between mouse and zebrafish regarding the role of catecholamines during HSPC specification, both model systems provide evidence that these neurotransmitters are positive regulators of HSPCs during development.
Concluding Remarks and Future Perspectives
We now have unprecedented genetic tools and microscopy techniques to functionally dissect the HSC microenvironment. Decades old anatomical data is converging with live imaging of the BM niche to reveal the specific locations, cellular behaviors, and dynamic responses of endogenous HSCs. We are moving closer to defining the functional unit of the HSC niche and where these discrete locations exist within the larger BM tissue. Although BM innervation was well described, only recently have we started to appreciate the complexity of HSC regulation by the nervous system. Peripheral nerves directly contact stromal cells, and via adrenergic signaling, control adhesion of HSCs to the niche. In addition, this system is connected to photic cues and therefore circadian rhythms. This timed daily release of HSCs into the circulation may be very important for tissue repair and regeneration. Stromal cells in the niche are not the only direct contacts with the nervous system, and innervation of arteriolar pericytes that create an interconnected syncytial network could provide a means of long-range signal transmission.
The concept of neural regulation of HSCs has expanded to include systemic neurotransmitters and hormones produced by the CNS and non-neuronal peripheral tissues. More recently, studies have found ontologically similar neural tissues that are incorporated into sites of hematopoiesis as various supportive niche cell types (e.g., peripheral nerves, Schwann cells, MSCs). Key future questions will include how the central and peripheral nervous systems coordinate to regulate HSCs. Also, how neurotransmitters exert their influence, either through neuronal-stromal cell contacts, or directly via cell surface neuroreceptors on HSCs, will answer some very important questions (see Outstanding Questions). Hopefully, our better understanding of the hematopoietic-neural interface will lead to transformative therapies for blood disease, possibly by novel application of neuromodulator drugs to improve HSC transplantation and regeneration of the BM niche.
Outstanding Questions.
Is HSC position relative to specific niche cell types important? Should this question be re-evaluated given that long-range signals may be transmitted via extended stromal cell projections, or a syncytial network of pericytes?
How does the nervous system regulate the size of the HSC niche? Does it globally control proliferation and regeneration, or innervate specific supportive cell types?
What is the role of neurotransmitters during specification of HSCs in the hemogenic endothelium of the embryo? Is their influence primarily global, local via peripheral nerves, or from non-neural sources?
What is the functional significance of neuroreceptors expressed on the surface of HSCs? What signals do they receive, given there is little evidence for direct peripheral nerve contact with BM HSCs?
To what extent is signaling integrated between niche cell types with shared ontogeny, such as peripheral nerves, Schwann cells, and MSCs? Also, what are the molecular and cellular determinants?
Highlights.
There are many ways in which the nervous system interacts with HSCs during development and into adulthood.
In the adult, neural regulation is thought to primarily influence HSC mobilization and regeneration after injury to the niche.
Our understanding of neural regulation of HSCs has extended beyond direct innervation of the niche by peripheral nerves, and now includes systemic signals controlled by the CNS and neural crest lineages that contribute to the niche.
Global signals that facilitate neural regulation of HSCs include serotonin and glucocorticoid hormones, both in the embryo during specification and in the adult BM.
Neural crest lineages contribute to sites of hematopoiesis, including the hemogenic endothelium in the embryo where HSCs first emerge, and to the fetal and adult BM microenvironment.
Acknowledgments
This work was supported by a Junior Faculty Scholar Award from the American Society of Hematology (ASH), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under award number K01DK103908.
Glossary
- Acute myelogenous leukemia (AML)
blood cancer with increased defective myeloid cells in the bone marrow; also called acute myeloid leukemia.
- Aorta-gonad-mesonephros (AGM)
region of embryo, including the dorsal aorta and its ventral wall, that is made up of hemogenic endothelium and gives rise to HSCs.
- Catecholamine
monoamine neurotransmitters including epinephrine (adrenaline), norepinephrine (noradrenaline), and dopamine.
- CXCL12
chemokine (C-X-C Motif) Ligand 12 [Stromal Cell-Derived Factor 1 (SDF-1)] chemokine protein that binds CXCR4 receptor and is required for HSC maintenance in the bone marrow niche.
- CXCL12-abundant reticular (CAR) cells
fibroblastic support cells in the bone marrow niche that contact blood vessels and hematopoietic cells.
- CXCR4
chemokine (C-X-C Motif) Receptor 4, a receptor for CXCL12 that is required for HSC homing to the bone marrow niche.
- Decentralized
describes bone marrow that is below the level of spinal cord injury and lacks normal supraspinal activity.
- Efferent nerve terminals
the termination of nerves that transmit signals from the central nervous system to limbs and organs.
- Endosteum
tissue that lines the inner surface of bone.
