Abstract
IL-15 is a proinflammatory cytokine that plays an essential role in the development and activation of natural killer (NK) cells. Adipose tissue acts as an endocrine organ that secretes cytokines and is an important reservoir for lymphocytes. We hypothesized that activation of the IL-15 signaling in adipose tissue will activate and expand the NK cell population and control tumor growth. We recently developed an adipocyte-targeting recombinant adeno-associated viral (rAAV) vector with minimal off-target transgene expression in the liver. Here, we used this rAAV system to deliver an IL-15/IL-15Rα complex to the abdominal fat by intraperitoneal (i.p.) injection. Adipose IL-15/IL-15Rα complex gene transfer led to the expansion of NK cells in the adipose tissue and spleen in normal mice without notable side effects. The i.p. injection of rAAV-IL-15/IL-15Rα complex significantly suppressed the growth of Lewis lung carcinoma implanted subcutaneously and exerted a significant survival advantage in a B16-F10 melanoma metastasis model. The antitumor effects were associated with the expansion of the NK cells in the blood, spleen, abdominal fat, and tumor, as well as the enhancement of NK cell maturity. Our proof-of-concept preclinical studies demonstrate the safety and efficacy of the adipocyte-specific IL-15/IL-15Rα complex vector as a novel cancer immune gene therapy.
Keywords: IL-15/IL-15Rα, adipocyte, rAAV, cancer, gene therapy, NK cell, visceral fat, immune therapy
Graphical Abstract

Cao and colleagues have designed a rAAV vector that selectively overexpresses IL-15/IL-15Rα in the adipocytes. A single intraperitoneal injection of this vector exhibits sustained levels of IL-15/IL-15Rα in the circulation with a strong safety profile and significant anti-tumor activity, supporting the pursuit of this mode of immunotherapy.
Introduction
Natural killer (NK) cells are part of the innate immune system and play an essential role in cancer immune surveillance by killing malignant cells and suppressing tumor growth.1, 2 NK cells are dependent on the presence of the cytokine interleukin (IL)-15, which supports their development and triggers their proliferation, motility, activation, and cytotoxic effector molecule expression.3, 4, 5 In addition, IL-15 maintains the survival of CD8 memory cells and induces the proliferation, survival, and function of effector CD8 T cells.6, 7 IL-15 shares many structural features with IL-2 that include the use of the two common receptor (R) subunits (IL-2/IL-15Rβ and IL-2/IL-15Rγ) and similar intracellular signaling. The high-affinity form of the IL-15R is a cytokine-specific Rα subunit, IL-15Rα.8 The primary mechanism by which IL-15 exerts its effect on NK and T cells is by trans-presentation of the IL-15/IL-15Rα complex expressed on the membrane of activated monocytes and dendritic cells to the IL-2R/IL-15Rβ and -γ heterodimer expressed on effector T, B, and NK cells.9
Increasing evidence from basic research and clinical studies has demonstrated promising antitumor effects of NK-cell-based immunotherapy. Hence, IL-15 signaling may be a tool for cancer immunotherapy by enhancing the antitumor response of NK and CD8 T cells. The use of IL-15, alone or in combination with vaccines and drug treatments, has proven effective in treating tumors in animal models.10 However, the IL-15 protein has shown several limitations, such as the short half-life and low biological activity in vivo,11, 12 possibly contributing to modest antitumor responses in patients.13 To address this limitation, IL-15/IL-15Rα complex or IL-15/IL-15Rα complex derivatives such as ALT-803 have been developed14, 15 and have been shown to be superior to IL-15 alone due to their extended half-life; longer persistence in tissues; and enhanced antitumor effect in cancer models of murine multiple myeloma,16 rat bladder cancer,14 murine glioblastoma,17 murine breast and colon cancer,18 and human ovarian cancer.19
Recombinant adeno-associated virus (rAAV) vectors are attractive gene delivery vehicles for both basic research and clinical gene therapy of genetic and acquired diseases due to their gene delivery efficacy, lack of pathogenicity, and strong safety profile.20 We developed a dual-cassette AAV vector system which, when packaged to the engineered hybrid serotype Rec2, selectively transduces adipose tissues with minimal off-target transgene expression in the liver. A single intraperitoneal (i.p.) injection of the adipose-targeting AAV vector containing leptin at a low dose completely rescues the metabolic syndromes of leptin-deficient ob/ob mice.21 Whether adipose tissue could be targeted for cancer therapy has yet to been assessed. Previous studies have shown that adipocyte IL-15 plays an essential role in regulating NK cell development locally and systemically.22 We hypothesized that increasing the expression of the IL-15/IL-15Rα complex in the adipose tissue, using gene therapy for cancer control, could be an alternative to other forms of IL-15 delivery, resulting in less toxicity and lowering the peak dose of serum IL-15. To test this hypothesis, we synthesized an IL-15/IL-15Rα complex transgene linking IL-15 and IL-15Rα with a 2A sequence23, 24, 25 and packaged this with the novel adipose-targeting rAAV vector. Two cancer models—a subcutaneous implantation of Lewis lung carcinoma (LLC) and a metastatic model with i.p. injection of B16-F10 melanoma26—were used in the proof-of-concept studies.
