Abstract
Background
The exact contribution of congenital cytomegalovirus infection (cCMVI) to permanent hearing loss (HL) in highly seropositive populations is unknown. We determined the contribution of cCMVI to HL and estimated the effectiveness of newborn hearing screening (HS) in identifying neonates with CMV-related HL.
Methods
A total of 11 900 neonates born from a population with ≥97% maternal seroprevalence were screened for cCMVI and HL. cCMVI was confirmed by detection of CMV-DNA in saliva and urine at age <3 weeks.
Results
Overall, 68 (0.6%; 95% confidence interval [CI], 0.4–0.7) neonates were identified with cCMVI. Of the 91 (0.8%) newborns who failed the HS, 24 (26.4%) were confirmed with HL, including 7 (29.2%; 95% CI, 17.2–59.3) with cCMVI. Another newborn with cCMVI passed the HS but was confirmed with HL at age 21 days. Of the 62 neonates with cCMVI who underwent a complete hearing evaluation, 8 (12.9%; 95% CI, 6.7–23.4) had HL and most (7/8; 87.5%; 95% CI, 46.6–99.7) were identified by HS. The rate of CMV-related HL was 8 per 11 887 neonates (0.7 per 1000 live births). The prevalence ratio of HL among neonates with cCMVI compared to CMV-uninfected neonates was 89.5 (95% CI, 39.7–202.0). No late-onset cCMVI-related HL was detected during a median follow-up of 36 months.
Conclusions
cCMVI is an important cause of HL in childhood in all settings. Integrating targeted cCMVI screening among neonates who fail a HS could be a reasonable, cost-effective strategy to identify newborns with early-onset cCMVI-related HL.
Keywords: cytomegalovirus, congenital infection, hearing screening, CMV screening, HL
A total of 11 900 infants were screened for congenital cytomegalovirus infection (cCMVI) and hearing. About one-third of permanent hearing impairment in the neonatal period could be attributed to cCMVI. Neonatal hearing screening identified most infants with CMV-related hearing loss.
(See the Editorial Commentary by Demmler Harrison on pages 1385–7.)
Sensorineural hearing loss (SNHL) is the most common sequela in children with congenital cytomegalovirus infection (cCMVI) [1–3]. Nonetheless, defining the exact contribution of cCMVI in permanent HL in infancy has been challenging because around 90% of newborns with cCMVI do not exhibit any abnormal clinical findings (asymptomatic infection). In addition, the need for testing of neonates’ specimens (urine or saliva) within 2 to 3 weeks of birth for cCMVI confirmation poses further challenges in defining the contribution of cCMVI to permanent HL.
Retrospective testing of stored samples such as dried blood spots (DBS) obtained at birth has suggested that a significant proportion of unexplained SNHL identified after the neonatal period is secondary to cCMVI [4–6]. However, because of the limitations of using stored DBS for cCMVI diagnosis, the true contribution of cCMVI to HL may be underestimated. Therefore, it is necessary to evaluate neonates with and without cCMVI by integrating systematic screenings for cCMVI and hearing to identify newborns with early-onset HL in addition to monitoring newborns who passed their hearing screening (HS) to detect late-onset HL. In this respect, no studies have definitively evaluated the role of cCMVI in permanent HL in childhood. Further, no population-based data are available for CMV highly seroprevalent populations.
We aimed to determine the contribution of cCMVI to permanent early HL in newborns born to women from a highly seropositive population and to define the proportion of neonates with cCMVI who eventually develop late-onset SNHL. By screening neonates for cCMVI and hearing, we were able to determine the proportion of newborns with CMV-related HL who would be identified using the strategy of targeted screening of newborns who failed universal HS for cCMVI.
METHODS
As part of the Brazilian Cytomegalovirus Hearing and Maternal Secondary Infection Study, in a prospective study, 11 900 neonates born from September 2013 to April 2017 were screened for cCMVI and hearing impairment. The study was carried out at 2 public maternity centers: the Reference Center of Women’s Health and the Clinical University Hospital at Ribeirão Preto Medical School. High and almost universal (97%; 95% confidence interval [CI], 95.8–98.0) maternal CMV seroprevalence, with similar age-specific seroprevalence in pregnant women from age 12 to 46 years, has been previously demonstrated in this population [7]. In this study, mothers were not tested for CMV antibodies during gestation or at delivery. The University Hospital Research Ethics Committee approved the study, and written informed consent was obtained from all participants.