- 5-Fluorouracil (5FU)
nucleoside metabolic inhibitor used to ablate proliferating cells while inducing endogenous HSCs to repopulate the bone marrow.
- Glucocorticoid (GC) hormones
adrenal steroid hormones that control a variety of physiological processes.
- Granulocyte-colony stimulating factor (G-CSF)
glycoprotein that stimulates bone marrow production of granulocytes and releases HSCs into the bloodstream.
- Hematopoietic stem cells (HSCs)
blood stem cells that can engraft long term, self-renew, and give rise to all blood lineages.
- Hematopoietic stem and progenitor cells (HSPCs)
population that is less well-defined than HSCs, including both stem cells and more differentiated progenitors.
- 6-Hydroxydopamine (6-OHDA)
neurotoxin that selectively destroys dopaminergic and noradrenergic neurons.
- Hypothalamic-pituitary-adrenal (HPA) axis
neuroendocrine system made up of hypothalamus, pituitary gland, and adrenal glands that regulates many body processes.
- Mesenchymal stem cells (MSCs)
multipotent stromal cells that can give rise to bone, cartilage, and fat cells.
- Mobilization
movement of stem cells from the bone marrow into the blood.
- Myelinated
refers to nerve fibers that are surrounded by a myelin sheath derived from Schwann cells.
- Myeloproliferative neoplasms (MPNs)
blood disease characterized by overproduction of white or red blood cells, or platelets.
- Osteoblast
mesenchymal cell involved in bone formation.
- Osteoclast
a bone cell that absorbs bone tissue.
- Osteocyte
osteoblast-derived bone cell embedded in matrix.
- Pericytes
broadly defined perivascular support cells that surround blood vessels.
- Peripheral nervous system
made up of the nerves and ganglia outside of the central nervous system.
- Schwann cells
glial cells that support and myelinate axons of the peripheral nervous system; there are also nonmyelinating Schwann cells.
- Stem cell niche
anatomical location of support cells that maintain and regulate stem cells.
- Sympathectomy
surgical cutting of sympathetic nerve or ablation by neurotoxin.
- Sympathetic nervous system (SNS)
part of autonomic nervous system (also includes parasympathetic nervous system) that regulates homeostasis and prepares body for physical and mental activity.
- Syncytium
referring to a tightly bound network of cells that distributes an excitatory signal from one cell to others through interconnections; often used to describe cardiac muscle.
References
- 1.Muller AM et al. (2016) Hematopoietic stem cells in regenerative medicine: astray or on the path? Transfus. Med. Hemother 43, 247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prendergast AM and Essers MA (2014) Hematopoietic stem cells, infection, and the niche. Ann. N. Y. Acad. Sci 1310, 51–57 [DOI] [PubMed] [Google Scholar]
- 3.Pham K et al. (2014) Polarized cells, polarized views: asymmetric cell division in hematopoietic cells. Front. Immunol 5, 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovtonyuk LV et al. (2016) Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenviron-ment. Front. Immunol 7, 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane GM et al. (2017) Adult haematopoietic stem cell niches. Nat. Rev. Immunol 4, 7 [DOI] [PubMed] [Google Scholar]
- 6.Wei Q and Frenette PS (2018) Niches for hematopoietic stem cells and their progeny. Immunity 48, 632–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melani M and Weinstein BM (2010) Common factors regulating patterning of the nervous and vascular systems. Annu. Rev. Cell Dev. Biol 26, 639–665 [DOI] [PubMed] [Google Scholar]
- 8.Calvo W (1968) The innervation of the bone marrow in laboratory animals. Am. J. Anat 123, 315–328 [DOI] [PubMed] [Google Scholar]
- 9.Mignini F et al. (2003) Autonomic innervation of immune organs and neuroimmune modulation. Auton. Autacoid Pharmacol 23, 1–25 [DOI] [PubMed] [Google Scholar]
- 10.Damm EW and Clements WK (2017) Pdgf signalling guides neural crest contribution to the haematopoietic stem cell specification niche. Nat. Cell Biol 19, 457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwan W et al. (2016) The central nervous system regulates embryonic HSPC production via stress-responsive glucocorticoid receptor signaling. Cell Stem Cell 19, 370–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce H et al. (2017) Cholinergic signals from the CNS regulate G-CSF-mediated HSC mobilization from bone marrow via a glucocorticoid signaling relay. Cell Stem Cell 20, 648–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isern J et al. (2014) The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. eLife 3, e03696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawahara G and Osada M (1964) Studies on the innervation of bone marrow with special reference to the intramedullary nerve fibers in dog and goat. Arch. Histol. Jpn 24, 471–487 [DOI] [PubMed] [Google Scholar]
- 15.Felten SY and Olschowka J (1987) Noradrenergic sympathetic innervation of the spleen: II: Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J. Neurosci. Res 18, 37–48 [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki K and Allen TD (1990) Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: The “neuroreticular complex”. Am. J. Anat 187, 261–276 [DOI] [PubMed] [Google Scholar]
