Abstract
The size of sexually selected weapons and their performance in battle are both critical to reproductive success, yet these traits are often in opposition. Bigger weapons make better signals. However, due to the mechanical properties of weapons as lever systems, increases in size may inhibit other metrics of performance as different components of the weapon grow out of proportion with one another. Here, using direct force measurements, we investigated the relationship between weapon size and weapon force production in two hindleg weapon systems, frog-legged beetles (Sagra femorata) and leaf-footed cactus bugs (Narnia femorata), to test for performance tradeoffs associated with increased weapon size. In male frog-legged beetles, relative force production decreased as weapon size increased. Yet, absolute force production was maintained across weapon sizes. Surprisingly, mechanical advantage was constant across weapon sizes and large weaponed males had disproportionately large leg muscles. In male leaf-footed cactus bugs, on the other hand, there was no relationship between weapon size and force production, likely reflecting the importance of their hindlegs as signals rather than force-producing structures of male-male competition. Overall, our results suggest that when weapon force production is important for reproductive success, large weaponed animals may overcome mechanical challenges by maintaining proportional lever components and investing in (potentially costly) compensatory mechanisms.
Introduction
Animal weapons have a history of strong selection for large size [1–17]. This, in part, results from their role as signals to potential mates [18–20] and rival males [19,21–26] where weapon size functions as an honest signal that captures the genetic and environmental variation underlying individual fitness (hereafter referred to simply as “quality”). Large weapons make the best signals [27,28]. However, as selection pushes weapons toward larger sizes, they face intrinsic, mechanical challenges that impede their performance as fighting tools [19,29–31]. This is because animal weapons, like many other mechanical traits (e.g., jaws of fishes [32–34] or jumping legs in insects [35–37], are lever systems, the components of which must appropriately interact to achieve high performance (e.g., Fig 1C–1E).
Fig 1.
A) Mating S. femorata (male on top, photo: D. O’Brien). B) Male N. femorata (photo: R. Boisseau). C-E) Illustrations of lever systems. C) S. femorata hindlimb. D) Simplified machine. E) N. femorata hindlimb. Components of lever systems color coded across all structures (Lin = input lever (brown), Lout = output lever (dark blue), Fin = force in (orange), Fout = force out (light blue), fulcrum (light red)). All are best described as 3rd order levers.
All lever systems are composed of a fulcrum (i.e., the pivot about which the lever turns), an “input” lever arm (Lin), an “output” lever arm (Lout), an input force (Fin), and an output force (Fout). (The relationships between these components are represented by Eq 1).
| (1) |
The components of lever systems must remain in proportion to maintain force output (Fout) [29,30,38]. While increased weapon size may be favored by selection acting toward more efficient signaling or increased reach during combat, variation in the strength of selection and/or constraint experienced by lever components may cause them to scale disproportionally with one another. If, for example, external structures (Lout—horns, antlers, etc.) are free to become large while internal structures (Lin and/or Fin—tendons, bone, muscle, etc.) are architecturally constrained in their growth, as selection acts to increase overall weapon size, Lout may scale with body size at a faster rate than Fin and/or Lin. When this occurs, the mechanical advantage of the lever system will decrease and weapon force output (Fout) will suffer [19,29,38].
The mechanical limits of lever systems should impede overall trait performance [19,29,30,38]. Large weapons may make compelling signals and even limit the frequency of combat [22,26,39,40]. However, the largest males in a population will still be tested by similarly armed opponent [22,26,39–44]. When this occurs, weapons need to perform well. If not, animals could sustain severe damage and/or death, thereby eliminating their reproductive potential [45,46]. Large traits that function only as signals or deterrents are not sustainable in the context of animal contests. For this reason, animal weapons often represent a selective balance between the need for large, conspicuous signals and strong, force-generating weapons [19,47].
To date, several studies have quantified the relationship between weapon size and mechanical performance [25,29,38,48–58]. Yet, the majority of these studies have focused on one of three ecological/evolutionary scenarios: the claws of crustaceans [49–51,55,57–59], jaws of lizards [25,48,52,60], or weapons that do not function as signals [54,56]. Since the relative importance of signaling and fighting may vary considerably depending on the ecology of the species, further work is necessary to understand how the relationship between weapon size and force production varies across taxa and context and how this variation influences the evolution of sexually selected weapons and signals.
Here, we evaluate weapon performance as a function of weapon size in two species with sexually selected hindleg weapons, frog-legged beetles (Sagra femorata: Fig 1A) and leaf-footed cactus bugs (Narnia femorata: Fig 1B). We first demonstrate that, for both species, large weapons are essential for competitive success and function as signals of male quality. In this context, we provide the first analysis of fighting success and escalation in the frog-legged beetle. Then, using a strain gauge force-transducer, we measure how weapon force production varies across the natural range of weapon sizes to better understand the balance between selection for increased weapon size and performance. In addition, we measure input lever arm length (Lin), output lever arm length (Lout), and muscle mass (estimate of Fin) in these weapons to evaluate patterns of constraint and compensation involved in maintaining weapon force output. We predicted that large weapons would have relatively (if not absolutely) lower force production than smaller ones (i.e., the “paradox of the weakening combatant” (sensu [31]). This would result from decreasing mechanical advantage as weapons become large, which should in turn decrease relative force production (Fout).
Materials and methods
Study species, weapon use, and fighting success
Male frog-legged beetles (Coleoptera, Chrysomelidae, Sagra femorata, Dury) have large, sexually dimorphic hindlegs that are used by males in one-on-one battle over direct access to females (Fig 1A). During combat, males attack one another, using their hindlegs to squeeze rival males, pry apart copulating pairs, and steal mates [61,62]. Previous work has demonstrated that males with large weapons have greater reproductive success than smaller weaponed rivals [62]. However, the role weapon size plays in fighting success has never been explicitly tested. Furthermore, it is unclear if the weapons of S. femorata also function as signals of quality and, by extension, if the paradox of the weakening combatant applies to this species [31]. Below, we analyze 104 naturally observed competitive interactions between male S. femorata to demonstrate that a) large weapons are essential for competitive success in this species and b) competition escalates in a manner consistent with weapons that also function as signals.
Observations of male-male competition were collected in tandem with measures of mating success reported in [62]. Briefly, a wild population of frog-legged beetles was observed for two breeding seasons. Prior to each season, adult beetles were marked with unique identification numbers. Throughout each season, two researchers scanned the population at regular intervals and recorded all reproductive behavior (including male-male competition). Overall, 104 antagonistic, male-male interactions were observed. Interactions began as males aggressively approached one another, progressed to one of five “escalation levels”, and ended when one contestant either retreated or was forcibly removed from the fighting area. Escalation levels were defined as follows: Level 1) non-violent interaction, Level 2) violent interaction without full combat, Level 3) full combat of mild intensity, Level 4) full combat of high intensity where one contestant retreats, Level 5) full combat of high intensity where one contestant is forcibly removed from the fighting area. Escalation levels were defined prior to analysis. For each interaction, winner, loser, escalation level, and the body (elytra length; EL) and weapon (femur length; FL) size of each contestant were recorded.