Congenital CMV Screening and Diagnosis
Neonates were screened for cCMVI by CMV-DNA detection in saliva specimens using a polymerase chain reaction assay [8]. Positive saliva results were confirmed by testing urine collected within 3 weeks after birth. Newborns with CMV-DNA detection in at least 2 saliva and 1 urine samples were defined as having cCMVI.
All neonates with cCMVI were prospectively followed with clinical and audiological evaluation every 6 months. The definition of symptomatic cCMVI was based on the recent Consensus Recommendations [9].
Hearing Screening, Audiological Assessment, and Outcomes
All newborns were classified as well babies or high-risk babies for HS according to the Joint Committee on Infant Hearing guidelines [10]. High-risk babies including those with cCMVI underwent HS using both otoacoustic emission (OAE) and automated auditory brainstem evoked responses (AABR-35dBHL). Well babies and the CMV-noninfected neonates underwent a 1-step screening by OAE; those who failed OAE were tested using AABR. Those newborns who failed HS were rescreened within 30 days using both OAE and AABR. Newborns who passed OAE in both ears or those who passed AABR in both ears were considered to have passed screening. Babies who failed rescreening in 1 or both ears or those who tested positive for cCMVI underwent diagnostic audiological assessment.
Audiological assessments for confirmation of hearing status included ABR by click and frequency-specific tone-burst stimuli, transient evoked OAE (TEOAE), and acoustic immittance measures. Hearing loss was defined as ≥25 dB hearing level (HL) for the ABR frequency-specific responses for the corrected tone burst and classified as mild (26-40dB), moderate (41-60dB), severe (61-80dB), or profound (over 81dB) on the basis of hearing thresholds averaged at 0.5, 1, 2, and 4 kHz frequencies [11].
CMV-infected children with normal hearing were scheduled to have behavioral hearing evaluation at 7, 12, 18, 24, 30, and 36 months and then annually until age 6 years. Ear-specific hearing results were sought at each visit, including visual reinforcement audiometry (age 6–12 months) or play audiometry (age 2–4 years). However, when a child would not tolerate head phones, sound field testing was performed and OAEs were used to exclude unilateral loss. After age 4 years, standard audiometry with an air-conduction transducer was applied. When HL was identified, tympanometry was performed to determine whether the loss was permanent sensorineural or transient conductive. ABR was performed when clinically indicated.
Hearing evaluation by behavior audiometry was defined by pure tone average (PTA) based on hearing thresholds at 0.5, 1, 2, and 4 kHZ frequencies for each ear. A PTA of a minimum response of 30 dB (free field) and 20 dB (using phones) was considered normal. Hearing loss was classified as mild (26–40 dB), moderate (41–60 dB), severe (61–80 dB), and profound (>81 dB) [12].
Hearing status during follow-up was considered normal in children with hearing thresholds ≤30 dB and bilateral TEOAE responses. Progressive HL was defined as worsening auditory thresholds with at least 10 dB in successive hearing tests with normal middle ear function. Hearing loss was considered late-onset when the loss was detected after the first month of life in 2 successive tests with normal middle ear function in children who passed the HS and had an initial normal complete audiological evaluation.
Data Analyses
Data were analyzed using SAS 9.4 (SAS Institute Inc., Cary, NC). The prevalence of HL and the 95% exact CI were calculated. Prevalence ratios and their 95% CIs among newborns with and without cCMVI were estimated using a log-binomial regression model.
RESULTS
The study population included 11 900 neonates who underwent screening for cCMVI and hearing. The general characteristics of newborns and their mothers are shown in Table 1.
Table 1.
