Abstract
The small Indian mongoose (Herpestes auropunctatus) was introduced to Japanese islands and has impacted on the island’s biodiversity. Population control has been attempted through capturing but its efficiency has rapidly declined. Therefore, new additional control methods are required. Our focus has been on the immunocontraceptive vaccines, which act in an especially species-specific manner. The amino-acid sequence of the mongoose ovum zona pellucida protein 3 (ZP3) was decoded and two types of synthetic peptides (A and B) were produced. In this study, these peptides were administered to mongooses (each n=3) and the sera were collected to verify immunogenicity using ELISA and IHC. Treated mongoose sera showed an increasing of antibody titer according to immunizations and the antigen-antibody reactions against the endogenous mongoose ZP. In addition, IHC revealed that immune sera absorbed with each peptide showed a marked reduction in reactivity, which indicated the specificity of induced antibodies. These reactions were marked in peptide A treated mongoose sera, and the antibody titer of one of them lasted for at least 21 weeks. These results indicated that peptide A was a potential antigen, inducing autoantibody generation. Moreover, immunized rabbit antibodies recognized mongoose ZP species-specifically. However, the induction of robust immune memory was not observed. Also, the actual sterility effects of peptides remain unknown, it should be verified as a next step. In any case, this study verified synthetic peptides we developed are useful as the antigen candidates for immunocontraception of mongooses.
Keywords: fertility control, immunocontraception, invasive alien species, mongoose, zona pellucida
The small Indian mongoose (Herpestes auropunctatus) is a carnivorous animal mainly native to the region expanding from West to Southeast Asia [35, 46]. The mongoose is listed among the “100 of the World’s Worst Invasive Alien Species” published by the invasive species specialist group of the IUCN [18]. This is the result of introducing the small Indian mongoose intentionally to 76 islands throughout the world, which has threatened the native ecosystems [3, 18, 33]. In Japan, ten-odd individuals of the small Indian mongoose were introduced to Okinawa Island in Japan in 1910 and brought in Amami-oshima Island in 1979, with the aim of biologically controlling Habu snakes and house rats [15, 45, 49, 51].
Okinawa Island and Amami-oshima Island are both located in the sub-tropical region, which has endemic ecosystems and uniquely evolved fauna. The lack of remarkable natural enemies and the comfortable climate easily allowed the mongoose population to establish its habitat and spread, which resulted in a large impact on the island’s biodiversity. Specifically, preying on the Amami rabbit (Pentalagus furnessi), Ryukyu long-furred rat (Diplothrix legata), Ryukyu robin (Erithacus komadori), Ryukyu tree lizard (Japalura polygonata), and other rare species, and the competition with species that prey on the same food resources such as Okinawa rail (Gallirallus okinawae) largely impacted the islands [36, 38, 49, 51]. Not only impacts on ecosystems but also social impacts such as risk of leptospirosis transmission and damage to poultry by mongooses were reported [14, 16, 30, 52].
The Ministry of the Environment Government of Japan (MOE) has conducted control of mongoose on both islands in cooperation with the local governments, starting from the year 2000 [34]. Further, An Invasive Alien Species Act was carried out in 2005, and small Indian mongoose was legally designated as an invasive alien species in Japan [32]. The MOE organized a group of experts in mongoose capturing called “Mongoose Busters” on each island and have succeeded in reducing the mongoose population drastically [34]. However, achieving a low mongoose population density has also lowered the capture efficiency. Therefore, the complete eradication of the mongoose population is not yet to be achieved. The other factors suppressing the capture efficiency are the increase in by-catch of native species and the appearance of trap-shy individuals, who are highly cautious of traps.
Therefore, new methods for mongoose population control are required. We focused on the immunocontraceptive methods, which induce immunological sterility by treatment with contraceptive vaccines. Immunocontraception in wildlife was initially established in 1980s to control wild horses in the U.S.A. [23, 28], and has since been applied to various mammals (e.g., cat, African elephant, and some ungulates), due to a growing concern for animal welfare [9, 24, 26]. Immunocontraception is a technique for suppressing reproduction of the target species, in which antibodies induced by the contraceptive vaccines inhibit the activity of reproduction-related factors as autoantigens. In these studies, nonspecies-specific agents such as porcine zona pellucida (PZP) or GnRH preparations, some of which have already been made into products (e.g., SpayVacTM, GonaConTM), have been used as direct injectable contraceptive vaccines [27, 29, 31]. On the other hand, species-specific agents have also been developed with remote injection to control the free-ranging population [8, 19]. Since the present study targeted on free-ranging invasive animals, it was not realistic to treat mongooses with any direct injectable agents under visual recognition or capture. Furthermore, the effects on non-target species had to be minimized. Therefore, the overall objective of this study was to develop mongoose-specific immunocontraceptive agents.
We focused on zona pellucida 3 glycoprotein (ZP3) as vaccine antigens, which has high species-specificity and plays an important role in the primary binding of sperms and oocytes [2, 4]. Manufacturing of vaccine candidates was necessary to clarify whether these agents would induce antibody production in mongoose and whether, the induced antibody, would recognize the mongoose endogenous zona pellucida (ZP) as biological antigens. Additionally, we tried to identify the species-specific effects of these peptides.
MATERIALS AND METHODS
Synthesis of antigen peptides
We started by decoding the full-length sequence of mongoose ZP3. Briefly, RNA was extracted from mongoose ovary, sampled from individuals culled in MOE control program. PCR products were obtained with reverse transcriptase reaction method and RACE method; RACE-PCR products were then amplified by TA cloning. After the sequence reaction, base sequence analysis was performed by the Division of Genomics Research of Life Science Research Center of Gifu University. Thereafter, amino-acid sequences were predicted from the decoded mongoose ZP3 gene sequence and homology of the sperm-oocyte binding site was compared with known ZP3 sequences from cat (Felis silvestris catus), dog (Canis lupus familiaris), stoat (Mustela ermine), mouse (Mus musculus), and humans (Homo sapiens).
