Abstract
Isolating active mesenchymal stem cells from a heterogeneous population is an essential step that determines the efficacy of stem cell therapy such as for osteoarthritis. Nowadays, the gold standard of cell sorting, fluorescence-activated cell sorting, relies on labelling surface markers via antibody–antigen reaction. However, sorting stem cells with high stemness usually requires the labelling of multiple biomarkers. Moreover, the labelling process is costly, and the high operating pressure is harmful to cell functionality and viability. Although label-free cell sorting, based on physical characteristics, has gained increasing interest in the past decades, it has not shown the ability to eliminate stem cells with low stemness. Cell motility, as a novel sorting marker, is hence proposed for label-free sorting active stem cells. Accumulating evidence has demonstrated the feasibility in manipulating directional cell migration through patterning the biophysical, biochemical or both gradients of the extracellular matrix. However, applying those findings to label-free cell sorting has not been well discussed and studied. This review thus first provides a brief overview about the effect of biophysical and biochemical gradients of the extracellular matrix on cell migration. State-of-the-art fabrication techniques for generating such gradients of hydrogels are then introduced. Among current research, the authors suggest that hydrogels with dual-gradients of biochemistry and biophysics are potential tools for accurate label-free cell sorting with satisfactory selectivity and efficiency.
Translational potential of this article
The reviewed label-free cell sorting approaches enable us to isolate active cell for cytotherapy. The proposed system can be further modified for single-cell analysis and drug screening.
Keywords: Gradients, Cell sorting, Hydrogels, Stem cells
Introduction
Accurate cell sorting is of significance in a variety of biomedical applications including cytotherapy. When referring to accurate cell sorting, it suggests not only isolating target cell types from a heterogeneous population but also sorting healthy and active target cells out of senescent cells. The need for such accurate cell sorting can be further illustrated by its application in mesenchymal stem cell (MSC) therapy. MSCs are originally identified from bone marrow–derived stromal cells with multipotency to differentiate into certain tissue types, such as bone and cartilage [8], [4]. They have been intensively investigated in the field of regeneration therapy, especially in orthopaedic surgery [12]. For example, MSC therapy holds a good promise for osteoarthritis, a prevalent debilitating age-related bone and joint problem [1]. Yet, the clinical performance of MSC therapy is still unsatisfactory because of hugely variable results and low reproductivity [32]. The intrinsic heterogeneity and uncertain cellular status of MSCs are the major concerns for cytotherapy, which should be carefully addressed in clinical practice in the future.
A successful MSC therapy relies on a sufficient population of pure and active MSCs. However, MSCs are scarce in the human body and are mixed with other types of cells [33], [41], [54]. Even for a purified cell population, the difference in cellular status, young and active or senescent stage, will also affect their differentiation potential and ultimately therapeutic efficacy [60]. Not surprisingly, senescent MSCs exhibited a decreased stemness [10]. The decreased stemness leads to reduced differential potential, ending up with poor therapeutic efficacy. By contrast, MSCs with low senescence index tend to perform better in tissue regeneration. This is evident in the case of cell-based therapy for cartilage regeneration where the group with a smaller ratio of senescent MSCs demonstrated a better chondrogenic potential [68]. Therefore, the quality of the cells plays an essential role in therapeutic efficacy. There is an urgent yet unmet need to develop a cost-effective and accurate sorting approach for MSCs with higher stemness in an attempt to achieve a better clinical outcome.
Currently, cell sorting strategies can be classified into either label-based or label-free techniques. A typical example of label-based cell sorting is fluorescence-activated cell sorting (FACS). In the FACS system, targeted cells are first bound to fluorescent detection antibodies according to their surface biomarkers. Based on the fluorescent signals, the cells are separated one after another. Although FACS has been considered as the “gold standard” in cell sorting, its high operating pressure may induce harm to the functionality and viability of sample cells. In addition, it is costly to operate because of the labelling process. Moreover, it relies strongly on the existing studies about the surface markers of target cells. However, a single biomarker that accounts for MSCs and their stemness has not been discovered yet. Hence, multiple biomarkers shall be labelled at the same time in the FACS system for stem cell isolation. Moreover, MSCs from different species have different surface markers for sorting: cluster of differentiation (CD146), CD105, alkaline Phosphatase (ALP), STROMAL-1 (STRO-1) and vascular cell adhesion molecule 1 (VCAM1) in humans and CD105, CD90 and VCAM1 in the mouse [4]. Even for human MSCs, surface markers still vary with their origins. Data suggest that specific classes of tissue progenitors from different tissues have specific surface markers. For instance, STRO-1 is expressed in MSCs from the bone marrow but absent in adipose tissue MSCs [52], [16]. All these factors and variation make label-based cell sorting of MSCs a complex and costly method. Considering the aforementioned limitations of label-based methods, isolating cells without labelling is preferred. Existing label-free strategies distinguish cells based on the physical features of cells such as size, deformability and intrinsic polarisability [49], [3]. Without labelling, this method is generally high in throughput and low in cost. Although MSCs can be isolated from the bone marrow by their size differences in a recent work carried out by Lee et al [26], cells with low multipotency are hardly eliminated because cell size is not necessarily a marker for stemness [27]. Hence, existing label-free methods based on physical features of cells are not able to isolate young and active MSCs from a heterogeneous population. A new separation marker is needed aside from cell size and surface markers.
