Abstract
Rubratoxin A, a potent inhibitor of PP2A, is known to suppress smooth muscle contraction. The inhibitory role of PP2A in smooth muscle contraction is still unclear. In order to clarify the regulatory mechanisms of PP2A on vascular smooth muscle contractility, we examined the effects of rubratoxin A on the Ca2+-induced contraction of β-escin skinned carotid artery preparations from guinea pigs. Rubratoxin A at 1 µM and 10 µM significantly inhibited skinned carotid artery contraction at any Ca2+ concentration. The data fitting to the Hill equation in [Ca2+]-contraction relationship indicated that rubratoxin A decreased Fmax-Ca2+ and increased [Ca2+]50, indices of Ca2+ sensitivity for the force and myosin-actin interaction, respectively. These results suggest that PP2A inhibition causes downregulation of the myosin light chain phosphorylation and direct interference with myosin-actin interaction.
Keywords: vascular smooth muscle, protein phosphatase 2A, rubratoxin A, Ca2+ sensitivity, contractile proteins
Introduction
In smooth muscle cells, the phosphorylation level of myosin regulatory light chain (MLC) is thought to essentially regulate contractility. Activation of Ca2+/calmodulin-dependent MLC kinase (MLCK) increases phosphorylated MLC, resulting in contraction, while inactivation of MLCK decreases phosphorylated MLC, leading to muscle relaxation (1).
Another mechanism, known as Ca2+-sensitization/desensitization, also plays a role in the regulation of smooth muscle contractility (1). In this mechanism, the relation between MLC phosphorylation level and intracellular Ca2+ concentration is altered by inactivating/activating MLC phosphatase (MLCP), a type 1 serine/threonine protein phosphatase (PP1), activity (2). For instance, inhibition of PP1 by tautomycin induced smooth muscle contractions even in the absence of Ca2+ with an accompanying rise in the MLC phosphorylation level (3, 4).
Although the role of MLCP in the regulation of smooth muscle contraction has been well documented (1, 2), the role of type 2A serine/threonine protein phosphatase (PP2A), another serine/threonine phosphatase, is still unclear (5). Okadaic acid (OA), a potent PP2A/PP1 inhibitor, has been used to study PP2A in smooth muscle preparations. An interesting feature of OA is that it has dual effects on smooth muscle contractility (see reviews in refs 5 and 6). OA at lower concentration (< 1 μM) relaxed pre-contracted smooth muscle preparations, while it induced muscle contraction by itself at higher concentration (7,8,9,10,11,12). Considering that OA inhibits PP2A (Ki = 34 pM) more potently than PP1 (Ki = 147,000 pM) (13), the relaxing effect at lower concentrations of OA is likely due to inhibition of PP2A, while the contracting effect at higher concentration is due to PP1 inhibition (11, 12) as discussed above. According to this, we can assume that not only PP1, but also PP2A plays a role in force development and/or maintenance in smooth muscle cells (14).
The next question is how PP2A is involved in the development and/or maintenance of smooth muscle contraction. Considering that membrane permeabilization by saponin or Triton X-100 diminished the OA’s relaxing effect (15), one can assume that PP2A controls intracellular Ca2+ concentration. However, this does not seem to be the primary role of PP2A because Ishida et al. showed that OA could attenuate a Ca2+-ionophore-induced contraction in bovine ciliary muscle and guinea pig taenia cecum (14). Watanabe and Takano-Ohmuro showed that extensive, but not moderate skinning, diminished the relaxing effect in guinea pig hepatic portal vein, suggesting that the loss of PP2A itself and/or some factors that would work with it would be the cause of the absence of OA’s relaxing effect in skinned preparations (15). Another possible cause would be that strong inhibition of MLCP by OA masked the relaxing effect induced by inhibition of PP2A. These possibilities cannot be examined by conventional PP2A inhibitors because those can also inhibit PP1.
