Abstract
Rationale:
The coexistence of a tuberculous aortic pseudoaneurysm and Pott disease in patients with a history of tuberculosis (TB) is relatively rare, and the treatment strategies remain still controversial.
Patient concerns:
A 57-year-old female patient with a history of primary pulmonary TB presented with symptoms of breathlessness, chest pain, weight loss, and fever. Magnetic resonance imaging (MRI) and computed tomography (CT) showed a thoracic aortic pseudoaneurysm secondary to Pott disease at T11/12 level.
Diagnoses:
Tuberculous pseudoaneurysm at the descending thoracic aorta associated with tuberculous vertebral osteomyelitis.
Interventions:
We originally planned a combined surgery consisting of posterior spine stabilization, anterior excision of the infected field, and aortic reconstruction. When we surgically stabilized the posterior spine, unexpectedly, the pseudoaneurysm ruptured. Immediately, we terminated the surgery and appropriately placed an endovascular stent graft, which successfully rescued the patient.
Outcomes:
When the patient's conditions were stable, we anteriorly debrided all infected tissues and then performed a spinal fusion by grafting autologous iliac bone. After the debridement and spinal fusion, we arranged a 1-year anti-tuberculous chemotherapy for this patient and performed a 24-month follow-up. This patient had no signs of recurrent infection during the follow-up.
Lessons:
For the patients with tuberculous aortic aneurysm(s) complicated with vertebral osteomyelitis, the endovascular repair of an aneurysm(s) should be considered a conventional therapy before the spine surgery, lowering the risk of aortic aneurysm rupture. Meanwhile, minimally invasive endovascular stent graft combined with anti-tuberculosis drugs may be considered one of the therapeutic regimens for the patients whose conditions are not suitable for open surgery, such as age, weakness, or severe organ failure.
Keywords: case report, endovascular repair, Pott disease, thoracic aorta, tuberculous pseudoaneurysm
1. Introduction
Tuberculous spondylitis (also known as Pott disease), one of the complications of tuberculosis (TB), is tuberculous arthritis of the vertebral body and intervertebral joints. The disease was first described by Percival Pott in 1779,[1] and its morbidity is approximately 1% among TB patients.[2,3] This disease commonly occurs in immunocompromised patients, dystrophia persons, and especially, elderly patients. Recently, TB has resurged in Asia and Africa, which caused an increased incidence of Pott disease.[4] However, it is clinically uncommon that an aneurysm coexists with Pott disease at the aorta near to the intervertebral joints, which greatly increases the risk of a ruptured of the tuberculous aortic aneurysm (TAA) during surgery. Indeed, 3 TAA ruptures during surgery have been reported.[5–7]
In this report, we describe a Pott disease case of a TAA rupture at the thoracic aorta when posteriorly stabilizing the spine. This patient was successfully treated by placing an endovascular stent graft. Here, we described the therapy procedures. Along with reported Pott disease cases, we propose some treatment principles for Pott disease patients.
2. Case report
The patient consents to this case report being published. This study was approved by the ethical committee of the Affiliated Hospital of Kunming University of Science and Technology, Kunming (Ethical approval number: 7131095).
A 57-year-old woman with primary pulmonary TB presented to the department of internal medicine with symptoms of breathlessness, chest pain, weight loss, and fever. A computed tomography (CT) scan showed vertebrae lesions with a paravertebral abscess in the T11/12 vertebrae (Fig. 1A and B). The patient was transferred to the department of orthopedic surgery for further clinical examinations.
Figure 1.