Cellular migration could be used as a new clue for cell sorting. The direction and speed of the migration, especially in stem cells, are affected and regulated by biophysical and biochemical gradients of the extracellular matrix (ECM) [21], [62]. At the same time, such directionality and speed decrease with the level of senescent status [40], [9]. As mentioned, senescent stem cells are a population of cells with low stemness having limited therapeutic efficacy in regenerative medicine. Hence, senescent MSCs with low motility are likely to be associated with low stemness. The difference in the direction and speed among diverse types and status of cells enables cell migration to be a novel marker for label-free sorting. In a natural MSC population, only the target and active cells would migrate fast and directionally in response to the designed patterns of the biochemical and biophysical gradients in the ECM. To establish such a well-defined ECM, hydrogels provide a suitable solution. Hydrogel scaffolds are flexible enough to be embedded with various biophysical and biochemical gradients through various techniques, such as photolithography, micromolding and 3D printing [64], [70], [71]. A hydrogel scaffold with a desired pattern of gradients will guide target cells to an intended site. With such a design, accurately sorting target stem cells from a heterogeneous population can be achieved, improving the therapeutic efficacy.
Although there are abundant studies on cell migration under biophysical or biochemical gradients and the fabrication of gradients, a gap remains about how to combine them for cell sorting. In this mini-review, the discussion on the feasibility of this approach will start by introducing the influence of ECM biochemical and biophysical gradients on cell migration, in terms of directionality and speed. Afterwards, we will introduce and compare existing methods for fabrication of hydrogels with these gradients. The feasibility of using hydrogels with gradients for stem cell sorting will then be deliberated through analysing the existing research on cell migration over gradient ECM. As cellular migration is common behaviour to most of the cells, existing examples of migration-based cell sorting regarding both MSCs and other cell types will be discussed. Finally, the research gap between cell migration and cell sorting will be filled by proposing the design of hydrogel scaffolds with gradients for accurate label-free stem cell sorting.
Effect of ECM factors on cell migration
Cell migration through 3D ECM is essential for many physiological and pathological processes, including tissue morphogenesis, tissue repair and regeneration, immune responses and wound healing [5], [13]. All these processes are crucial for satisfactory therapeutic efficacy of stem cell therapy. Such a kind of migration can be considered as a directional movement of a single cell or group of collective cells in response to external signals [30]. A comprehensive understanding of these processes will help design the cell sorting strategies better.
When cells migrate, they must generate traction forces against the substrates, which involves numerous procedures. Briefly, it can be described by three steps: directional sensing, polarization, and movement [46], [55]. Cells spread broad lamellipodia or spike-like filopodia that contact with the adhesion sites (fibronectin, collagen, laminin, vitronectin and fibrin) of the ECM via an ECM–integrin–cytoskeleton linkage to gain a strong enough traction force [48]. Therefore, such a process is mediated by integrins together with other adhesion receptors through interactions between the cytoskeleton and ECM [14]. These receptors have the affinity to the molecules such as fibronectin, collagen, laminin, vitronectin and fibrin in ECM the [22], [69]. Concurrently, the ECM provides adhesion sites supporting the cells to exert forces against biophysical barriers to migrate. Throughout this process, the ECM plays an important role by its biophysical and biochemical signals.
ECM acts as biophysical cues to determine cell migration directions .The rigidity of the ECM acts as biophysical cues to determine cell migration directions. Lo and his group first described the migration of healthy 3T3 fibroblasts controlled by gradients of substrate rigidity [30]. 3T3 fibroblasts showed preference of a stiffer substrate over the soft part, which was named as “durotaxis” in their study. The directional movement of cells to a stiffer site is mediated through the focal adhesion kinase (FAK)/phosphopaxillin/vinculin signalling pathway [42]. These results suggest that the biophysical property, particularly stiffness, of the ECM can determine the direction of cell migration.
For biochemical factors, cells sense a concentration gradient of a specific chemical and migrate to the high-concentration end along the gradient, which is known as chemotaxis. For example, leukocytes, including monocytes, lymphocytes, eosinophils, and neutrophils, can be attracted by formyl peptides to aggregate, and this can result in an immune response towards an infection site [38], [73]. The chemokines (e.g., formyl peptides) are homoeostatic or inflammatory and implicated in the cell migration process associated with tissue development, maintenance, and repair [31], [24]. Both inflammatory and homoeostatic chemokines regulate the mobility of cells by interacting with G protein–linked transmembrane receptors called chemokine receptors. As the ECM serves as a container or medium to store or diffuse chemokines, the biochemical gradients inside will induce directional migration of the target cells.
Design and fabrication of hydrogels with biophysical and biochemical gradients
In the literature, gradients are generally understood to be structures with a gradually spatio-temporal transition in at least one of their properties [15]. Hydrogels are intensively used as the artificial ECM in the investigation of cell–matrix interaction because of their remarkable biocompatibility and adjustability. A variety of methods have been developed to establish biophysical and biochemical gradients in hydrogel scaffolds as they are the two major gradients widely studied in the ECM.
Biophysical gradients
Biophysical gradients mainly refer to gradual changes in stiffness, stress/strain or porosity/pore size of hydrogels. Because of their influence on intracellular signalling and transfer of nutrients and metabolites, gradients in porosity/pore size are less studied in cell migration [64]. On the contrary, both stiffness and stress/strain gradients have been widely studied for their influence on cell migration [43], [56], [72]. Consequently, this review will focus on the biophysical gradients in stiffness and stress/strain.