- 17.De Mello WC (2016) Exchange of chemical signals between cardiac cells. Fundamental role on cell communication and metabolic cooperation. Exp. Cell Res 346, 130–136 [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama T et al. (2006) Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988 [DOI] [PubMed] [Google Scholar]
- 19.Rouleau MF et al. (1990) Characterization of the major parathyroid hormone target cell in the endosteal metaphysis of rat long bones. J. Bone Miner. Res 5, 1043–1053 [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Nieto D et al. (2012) Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood 119, 5144–5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schajnovitz A et al. (2011) CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat. Immunol 12, 391–398 [DOI] [PubMed] [Google Scholar]
- 22.Tabarowski Z et al. (1996) Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem. 98, 453–457 [DOI] [PubMed] [Google Scholar]
- 23.Kunisaki Y et al. (2013) Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itkin T et al. (2016) Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding L et al. (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Méndez-Ferrer S et al. (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asada N et al. (2017) Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol 19, 214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki S et al. (2011) Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158 [DOI] [PubMed] [Google Scholar]
- 29.Acar M et al. (2015) Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afan AM et al. (1997) Bone marrow innervation regulates cellular retention in the murine haemopoietic system. Br. J. Haematol 98, 569–577 [DOI] [PubMed] [Google Scholar]
- 31.Benestad HB et al. (1998) No neuronal regulation of murine bone marrow function. Blood 91, 1280–1287 [PubMed] [Google Scholar]
- 32.Takase B and Nomura S (1957) Studies on the innervation of the bone marrow. J. Comp. Neurol 108, 421–443 [DOI] [PubMed] [Google Scholar]
- 33.Takeda S et al. (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 [DOI] [PubMed] [Google Scholar]
- 34.Asada N et al. (2013) Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell 12, 737–747 [DOI] [PubMed] [Google Scholar]
- 35.Kondo H et al. (2005) Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J. Biol. Chem 280, 30192–30200 [DOI] [PubMed] [Google Scholar]
- 36.Katayama Y et al. (2006) Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 [DOI] [PubMed] [Google Scholar]
- 37.Kollet O et al. (2006) Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med 12, 657–664 [DOI] [PubMed] [Google Scholar]
- 38.Adams GB et al. (2006) Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 [DOI] [PubMed] [Google Scholar]
- 39.Méndez-Ferrer S et al. (2008) Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447 [DOI] [PubMed] [Google Scholar]
- 40.Lucas D et al. (2013) Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat. Med 19, 695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iversen PO et al. (2000) Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood 96, 2081–2083 [PubMed] [Google Scholar]
- 42.Maestroni GJ and Conti A (1994) Modulation of hematopoiesis via alpha 1-adrenergic receptors on bone marrow cells. Exp. Hematol 22, 313–320 [PubMed] [Google Scholar]
- 43.Maestroni GJ et al. (1992) Effect of adrenergic agents on hematopoiesis after syngeneic bone marrow transplantation in mice. Blood 80, 1178–1182 [PubMed] [Google Scholar]
- 44.Honma S (2018) The mammalian circadian system: a hierarchical multi-oscillator structure for generating circadian rhythm. J. Physiol. Sci 68, 207–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Méndez-Ferrer S et al. (2010) Cooperation of β2- and β3- adrenergic receptors in hematopoietic progenitor cell mobilization. Ann. N. Y. Acad. Sci 1192, 139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ara T et al. (2003) Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19, 257–267 [DOI] [PubMed] [Google Scholar]
- 47.Greenbaum A et al. (2013) CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding L and Morrison SJ (2013) Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arranz L et al. (2014) Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512, 78–81 [DOI] [PubMed] [Google Scholar]
- 50.Hanoun M et al. (2014) Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell 15, 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James C et al. (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434, 1144–1148 [DOI] [PubMed] [Google Scholar]
- 52.Kralovics R et al. (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med 352, 1779–1790 [DOI] [PubMed] [Google Scholar]
- 53.Levine RL et al. (2005) Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and mye-loid metaplasia with myelofibrosis. Cancer Cell 7, 387–397 [DOI] [PubMed] [Google Scholar]
- 54.Baxter EJ et al. (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders.Lancet 365,1054–1061 [DOI] [PubMed] [Google Scholar]
- 55.Duarte D et al. (2018) Inhibition of endosteal vascular niche remodeling rescues hematopoietic stem cell loss in AML. Cell Stem Cell 22, 64–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sipkins DA et al. (2005) In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colmone A et al. (2008) Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 322, 1861–1865 [DOI] [PubMed] [Google Scholar]