Characteristics of 11 900 neonates Screened for Congenital Cytomegalovirus Infection and Hearing
| Characteristic | Total (N = 11 900) |
|---|---|
| Maternal age (range), y | 26 (12–50) |
| Parity (primiparous) | 4586 (38%) |
| Gestational age | |
| ≥37 weeks | 10 312 (86.6%) |
| <37 weeks | 1587 (13.4%) |
| Sex | |
| Male | 6176 (51.9%) |
| Female | 5717 (48.0%) |
| Indeterminate | 7 (0.1%) |
| Birth weight, median (range), g | 3180 (490–5540) |
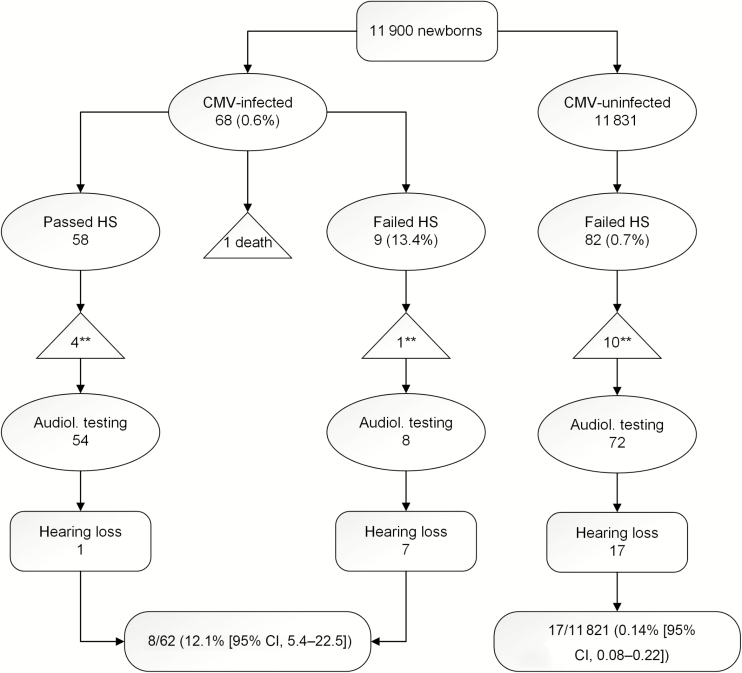
Screening and referral audiological data for newborns with and without cCMVI are shown in Figure 1. Of the 11 900 neonates, 68 (0.6%; 95% CI, 0.4–0.7) were positive for cCMVI (1 died before the HS), 91 (0.8%) did not pass the HS, and 24 (26.4%) were confirmed with early permanent HL. About one-third of newborns with permanent HL identified on HS and confirmed on diagnostic evaluation had cCMVI (7/24; 29.2%; 95% CI, 17.2–59.3). Of the 62 neonates with cCMVI who underwent complete hearing evaluation, HL was detected in 8 (12.9%; 95% CI, 6.7–23.4) newborns (5 bilateral and 3 unilateral). All newborns with HL except 1 (7/8; 87.5%; 95% CI, 46.6–99.7) were identified by HS after birth. One newborn (12.5%) who passed the HS but was confirmed with HL at age 21 days. Among neonates without cCMVI, permanent HL (14 bilateral and 3 unilateral) was confirmed in 17/11 821 (0.14%; 95% CI, 0.08–0.22). The prevalence ratio of HL among neonates with cCMVI compared to those CMV-uninfected neonates was 89.5% (95% CI, 39.7–202.0).
Figure 1.
Hearing screening and confirmatory audiological testing according to congenital CMV infection status. Abbreviations: CI, confidence interval; CMS, cytomegalovirus; HS, hearing screening. ** indicates lost to follow-up.
In total, permanent HL (congenital and early-onset) was detected in 25 neonates, a prevalence rate of 2 per 1000 live births (95% CI, 1.3–3.0). As shown in Table 2, 8/25 (32%) neonates with permanent HL were CMV-infected. Among all neonates with permanent HL, 26% of neonates with bilateral HL and 50% of those with unilateral HL were diagnosed with cCMVI.
Table 2.
Contribution and Characteristics of Congenital Cytomegalovirus Infection–related Hearing Loss to Overall Hearing Loss
| HL | Proportion (%; 95% Confidence Interval) |
|---|---|
| CMV-related /all cases of HL | 8/25 (32; 14.9–53.5) |
| Unilateral | 3/6 (50; 11.8–88.2) |
| Bilateral | 5/19 (26; 9.1–51.2) |
| CMV-related HL/all cases of congenital CMV infection | 8/62 (12.9; 5.7–23.8) |
| Asymptomatic | 4/54 (7.4; 2.0–17.6) |
| Symptomatic | 4/8 (50; 17.4–82.5) |
| CMV-related HL identified by hearing screening | 7/8 (87.5; 46.6–99.7 |
Abbreviations: CMV, cytomegalovirus; HL, hearing loss.