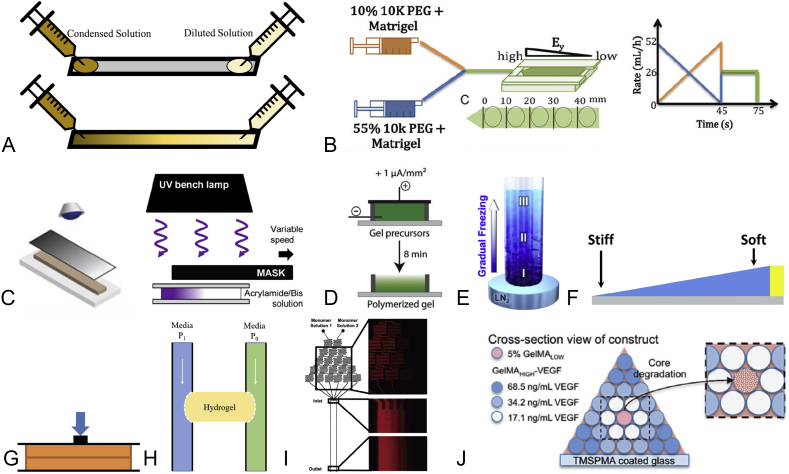
Stiffness is normally reflected as Young's modulus and can be achieved by a variety of methods. The simplest method of achieving is automatic diffusion. A gradient of crosslinker is formed by diffusing molecules in a condensed solution all the way down to the dilute area (Figure 1A). In the process of crosslinking, the site with more crosslinkers will become stiffer. Although a gradient as steep as 142.6 kPa/mm was created in this way, it took hours to complete [19]. A similar but faster approach is dynamic mixture, in which gradients could be generated in only 45s through mixing solutions of high and low concentration with dynamic speeds (Figure 1B). Besides, for photo-crosslinking hydrogels, covering the hydrogel precursor with either stationary or sliding greyscale masks has shown a capability in creating gradients effectively (Figure 1C). For example, a linear stiffness of 170 kPa/mm could be produced within 4 min by sliding a greyscale mask over the hydrogel precursor [57]. Presented in Figure 1D, applying an external current to the hydrogel whose gelation is pH dependent for 8 min leaded to the formation of stiffness gradients [53]. In the same manner, a temperature gradient throughout the thermosensitive hydrogel precursor during 30-min gelation resulted in a stiffness gradient, as shown in Figure 1E [37]. Apart from previous methods based on the concentration gradients of precursors or gradients in the crosslinking level, the structure of the hydrogel bulk can bring about stiffness gradients as well. In the recent work carried out by Shu et al [50], the stiffness gradient resulted from the thickness gradients in the structure of the hydrogel bulk, in which the stiffness decreased with thickness (Figure 1F).
Figure 1.
(A) Gradients induced by automatic diffusion [19]; (B) Gradients induced by dynamic mixture [34]; (C) Gradients induced by stationary greyscale mask [47]/sliding greyscale mask [57]; (D) Gradients controlled by current [53]; (E) Gradients controlled by temperature gradients [37]; (F) Gradients induced by shape [50]; (G) Gradients induced by compression-relaxation [11]; (H) Gradients induced by interstitial flow. P1≠P0 and the stress gradient was applied on the gel (yellow part) [43]. (I) Gradients controlled by microfluidics [6]; (J) Gradients built by 3D printing [7]. VGEF = vascular endothelial growth factor; PEG = polyethylene glycol; TMSPMA = 3-(Trimethoxysilyl)propyl methacrylate; GelMA = gelatin methacryloyl.
Different from stiffness gradients emphasise on the intrinsic properties of the hydrogel, stress/strain gradients rely on an external stimulus to generate an uneven distribution of stress/strain. Both radial and linear stress gradients can be established by compression-relaxation of the gel or introducing interstitial flow. The compression–relaxation constructs a radial stress gradient by compressing at the centre of the hydrogel (Figure 1G). The gradient was induced by a variation in the thickness resulted from the compression [11]. On the other hand, the interstitial flow going through the gel (yellow part in Figure 1H) will bring about the stress gradients. The gradient can be more precisely controlled by the interstitial flow from a microfluidic system [43]. As for the strain gradient, several methods have been proved to be practical, including direct stretching and vacuum sucking [63], [51].
Biochemical gradients
Growth factors and extracellular proteins are two molecules commonly used in biochemical gradient hydrogels. The biochemical gradient is usually characterized by the concentration of the embedded molecules in hydrogels. Similar to stiffness gradients, automatic diffusion is applicable for creating a desired pattern of concentration distribution [59]. Diffusion normally starts after the hydrogel has gelled. However, sustaining solution supply is necessary to prevent the formation of an equilibrium concentration because the molecules are not immobilised in this way [25]. Biochemical substances can also be immobilised in the gradients generated by dynamic diffusion, microfluidics or 3D printing. For dynamic mixture, molecules are first mixed with hydrogel precursors and are entrapped after hydrogel gelation, retaining the desired gradient pattern [20], [28]. Similar to biophysical gradients, dynamic mixture reduces the time cost for fabricating biochemical gradients significantly from hours to minutes compared with automatic diffusion. Similarly, a tree-shaped microfluidics system is also able to mix the target molecule solution into a desired concentration gradient (Figure 1I). The gradient is established right after the solution flows through the channel. The biochemical molecule can be either directly immobilised onto the substrate at the output end or conjugated with the hydrogel precursor [17], [29]. However, the concentration of the molecule increases outlet by outlet of the microfluidic device. As a result, gradients produced are more similar to an integration of different concentrated hydrogel strips rather than a scaffold of a continuous gradient. Apart from the aforementioned techniques, 3D printing can also construct a biochemical gradient by extruding different concentrations of the material for each layer as illustrated in Figure 1J [7].
Hydrogels with gradients for stem cell sorting
As mentioned previously, both biophysical and biochemical gradients of the ECM have an impact on cell migration, especially in terms of directionality and speed. Although how cells migrate under different patterns of gradients has been exhaustively studied, there are limited studies that apply such features to cell sorting. Published work about directional migration under either single or dual gradients has exhibited the possibility and applicability of such an idea. Theoretically, by seeding cells onto/into a hydrogel scaffold with certain gradients, directional migration of target cells will be initiated. While directional migration isolates a particular population of MSCs, targets cells with less stemness tend to lag behind the active MSCs, which leads to the failure of reaching the collection site. Consequently, the label-free cell sorting approach based on cell migration can be attained.