A recent study by Wada et al. (16) showed that rubratoxin A, a Penicillium rubrum produced mycotoxin, was a specific PP2A inhibitor. Rubratoxin A inhibited carbachol-induced contraction in intact bovine ciliary muscle (14). It also suppressed ionomycin-induced Ca2+-dependent contraction in bovine ciliary muscle and guinea pig taenia cecum, suggesting that modulation of intracellular Ca2+-dependent pathways by rubratoxin A causes suppression of the smooth muscle contraction (14). By utilizing the advantages of rubratoxin A to specifically inhibit PP2A and of preparations moderately skinned with β-escin to precisely control the intracellular Ca2+ concentration, we examined the role of PP2A in contractile response to the clamped Ca2+ concentrations in the smooth muscle of guinea pig carotid artery preparations.
Materials and Methods
Animal experiments were performed at Tokyo Metropolitan University at Arakawa. All experimental procedures were performed according to the “Guideline for Proper Conduct of Animal Experiments” approved by the Science Council of Japan, and were carried out under the rules and regulations of the research ethics committee of Tokyo Metropolitan University. Also, Tokyo Metropolitan University approved all procedures involving animals (A28-1, A29-1, A30-20). Male Hartley guinea pigs weighing approximately 250 g were sacrificed under deep anesthesia with pentobarbital (Somnopentyl, Kyoritsu Seiyaku Co., Tokyo, Japan). The carotid arteries were removed and immersed in normal extracellular solution (NES). A small muscle layer strip (0.3–0.4 mm wide and 1.0–1.5 mm long) was prepared by cutting off the carotid artery. The preparation was attached to a pair of tungsten wires with silk thread monofilaments, one of which was connected to a force transducer (ULA-10G, Minebea Mitsumi Inc., Kanagawa, Japan) to measure isometric tension (17). A bubble plate system with six wells (0.135 ml each) was used to change the solution quickly (18).
The skinning (cell membrane permeabilization) procedure was essentially the same as that described by Hashimoto et al. (17). The preparation in the NES was moved into artificial intracellular solution without Ca2+ (relaxing solution), causing relaxation to near the resting level. An intact carotid arterial smooth muscle preparation was treated for 10 min with 600 µM β-escin (Sigma, St. Louis, MO, USA) in the relaxing solution. Experimental temperature was maintained at 30.0 ± 1.0 °C.
Experimental procedure
The skinned preparation was firstly immersed in 10 µM Ca2+ solution for 15 min to elicit Ca2+-induced contraction (control contraction). Then, the preparation was relaxed by changing the solution with a Ca2+-free relaxing solution containing 10 mM EGTA to lower the intracellular Ca2+ quickly. After 8 min exposure to the relaxing solution, various concentrations of rubratoxin A (10 nM, 100 nM, 1 µM and 10 µM) were added to the relaxing solution and the preparation incubated for 2 min. Finally, the preparation was contracted with various concentrations of Ca2+ solution (1 µM, 2 µM, 3 µM and 10 µM) in the absence or presence of rubratoxin A (test contraction). In the test contractions without rubratoxin A, 1% of dimethylsulfoxide (DMSO, Sigma) was added to the solution.
Solutions and chemical
NES contained 150 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 5 mM 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulphonic acid (HEPES, Nacalai Tesque, Kyoto, Japan), 50 μU/ml insulin (Sigma), and pH was adjusted with Tris (hydroxymethyl) aminomethane (Tris; Nacalai Tesque) /H2O to pH 7.40 at 25 °C. Contents of the artificial intracellular solutions for skinned preparations were 0.85 mM Mg (methanesulfonate)2 (Tokyo Chemical Industry, Tokyo, Japan), 1 mM MgATP (1.35 mM total ATP Na2) (Roche, Indianapolis, IN, USA), 20 mM creatine phosphate Na2 (CrP; Nacalai Tesque), 10 mM etylene glycole-bis (2-aminoetyl) tetraacetic acid (EGTA; Nacalai Tesque). K (methanesulfonate) (Tokyo Chemical Industry), was added to the solutions to keep the ionic strength at 200 mM, and pH was adjusted with 20 mM 1,4-piperazinediethanesulophonic acid (PIPES; Nacalai Tesque) and KOH (Wako Pure Chemicals) to 7.0 at 25 °C, which were prepared according to the method of Horiuti (18). The relaxing solution contained 10 mM EGTA without Ca2+, and solutions of various Ca2+ concentrations (1 to 10 µM Ca2+) were prepared by mixing the CaEGTA solution containing 10 mM EGTA and 10 mM Ca (methanesulfonate)2 (Tokyo Chemical Industry), in the appropriate proportion with addition of 1 µM calmodulin (Wako Pure Chemicals, Osaka, Japan). The apparent dissociation constant of Ca2+ -EGTA was assumed to be 106.4/M−1. Rubratoxin A (Microbial Chemistry Research Foundation, Tokyo, Japan) was dissolved into DMSO.