Through the above steps, two types of peptides, namely peptide A (CGLPGHSRRLSHPERPRRK) and peptide B (CHPERPRRKLAPRSRNRRH), which have a homologous amino-acid sequence to a part of the mongoose ZP3 sperm-oocyte binding site (both 19 AA residues) were designed. These peptides were synthesized and conjugated with keyhole limpet hemocyanin (KLH) on the cysteine residue in order to promote antigenicity (Sigma-Aldrich Japan K.K.; Tokyo, Japan). Each peptide was assumed to include two epitope regions. One of them has common amino-acid sequence between peptide A and B.
Animals
Nine female small Indian mongooses were captured in Nago city, Okinawa using box traps in the MOE mongoose control project [34]. They were all determined as mature by their head/body length (265–290 mm) and body weight (275–487 g) [37]. The animals were acclimatized to captivity for at least 2 weeks, and were housed in individual cages (L 230 × H 270 × W 600 mm) in the outdoor rearing facility at the Yambaru wildlife conservation center of MOE. All animals were fed dog food and had free access to water at all times. The mongooses were randomly assigned to three groups: group A, which received peptide A (n=3; A-1~3), group B, which received peptide B (n=3; B-1~3), and control group C treated with phosphate-buffer saline (PBS; n=3; C-1~3). No animals showed noticeable body weight loss or behavioral abnormality during the experiment.
Immunization and blood sampling
Starting from February 2014, the animals were treated with peptides (150 µg/150 µl) emulsified with the same volume of Freund’s incomplete adjuvant (TiterMax® Gold, TiterMax U.S.A. Inc., Norcross, GA, U.S.A.) or 150 µl of PBS with the same volume of adjuvant as control under anesthesia. Immunization was performed four times at an interval of 2 weeks in several dorsal subcutaneous sites. Two of the animals (A-1 and B-2) were used for the confirmation of the antibody’s durability. Subsequently, after a decrease in the antibody titer (83 weeks after the first immunization), these two animals were treated with a single immunization to evaluate the immune memory. The mongooses were anesthetized with intramuscular injection of ketamine hydrochloride (0.5 mg/head; Ketalar, Daiichi Sankyo, Tokyo, Japan) and medetomidine hydrochloride (0.07 mg/head; Domitor, Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan) using a special box cage. Subsequently, the animals were treated with atipamezole hydrochloride (0.35 mg/head; Antisedan, Nippon Zenyaku Kogyo Co., Ltd.) to promote recovery from sedation. Blood (1 ml) was collected via cardiocentesis at the time of immunization (except the second immunization) and a week after the fourth immunization. Blood samples from A-1 and B-2 were collected continuously at an interval of approximately 15 weeks and at the time of reimmunization. Blood samples were kept overnight at 4°C, and were subsequently centrifuged for 10 min at 10,000 rpm, and the serum was separated. All serum samples were subdivided and stored at −80°C until further analysis. All animals, except A-1 and B-2 were euthanized 1 week after the last immunization via exsanguination under anesthesia, and the ovaries were collected for pathological analysis. Euthanasia was performed in accordance with the guideline of The American Veterinary Medical Association (AVMA; 2007).
Enzyme-linked immunosorbent assay (ELISA)
The amount of serum antibodies against synthetic peptides was measured by using indirect ELISA. Briefly, 96-well microplates (#3590, Corning Inc., Corning, NY, U.S.A.) were coated with 100 µl of peptide A or B in carbonate-bicarbonate buffer (C3041, Sigma-Aldrich Co., St. Louis, MO, U.S.A.) and incubated for 1 hr at room temperature. After washing three times with 200–300 µl/well of PBS containing 0.05% Tween®20 (Sigma-Aldrich; PBS-T), nonspecificity was blocked with 3% skim milk in PBS (200 µl/well) overnight at 4°C. After washing three times with PBS-T, mongoose serum in PBS (1:100; 100 µl/well) was added as the primary antibody and incubated for 1 hr at room temperature. After washing three times with PBS-T (300–400 µl/well), horseradish peroxidase (HRP)-labeled goat anti-Ferret IgG antibody (1:2,500; 1 mg/ml; Bethyl laboratories Inc., Montgomery, AL, U.S.A.) in PBS (100 µl/well) was added and incubated for 1 hr at room temperature; cross-reactivity to mongoose IgG has been previously reported [17]. After washing three times with PBS-T (200–300 µl/well), tetramethyl benzidine (100 µl/well; TMB Substrate Regent Set, BD, Franklin Lakes, NJ, U.S.A.) was added to induce a chromogenic reaction for 20 min, and the reaction was subsequently stopped by 1 M of H2SO4. The absorbance was measured at dual wavelengths, 450/655 nm using a microplate reader (Model 680, Bio-Rad Laboratories, Hercules, CA, U.S.A.).
All sera were tested in triplicate on a plate to verify the technical validity of the experiment, and the average optical density (OD) and standard deviation (SD) of sera were calculated. An average OD + 3 SD of all control sera was set as the cut-off value.
IHC–labeled streptavidin-biotin (LSAB) method
Immunohistochemical analysis was performed on sections (4 µm) from fixed and paraffin-embedded non-immunized mongoose ovaries obtained from three mature female mongooses, other than from immunized individuals, captured under the same MOE control program, to verify the recognition of endogenous ZP by induced mongoose antibodies. Mongooses were then euthanized under anesthesia by exsanguination and ovaries were collected and fixed in 4% neutral buffered formalin.
