Summary
Small cell neuroendocrine (SCN) cancers (SCNCs) are an aggressive cancer subtype. Transdifferentiation towards a SCN phenotype has been reported as a resistance route in response to targeted therapies. Here, we identified a convergence to a SCN state that is widespread across epithelial cancers and is associated with poor prognosis. More broadly, non-SCN metastases have higher expression of SCN-associated transcription factors than non-SCN primary tumors. Drug sensitivity and gene dependency screens demonstrate that these convergent SCNCs have shared vulnerabilities. These common vulnerabilities are found across unannotated SCN-like epithelial cases, small round blue cell tumors, and unexpectedly in hematological malignancies. The SCN convergent phenotype and common sensitivity profiles with hematological cancers can guide treatment options beyond tissue-specific targeted therapies.
Keywords: small cell neuroendocrine, pan-cancer signatures, drug sensitivity screen, RNA interference screen, hematological malignancies, pharmacogenomics
Graphical Abstract:

Balanis et al. identify a small cell neuroendocrine (SCN) state across various epithelial cancer types that shares genome-wide expression, methylation, and copy number alteration patterns and associates with poor prognosis. SCN cancers have common vulnerabilities that, unexpectedly, are shared with blood cancers.
Introduction
Small cell neuroendocrine cancers (SCNCs) are highly aggressive and arise in multiple tissues, commonly reported in lung and therapy-refractive prostate cancers. SCNCs across tissues share morphology and marker-based histology such as high nuclear to cytoplasm ratios, frequent mitotic figures, and granular chromatin (Klimstra et al., 2015). At the molecular level, TP53 and RB1 loss and/or inactivating mutations are essentially obligatory for SCNCs of the lung and highly enriched in SCNCs of the prostate (Beltran et al., 2016; George et al., 2015). Additionally, SCNCs express common neuroendocrine markers such as chromogranin A (CHGA) and synaptophysin (SYP) (Oberg et al., 2015).
The cell of origin of SCNCs across tissues is unclear. One proposed mechanism of origin is through transdifferentiation from a non-neuroendocrine cell lineage, which has been observed in patient tumors and in experimental models (Mu et al., 2017; Niederst et al., 2015; Yang et al., 2018). Transdifferentiation into SCLC can be a mechanism for treatment resistance to EGFR kinase inhibitors for lung adenocarcinomas (LUAD) (Lee et al., 2017; Marcoux et al., 2018; Niederst et al., 2015). De novo neuroendocrine prostate cancers (NEPC) are rare (approximately 1% of cases). As in lung, transdifferentiation from a prostate adenocarcinoma (PRAD) to an SCN state, in response to androgen suppression therapy, has been reported (Beltran et al., 2016), with 25–40% of resulting resistant tumors expressing neuroendocrine markers (Alanee et al., 2015; Watson et al., 2015). Thus, across multiple tissues types, adenocarcinomas have been observed to escape targeted therapy through evolution to a SCN-like phenotype, highlighting the need to characterize and develop therapies for this aggressive cancer outcome.
There are currently no effective therapies for SCNCs, and SCNC typically leads to early and widespread metastases (Wong et al., 2009). Etoposide- and platinum-based chemotherapies are the primary first-line treatment modalities (Klimstra et al., 2015; Nadal et al., 2014) but they are only transiently effective, 5-year survival rates of patient with SCN lung or prostate cancers are less than 20% (Alanee et al., 2015). Here we sought to provide a molecular and functional underpinning for the observed pathology-based similarities among SCNCs arising in multiple tissue types.