Data analysis of the mechanical properties
The developed tension levels of the test contraction of skinned preparations were expressed as; relative tension = (an observed tension of the test contraction – the basal tension) / (the control tension – basal tension).
Ca2+ sensitivity for the Ca2+-induced contraction of skinned preparations was also estimated by data fitting to the Hill equation with the program Kaleida Graph (Synergy Software, Regarding, PA, USA) using the Levenberg-Marquardt algorithm; Relative force=Fmax-Ca2+×[Ca2+]N/ ([Ca2+]50N+[Ca2+]N), where Fmax-Ca2+ is the maximum Ca2+-activated force at 10 µM Ca2+, and [Ca2+]50 denotes Ca2+ concentration for the half maximal Ca2+ activated force. The Hill coefficient (N) is a measure of the slope.
Latency of the Ca2+-induced contraction of skinned preparations was defined as the time when the activated force reached 1% of the maximal contraction value.
Statistical analysis
The results are presented as means ± standard errors (S.E.M.). The statistical hypotheses on the differences between means were tested with the Student’s t test for paired samples unless noted otherwise. The null hypothesis was rejected when P was less than 0.05.
Results
Effects of rubratoxin A on the Ca2+-induced contraction of skinned preparations
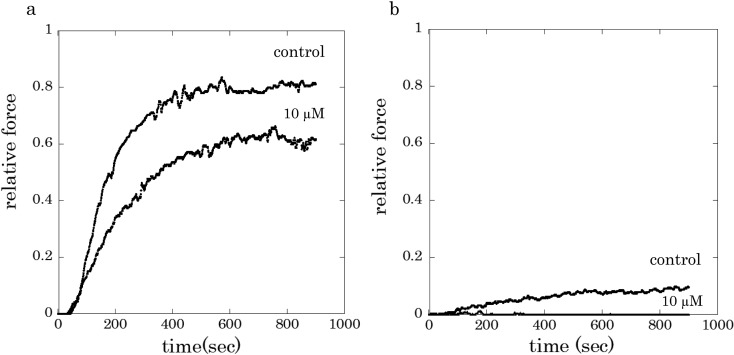
Figure 1 shows typical tension traces of β-escin skinned preparations of carotid artery smooth muscle from guinea pigs. When a muscle preparation was activated with 10 µM Ca2+ and 1 µM calmodulin, after short period latency, the active tension gradually developed and reached a sustained level within 720 sec. In the presence of rubratoxin A at concentration of 10 µM, Ca2+-induced contraction was suppressed as shown in a more gradual force development and smaller sustained force, when compared to control (Fig. 1a). The presence of 10 μM rubratoxin A nearly abolished the contractile response to 1 μM Ca2+ (Fig. 1b). The force inhibitory effects of rubratoxin A on the Ca2+ -induced contraction was still observed even after functional destruction of intracellular Ca2+ store with Ca2+ ionophore A23187 (data not shown).
Fig. 1.
Typical force traces of skinned carotid artery muscle preparations activated with 10 µM Ca2+ (a) or 1 µM Ca2+ (b), with 1 µM calmodulin. Rubratoxin A (10 µM) was applied for 2 min before exposure to Ca2+ subsequently for 15 min.