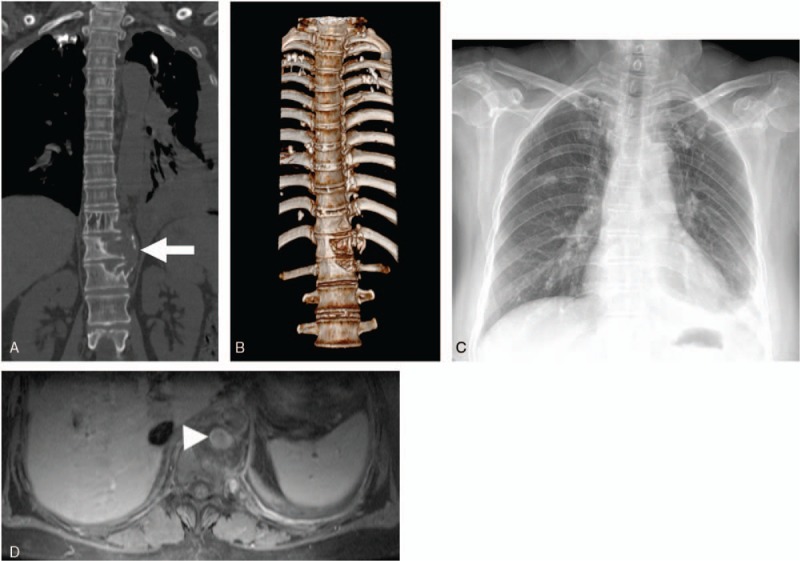
A, Coronal CT scan shows left-side vertebral destruction with a paravertebral abscess (arrow). B, Three-dimensional CT scan shows the vertebral destruction in the 11th to 12th thoracic vertebrae. C, Anteroposterior radiograph of the chest shows a widened mediastinum and left pleural effusion. D, Axial enhanced CT scan shows that a tuberculous pseudoaneurysm (arrow-head) of the descending thoracic aorta was surrounded by a paravertebral abscess. CT = computed tomography.
This patient exhibited slightly raised C-reactive protein levels (CRP: 10.1 mg/L) and erythrocyte sedimentation rate (ESR: 43 mm/h), positive for tubercle bacillus antibody and interferon gamma release, but negative for anti-treponemal antibodies and human immunodeficiency virus (HIV) serology. White cell count, renal and liver function, and electrolytes were normal. Tuberculin skin test was strongly positive, and sputum cultures for acid-fast bacilli were negative. Chest x-ray showed a widened mediastinum and a left pleural effusion consistent with pathological features of pulmonary TB (Fig. 1C). Hydrothorax biochemistry analysis showed elevated total cell count (3.15 × 109/L), predominant monocytes, raised levels of lactate dehydrogenase (LDH: 334 U/L) and adenosine deaminase (ADA: 98 U/L), and positive in rivalta test. The patient received a standard anti-tuberculosis chemotherapy treatment (po QD: Isoniazid, 300 mg; rifampicin, 450 mg; pyrazinamide, 1500 mg; and ethambutol, 750 mg). A contrast-enhanced CT scan demonstrated a left paravertebral abscess, osteomyelitis, and a 2.0 × 2.5 cm2 pseudoaneurysm on the suprarenal aorta at the T11 level (Fig. 1D). Magnetic resonance imaging (MRI) further confirmed the presence of a saccular dilatation of the posterior thoracic aorta surrounded by a paravertebral abscess (Fig. 2A and B).
Figure 2.
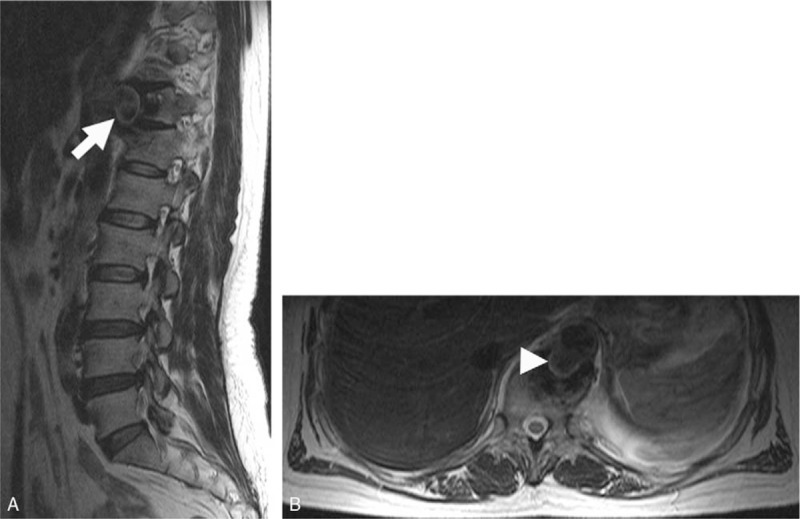
A, Sagittal MRI scan indicates the vertebral erosion with a paravertebral abscess in the 11th to 12th thoracic vertebrae (arrow). B, Axial MRI scan shows a descending thoracic aortic saccular pseudoaneurysm (arrow-head). MRI = magnetic resonance imaging.