Single gradient
The single gradient can be broadly defined as having solely biophysical or biochemical gradients in the hydrogel scaffold. There are plenty of studies focussing on cell migration under single gradients (Table 1). In the field of biochemical gradients, various molecules such as growth factors and chemokines have been used to guide cell migration. Owing to cellular chemotaxis, cells are intended to move towards the side with higher biomolecule concentration. In a platform with epidermal growth factor (EGF) gradients, adipose-derived stem cells migrated to the high EGF concentration were sorted out as higher chemotaxis for EGFs [35]. In this study, the cells were primary cells isolated from rat subcutaneous adipose tissues. The selected stem cells with higher chemotaxis for EGFs are believed to have better stem cell homing to epidermal tissues that enhance the performance of cell therapy. For human bone marrow MSCs, they have chemotaxis towards a variety of tissue growth factors more than EGFs such as platelet-derived growth factor-AB, hepatocyte growth factor, vascular endothelial growth factor and insulin-like growth factor-1 [44]. Through altering the chemoattractant used, different subpopulations of stem cells can be isolated for regenerating the corresponding tissues. It should be noted that the threshold and saturation exist in chemotaxis, which means that only the gradient within a certain range is able to stimulate directional cell migration [67]. Although biochemical gradients allow high-selectivity isolation of subpopulation of active stem cells for different tissues, it is low in efficiency compared with the traditional label-based approach FACS. FACS takes typically 12–24 h whereas the directional migration of cells in biochemical gradients was reported to commence on the second day after cell seeding [59].
Table 1.
Migration behavioural analyses using gradients.
| Reference | Journal | Gradient pattern | Cell sources | Selectivity | Efficiency |
|---|---|---|---|---|---|
| Biochemical gradients | |||||
| [17] | Langmuir | Surface gradient of laminin at 10/15/18/34 pg/dm2 ·μm | Cell line of rat small intestine epithelial cell | Over 60% cells migrated towards the higher concentrated area. | Cells migrated at a velocity of 8–12 μm/h. |
| [65] | Science | Relative concentration of interstitial CCL21 from 1 to 0.4 over 100 μm | Mature dendritic cells derived from mice bone marrow | N.A. | Cells migrated at a velocity of 60–120 μm/h; The velocity reduced with the increase in chemokines. |
| [67] | Small | Linear gradient of CXCL12 at 44 ng/mL·mm | Cell line of neural stem cell | N.A. | Cells migrated at a velocity around 51 μm/h. |
| [35] | RSC Advances | Linear gradient of epidermal growth factor at 57 ng/mL·mm | Primary stem cell derived from adipose tissues | 83% cells migrated towards the biochemical gradients. | Significant cell migration to extraction target region after 24 h |
| [59] | Scientific Reports | Epidermal growth factor (0–50 ng/mL) | Cell line of breast cancer cells | N.A. | Cells migrated at a velocity around 9.6 μm/h. |
| Biophysical gradients | |||||
| [30] | Biophysical Journal | Stiffness gradient from 140 to 300 kdyn/cm2 | Cell line of fibroblasts | N.A. | Cell migration velocity increased from 26.4 to 32.4 μm/h with increase in stiffness. |
| [36] | Acta Biomaterialia | Linear stiffness gradient at 2 kPa/mm | Cell Line of Macrophages | Most of cells located to stiffer areas after 48 h. | |
| [43] | Proceedings of the National Academy of Sciences of the United States of America | Stress gradient caused by interstitial flow | Cell line of breast cancer cells | 47% of cells migrated against the flow (flow rate: 0.3 μm/s); 24% of cells migrated against the flow (flow rate: 3 μm/s). |
Cells migrated at a velocity around 6 μm/h. |
| [62] | Biotechnology Journal | Stiffness (physiological gradient of 1 Pa/μm; pathological gradient of 10 Pa/μm; and step gradient of 100 Pa/μm) | Cell line of mesenchymal stem cells | N.A. | Physiological gradients: 3.0 ± 0.7 μm/h; pathological gradients: 6.2 ± 0.6 μm/h; Step gradients: 18.0 ± 0.7 μm/h. |
| [19] | Proceedings of the National Academy of Sciences of the United States of America | Linear stiffness gradient at 72 kPa/mm | Cell line of vascular smooth muscle cells | N.A. | Cells migrated at a velocity around 14 μm/h. |
| Dual gradients | |||||
| [23] | ACS Biomaterials Science & Engineering | Step stiffness gradient from 46.7 kPa to 126.7 kPa; Step surface gradient of collagen from (1) 12.7 to 3.5 molecule/μm2 (2) 23.9 to 3.5 molecule/μm2 |
Cell line of fibroblasts | (1) 62% of cells migrated to soft but high collagen concentration areas; (2) 73% of cells migrated to soft but high collagen concentration areas. |
(1) Cells migrated at a velocity around 11.10 μm/h; (2) Cells migrated at a velocity around 8.54 μm/h. |
N.A.: The study was conducted on a single-cell level. Hence, the data for selectivity are not applicable.
Stiffness gradients sort cells in a more efficient manner as directional migration of cells was observed within the first 24 h [66], [19]. Cells can be sorted based on their tendency to migrate to the environment of optimal stiffness. Nevertheless, because the porosity of a scaffold reduces with the rise in stiffness, biocompatibility of using stiffness gradients solely becomes a problem for a highly stiff scaffold [21]. As a result, it may be difficult to perform further analysis such as screening on sorted cells inside the hydrogel. There is also some evidence showing that stress/strain gradients could guide cell migration. Breast cancer cells were observed to migrate against the stress gradient generated by interstitial flow when they were in a large group in high density [43]. However, such behaviour seems to be density related because an opposite result appeared when the cells were in low density. Thus, it may be difficult to sort the target cell line out when it is in low density. Another crucial drawback of biophysical gradients regarding cell sorting is the selectivity. Durotaxis is common to most kinds of cells. In the existing studies on cell migration under biophysical gradients, cell sources are mainly cell lines. Hence, there is a lack of evidence on proving the possibility and feasibility of cell sorting based on biophysical gradients only. Instead of directly from a heterogeneous population, this method could be more effective for isolating stem cells with high-stemness from a homogeneous population.