Results
SCNCs across tissues converge on a common molecular phenotype
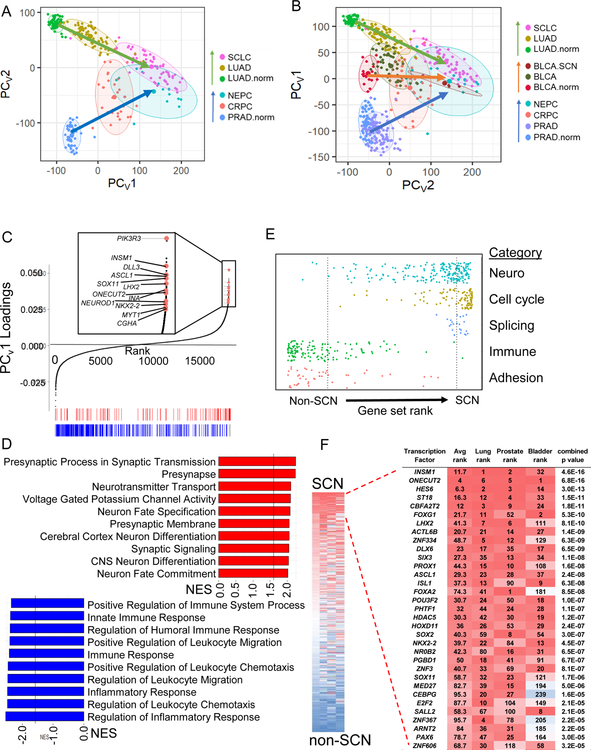
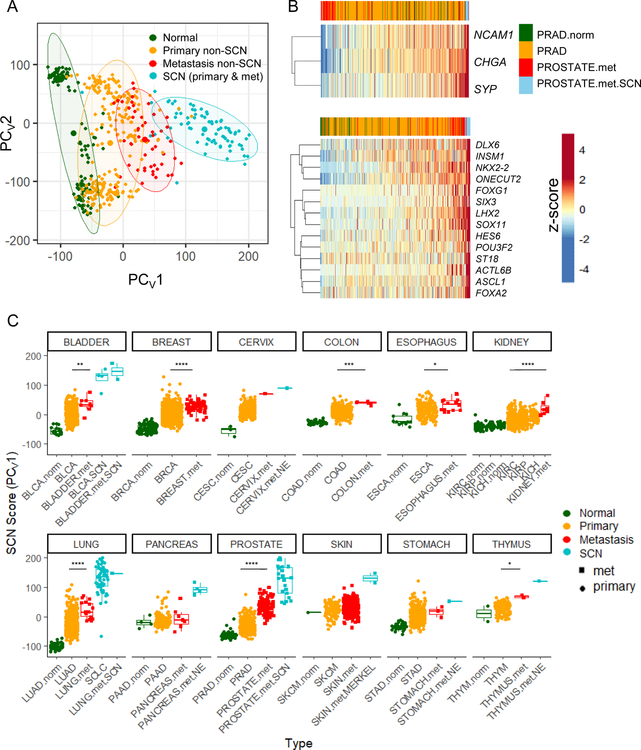
The advent of global molecular profiling of SCNCs (Beltran et al., 2016; George et al., 2015; Robertson et al., 2018) allows a molecular understanding of pan-tissue SCNC similarities. An unsupervised principal component analysis (PCA) performed on RNA-seq data of tumor biopsies of LUAD, castration-resistant prostate adenocarcinoma (CRPC-adeno), SCLC, and castration-resistant neuroendocrine prostate cancer (NEPC), along with normal lung and prostate tissues, revealed a strongly convergent expression signature (Figure 1A, Table S1), as previously reported in comparisons of normal tissue and SCN tumors (Park et al., 2018). We further find that as tumor cell states progress along a transdifferentiation trajectory from adenocarcinoma to SCNC (arrows), the tumors become increasingly independent of tissue of origin, with SCNCs of different tissues more similar to each other than adenocarcinomas of different tissues.
Figure 1. Pan-cancer convergence of SCN carcinoma.
