ABSTRACT
We carried out a retrospective observational study of 264 HER2-positive advanced breast cancer (ABC) patients to explore the efficacy of first-line treatment with pertuzumab/trastuzumab/taxane in real-world setting. Survival data were analyzed by Kaplan Meier curves and log rank test.
Median follow-up, length of pertuzumab/trastuzumab/taxane treatment and of pertuzumab, trastuzumab maintenance were 21, 4 and 15 months, respectively. The response rate was 77.3%, and the clinical benefit rate 93.6%. Median progression-free survival (mPFS) was 21 months, and median overall survival (mOS) was not reached.
When comparing patients by trastuzumab-pretreatment, similar PFS were observed, although a longer OS was reached in trastuzumab-naïve patients (p = 0.02). Brain metastases at baseline and their development in course of therapy were associated with significantly shorter PFS (p = 0.0006) and shorter OS, although at a not fully statistically relevant extent (p = 0.06).
The addition of maintenance endocrine therapy (ET) to pertuzumab/trastuzumab maintenance was associated with longer PFS (p = 0.0001), although no significant differences were detected in OS (p = 0.31). Results were confirmed by propensity score analysis (p = 0.003 and p = 0.46, respectively).
In multivariate models, longer PFS was related to lower Performance Status (PS) (p = 0.07), metastatic stage at diagnosis (p = 0.006) and single metastatic site (p < 0.0001). An OS advantage was observed with lower PS (p < 0.0001), single metastatic site (p = 0.004), no prior exposure to trastuzumab (p = 0.004) and response to pertuzumab-based treatment (p = 0.003). Our results confirm that trastuzumab/pertuzumab/taxane is the standard of care as first-line treatment of patients with HER2-positive ABC even in the real-world setting. Moreover, the double-maintenance therapy (HER2 block and ET) is strongly recommended when feasible.
Keywords: metastatic breast cancer, pertuzumab, trastuzumab, maintenance, HER2, first-line treatment, endocrine therapy
Introduction
Human epidermal growth factor receptor 2 (HER2) overexpression and gene amplification are represented in approximately 15–20% of breast cancers and are associated with increased aggressiveness, poor prognosis and shorter survival.1 In the advanced setting, the combination of chemotherapy, particularly taxanes and trastuzumab, a monoclonal antibody directed against membrane HER2 receptor, is able to determine high response rate and prolonged survival.2,3 Since the use of anthracycline in combination with trastuzumab showed increased risk of cardiotoxicity,1,4 alternative trastuzumab-based combinations have been used and have shown some efficacy in the advanced setting, e.g., vinorelbine, capecitabine, gemcitabine, letrozole or anastrozole.5-10
Trastuzumab-based triple combinations (i.e. trastuzumab + 2 chemotherapeutic agents) did not show advantage over double combinations (trastuzumab + 1 chemotherapeutic drug),11,12 thus, the standard first-line treatment was the combination of a taxane and trastuzumab. In the Cleopatra phase III randomized trial, Pertuzumab, a novel HER2 targeted humanized monoclonal antibody, showed, in combination with trastuzumab and docetaxel, a clear advantage both in progression free survival (PFS) and overall survival (OS) compared with trastuzumab and docetaxel.13-15 These results led to the approval of this regimen as definite first-line treatment for advanced HER2 positive breast cancer. Subsequently, a phase II study confirmed the efficacy and safety of pertuzumab, trastuzumab and weekly paclitaxel, as first and second-line treatment for advanced disease.16
So far, the combination of trastuzumab/pertuzumab and a taxane is considered the gold standard of first-line treatment in HER2 positive advanced breast cancer. Few and generally limited in size preliminary reports in patients treated outside of clinical trials seem to confirm the efficacy and tolerability of this regimen.17,18 Since patients treated in prospective randomized trials are usually selected based on strict inclusion/exclusion criteria, and do not necessary exemplify the real-life setting, we carried out a large retrospective observational study of advanced HER2 positive breast cancer patients treated as first-line treatment with pertuzumab/trastuzumab/taxane combination at several Italian cancer centres.
Results
Overall, we retrospectively evaluated 264 HER2 positive advanced breast cancer patients, treated with pertuzumab/trastuzumab and taxanes (docetaxel, 205/264, paclitaxel, 59/264), from September 2012 through August 2017 at 22 Italian cancer centres.
Main patient and tumor characteristics are listed in Table 1. In brief, median age was 53 years, median ECOG PS was 0. Ninety-seven patients (97/264; 36.7%) were pre- and 167/264 (63.3%) patients were post-menopausal at baseline. Seventy-nine (79/264) patients had tumors with both hormonal receptors negative, 53/264 patients had tumor expressing one hormonal receptor, and 132/264 patients had “triple positive” (ER, PgR, HER2 positive) tumors.
Table 1.