Male leaf-footed cactus bugs (Hemiptera, Coreidae, Narnia femorata, Stål) also have enlarged, sexually dimorphic hindlegs that are used in male-male competition over reproductive territories (Fig 1B). During fights, rival males back up to one another and use their weapons to squeeze opponents and pull them away from potential mates [26,63–65]. Overall, large weapons offer a competitive advantage over smaller ones. Males with the largest weapons tend to win the most fights and, as a result, have the greatest reproductive success [26,63]. The hindlegs of leaf-footed cactus bugs also function as signals. These weapons are conditionally dependent indicators of male quality [65–68] and male-male interactions escalate more frequently when competitors are similarly matched in weapon size [26], a pattern consistent with classic predictions for weapon-signals [39–44,69]. Similar to frog-legged beetles, the hindlegs of leaf-footed cactus bugs act both as tools of combat and signals of quality. As a result, these weapons are subject to selective conflict between these two functions and, by extension, the paradox or the weakening combatant [31].
Squeezing force
For analyses of squeezing force, adult S. femorata were collected from a wild population in Matsuzaka, Mie Prefeture, Japan. Upon capture, measurements of elytra length (body size) and femur length (weapon size [62]) were collected using digital calipers. Animals were housed in 150 ml plastic cups at 25°C and fed Kudzu (Pueraria spp.) leaves ad libitum. Juvenile leaf-footed cactus bugs were initially collected from a wild population in Gainesville, Florida, USA. Juveniles were shipped to Missoula, Montana, USA where they were housed in 500 mL plastic cups at 28°C and fed cactus fruit and pads (Opuntia spp.) ad libitum. Measurements of body length (body size) and femur area (weapon size [65]) were collected for each adult using photographs and ImageJ 1.50i software (NIH, USA).
Squeezing force of hindleg weapons was collected using a full bridge, strain gauge force transducer (S1 Fig). The transducer was composed of two needles, which were attached to parallel metal plates. These plates were constructed of flexible brass, which bent as the animal squeezed the needles. Bending of the brass plates (i.e., squeezing force) was recorded using attached strain gauges (model EA-06-062AQ-350, Vishay Measurements Group, NC USA) and was transmitted to a computer (Dell Vosro 220, Dell, TX USA) via amplifier (model 2160 Vishay Measurements Group, NC USA) and AD converter (PowerLab 8sp, ADinstruments, Sydney Australia). Raw values were collected as a change in voltage and converted to a measure of force (N). All measures were recorded in Lab Chart v7.2 (ADinstruments, Sydney AUS).
The relationship between force and measured voltage was identified as non-linear during subsequent analyses, thereby overestimating squeezing force for the largest weapons (particularly large weaponed S. femorata). The force transducer was therefore calibrated across a range of known weight (2g–100g), a curvilinear ordinary least squares (OLS) regression was fit to the data, and the equation of the best fit curvilinear line (y = 93.362x−10.239x2 + 36; F2,4 = 1646; p < 0.001) was used to correct raw voltage output to accurate force measures. Corrected measures are reported here.
During squeezing trials, animals were held by an observer at the thorax and a single hindleg was placed near the force transducer. For both species, closing force was measured at the most distal point of the true output lever (Lout). In S. femorata, Lout is equal to the linear distance from the center of the femur-tibia joint (fulcrum) to the distal spine of the tibia (Fig 1C, Figure A in S2 Fig). In N. femorata, Lout is equal to the linear distance from the center of the femur-tibia joint (fulcrum) to the most distal point on the widened “leaf” of the tibia (Fig 1E, Figure B in S2 Fig). Leg placement during squeezing measures aimed to mimic leg position during male-male competition, estimated through personal observation and video recording [D. O’Brien; Miller Lab, University of Florida]. While the animal was squeezing, a second observer annotated each “squeeze”, sorting acceptable “maximum force squeezes” (i.e., performance opportunities, see [70]) from inadequate ones (e.g., poor leg placement on the needles) and removing noise (e.g., insect leg bumping into the needle rather than squeezing it). Even so, due to a lack of cooperation from the animals (especially N. femorata), there was appreciable variation in leg placement across trials.
Maximum squeezing force was measured across two 2–4 minute trails per insect, during which an average of 8.261 acceptable "maximum force squeezes” were collected. The mean value of all acceptable squeezes for a single animal was calculated as that animal’s overall maximum squeezing force. This measure of maximum squeezing force was used in place of a single, raw measure of maximum squeezing force to better account for variation in animal performance. Insects that held onto the transducer and squeezed constantly throughout either trial were removed from the analysis, ensuring that “squeezing endurance” was not measured in place of maximum squeezing force.
Dissections (muscle mass and measures of Lin and Lout)
Hindleg muscle mass was collected from a subset of S. femorata (males: n = 88, females: n = 85) and all N. femorata used in squeezing analyses. Whole hindlegs (S. femorata) and femurs (N. femorata) were dissected, dried at 70°C, and weighed. After initial weighing, muscle was digested by fully submerging the leg in 10% KOH and incubating at 70°C for 12 (S. femorata) or 8 (N. femorata) hours to ensure total dissolution of soft tissues [71]. After digestion, hindlimbs were dried at 70°C and weighed a second time. The difference between the first and second weighing was taken as an estimate of dry muscle mass. Muscle mass was taken from a single leg (leg used in squeezing trial when available).
Hindlegs were dissected in a subset of S. femorata (n = 27) to determine the precise internal structure of the leg and to gain accurate measures of Lin and Lout (Fig 1C, Figure A in S2 Fig). Lin was identified as the linear distance from the center of the femur-tibia joint to the muscle attachment sclerite of the tibia. Lout was identified as the linear distance from the center of the femur-tibia joint to the distal spine of the tibia. Measurements of Lin and Lout were collected using photographs of dissected legs and ImageJ 1.50i software (NIH, USA). From these measures, the relationships between Lout and tibia length and Lin and tibia length were calculated using ordinary least squares regression [72]. There were no significant sex differences in these relationships (95% CI intercept Lin for males [-0.227, 0.972] and females [-0.294, 0.691], 95% CI slope Lin for males [-0.29, 0.11] and females [-0.038, 0.135], 95% CI intercept Lout for males [-2.144, 3.397] and females [-0.855, 1.11], 95% CI slope Lout for males [0.554, 1.197] and females [0.776, 1.086]). Thus, male and female data were combined into the two regressions reported here (Lin: y = 0.079x + 0.03, F1,24 = 91.26, p < 0.0001; Lout: y = 0.903x + 0.39, F1,24 = 795.8, p < 0.0001). Equations from these regressions were then used to estimate Lin and Lout for every beetle using measures of tibia length described above.
Similarly, hindlegs of N. femorata were dissected to identify exact measures of Lin and Lout. Lin was identified as the linear distance from the center of the femur-tibia joint to the attachment point of the flexor muscle on the tibia (Fig 1E, Figure B in S2 Fig). Lout was identified as the linear distance from the center of the femur-tibia joint to the most distal point on the widened “leaf” of the tibia. Both Lin and Lout were directly measured in all animals using photographs of dissected legs and ImageJ 1.50i software (NIH, USA).
Statistical analyses
All statistical analyses were performed in R 3.3.2 (R Core Development Team 2016). To analyze competitive interactions between male S. femorata, “Male A” was identified as the male approached by a competitor and the approaching competitor was identified as “Male B”. Logistic regression was used to assess interaction outcome (Male A or B wins) in relation to the difference in competitor weapon size (FLA − FLB). In addition, a mixed effects model (R package lme4 [73]) was used to determine the role weapon size plays in competitive success while controlling for repeated measures of the same individual across multiple interactions. This model included weapon size as a fixed effect, and interaction number and competitor as random effects. Finally, a generalized linear model (family = “Gaussian”) was used to assess the relationship between escalation level and absolute difference in competitor weapon size (|FLA − FLB|).