Results
rAAV-Mediated Gene Delivery of IL-15/IL-15Rα Complex to Visceral Fat Shows No Notable Toxicity
We have previously reported that a dual-cassette AAV serotype Rec2 vector system achieved highly selective transduction of visceral fat while severely restricting off-target transduction of liver via i.p. injection.21 As shown in Figure 1A, this AAV vector harbors two expression cassettes: one using the chicken β-actin (CBA) promoter to drive transgene expression and the other using the liver-specific albumin promoter to drive a microRNA-targeting the WPRE sequence that only exists in this AAV vector. We cloned the IL-15 and IL-15Rα cDNA sequence to this vector using a 2A sequence to express these two proteins from one transcript to form a functional complex. Empty vector containing identical backbone without the transgene was used as a control. The mice received either Rec2-IL-15/IL-15Rα complex or Rec2-empty (2 × 1010 viral genome [vg] per mouse, i.p. injection) and were monitored for 5 weeks. Adipocytes from visceral adipose tissue (VAT) exhibited an ∼200-fold increase of IL-15 mRNA in IL-15/IL-15Rα-complex-treated mice (Figure 1B). The IL-15/IL-15Rα complex protein level in serum was 65% higher in IL-15/IL-15Rα-complex-treated mice than in control mice (Figure 1C). Body weight and food intake were measured weekly, and no differences were observed (Figures 1D and 1E). Rectal temperatures were recorded at 5 weeks and showed no difference (Figure 1F). At 5 weeks post-rAAV injection, mice were sacrificed, blood and serum were collected, and liver function tests were obtained. No significant changes were found in the liver function panel (Figure 1G), spleen weight (data not shown), absolute number of splenocytes (Figure 1H), or complete blood count with differentials (Figure 1I).
Figure 1.
Adipocyte Gene Transfer of the IL-15/IL-15Rα Complex Shows No Notable Toxicity
(A) Schematic of AAV vectors. (B) mRNA expression level of the IL-15/IL-15Rα complex in VAT adipocytes. (C) Protein level of the IL-15/IL-15Rα complex in serum. (D) Weight change. (E) Food intake. (F) Body temperature. (G) Concentration of ALP, ALT, AST, and GGT in serum. (H) Absolute number of splenocytes. (I) Complete blood count with differentials. Data represent mean ± SEM; n = 5 per group. *p < 0.05; ***p < 0.001.
Adipose Gene Transfer of the IL-15/IL-15Rα Complex Expands NK Cells in Spleen and VAT
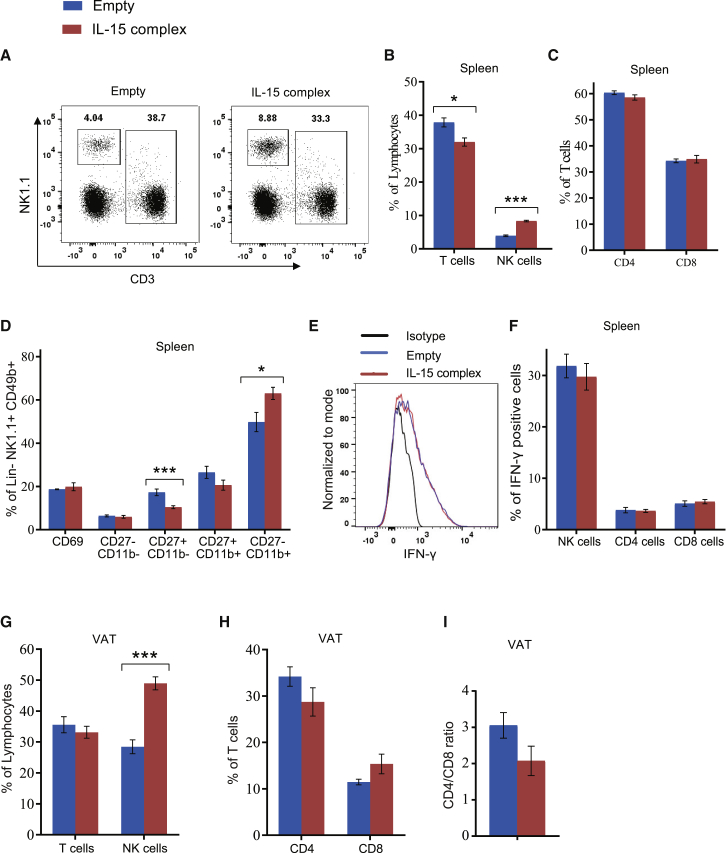
Further studies were undertaken to characterize the IL-15/IL-15Rα-complex-mediated lymphocyte profile. In spleen, the percentage of NK cells was approximately 2-fold higher in the IL-15/IL-15Rα-complex-treated mice than in the control-treated mice. The absolute number of NK cells was significantly increased in the spleen by 2-fold in the IL-15/IL-15Rα-complex-treated mice versus the control group (2.62 ± 0.22 [versus 1.24 ± 0.14] × 106 NK cells, p < 0.01). In contrast, the overall T cell percentage was reduced by 4% (Figures 2A and 2B), yet there was no change in the percentage of CD4 or CD8 T cells (Figure 2C). No significant change in CD69 expression was observed in NK cells in the IL-15/IL-15Rα-complex-treated mice (Figure 2D). Mouse NK cell subsets are defined by the surface density of CD27 and CD11b and classified as precursor (I, CD11blowCD27low), immature (II, CD11blowCD27high), proinflammatory (III, CD11bhighCD27high), or cytotoxic (IV, CD11bhighCD27low).27 IL-15/IL-15Rα complex treatment preferentially decreased the stage II NK cell subset and increased the stage IV NK cell subset (Figure 2D). An in vitro stimulation experiment showed that treatment with the IL-15/IL-15Rα complex had no effects on interferon (IFN)-γ secretion from NK, CD4, or CD8 cells (Figures 2E and 2F). Moreover, no changes in the splenic regulatory T cells or in the VAT were observed (data not shown). In VAT, the IL-15/IL-15Rα-complex-treated mice showed a doubling of the percentage of NK cells and a 3-fold increase in the absolute number of NK cells (150 ± 16.7 [versus 47 ± 7.3] × 103 NK cells, p < 0.01), while it had no significant effect on the percentage of total T cells or the CD4/CD8 ratio (Figure 2G).
Figure 2.
Adipocyte Gene Transfer of the IL-15/IL-15Rα Complex Expands NK Cells in Spleen and VAT
(A) Representative flow cytometry of NK cells and T cells in spleen. (B) Percentage of NK cells and T cells in spleen. (C) Percentage of CD4 or CD8 T cells in spleen. (D) Percent CD69 expression overall and subsets of splenic NK cells. (E) Representative flow cytometry of IFN-γ level within splenic NK cells. (F) IFN-γ expression on NK, CD4, and CD8 cells in spleen after 6 h in vitro stimulation. (G) Percentage of T cells and NK cells in VAT. (H) Percentage of CD4 and CD8 T cells in VAT. (I) CD4/CD8 ratio in VAT. Data represent mean ± SEM; n = 5 per group. *p < 0.05; ***p < 0.001.