(A) Varimax-rotated PCA (PCAv) of adjacent normal (norm), adenocarcinoma, and SCNC for lung and prostate patient tumors. Ellipses represent 80% confidence regions. (B) PCAv of samples in (A) and bladder patient tumor data. TCGA BLCA includes 4 SCN samples that were labeled separately (BLCA.SCN). PC1 and PC2 are reversed to show the SCN signature on the x-axis as in panel A. (C) Gene loadings and selected top SCN-related genes of the varimax-rotated first principal component of the PCA (PCV1) of panel A. Enrichment analysis (GSEA) was run on this ranked gene list. Shown along the bottom are the top gene sets in the ‘neuro’ (red) and ‘immune’ (blue)categories, also shown in panel D. (D) Top 10 gene sets from ‘neuro’ and ‘immune’ gene setcategories. Lines mark false discovery rate (FDR) q value of 0.05. (E) Distribution of gene sets from the C5 MSigDB collection ranked by normalized enrichment score (NES). All listed categories enrichments are nominally significant (p value < 0.001) by Kolmogorov-Smirnov test. Dashed lines mark FDR q-value < 0.05 for individual gene sets in each direction. (F) Average rank ordering of VIPER activities across the three tissue types (left) and zoom in to top of rank based inferred activity in each cancer separately (right). Combined p value across the three tissues by Stouffer’s method. See also Figure S1 and Table S1
When included in the PCA analysis, four samples of SCN bladder cancer (BLCA) in the TCGA (Robertson et al., 2018) also support convergence of SCN cases, with the result reflecting a developmental landscape for the three tissues (Figure 1B). Hierarchical clustering supported that SCNCs of these three tissues were more similar to each other than to their non-SCNC counterparts (Figure S1A). Genes strongly contributing to the SCN signature include SCN-associated genes such as CHGA and INSM1, and genes related to neural transcriptional programs such as ASCL1, NEUROD1, SEZ6, INA, and NKX2–2 (Figure 1C, Table S1). It is important to note that any one cancer incidence may contain only a subset of these markers (Figure S1B) and hence be missed by traditional classification schemes based on only a few markers (Oberg et al., 2015).
Enrichment analysis revealed the functional categories of SCN signature-associated genes. Enriched on the SCN side were gene sets related to neuron development and function, to splicing, and to cell cycle whereas de-enriched gene sets included those related to adhesion and to immune response and inflammation (hereafter referred to as immune gene sets) (Figure 1D–1E). To determine the contribution of proliferation genes on convergence, we removed proliferation and proliferation-associated genes, and found that the convergence of SCNCs across tissues was maintained (Figure S1C). Enrichment analysis on ranked genes from the proliferation gene-removed PCA confirmed the loss of enrichment of cell cycle gene sets, and the maintenance of enrichment of neuronal gene sets (Figure S1D). Gene expression-based PCA defined on LUAD and SCLC cell lines in the Cancer Cell Line Encyclopedia (CCLE) faithfully predicted SCN cases of lung tumors (Figure S1E), but not other transitions such as melanoma dedifferentiation (Tsoi et al., 2018) (Figure S1F), supporting cell lines as an informative model for the interrogation of the SCN and non-SCN dichotomy. Interestingly, immune gene sets were also de-enriched in SCLC cell lines, indicating that the reduction of canonical immune and inflammation mediators in the SCN state is in part cancer cell-intrinsic (Figure S1G).
Inference of transcription factor activity from a data-guided transcription network across lung, prostate, and bladder SCN and non-SCN tumor datasets revealed a strong similarity across SCNCs in these tissues (Figure 1F). The transcription factor network identified included multiple factors central to neural development and brain patterning, such as LHX2, HES6, PROX1, PAX6, MYT1, and NKX2–2 (Table S1).