The rate of permanent HL resulting from cCMVI was 8 per 11 887 neonates, or 0.7 per 1000 live births.
Characteristics of Newborns With cCMVI and Permanent Congenital or Early-onset HL Findings
Of the 68 neonates with cCMVI, 8 (11.8%) had clinical findings suggestive of congenital infection at birth consistent with moderate to severe cCMV disease. Hepatosplenomegaly was the most common finding (6/8; 75%) followed by neonatal hepatitis with elevated transaminases or direct bilirubin (5/8; 62.5%) and thrombocytopenia with petechiae (4/8; 50%). All symptomatic neonates except 1 had neurological abnormalities. Seven symptomatic newborns were treated with antiviral therapy from the neonatal period. The 3 neonates identified at the beginning of the study received intravenous ganciclovir for 6 weeks, and the remaining 4 received oral valganciclovir for 6 months. One extreme preterm newborn died before complete evaluation for categorization as symptomatic but likely had disseminated cCMV disease and received intravenous ganciclovir. The remaining 59 neonates were asymptomatic. As shown in Table 2, SNHL was detected in 4/54 (7.4%) asymptomatic and in 4/8 (50%) symptomatic CMV-infected neonates.
HS of newborns with cCMVI was performed at a median age of 1 day (range, 1–44), and HL was confirmed at a median age of 28 days (range, 8–120). Five infants had audiological assessments at age >30 days. Four of them had failed HS in both ears and 1 failed in the left ear.
Table 3 shows clinical and detailed audiological data for the 8 neonates with hearing impairment according to the presence of cCMV disease detected at birth. Four of them had moderate to severe cCMV disease at birth, failed the HS, and were diagnosed with severe to profound bilateral HL. Further, they had additional risk factors for HL, including intravenous aminoglycoside therapy (2 neonates) and a stay in a neonatal intensive care unit >5 days (2 newborns). The remaining 4 infants with HL were asymptomatic and had no other risk factors for HL. Three of them also failed the HS and were diagnosed with profound unilateral HL. The remaining asymptomatic neonate was screened only with OAE at age 2 days because the diagnosis of cCMVI was not known and passed HS in both ears. However, after diagnosis of cCMVI, the audiological reassessment at age 21 days identified failure of OAE and ABR tone burst in the left ear, and profound unilateral SNHL was confirmed.
Table 3.
Audiological Evaluation Data in 8 Children With Congenital Cytomegalovirus Infection and Hearing Loss According to Clinical Presentation at Birth and Follow-up
| Patient No. | Congenital Cytomegalovirus Disease | Hearing Screening | HL Confirmation | Hearing Follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age (Days) | Otoacoustic Emissions | Automated Auditory Brainstem | Age (Days) | Tone-burst ABR | Age at Last Testing (Months) | Tone-burst ABR | HL Threshold | ||
| 1 | Severea | 6 | Bilateral failure | Bilateral failure | 42 | Bilateral moderate/ severe | 48 | L: moderate/severe | Progressive (at age 21 months) |
| R: profound | |||||||||
| 2 | Severea | 30 | Bilateral failure | Bilateral failure | 62 | Bilateral profound | 36 | Bilateral profound | Stable |
| 3 | Severeb | 9 | Bilateral failure | Bilateral failure | 69 | Bilateral moderate/ severe | 30 | Bilateral moderate/ severe | Stable |
| 4 | Severeb | 44 | Bilateral failure | Bilateral failure | 120 | Bilateral profound | 18 | Bilateral profound | Stable |
| 5 | Asymptomatic | 17 | L: pass | Bilateral failure | 19 | L: mild | 48 | Bilateral severe/ profound | Progressive (at age 8 months) |
| R: fail | R: severe/profound | ||||||||
| 6 | Asymptomatic | 2 | L: fail | Not done | 21 | L: moderate/severe | 36 | L: moderate/severe | Stable |
| R: pass | |||||||||
| R: normal | R: normal | ||||||||
| 7 | Asymptomatic | 2 | L: fail | L: fail | 63 | L: profound | 36 | L: profound | Stable |
| R: pass | R: pass | ||||||||
| R: normal | R: normal | ||||||||
| 8 | Asymptomatic | 1 | Bilateral pass | Not done | 21 | L: normal | 30 | L: normal | Stable |
| R: profound | |||||||||
| R: profound (at age 21 days) |
Bold font indicates HL threshold changes.
