Abstract
Introduction
Patients affected by the behavioral variant frontotemporal dementia (bvFTD) frequently experience, at a delayed onset, abnormal eating behavior involving increased food intake. Although delusional food-related symptoms have attracted much attention, the behavioral and neural features of food aversion manifestations in bvFTD remain poorly documented.
Methods
We describe the rare case of a patient with bvFTD presenting with lack of interoception for swallowing and digestion, coupled with a dramatic food aversion at onset. We also compared his MRI scan to 84 healthy individuals using a voxel-based morphometry approach.
Results
We found gray matter density reductions involving the postcentral gyrus bilaterally, insulae, and right medial orbitofrontal cortex.
Discussion
Our results shed new light on the behavioral and neuroanatomical features of food aversion and interoception deficits in bvFTD, suggesting that besides orbitofrontal cortex, also a distributed system associated with interoception might play a role in such behavioral manifestation.
Keywords: Behavioral variant frontotemporal dementia, Interoception, Eating disturbances, Insula, Voxel-based morphometry
1. Introduction
Frontotemporal dementia (FTD) embraces a broad spectrum of behavioral disorders, categorized in three main phenotypes: a behavioral (bvFTD), semantic, and language variants [1], [2]. The bvFTD is one of the most common manifestations of FTD, and according to the recently revised diagnostic criteria [3], at least three of six core symptoms should be present. There are some earlier manifestations concerning (1) behavioral disinhibition, (2) apathy or inertia, (3) loss of sympathy or empathy, (4) perseverative or compulsive behavior, and other later symptoms such as (5) executive dysfunctions and (6) hyperorality or dietary changes.
The phenomenology of eating disturbances in bvFTD is complex and poorly documented. Patients with bvFTD may show increased food intake with pathological sweet preference, altered flavor processing, swallowing difficulties, and hyperphagia [4]. Marked hyperphagia is specifically distinctive of bvFTD [5]. Such symptoms may cause an increase in weight in patients with bvFTD, who present with a higher body mass index compared with other types of dementia [6]. Evidence on the neural correlates of such symptoms is scarce. It has been shown that pathological sweet preference and increased food consummation could be associated with a right hemisphere dysfunctional networks involving the insula and frontostriatal regions [5], [7], [8].
If on the one hand much attention has been paid to “productive” food-seeking behaviors, food aversion with weight loss have rarely been studied [4], [9], and its neural correlates are currently unknown. Thompson et al. [10] have described two cases of food aversion in frontotemporal lobar degeneration. Notably, their patients were affected by semantic dementia, and food-related symptoms appeared at a late stage of the pathology. Respectively at 7 and 5 years from onset, the patients presented with symptoms-like anorexia and bulimia nervosa. The neuroanatomical pattern has not been reported.
Here, we describe an intriguing case of a patient with bvFTD presenting with a food aversion at onset, involving interoceptive self-awareness concerning food intake and digestion, coupled with a dramatic weight loss. The case is summarized, and possible neuroanatomical correlates discussed [11].
2. Methods
2.1. Case report
MT is a 55 year-old man, with 13 years of education. In March 2015, he was diagnosed with bvFTD. The patient showed symptoms involving the cognitive (executive and attentional dysfunctions) and affective-emotional domains (diminished social interest, apathy, and low empathy). At the neurological examination, no focal symptoms were found, including no signs of dysphagia (Gugging Swallowing Screen [GUSS]). At the neuropsychological assessment, MT showed executive (cognitive flexibility, phonemic fluency, working memory), attentional (both sustained and divided), episodic (no hippocampal amnesic syndrome), and visuospatial memory deficits (see Table 1). At the Neuropsychiatric Inventory, his wife indicated the presence of delusions, anxiety, agitation, wandering, and appetite/eating disorders (see Table 1). In particular, for the appetite/eating dimension, his wife reported a loss of appetite and a severe weight loss. Intriguingly, he claimed to be unable to perceive “food in his stomach,” and therefore he supposed to be unaware of his sense of satiety. Furthermore, MT referred a lack of self-perception of swallowing and “digestion.” These behavioral signs were coupled with severe anxiety and obsessive thoughts concerning food and eating that caused the significant body weight loss. The patient claimed: “I could not feel food in my stomach” or “It does not seem to me that I have had lunch.” Besides, his wife referred that he manifested with severe anxiety and intense anticipatory fear regarding the subsequent lack of perception for food digestion: “What if I cannot feel anything?” or “What if I will not be able to swallow my meal?” declaring “I am never hungry” to possibly avoid eating at mealtime. The patient did not show abnormal eating behavior such as putting too much food in his mouth at once, either a change in food preference. He also did not develop eating behaviors such as eating the same types of food each day or eating the food in the same order.