Ordinary least squares (OLS) regression was used to assess all scaling relationships [72] and all data were log10 transformed prior to analysis. For both species and in both sexes, weapon size (S. femorata, femur length; N. femorata, femur area), Lin, Lout, and muscle mass were regressed on body size (S. femorata: elytra length, N. femorata: body length) in separate models.
Maximum squeezing force was regressed on weapon size in both species and both sexes to assess overall weapon force output. For male S. femorata, linear models with interaction terms between weapon size and muscle mass were constructed to further explore the effect of weapon size, muscle mass, and their interaction on squeezing force. Differences in maximum squeezing force between sexes were calculated using t-tests.
To determine whether the observed increase in muscle mass relative to body size represented a compensatory mechanism, 95% confidence intervals were generated from the OLS regression and used to compare the observed scaling relationship between muscle mass and body size to the expected, isometric relationship (β0 = 3 for volumetric measures). If the observed slope was greater than expected (i.e., β > 3), it was considered a compensatory mechanism [38].
Finally, since mechanical advantage is expected to decrease in the absence of compensation as weapons grow large [29,38], log10 mechanical advantage ([Lin]/[Lout]) was regressed against weapon size.
Results
Fighting success in S. femorata
In S. femorata, large weaponed males won 69.15% of competitive interactions and this trend held after controlling for repeated measures of contestants across multiple interactions (β = 0.452 ± 0.155, z = 2.92, p < 0.01). In addition, the probability that the larger weaponed male won increased as the difference in competitor weapon size increased (z95 = 2.672, p < 0.01; Fig 2A). Overall, weapon size appears to be a key component of competitive success where large, strong weapons offer an advantage over smaller ones. In addition, these weapons seem to function as competitive signals. Across interactions, escalation level decreased as absolute difference in competitor weapon size increased (F2,101 = 8.658, p < 0.001; Fig 2B). This is consistent with classic contest theory [39–44,69] and recent empirical evidence [22,26], which predicts distinct patterns of escalation when weapons are also used as signals. Overall, the hindlegs of S. femorata act as both weapons of male-male battle and signals of quality and are therefore subject to conflict between these two functions.
Fig 2. Interaction outcome and escalation in S. femorata.
A) Interaction outcome. Red line represents logistical regression of interaction outcome (Male A or B wins) in relation to the difference in competitor weapon size (Femur length; FLA − FLB). Positive x values indicate Male A had a larger weapon size than the opponent. Negative x values indicate that Male B had a larger weapon size than the opponent. B) Interaction escalation. Red line represents generalized linear model between interaction escalation level and absolute difference in weapon size (Femur length; |FLA − FLB|).
Squeezing force
In male S. femorata, maximum squeezing force increased hypoallometrically with weapon size (Fig 3A; Table 1). There was no significant interaction between muscle mass and weapon size on maximum squeezing force (t84 = 0.669, p = 0.505). In female S. femorata, there was no significant relationship between maximum squeezing force and weapon size (Fig 3C; Table 1). In S. femorata, maximum squeezing force was higher in males than in females (meanmale = 0.338N; meanfemale = 0.109N; t113.42 = 15.996, p < 0.0001).
Fig 3. Relationship between maximum squeezing force and weapon size for A) male S. femorata, B) male N. femorata, C) female S. femorata, and D) female N. femorata.
Red lines represent OLS regression. Shaded areas represent 95% confidence intervals around OLS regressions. Lines omitted for non-significant regressions.
Table 1. Models for squeezing force analyses (y ~ x format).
SE = standard error. R2 = adjusted R2. S. femorata: weapon size = femur length, body size = elytra length. N. femorata: weapon size = femur area, body size = body length.
| Weapon size | |||||||||
| Model | Intercept | SE | Slope | SE | n | R2 | F(df) | p value | |
| S. femorata (male) | weapon size ~ body size | -0.443 | 0.048 | 1.267 | 0.042 | 95 | 0.906 | 903.6 1,93 | < 0.0001 |
| S. femorata (female) | weapon size ~ body size | -0.29 | 0.063 | 1.036 | 0.057 | 99 | 0.769 | 327 1,97 | < 0.0001 |
| N. femorata (male) | weapon size ~ body size | -2.143 | 0.199 | 2.849 | 0.1967 | 39 | 0.846 | 209.8 1,37 | < 0.0001 |
| N. femorata (female) | weapon size ~ body size | -1.516 | 0.1491 | 2.12 | 0.141 | 44 | 0.841 | 227.6 1,42 | < 0.0001 |
| Muscle mass | |||||||||
| Model | Intercept | SE | Slope | SE | n | R2 | F(df) | p value | |
| S. femorata (male) | muscle mass ~ body size | -2.912 | 0.199 | 3.46 | 0.176 | 88 | 0.819 | 387.9 1,86 | < 0.0001 |
| S. femorata (female) | muscle mass ~ body size | -2.975 | 0.278 | 3.278 | 0.251 | 85 | 0.672 | 170.4 1,83 | < 0.0001 |
| N. femorata (male) | muscle mass ~ body size | -0.382 | 0.756 | 3.027 | 0.745 | 39 | 0.252 | 16.51 1,37 | < 0.001 |
| N. femorata (female) | muscle mass ~ body size | -0.936 | 0.322 | 3.438 | 0.304 | 44 | 0.71 | 128.2 1,42 | < 0.0001 |
| Lin | |||||||||
| Model | Intercept | SE | Slope | SE | n | R2 | F(df) | p value | |
| S. femorata (male) | Lin ~ body size | -1.316 | 0.179 | 1.023 | 0.159 | 95 | 0.301 | 41.56 1,93 | < 0.0001 |
| S. femorata (female) | Lin ~ body size | -0.481 | 0.728 | 0.166 | 0.066 | 100 | 0.051 | 6.327 1,98 | 0.014 |
| N. femorata (male) | Lin ~ body size | -1.795 | 0.246 | 1.324 | 0.243 | 39 | 0.421 | 29.66 1,37 | < 0.0001 |
| N. femorata (female) | Lin ~ body size | -2.036 | 0.204 | 1.483 | 0.192 | 44 | 0.576 | 59.39 1,42 | < 0.0001 |
| Lout | |||||||||
| Model | Intercept | SE | Slope | SE | n | R2 | F(df) | p value | |
| S. femorata (male) | Lout~ body size | -0.248 | 0.178 | 1.016 | 0.157 | 95 | 0.302 | 41.7 1,93 | < 0.001 |
| S. femorata (female) | Lout~ body size | 0.582 | 0.722 | 0.164 | 0.065 | 100 | 0.051 | 6.318 1,98 | 0.014 |
| N. femorata (male) | Lout~ body size | -0.085 | 0.111 | 0.719 | 0.11 | 39 | 0.526 | 43.15 1,37 | < 0.0001 |
| N. femorata (female) | Lout~ body size | -0.469 | 0.139 | 1.048 | 0.131 | 44 | 0.596 | 64.29 1,42 | < 0.0001 |
| Mechanical advantage | |||||||||
| Model | Intercept | SE | Slope | SE | n | R2 | F(df) | p value | |
| S. femorata (male) | mechanical adv. ~ weapon size | -0.957 | 0.367 | -0.101 | 0.372 | 13 | -0.087 | 0.074 1,11 | 0.791 |
| S. femorata (female) | mechanical adv. ~ weapon size | -0.786 | 0.329 | -0.382 | 0.444 | 13 | -0.022 | 0.742 1,11 | 0.408 |
| N. femorata (male) | mechanical adv. ~ weapon size | -1.268 | 0.052 | 0.227 | 0.07 | 39 | 0.206 | 10.6 1,36 | 0.002 |
| N. femorata (female) | mechanical adv. ~ weapon size | -1.221 | 0.068 | 0.157 | 0.093 | 44 | 0.042 | 2.855 1,41 | 0.01 |
| Squeezing force | |||||||||
| Model | Intercept | SE | Slope | SE | n | R2 | F(df) | p value | |
| S. femorata (male) | Squeezing force ~ weapon size | 1.183 | 0.134 | 0.63 | 0.135 | 95 | 0.18 | 21.68 1,93 | < 0.001 |
| S. femorata (female) | Squeezing force ~ weapon size | 1.531 | 0.01 | 0.153 | 0.116 | 100 | 0.007 | 1.736 1,98 | 0.191 |
| N. femorata (male) | Squeezing force ~ weapon size | -1.592 | 0.306 | 0.353 | 0.409 | 39 | -0.007 | 0.756 1,36 | 0.39 |
| N. femorata (female) | Squeezing force ~ weapon size | -2.241 | 0.272 | 1.289 | 0.369 | 44 | 0.211 | 12.21 1,41 | < 0.01 |
In male N. femorata, there was no significant relationship between maximum squeezing force and weapon size (Fig 3B; Table 1) [38]. In females, maximum squeezing force increased isometrically with weapon size (Fig 3D; Table 1). There was no significant difference in maximum squeezing force between sexes in N. femorata (t96.286 = -0.0396, p = 0.693).