Main baseline characteristics of the study population (N:264).
| Characteristics | Patients, N (%) |
|---|---|
| Age | |
| Median (range) | 53 (29–80) |
| Histology | |
| Ductal | 234 (88.6) |
| Lobular | 9 (3.4) |
| Other | 21 (8.0) |
| Metastatic at diagnosis | |
| Yes | 119 (45.1) |
| Not | 145 (54.9) |
| Grading | |
| 1–2 | 82 (31.1) |
| 3 | 141 (53.4) |
| Unknown | 41 (15.5) |
| ER and/or PgR positive at initial diagnosis | |
| Yes | 183 (69.3) |
| Not | 81 (30.7) |
| HER2-positive at initial diagnosis | |
| Yes | 225 (85.2) |
| No | 39 (14.8) |
| HER2-status at study entry | |
| IHC 2+ (ISH amplified) | 57 (21.6) |
| IHC 3+ | 156 (59.1) |
| Positive, unknown | 51 (19.3) |
| Subtype at study entry | |
| Triple-positive | 132 (50) |
| ER or PgR positive | 53 (20.1) |
| ER and PgR negative | 79 (29.9) |
| ECOG Performance Status | |
| 0 | 185 (70.1) |
| 1 | 69 (26.1) |
| 2 | 10 (3.8) |
| Neoadjuvant chemotherapy | |
| Yes | 53 (20.1) |
| No | 211 (79.9) |
| Adjuvant chemotherapy | |
| Yes | 92 (34.8) |
| No | 172 (65.2) |
| Neoadjuvant trastuzumab | |
| Yes | 30 (11.4) |
| No | 234 (88.6) |
| Adjuvant trastuzumab | |
| Yes | 71 (26.9) |
| No | 193 (73.1) |
| Adjuvant hormonal therapy | |
| Yes | 89 (33.7) |
| No | 175 (66.3) |
| Adjuvant radiotherapy | |
| Yes | 87 (33.0) |
| No | 175 (67.0) |
| Metastatic sites | |
| Visceral | 163 (61.7) |
| Brain | 21 (7.9) |
| Bone-only | 34 (12.9) |
| Number of metastatic sites | |
| 1 | 137 (51.9) |
| 2 | 74 (28.0) |
| ≥3 | 53 (20.1) |
| Type of taxane associated with pertuzumab-trastuzumab | |
| Paclitaxel weekly | 59 (22.3) |
| Docetaxel every three weeks | 205 (77.7) |
Abbreviations: N, number; ER estrogen receptor, PgR, progesterone receptor
One hundred forty-five (145/264) patients had received previous neoadjuvant (53/264) or adjuvant (92/264) chemotherapy regimens, inclusive of trastuzumab in 30/53 and 71/92 patients, respectively. Ten (10/264) patients had not received previous trastuzumab in the early setting because diagnosed before its registration. Thirteen (13/264) patients had received endocrine therapy without chemotherapy, in combination with trastuzumab, in the adjuvant setting.
Among the 264 patients evaluated, 145 (54.9%) had recurrent disease, with a median disease-free interval of 53 months (range, 7–342), whereas 119 patients (45.1%) had de novo metastatic disease. At treatment starting, 61.7% of our patients had visceral metastases, 12.9% bone-only lesions, 7.9% asymptomatic brain metastases, and 48.1% had multiple metastatic sites.
Treatment received
All the patients received pertuzumab-based regimens as first-line chemotherapy treatment for HER2 positive advanced disease, and were evaluable for treatment efficacy. Median follow up was 21 months (range, 3–59). Median pertuzumab/trastuzumab and chemotherapy duration was 4 months (range, 1–16), with a median number of chemotherapy cycles of 6 (range, 1–9). In more detail, the median number of administered docetaxel cycles was 6 (range, 1–20) and the median number of weekly paclitaxel administrations was 16 (range, 2–33). Discontinuation of taxanes was most commonly due to cumulative haematological toxicity.
After the discontinuation of chemotherapy, 234 (88.6%) patients received pertuzumab/trastuzumab maintenance therapy, with a median duration of 15 months (range, 2–43), with 46% of the patients having been treated for more than 12 months in absence of disease progression. Endocrine maintenance treatment together with pertuzumab/trastuzumab administration was given to 103 (39%) patients, who represented the 55.6% of the endocrine receptor positive tumors. Brain radiotherapy was administered in 30 patients on maintenance therapy with pertuzumab and trastuzumab, while 10 patients had already been treated with radiation therapy for brain metastases before starting the first-line treatment. Through the follow up period, pertuzumab/trastuzumab maintenance treatment was discontinued in 144 (54.6%) patients. The most common reasons for discontinuation were disease progression (109 patients), toxicity (10 patients), patient decision (8 patients) or medical decision (17 patients).
Efficacy
Overall, we observed 40 complete responses (CR) (15.2%) and 169 partial responses (PR) (64%), for an overall response rate (ORR) of 77.3% (95%CI, 72.2–82.3) (Table 2). A stable disease (SD) was recorded in 15.5% of these patients. A clinical benefit (CB), defined as response or stable disease lasting at least 6 months, was observed in 247 patients (93.6%, 95%CI, 90.6–96.5). As showed in Table 3, not fully significant differences were observed when we analyzed objective responses according to ER/PgR status (p = 0.06).