Taken together, neither biochemical nor biophysical gradient methods can purify stem cells effectively on their own. Although biochemical gradients may have high selectivity in isolating cells, they are time-consuming. Although biophysical gradients require less time, they do not have high selectivity because durotaxis is common to most kinds of cells. Therefore, the combination of biochemical and biophysical gradients as dual gradients could be a better solution.
Dual gradients
Dual gradients can be described as embedding both biophysical and biochemical gradients into one hydrogel scaffold at the same time. The reason for the combination of the two factors is that both of them play an essential role in modulating stem cell migration [21]. The gradient of chemoattractants is responsible for isolating a specific subpopulation of stem cells. On top of its selectivity, it is found to be more predominant in directing cell migration in a dual gradient system [18]. This is because chemotaxis is more predominant in directing cell migration as observed by Hale et al [18]. In that study, the direction of the biochemical (i.e., matrix protein) gradient was opposite to that of the biophysical gradient (i.e., stiffness). As the biochemical gradient increased, cells migrated to the site with high protein concentration, even though it was softer, following the biochemical gradient but opposing the biophysical gradient. In consequence, it is reasonable to sort out a certain cell type using the corresponding chemoattractants. As for the stiffness gradient, it is mainly for distinguishing cells with various motilities. The stiffness of the matrix is primarily regulating cell migration speed as it directly affects the spreading area of cells [39]. In the meantime, the migration speed of senescent stem cells in vitro is three times as slow as the normal stem cells [2]. The senescent stem cells are hence eliminated as they will lag behind the active stem cells in their migration on the scaffold with the stiffness gradient.
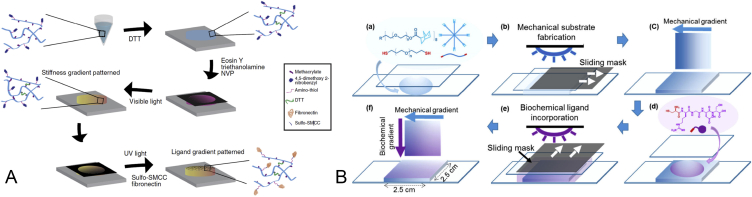
Several studies have claimed the success of creating dual gradients [23], [58], [45], [61]. To the best of our knowledge, most of the current studies on dual gradients used the photomask method to establish the biochemical and biophysical gradients. Because the two individual gradients are formed by light exposure, it is essential to prevent interference between the gradients. In the work carried out by Rape et al [45], two lights of distinct wavelength were used to stimulate the formation of two individual gradients (Figure 2A). Another solution proposed in the work by carried out by Tong et al [58] was to add the precursor of the second gradient after the gelation of the first gradient (Figure 2B). Although the present studies can generate a well-defined dual gradient hydrogel, the photo-crosslinking method limits its available materials. Especially, most of the biochemical molecules are not photosensitive. Such a method is further constrained as it requires the selection of crosslinkers with two distinctive initiating wavelengths to prevent interference.
Figure 2.
(A) Two Distinct stimulating light to generate dual gradients [45] (B) Overlaying the second gradient on the first gradient. SMCC = sulfo-sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate; DTT = dithiothreitol; NVP = N-vinylpyrrolidone.
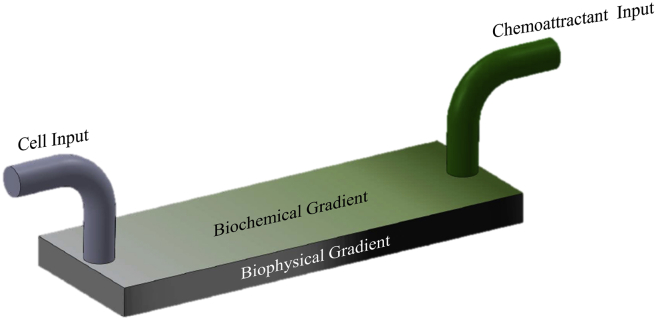
To address the aforementioned problems of making well-defined dual gradients, a combination of photomask and diffusion is suggested. A photomask is advanced in generating gradients in a customized pattern. In the meantime, automatic diffusion is the simplest method for the establishment of gradients applicable for almost all materials. The stiffness gradient can be created by a linear greyscale mask, whose greyscale decreases from one side to the other. Consequently, the hydrogel scaffolds will contain linear gradients of both biochemistry and biophysics. As shown in Figure 3, the chemoattractant for the target stem cells is pumping from one end of the hydrogel chip, establishing a biochemical gradient. For example, insulin-like growth factor-1 can be constructed for isolating active MSCs for orthopaedic surgery, which is a chemoattractant for MSCs and an essential hormone in bone growth. Only the target stem cells with the complementary receptor will migrate along the biochemical gradient. Because there is a stiffness gradient, senescent stem cells with less motility are likely to stop midway. As a result, only the active target cells are able to migrate across the stiffness gradients, reaching the end with the highest concentration of the chemoattractant. Because the cells are isolated by their own migration, it is reasonable to believe that these cells are still active and suitable for follow-up applications. Similar to what has been discussed by Natarajan et al., this kind of cells selected by migration would have a better therapeutic efficacy [35]. Such a design is believed to be capable of sorting out the target cells while eliminating the senescent subjects.
Figure 3.
Design of the dual gradients hydrogel scaffold.