According to the general methods, sections were deparaffinized and rehydrated using xylene and alcohol gradient. At this stage, one section was used for hematoxylin and eosin staining (HE) to observe the tissue structure. For IHC, antigen retrieval was performed in other sections by incubating them in citrate buffer solution (pH 5.4) in a warm bath (90°C) for 40 min. After cooling down for 20 min at room temperature, sections were washed three times with PBS (5 min), and the endogenous peroxidase activity was inhibited with 3% H2O2 for 15 min at room temperature. After washing three times with PBS (5 min), the slides were treated with 3% bovine serum albumin (BSA; A3590, Sigma-Aldrich Co.) in PBS (100 µl/section) and incubated overnight at 4°C to block nonspecific reactions. Subsequently, the sections were incubated with primary antibody, i.e., either serum from the treated mongooses, diluted at 1:50 with 3% BSA in PBS (100 µl/section) for 1 hr at room temperature. After washing three times with PBS (5 min), the sections were incubated with HRP-labeled protein A (1:1,000, 100 µl/section; ab7456, Abcam, Cambridge, U.K.) for 1 hr at room temperature. After washing in PBS, sections were incubated with streptavidin (1:200, 100 µl/section; Extravidin®, Sigma-Aldrich Co.) in PBS for 1 hr at room temperature to amplify the avidin-biotin reaction. Subsequently, the sections were washed in PBS, incubated in 3,3ʹ-diaminobenzidin (DAB; Dako, Santa Clara, CA, U.S.A.) for 1 min to induce a chromogenic reaction, and counterstained with hematoxylin. All slides were sealed through a dehydrating and permeating operation.
To evaluate the specificity of the antibody, an absorption test was performed, in which absorbed sera with immunized peptide (e.g., A-1 serum-peptide A) were used as the primary antibody. Each serum and the same volume of peptide were co-incubated in PBS (1:50 diluent) for 1 hr at room temperature.
Additionally, to estimate the species-specificity of antibodies induced by peptides, IHC using immunized rabbit sera and ovary sections of various animals was performed. Briefly, the same peptides (peptide A or B) were administrated to rabbits (each n=2; a-1, 2 and b-1, 2) 5 times every two weeks and sera were collected continuously. Immunized rabbit sera were used as the primary antibody (1:100 with 3% BSA in PBS, 100 µl/section). Ovary sections from Mongoose, Japanese marten (Martes melampus melampus), cat (Felis silvestris catus), dog (Canis lupus familiaris), Japanese wild boar (Sus scrofa), and rabbit (Oryctolagus cuniculus) were used (fixed with 4% neutral buffered formalin, 4 µm). Ovary samples were collected from spayed cats and dogs, captured wild animals based on permission, and experimental animals. Every captured or experimental animal were euthanized in accordance with the guideline of AVMA appropriately and ovaries from them were treated as ones from mongooses. Other experimental conditions were the same as described above.
RESULTS
Comparisons of ZP3 homology between mongoose and the other animals
Decoded base sequence of mongoose ZP3 reached 1,278 bp and it is assumed to be translated into a 426 AA sequence. The homologies between the mongoose ZP3 sperm-oocyte binding site and the ones from cat, dog, stoat, mouse and human were 47.8, 60.9, 60.9, 17.4, and 34.8%, respectively.
Antigenicity of the synthetic peptides
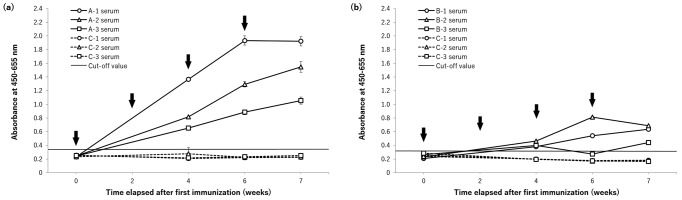
Both groups of animals treated with synthetic peptides showed a higher antibody titer than the pre-immunized serum or control animals (Fig. 1). In particular, animals in group A showed a significantly higher antibody titer, notwithstanding some individual variation, which increased mostly according to immunization (Fig. 1a). On the other hand, the antibody titer in group B was generally positive, but lower than that in group A; animal B-3 displayed a temporal decrease in the titer, which was less than the cut-off value (Fig. 1b).
Fig. 1.
Antibody titers (absorbance at 450–655 nm; mean ± SD) in mongoose sera following immunization with peptide A (a) and peptide B (b). Horizontal lines mean the cut-off value calculated from each control on same microplate: cut-off value=mean antibody titer of control + 3 SD. Arrows indicate the times of immunization.
Longevity of the antibody titer and intensity of the immune memory
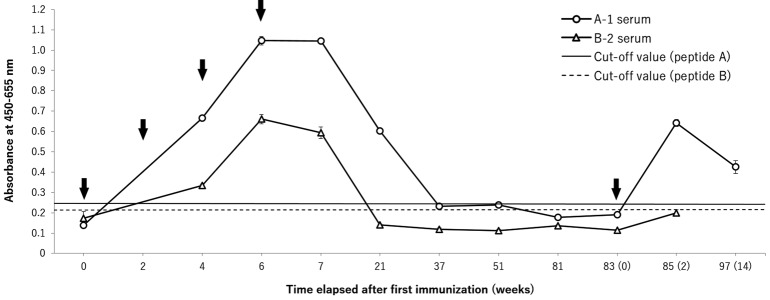
Blood samples were continuously collected over time from two animals followed-up for their antibody titer (A-1 and B-2), which exhibited the higher titer in each group. The antibodies’ longevity and the reaction against single reimmunization are shown in Fig. 2. The antibody titer of both animals gradually declined after the last immunization and became lower than each cut-off value, and stabilized subsequently at a low value. The antibody titer of the A-1 serum lasted for at least 21 weeks from the first immunization; whereas, that of the B-2 mongoose lasted for at least 7 weeks.