Morphological measures of lever components
A summary of all morphological measures is provided in S1 Table. In S. femorata, weapon size increased hyperallometrically with body size in males and isometrically in females (Fig 4A and 4C; Table 1). Lin increased isometrically with body size in males and hypoallometrically with body size in females (Fig 5; Table 1). Lout increased isometrically with body size in males and hypoallometrically with body size in females (Fig 5; Table 1). There was no signifcant relationship between mechanical advantage and weapon size in males or females (Fig 5; Table 1).
Fig 4. Relationship between weapon size and body size for A) male S. femorata, B) male N. femorata, C) female S. femorata, and D) female N. femorata.
Red lines represent OLS regression. Shaded areas represent 95% confidence intervals around OLS regressions.
Fig 5. Relationships between lever components/mechanical advantage and body size for A) S. femorata males, B) S. femorata females, C) N. femorata males, and D) N. femorata females.
Top: Lin vs. body size. Middle: Lout vs. body size. Bottom: mechanical advantage vs. body size. Red lines represent OLS regression. Shaded areas represent 95% confidence intervals around OLS regressions. Lines omitted for non-significant regressions.
In N. femorata, weapon size increased hyperallometrically with body size in males and isometrically with body size in females (Fig 4B and 4D; Table 1). Lin increased increased hyperallometrically with body size in both males and females (Fig 5; Table 1). Lout increased hypoallometrically with body size in males and isometrically with body size in females (Fig 5; Table 1). Mechanical advantage increased hypoallometrically with weapon size in both males and females (Fig 5; Table 1).
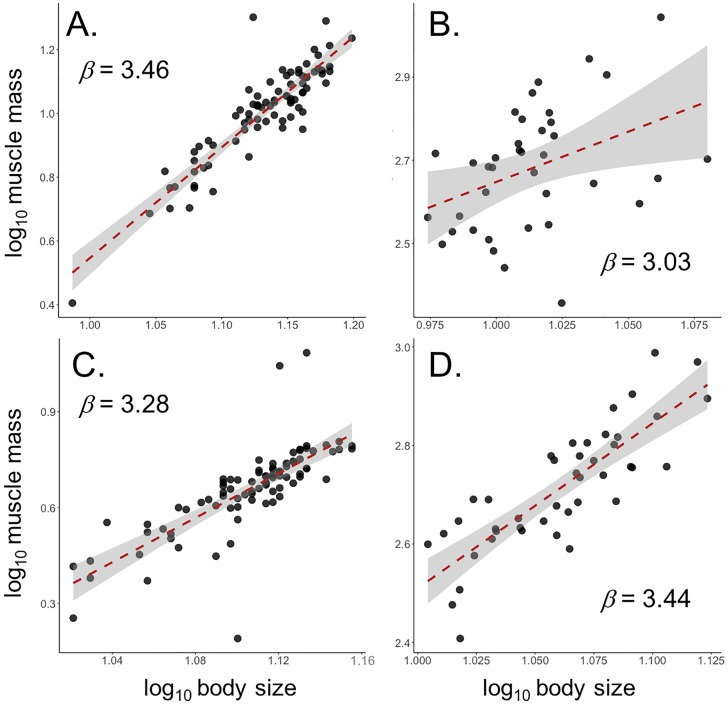
In male S. femorata, muscle mass increased hyperallometrically with body size, which is consistant with a compensatory mechanism (β = 3.406 ± 0.176, F1,86 = 387.9, p < 0.0001; Fig 6A; Table 1). In females, muscle mass also increased hyperallometrically with body size ((β = 3.278 ± 0.251, F1,83 = 170.4, p < 0.0001; Fig 6C; Table 1).
Fig 6. Relationship between hindlimb muscle mass and body size for A) male S. femorata, B) male N. femorata, C) female S. femorata, and D) female N. femorata.
Red lines represent OLS regression. Shaded areas represent 95% confidence intervals around OLS regressions.
In male N. femorata, muscle mass scaled isometrically with body size (males: β = 3.027 ± 0.745, F1,37 = 16.51, p < 0.001; Fig 6B; Table 1). Muscle mass scaled hyperallometrically with body size in female N. femorata (β = 3.438 ± 0.304, F1,42 = 128.2, p < 0.0001; Fig 6D; Table 1).
Discussion
We measured weapon force output as a function of weapon size in two wild, sexually selected weapon systems, frog-legged beetles (S. femorata) and leaf-footed cactus bugs (N. femorata). In frog-legged beetles, weapon force output increased hypoallometrically (β = 0.630 ± 0.135) with weapon size, suggesting large weaponed males have relatively lower, but absolutely higher, force production than smaller rivals (Fig 3A; Table 1). As weapons grow large, mechanical advantage (and therefore weapon force output) is predicted to decrease in the absence of compensation and limit the relationship between weapon size and weapon force output(Eq 1; [29,38]In frog-legged beetles, however, mechanical advantage was maintained across all animals and absolute force production increased with weapon size (Figs 3A and 5; Table 1). This suggests frog-legged beetles employ one or more compensatory mechanism, which partially mitigates the mechanical limits predicted to hinder large weapon sizes.
Here, we identified two potential compensatory mechanisms, proportional growth of weapon/hindleg lever components and disproportionate growth of femur muscle mass. Overall, male frog-legged beetles do not experience mechanical disadvantage as weapons grow large, since they compensate for the increase in output lever length associated with increased in weapon size by similarly increasing input lever length. Male frog-legged beetles display longer input and output levers than females, which result in constant mechanical advantage across weapon sizes and between sexes (Fig 5; Table 1; S1 Table).
In addition, in male frog-legged beetles, femur muscle mass (Fin) increased hyperallometrically with body size (β > 3; Fig 6A; Table 1), which is consistent with compensatory mechanisms identified in other systems [38]. It should be noted, however, that both absolute and relative weapon force output should increase with weapon size, given disproportionate muscle growth and the observed maintenance of mechanical advantage (Fig 5; Table 1). Clearly, there are as-yet undiscovered limits to weapon force production in this system (mechanical and/or behavioral), and further work is necessary to uncover why exactly weapon force output scales hypoallometrically in the frog-legged beetle.