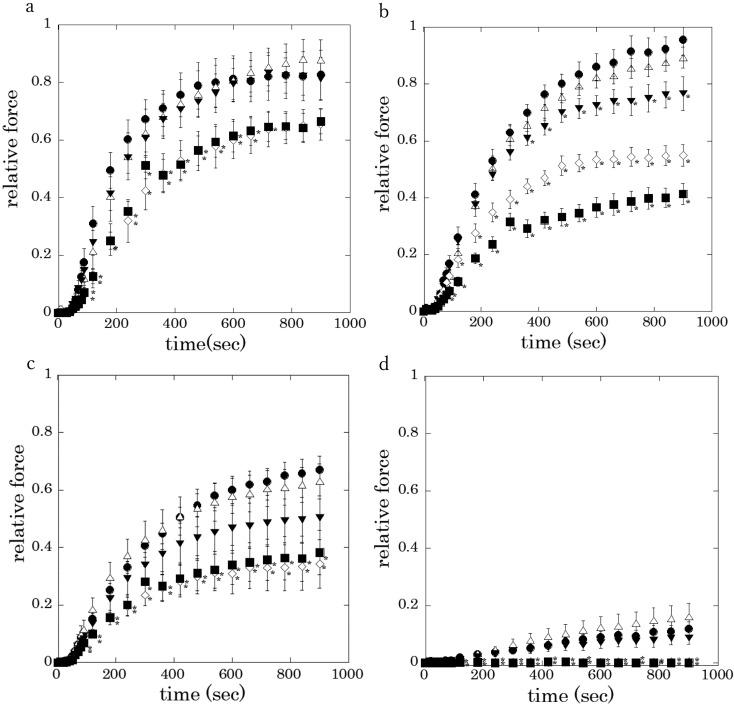
The averaged active tension level at 60–80 sec after Ca2+ stimulation was significantly suppressed in a concentration dependent manner in the presence of rubratoxin A at 1 and 10 μM (Fig. 2). It is noted that rubratoxin A at the tested lower concentration of 100 nM significantly inhibited 3 µM Ca2+-induced contraction (Fig. 2b, closed triangle).
Fig. 2.
Effects of rubratoxin A on the Ca2+-induced contraction (a: 10 µM
Ca2+, b: 3 µM, c: 2 µM, d: 1 µM). 1% DMSO without rubratoxin A
(control:  ), 10 nM (
), 10 nM ( ), 100 nM (
), 100 nM ( ), 1 µM (
), 1 µM ( ), or 10 µM (
), or 10 µM ( ) rubratoxin
A was added to the artificial intracellular solutions. Values are the means ± S.E.M.
of 4–7 experiments. Asterisk indicates significant difference of the active force
compared with that of controls, where P values are less than
0.05.
) rubratoxin
A was added to the artificial intracellular solutions. Values are the means ± S.E.M.
of 4–7 experiments. Asterisk indicates significant difference of the active force
compared with that of controls, where P values are less than
0.05.
Effects of rubratoxin A on Ca2+ sensitivity for the Ca2+-induced contraction of
skinned preparations
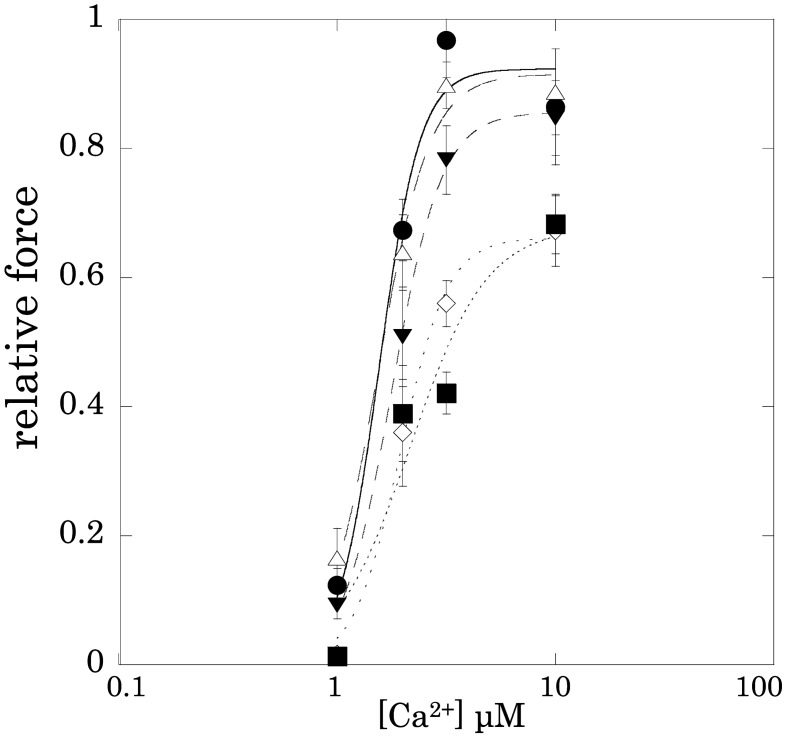
Figure 3 shows the effects of rubratoxin A on the relationship between Ca2+ concentration and active tension. Rubratoxin A at 1 µM as well as at 10 µM significantly inhibited skinned carotid artery contraction at any Ca2+ concentration. The data fitting to the Hill equation indicated that rubratoxin A at 1 and 10 μM apparently decreased Fmax-Ca2+ with raising [Ca2+]50, indices of the Ca2+ sensitivity for active tension (Table 1). Rubratoxin A at 10 µM also decreased the Hill coefficient N (Hill’s N, Table 1).