Fig. 2.
Durability of antibody titers (absorbance at 450–655 nm; mean ± SD) in mongoose following immunization with peptide A (A-1) and peptide B (B-2) and degree of immune memory against reimmunization with each peptides. The cut-off value was calculated from control on each peptide A (horizontal solid line) and peptide B (horizontal broken line): cut-off value=mean antibody titer of each control + 3 SD. Arrows indicate the times of immunization.
Regarding the immune memory, the degree of the increase in the antibody titer against single reimmunization after the antibody became stably low was evaluated. The inclination of the A-1 antibody titer to increase by reimmunization corresponded to two times that of the former four times of immunization. The B-2 antibody titer only slightly increased against the reimmunization, but remained under the cut-off value.
Histological findings of immunized mongoose ovaries
No infiltration of inflammatory cells nor defections were observed in HE-stained ovary tissues in the experimental animals compared with control ovaries (data not shown).
Antigen recognition by the antibodies
Each sera containing antibodies induced by ZP3 peptides were tested as the primary antibody by IHC. Based on the results, ZP-specific positive reactions were observed in the serum of both experimental groups, in comparison with serum from the control animals (Fig. 3). Marked positive reactions were observed in the A-2 serum (Fig. 3b), while the A-1 and A-3 sera showed a moderate positive reaction (Fig. 3a, 3c). The sera of animals immunized with peptide B showed a negative to mild positive reaction (Fig. 3d–f). The positive reactions were observed at every stage of follicles containing ZP, namely the secondary, antral, and atretic follicles (Fig. 4). Because of the nature of this experimental condition, especially in ovary blood vessels, diffused nonspecific reactions were observed; however, these were minimal to ignorable compared with the positive reactions.
Fig. 3.

Immunoreactivity of immunized mongoose sera with synthetic peptides against normal mongoose ovary in IHC. a: A-1 antiserum, b: A-2 antiserum, c: A-3 antiserum, d: B-1 antiserum, e: B-2 antiserum, f: B-3 antiserum, g: C-1 serum (control), and h: HE-stained normal mongoose ovary. Arrowheads: zona pellucida. Bars=50 µm.
Fig. 4.

Immunoreactivity of the A-2 mongoose antiserum against zona pellucida of various stages of follicles. PF: primary follicle, SF: secondary follicle, TF: tertiary follicle, GF: Graafian follicle, and AF: atretic follicle. Arrowheads: zona pellucida. Bar=100 µm.
Specificity of antigen-antibody reaction
To confirm the specificity of the antigen-antibody reaction, sera absorbed with the peptide were tested using IHC. Based on the results, the positive reactions disappeared or decreased in the absorbed sera (Fig. 5). The A-2 serum showed the strongest reaction in IHC, and, when co-incubated with peptide A, lost the specific positive reactions with ZP, but not the nonspecific reactions (Fig. 5a, 5b). In the group B, the positive reactions did not disappear completely, but became weaker in the absorbed serum than in the non-absorbed serum (Fig. 5c, 5d).
Fig. 5.
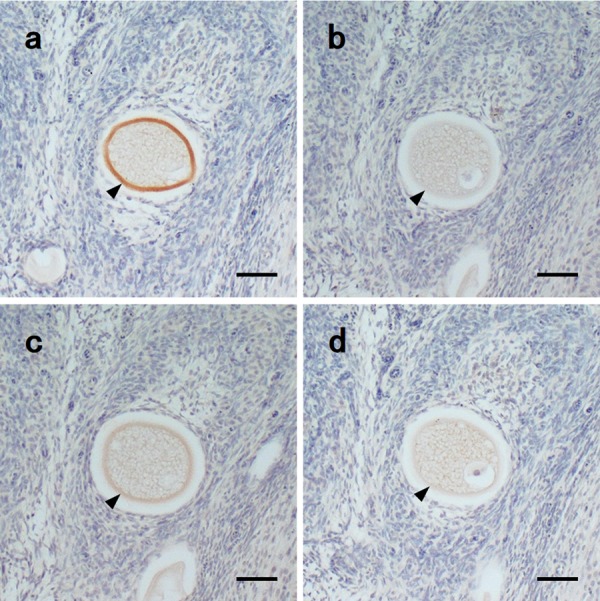
Immunoreactivity of immunized mongoose sera with synthetic peptides and absorbed sera with each peptide against normal mongoose ovary in IHC. a: A-2 antiserum, b: A-2 antiserum absorbed with peptide A, c: B-1 antiserum, and d: B-1 antiserum absorbed with peptide B. Arrowheads: zona pellucida. Bars=50 µm.
Species-specificity of induced rabbit antibodies
Antigen-antibody reactions were observed in each serum sample derived from rabbits immunized with peptide A or B, and the reactions were specific to mongoose ovary (Fig. 6). In the mongoose ovary section, rabbit antibodies bound to the ZP. The reaction did not depend on the stage of follicles (Fig. 6a). The positive reaction was remarkable in rabbit sera immunized with peptide A than in those immunized with peptide B (results not shown).
Fig. 6.

Immunoreactivity of immunized rabbit sera (a-2) with synthetic peptides A against ovary of various animal species in IHC. a: small Indian mongoose, b: Japanese marten, c: cat, d: dog, e: Japanese wild boar, and f: rabbit. Arrowheads: zona pellucida. Bars=50 µm.
DISCUSSION
According to the ELISA results, the immune response to the synthetic peptides A and B was confirmed in mongooses. Since peptides without KLH conjugation were used as a coated antigen, the induced antibodies were considered as the specific reactions against each peptide rather than the carrier protein. The capacity of peptide A to induce antibodies seemed to be superior to that of peptide B, even though these two synthetic peptides were suspected to include a common epitope sequence. However, there is a possibility that another epitope region in peptide A may be relatively excellent in the antigen presentation capacity of the immune system of mongoose.