Table 2.
Best responses to pertuzumab-based treatment.
| Best responses, Number (%) | |
|---|---|
| Complete response | 40 (15.2) |
| Partial response | 169 (64) |
| Stable Disease | 41 (15.5) |
| Progressive Disease | 14 (5.3) |
| Total | 264 (100) |
Table 3.
Best responses to pertuzumab-based treatment according to molecular subtype.
|
Responses |
||||
|---|---|---|---|---|
| Complete response N (%) |
Partial response N (%) |
Stable Disease N (%) |
Progressive Disease N (%) |
|
| Triple-positive | 14 (10.6) | 89 (67.4) | 22 (16.7) | 7 (5.3) |
| ER or PgR positive | 6 (11.3) | 38 (71.7) | 8 (15.1) | 1 (1.9) |
| ER and PgR negative | 20 (25.3) | 42 (53.2) | 11 (13.9) | 6 (7.6) |
| Chi square test: p = 0.06 | ||||
Abbreviations: N, number; ER estrogen receptor, PgR, progesterone receptor
Among those patients who were metastatic at diagnosis (119), we observed 18 CR (15.1%) and 81 PR (68.1%), for an ORR of 83.2% (95%CI, 76.5–89.9), and a CB rate of 95.8% (95%CI, 92.2–99.4). Among the 75 patients having received previous neoadjuvant/adjuvant trastuzumab, we observed 12 CR (16%), and 46 PR (61.3%), with an ORR of 77.3% (95%CI, 67.8–86.8). Stable disease was recorded in 15 patients (20%), and CB was reported in 72 patients (96%; 95%CI, 91.6–100). No differences in ORR emerged when we compared trastuzumab-naïve and pretreated patients (p = 0.64).
Overall response rate was slightly higher in patients with visceral metastases (83.4%), compared with other metastatic sites (74.6%), or bone-only involvement (67.6%), although at a not statistically significant extent (p = 0.07). In patients with baseline brain metastases (21 patients) ORR was 52.4%, while it was 81.5% in their counterpart (p = 0.002).
In our case series, a significant difference in response rate was observed between the type of taxane administered in combination with pertuzumab/trastuzumab, being ORR 82.9% with docetaxel and 66.1% with paclitaxel (p = 0.005).
All but 30 patients (27 due to progressive disease, 1 due to cardiotoxicity, 2 lost to follow-up) after chemotherapy discontinuation had received trastuzumab/pertuzumab as maintenance therapy. Overall, 37/234 patients (15.8%) further improved their response (Supplementary Table 1).
Among the 169 patients with ER and/or PgR positive tumors having received pertuzumab/trastuzumab maintenance, 103 (60.9%) patients received in addition endocrine maintenance therapy. Among them, 18 patients (17.5%) showed further improvement of response. Conversely, in 66 patients receiving only pertuzumab/trastuzumab without hormonal treatment, response rate was further improved in 8 cases (12.1%) (p = 0.39). In this subgroup of patients, the median length of trastuzumab/pertuzumab maintenance therapy was 8 months (range, 1 to 37), with a median length of maintenance endocrine therapy of 11 months (range, 1 to 55). Central nervous system as site of disease progression was observed in 33 patients without previous brain metastases (13.6%), and in 12 patients (57.1%) with baseline brain metastases.
Long-term outcomes
Overall, in these 264 patients, median PFS was 21 months (95%CI, 17–25), whereas median OS was not reached (Figure 1). Rates of 1-year PFS and 2-year OS were also computed and reported in Table 4. No statistical differences were observed when we analyzed PFS and OS according to hormonal receptors expression or not (p = 0.57 and p = 0.23, respectively). Comparable median PFS were reached when groups were compared by trastuzumab pretreatment, being 17 months in pretreated and 23 months in naïve patients (p = 0.10). Conversely, a longer OS was observed in trastuzumab naïve patients (p = 0.02). Visceral involvement or bone-only metastases did not impact PFS (p = 0.40 and 0.10, respectively) or OS (p = 0.58 and 0.70, respectively).
Figure 1.
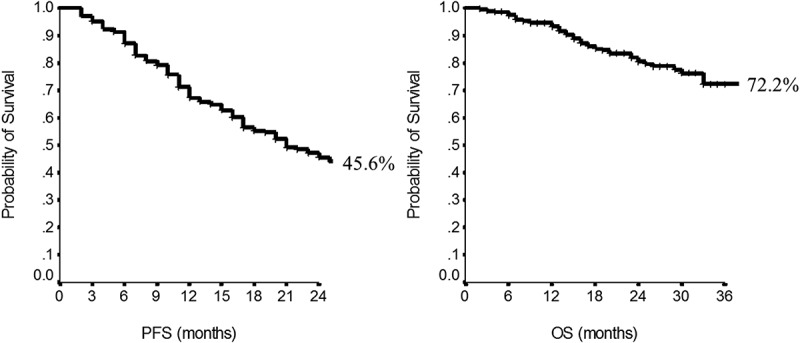
Progression-Free Survival (a) and Overall Survival (b) in the overall population.