Abbreviations: HL, hearing loss; L, left ear; R, right ear.
aTreated with ganciclovir for 42 days.
bTreated with valganciclovir for 6 months.
Hearing Follow-up of Children With cCMVI
Hearing status was monitored periodically in 57/62 (91.9%) infants (8 with early HL and 49 with normal hearing) at the median age of 36 months (range, 18–48). The median number of audiological tests for each child was 5 (range, 3–8). Forty-two (73.7%) children had completed at least 36 months of follow-up. The median duration of follow-up (36 months) did not differ between children with and without HL.
As shown in Table 3, among children with early-onset HL, worsening of hearing thresholds in the better ear was observed in a single child with symptomatic cCMVI at age 21 months. The remaining 3 symptomatic children had stable hearing thresholds over time. All of these children are in hearing rehabilitation with hearing aids and are being evaluated for cochlear implantation. Additionally, progression of HL was observed in 1 of the 4 asymptomatic children in whom the hearing threshold in the better ear declined from 30 dB to no response at 100 dB at age 8 months; rehabilitation with hearing aids and bilateral cochlear implants were indicated. The remaining 3 asymptomatic children with unilateral profound HL did not show progressive impairment in the normal ear, and no rehabilitation intervention has been needed to date.
Among 49 children with cCMVI with normal hearing at baseline (3 symptomatic and 46 asymptomatic), no late-onset CMV-related HL was detected during a median follow-up of 36 months. Although 19/49 (38%) children exhibited conductive HL at 1 or more visits due to otitis media with effusion, as documented by tympanometry and no response to TEOAE, all of these children recovered and had normal hearing at their last follow-up visit. Stable hearing thresholds were observed in the remaining 30 children.
DISCUSSION
We prospectively screened approximately 12 000 newborns from a highly seropositive population for cCMVI and HL to determine the exact contribution of cCMVI to permanent hearing impairment in neonates and to estimate the effectiveness of newborn HS in identification of neonates with cCMVI and congenital or early-onset HL.
Our findings demonstrate that about one-third of permanent HL detected in the neonatal period could be attributed to cCMVI. In addition, newborn HS identified most (87.5%) infants with CMV-related HL. Furthermore, we did not observe late-onset HL in children with cCMVI during a median follow-up of 36 months.
Previous studies have prospectively evaluated the prevalence of SNHL that could be attributed to cCMVI. Hicks et al [13] verified the utility of a risk factor–based HS to identify HL resulting from cCMVI and found that only 34 (20%) of 167 infants with cCMVI and 2 of the 14 with CMV-related HL could be detected using this approach. A large North American cohort of approximately 100 000 patients [14] and a smaller cohort (n = 1720) from rural India [15] estimated the contribution of CMV-related HL in newborns who underwent both hearing and cCMVI screening. Similar to our findings, they reported that HS failure was more than 2-fold higher in CMV-infected than in CMV-uninfected newborns, highlighting that cCMVI-related HL occurs in all settings. Our finding that 12% of newborns with cCMVI had congenital or early-onset HL is similar to that reported in previous studies [14, 15]. While Dar et al [15] found the prevalence of HL to be 20-fold higher in neonates with cCMVI than in those without, we found it to be 90-fold higher, primarily because of the lower prevalence of HL in CMV-uninfected neonates (1.3/1000 vs 5/1000) in our study, demonstrating that the overall prevalence of HL varies in different populations.
Since early detection of CMV-infected neonates with HL could improve outcomes by means of antivirals and/or hearing interventions [16], it is important to identify children with CMV-related HL early in life. By using both OAE and AABR for screening of CMV-infected neonates for HL, we were able to identify most children with CMV-related HL (87.5%, 95% CI, 46.6–99.7). However, many hospitals screen newborns using only OAE as the initial screening method, and only those who fail OAE undergo AABR testing because of the cost and personnel time. Our study, which combined both OAE and AABR screening, was more effective in identifying newborns with CMV-related HL compared to the findings from the CHIMES study [14] in which only 57% (95% CI, 39–73) of neonates with early-onset HL were identified on newborn HS. In addition to the technical challenges of carrying out OAE testing of neonates, which can lead to false-positive results, HS that uses only OAE might miss a neonate with HL at some frequencies. The false-negative rate of OAE testing was estimated to be 1 per 1000 ears based on adults with moderate to severe–profound HL, and this rate can be higher for newborns [17]. Another possibility for missing cCMVI-related HL using only OAE is the fact that the pathogenesis of CMV-related HL is not yet completely understood nor is it known if there is a proportion of cCMV-infected newborns who might have auditory neuropathy but normal hair cell function who would likely be missed by OAE. However, previous studies have not reported auditory neuropathy in neonates with cCMVI [14, 18].