The shared SCN gene expression signature has an epigenomic basis
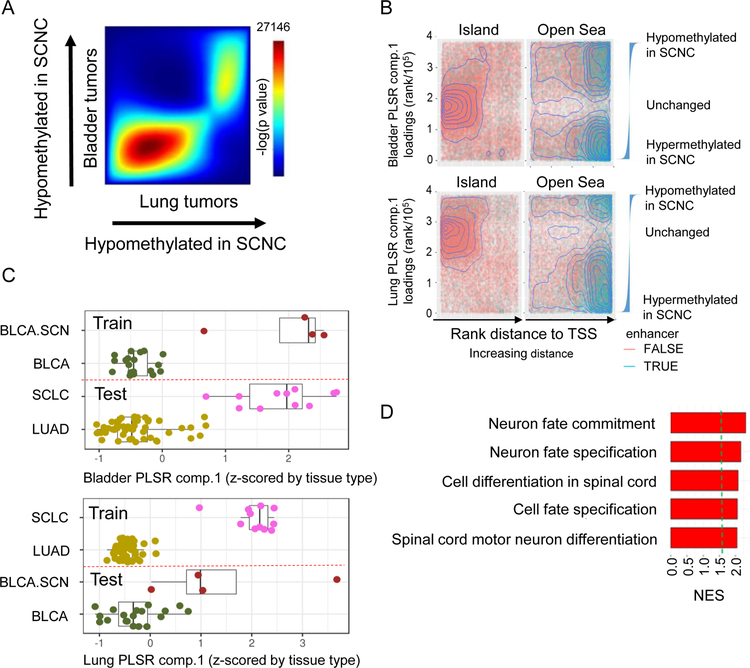
Signature overlap analysis using rank-rank hypergeometric overlap (RRHO) on Partial Least Squares Regression (PLSR) loadings-based signatures, created individually from either lung or bladder cancers, showed that the DNA methylation signatures of the SCN transition were highly similar (Figure 2A) (Plaisier et al., 2010). To characterize the methylation sites distinguishing SCNCs from their adenocarcinoma counterparts, we plotted methylation sites by their rank in the PLSR loadings. This analysis revealed that sites within open-sea regions (transcriptional start site (TSS) distal), rather than CG island regions (TSS proximal), are important in contribution to the SCN to non-SCN distinction (1-sided KS-test p value < 2.2 × 10 −16) (Figure 2B). Distinct from the canonical role of DNA methylation in regulating transcription at gene promoters, these distal methylation changes that delineate the epigenetic differences between SCNCs and non-SCNCs are consistent with enhancer-based regulation of gene expression programs (Sur and Taipale, 2016).
Figure 2. An epigenetic basis for the shared SCN gene expression signature.
(A) RRHO heat map of lung and bladder SCN signatures defined by PLSR loadings. Number shown is the maximum -log10(p value) of the RRHO heatmap. (B) Enrichment analysis of methylation sites that define the lung or bladder SCN versus non-SCN dichotomy (random, representative sample of 100K sites shown for visibility). X-axis is the increasing (arrows) rank distance of the CG site from the nearest TSS. Y-axis is the rank value of individually-run lung or bladder PLSR component 1 loadings, thus extreme values represent sites with differential methylation between SCN and non-SCN tumors. Waterfall plots show the relative values of the ranked loadings. (C) PLSR analysis of DNA methylation data from patient bladder SCN and non-SCN tumor samples, and projection of lung LUAD and SCLC tumor samples onto this framework, or vice versa. PLSR component 1 is z-scored by tissue type. Dashed red lines separate training data (above line) from testing data (below line). Lines inside boxplots represent the 25th, 50th, and 75th quantiles. Whiskers extend to 1.5 the interquartile range. (D) Top 5 enrichment analysis (GSEA) terms for genes hypomethylated in SCN (averaged lung, prostate, and bladder PLSR component 1 loadings). Dashed green line is at FDR p value = 0.05. See also Figure S2.