Fig. 3.
Effects of rubratoxin A on the Ca2+concentration-relative tension
relationship. 1% DMSO without rubratoxin A (control:  ), 10 nM (
), 10 nM ( ), 100 nM (
), 100 nM ( ), 1 µM
(
), 1 µM
( ), or 10
µM (
), or 10
µM ( ) rubratoxin A was added to the artificial intracellular solutions.
) rubratoxin A was added to the artificial intracellular solutions.
Table 1. Effects of rubratoxin A (10 nM – 10 μM) on the Fmax-Ca2+, Hill coefficient, and [Ca2+]50 of the β-escin skinned carotid artery preparations of the guinea pig.
| concentration | control | 10 nM | 100 nM | 1 μM | 10 μM |
| Fmax-Ca2+ | 0.923 ± 0.084 | 0.915 ± 0.050 | 0.856 ± 0.018 | 0.662 ± 0.036 | 0.678 ± 0.160 |
| Hill’s N | 4.612 ± 1.877 | 3.701 ± 0.782 | 3.826 ± 0.344 | 4.061 ± 0.999 | 2.490 ± 1.898 |
| [Ca2+]50 μM | 1.560 ± 0.212 | 1.542 ± 0.125 | 1.788 ± 0.049 | 1.953 ± 0.118 | 2.168 ± 0.714 |
The relationship between Ca2+concentration and Ca2+-induced tension development in the absence and presence of rubratoxin A was fitted to the Hill equation. Fmax-Ca2+, tension level of the maximal Ca2+-induced contraction; Hill’s N, Hill coefficient; [Ca2+]50, Ca2+ concentration of the half-maximal Ca2+-induced tension.
Effects of rubratoxin A on latency of the Ca2+-induced contraction of skinned preparations
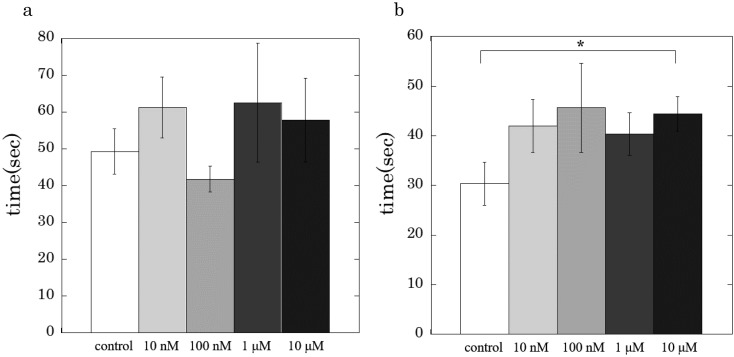
Figure 4 shows effects of rubratoxin A on the latency of Ca2+-induced contraction of the skinned carotid artery. The presence of rubratoxin A at 10 µM significantly prolonged the latency of the skinned artery contraction activated with 2 µM Ca2+ (Fig. 4b), but not with 10 µM Ca2+ (Fig. 4a). It should be noted that the latency of 1 µM Ca2+-induced contraction in the presence of rubratoxin A at 1 or 10 μM was not measurable, i.e., the latent time might be more than 720 sec of the measurement period, since no active tension developments were observed in the presence of rubratoxin A (Fig. 2d).