Table 4.
1-year PFS and 2-year OS according to tumor and patient characteristics.
| Characteristics | N | 1-year PFS | p | 2-year OS | p |
|---|---|---|---|---|---|
| Overall | 264 | 67.2 | - | 80.5 | - |
| Molecular subtype | |||||
| Triple-positive | 53 | 72.9 | 87.8 | ||
| ER or PgR positive | 132 | 66.7 | 80.1 | ||
| ER and PgR negative | 79 | 63.7 | 0.57 | 76.3 | 0.23 |
| Trastuzumab pretreatment | |||||
| yes | 75 | 58.0 | 67.5 | ||
| no | 189 | 70.8 | 0.10 | 85.6 | 0.02 |
| Visceral involvement | |||||
| Yes | 163 | 62.8 | 79.6 | ||
| No | 101 | 74.5 | 0.4 | 81.7 | 0.58 |
| Bone-only metastases | |||||
| Yes | 34 | 76.3 | 85.0 | ||
| No | 230 | 65.9 | 0.10 | 79.9 | 0.70 |
| Brain metastases | |||||
| No | 210 | 69.6 | 83.9 | ||
| Yes, pretreatment | 21 | 70.8 | 77.7 | ||
| Yes, during pertuzumab treatment | 33 | 51.0 | 0.0006 | 65.6 | 0.06 |
| Type of taxane administered | |||||
| Docetaxel | 205 | 65.0 | 79.4 | ||
| Paclitaxel | 59 | 74.8 | 0.44 | 84.7 | 0.47 |
| Maintenance ET | |||||
| Yes | 103 | 83.2 | 89.5 | ||
| No | 67 | 59.6 | 0.0001 | 76.9 | 0.31 |
| HER2 status | |||||
| 3+ | 156 | 73.1 | 85.1 | ||
| 2+, amplified | 57 | 60.7 | 0.18 | 63.9 | 0.002 |
| HER2 2+, maintenance ET | |||||
| Yes | 26 | 79.8 | 85.4 | ||
| No | 16 | 56.3 | 0.12 | 49.2 | 0.15 |
| HER2 3+, maintenance ET | |||||
| Yes | 58 | 86.7 | 87.5 | ||
| No | 33 | 61.7 | 0.02 | 82.4 | 0.62 |
Abbreviations: ET, endocrine therapy; PFS, progression free survival; OS, overall survival
In patients without brain metastases at baseline we observed a longer median PFS (26 months; 95%CI, 22–30), compared to patients with known brain metastases (20 months; 95%CI 13–27) or patients who developed brain metastases in course of pertuzumab-based treatment (13 months; 95%CI, 9–17) (p = 0.0006). Accordingly, rates of 2-year OS were 83.9% in patients without brain metastases at baseline, 77.7% in patients with documented brain metastases, and 65.6% in patients who developed brain metastases while on treatment.
The type of taxane administered in combination with pertuzumab/trastuzumab had no significant impact on long-term outcomes, being PFS and OS similar (p = 0.44 and p = 0.47, respectively). Maintenance endocrine therapy, added to maintenance pertuzumab and trastuzumab, was associated with longer median PFS, being 28 months (95%CI, 26–31), versus 17 (95%CI, 13–21) months in patients without maintenance endocrine therapy, respectively (p = 0.0001) (Figure 2(a,b)). Conversely, no differences have been observed to date in OS according to maintenance endocrine treatment (p = 0.31). Results were confirmed when adjusting for propensity score, with a median PFS of 17 months (95%CI, 12–22) in patients who did not receive hormonal therapy and 29 months (95%CI, 25–33) in patients who received hormonal therapy (p = 0.003). No differences were observed in OS according to maintenance endocrine treatment (p = 0.46) (Figure 2(c,d)).
Figure 2.

Progression-free survival (PFS, a) and overall survival (OS, b) according to administration of maintenance hormonal therapy and adjusted for propensity score (PFS, c; OS, d) .
No significant differences were observed in median PFS according to HER2 hyperexpression/amplification (p = 0.18). Conversely, HER2 status impacted OS, with rates of 2-year OS of 63.9% in patients with HER2 2+/amplified tumors and 85.1% in patients with HER2 3+ tumors. In patients with HER2 2+/amplified tumors, no differences in median PFS were observed depending on double maintenance treatment (p = 0.12). Conversely, in the HER2 3+ subset, maintenance endocrine therapy added to double HER2 block led to a longer PFS (p = 0.02). In terms of OS, no statistical differences were seen, even if patients with HER2 2+/amplified tumors who received maintenance endocrine therapy showed a non-significantly longer OS (p = 0.15). In patients with HER2 3+ tumors, endocrine maintenance treatment had no impact in terms of OS (p = 0.62) (Table 4).