The longevity of the antibody titers was at least 21 and 7 weeks in A-1 and B-2, respectively. Moreover, peptide A exhibited a longer antigenicity than peptide B. Abe (1995) reported that the life span of small Indian mongoose is 2 years or less in Amami-oshima Island [1], while the female estrus season spans mainly from March to August and generally occurs at 1 year of age [37]. Considering these reproductive biological characterizations, a single immunization by an 5-month lasting vaccine in spring would be applicable to keep the antibody titer for a most of breeding season. However, it must be noted that these results occurred after multiple direct immunizations of an antigen peptide amplified antigenicity and not suggested longevity of fertility suppressive effects furthermore in the oral vaccination. Prolongations of the lifespan and/or the breeding season were also reported in some species treated with contraceptive vaccines [22, 41, 50]; hence, the effect of increased longevity and breeding season on the duration of the contraceptive effect would have to be considered.
We did not observe any significant results in the immune memory test to be able to judge whether the immune memory had been induced. It is worth noting that the induction of immune memory is nonessential but helpful to solid fertility control. The immune memory may be improved by variations of the vaccination route or by additional modification of the antigen agents [13].
Although not shown, no inflammation or other histological disorders were observed in the ovary tissues of all treated animals. The peptides evaluated in this study did not seem to affect the ovary or oocytes histologically, although previous studies have indicated the presence or absence of ovaritis [7, 12, 20]. The correlation between ovaritis and contraceptive effect is still unknown, while absence of disorders is important from the perspective of animal welfare [21, 39, 41]. Although considered invasive alien species, the pain attendant on treatment should be minimized [40].
It is well established that ZP consists of three to four types of glycoproteins containing a ZP module (ZP 1–4); it is synthesized by oocytes from the secondary follicle period and is post-translationally modified with a sugar chain [2, 4]. Furthermore, ZP3 is a principal glycoprotein that is implicated in the primary binding of sperms and has a high species-specificity [2, 4]. Antibodies induced by treatment with synthesized ZP3 peptides bound to mongoose ZP, and recognized ZP at every stage of follicles forming ZP. Moreover, these antigen-antibody responses were specific based on the experiment using antibodies absorbed with antigen peptides. These results indicated that the exogenous antigens stimulated a specific in vivo response to endogenous ZP proteins in the mongooses, and that the ZP3 epitope regions targeted by the antibodies are expressed from an early ZP stage, which is unsusceptible to its maturation. Ideally, control sera in the absorption test should be co-incubated with unrelated peptide to immunization. However, all sera were diluted with a blocking solution that contained more miscellaneous proteins. Thus, the possibility that the peptide solution impeded the antigen-antibody responses nonspecifically would be rejected.
Table 1 summarized the results of the ELISA and IHC assays. There was a general correlation between the treated groups in the strength of the reaction in both assays, within group variation; for example, the A-1 serum showed the highest antibody titer in ELISA and the second strongest response in IHC. These discrepancies may be due to the differences of the target antigen or the other conditions between ELISA and IHC. The combination of tissues and primary antibodies originating from the same tissue species, i.e., mongoose-mongoose, should be avoided in IHC. The secondary antibodies also bind to endogenous immunoglobulin, which results in nonspecific reactions [11]. However, immune sera had high specificity to mongoose ZP enough. In addition, by appropriately adjusting the dilution concentration and incubation condition of the sera and secondary antibodies, the nonspecific reactions were minimized and the specific reactions were potentiated.
Table 1. Summarized the results of ELISA and histological analysis.
| Group name (treatment) | Individual No. | Antibody titer (absorbance at 450–655 nm) | Reactibity in IHC |
Pathological findings |
|
|---|---|---|---|---|---|
| Pre-immuned | Immuned (4 times) | ||||
| Group A (peptide A) | A-1 | 0.231 ± 0.017 | 1.922 ± 0.067 | ++ | ND |
| A-2 | 0.242 ± 0.021 | 1.548 ± 0.079 | +++ | – | |
| A-3 | 0.244 ± 0.011 | 1.056 ± 0.052 | ++ | – | |
| Group B (peptide B) | B-1 | 0.208 ± 0.004 | 0.637 ± 0.012 | + | – |
| B-2 | 0.229 ± 0.013 | 0.689 ± 0.001 | ± | ND | |
| B-3 | 0.262 ± 0.026 | 0.442 ± 0.009 | – | – | |
| Group C (control) | C-1 | 0.257 ± 0.004 (peptide A)/0.254 ± 0.019 (peptide B) | – | – | |
| C-2 | 0.234 ± 0.010 (peptide A)/0.239 ± 0.005 (peptide B) | – | – | ||
| C-3 | 0.253 ± 0.014 (peptide A)/0.288 ± 0.029 (peptide B) | – | – | ||
+++: marked, ++: moderate, +: mild, ±: mild to negative, –: negative, ND: no data.
The synthetic peptides used in this study, especially peptide A, seemed useful as vaccine candidates. As a next step, we have to evaluate the actual fertility suppressive effect of the peptides. Only after a successful inhibition of pregnancy may we discuss whether the quantity of the antibody titer is effective or not. Although it has not been explained completely, the actual inhibitory pathway for fertilization by immunocontraception is assumed to be the immune response against the oocytes in follicles and/or ovulated [20]. Nevertheless, there is likely to be a correlation between the antibody titer and the fertilization suppressive effect. However, a previous immunocontraceptive study in mice has reported no correlation between them [12].