Male leaf-footed cactus bugs showed no significant relationship between weapon force output (Fout) and weapon size (Fig 3B; Table 1). This result was surprising given the observed increase in mechanical advantage with weapon size (Fig 5; Table 1). However, leg muscles remained proportional across all weapon sizes (Fig 6B; Table 1), which may explain why weapon force output did not increase with weapon size in males of this species. This result was unexpected given the established role hindleg weapons play in male-male competition [26,63], and the maintenance of mechanical advantage across weapon sizes. One explanation for this trend is that these hindlegs may be under relatively weak selection for increased force production in the context male-male combat. Instead, the hindlegs of leaf-footed cactus bugs may serve a greater role as intersexual signals of male quality, a behavioral context in which weapon force output is not an important component of fitness and hindlimb area, rather than force production, is under strong selection for increased size. Indeed, previous work suggests hindleg area is an honest indicator of overall quality [65–68] and recent studies have detected directional selection for increased hindleg area in the wild [63]. If true, then focal animals may have been unwilling to perform at full capacity during squeezing trails, since their hindlegs function primarily as display signals rather than weapons.
Alternatively, the ability to squeeze an opponent between both femurs, rather than between the femur and tibia of a single leg (as measured here), may be the most relevant metric of fighting success in this system (personal observation; Miller lab, University of Florida). Either scenario would result in an underestimation of weapon force output and could explain the observed non-significant relationship between weapon size and weapon force output. While we maintain our measures of weapon size, Lin, and Lout, are relevant in this system and to understanding the forces produced by these weapons, further investigation is necessary to establish exactly how weapon length and force production influence the outcome of male-male competition in the leaf-footed cactus bug, and what role, if any, these traits play in overall reproductive success.
Compensatory muscle growth and honest signaling in the frog-legged beetle
Sexually selected weapons act as signals of quality and weapons of male-male battle. In both contexts, honesty is essential. Weapon size must honestly display quality to potential mates [18–20] and fighting ability to rival males [19,21–24,26,48] and, when tested in combat by similarly armed opponents, large weapons must produce sufficient force [69]. If not, receivers are predicted to focus to other, more reliable indicators of quality/fighting ability and selection for large weapons/signals should relax. Honesty in sexually selected weapons can be maintained through several mechanisms, including exquisite sensitivity to stress [74], parasite load [75,76], environmental condition [77], and intrinsic cost associated large structures [78,79]. The latter is particularly relevant to weapon systems where large, conspicuous structures often hinder the animals that bear them [80–85]. When present, the costs of sexually selected weapons typically increase with trait size, so only the largest animals can develop and wield large weapons and high quality signals are restricted to high quality males [28,78,79,86].
We suggest the compensatory muscle growth identified in frog-legged beetles comes at a cost and, through that cost, functions as mechanism of honesty. Muscle is notoriously expensive to develop [87–89] and maintain [80,82,90–94]. In preserving absolute weapon force output through compensatory muscle growth, frog-legged beetles may experience added metabolic [80,94] and locomotor [80,82] strain. For example, fiddler crabs with large, muscular claws suffer from disproportionally high resting metabolic rates [80,94], while stag beetles with large mandibles experience decreased flight performance resulting from their heavy, muscular jaws [82]. Such costs are consistent with theoretical models of handicap and indicator traits, where cost helps maintain the honesty/integrity of sexually selected traits as signals [78,79,86,95–98]. We therefore suggest that compensation for mechanical disadvantage through muscle growth may contribute to the integrity of weapon size as an honest indicator of quality and fighting ability in this system.
Conclusion
The size of sexually selected weapons is critical to their role as honest signals. Weapons signal overall quality to potential mates and display fighting prowess to rival males. In both contexts, large traits are favored. However, selection for large, conspicuous signals is likely balanced by the need for weapons to perform well during combat. Here, we analyzed the relationship between weapon size and weapon force production (i.e., performance) in two systems, frog-legged beetles (S. femorata) and leaf-footed cactus bugs (N. femorata). In male frog-legged beetles, weapon force output scaled hypoallometrically with weapon size. This is partially consistent with lever theory, where both absolute and relative force output are predicted to decrease as weapons become large [29,38]. However, absolute force output appears to be maintained in this system through the maintenance of mechanical advantage across all weapon sizes and a disproportionally steep scaling relationship between leg muscle mass and body size. Alternatively, male leaf-footed cactus bugs showed no relationship between weapon size and force production, potentially reflecting the importance of hindleg area as an intersexual display of male quality rather than a force-producing weapon of male-male competition.
Overall, we suggest that when weapon force production is an important component of reproductive success, and animals experience mechanical limits to weapon force production, the evolution of compensatory mechanisms is likely (reviewed in [99]). We also suggest that some compensatory mechanisms, such as muscle growth in the frog-legged beetle, could enhance signal honesty in the context of sexual selection, both by disproportionately increasing metabolic or other costs associated with the largest male weapons and by maintaining fight performance at even the largest weapon sizes. Clearly, more work is required to understand the realized cost of heavily muscled weapons, how this influences individual fitness in the wild, and the ubiquity of the trends described here.
Supporting information
(DOCX)
A) Rigid metal bar used to stabilize the transducer stationary during trials. B) Flexible, brass arms that bend during squeezing trials. C) Needles that the animals squeeze during trials. Squeezing force (red) causes deformation in brass arms (B). Deformation is recorded by strain gauges (blue) in a full bridge configuration, as they are placed under tension (T1 and T2) and compression (C1 and C2).
(TIFF)
(TIFF)
Acknowledgments
We thank Bret Tobalske for designing and building the force transducer and technical support, Sarah Solie for her assistance in animal capture, observation of antagonistic interactions, and analyses for squeezing force in Sagra femorata, The Miller Lab (University of Florida) for collection of Narnia femorata and assistance in early planning of the project, Teruyuki Niimi, Hiroki Gotoh, Masako Katsuki, and Yasu Okada for their support in Japan, and Doug Emlen, Sheila Patek, Art Woods, and Steve Lane, for feedback on the project and manuscript.