In multivariate analysis (Table 5), a PFS benefit was associated with lower PS (p = 0.07), metastatic setting at diagnosis (p = 0.006) and presence of a single metastatic site (p < 0.0001). Parameters related to an OS advantage were lower PS (p < 0.0001), presence of a single metastatic site (p = 0.004), no prior treatment with trastuzumab (p = 0.004) and a clinical response to pertuzumab-based treatment (p = 0.003).
Table 5.
Multivariate analysis.
| PFS | HR | IC95% | P |
|---|---|---|---|
| Performance status (1–2 vs 0) | 1.39 | 0.97–1.99 | 0.07 |
| Metastases at diagnosis (no vs yes) | 1.63 | 1.15–2.32 | 0.006 |
| N° of metastatic sites (≥2 vs 1) |
2.04 |
1.44–2.89 |
<0.0001 |
| OS |
HR |
IC95% |
P |
| Performance status (1–2 vs 0) | 3.09 | 1.72–5.56 | <0.0001 |
| N° of metastastatic sites (≥2 vs 1) | 2.49 | 1.34–4.61 | 0.004 |
| Pretreatment with Trastuzumab (yes vs no) | 2.36 | 1.31–4.25 | 0.004 |
| Response to treatment (no vs yes) | 2.54 | 1.38–4.65 | 0.003 |
Abbreviations: HR, hazard ratio; IC95%, confidence interval; N°, number; PFS, progression free survival; OS, overall survival
Overall, no new safety concerns were identified. The most common adverse events were diarrhea, rash, mucosal inflammation and pruritus. No episodes of febrile neutropenia were reported. After the discontinuation of taxanes, the incidence of all adverse events decreased considerably. Unfortunately, we are unable to provide more extensive information on safety.
Regarding cardiotoxicity, all the recruited patients had a baseline a LVEF > 50%. Overall, 25 patients (9.4%) experienced cardiac toxicity of any grade. Cardiotoxicity resulted in permanent discontinuation of treatment in 9 patients (3.4%).
Discussion
Our analysis supports the efficacy of first-line treatment with pertuzumab, trastuzumab and docetaxel/paclitaxel in a non-selected population of HER2 positive advanced breast cancer patients, with a response rate of 77.3%, a CB of 93.6%, and a median PFS of 21 months. Our results are consistent with the findings from the Cleopatra trial.14
There are some differences between our patient population and patients enrolled in the Cleopatra trial. In regard to patients pretreated with trastuzumab in the early setting, in our study they were 75 (28.4%), whereas in the pivotal trial they were 47 (11.7%). Moreover, in our patient population there were less patients with visceral lesions (61.7% versus 78.1%), more patients had hormonal receptor positive disease (70.1% versus 47.0%), and 103 (39%) patients received maintenance endocrine treatment, which was not allowed in the Cleopatra trial. Furthermore, patients with baseline brain metastases were included in our analysis, whereas were excluded from the registrative trial. In our patient population the use of docetaxel was associated with higher ORRs compared to paclitaxel, i.e., 82.9% versus 66.1% (p = 0.005). This latter difference did not translate into long-term outcome, and may be related to different tumor and patient characteristics, or to the choice of paclitaxel in frail patients with more extensive metastatic involvement.
Overall, maintenance endocrine therapy was added to maintenance pertuzumab/trastuzumab in 103 patients. As expected, this subgroup of patients, receiving both maintenance treatments, had the most favorable long-term outcome, with a median PFS of 28 months, and a 2-year OS of 89.5%. The use of a propensity score minimized the chances that the differences observed between the groups compared were driven by unevenly distributed baseline characteristics for the patients included, and increased our confidence in this study results. These latter data are not comparable with the results from the pivotal trial, since in the Cleopatra trial maintenance endocrine treatment was not allowed. We thus relied on the phase II PERTAIN study, which evaluated the benefit of adding pertuzumab to trastuzumab plus an aromatase inhibitor in women with locally advanced or metastatic hormone receptor–positive, HER2-positive breast cancer. Compared to trastuzumab plus endocrine therapy alone, the addition of pertuzumab yielded a statistically significant 3-month prolongation of PFS and more durable responses19.
The differences observed in long-term outcomes by HER2 status (2+ amplified or 3+) in the present trial are probably related to the observational nature of the study and limited number of patients included in this subset analysis. Our confidence toward this specific result is additionally lessened by the high number of participating centers and lack of a centralized revision for HER2 status.
When globally considered, our results favorably compare with those from the registrative trial. Nevertheless, it is worth underlying that the present findings are observed in an unselected patient population. As such, our study patients showed comorbidities (more often represented by hypertension, diabetes, obesity and hypothyroidism), and/or involvement of unfavorable metastatic sites, such as brain metastases at baseline. In addition, our analysis included data on a considerable number of patients with early recurrence or who recurred after previous trastuzumab in the early setting.
We reported a comparable efficacy of the regimen of interest in subgroups differing by trastuzumab-pre-treatment, with a median PFS of 17 months, compared with 23 months observed in trastuzumab naïve patients (p = 0.10), even if a higher rate of 2-year OS (85.6% vs 67.5%) was observed in trastuzumab naïve patients.