Table 1.
Results of the neuropsychological and neuropsychiatric evaluations
| Test | MT's score |
|---|---|
| Neuropsychological assessment | |
| Mini-Mental State Examination | 20∗ |
| Attentional Matrices | 41 |
| Trail Making Test | |
| A | 92∗ |
| B | 324∗ |
| Digit Span | |
| Forward | 4 |
| Backward | 2∗ |
| Corsi Span | 4 |
| Phonemic fluency | 5∗ |
| Semantic fluency | 24 |
| Naming Test | 57 |
| Free and Cued Selective Reminding | |
| Immediate recall | 10∗ |
| Delayed recall | 3∗ |
| Sensibility index | 0.92 |
| Rey Complex Figure Copy | |
| Copy | 34 |
| Delayed recall | 3.5∗ |
| Neuropsychiatric Inventory | |
| Delusions | F = 5; S = 3; D = 5 |
| Agitation/aggression | F = 2; S = 2; D = 3 |
| Depression/dysphoria | F = 2; S = 2; D = 3 |
| Anxiety | F = 4; S = 3; D = 4 |
| Apathy/indifference | F = 4; S = 3; D = 5 |
| Motor disturbance | F = 2; S = 2; D = 3 |
| Appetite/eating | F = 4; S = 3; D = 5 |
NOTE. For the Neuropsychiatric Inventory subscores, the F stands for frequency, S for severity, and D for distress of the symptomatology.
A clinical deficit.
2.2. Neuroimaging
The patient underwent two high-resolution volumetric MRI T1-weighed examinations (collected over 1-year period). The MRI scans were performed on a 1.5-T GE Signa scanner. A high-resolution T1-weighted anatomical scan was acquired using a 3D-SPGR sequence (flip angle = 20°, TE = 2.92 ms, TR = 9.16 ms, acquisition matrix: 256 × 256; slice thickness = 1 mm, interslice gap = 0 mm, and voxel size = 1 × 1 × 1 mm). The volumetric MRI scans included 150 slices acquired on oblique sections parallel to the AC-PC line to cover the entire brain volume.
Data were analyzed using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk), with an optimized voxel-based morphometry (VBM) protocol. The analyses were based on segmented, modulated, 10 × 10 × 10 mm smoothed, gray matter scans. The patient's MRIs were compared to the data of 84 healthy controls (age range: 18–84; mean age: 38.4 years) scanned with the same MRI protocol. A voxel-by-voxel comparison of gray matter density was performed using the t-statistics after removing the effect of global gray matter estimates using proportional scaling, the effect of the age of the participants being covaried out. The two scans of the patient were modeled separately in the design matrix. We describe only results meeting either a voxel-level P < .05 family-wise error rate–corrected threshold or a P < .001 uncorrected threshold belonging to clusters significant at P < .05 family-wise error rate corrected for their spatial extent.
3. Results
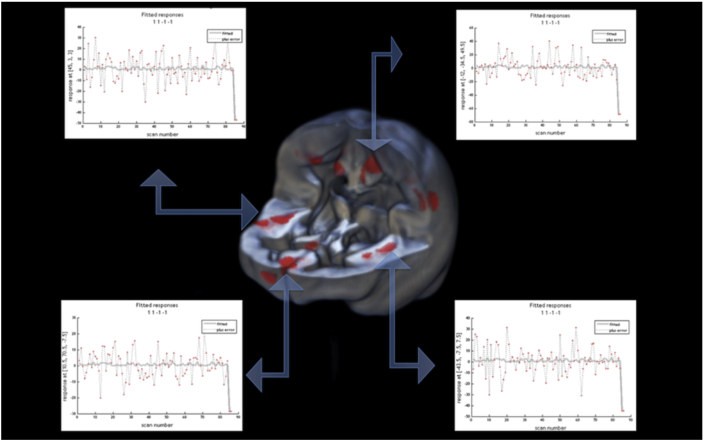
At a clinical level, the MRIs did not show any signs of gray matter density alterations. We then analyzed the two MRI scans using VBM. MT showed significant gray matter density reductions involving the dorsal medial subdivision of the postcentral gyrus bilaterally (X = −12; Y = −34; Z = 49; z-score = 5.9; X = 14; Y = −22; Z = 42; z-score = 4.2), the insulae (X = 45; Y = 3; Z = 3; z-score = 4.9; X = −44; Y = −7; Z = 7; z-score = 4.7), and right medial orbitofrontal cortex (X = 10; Y = 70; Z = −8; z-score = 4.6) (see Fig. 1).