Compared with other existing methods, the proposed method has a better performance in selectivity, cost and efficiency. Taking advantages of intrinsic chemotaxis, the proposed method eliminates the labour-intensive process of labelling multiple biomarkers required in FACS. Because cells are passively sorted by the machine after labelling in FACS, dead but labelled cells can be mistaken as target cells. In addition, the adhesion test is still required after FACS on stem cells. In contrast to that, the proposed approach relies on the active and directional migration of cells on a hydrogel scaffold, and it owns better selectivity over FACS and waives the need for the adhesion test. Moreover, the cost is significantly reduced as only a constant supply of chemoattractants is needed to operate this device, whereas a bulky machine and numbers of antibodies are needed for FACS. According to the published work, the hydrogel scaffold with dual gradients could be fabricated within an hour. Meanwhile, the time for cell migration is expected to be around 10–24 h because the cell migration velocity is around tens of micrometre per hour. With the improvement in selectivity and similar operation time, the overall efficiency is increased. As for label-free methods based on biophysical properties of cells such as size, the precise selection of chemoattractant for this dual gradient method is able to isolate sub-population cells with higher selectivity. In short, the novel mechanism of the proposed method opens up a cost-effective and accurate isolation approach for MSCs to improve the therapeutic efficacy of cytotherapy.
Conclusion
Isolating target stem cells from a heterogeneous mixture is a vital step in stem cell therapy. As the biochemical and biophysical gradients from the ECM have an effect on cell migration in terms of directionality and speed, they can be applied for label-free cell sorting. With the development of gradients fabrication, biochemical and biophysical gradient of different patterns can be created solely or dually. Therefore, hydrogels with gradients in the ECM provide a promising strategy for effective cell sorting. A well-defined dual gradient hydrogel chip including stiffness and biochemical molecules is believed to sort out desired and active population of cells without labelling. Such design is potential to be applied to sort out active MSCs and contributes to the improvement of their application in therapy.
Conflicts of interest
None.
Acknowledgements
The authors would like to thank Y. Y. Kwan for the assistance in language editing. This research was supported by Research Grants Council Early Career Scheme by University Grants Committee (PolyU 251008/18M).
References
- 1.Barry F., Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9:584–594. doi: 10.1038/nrrheum.2013.109. [DOI] [PubMed] [Google Scholar]
- 2.Bertolo A., Gemperli A., Gruber M., Gantenbein B., Baur M., Pötzel T., Stoyanov J. In vitro cell motility as a potential mesenchymal stem cell marker for multipotency. Stem cell Transl Med. 2015;4:84–90. doi: 10.5966/sctm.2014-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhagat A.A.S., Bow H., Hou H.W., Tan S.J., Han J., Lim C.T. Microfluidics for cell separation. Med Biol Eng Comput. 2010;48:999–1014. doi: 10.1007/s11517-010-0611-4. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P., Cao X., Frenette P.S., Mao J.J., Robey P.G., Simmons P.J., Wang C.-Y. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks P.C., Strömblad S., Sanders L.C., Von schalscha T.L., Aimes R.T., Stetler-Stevenson W.G., Quigley J.P., Cheresh D.A. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 6.Burdick J.A., Khademhosseini A., Langer R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir. 2004;20:5153–5156. doi: 10.1021/la049298n. [DOI] [PubMed] [Google Scholar]
- 7.Byambaa B., Annabi N., Yue K., Trujillo-DE Santiago G., Alvarez M.M., Jia W.T., Kazemzadeh-Narbat M., Shin S.R., Tamayol A., Khademhosseini A. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 9.Carlos Sepúlveda J., Tomé M., Eugenia Fernández M., Delgado M., Campisi J., Bernad A., González M.A. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem cells. 2014;32:1865–1877. doi: 10.1002/stem.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choumerianou D.M., Martimianaki G., Stiakaki E., Kalmanti L., Kalmanti M., Dimitriou H. Comparative study of stemness characteristics of mesenchymal cells from bone marrow of children and adults. Cytotherapy. 2010;12:881–887. doi: 10.3109/14653249.2010.501790. [DOI] [PubMed] [Google Scholar]
- 11.Dolan G.K., Yakubov G.E., Bonilla M.R., Lopez-Sanchezb P., Stokes J.R. Friction, lubrication, and in situ mechanics of poroelastic cellulose hydrogels. Soft Matter. 2017;13:3592–3601. doi: 10.1039/c6sm02709a. [DOI] [PubMed] [Google Scholar]
- 12.Elbuluk A., Einhorn T.A., Iorio R. A comprehensive review of stem-cell therapy. JBJS Rev. 2017;5 doi: 10.2106/JBJS.RVW.17.00002. [DOI] [PubMed] [Google Scholar]
- 13.Friedl P., Hegerfeldt Y., Tusch M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol. 2009;10:445. doi: 10.1387/ijdb.041821pf. [DOI] [PubMed] [Google Scholar]
- 14.Geiger B., Yamada K.M. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol. 2011;3:a005033. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genzer J., Bhat R.R. Surface-bound soft matter gradients. Langmuir. 2008;24:2294–2317. doi: 10.1021/la7033164. [DOI] [PubMed] [Google Scholar]
- 16.Gronthos S., Franklin D.M., Leddy H.A., Robey P.G., Storms R.W., Gimble J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 17.Gunawan R.C., Silvestre J., Gaskins H.R., Kenis P.J., Leckband D.E. Cell migration and polarity on microfabricated gradients of extracellular matrix proteins. Langmuir. 2006;22:4250–4258. doi: 10.1021/la0531493. [DOI] [PubMed] [Google Scholar]
- 18.Hale N., Yang Y., Rajagopalan P. Cell migration at the interface of a dual chemical-mechanical gradient. ACS Appl Mater Interfaces. 2010;2:2317–2324. doi: 10.1021/am100346k. [DOI] [PubMed] [Google Scholar]