Fig. 4.
Effects of rubratoxin A (10 μM) on the latent time of the Ca2+-induced contraction (a: 10 µM Ca2+, b: 2 µM). Values are the means ± S.E.M. of 4–7 experiments. Asterisk indicates a significant difference of the latency compared with that of control, where P values are less than 0.05.
Discussion
In the present study, we examined the role of PP2A in the regulation of smooth muscle contraction by utilizing a novel PP2A specific inhibitor, rubratoxin A (16), and β-escin skinned carotid artery preparations. We found that rubratoxin A at 1 µM and higher significantly suppressed Ca2+-induced contraction at any concentrations of Ca2+. Rubratoxin A at 100 nM also suppressed Ca2+-induced contraction at concentrations of 3 µM Ca2+ or less (Fig. 2). At these concentrations, rubratoxin A did not inhibit PP1 at all (16). These results indicate that PP2A plays a role in force development and/or maintenance of smooth muscle contraction.
In the previous studies, extensive skinning treatment with saponin or Triton X-100 diminished the relaxing effect of OA (15). These results raised possibilities that 1) PP2A would be involved in the control of intracellular Ca2+ concentration, 2) strong inhibition of MLCP by OA would mask the relaxing effect induced by inhibition of PP2A, or 3) PP2A or its target regulatory protein(s) would be lost during permeabilization. The present result contradicts the first one since rubratoxin A could inhibit the β-escin skinned carotid arteries at a constant Ca2+ concentration. Also, the second one would not be at least the primary reason why OA’s relaxing effect was diminished in the extensively skinned preparations with saponin or Triton X-100, because irreversible relaxing effects was not retained after washing out high concentration OA (15) at which the agent induced reversible contraction in Triton X-100 skinned preparations (6, 19), but retained in alpha toxin skinned preparations (15, 20).
The potency of the force inhibiting effects of rubratoxin A was stronger at lower concentrations of Ca2+ (Fig. 1), and therefore the agent decreased [Ca2+]50, an index of the Ca2+ sensitivity for the force. This result indicates that rubratoxin A enhances MLCP activity and/or suppresses MLCK activity through PP2A inhibition.
In a previous study, phosphorylation of CPI-17 through the PKC activated pathway decreased MLCP activity and enhanced the Ca2+ sensitivity (21). Furthermore, PP2A and PKCα were physically associated in mast cells (22). Therefore, it might be possible that rubratoxin A suppresses the PKC and CPI-17 dependent inhibition of MLCP activity resulting in the decrease in the Ca2+ sensitivity for the force. In fact, 1 μM OA, at which the agent acts a PP2A inhibitor on the smooth muscle, induced phosphorylation of both CPI-17 and PKC in the canine cerebral artery (23).
On the other hand, Ishida et al. showed that PKC inhibition with Gö6983 failed to affect the inhibition of ciliary muscle contraction caused by 1 μM OA (14). Furthermore, no signaling pathways which induce MLC phosphorylation other than Ca2+-calmodulin were activated in the present experimental condition. Therefore, it remains to be concluded whether rubratoxin A acts on the PKC and CPI-17 dependent MLCP regulatory mechanisms, inducing the suppression of the Ca2+-induced contraction of the skinned carotid artery.
An alternative possible mechanism of rubratoxin A-induced Ca2+ desensitization in the skinned carotid artery is inhibition of the MLCK activity. In fact, time latency to the force development which might reflect MLCK activity and/or direct activation of myosin ATPase (24) was significantly delayed in the presence of rubratoxin A at 10 μM in the case of 2 µM Ca2+ (Fig. 4). The same result was also obtained even when tautomycin, a MLCP inhibitor, was added (data not shown). Therefore, rubratoxin A induced modulation of PP2A dependent MLCK regulatory mechanisms may partially inactivate MLCK.