Adipose IL-15/IL-15Rα Complex Gene Therapy Suppresses LLC Growth
To investigate the effect of IL-15/IL-15Rα complex gene transfer into VAT on the growth of a distant tumor, LLC cells were implanted subcutaneously on the flank 5 weeks after i.p. injection of Rec2-IL-15/IL-15Rα complex or Rec2-empty. The IL-15/IL-15Rα-complex-treated mice showed an approximately 50% reduction in tumor weight and tumor size (Figures 3A–3C). The immune profile was characterized in tumor, spleen, and VAT. In the tumors of IL-15/IL-15Rα-complex-treated mice, the tumor-infiltrating lymphocytes (TILs) showed an approximately 25% increase in the percentage of NK cells, whereas there was no change in the percentage of T cells (Figures 3C and 3D). There was no change in the percentage of CD4 or CD8 T cells within the tumors (Figure 3E). In the spleen, IL-15/IL-15Rα-complex-treated mice selectively increased NK cell percentage by 2-fold but showed no change in total percentage of T cells (Figure 3F). The absolute number of splenic NK cells was significantly increased in the IL-15/IL-15Rα-complex-treated group compared to that in the control group (2.72 ± 0.26 [versus 1.11 ± 0.08] × 106 NK cells, p < 0.001). Of note, IL-15/IL-15Rα-complex-treated mice showed a significantly reduced percentage of CD4 T cells and increased CD8 T cells within the spleen, thereby reducing the CD4/CD8 ratio (Figure 3G). Furthermore, IL-15/IL-15Rα-complex-treated mice showed a decrease in the percentage of stage I, II, and III splenic NK cells but an increase in stage IV NK cells (Figure 3H), which have the highest cytotoxic capacity.28 In the VAT, IL-15/IL-15Rα-complex-treated mice also showed an increase in the percentage and absolute number of NK cells (absolute numbers: 235 ± 24 [versus 93 ± 5.4] × 103, p < 0.01), and a reduced CD4/CD8 ratio (Figures 3I–3K).
Figure 3.
Adipocyte IL-15/IL-15Rα Complex Gene Therapy Suppresses LLC Growth
(A) LLC tumors dissected out of sacrificed mice 19 days post-implantation. (B) Tumor mass. (C) Representative flow cytometry of NK cells and T cells in tumor. (D) The percentage of NK cells and T cells in tumor. (E) The percentage of CD4 or CD8 T cells in the tumor. (F) Percentage of T cells and NK cells in the spleen. (G) Percentage of CD4 or CD8 T cells in the spleen. (H) The percentage of CD69 expression on splenic NK cells and the percentage of NK cell subsets in spleen. (I) Percentage of NK cells and T cells in VAT. (J) Percentage of CD4 and CD8 T cells in VAT. (K) CD4/CD8 ratio in VAT. Data represent mean ± SEM; n = 10 per group. *p < 0.05; ***p < 0.001.
Adipose IL-15/IL-15Rα Complex Gene Therapy Prolongs Survival in a Mouse Model of B16-F10 Metastatic Melanoma
To determine whether IL-15/IL-15Rα complex gene therapy could exert survival benefit, B16-F10 melanoma cells were intraperitoneally implanted 5 weeks after the i.p. injection of Rec2- IL-15/IL-15Rα complex. Treatment with the IL-15/IL-15Rα complex significantly extended the median survival by approximately 40% (18 days for control-treated mice and 25 days for IL-15/IL-15Rα-complex-treated mice, p < 0.001; Figure 4A).
Figure 4.
Adipocyte IL-15/IL-15Rα Complex Gene Therapy Prolongs Survival in a Preclinical Mouse Model of Metastatic Melanoma
(A) Kaplan-Meier survival curve analysis of mice treated via i.p. injection with IL-15/IL-15Rα complex gene therapy or with vector control, followed by i.p. injection of B16-F10 melanoma cells. (B) In vivo bioluminescence imaging of luciferase-expressing B16-F10 at day 10 post-tumor implantation in a separate experiment. (C) Total bioluminescence activity (photons per second). (D) Images of all mice at sacrifice 11 days post-tumor implantation. White arrowheads point to large melanoma tumors. (E) VAT adipocyte IL-15/IL-15Rα complex expression. Data represent mean ± SEM; n = 10 per group. ***p < 0.001.
Adipose IL-15/IL-15Rα Complex Gene Therapy Elicits Antitumor and Immune Responses in a Model of Metastatic Melanoma
To examine the tumor burden, immune profiles, and survival in the preclinical model of metastatic melanoma, we repeated the i.p. Rec2-IL-15/IL-15Rα complex gene therapy and measured the tumor burden by luciferase imaging 10 days after melanoma implantation. The luciferase signal intensity was 60% lower in IL-15/IL-15Rα-complex-treated mice when compared to vector-control-treated mice (Figures 4B and 4C). Multiple large tumors were observed in all of the control-treated mice on day 10 (Figure 4D). In contrast, significantly smaller tumors were found in all of the IL-15/IL-15Rα-complex-treated mice (all mice were shown in Figure 4D). The adipocyte overexpression of the IL-15/IL-15Rα complex was confirmed by qRT-PCR (Figure 4E).