- 58.Ishikawa F et al. (2007) Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat. Biotechnol 25, 1315–1321 [DOI] [PubMed] [Google Scholar]
- 59.Hérault A et al. (2017) Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature 544, 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hawkins ED et al. (2016) T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature 538, 518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffman CM and Calvi LM (2014) Minireview: complexity of hematopoietic stem cell regulation in the bone marrow microenvironment. Mol. Endocrinol 28, 1592–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steidl U et al. (2004) Primary human CD34+ hematopoietic stem and progenitor cells express functionally active receptors of neuromediators. Blood 104, 81–88 [DOI] [PubMed] [Google Scholar]
- 63.Spiegel A et al. (2007) Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat. Immunol 8, 1123–1131 [DOI] [PubMed] [Google Scholar]
- 64.Zangiacomi V et al. (2009) Human cord blood-derived hematopoietic and neural-like stem/progenitor cells are attracted by the neurotransmitter GABA. Stem Cells Dev. 18, 1369–1378 [DOI] [PubMed] [Google Scholar]
- 65.Fonseca-Pereira D et al. (2014) The neurotrophic factor receptor RET drives haematopoietic stem cell survival and function. Nature 514, 98–101 [DOI] [PubMed] [Google Scholar]
- 66.Galve-Roperh I et al. (2013) Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res 52, 633–650 [DOI] [PubMed] [Google Scholar]
- 67.Lv J et al. (2017) 5-Hydroxytryptamine synthesized in the aortagonad-mesonephros regulates hematopoietic stem and progenitor cell survival. J. Exp. Med 214, 529–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang M et al. (2007) Promoting effects of serotonin on hematopoiesis: ex vivo expansion of cord blood CD34+ stem/progenitor cells, proliferation of bone marrow stromal cells, and antiapoptosis. Stem Cells 25, 1800–1806 [DOI] [PubMed] [Google Scholar]
- 69.North TE et al. (2007) Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knecht AK and Bronner-Fraser M (2002) Induction of the neural crest: a multigene process. Nat. Rev. Genet 3, 453–461 [DOI] [PubMed] [Google Scholar]
- 71.Bertrand JY et al. (2010) Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boisset J-C et al. (2010) In vivo imaging of haematopoietic cells emerging from themouseaortic endothelium.Nature 464,116–120 [DOI] [PubMed] [Google Scholar]
- 73.Kissa K and Herbomel P (2010) Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115 [DOI] [PubMed] [Google Scholar]
- 74.Fitch SR et al. (2012) Signaling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell 11, 554–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lumb R and Schwarz Q (2015) Sympathoadrenal neural crest cells: the known, unknown and forgotten? Dev. Growth Differ 57, 146–157 [DOI] [PubMed] [Google Scholar]
- 76.An M et al. (2002) Differentiation and maturation of zebrafish dorsal root and sympathetic ganglion neurons. Ann. N. Y. Acad. Sci 446, 267–275 [DOI] [PubMed] [Google Scholar]
- 77.Tamplin OJ et al. (2015) Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160, 241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murayama E et al. (2006) Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 25, 963–975 [DOI] [PubMed] [Google Scholar]
- 79.Levi F and Schibler U (2007) Circadian rhythms: mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol 47, 593–628 [DOI] [PubMed] [Google Scholar]
- 80.Reppert SM and Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418, 935–941 [DOI] [PubMed] [Google Scholar]
- 81.Lucas D et al. (2008) Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell 3, 364–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levesque JP et al. (2003) Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J. Clin. Invest 111, 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petit I et al. (2002) G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol 3, 687–694 [DOI] [PubMed] [Google Scholar]
- 84.Broxmeyer HE et al. (2005) Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med 201, 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Y et al. (2017) Uncovering the mystery of opposite circadian rhythms between mouse and human leukocytes in humanized mice. Blood 130, 1995–2005 [DOI] [PubMed] [Google Scholar]
- 86.Cote F et al. (2003) Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl. Acad. Sci. U. S. A 100, 13525–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zill P et al. (2009) Predominant expression of tryptophan hydroxylase 1 mRNA in the pituitary: a postmortem study in human brain. Neuroscience 159, 1274–1282 [DOI] [PubMed] [Google Scholar]
- 88.Zhang P et al. (2015) G protein-coupled receptor 183 facilitates endothelial-to-hematopoietic transition via Notch1 inhibition. Cell Res. 25, 1093–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L et al. (2013) Fev regulates hematopoietic stem cell development via ERK signaling. Blood 122, 367–375 [DOI] [PubMed] [Google Scholar]
- 90.Zeinali F et al. (2013) Serotonin in blood: assessment of its origin by concomitant determination of beta-thromboglobulin (platelets) and chromogranin A (enterochromaffin cells). Scand. J. Clin. Lab. Invest 73, 148–153 [DOI] [PubMed] [Google Scholar]