Data Availability
All data are available through Harvard Dataverse: https://doi.org/10.7910/DVN/VZGYOI. Citation: O'Brien, Devin M;Boisseau, Romain P, 2018, "Replication Data for: Overcomic mechanical adversity in extreme hindleg weapons", https://doi.org/10.7910/DVN/VZGYOI, Harvard Dataverse, V1.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Willisch CS, Biebach I, Marreros N, Ryser-Degiorgis M-P, Neuhaus P. Horn growth and reproduction in a long-lived male mammal: no compensation for poor early-life horn growth. Evol Biol. 2015;42: 1–11. [Google Scholar]
- 2.Eberhard WG. The function of horns in Podischnus agenor (Dynastinae) Sexual selection and reproductive competition in insects. Academic Press; UK; 1979. pp. 231–258. [Google Scholar]
- 3.Conner J. Field measurements of natural and sexual selection in the fungus beetle, Bolitotherus cornutus. Evolution. 1988; 736–749. 10.1111/j.1558-5646.1988.tb02492.x [DOI] [PubMed] [Google Scholar]
- 4.Hongo Y. Evolution of male dimorphic allometry in a population of the Japanese horned beetle Trypoxylus dichotomus septentrionalis. Behav Ecol Sociobiol. 2007;62: 245–253. 10.1007/s00265-007-0459-2 [DOI] [Google Scholar]
- 5.Crespi B J. Territoriality and fighting in a colonial thrips, Hoplothrips pedicularius, and sexual dimorphism in Thysanoptera. Ecol Entomol. 1986;11: 119–130. [Google Scholar]
- 6.Burkhardt D, de la Motte I. Physiological, behavioural, and morphometric data elucidate the evolutive significance of stalked eyes in Diopsidae (Diptera). Entomol Gen. 1987;12: 221–233. [Google Scholar]
- 7.Burkhardt D, de la Motte I. Big ‘antlers’ are favoured: female choice in stalk-eyed flies (Diptera, Insecta), field collected harems and laboratory experiments. J Comp Physiol A. 1988;162: 649–652. 10.1007/BF01342640 [DOI] [Google Scholar]
- 8.Zeh DW. Aggression, density, and sexual dimorphism in chernetid pseudoscorpions (Arachnida: Pseudoscorpionida). Evolution. 1987; 1072–1087. 10.1111/j.1558-5646.1987.tb05877.x [DOI] [PubMed] [Google Scholar]
- 9.Petrie M. Intraspecific variation in structures that display competitive ability: large animals invest relatively more. Anim Behav. 1988;36: 1174–1179. [Google Scholar]
- 10.Fisher RA. The genetical theory of natural selection. Рипол Классик; 1958. [Google Scholar]
- 11.West-Eberhard MJ. Sexual selection, social competition, and evolution. Proc Am Philos Soc. 1979;123: 222–234. [Google Scholar]
- 12.West-Eberhard MJ. Sexual Selection, Social Competition, and Speciation. Q Rev Biol. 1983;58: 155–183. [Google Scholar]
- 13.Green AJ. Positive allometry is likely with mate choice, competitive display and other functions. Anim Behav. 1992;43: 170–172. 10.1016/S0003-3472(05)80086-7 [DOI] [Google Scholar]
- 14.Kruuk LEB, Slate J, Pemberton JM, Brotherstone S, Guinness F, Clutton-Brock T. Antler Size in Red Deer: Heritability and Selection but No Evolution. Evolution. 2002;56: 1683–1695. 10.1111/j.0014-3820.2002.tb01480.x [DOI] [PubMed] [Google Scholar]
- 15.Bonduriansky R, Day T. The Evolution of Static Allometry in Sexually Selected Traits. Evolution. 2003;57: 2450–2458. 10.1111/j.0014-3820.2003.tb01490.x [DOI] [PubMed] [Google Scholar]
- 16.Bonduriansky R, Rowe L. Sexual selection, genetic architecture, and the condition dependence of body shape in the sexually dimorphic fly Prochyliza xanthostoma (Piophilidae). Evolution. 2005;59: 138–151. [PubMed] [Google Scholar]
- 17.Kokko H, Brooks R, McNamara JM, Houston AI. The sexual selection continuum. Proc R Soc B Biol Sci. 2002;269: 1331–1340. 10.1098/rspb.2002.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanpé C, Gaillard J-M, Kjellander P, Liberg O, Delorme D, Hewison AJ. Assessing the intensity of sexual selection on male body mass and antler length in roe deer Capreolus capreolus: is bigger better in a weakly dimorphic species? Oikos. 2010;119: 1484–1492. [Google Scholar]
- 19.Dennenmoser S, Christy JH. The design of a beautiful weapon: compensation for opposing sexual selection on a trait with two functions. Evolution. 2013;67: 1181–1188. 10.1111/evo.12018 [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson GS, Reillo PR. Female Choice Response to Artificial Selection on an Exaggerated Male Trait in a Stalk-Eyed Fly. Proc R Soc Lond B Biol Sci. 1994;255: 1–6. 10.1098/rspb.1994.0001 [DOI] [Google Scholar]
- 21.Clutton-Brock TH, Albon SD, Gibson RM, Guinness FE. The logical stag: Adaptive aspects of fighting in red deer (Cervus elaphus L.). Anim Behav. 1979;27, Part 1: 211–225. 10.1016/0003-3472(79)90141-6 [DOI] [Google Scholar]
- 22.Barrette C, Vandal D. Sparring, relative antler size, and assessment in male caribou. Behav Ecol Sociobiol. 1990;26: 383–387. 10.1007/BF00170894 [DOI] [Google Scholar]
- 23.Panhuis TM, Wilkinson GS. Exaggerated male eye span influences contest outcome in stalk-eyed flies (Diopsidae). Behav Ecol Sociobiol. 1999;46: 221–227. 10.1007/s002650050613 [DOI] [Google Scholar]
- 24.Painting CJ, Holwell GI. Exaggerated rostra as weapons and the competitive assessment strategy of male giraffe weevils. Behav Ecol. 2014; aru119. 10.1093/beheco/aru119 [DOI] [Google Scholar]
- 25.Lappin AK, Husak JF. Weapon Performance, Not Size, Determines Mating Success and Potential Reproductive Output in the Collared Lizard (Crotaphytus collaris.). Am Nat. 2005;166: 426–436. 10.1086/432564 [DOI] [PubMed] [Google Scholar]
- 26.Nolen ZJ, Allen PE, Miller CW. Seasonal resource value and male size influence male aggressive interactions in the leaf footed cactus bug, Narnia femorata. Behav Processes. 2017;138: 1–6. 10.1016/j.beproc.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 27.Fromhage L, Kokko H. Sexually selected traits evolve positive allometry when some matings occur irrespective of the trait. Evolution. 2014;68: 1332–1338. 10.1111/evo.12349 [DOI] [PubMed] [Google Scholar]
- 28.Biernaskie JM, Grafen A, Perry JC. The evolution of index signals to avoid the cost of dishonesty. Proc R Soc Lond B Biol Sci. 2014;281: 20140876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goyens J, Dirckx J, Dierick M, Van Hoorebeke L, Aerts P. Biomechanical determinants of bite force dimorphism in Cyclommatus metallifer stag beetles. J Exp Biol. 2014;217: 1065–1071. 10.1242/jeb.091744 [DOI] [PubMed] [Google Scholar]
- 30.Goyens J, Dirckx J, Aerts P. Jaw morphology and fighting forces in stag beetles. J Exp Biol. 2016;219: 2955–2961. 10.1242/jeb.141614 [DOI] [PubMed] [Google Scholar]
- 31.Levinton JS, Allen BJ. The paradox of the weakening combatant: trade-off between closing force and gripping speed in a sexually selected combat structure. Funct Ecol. 2005;19: 159–165. 10.1111/j.0269-8463.2005.00968.x [DOI] [Google Scholar]