In contrast to previous studies that reported late-onset HL ranging from 5% to 38% of children with cCMVI [19–22], we did not observe late-onset SNHL in our study participants during the first 36 months of life. Although there is no consensus on the timing of the onset of HL for the deficit to be classified as delayed, we defined HL detected after the first month of life following a prior pass on HS and normal hearing evaluation. It has been shown that cCMVI-related SNHL can be delayed by up to 6 or more years [1]. Although the majority of CMV-related HL occurs within the first 3 years of life, the median age at late-onset HL in children with asymptomatic cCMVI was reported to be 44 months [20]. Therefore, it is possible that the duration of follow-up was not sufficient in our study to detect all late-onset losses. Nevertheless, late-onset HL appears to be less frequent in our population. To identify HL that occurs after the first few years of life, the study children continued to be monitored with periodical hearing evaluations. Progressive SNHL was found in 2 study children (25%), which is lower than previously reported rates of 50% [23] in children with asymptomatic cCMVI. Although conductive HL caused by middle ear dysfunction was very frequent, affecting 35% of study children with cCMVI during follow-up, all of them had a normal hearing evaluation at their last follow-up visit.
In summary, our results reinforce that cCMVI is an important cause of permanent hearing impairment in childhood, even in neonates born to mothers from a high CMV seroprevalence population [24]. We estimate that cCMVI accounts for about one-third of all cases of bilateral congenital or early-onset permanent HL and about half of all cases of unilateral SNHL in this population. Further, HL is 90-fold higher in neonates with cCMVI compared to uninfected neonates, and 0.7 per 1000 live births in this population will suffer from CMV-related HL. These findings emphasize the need for public health measures to prevent cCMVI and to identify these children early in life to improve outcomes. We also demonstrate the effectiveness of targeted screening of neonates who fail newborn HS for cCMVI in the identification of most newborns with CMV-related SNHL as reported by other investigators [25, 26]. Therefore, integrating targeted cCMVI screening in newborns who fail HS could be a reasonable cost-effective strategy to identify newborns with early onset HL in this population. The rates of late-onset HL in children with cCMVI seems to be lower when compared to previous studies, suggesting that the natural history of SNHL related to cCMVI might be different in this population.
Notes
Acknowledgments. The authors are grateful for the support of the Pediatric Infectious Diseases Laboratory technicians and the staff members of the Study Center on Maternal, Perinatal and Infant’s Infection from Ribeirão Preto Medical School. They acknowledge Dr Bruno C.P. Lopes for his help with audiological exams and Davi C. Aragon for assisting with data analysis.
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number 2R01HD061959-07A2 to W. J. B.) and Fundação de Amparo à Pesquisa do Estado de São Paulo (grant number 2013/06579-0 to M. M. M. P.).