Data concerning the efficacy of trastuzumab-based first-line treatment in patients relapsing after neo/adjuvant trastuzumab are still controversial, even if there is a trend towards a lower benefit in patients recurring after adjuvant/neoadjuvant trastuzumab.20,21 The combination of pertuzumab and trastuzumab seems to be superior to the use of one single HER2 targeted agent.13,14,22 Indeed, pertuzumab is thought to potentiate trastuzumab effect by preventing dimerization of HER2, stimulating antibody-dependent cell-mediated cytotoxicity (ADCC) and, due to the different HER2 epitopes as site of binding, pertuzumab is supposed to exert synergistic action with a more complete block of HER2 signaling.23,24 A phase II study with pertuzumab and trastuzumab without chemotherapy in 66 pretreated patients who developed disease progression while on trastuzumab-based therapy showed ORR and CB of 24.2% and 50%, with a median PFS of 5.5 months, confirming the efficacy of adding pertuzumab to trastuzumab even in heavily pretreated patients progressing under trastuzumab-based regimens.25 In the Cleopatra trial, the small subset of patients previously exposed to trastuzumab in the early setting, i.e., 88 patients, had substantially the same favorable outcome in PFS (HR 0.62) than trastuzumab naïve patients (HR 0.60),13 thus suggesting the advantage of adding pertuzumab even in patients failing early trastuzumab-based treatments. Moreover, in the registrative trial, there is an OS advantage in the pertuzumab arm even in trastuzumab pretreated patients (HR 0.68).14
In our patient population there was a subset of early recurred patients (while on or before 12 months from adjuvant trastuzumab completion, N:14; 5.3%), whereas in the Cleopatra trial there was a 12 month-interval between trastuzumab end and study enrollment. Notwithstanding these differences, even in this subset of patients, treatment did not show lower efficacy. Unfortunately, we do not have data on subsequent treatment-lines, which may have influenced OS results.
Another key point is the length of the taxane-based chemotherapy associated with pertuzumab/trastuzumab administration. In our study, the median duration of docetaxel therapy was shorter than in the Cleopatra trial (6 vs 8 cycles, respectively), with similar results in efficacy. In the registrative study, at least six cycles of docetaxel were recommended (13). In this setting, the use of chemotherapy is not a matter of debate. However, decisions concerning the number of docetaxel cycles should be taken at the individual patient level and in light of considerations pertinent to the performance status, burden of disease, and toxicity. Moreover, maintenance therapy with pertuzumab and trastuzumab extends the efficacy of treatment without relevant adverse events. In HER2+/ER+ tumors, our data also show that the addition of endocrine maintenance therapy to pertuzumab/trastuzumab improves long term outcomes and represents a suitable option in patients who do not tolerate chemotherapy.
A retrospective study has recently evaluated 155 patients treated with trastuzumab/pertuzumab and a taxane as first-line treatment at 8 Italian centers, and compared results with those from the Cleopatra trial.17 Patients enrolled in this observational trial had more frequently endocrine positive tumors, less visceral metastases and more patients had received adjuvant trastuzumab. The median number of docetaxel cycles was 7, and median PFS was 27.8 months, with no relevant differences in term of efficacy across the subgroups analyzed. This latter PFS estimate is higher than that in the registrative trial, possibly reflecting differences in tumors and patients characteristics, endocrine maintenance treatment, but also different timing in disease re-evaluation between randomized trials and clinical practice.
Another retrospective observational study investigated the outcome of HER2 positive advanced breast cancer patients treated in real-world oncology practice with pertuzumab as first-line treatment.18 Two hundred and forty-nine (249) patients received trastuzumab, pertuzumab and taxane as first-line for advanced disease. Sixty-one percent (61%) had previously received neoadjuvant/adjuvant trastuzumab, and 60% of these patients had hormonal receptor positive tumors. The median number of pertuzumab cycles was 11 (range, 1–41), with a median duration of 7.3 months (range, 0.7–29). Two-hundred and twenty-six patients received docetaxel, 21 paclitaxel, and 2 nab-paclitaxel. The median number of docetaxel cycles was 6 (range, 1–27). Subsequently, about 25% of patients with hormonal receptor positive tumors received endocrine therapy. Median PFS was 16.9 months, with an estimated 1 year PFS rate of 62%.