- 32.Westneat MW. A biomechanical model for analysis of muscle force, power output and lower jaw motion in fishes. J Theor Biol. 2003;223: 269–281. [DOI] [PubMed] [Google Scholar]
- 33.Westneat MW. Evolution of levers and linkages in the feeding mechanisms of fishes. Integr Comp Biol. 2004;44: 378–389. 10.1093/icb/44.5.378 [DOI] [PubMed] [Google Scholar]
- 34.Waltzek TB, Wainwright PC. Functional morphology of extreme jaw protrusion in neotropical cichlids. J Morphol. 2003;257: 96–106. 10.1002/jmor.10111 [DOI] [PubMed] [Google Scholar]
- 35.Sutton GP, Burrows M. Biomechanics of jumping in the flea. J Exp Biol. 2011;214: 836–847. 10.1242/jeb.052399 [DOI] [PubMed] [Google Scholar]
- 36.McHenry MJ. There is no trade-off between speed and force in a dynamic lever system. Biol Lett. 2011;7: 384–386. 10.1098/rsbl.2010.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burrows M, Wolf H. Jumping and kicking in the false stick insect Prosarthria teretrirostris: kinematics and motor control. J Exp Biol. 2002;205: 1519–1530. [DOI] [PubMed] [Google Scholar]
- 38.Mills MR, Nemri RS, Carlson EA, Wilde W, Gotoh H, Lavine LC, et al. Functional mechanics of beetle mandibles: Honest signaling in a sexually selected system. J Exp Zool Part Ecol Genet Physiol. 2016;325: 3–12. 10.1002/jez.1961 [DOI] [PubMed] [Google Scholar]
- 39.Smith JM, Parker GA. The logic of asymmetric contests. Anim Behav. 1976;24: 159–175. 10.1016/S0003-3472(76)80110-8 [DOI] [Google Scholar]
- 40.Maynard Smith J, Price GR. The Logic of Animal Conflict. Nature. 1973;246: 15. [Google Scholar]
- 41.Parker GA. Assessment strategy and the evolution of fighting behaviour. J Theor Biol. 1974;47: 223–243. [DOI] [PubMed] [Google Scholar]
- 42.Parker GA, Rubenstein DI. Role assessment, reserve strategy, and acquisition of information in asymmetric animal conflicts. Anim Behav. 1981;29: 221–240. [Google Scholar]
- 43.Maynard Smith J. The theory of games and the evolution of animal conflicts. J Theor Biol. 1974;47: 209–221. 10.1016/0022-5193(74)90110-6 [DOI] [PubMed] [Google Scholar]
- 44.Maynard Smith J. Evolution and the Theory of Games: In situations characterized by conflict of interest, the best strategy to adopt depends on what others are doing. Am Sci. 1976;64: 41–45. [PubMed] [Google Scholar]
- 45.McCullough EL. Mechanical limits to maximum weapon size in a giant rhinoceros beetle. Proc R Soc Lond B Biol Sci. 2014;281: 20140696. 10.1098/rspb.2014.0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones AR. Chela injuries in the fiddler crab, Uca burgersi holthuis. Mar Behav Physiol. 1980;7: 47–56. 10.1080/10236248009386970 [DOI] [Google Scholar]
- 47.McCullough EL, Miller CW, Emlen DJ. Why Sexually Selected Weapons Are Not Ornaments. Trends Ecol Evol. 2016; [DOI] [PubMed] [Google Scholar]
- 48.Lappin AK, Brandt Y, Husak JF, Macedonia JM, Kemp DJ. Gaping displays reveal and amplify a mechanically based index of weapon performance. Am Nat. 2006;168: 100–113. 10.1086/505161 [DOI] [PubMed] [Google Scholar]
- 49.Bywater CL, Angilletta MJ, Wilson RS. Weapon size is a reliable indicator of strength and social dominance in female slender crayfish (Cherax dispar). Funct Ecol. 2008;22: 311–316. 10.1111/j.1365-2435.2008.01379.x [DOI] [Google Scholar]
- 50.Claverie T, Smith IP. Functional significance of an unusual chela dimorphism in a marine decapod: specialization as a weapon? Proc R Soc B Biol Sci. 2007;274: 3033–3038. 10.1098/rspb.2007.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Claverie T, Chan E, Patek SN. Modularity and Scaling in Fast Movements: Power Amplification in Mantis Shrimp. Evolution. 2011;65: 443–461. 10.1111/j.1558-5646.2010.01133.x [DOI] [PubMed] [Google Scholar]
- 52.Husak JF, Lappin AK, Fox SF, Lemos-Espinal JA. Bite-Force Performance Predicts Dominance in Male Venerable Collared Lizards (Crotaphytus antiquus). Copeia. 2006;2006: 301–306. [Google Scholar]
- 53.Lailvaux SP, Hathway J, Pomfret J, Knell RJ. Horn size predicts physical performance in the beetle Euoniticellus intermedius (Coleoptera: Scarabaeidae). Funct Ecol. 2005;19: 632–639. 10.1111/j.1365-2435.2005.01024.x [DOI] [Google Scholar]
- 54.Paul J, Gronenberg W. Optimizing force and velocity: mandible muscle fibre attachments in ants. J Exp Biol. 1999;202: 797–808. [DOI] [PubMed] [Google Scholar]
- 55.Sneddon LU, Huntingford FA, Taylor AC. Weapon size versus body size as a predictor of winning in fights between shore crabs, Carcinus maenas (L.). Behav Ecol Sociobiol. 1997;41: 237–242. 10.1007/s002650050384 [DOI] [Google Scholar]
- 56.Tanaka Y, Hisada M. The hydraulic mechanism of the predatory strike in dragonfly larvae. J Exp Biol. 1980;88: 1–19. [Google Scholar]
- 57.Walter GM, van Uitregt VO, Wilson RS. Social control of unreliable signals of strength in male but not female crayfish, Cherax destructor. J Exp Biol. 2011;214: 3294–3299. 10.1242/jeb.056754 [DOI] [PubMed] [Google Scholar]
- 58.Wilson RS, James RS, Bywater C, Seebacher F. Costs and benefits of increased weapon size differ between sexes of the slender crayfish, Cherax dispar. J Exp Biol. 2009;212: 853–858. 10.1242/jeb.024547 [DOI] [PubMed] [Google Scholar]
- 59.Wilson RS, Angilletta MJ, James RS, Navas C, Seebacher F. Dishonest signals of strength in male slender crayfish (Cherax dispar) during agonistic encounters. Am Nat. 2007;170: 284–291. 10.1086/519399 [DOI] [PubMed] [Google Scholar]
- 60.Huyghe K, Vanhooydonck B, Scheers H, Molina-Borja M, Van Damme R. Morphology, performance and fighting capacity in male lizards, Gallotia galloti. Funct Ecol. 2005;19: 800–807. [Google Scholar]
- 61.Katsuki M, Yokoi T, Funakoshi K, Oota N. Enlarged hind legs and sexual behavior with male-male interaction in Sagra femorata. Entomol Sci. In Press;In Press. [Google Scholar]
- 62.O’Brien DM, Katsuki M, Emlen DJ. Selection on an extreme weapon in the frog-legged leaf beetle (Sagra femorata). Evolution. 2017;71: 2584–2598. 10.1111/evo.13336 [DOI] [PubMed] [Google Scholar]
- 63.Procter DS, Moore AJ, Miller CW. The form of sexual selection arising from male–male competition depends on the presence of females in the social environment. J Evol Biol. 2012;25: 803–812. 10.1111/j.1420-9101.2012.02485.x [DOI] [PubMed] [Google Scholar]
- 64.Addesso KM, Short KA, Moore AJ, Miller CW. Context-dependent female mate preferences in leaf-footed cactus bugs. Behaviour. 2014;151: 479–492. [Google Scholar]
- 65.Sasson DA, Munoz PR, Gezan SA, Miller CW. Resource quality affects weapon and testis size and the ability of these traits to respond to selection in the leaf-footed cactus bug, Narnia femorata. Ecol Evol. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller CW, McDonald GC, Moore AJ. The tale of the shrinking weapon: seasonal changes in nutrition affect weapon size and sexual dimorphism, but not contemporary evolution. J Evol Biol. 2016;29: 2266–2275. 10.1111/jeb.12954 [DOI] [PubMed] [Google Scholar]