Next, we further characterized the immune profile. In blood, treatment with the IL-15/IL-15Rα complex gene therapy increased the percentage of NK cells by ∼2-fold without proportional change for the total percentage of T cells (Figure 5A). There was no percent change in CD4 or CD8 T cell subsets (Figure 5B). Consistent with the findings in mice treated without tumor (Figure 2D) and in the LLC mouse model (Figure 3H), treatment with the IL-15/IL-15Rα complex gene therapy shifted the NK cell development toward the most mature stage, with a reduction of stage II and an increase of stage IV (Figure 5C). IL-15/IL-15Rα-complex-treated mice displayed a significantly higher CD44 expression on CD4 and CD8 T cells compared to vector-control-treated mice (Figure 5C), which is a marker indicative of effector-memory T cells.29, 30 The spleen immune phenotype was similar to that of blood (Figures 5E–5G), and the absolute number of NK cells was significantly increased in the IL-15/IL-15Rα-complex-treated mice versus the control group (2.55 ± 0.10 [versus 1.17 ± 0.11] × 106, p < 0.001). Of note, CD69 expression on NK cells in the spleen was significantly lower in IL-15/IL-15Rα-complex-treated mice (Figure 5F). In vitro stimulation with the IL-15/IL-15Rα complex showed a significant increase of IFN-γ secretion for NK, CD4, and CD8 T cells taken from the mice treated with the IL-15/IL-15Rα complex compared to controls (Figures 5H and 5I). Within the tumors taken from mice treated with IL-15/IL-15Rα complex gene therapy, the percentages of T cells and NK cells were significantly higher when compared with tumors from vector-control-treated mice; NK cell percentage increased approximately 4-fold, whereas the T cell increase was restricted to the CD8+ subset with a percentage increase of approximately 50% (Figures 6A–6C). Interestingly, VAT showed a significant increase in the percentage and absolute number of NK cells in the IL-15/IL-15Rα-treated group versus those of the control group (absolute numbers: 181 ± 11.2 [versus 106 ± 8.3] × 103, p < 0.001), but a significant decrease in the percentage of T cells, restricted to the CD8+ subset (Figures 6D–6F).
Figure 5.
IL-15/IL-15Rα Complex Gene Therapy Induces an Antitumor Immune Phenotype in a Preclinical Mouse Model of Metastatic Melanoma
(A) Percentage of NK cells and T cells in blood. (B) The percentage of CD4 or CD8 T cells in blood. (C) The percentage of NK cell subsets in blood. (D) The percentage of CD44 expressed on CD4 or CD8 T cells in blood. (E) The percentage of NK cells and T cells in spleen. (F) The percentage of CD69 expression on splenic NK cells and the percentage of NK cell subsets in spleen. (G) The percentage of CD4 or CD8 T cells in spleen. (H) Representative flow cytometry of IFN-γ in splenic NK cells. (I) IFN- γ expression in NK, CD4, and CD8 cells from spleen after 6 h in vitro stimulation. Data represent mean ± SEM; n = 10 per group. *p < 0.05; ***p < 0.001.
Figure 6.
IL-15/IL-15Rα Complex Gene Therapy Regulates Tumor and VAT Immune Cells in Melanoma-Bearing Mice
(A) Representative flow cytometry illustrating the percentage of NK cells and T cells in tumor. (B) Percentage of NK cells and T cells in tumor. (C) Percentage of CD4 and CD8 T cells in tumor. (D) Representative flow cytometry illustrating percentage of NK cells and T cells in VAT. (E) The percentage of NK cells and T cells in VAT. (F) The percentage of CD4 and CD8 T cells in VAT. Data represent mean ± SEM; n = 10 per group. *p < 0.05; ***p < 0.001.
Discussion
Adipose tissue is a multifunctional organ that regulates whole-body metabolic homeostasis and produces scores of adipokines, cytokines, and other endocrine substances. Although adipose tissue is among the most abundant tissues and more accessible compared to liver, gene therapies targeting adipose tissue are scarce, largely due to the lack of efficient gene delivery vehicles. We have recently developed a rAAV serotype vector system coupled with a dual-cassette design that achieves high transduction of multiple visceral fat depots by i.p. injection at a dose of 1 to 2 orders lower than commonly used doses for systemic gene delivery with minimal off-target transduction of the liver. This new rAAV vector system paves the way to target adipose tissue for gene therapy of genetic and acquired diseases.21 Our study demonstrated that rAAV-mediated gene delivery of the IL-15/IL-15Rα complex to visceral fat at a low dose effectively stimulated NK cells, suppressed tumor progression, and prolonged survival in preclinical animal models of cancer. To our knowledge, this is the first attempt to target adipose tissue for gene therapy of cancer.
IL-15 plays an essential role in both the innate and adaptive immunity and is co-expressed with IL-15Rα to form a protein complex in antigen-presenting cells, hence activating NK and T cells by trans-presentation.8, 9, 10 Previous studies have shown that the soluble IL-15/IL-15Rα complex delivers a 50-fold more potent immune stimulatory effect than IL-15 alone31 and is more efficacious in treating solid and metastatic tumors in animal models.9, 32, 33 One concern with IL-15 protein administration is side effects such as neutropenia and hypotension, which are most likely due to the spike in the serum concentration of IL-15.34, 35 An IL-15 superagonist is reported to induce weight loss, hypothermia, and liver injury in mice similar to those reported in humans.36 Several reports using plasmids to overexpress IL-15 or both IL-15 and IL-15Rα have shown no apparent liver toxicity but have shown enlarged spleens due to an increase in splenocytes.15, 37
In our study, adipose IL-15/IL-15Rα complex gene delivery resulted in an ∼200-fold increase of IL-15 mRNA in the transduced adipose depot, along with an approximately 2-fold increase of the IL-15/IL-15Rα complex protein in the serum with no changes in behavior, weight, body temperature, organ size, or liver toxicity. We achieved elevated levels of the IL-15/IL-15Rα complex 5 weeks after a single administration of the construct, resulting from continuous production of the IL-15/IL-15Rα complex by the adipose tissue and without measurable toxicity. The IL-15/IL-15Rα complex was capable of inducing a significant percent increase of NK cells in the spleen, VAT, tumor, and blood, as well as percent increase in CD8 and CD4 memory T cells in the blood. Therefore, a single i.p. treatment with the IL-15/IL-15Rα complex gene therapy targeting adipose tissue appears as an effective way to target immune effector cells while suppressing tumor growth and to prolong survival without any apparent toxicity.