- 19.Hartman C.D., Isenberg B.C., Chua S.G., WONG J.Y. Vascular smooth muscle cell durotaxis depends on extracellular matrix composition. Proc Natl Acad Sci U S A. 2016;113:11190–11195. doi: 10.1073/pnas.1611324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill M.C., Nguyen M.K., Jeon O., Alsberg E. Spatial control of cell gene expression by siRNA gradients in biodegradable hydrogels. Adv Healthc Mater. 2015;4:714–722. doi: 10.1002/adhm.201400458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang N.F., Li S. Regulation of the matrix microenvironment for stem cell engineering and regenerative medicine. Ann Biomed Eng. 2011;39:1201–1214. doi: 10.1007/s10439-011-0297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hynes R.O., Naba A. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain G., Ford A.J., Rajagopalan P. Opposing rigidity-protein gradients reverse fibroblast durotaxis. ACS Biomater Sci Eng. 2015;1:621–631. doi: 10.1021/acsbiomaterials.5b00229. [DOI] [PubMed] [Google Scholar]
- 24.Le Y., Zhou Y., Iribarren P., Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- 25.Lee K.H., Lee K.H., Lee J., Choi H., Lee D., Park Y., Lee S.H. Integration of microfluidic chip with biomimetic hydrogel for 3D controlling and monitoring of cell alignment and migration. J Biomed Mater Res. 2014;102:1164–1172. doi: 10.1002/jbm.a.34772. [DOI] [PubMed] [Google Scholar]
- 26.Lee L.M., Rosano J.M., Wang Y., Klarmann G., Garson C.J., Prabhakarpandian B., Pant K., Alvarez L.M., LAI E. Label-free mesenchymal stem cells enrichment from bone marrow samples by inertial microfluidics. Analyt Methods. 2018;10(7) [Google Scholar]
- 27.Lee W.C., Shi H., Poon Z., Nyan L.M., Kaushik T., Shivashankar G., Chan J.K., Lim C.T., Han J., Van Vliet K.J. Multivariate biophysical markers predictive of mesenchymal stromal cell multipotency. Proc Natl Acad Sci Unit States Am. 2014;111:E4409–E4418. doi: 10.1073/pnas.1402306111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim H.J., Mosley M.C., Kurosu Y., Smith Callahan L.A. Concentration dependent survival and neural differentiation of murine embryonic stem cells cultured on polyethylene glycol dimethacrylate hydrogels possessing a continuous concentration gradient of n-cadherin derived peptide His-Ala-Val-Asp-Lle. Acta Biomater. 2017;56:153–160. doi: 10.1016/j.actbio.2016.11.063. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z.B., Xiao L.D., Xu B.J., Zhang Y., Mak A.F.T., Li Y., Man W.Y., Yang M. Covalently immobilized biomolecule gradient on hydrogel surface using a gradient generating microfluidic device for a quantitative mesenchymal stem cell study. Biomicrofluidics. 2012;6 doi: 10.1063/1.4704522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo C.M., Wang H.B., Dembo M., Wang Y.L. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luther S.A., Bidgol A., Hargreaves D.C., Schmidt A., Xu Y., Paniyadi J., Matloubian M., Cyster J.G. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 32.Mcintyre J.A., Jones I.A., Han B., Vangsness C.T., JR. Intra-articular mesenchymal stem cell therapy for the human joint: a systematic review. Am J Sports Med. 2017 doi: 10.1177/0363546517735844. 0363546517735844. [DOI] [PubMed] [Google Scholar]
- 33.Mcleod C., Mauck R. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cells Mater. 2017;34:217–231. doi: 10.22203/eCM.v034a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosley M.C., Lim H.J., Chen J., Yang Y.H., Li S., Liu Y., Smith callahan L.A. Neurite extension and neuronal differentiation of human induced pluripotent stem cell derived neural stem cells on polyethylene glycol hydrogels containing a continuous Young's Modulus gradient. J Biomed Mater Res A. 2017;105:824–833. doi: 10.1002/jbm.a.35955. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan K., Tian C., Xiang B., Chi C., Deng J.X., Zhang R.D., Freed D.H., Arora R.C., Tian G.H., Lin F. Selection of chemotactic adipose-derived stem cells using a microfluidic gradient generator. RSC Adv. 2015;5:6332–6339. [Google Scholar]
- 36.Nemir S., Hayenga H.N., West J.L. PEGDA hydrogels with patterned elasticity: novel tools for the study of cell response to substrate rigidity. Biotechnol Bioeng. 2010;105:636–644. doi: 10.1002/bit.22574. [DOI] [PubMed] [Google Scholar]
- 37.Oh S.H., An D.B., Kim T.H., Lee J.H. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016;35:23–31. doi: 10.1016/j.actbio.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Ono S.J., Nakamura T., Miyazaki D., Ohbayashi M., Dawson M., Toda M. Chemokines: roles in leukocyte development, trafficking, and effector function. J Allergy Clin Immunol. 2003;111:1185–1199. doi: 10.1067/mai.2003.1594. [DOI] [PubMed] [Google Scholar]
- 39.Pelham R.J., Wang Y.-L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci Unit States Am. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillip J.M., Wu P.-H., Gilkes D.M., Williams W., Mcgovern S., Daya J., Chen J., Aifuwa I., Lee J.S., Fan R. Biophysical and biomolecular determination of cellular age in humans. Nat Biomed Eng. 2017;1:0093. doi: 10.1038/s41551-017-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phinney D.G. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle. 2007;6:2884–2889. doi: 10.4161/cc.6.23.5095. [DOI] [PubMed] [Google Scholar]