Our team planned to perform a combined surgery, that is, the combination of a posterior vertebra stabilization surgery that stabilizes the spine and an anterior open-chest surgery that extensively excises infected tissues and pseudoaneurysm, aortic reconstruction, and intervertebral fusion (autologous iliac bone graft). According to this plan, we first performed a vertebra stabilization surgery by placing a double-screw and double-rod system on T9 to L2 vertebra. When the posterior vertebra stabilization was nearly finished, the patient progressively lost normal hemodynamics (heart rate increased to 150 beats/min, and blood pressure dropped to 85/50 mmHg), which was accompanied by a remarkable decline of hemoglobin from preoperative 12.1 to 4.1 g/dL, implying, tuberculous pseudoaneurysm rupture. Immediately, we administrated 8 erythrocyte concentrates to this patient, and performed a thoracoabdominal angiography. We observed a pseudoaneurysm rupture on the lateral wall of the descending thoracic aorta at the T11 level (Fig. 3A). Therefore, we performed an endovascular treatment by positioning an Ankura thoracic stent graft (28 mm at diameter; 160 mm at length; Lifetech, Shenzhen, China) without covering the orifice of the bilateral renal arteries. Arteriography showed complete blockage of the orifice of pseudoaneurysm rupture with no residual blood flow to the pseudoaneurysm sac (Fig. 3B). The patient was then transferred to the intensive care unit of our hospital, and we suspended the anterior open-chest surgery.
Figure 3.
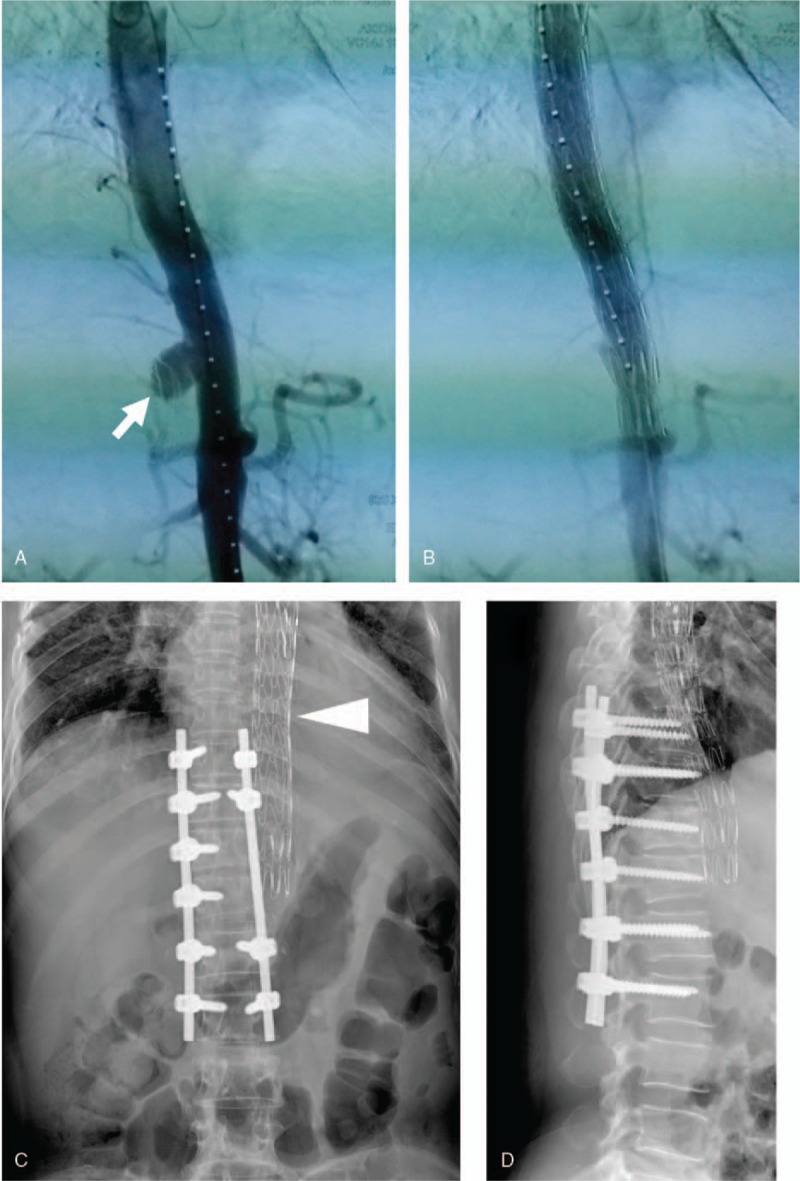
A, Thoracoabdominal angiography shows a ruptured tuberculous pseudoaneurysm at the descending thoracic aorta at the T11/12 level (arrow). B, Thoracoabdominal angiography shows that the pseudoaneurysm was successfully repaired by an endovascular stent graft placement. X-ray images show internal fixation of the spine and implanted endovascular stent (arrow-head) 24 months after surgery. C, Anteroposterior view. D, Lateral view.