- 67.Cirino LA, Miller CW. Seasonal Effects on the Population, Morphology and Reproductive Behavior of Narnia femorata (Hemiptera: Coreidae). Insects. 2017;8: 13 10.3390/insects8010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joseph PN, Sasson DA, Allen PE, Somjee U, Miller CW. Adult nutrition, but not inbreeding, affects male primary sexual traits in the leaf-footed cactus bug Narnia femorata (Hemiptera: Coreidae). Ecol Evol. 2016;6: 4792–4799. 10.1002/ece3.2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hardy IC, Briffa M. Animal contests. Cambridge University Press; 2013. [Google Scholar]
- 70.Adolph SC, Pickering T. Estimating maximum performance: effects of intraindividual variation. J Exp Biol. 2008;211: 1336–1343. 10.1242/jeb.011296 [DOI] [PubMed] [Google Scholar]
- 71.Holwell GI, Winnick C, Tregenza T, Herberstein ME. Genital shape correlates with sperm transfer success in the praying mantis Ciulfina klassi (Insecta: Mantodea). Behav Ecol Sociobiol. 2010;64: 617–625. [Google Scholar]
- 72.Kilmer JT, Rodríguez RL. Ordinary least squares regression is indicated for studies of allometry. J Evol Biol. 2016; n/a–n/a. 10.1111/jeb.12986 [DOI] [PubMed] [Google Scholar]
- 73.Bates D, Machler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67 doi: 10.18637/jss.v067.i01 [Google Scholar]
- 74.Topiński P. Abnormal antler cycles in deer as a result of stress inducing factors. Acta Theriol (Warsz). 1975;20: 267–279. [Google Scholar]
- 75.Ezenwa VO, Jolles AE. Horns honestly advertise parasite infection in male and female African buffalo. Anim Behav. 2008;75: 2013–2021. [Google Scholar]
- 76.Hamilton WD, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218: 384–387. [DOI] [PubMed] [Google Scholar]
- 77.Zeh DW, Zeh JA. Condition-dependent sex ornaments and field tests of sexual-selection theory. Am Nat. 1988;132: 454–459. [Google Scholar]
- 78.Eshel I, Volovik I, Sansone E. On Fisher-Zahavi’s handicapped sexy son. Evol Ecol Res. 2000;2: 509–523. [Google Scholar]
- 79.Zahavi A. Mate selection—a selection for a handicap. J Theor Biol. 1975;53: 205–214. [DOI] [PubMed] [Google Scholar]
- 80.Allen BJ, Levinton JS. Costs of bearing a sexually selected ornamental weapon in a fiddler crab. Funct Ecol. 2007;21: 154–161. [Google Scholar]
- 81.Emlen DJ. Costs and the diversification of exaggerated animal structures. Science. 2001;291: 1534–1536. [DOI] [PubMed] [Google Scholar]
- 82.Goyens J, Van Wassenbergh S, Dirckx J, Aerts P. Cost of flight and the evolution of stag beetle weaponry. J R Soc Interface. 2015;12: 20150222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawano K. Cost of evolving exaggerated mandibles in stag beetles (Coleoptera: Lucanidae). Ann Entomol Soc Am. 1997;90: 453–461. [Google Scholar]
- 84.Moczek AP, Emlen DJ. Male horn dimorphism in the scarab beetle, Onthophagus taurus: do alternative reproductive tactics favour alternative phenotypes? Anim Behav. 2000;59: 459–466. 10.1006/anbe.1999.1342 [DOI] [PubMed] [Google Scholar]
- 85.Moczek AP, Nijhout HF. Trade-offs during the development of primary and secondary sexual traits in a horned beetle. Am Nat. 2004;163: 184–191. 10.1086/381741 [DOI] [PubMed] [Google Scholar]
- 86.Johnstone RA. Sexual Selection, Honest Advertisement and the Handicap Principle: Reviewing the Evidence. Biol Rev. 1995;70: 1–65. 10.1111/j.1469-185X.1995.tb01439.x [DOI] [PubMed] [Google Scholar]
- 87.Joubert DM. An analysis of factors influencing post-natal growth and development of the muscle fibre. J Agric Sci. 1956;47: 59–102. 10.1017/S0021859600039794 [DOI] [Google Scholar]
- 88.Dauncey MJ, Gilmour SR. Regulatory factors in the control of muscle development. Proc Nutr Soc. 1996;55: 543–560. [DOI] [PubMed] [Google Scholar]
- 89.Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep: II. Skeletal muscle growth and development. J Anim Sci. 2000;78: 50–61. 10.2527/2000.78150x [DOI] [PubMed] [Google Scholar]
- 90.Beltman JGM, Van Der Vliet MR, Sargeant AJ, De Haan A. Metabolic cost of lengthening, isometric and shortening contractions in maximally stimulated rat skeletal muscle. Acta Physiol Scand. 2004;182: 179–187. 10.1111/j.1365-201X.2004.01338.x [DOI] [PubMed] [Google Scholar]
- 91.Hortobágyi T, Finch A, Solnik S, Rider P, DeVita P. Association Between Muscle Activation and Metabolic Cost of Walking in Young and Old Adults. J Gerontol Ser A. 2011;66A: 541–547. 10.1093/gerona/glr008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Casey TM. A Comparison of Mechanical and Energetic Estimates of Flight Cost for Hovering Sphinx Moths. J Exp Biol. 1981;91: 117–129. [Google Scholar]
- 93.Dickinson MH, Lighton JRB. Muscle efficiency and elastic storage in the flight motor of Drosophila. Sci Wash. 1995;268: 87. [DOI] [PubMed] [Google Scholar]
- 94.Bywater CL, White CR, Wilson RS. Metabolic incentives for dishonest signals of strength in the fiddler crab Uca vomeris. J Exp Biol. 2014;217: 2848–2850. 10.1242/jeb.099390 [DOI] [PubMed] [Google Scholar]
- 95.Iwasa Y, Pomiankowski A, Nee S. The evolution of costly mate preferences II. The’handicap’principle. Evolution. 1991; 1431–1442. 10.1111/j.1558-5646.1991.tb02646.x [DOI] [PubMed] [Google Scholar]
- 96.Iwasa Y, Pomiankowski A. Good parent and good genes models of handicap evolution. J Theor Biol. 1999;200: 97–109. 10.1006/jtbi.1999.0979 [DOI] [PubMed] [Google Scholar]
- 97.Pomiankowski A. Sexual selection: The handicap principle does work–sometimes. Proc R Soc Lond B Biol Sci. 1987;231: 123–145. [Google Scholar]
- 98.Smith JM. Sexual selection and the handicap principle. J Theor Biol. 1976;57: 239–242. [DOI] [PubMed] [Google Scholar]
- 99.Husak JF, Swallow JG. Compensatory traits and the evolution of male ornaments. Behaviour. 2011;148: 1–29. 10.1163/000579510X541265 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
A) Rigid metal bar used to stabilize the transducer stationary during trials. B) Flexible, brass arms that bend during squeezing trials. C) Needles that the animals squeeze during trials. Squeezing force (red) causes deformation in brass arms (B). Deformation is recorded by strain gauges (blue) in a full bridge configuration, as they are placed under tension (T1 and T2) and compression (C1 and C2).
(TIFF)
(TIFF)
Data Availability Statement
All data are available through Harvard Dataverse: https://doi.org/10.7910/DVN/VZGYOI. Citation: O'Brien, Devin M;Boisseau, Romain P, 2018, "Replication Data for: Overcomic mechanical adversity in extreme hindleg weapons", https://doi.org/10.7910/DVN/VZGYOI, Harvard Dataverse, V1.