A transgenic mouse line overexpressing a high dose of endogenous IL-15 is associated with tumor formation.38 This could be related to chronic exposure to high levels of IL-15, the strain of mouse (FVB), neither, or both. We have identified the human NKT cell most susceptible to chronic IL-15 stimulation39 and could monitor for the expansion of this small subset under conditions of chronic IL-15/IL-15Rα complex production resulting from selective expression of the complex in adipocytes in future studies.
Treatment with the IL-15/IL-15Rα complex gene therapy induced an increase in both the percentage and absolute number of splenic and adipose NK cells when compared to vector-control-treated mice, and there was a preferential percent increase in the cytotoxic (CD11bhighCD27low) NK subset in the spleen. These increases, along with a consistent increase in percent NK cells in the tumor, were associated with an antitumor effect in each of the tumor models that were studied in this report; however, depletion of the NK cell population to establish a cause and effect was not pursued in this study.
There are several explanations for the increase of NK cells in the spleen in the absence of tumor implantation. One is the increase in the circulating level of the IL-15/IL-15Rα complex that can promote the survival, proliferation, and differentiation of mouse NK cells toward the most potent cytotoxic subset with a longer lifespan.38, 40, 41 The other possibility is that the increase of IL-15/IL-15Rα complex in the adipose tissue supports the development and proliferation of adipose resident NK cells that can leave the adipose tissue and populate other organs such as the spleen.22 Of note, in the absence of cancer, adipose IL-15/IL-15Rα complex gene therapy expanded the NK population in the spleen modestly (∼2 fold) without toxicity, compared to four repeated i.p. infusions of IL-15/IL-15Rα complex protein, which induced a 16-fold increase in NK cells associated with toxicity and organomegaly,36 or hepatic IL-15/IL-15Rα complex gene therapy delivered intravenously (i.v.), which induced a 46.9-fold increase in NK cells associated with organomegaly.15, 37 Furthermore, the mice in our study that did not have tumors but were treated with IL-15/IL-15Rα complex gene therapy had NK cells that were not activated, as evidenced by the absence of an increase in NK cell IFN-γ production upon stimulation when compared to mice treated with the empty vector control. In contrast, when challenged by a tumor, mice treated with IL-15/IL-15Rα complex gene therapy displayed a robust immune response characterized by a relative expansion of NK cells in the spleen, adipose tissue, blood, and tumor; a relative increase in the percentage of CD8 and CD4 memory cells in the blood; and a significant increase in the expression of IFN-γ when compared to vector-control-treated mice. In addition, our study was consistent with other studies in which the treatment of IL-15/IL-15Rα complex did not alter the overall number of regulatory T cells.6, 42
Encouraged by the promising results of this proof-of-concept study, we are expanding the adipose IL-15/IL-15Rα complex gene therapy to additional solid tumor and leukemia models and assessing the therapeutic efficacy in established cancer models of implantation as well as oncogene-induced cancer models.
Obesity is linked to an increased risk of numerous types of cancers43, 44, 45 and to a poorer prognosis. Obesity is also associated with adipose dysfunction, including disturbance of adipose resident immune cells, which may partially underlie the link with cancer. Thus, it will be interesting to investigate the immune, metabolic, and anticancer effects of adipose IL-15/IL-15Rα complex gene therapy in the context of obesity.
This proof-of-concept study does not address whether the selective expression and continuous release of the IL-15/IL-15Rα complex by adipocytes may or may not have advantages over daily subcutaneous administration of the IL-15/IL-15Rα complex in humans. However, the two approaches would likely have distinct clinical consequences for patients. For example, the daily subcutaneous administration of the complex relies on patient compliance because of self-administration, and that could impact anti-tumor efficacy, whereas this would not be an issue with IL-15/IL-15Rα production resulting from selective expression of the complex in adipocytes; local pain, redness, and swelling at the sites of subcutaneous injection of the IL-15/IL-15Rα complex could lead to poor compliance with administration, and this would not be an issue with IL-15/IL-15Rα production resulting from selective expression of the complex in adipocytes; pharmacokinetics could be distinct between the two methods of delivery and result in clinically meaningful differences in anti-tumor efficacy; and preferential expansion of adipose NK cells as the result of IL-15/IL-15Rα production from selective expression of the complex in adipocytes could lead to differences in therapeutic uses and efficacy. Acute toxicities could be easily managed by more readily by subcutaneous administration, while this may be difficult with IL-15/IL-15Rα production resulting from selective expression of the complex in adipocytes. Moreover, a recent study indicated that continuous in vitro and in vivo treatment of NK cells with IL-15 alone resulted in decreased NK cell viability and a NK cell-cycle arrest gene expression pattern.46 No studies were conducted with the IL-15/IL-15Rα complex, and similar findings of NK cell exhaustion were not reported in the first-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation.47 It will be interesting to see whether similar patterns of NK cell exhaustion are seen with chronic administration of the IL-15/IL-15Rα complex or with the IL-15 superagonist complex ALT-803, and, if seen, this would certainly limit the use of IL-15/IL-15Rα therapy resulting from selective expression of the complex in adipocytes. Future studies are required to evaluate the translational potential of the adipose-targeting IL-15/IL-15Rα gene therapy.
In summary, our study demonstrated that i.p. administration of a single dose of the adipose-targeting rAAV vector selectively transduced the visceral fat depots to overexpress the IL-15/IL-15Rα complex, leading to a relative expansion of NK cells, inhibition of tumor progression, and a significant survival benefit in a preclinical model of cancer, without demonstrable toxicity. The potent antitumor effect and lack of toxicity in mouse models of cancer validate the concept of IL-15/IL-15Rα complex gene therapy targeting the adipose tissue and provide a strong rationale for further testing in combination with other treatment modalities.