Five days later, we sequentially performed a debridement of all infected tissues, a partial corpectomy of the T11 and T12 vertebrae, a meticulous removal of the necrotic tissues in the spinal canal, and, followed by an intervertebral fusion. Histopathological observations of the resected lesions exhibited typical caseous necrosis and chronic granulomatous inflammation with marked accumulation of epithelioid cells and multinucleated giant cells (Fig. 4A and B). The patient recovered rapidly and was discharged 18 days after the surgery. The patient was then treated with anti-tuberculous chemotherapy for 1 year, and we arranged a 24-month follow-up. During this follow-up, the patient had no signs of recurrent infection and showed a fixed vertebra and precisely placed thoracic stent on imaging (Fig. 3C and D).
Figure 4.
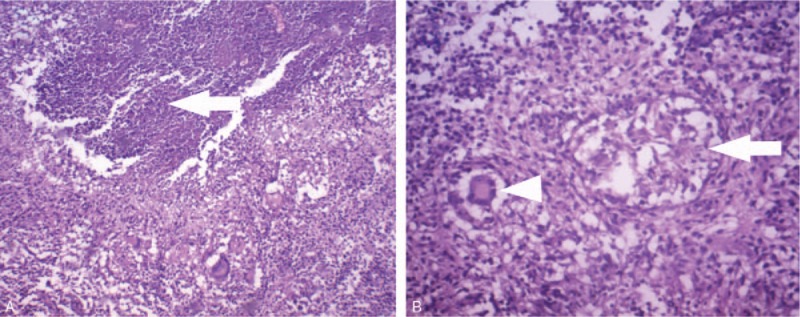
A, Hematoxylin & eosin (H&E) staining of the resected tissues shows typical caseous necrosis (arrow) consistent with the pathological features of spinal TB (original magnification, ×40). B, Photomicrograph shows accumulation of epithelioid cells (arrow) and multinucleated giant cells (arrow-head) (H&E stain; original magnification, ×100). TB = tuberculosis.
3. Discussion
In patients with TB, the coexistence of TAA and tuberculous spondylitis are relatively rare. Since Long et al[8] reviewed advances in tuberculous mycotic aneurysm of the aorta in 1999, only 23 patients with tuberculous pseudoaneurysm were reported, of whom 22 had pseudoaneurysm secondary to tuberculous spondylitis.[5–7,9–20] We reviewed these case reports and found only 3 patients with a TAA rupture during the surgery (Table 1).[5–7] Together with the present case, a total of only 4 cases have been reported.
Table 1.
Review of literature about a ruptured TAA secondary to Pott disease during surgery.

Clinically, once the complications of TAA and Pott disease are diagnosed, treatments should start immediately. The current regimen to treat TAA complicated with vertebral erosion is the combination of anti-tuberculosis chemotherapy and anterior open-chest surgery.[15,21] This therapeutic strategy aims to stabilize the spine, extensively debride infected tissues, reconstruct aorta in situ, and perform intervertebral fusion. The anterior surgery of anterior spine stabilization and intervertebral fusion has been considered as the “gold standard” procedure, but this surgery has potentially lethal risk of a disseminated TB infection due to the use of a metallic implant (titanium mesh, screw, and rod) in the infected field. Therefore, it is necessary to optimize anterior and posterior spine stabilization and fusion, avoiding lethal risks during the therapy.[22,23]
The case in this report showed the combination of TAA with marked tuberculous spondylitis according to our initial diagnosis. We, therefore, planned to perform the combination of posterior spine stabilization and, anterior open-chest debridement surgery. Unexpectedly, during our posterior spine stabilization surgery, TAA ruptured and caused emergency conditions of hemodynamic instability. We immediately placed a thoracic stent graft and eventually completed whole combination surgery. However, this combination surgery did not include aortic resection and reconstruction. To reduce surgical trauma, we abandoned the original surgery plan and chose to leave the thoracic stent in place on the following anterior surgery. During the 24-month follow-up, no recurrent infection occurred, no fixation loosening was found, and complete spinal interbody fusion was obtained.