A recent metanalysis has evaluated the efficacy and safety of pertuzumab-based treatment in advanced HER2 positive breast cancer patients. It included 5 randomized clinical trials involving 3,742 patients. Overall, the regimen pertuzumab/trastuzumab/docetaxel was associated with a significant reduction in death (HR: 067, 95%CI, 0.57–0.78) and an improved OS (HR 0.66, 95%CI 0.35–0.67), and PFS (HR 0.64, 95%CI 0.58–0.71), without a significant increase in cardiotoxicity.26
Our study has some limitations. First, the length of follow up was relatively short and overall insufficient to draw firm conclusions in terms of OS. More generally, our results must be interpreted in light of multiple biases and weaknesses mainly, though not exclusively, stemming from our study design, i.e., retrospective, observational design. Our study further lacked detailed data on toxicity and patients’ co-morbidities. Missing data represent a common limitation in the conduct of studies including patients from real life setting, since the systematic collection of data may not easily comply with the activities related to the clinical routine. Our study also has some relevant strengths, since it provides evidence in support of the activity of trastuzumab, pertuzumab and a taxane-regimen in real-world practice and, to our knowledge, it is the largest observational case series made available thus far. Moreover, our results suggest the relevant and favorable impact of endocrine maintenance treatment in patients with endocrine receptor positive tumors, and in patients with prior exposure to trastuzumab in the early setting.
Our results support the administration of trastuzumab, pertuzumab, and taxane as first-line treatment of patients with HER2 positive advanced breast cancer in every-day clinical practice. Furthermore, to our knowledge, this is the study with the largest number of patients with available data on the combination of HER2 double-block and hormonal therapy as a maintenance strategy. Our data fully support that the use of all treatments available to the clinicians in this subset of patients positively affects the outcome of our patients.
Patients and methods
We retrospectively identified 264 consecutive Caucasian, female patients from the real life setting who received pertuzumab/trastuzumab and taxanes (docetaxel or paclitaxel) at 22 Italian oncologic centres. None of these patients had been previously included in randomized clinical trials. The follow-up was stopped in September 2017, that is, when a median follow-up of at least 12 months was reached, and statistical analysis performed. Our primary objective was to evaluate the efficacy of pertuzumab, trastuzumab and taxane (docetaxel/paclitaxel)-based regimens in a non-selected patient population. Secondarily, we aimed to explore the efficacy of pertuzumab-based treatment in pre-defined subsets of patients identified according to patient- and disease-related key features.
Pertuzumab, trastuzumab and docetaxel or paclitaxel, were administered intravenously according to the current guidelines, until disease progression or death, unacceptable toxicity, or patient refusal. Patients not progressing while on chemotherapy treatment were usually given maintenance pertuzumab and trastuzumab. Patients with hormonal receptor positive tumors were included in the analysis independently on the administration of maintenance endocrine therapy. Treatment efficacy was evaluated every 3 months as standard practice according to RECIST criteria. Our study was approved by the Ethic Committees of the coordinating and satellite centres and conducted according to the Helsinki Declaration. All the patients released a written informed consent.
Data collection
Medical records were retrieved for demographic, clinical and molecular features, previous treatments and related outcomes, number and site of metastases at the time of treatment starting, objective response, date at disease progression and date at last follow-up or death. Pathology assessment was performed in surgical specimens of primary tumors at the participating centers. When missing, the molecular features were centrally evaluated in formalin-fixed, paraffin-embedded tissue sections. In 93 patients (35.2%), a re-biopsy of a single metastatic lesion was performed. HER2 status was evaluated by immunohistochemistry, and expression level 3+ (DAKO Herceptest) was considered positive. Fluorescence, chromogenic, or silver in situ hybridization (FISH, CISH, SISH, respectively) were performed to identify HER2 amplification in case of HER2 2+ staining. Anonymized data were entered into a dedicated database.
Statistical analysis
Variables were assessed by Pearson Chi-Square test or Fisher Exact test. Their impact on survival was tested in Cox uni/multivariate models. The multivariate Cox hazard model was built using stepwise regression (forward selection). Enter and remove limit were p = 0.10 and p = 0.15. The following variables were considered: age, ECOG Performance Status (PS), histology, ki67 percent expression (%), molecular subtype, stage at diagnosis, type of surgery, adjuvant and neoadjuvant treatment, disease-free survival, trastuzumab pretreatment, type and number of metastatic sites and objective response. Survival was addressed by the Kaplan–Meier method and log-rank test. Significance was defined at p ≤ 0.05 level.
The effect of covariates potentially acting as confounders in a non-randomized cohort was minimized by propensity score match, which allowed to create patient groups who were similarly likely to receive a given treatment based on their baseline characteristics.27 The SPSS software was used for statistical evaluations (SPSS version 21.0, SPSS Inc., Chicago, Illinois, USA).
Funding Statement
This work was supported by Consorzio Interuniversitario Nazionale per la Bio-Oncologia (CINBO).