Materials and Methods
Mice
Male C57/BL6 mice (6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA, USA) and housed in temperature-controlled (22–23°C) and humidity-controlled rooms with food and water ad libitum. The mice were fed a normal chow diet (NCD; 11% fat; caloric density, 3.4 kcal/g; Envigo Teklad). All animal experiments were performed in accordance to the guidelines approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
AAV Vector Construction and Package
Mouse IL-15 cDNA (GenBank: NM_001254747.4) and IL-15Rα (GenBank: NM_001271497.1) were synthesized by Integrated DNA Technologies (IDT). The stop codon was removed from the IL-15 cDNA sequence; subsequently, viral 2A sequence (GGC AGT GGA GAG GGC AGA GGA AGT CTG CTA ACA TGC GGT GAC GTC GAG AAT CCT GGC CCA) was inserted between the IL-15 and IL-15Rα genes. The synthesized DNA fragment contains XhoI (CTCGAG) and SacI (GAGCTC) sequences at the 5′ and 3′ termini, respectively. After cloning into the rAAV vector, the sequence of the insert was verified by sequencing. The cDNA was subcloned into a novel AAV plasmid of dual cassettes that restricts off-target transduction in liver.21 The rAAV plasmid contains a vector expression cassette consisting of the cytomegalovirus (CMV) enhancer and CBA promoter, woodchuck posttranscriptional regulatory element (WPRE) enhancing transgene expression, and bovine growth hormone (bGH) poly(A) flanked by AAV2 inverted terminal repeats. Engineered hybrid serotype Rec2 vectors were packaged and purified as described previously.48 The control vector contained the identical backbone with no transgene and was termed Rec2-empty.
Measurement of the IL-15/IL-15Ra Complex
The amount of IL-15/IL-15Rα complex in serum was measured using the IL-15/IL-15Rα Complex Mouse ELISA Kit (Invitrogen).
LLC Experiment
Male C57BL/6 mice, 6 weeks of age, were randomized to receive Rec2-IL-15/IL-15Rα complex or Rec2-empty (2 × 1010 vg per mouse, i.p. injection in 100 μL AAV dilution buffer). After 5 weeks of AAV injection, LLC cells (ATCC) were subcutaneously implanted (3 × 105 cells per mouse; n = 10 per group). The mice were sacrificed on day 19 after tumor implantation.
B16-F10 Melanoma Experiment and Survival Study
Male C57BL/6 mice, 6 weeks of age, were randomized to receive Rec2-IL-15/IL-15Rα complex or Rec2-empty (2 × 1010 vg per mouse, i.p. injection). Five weeks after AAV injection, luciferase-tagged B16-F10 melanoma cells (Imanis Life Sciences) were intraperitoneally implanted (1 × 105 cells per mouse, n = 10 per group). The luciferase imaging was performed on day 10 after tumor implantation, and the mice were sacrificed on day 11 for immune profiling. For the survival study, the same experiment was repeated, and the mice were kept until moribund, at which time they were sacrificed.
Luciferase Imaging
D-luciferin was administered via i.p. injection into each mouse at a dose of 150 mg/g body weight. After 70 min, the mice were anesthetized in an isoflurane chamber and then placed in a warm and light-tight scan chamber. The bioluminescence imaging was generated using the IVIS Lumina II from Caliper Life Sciences (Small Animal Imaging Core Facility of Ohio State University [OSU]) and presented by total flux (photons per second).
Isolation of Leukocytes and Flow Cytometry
Mice were anesthetized with 2.5% isoflurane followed by decapitation, and then truncal blood was collected. The spleen, tumor, and VAT were dissected and weighed. The blood sample was treated twice with ammonium chloride solution (Stem Cell Technologies) to lyse the red blood cells (RBCs), washed, and then suspended in fluorescence-activated cell sorting (FACS) buffer (1% fetal bovine serum [FBS] in PBS). Spleens were mechanically dissociated through a 70-μM strainer to obtain single-cell suspension, and RBCs were lysed with ammonium chloride solution and then washed and resuspended in FACS buffer. The VAT was dissected and minced into small pieces in Kreb-Ringer HEPES buffer (pH 7.4). Collagenase (1 mg/mL, Sigma C6885) was added to all tissues and incubated for 45 min at 37°C with shaking. The mixture was centrifuged to separate the floating adipocytes from the adipose stromal vascular fraction (SVF). The SVF pellet was treated with ammonium chloride solution to lyse the RBCs, washed, and resuspended in FACS buffer. To obtain leukocytes from the tumor, LLC or melanoma tissue samples were minced into small pieces. Collagenase type II (1 mg/mL, Sigma) was added to all tissues and incubated for 45 min at 37°C with shaking. Cells were filtered using a 70-μM strainer and washed twice with RPMI, and ammonium chloride solution was added to lyse the RBCs. Leukocytes were pre-enriched using density gradient centrifugation. The interlayer cells were collected, washed with RPMI, and resuspended in FACS buffer. Cells were counted using the Cellometer Auto 2000 (Nexcelom Bioscience). For surface staining, cells were stained with a fluorescent-dye-conjugated antibody with the appropriate surface markers for 20 min. The antibodies used for flow cytometry immunophenotyping are listed in Table S1.
For the analysis of IFN-γ production by NK and T cells, 2 × 106 splenocytes were stimulated for 6 h in RPMI medium containing phorbol 12-myristate 13-acetate (PMA) (81 nM), ionomycin (eBioscience, 1.34 mM), IL-2 (NIH, 150 U/mL), and Brefeldin A (BD Biosciences) at 37°C. Cells were first stained for surface markers. After fixation and permeabilization using a Foxp3 staining buffer set (eBiosciences), cells were stained for intracellular IFN-γ and its corresponding isotype control, immunoglobulin G (IgG)1κ.
Cell events were acquired using BD LSR II flow cytometry (BD Biosciences), and the results were analyzed using FlowJo software, v10 (Tree Star).
In Vivo Toxicity Analysis
Male C57BL/6 mice (6 weeks of age) were randomized to receive Rec2-IL15/IL15Rα complex or Rec2-empty (2 × 1010 vg per mouse, i.p. injection) and were monitored for 5 weeks. Throughout that interval, the mice were assessed for clinical and behavioral changes, body weight, and food intake on a weekly basis. Rectal temperatures of mice at various time points after i.p. administration of Rec2 vectors were measured with a microprobe thermometer (Physitemp, model BAT-12) topped with lubricant. At 5 weeks after AAV injection, blood was collected for complete blood count and liver function panel by the Comparative Pathology and Mouse Phenotyping Shared Resources at The Ohio State University. Alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyltransferase (GGT) concentrations were measured as indices of liver injury.