PP2A might act not only on MLC phosphorylation/dephosphorylation, but by direct actin myosin interaction. In neuronal cells, PP2A directly regulates F-actin disassembly by interacting with dephosphorylating F-actin severing factors (25). Our present result, that rubratoxin A slightly but significantly inhibited the maximal Ca2+-activated contraction (Figs. 2a and 3), an index of the actin-myosin interaction, indicates that PP2A inhibition suppresses MLCP-independent regulation of actin-myosin interaction. Further studies are necessary to determine whether rubratoxin A directly inhibits actin-myosin interaction in carotid artery and other types of smooth muscle.
In summary, the present study shows that rubratoxin A decreased Ca2+ sensitivity of the force and suppressed the maximal Ca2+-induced contractile force, suggesting that PP2A inhibition causes both the downregulation of myosin phosphorylation and direct interference with myosin-actin interaction.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgement
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Sciences, Sports and Culture of Japan (to 23500475; 2011–2015 to MW and 16K08500; 2016–2018 to MW, and KT).
References
- 1.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83(4): 1325–58. doi: 10.1152/physrev.00023.2003 [DOI] [PubMed] [Google Scholar]
- 2.Hartshorne DJ, Ito M, Erdödi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem. 2004; 279(36): 37211–4. doi: 10.1074/jbc.R400018200 [DOI] [PubMed] [Google Scholar]
- 3.Hori M, Magae J, Han YG, Hartshorne DJ, Karaki H. A novel protein phosphatase inhibitor, tautomycin. Effect on smooth muscle. FEBS Lett. 1991; 285(1): 145–8. doi: 10.1016/0014-5793(91)80745-O [DOI] [PubMed] [Google Scholar]
- 4.Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, Somlyo AV. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J Biol Chem. 1992; 267(21): 14662–8. [PubMed] [Google Scholar]
- 5.Butler T, Paul J, Europe-Finner N, Smith R, Chan EC. Role of serine-threonine phosphoprotein phosphatases in smooth muscle contractility. Am J Physiol Cell Physiol. 2013; 304(6): C485–504. doi: 10.1152/ajpcell.00161.2012 [DOI] [PubMed] [Google Scholar]
- 6.Takai A. Effects of protein phosphatase inhibitors on smooth muscles. In: Bolton T, Tomita T, editors. Smooth Muscle Excitation. Academic Press; 1996. p. 277–90. [Google Scholar]
- 7.Takai A, Eto M, Hirano K, Takeya K, Wakimoto T, Watanabe M. Protein phosphatases 1 and 2A and their naturally occurring inhibitors: current topics in smooth muscle physiology and chemical biology. J Physiol Sci. 2018; 68(1): 1–17. doi: 10.1007/s12576-017-0556-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata S, Ishida Y, Kitano H, Ohizumi Y, Habon J, Tsukitani Y, Kikuchi H. Contractile effects of okadaic acid, a novel ionophore-like substance from black sponge, on isolated smooth muscles under the condition of Ca deficiency. J Pharmacol Exp Ther. 1982; 223(1): 135–43 [PubMed] [Google Scholar]
- 9.Ozaki H, Ishihara H, Kohama K, Nonomura Y, Shibata S, Karaki H. Calcium-independent phosphorylation of smooth muscle myosin light chain by okadaic acid isolated from black sponge (Halichondria okadai). J Pharmacol Exp Ther. 1987; 243(3): 1167–73. [PubMed] [Google Scholar]
- 10.Karaki H, Mitsui M, Nagase H, Ozaki H, Shibata S, Uemura D. Inhibitory effect of a toxin okadaic acid, isolated from the black sponge on smooth muscle and platelets. Br J Pharmacol. 1989; 98(2): 590–6. doi: 10.1111/j.1476-5381.1989.tb12633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashizawa N, Kobayashi F, Tanaka Y, Nakayama K. Relaxing action of okadaic acid, a black sponge toxin on the arterial smooth muscle. Biochem Biophys Res Commun. 1989; 162(3): 971–6. doi: 10.1016/0006-291X(89)90768-7 [DOI] [PubMed] [Google Scholar]