In this case, one possible reason for tuberculous pseudoaneurysm rupture is that posterior spine stabilization surgery might somehow squeeze aortic aneurysms because TAA and tuberculous spondylitis are adjacent. In addition, the rupture of aortic aneurysms often unpredictably happens even when TAA is only 10 mm in diameter.[24] In the situation with risk of the rupture of aortic aneurysms, it is, therefore, reasonable to repair TAA before spine surgery avoiding TAA rupture. At present, a less invasive endovascular repair device has been considered attractive in repairing TAA.[25,26] However, this endovascular repair does not allow extensive excision of necrotic tissues, which could restrict anti-tuberculosis medication efficiency.[9,27] The minimal invasive endovascular therapy may be more efficient before spine surgery. Thus, the endovascular repair in combination with, posterior spine stabilization, anterior open-chest debridement, and autologous iliac bone graft may represent a new promising strategy for the patients with TAA complicated with tuberculous spondylitis.
4. Conclusion
The patients with Pott disease complicated with TAA may be at high risk of the TAA rupture during spine surgery. Therefore, the repairment or stabilization of TAA is necessary before spine surgery, even TAA may be repaired first. For the patients with TAA complicated with severe vertebral erosion, endovascular stent grafting should be considered as a conventional therapy before spine surgery or a vital part of the surgical procedures.
Author contributions
Conceptualization: Weichao Li, Hongrong Li.
Data curation: Weichao Li, Xianrun Sun, Yong Yang.
Formal analysis: Weichao Li, Xianrun Sun.
Investigation: Weichao Li, Zengdong Meng.
Methodology: Zengdong Meng, Shaoping Yao.
Project administration: Hongrong Li, Yong Yang, Shaoping Yao.
Software: Yong Yang, Shaoping Yao.
Supervision: Xianrun Sun, Hongrong Li.
Visualization: Zengdong Meng.
Writing – original draft: Weichao Li.
Writing – review & editing: Shaoping Yao.
Footnotes
Abbreviations: ADA = adenosine deaminase, CRP = C-reactive protein, CT = computed tomography, ESR = erythrocyte sedimentation rate, HIV = human immunodeficiency virus, L = lumbar vertebra, LDH = lactate dehydrogenase, MRI = magnetic resonance imaging, T = thoracic vertebra, TAA = tuberculous aortic aneurysms, TB = tuberculosis.
This study was supported by grants from the National Natural Science Foundation of China (NSFC, No: 81860240), and the Applied Basic Research Foundation of Yunnan Province (No: 2018FB119). The authors thank Haoyue (Max) Zhang (Iowa City West High School, Iowa City 52246, IA, USA) for English editing.
The authors have no conflicts of interest to disclose.
References
- [1].Nussbaum ES, Rockswold GL, Bergman TA, et al. Spinal tuberculosis: a diagnostic and management challenge. J Neurosurg 1995;83:243–7. [DOI] [PubMed] [Google Scholar]
- [2].Jain AK. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br 2010;92:905–13. [DOI] [PubMed] [Google Scholar]
- [3].Huang B, Li CQ, Liu T, et al. Primary non-Hodgkin's lymphoma of the lumbar vertebrae mimicking tuberculous spondylitis: a case report. Arch Orthop Trauma Surg 2009;129:1621–5. [DOI] [PubMed] [Google Scholar]
- [4].Polley P, Dunn R. Noncontiguous spinal tuberculosis: incidence and management. Eur Spine J 2009;18:1096–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu WC, Kwak BK, Kim KN, et al. Tuberculous aneurysm of the abdominal aorta: endovascular repair using stent grafts in two cases. Korean J Radiol 2000;1:215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xue J, Yao Y, Liu L. Treatment of tuberculous aortic pseudoaneurysm associated with vertebral tuberculosis: a case series and a literature review. Medicine (Baltimore) 2018;97:e0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pluemvitayaporn T, Jindahra S, Pongpinyopap W, et al. Concomitant mycotic abdominal aortic aneurysm and lumbartuberculous spondylitis with cauda equine syndrome: a rare condition-a case report and literature review. Spinal Cord Ser Cases 2018;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Long R, Guzman R, Greenberg H, et al. Tuberculous mycotic aneurysm of the aorta: review of published medical and surgical experience. Chest 1999;115:522–31. [DOI] [PubMed] [Google Scholar]