Immunized rabbit sera with the same synthetic peptides showed mongoose-specific responses in IHC using ovary tissues of various animals, including rabbit. This result may mean a certain species-specificity of synthetic peptides. However, actual specificity of contraceptive effect should be mentioned after it has been proved that these synthetic peptides do not affect any animals other than mongoose. On that account, it has to be confirmed by the vaccination of synthetic peptides to various species; especially sympatric animals with mongoose in Japan. Rare species that are difficult to use for experiments are included within them. So, there is a necessity for obtaining a suitable permission or using some experimental animals as a substitute for them.
Remarkable differences in the immune response between animals were not observed in this small-scale study. However, the individual differences in the immune response possibility have a negative impact on the fertility control in the mongoose population. Selection pressure will be applied on the mongooses’ acquired resistance to the fertility suppressive effects of contraceptive vaccines, which can lead the dominant character of the mongoose population to vaccine resistance [42]. To avoid such “adverse effects of immunocontraceptive vaccines”, we have to identify the frequency of appearance of individuals potentially resistant to the fertility suppression and estimate the population suppressive effects of contraception under various patterns [42, 44].
To deliver an immunocontraceptive vaccine to free-roaming wildlife animals, which are particularly difficult to access, remote methods for vaccination (vaccine delivery systems) are necessary [43]. Some oral vaccine delivery agents have been already devised for immunocontraceptive vaccines (e.g., variety of virus vectors, bacterial ghosts, and liposomes) [5, 47, 48]. Particularly in Japan, the diffusion of recombinant biological vectors into the field is prohibited legally. Therefore, no proliferative delivery systems such as bacterial ghosts and liposomes have the potential to be applied to control the mongoose population [6, 10, 25, 48]. These should be selected after investigating further, the compatibility with vaccines and the cost effectiveness.
Acknowledgments
We are grateful to Katsushi Nakata and the other staffs of Yambaru Wildlife Conservation Center (Ufugi Nature Museum) for cooperation in this study. This work was supported by JSPS KAKENHI Grant Number 26430203, the Global Environment Research Fund (4-1401, Leader: K. Goka) of the Ministry of the Environment, Japan, 2014 and a grants-in-aid of The Inui Memorial Trust for Research on Animal Science, Japan.
REFERENCES
- 1.Abe S.1995. Age determination of mongoose on Amami-oshima Island based on eye lens weight. Chirimosu 6: 34–43(in Japanese). [Google Scholar]
- 2.Akatsuka K., Yoshida-Komiya H., Tulsiani D. R. P., Orgebin-Crist M. C., Hiroi M., Araki Y.1998. Rat zona pellucida glycoproteins: molecular cloning and characterization of the three major components. Mol. Reprod. Dev. 51: 454–467. doi: [DOI] [PubMed] [Google Scholar]
- 3.Bennett C. E., Wilson B. S., Desalle R.2011. DNA barcoding of an invasive mammal species, the small Indian mongoose (Herpestes javanicus; E. Geoffroy Saint-Hillaire 1818) in the Caribbean and Hawaiian Islands. Mitochondrial DNA 22: 12–18. doi: 10.3109/19401736.2010.542241 [DOI] [PubMed] [Google Scholar]
- 4.Bleil J. D., Wassarman P. M.1980. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell 20: 873–882. doi: 10.1016/0092-8674(80)90334-7 [DOI] [PubMed] [Google Scholar]
- 5.Cross M. L., Fleming S. B., Cowan P. E., Scobie S., Whelan E., Prada D., Mercer A. A., Duckworth J. A.2011. Vaccinia virus as a vaccine delivery system for marsupial wildlife. Vaccine 29: 4537–4543. doi: 10.1016/j.vaccine.2011.04.093 [DOI] [PubMed] [Google Scholar]
- 6.Cui X., Duckworth J. A., Lubitz P., Molinia F. C., Haller C., Lubitz W., Cowan P. E.2010. Humoral immune responses in brushtail possums (Trichosurus vulpecula) induced by bacterial ghosts expressing possum zona pellucida 3 protein. Vaccine 28: 4268–4274. doi: 10.1016/j.vaccine.2010.04.032 [DOI] [PubMed] [Google Scholar]
- 7.Curtis P. D., Richmond M. E., Miller L. A., Quimby F. W.2007. Pathophysiology of white-tailed deer vaccinated with porcine zona pellucida immunocontraceptive. Vaccine 25: 4623–4630. doi: 10.1016/j.vaccine.2007.03.033 [DOI] [PubMed] [Google Scholar]
- 8.Duckworth J. A., Wilson K., Cui X., Molinia F. C., Cowan P. E.2007. Immunogenicity and contraceptive potential of three infertility-relevant zona pellucida 2 epitopes in the marsupial brushtail possum (Trichosurus vulpecula). Reproduction 133: 177–186. doi: 10.1530/REP-06-0088 [DOI] [PubMed] [Google Scholar]
- 9.Fayrer-Hosken R. A., Grobler D., Van Altena J. J., Bertschinger H. J., Kirkpatrick J. F.2000. Immunocontraception of African elephants. Nature 407: 149. doi: 10.1038/35025136 [DOI] [PubMed] [Google Scholar]