- 12.Hirano K, Kanaide H, Nakamura M. Effects of okadaic acid on cytosolic calcium concentrations and on contractions of the porcine coronary artery. Br J Pharmacol. 1989; 98(4): 1261–6. doi: 10.1111/j.1476-5381.1989.tb12672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takai A, Sasaki K, Nagai H, Mieskes G, Isobe M, Isono K, Yasumoto T. Inhibition of specific binding of okadaic acid to protein phosphatase 2A by microcystin-LR, calyculin-A and tautomycin: method of analysis of interactions of tight-binding ligands with target protein. Biochem J. 1995; 306(Pt 3): 657–65. doi: 10.1042/bj3060657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida M, Takeya K, Miyazu M, Yoshida A, Takai A. Force-inhibiting effect of Ser/Thr protein phosphatase 2A inhibitors on bovine ciliary muscle. J Smooth Muscle Res. 2015; 51: 10–21. doi: 10.1540/jsmr.51.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe M, Takano-Ohmuro H. Extensive skinning of cell membrane diminishes the force-inhibiting effect of okadaic acid on smooth muscles of Guinea pig hepatic portal vein. Jpn J Physiol. 2002; 52(2): 141–7. doi: 10.2170/jjphysiol.52.141 [DOI] [PubMed] [Google Scholar]
- 16.Wada S, Usami I, Umezawa Y, Inoue H, Ohba S, Someno T, Kawada M, Ikeda D. Rubratoxin A specifically and potently inhibits protein phosphatase 2A and suppresses cancer metastasis. Cancer Sci. 2010; 101(3): 743–50. doi: 10.1111/j.1349-7006.2009.01438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto R, Yumoto M, Watanabe M, Konishi M, Haraoka J, Miki T. Differential effects of an expected actin-tropomyosin binding region of heat shock protein 20 on the relaxation in skinned carotid artery and taenia cecum from guinea pig. J Smooth Muscle Res. 2009; 45(1): 63–74. doi: 10.1540/jsmr.45.63 [DOI] [PubMed] [Google Scholar]
- 18.Horiuti K. Mechanism of contracture on cooling of caffeine-treated frog skeletal muscle fibres. J Physiol. 1988; 398: 131–48. doi: 10.1113/jphysiol.1988.sp017034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takai A. Okadaic acid. Protein phosphatase inhibition and muscle contractile effects. J Muscle Res Cell Motil. 1988; 9(6): 563–5. doi: 10.1007/BF01738761 [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, Nakano M. Force-inhibiting effect of okadaic acid on skinned rat uterus permeabilized with α-toxin. Pflugers Arch. 1995; 430(5): 754–6. doi: 10.1007/BF00386172 [DOI] [PubMed] [Google Scholar]
- 21.Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem. 2009; 284(51): 35273–7. doi: 10.1074/jbc.R109.059972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boudreau RT, Garduno R, Lin TJ. Protein phosphatase 2A and protein kinase Cα are physically associated and are involved in Pseudomonas aeruginosa-induced interleukin 6 production by mast cells. J Biol Chem. 2002; 277(7): 5322–9. doi: 10.1074/jbc.M108623200 [DOI] [PubMed] [Google Scholar]
- 23.Obara K, Mitate A, Nozawa K, Watanabe M, Ito Y, Nakayama K. Interactive role of protein phosphatase 2A and protein kinase Cα in the stretch-induced triphosphorylation of myosin light chain in canine cerebral artery. J Vasc Res. 2010; 47(2): 115–27. doi: 10.1159/000235966 [DOI] [PubMed] [Google Scholar]
- 24.Horiuti K, Somlyo AV, Goldman YE, Somlyo AP. Kinetics of contraction initiated by flash photolysis of caged adenosine triphosphate in tonic and phasic smooth muscles. J Gen Physiol. 1989; 94(4): 769–81. doi: 10.1085/jgp.94.4.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman A, Taleski G, Sontag E. The protein serine/threonine phosphatases PP2A, PP1 and calcineurin: A triple threat in the regulation of the neuronal cytoskeleton. Mol Cell Neurosci. 2017; 84: 119–31. doi: 10.1016/j.mcn.2017.01.005 [DOI] [PubMed] [Google Scholar]