Statistical Analysis
Values are expressed as mean ± SEM. All data obtained were analyzed on GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). Kaplan-Meier survival curves were generated using GraphPad Prism, and statistical differences were assessed via log-rank and Wilcoxon analyses. Student’s t test was utilized for group comparison, and significance was defined as *p < 0.05, **p < 0.01, and ***p < 0.001.
Author Contributions
Conceptualization, R.X., A.G.M., M.A.C., and L.C.; Methodology Development: R.X., A.G.M., M.A.C., and L.C.; Data Acquisition: R.X., A.G.M., W.H., L.A.C., R.K.W., N.J.Q., Y.Y., and H.C.M.; Writing and Editing Manuscript: R.X., A.G.M., M.A.C., and L.C.; Funding Acquisition, M.A.C. and L.C.
Conflicts of Interest
The vector construct21 has been submitted for patent filing by The Ohio State University; otherwise, we declare that none of the authors have any conflict of interest.
Acknowledgments
This work was done in Columbus, OH, USA. This work was supported by NIH grants AG041250, CA166590, CA178227, and CA163640 to L.C. and CA163205, CA210087, CA95426, and CA068458 to M.A.C.
Footnotes
Supplemental Information can be found with this article online at https://doi.org/10.1016/j.ymthe.2019.02.011.
Contributor Information
Michael A. Caligiuri, Email: mcaligiuri@coh.org.
Lei Cao, Email: lei.cao@osumc.edu.
Supplemental Information
References
- 1.Street S.E., Hayakawa Y., Zhan Y., Lew A.M., MacGregor D., Jamieson A.M., Diefenbach A., Yagita H., Godfrey D.I., Smyth M.J. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J. Exp. Med. 2004;199:879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitvogel L., Tesniere A., Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 3.Carson W.E., Giri J.G., Lindemann M.J., Linett M.L., Ahdieh M., Paxton R., Anderson D., Eisenmann J., Grabstein K., Caligiuri M.A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vosshenrich C.A., Ranson T., Samson S.I., Corcuff E., Colucci F., Rosmaraki E.E., Di Santo J.P. Roles for common cytokine receptor γ-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J. Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 5.Fehniger T.A., Caligiuri M.A. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 6.Cheng L., Du X., Wang Z., Ju J., Jia M., Huang Q., Xing Q., Xu M., Tan Y., Liu M. Hyper-IL-15 suppresses metastatic and autochthonous liver cancer by promoting tumour-specific CD8+ T cell responses. J. Hepatol. 2014;61:1297–1303. doi: 10.1016/j.jhep.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schluns K.S., Williams K., Ma A., Zheng X.X., Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 8.Waldmann T.A. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol. Res. 2015;3:219–227. doi: 10.1158/2326-6066.CIR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois S., Mariner J., Waldmann T.A., Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 10.Waldmann T.A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H., Carrasquillo J.A., Paik C.H., Waldmann T.A., Tagaya Y. Differences of biodistribution, pharmacokinetics, and tumor targeting between interleukins 2 and 15. Cancer Res. 2000;60:3577–3583. [PubMed] [Google Scholar]
- 12.Zamai L., Ponti C., Mirandola P., Gobbi G., Papa S., Galeotti L., Cocco L., Vitale M. NK cells and cancer. J. Immunol. 2007;178:4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 13.Conlon K.C., Lugli E., Welles H.C., Rosenberg S.A., Fojo A.T., Morris J.C., Fleisher T.A., Dubois S.P., Perera L.P., Stewart D.M. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes-Giacoia E., Miyake M., Goodison S., Sriharan A., Zhang G., You L., Egan J.O., Rhode P.R., Parker A.S., Chai K.X. Intravesical ALT-803 and BCG treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and NK cell expansion. PLoS ONE. 2014;9:e96705. doi: 10.1371/journal.pone.0096705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubinstein M.P., Kovar M., Purton J.F., Cho J.H., Boyman O., Surh C.D., Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15Ralpha. Proc. Natl. Acad. Sci. USA. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W., Jones M., Liu B., Zhu X., Johnson C.B., Edwards A.C., Kong L., Jeng E.K., Han K., Marcus W.D. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor αSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 2013;73:3075–3086. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathios D., Park C.K., Marcus W.D., Alter S., Rhode P.R., Jeng E.K., Wong H.C., Pardoll D.M., Lim M. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int. J. Cancer. 2016;138:187–194. doi: 10.1002/ijc.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim P.S., Kwilas A.R., Xu W., Alter S., Jeng E.K., Wong H.C., Schlom J., Hodge J.W. IL-15 superagonist/IL-15RαSushi-Fc fusion complex (IL-15SA/IL-15RαSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget. 2016;7:16130–16145. doi: 10.18632/oncotarget.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felices M., Chu S., Kodal B., Bendzick L., Ryan C., Lenvik A.J., Boylan K.L.M., Wong H.C., Skubitz A.P.N., Miller J.S., Geller M.A. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol. Oncol. 2017;145:453–461. doi: 10.1016/j.ygyno.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santiago-Ortiz J.L., Schaffer D.V. Adeno-associated virus (AAV) vectors in cancer gene therapy. J. Control. Release. 2016;240:287–301. doi: 10.1016/j.jconrel.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang W., Liu X., Queen N.J., Cao L. Targeting visceral fat by intraperitoneal delivery of novel AAV serotype vector restricting off-target transduction in liver. Mol. Ther. Methods Clin. Dev. 2017;6:68–78. doi: 10.1016/j.omtm.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liou Y.H., Wang S.W., Chang C.L., Huang P.L., Hou M.S., Lai Y.G., Lee G.A., Jiang S.T., Tsai C.Y., Liao N.S. Adipocyte IL-15 regulates local and systemic NK cell development. J. Immunol. 2014;193:1747–1758. doi: 10.4049/jimmunol.1400868. [DOI] [PubMed] [Google Scholar]