- 10.Ganeshpurkar A., Ganeshpurkar A., Pandey V., Agnihotri A., Bansal D., Dubey N.2014. Harnessing the potential of bacterial ghost for the effective delivery of drugs and biotherapeutics. Int. J. Pharm. Investig. 4: 1–4. doi: 10.4103/2230-973X.127733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster T., Randolph-Habecker J.2014. A flexible mouse-on-mouse immunohistochemical staining technique adaptable to biotin-free reagents, immunofluorescence, and multiple antibody staining. J. Histochem. Cytochem. 62: 197–204. doi: 10.1369/0022155413511620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy C. M., Beaton S., Hinds L. A.2008. Immunocontraception in mice using repeated, multi-antigen peptides: immunization with purified recombinant antigens. Mol. Reprod. Dev. 75: 126–135. doi: 10.1002/mrd.20745 [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Medrano J. H., Williams R. W., van Drunen Littel-van den Hurk S., Peters A. R., Hannant D., Campbell B. K., Webb R.2013. Early postnatal immunisation against gonadotrophin-releasing hormone induces a high but differential immune response in heifer calves. Res. Vet. Sci. 95: 472–479. doi: 10.1016/j.rvsc.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 14.Higa H. H., Fujinaka I. T.1976. Prevalence of rodent and mongoose leptospirosis on the Island of Oahu. Public Health Rep. 91: 171–177. [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda T., Yamada F.2011. Overseas countermeasures against alien mammals. pp. 59–76 In: Invasive Alien Mammals in Japan: Biology of Control Strategy and Conservation, 1st ed. (Yamada, F., Ikeda, T. and Ogura, G. eds.), University of Tokyo Press, Tokyo (in Japanese). [Google Scholar]
- 16.Ishibashi O., Ogura G.2012. Present situation of mongoose in Japan and survey of Leptospira in mongoose. Chikyu Kankyou. 17: 193–202 (in Japanese). [Google Scholar]
- 17.Ishibashi O., Sumida K., Iizuka S., Sudo K., Yamashita K., Ogura G., Sunagawa K., Nakada T.2008. Selection and test of the serodiagnostic test with IgG-ELISA method in the small Asian mongoose. The Science Bulletin of the Faculty of Agriculture. University of the Ryukyus. 55: 7–10(in Japanese with English abstract). [Google Scholar]
- 18.Invasive Species Specialist Group2011. Groval Invasive Species Datebase. http://www.iucngisd.org/gisd/species.php?sc=86 [accessed on November 5, 2017].
- 19.Ji W., Clout M. N., Sarre S. D.2000. Responses of male brushtail possums to sterile females: implications for biological control. J. Appl. Ecol. 37: 926–934. doi: 10.1046/j.1365-2664.2000.00546.x [DOI] [Google Scholar]
- 20.Joonè C. J., Schulman M. L., Bertschinger H. J.2017. Ovarian dysfunction associated with zona pellucida-based immunocontraceptive vaccines. Theriogenology 89: 329–337. doi: 10.1016/j.theriogenology.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 21.Kirkpatrick J. F., Turner A.2002. Reversibility of action and safety during pregnancy of immunization against porcine zona pellucida in wild mares (Equus caballus). Reprod. Suppl. 60: 197–202. [PubMed] [Google Scholar]
- 22.Kirkpatrick J. F., Turner A.2007. Immunocontraception and increased longevity in equids. Zoo Biol. 26: 237–244. doi: 10.1002/zoo.20109 [DOI] [PubMed] [Google Scholar]
- 23.Kirkpatrick J. F., Turner J. W.1991. Compensatory reproduction in feral horses. J. Wildl. Manage. 55: 649–652. doi: 10.2307/3809514 [DOI] [Google Scholar]
- 24.Kirkpatrick J. F., Calle P. P., Kalk P., Liu I. K. M., Turner J. W.1996. Immunocontraception of captive exotic species. II. Formosan sika deer (Cervus nippon taiouanus), axis deer (Cervus axis), Himalayan tahr (Hemitragus jemlahicus), Roosevelt elk (Cervus elaphus roosevelti), Reeves’ muntjac (Muntiacus reevesi), and sambar deer (Cervus unicolor). J. Zoo Wildl. Med. 27: 482–495. [Google Scholar]
- 25.Langemann T., Koller V. J., Muhammad A., Kudela P., Mayr U. B., Lubitz W.2010. The Bacterial Ghost platform system: production and applications. Bioeng. Bugs 1: 326–336. doi: 10.4161/bbug.1.5.12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy J. K., Mansour M., Crawford P. C., Pohajdak B., Brown R. G.2005. Survey of zona pellucida antigens for immunocontraception of cats. Theriogenology 63: 1334–1341. doi: 10.1016/j.theriogenology.2004.07.015 [DOI] [PubMed] [Google Scholar]
- 27.Levy J. K., Friary J. A., Miller L. A., Tucker S. J., Fagerstone K. A.2011. Long-term fertility control in female cats with GonaCon™, a GnRH immunocontraceptive. Theriogenology 76: 1517–1525. doi: 10.1016/j.theriogenology.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 28.Liu I. K., Bernoco M., Feldman M.1989. Contraception in mares heteroimmunized with pig zonae pellucidae. J. Reprod. Fertil. 85: 19–29. doi: 10.1530/jrf.0.0850019 [DOI] [PubMed] [Google Scholar]
- 29.Mask T. A., Schoenecker K. A., Kane A. J., Ransom J. I., Bruemmer J. E.2015. Serum antibody immunoreactivity to equine zona protein after SpayVac vaccination. Theriogenology 84: 261–267. doi: 10.1016/j.theriogenology.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 30.Middleton C. R., Ansdell V. E., Sasaki D. M.2001. Of mice and mongooses ... a history of leptospirosis research in Hawaii. Hawaii Med. J. 60: 179–181, 184–186. [PubMed] [Google Scholar]
- 31.Miller L. A., Johns B. E., Killian G. J.2000. Immunocontraception of white-tailed deer with GnRH vaccine. Am. J. Reprod. Immunol. 44: 266–274. doi: 10.1111/j.8755-8920.2000.440503.x [DOI] [PubMed] [Google Scholar]
- 32.Ministory of the Environment Government of Japan 2016. List of Regulated Living Organisms under the Invasive Alien Species Act. https://www.env.go.jp/nature/intro/2outline/files/siteisyu_list_e.pdf [accessed on November 5, 2017].