Acknowledgments
We thank Ana Maria Edlisca and Rosa Carbone for editorial assistance and data managing.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL.. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001. 15;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Antón A, Lluch A, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 4.Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, Gelman R, Winer EP. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007;110(5):965–972. doi: 10.1002/cncr.22885. [DOI] [PubMed] [Google Scholar]
- 6.Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, Karlsson P, Tange UB, Sørensen PG, Møller S, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol. 2011;29(3):264–271. doi: 10.1200/JCO.2010.30.8213. [DOI] [PubMed] [Google Scholar]
- 7.Fountzilas G, Christodoulou C, Tsavdaridis D, Kalogera-Fountzila A, Aravantinos G, Razis E, Kalofonos HP, Papakostas P, Karina M, Gogas H, et al. Paclitaxel and gemcitabine, as first-line chemotherapy, combined with trastuzumab in patients with advanced breast cancer: a phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG). Cancer Invest. 2004;22(5):655–662. [DOI] [PubMed] [Google Scholar]
- 8.Yardley DA, Burris HA 3rd, Simons L, Spigel DR, Greco FA, Barton JH, Shipley D, Drosick D, Hainsworth JD. A phase II trial of gemcitabine/carboplatin with or without trastuzumab in the first-line treatment of patients with metastatic breast cancer. Clin Breast Cancer. 2008;8(5):425–431. doi: 10.3816/CBC.2008.n.051. [DOI] [PubMed] [Google Scholar]
- 9.Huober J, Fasching PA, Barsoum M, Petruzelka L, Wallwiener D, Thomssen C, Reimer T, Paepke S, Azim HA, Ragosch V, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer: results of the eLEcTRA trial. Breast. 2012;21(1):27–33. doi: 10.1016/j.breast.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27(33):5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 11.Perez EA, Suman VJ, Rowland KM, Ingle JN, Salim M, Loprinzi CL, Flynn PJ, Mailliard JA, Kardinal CG, Krook JE, et al. Two concurrent phase II trials of paclitaxel/carboplatin/trastuzumab (weekly or every-3-week schedule) as first-line therapy in women with HER2-overexpressing metastatic breast cancer: NCCTG study 983252. Clin Breast Cancer. 2005;6(5):425–432. doi: 10.3816/CBC.2005.n.047. [DOI] [PubMed] [Google Scholar]
- 12.Valero V, Forbes J, Pegram MD, Pienkowski T, Eiermann W, von Minckwitz G, Roche H, Martin M, Crown J, Mackey JR, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol. 2011;29(2):149–156. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 13.Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swain SM, Kim SB, Cortés J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a multicenter, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth LM, Iyengar NM, Chen MF, Popper SM, Patil S, Wasserheit-Lieblich C, Argolo DF, Singh JC, Chandarlapaty S, Sugarman SM, et al. Weekly paclitaxel with trastuzumab and pertuzumab in patients with HER2-overexpressing metastatic breast cancer: overall survival and updated progression-free survival results from a phase II study. Breast Cancer Res Treat. 2016;158(1):91–97. doi: 10.1007/s10549-016-3851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Placido S, Giuliano M, Schettini F, Von Arx C, Buono G, Riccardi F, Cianniello D, Caputo R, Puglisi F, Bonotto M, et al. Human epidermal growth factor receptor 2 dual blockade with trastuzumab and pertuzumab in real life: italian clinical practice versus the CLEOPATRA trial results. Breast. 2018;38:86–91. doi: 10.1016/j.breast.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Robert NJ, Goertz HP, Chopra P, Jiao X, Yoo B, Patt D, Antao V. HER2-positive metastatic breast cancer patients receiving pertuzumab in a community oncology practice setting: treatment patterns and outcomes. Drugs Real World Outcomes. 2017;4(1):1–7. doi: 10.1007/s40801-016-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arpino G, Ferrero J-M, de la Haba-Rodriguez J, et al. Primary analysis of PERTAIN: A randomized, two-arm, open-label, multicenter phase II trial assessing the efficacy and safety of pertuzumab given in combination with trastuzumab plus an aromatase inhibitor in first-line patients with HER2-positive and hormone receptor-positive metastatic or locally advanced breast cancer. 2016 San Antonio Breast Cancer Symposium, Abstract S3–04, 2016 Dec 8. [Google Scholar]
- 20.Rier HN, Levin MD, van Rosmalen J, Bos MMEM, Drooger JC, de Jong P, Portielje JEA, Elsten EMP, Ten Tije AJ, Sleijfer S, et al. First-line palliative HER2-targeted therapy in HER2-positive metastatic breast cancer is less effective after previous adjuvant trastuzumab-based therapy. Oncologist. 2017;22(8):901–909. doi: 10.1634/theoncologist.2016-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambertini M, Ferreira AR, Poggio F, Puglisi F, Bernardo A, Montemurro F, Poletto E, Pozzi E, Rossi V, Risi E, et al. Patterns of care and clinical outcomes of first-line trastuzumab-based therapy in HER2-positive metastatic breast cancer patients relapsing after (neo)adjuvant trastuzumab: an italian multicenter retrospective cohort study. Oncologist. 2015;20(8):880–889. doi: 10.1634/theoncologist.2015-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a multicenter, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 23.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 24.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5(4):317–328. [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, Gelmon KA, Verma S, Wardley A, Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28(7):1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian T, Ye J, Zhou S. Effect of pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer: A meta-analysis. Int J Clin Pharmacol Ther. 2017;55(9):720–727. doi: 10.5414/CP202921. [DOI] [PubMed] [Google Scholar]
- 27.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