Fig. 1.
Areas of significant reduction of gray matter density (P < .05 cluster corrected; forming cluster threshold P < .001) overlapped on a 3D rendering of an averaged VBM gray matter template. The last two points of the data plots represent the data points of the two MRI examinations in MT. The preceding data points are derived from 84 healthy controls.
4. Discussion
Patients with bvFTD commonly experience productive symptoms involving increase in appetite, binges, or overeats at mealtime [4]. As a consequence, superior calories intake and weight increase in bvFTD compared with other types of dementia have been reported [5]. These symptoms may appear later with the evolution of the pathology and progress at a higher rate four years after the onset [12]. To our knowledge, this is the first case of lack of interoception for swallowing and digestion with a decreased food intake and weight loss at onset in a patient with bvFTD.
On the basis of the VBM results, one might speculate that MT's dramatic food-related behavioral signs are the result of dysfunctions involving specific brain networks. We found gray matter density reductions in the insula, a region typically associated with the representation of the one's own body and interoceptive self-awareness [13], [14]. Furthermore, reductions of the medial orbitofrontal cortex may explain the patient food aversion. It has been previously demonstrated that such brain area is involved in food intake and food reward [15]. Finally, the lack of perception of food swallowing could be due to the selective atrophy found in the postcentral gyri. Indeed, neural activity in the dorsal medial somatosensory cortices, in stereotactic coordinates almost identical to our foci of gray matter reduction, has been reported during swallowing perception in an fMRI study on healthy subjects [16].
So far, there are only a few available studies using the VBM approach, which have specifically investigated only delusional eating disturbances in FTD. Pathological sweet preference and hyperphagia have been associated with atrophy of the posterolateral orbitofrontal cortex bilaterally, right anterior insula, and anterolateral orbitofrontal cortex bilaterally [7]. Another study has shown that overeating behavior in six patients with FTD was associated with atrophy of the insular, and the orbitofrontal-striatal circuit in the right hemisphere [8]. Only one recent study has demonstrated that increased calories intake in patients with bvFTD was correlated with a decrease in gray matter density in the cingulate cortices, inferior temporal structures extending posteriorly, the thalami, right hippocampus, right cerebellum, occipital cortex, and lingual gyrus [5]. In this background, we complemented previous findings confirming the role of the insula and orbitofrontal cortex in eating disturbances in bvFTD. We further suggested that other brain areas involved in interoception might play a role in bvFTD symptoms.
Interoception influences cognition more deeply than hitherto considered, and its role in the neurobiological changes occurring in bvFTD has been recently discussed [17]. Van den Stock and Kumfor [17] have proposed a multiple domains deficit framework for bvFTD involving interoception, emotion, and social cognition. The authors have hypothesized that the degeneration of insular regions may underlie the neuropsychological deficits at these cognitive domains, usually present in patients with bvFTD. Interestingly, it has also been demonstrated that patients with other variants of FTD (e.g., semantic dementia) may present with the inability to identify bodily sensations. Gan et al. [18] have shown that somatic symptoms are misinterpreted in these patients, inducing a persistent concern about somatic sensations. Equally, in other pathological populations, interoceptive awareness deficits were associated with difficulties in recognizing emotions from internal bodily sensation (alexithymia) [19]. In this vein, the present case provides further evidence for the multiple domains deficit account, as MT presented with interoceptive and emotional dysfunctions, with reduced gray matter in the insulae. Together with the explicitly verbalized interoception deficits, the patient was less affectionate and lacking in emotions when compared with his usual self, and lost interest in friends and family members. It is also interesting to note that given the lack of interoception, one would expect the patient to gain weight. In fact, although interoception deficits have been found in both anorexia and obesity, they have been recently demonstrated to negatively correlate with the body mass index [20]. In our case, the patient lost weight probably due to anticipatory anxiety and obsessive thoughts related to the act of nutrition.
The origin of eating disturbances in bvFTD remains to be clarified, and although limited to a single case study, our results add another piece to the puzzle, informing on possible behavioral and neuroanatomical alterations in food aversion symptoms in bvFTD.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. Although food aversion and interoception in patients affected by the behavioral variant frontotemporal dementia (bvFTD) are not yet as widely studied, like other aspects of FTD, there have been a few publications describing food seeking in FTD. These relevant works are appropriately cited.