- [9].Dahl T, Lange C, Ødegård A, et al. Ruptured abdominal aortic aneurysm secondary to tuberculous spondylitis. Int Angiol 2005;24:98–101. [PubMed] [Google Scholar]
- [10].Falkensammer J, Behensky H, Gruber H, et al. Successful treatment of a tuberculous vertebral osteomyelitis eroding the thoracoabdominal aorta: a case report. J Vasc Surg 2005;42:1010–3. [DOI] [PubMed] [Google Scholar]
- [11].Takahashi Y, Sasaki Y, Shibata T, et al. Descending thoracic aortic aneurysm complicated with severe vertebral erosion. Eur J Cardiothorac Surg 2007;31:941–3. [DOI] [PubMed] [Google Scholar]
- [12].Hussein H, Azizi ZA. Tuberculous aortic pseudoaneurysm treated with in situ silver-impregnated vascular inlay graft. Asian J Surg 2008;31:87–9. [DOI] [PubMed] [Google Scholar]
- [13].Li FP, Wang XF, Xiao YB. Endovascular stent graft placement in the treatment of a ruptured tuberculous pseudoaneurysm of the descending thoracic aorta secondary to Pott's disease of the spine. J Card Surg 2012;27:75–7. [DOI] [PubMed] [Google Scholar]
- [14].Jain AK, Chauhan RS, Dhammi IK, et al. Tubercular pseudoaneurysm of aorta: a rare association with vertebral tuberculosis. Spine J 2007;7:249–53. [DOI] [PubMed] [Google Scholar]
- [15].Choudhary SK, Bhan A, Talwar S, et al. Tubercular pseudoaneurysms of aorta. Ann Thorac Surg 2001;72:1239–44. [DOI] [PubMed] [Google Scholar]
- [16].Pimple MK, Narlawar RS, Bapat MR. Mycotic aneurysm of the descending thoracic aorta with intraspinal extension-a case report. Acta Orthop Scand 2002;73:597–600. [DOI] [PubMed] [Google Scholar]
- [17].Han JT, Zhao J, Luan JY, et al. A case of multiple tuberculous aneurysm of the abdominal aorta. Beijing Da Xue Xue Bao Yi Xue Ban 2007;39:361–4. [PubMed] [Google Scholar]
- [18].Villegas MO, Mereles AP, Tamashiro GA, et al. Endovascular treatment of an aortoiliac tuberculous pseudoaneurysm. Cardiovasc Intervent Radiol 2013;36:540–4. [DOI] [PubMed] [Google Scholar]
- [19].Liu S, Xing HX, Bao JM. Successful in situ reconstruction with a prosthetic graft in tuberculous pseudoaneurysm of abdominal aorta: two case reports. Kuwait Med J 2014;46:65–9. [Google Scholar]
- [20].Zhang C, Chen B, Gu Y, et al. Tuberculous abdominal aortic pseudoaneurysm with renal and vertebral tuberculosis: a case and literature review. J Infect Dev Ctries 2014;8:1216–21. [DOI] [PubMed] [Google Scholar]
- [21].Park SC, Moon IS, Koh YB. Tuberculous pseudoaneurysm of the descending thoracic aorta. Ann Vasc Surg 2010;24:417.e11–3. [DOI] [PubMed] [Google Scholar]
- [22].Talu U, Gogus A, Ozturk C, et al. The role of posterior instrumentation and fusion after anterior radical debridement and fusion in the surgical treatment of spinal tuberculosis: experience of 127 cases. J Spinal Disord Tech 2006;19:554–9. [DOI] [PubMed] [Google Scholar]
- [23].Ma YZ, Cui X, Li HW, et al. Outcomes of anterior and posterior instrumentation under different surgical procedures for treating thoracic and lumbar spinal tuberculosis in adults. Int Orthop 2012;36:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].De Prophetis N, Armitage HV, Triboletti ED. Rupture of tuberculous aortic aneurysm into lung. Ann Surg 1959;150:1046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clough RE, Topple JA, Zayed HA, et al. Endovascular repair of a tuberculous mycotic thoracic aortic aneurysm with a custom-made device. J Vasc Surg 2010;51:1272–5. [DOI] [PubMed] [Google Scholar]
- [26].Dogan S, Memis A, Kale A, et al. Endovascular stent graft placement in the treatment of ruptured tuberculous pseudoaneurysm of the descending thoracic aorta: case report and review of the literature. Cardiovasc Intervent Radiol 2009;32:572–6. [DOI] [PubMed] [Google Scholar]
- [27].Hatem CM, Kantis GA, Christoforou D, et al. Tuberculous aneurysm of the descending thoracic aorta. J Thorac Cardiovasc Surg 2002;123:373–4. [DOI] [PubMed] [Google Scholar]