- 23.Donnelly M.L., Hughes L.E., Luke G., Mendoza H., ten Dam E., Gani D., Ryan M.D. The ‘cleavage’ activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’ sequences. J. Gen. Virol. 2001;82:1027–1041. doi: 10.1099/0022-1317-82-5-1027. [DOI] [PubMed] [Google Scholar]
- 24.Lewis J.E., Brameld J.M., Hill P., Barrett P., Ebling F.J., Jethwa P.H. The use of a viral 2A sequence for the simultaneous over-expression of both the vgf gene and enhanced green fluorescent protein (eGFP) in vitro and in vivo. J. Neurosci. Methods. 2015;256:22–29. doi: 10.1016/j.jneumeth.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan M.D., King A.M., Thomas G.P. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J. Gen. Virol. 1991;72:2727–2732. doi: 10.1099/0022-1317-72-11-2727. [DOI] [PubMed] [Google Scholar]
- 26.Cha J., Roomi M.W., Ivanov V., Kalinovsky T., Niedzwiecki A., Rath M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int. J. Oncol. 2013;42:55–64. doi: 10.3892/ijo.2012.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiossone L., Chaix J., Fuseri N., Roth C., Vivier E., Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 28.Fu B., Wang F., Sun R., Ling B., Tian Z., Wei H. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology. 2011;133:350–359. doi: 10.1111/j.1365-2567.2011.03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baaten B.J., Li C.R., Deiro M.F., Lin M.M., Linton P.J., Bradley L.M. CD44 regulates survival and memory development in Th1 cells. Immunity. 2010;32:104–115. doi: 10.1016/j.immuni.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong K.L., Tang L.F., Lew F.C., Wong H.S., Chua Y.L., MacAry P.A., Kemeny D.M. CD44high memory CD8 T cells synergize with CpG DNA to activate dendritic cell IL-12p70 production. J. Immunol. 2009;183:41–50. doi: 10.4049/jimmunol.0803473. [DOI] [PubMed] [Google Scholar]
- 31.Stoklasek T.A., Schluns K.S., Lefrançois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epardaud M., Elpek K.G., Rubinstein M.P., Yonekura A.R., Bellemare-Pelletier A., Bronson R., Hamerman J.A., Goldrath A.W., Turley S.J. Interleukin-15/interleukin-15R α complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 33.Rhode P.R., Egan J.O., Xu W., Hong H., Webb G.M., Chen X., Liu B., Zhu X., Wen J., You L. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol. Res. 2016;4:49–60. doi: 10.1158/2326-6066.CIR-15-0093-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger C., Berger M., Hackman R.C., Gough M., Elliott C., Jensen M.C., Riddell S.R. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldmann T.A., Lugli E., Roederer M., Perera L.P., Smedley J.V., Macallister R.P., Goldman C.K., Bryant B.R., Decker J.M., Fleisher T.A. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117:4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y., Luan L., Rabacal W., Bohannon J.K., Fensterheim B.A., Hernandez A., Sherwood E.R. IL-15 superagonist-mediated immunotoxicity: role of NK cells and IFN-γ. J. Immunol. 2015;195:2353–2364. doi: 10.4049/jimmunol.1500300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang C.-M., Lo C.H., Shih Y.M., Chen Y., Wu P.Y., Tsuneyama K., Roffler S.R., Tao M.H. Treatment of hepatocellular carcinoma with adeno-associated virus encoding interleukin-15 superagonist. Hum. Gene Ther. 2010;21:611–621. doi: 10.1089/hum.2009.187. [DOI] [PubMed] [Google Scholar]
- 38.Fehniger T.A., Suzuki K., Ponnappan A., VanDeusen J.B., Cooper M.A., Florea S.M., Freud A.G., Robinson M.L., Durbin J., Caligiuri M.A. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J. Exp. Med. 2001;193:219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J., Mitsui T., Wei M., Mao H., Butchar J.P., Shah M.V., Zhang J., Mishra A., Alvarez-Breckenridge C., Liu X. NKp46 identifies an NKT cell subset susceptible to leukemic transformation in mouse and human. J. Clin. Invest. 2011;121:1456–1470. doi: 10.1172/JCI43242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy M.K., Glaccum M., Brown S.N., Butz E.A., Viney J.L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C.R. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koka R., Burkett P.R., Chien M., Chai S., Chan F., Lodolce J.P., Boone D.L., Ma A. Interleukin (IL)-15R[α]-deficient natural killer cells survive in normal but not IL-15R[α]-deficient mice. J. Exp. Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergamaschi C., Rosati M., Jalah R., Valentin A., Kulkarni V., Alicea C., Zhang G.M., Patel V., Felber B.K., Pavlakis G.N. Intracellular interaction of interleukin-15 with its receptor α during production leads to mutual stabilization and increased bioactivity. J. Biol. Chem. 2008;283:4189–4199. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- 43.Reeves G.K., Pirie K., Beral V., Green J., Spencer E., Bull D., Million Women Study Collaboration Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 45.Whitlock G., Lewington S., Sherliker P., Clarke R., Emberson J., Halsey J., Qizilbash N., Collins R., Peto R., Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felices M., Lenvik A.J., McElmurry R., Chu S., Hinderlie P., Bendzick L., Geller M.A., Tolar J., Blazar B.R., Miller J.S. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight. 2018;3:96219. doi: 10.1172/jci.insight.96219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romee R., Cooley S., Berrien-Elliott M.M., Westervelt P., Verneris M.R., Wagner J.E., Weisdorf D.J., Blazar B.R., Ustun C., DeFor T.E. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131:2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X., Magee D., Wang C., McMurphy T., Slater A., During M., Cao L. Adipose tissue insulin receptor knockdown via a new primate-derived hybrid recombinant AAV serotype. Mol. Ther. Methods Clin. Dev. 2014;1 doi: 10.1038/mtm.2013.8. article 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.