Potential conflicts of interest. S. B. reports grants from Meridian Bioscience, Inc., and personal fees from Merck outside the submitted work. K. B. F. reports personal fees from Merck outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fowler KB Congenital cytomegalovirus infection: audiologic outcome. Clin Infect Dis 2013; 57(Suppl 4):S182–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fowler KB, Boppana SB. Congenital cytomegalovirus infection. Semin Perinatol 2018; 42:149–54. [DOI] [PubMed] [Google Scholar]
- 3. Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics 2014; 134:972–82. [DOI] [PubMed] [Google Scholar]
- 4. Meyer L, Sharon B, Huang TC, et al. Analysis of archived newborn dried blood spots (DBS) identifies congenital cytomegalovirus as a major cause of unexplained pediatric sensorineural hearing loss. Am J Otolaryngol 2017; 38:565–70. [DOI] [PubMed] [Google Scholar]
- 5. Barbi M, Binda S, Caroppo S, Ambrosetti U, Corbetta C, Sergi P. A wider role for congenital cytomegalovirus infection in sensorineural hearing loss. Pediatr Infect Dis J 2003; 22:39–42. [DOI] [PubMed] [Google Scholar]
- 6. Korver AM, de Vries JJ, Konings S, et al. ; DECIBEL collaborative study group DECIBEL study: congenital cytomegalovirus infection in young children with permanent bilateral hearing impairment in the Netherlands. J Clin Virol 2009; 46(Suppl 4):S27–31. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto AY, Castellucci RA, Aragon DC, Mussi-Pinhata MM. Early high CMV seroprevalence in pregnant women from a population with a high rate of congenital infection. Epidemiol Infect 2013; 141:2187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamamoto AY, Mussi-Pinhata MM, Marin LJ, Brito RM, Oliveira PF, Coelho TB. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J Clin Virol 2006; 36:228–30. [DOI] [PubMed] [Google Scholar]
- 9. Rawlinson WD, Boppana SB, Fowler KB, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis 2017; 17:e177–88. [DOI] [PubMed] [Google Scholar]
- 10. Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics, 2007; 120:898–921. [DOI] [PubMed] [Google Scholar]
- 11. Stapells DR, Gravel JS, Martin BA. Thresholds for auditory brain stem responses to tones in notched noise from infants and young children with normal hearing or sensorineural hearing loss. Ear Hear 1995; 16:361–71. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization 2016. Grades of hearing impairment. Available at: http://www.who.int/pbd/deafness/hearing_impairment_grades/en/. Accessed 25 January 2019. [Google Scholar]
- 13. Hicks T, Fowler K, Richardson M, Dahle A, Adams L, Pass R. Congenital cytomegalovirus infection and neonatal auditory screening. J Pediatr 1993; 123:779–82. [DOI] [PubMed] [Google Scholar]
- 14. Fowler KB, McCollister FP, Sabo DL, et al. A targeted approach for congenital cytomegalovirus screening within newborn hearing screening. Pediatrics 2017; 139:e20162128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dar L, Namdeo D, Kumar P, et al. Congenital cytomegalovirus infection and permanent hearing loss in rural north Indian children. Pediatr Infect Dis J 2017; 36:670–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fletcher KT, Horrell EMW, Ayugi J, et al. The natural history and rehabilitative outcomes of hearing loss in congenital cytomegalovirus: a systematic review. Otol Neurotol 2018; 39:854–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White KR, Nelson LH, Munoz K. How many babies with hearing loss will be missed by repeated newborn hearing screening with transient evoked otoacoustic emissions due to statistical artifact? JEHDI 2016; 1:56–62. [Google Scholar]
- 18. Foulon I, Vleurinck L, Kerkhofs K, Gordts F. Hearing configuration in children with cCMV infection and proposal of a flow chart for hearing evaluation. Int J Audiol 2015; 54:714–9. [DOI] [PubMed] [Google Scholar]
- 19. Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr 1999; 135:60–4. [DOI] [PubMed] [Google Scholar]
- 20. Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol 2000; 11:283–90. [PubMed] [Google Scholar]
- 21. Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr 2008; 153:84–8. [DOI] [PubMed] [Google Scholar]
- 22. Goderis J, Keymeulen A, Smets K, et al. Hearing in children with congenital cytomegalovirus infection: results of a longitudinal study. J Pediatr 2016; 172:110–115.e2. [DOI] [PubMed] [Google Scholar]
- 23. Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr 1997; 130:624–30. [DOI] [PubMed] [Google Scholar]
- 24. de Vries JJ, van Zwet EW, Dekker FW, Kroes AC, Verkerk PH, Vossen AC. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: a population-based prediction model. Rev Med Virol 2013; 23:241–9. [DOI] [PubMed] [Google Scholar]
- 25. Gantt S, Dionne F, Kozak FK, et al. Cost-effectiveness of universal and targeted newborn screening for congenital cytomegalovirus infection. JAMA Pediatr 2016; 170:1173–80. [DOI] [PubMed] [Google Scholar]
- 26. Diener ML, Zick CD, McVicar SB, Boettger J, Park AH. Outcomes from a hearing-targeted cytomegalovirus screening program. Pediatrics. 2017; 139:e20160789. [DOI] [PubMed] [Google Scholar]