- 33.Morley C. G., Winder L.2013. The effect of the small Indian mongoose (Urva auropunctatus), island quality and habitat on the distribution of native and endemic birds on small islands within Fiji. PLoS One 8: e53842. doi: 10.1371/journal.pone.0053842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naha Nature Concervation Office, Ministory of the Environment Government of Japan. 2017. Result report of mongoose control project in North part of Okinawa Island of 2016 and execution plan of 2017. http://kyushu.env.go.jp/naha/pre_2017/2829.html (in Japanese) [accessed on November 2, 2017].
- 35.Nellis D. W.1989. Herpestes auropunctatus. Mamm. Species 342: 1–6. doi: 10.2307/3504091 [DOI] [Google Scholar]
- 36.Ogura G., Kawashima Y., Oda S.2003. Analysis of captured small Asian mongooses, and present situation of countermeasures and problems. J. Vet. Med. 56: 295–301(in Japanese). [Google Scholar]
- 37.Ogura G., Nonaka Y., Kawashima Y., Sakashita M., Nakachi M., Oda S.2001. Relationship between body size and sexual maturation, and seasonal change of reproductive activities in the female feral small Asian mongoose on Okinawa Island. Jpn. J. Zoo Wildl. Med. 6: 7–14(in Japanese with English abstract). doi: 10.5686/jjzwm.6.7 [DOI] [Google Scholar]
- 38.Ogura G., Sasaki T., Toyama M., Takehara K., Nakachi M., Ishibashi O., Kawashima Y., Oda S.2002. Food habits of the feral small Asian mongoose (Herpestes javanicus) and impacts on native species in the north part of Okinawa Island. Honyurui Kagaku 41: 53–62(Mammalian Science). [Google Scholar]
- 39.Powell D. M.1999. Preliminary evaluation of porcine zona pellucida (PZP) immunocontraception for behavioral effects in feral horses (Equus caballus). J. Appl. Anim. Welf. Sci. 2: 321–335. doi: 10.1207/s15327604jaws0204_6 [DOI] [PubMed] [Google Scholar]
- 40.Ramsey D.2005. Population dynamics of brushtail possums subject to fertility control. J. Appl. Ecol. 42: 348–360. doi: 10.1111/j.1365-2664.2005.01006.x [DOI] [Google Scholar]
- 41.Ransom J. I., Cade B. S., Hobbs N. T.2010. Influences of immunocontraception on time budgets, social behavior, and body condition in feral horses. Appl. Anim. Behav. Sci. 124: 51–60. doi: 10.1016/j.applanim.2010.01.015 [DOI] [Google Scholar]
- 42.Ransom J. I., Powers J. G., Thompson Hobbs N., Baker D. L.2014. Ecological feedbacks can reduce population-level efficacy of wildlife fertility control. J. Appl. Ecol. 51: 259–269. doi: 10.1111/1365-2664.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma S., Hinds L. A.2012. Formulation and delivery of vaccines: Ongoing challenges for animal management. J. Pharm. Bioallied Sci. 4: 258–266. doi: 10.4103/0975-7406.103231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suppo C., Naulin J. M., Langlais M., Artois M.2000. A modelling approach to vaccination and contraception programmes for rabies control in fox populations. Proc. Biol. Sci. 267: 1575–1582. doi: 10.1098/rspb.2000.1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toyama M., Ogura G.1998. In regard to the Introduction of the Common Grey Mongoose, Herpestes edwardsii into Okinawa in Okinawan Newspapers. Bulletin of the Historiographical Study on Okinawan History 4: 141–170(in Japanese). [Google Scholar]
- 46.Veron G., Patou M.L., Pothet G., Simberloff D., Jennings A. P.2007. Systematic status and biogeography of the Javan and small Indian mongooses (Herpestidae, Carnivora). Zool. Scr. 36: 1–10. doi: 10.1111/j.1463-6409.2006.00261.x [DOI] [Google Scholar]
- 47.Vos A., Kretzschmar A., Ortmann S., Lojkic I., Habla C., Müller T., Kaiser C., Hundt B., Schuster P.2013. Oral vaccination of captive small Indian mongoose (Herpestes auropunctatus) against rabies. J. Wildl. Dis. 49: 1033–1036. doi: 10.7589/2013-02-035 [DOI] [PubMed] [Google Scholar]
- 48.Walcher P., Cui X., Arrow J. A., Scobie S., Molinia F. C., Cowan P. E., Lubitz W., Duckworth J. A.2008. Bacterial ghosts as a delivery system for zona pellucida-2 fertility control vaccines for brushtail possums (Trichosurus vulpecula). Vaccine 26: 6832–6838. doi: 10.1016/j.vaccine.2008.09.088 [DOI] [PubMed] [Google Scholar]
- 49.Watari Y., Takatsuki S., Miyashita T.2008. Effects of exotic mongoose (Herpestes javanicsus) on the native fauna of Amami-Oshima Island, southern Japan, estimated by distribution patterns along the historical gradient of mongoose invasion. Biol. Invasions 10: 7–17. doi: 10.1007/s10530-007-9100-6 [DOI] [Google Scholar]
- 50.Williams J. S.1999. Compensatory Reproduction and dispersal in an introduced mountain goat population in central Montana. Wildl. Soc. Bull. 27: 1019–1024. [Google Scholar]
- 51.Yamada F., Sugimura K., Abe S., Handa Y.2000. Present status and conservation of the endangered Amami rabbit Pentalagus furnessi. Tropics 10: 87–92. doi: 10.3759/tropics.10.87 [DOI] [Google Scholar]
- 52.Yogi M., Ogura G., Ishibashi O., Kawashima Y., Sunagawa K., Oda S.2006. Damages by the mongoose in the poultry industry of Okinawa Island. J. Okinawan Anim. Husbandry 41: 5–13(in Japanese). [Google Scholar]