-
2.
Interpretation: Our results shed new lights on the behavioral and neuroanatomical features of food aversion and interoception deficits in bvFTD, suggesting that besides orbitofrontal cortex, also a distributed system associated with interoception might play a role in such behavioral manifestation.
-
3.
Future directions: The manuscript offers a framework for conducting additional studies on the behavioral and neural correlates of food aversion in patients with bvFTD and the generation of new hypotheses on the origin of eating disturbances in bvFTD, further emphasizing the role of interoceptive deficits.
Acknowledgments
Funding: This research did not receive grants from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
The authors declare no competing financial and nonfinancial interests.
References
- 1.Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rascovsky K., Hodges J.R., Kipps C.M., Johnson J.K., Seeley W.W., Mendez M.F. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord. 2007;21:S14–S18. doi: 10.1097/WAD.0b013e31815c3445. [DOI] [PubMed] [Google Scholar]
- 3.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeda M., Brown J., Holland a.J., Fukuhara R., Hodges J.R. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002;73:371–376. doi: 10.1136/jnnp.73.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed R.M., Irish M., Henning E., Dermody N., Bartley L., Kiernan M.C. Assessment of eating behavior disturbance and associated neural networks in frontotemporal dementia. JAMA Neurol. 2016;73:282–290. doi: 10.1001/jamaneurol.2015.4478. [DOI] [PubMed] [Google Scholar]
- 6.Faxén-Irving G., Fereshtehnejad S.-M., Falahati F., Cedergren L., Göranzon H., Wallman K. Body mass index in different dementia disorders: results from the Swedish Dementia Quality Registry (SveDem) Dement Geriatr Cogn Dis Extra. 2014;4:65–75. doi: 10.1159/000360415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitwell J.L., Sampson E.L., Loy C.T., Warren J.E., Rossor M.N., Fox N.C. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage. 2007;35:207–213. doi: 10.1016/j.neuroimage.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Woolley J.D., Gorno-Tempini M.L., Seeley W.W., Rankin K., Lee S.S., Matthews B.R. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69:1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- 9.Aiello M., Silani V., Rumiati R.I. You stole my food! Eating alterations in frontotemporal dementia. Neurocase. 2016;22:400–409. doi: 10.1080/13554794.2016.1197952. [DOI] [PubMed] [Google Scholar]
- 10.Thompson A.E., Clark C.N., Hardy C.J., Fletcher P.D., Greene J., Rohrer J.D. Two cases of food aversion with semantic dementia. Neurocase. 2016;22:312–316. doi: 10.1080/13554794.2016.1149592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnier J.J., Kienle G., Altman D.G., Moher D., Sox H., Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache. 2013;53:1541–1547. doi: 10.1111/head.12246. [DOI] [PubMed] [Google Scholar]
- 12.Le Ber I., Guedj E., Gabelle A., Verpillat P., Volteau M., Thomas-Anterion C. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129:3051–3065. doi: 10.1093/brain/awl288. [DOI] [PubMed] [Google Scholar]
- 13.Berlucchi G., Aglioti S.M. The body in the brain revisited. Exp Brain Res. 2010;200:25–35. doi: 10.1007/s00221-009-1970-7. [DOI] [PubMed] [Google Scholar]
- 14.Craig A.D. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 15.Berridge K.C., Kringelbach M.L. Pleasure Systems in the Brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Windel A.S., Mihai P.G., Lotze M. Neural representation of swallowing is retained with age. A functional neuroimaging study validated by classical and Bayesian inference. Behav Brain Res. 2015;286:308–317. doi: 10.1016/j.bbr.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Van den Stock J., Kumfor F. Behavioural variant frontotemporal dementia: at the interface of interoception, emotion and social cognition? Cortex. 2017 doi: 10.1016/j.cortex.2017.08.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Gan J.J., Lin A., Samimi M.S., Mendez M.F. Somatic symptom disorder in semantic dementia: the role of alexisomia. Psychosomatics. 2016;57:598–604. doi: 10.1016/j.psym.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Shah P., Hall R., Catmur C., Bird G. Alexithymia, not autism, is associated with impaired interoception. Cortex. 2016;81:215–220. doi: 10.1016/j.cortex.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert B.M., Pollatos O. Attenuated interoceptive sensitivity in overweight and obese individuals. Eat Behav. 2014;15:445–448. doi: 10.1016/j.eatbeh.2014.06.002. [DOI] [PubMed] [Google Scholar]