- 42.Plotnikov S.V., Pasapera A.M., Sabass B., Waterman C.M. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell. 2012;151:1513–1527. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polacheck W.J., Charest J.L., Kamm R.D. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc Natl Acad Sci USA. 2011;108:11115–11120. doi: 10.1073/pnas.1103581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponte A.L., Marais E., Gallay N., Langonné A., Delorme B., Hérault O., Charbord P., Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 45.Rape A.D., Zibinsky M., Murthy N., Kumar S. A synthetic hydrogel for the high-throughput study of cell-ECM interactions. Nat Commun. 2015;6:8129. doi: 10.1038/ncomms9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rappel W.J., Loomis W.F. Eukaryotic chemotaxis. Wiley Interdiscipl Rev Syst Biol Med. 2009;1:141–149. doi: 10.1002/wsbm.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sant S., Hancock M.J., Donnelly J.P., Iyer D., Khademhosseini A. Biomimetic gradient hydrogels for tissue engineering. Can J Chem Eng. 2010;88:899–911. doi: 10.1002/cjce.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheetz M.P., Felsenfeld D.P., Galbraith C.G. Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998;8:51–54. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 49.Shields C.W., Reyes C.D., Lopez G.P. Microfluidic cell sorting: a review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip. 2015;15:1230–1249. doi: 10.1039/c4lc01246a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shu Y.W., Chan H.N., Guan D.S., Wu H.K., Ma L. A simple fabricated thickness-based stiffness gradient for cell studies. Sci Bull. 2017;62:222–228. doi: 10.1016/j.scib.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Simmons C.S., Sim J.Y., Baechtold P., Gonzalez A., Chung C., Borghi N., Pruitt B.L. Integrated strain array for cellular mechanobiology studies. J Micromech Microeng. 2011;21 doi: 10.1088/0960-1317/21/5/054016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmons P.J., Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 53.Simona B.R., Hirt L., Demko L., Zambelli T., Voros J., Ehrbar M., Milleret V. Density gradients at hydrogel interfaces for enhanced cell penetration. Biomater Sci. 2015;3:586–591. doi: 10.1039/c4bm00416g. [DOI] [PubMed] [Google Scholar]
- 54.Squillaro T., Peluso G., Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 55.Stefanoni F., Ventre M., Mollica F., Netti P.A. A numerical model for durotaxis. J Theor Biol. 2011;280:150–158. doi: 10.1016/j.jtbi.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Sun X.M., Lang Q., Zhang H.B., Cheng L.Y., Zhang Y., Pan G.Q., Zhao X., Yang H.L., Zhang Y.G., Santos H.A., Cui W.G. Electrospun photocrosslinkable hydrogel fibrous scaffolds for rapid in vivo vascularized skin flap regeneration. Adv Funct Mater. 2017;27 [Google Scholar]
- 57.Sunyer R., Jin A.J., Nossal R., Sackett D.L. Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. PLoS One. 2012;7:e46107. doi: 10.1371/journal.pone.0046107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tong X.M., Jiang J., Zhu D.Q., Yang F. Hydrogels with dual gradients of mechanical and biochemical cues for deciphering cell-niche interactions. ACS Biomater Sci Eng. 2016;2:845–852. doi: 10.1021/acsbiomaterials.6b00074. [DOI] [PubMed] [Google Scholar]
- 59.Truong D., Puleo J., Llave A., Mouneimne G., Kamm R.D., Nikkhah M. Breast cancer cell invasion into a three dimensional tumor-stroma microenvironment. Sci Rep. 2016;6:34094. doi: 10.1038/srep34094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turinetto V., Vitale E., Giachino C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci. 2016;17:1164. doi: 10.3390/ijms17071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vega S.L., Kwon M.Y., Song K.H., Wang C., Mauck R.L., Han L., Burdick J.A. Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments. Nat Commun. 2018;9:614. doi: 10.1038/s41467-018-03021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vincent L.G., Choi Y.S., Alonso-Latorre B., Del Alamo J.C., Engler A.J. Mesenchymal stem cell durotaxis depends on substrate stiffness gradient strength. Biotechnol J. 2013;8 doi: 10.1002/biot.201200205. 472-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L., Li Y.H., Chen B., Liu S.B., Li M.X., Zheng L., Wang P.F., Lu T.J., Xu F. Patterning cellular alignment through stretching hydrogels with programmable strain gradients. ACS Appl Mater Interfaces. 2015;7:15088–15097. doi: 10.1021/acsami.5b04450. [DOI] [PubMed] [Google Scholar]
- 64.Wang L., Li Y.H., Huang G.Y., Zhang X.H., Pingguan-Murphy B., Gao B., Lu T.J., Xu F. Hydrogel-based methods for engineering cellular microenvironment with spatiotemporal gradients. Crit Rev Biotechnol. 2016;36:553–565. doi: 10.3109/07388551.2014.993588. [DOI] [PubMed] [Google Scholar]
- 65.Weber M., Hauschild R., Schwarz J., Moussion C., De vries I., Legler D.F., Luther S.A., Bollenbach T., Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 66.Wong J.Y., Velasco A., Rajagopalan P., Pham Q. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir. 2003;19:1908–1913. [Google Scholar]
- 67.Xu H., Heilshorn S.C. Microfluidic investigation of BDNF-enhanced neural stem cell chemotaxis in CXCL12 gradients. Small. 2013;9:585–595. doi: 10.1002/smll.201202208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin L., Wu Y., Yang Z., Tee C.A., Denslin V., Lai Z., Lim C.T., Lee E.H., Han J. Microfluidic label-free selection of mesenchymal stem cell subpopulation during culture expansion extends the chondrogenic potential in vitro. Lab Chip. 2018;18:878–889. doi: 10.1039/c7lc01005b. [DOI] [PubMed] [Google Scholar]
- 69.Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014:S20. doi: 10.1097/IJG.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao X., Lang Q., Yildirimer L., Lin Z.Y., Cui W.G., Annabi N., Ng K.W., Dokmeci M.R., Ghaemmaghami A.M., Khademhosseini A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv Healthc Mater. 2016;5:108–118. doi: 10.1002/adhm.201500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao X., Liu S., Yildirimer L., Zhao H., Ding R., Wang H., Cui W., Weitz D. Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv Funct Mater. 2016;26:2809–2819. [Google Scholar]
- 72.Zhao X., Sun X.M., Yildirimer L., Lang Q., Lin Z.Y., Zheng R.L., Zhang Y.G., Cui W.G., Annabi N., Khademhosseini A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017;49:66–77. doi: 10.1016/j.actbio.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zigmond S.H., Hirsch J.G. Leukocyte locomotion and chemotaxis. J Exp Med. 1973;137:387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]