Methylation sites distinguishing SCN from non-SCN tumors in one tissue could separate the same groupings in another tissue. When we projected lung cancer samples to a PLSR methylation signature based on the bladder dichotomy, we observed that these sites on average distinguished SCLC from LUAD (Figure 2C). Training on lung samples likewise predicted bladder samples, further highlighting the concordance of non-SCN versus SCN methylation profiles across tissues (Figure 2C). Projection of gene-summarized methylation values of lung tumor samples onto a PLSR analysis of lung cell lines confirmed concordance of methylation patterns between tumors and cell lines (Figure S2A). Pairwise RRHO analyses supported the methylation-based concordance of SCN tumors from lung, bladder, and prostate tissues (Figure S2B). Gene-based summarization of methylation sites across the three tissues revealed enrichment of neuronal development gene sets (Figure 2D), supporting that the pan-tissue convergent similarity in SCNCs is functionally maintained across their methylomes.
Selection for SCN-associated DNA copy number alteration signatures across tissue types
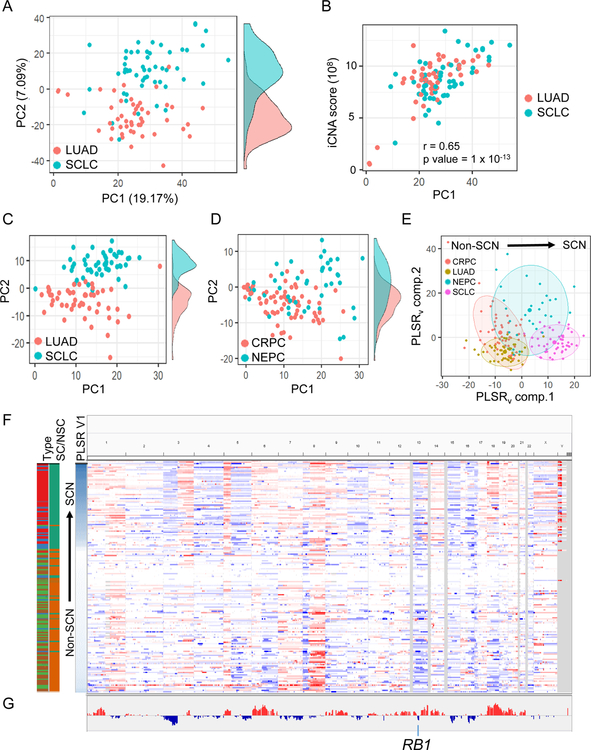
Recurrent genomic scars in cancer are reflective of selective forces that confer fitness advantages through copy number alterations (CNA) in distinct genomic regions (Graham et al., 2017). PCA of CNA patterns in SCLC and LUAD cell lines showed SCN-specific differences in amplification and deletion patterns along the second principal component (Figure 3A, Table S2), with the first principal component describing the overall degree of aneuploidy (Figure 3B). Projection of tumor samples onto this PCA framework demonstrated that CNA patterns in SCLC cell lines were reflected in both SCLC (Figure 3C) and NEPC (Figure 3D) patient tumors. Consistently amplified or deleted regions in SCN cases of the two tissue types, analyzed together (Figure 3E–3F) or independently (Figure 3G, Table S2), support that common selective forces act on SCN variants in both tissues. For example, SCNCs shared 1p amplification and 3p deletion (Figure 3F). As a positive control, we noted that RB1 was included in the consistent deletion region on chromosome 13 (Figure 3G). Furthermore, these specific CNA changes are seen in both de novo (lung) and treatment-induced transdifferentiation (prostate) SCN cases.
Figure 3. SCNCs of lung and prostate origin share DNA copy number alteration patterns.
(A) PCA on lung CCLE cell line CNA profiles. (Side tracks are density plots of points along PC2) (B) Cell line PC1 score reflects degree of aneuploidy (integrated CNA (iCNA) score). (C) Projection of lung tumors onto the cell line PCA of panel A. LUAD category randomly down-sampled for clarity. Cell lines are from the same batch. (D) Projection of prostate tumors onto PCA of panel A. (E) PLSR of lung and prostate tumor CNA profiles, regressed on SCN or non-SCN status. LUAD category randomly down-sampled to match numbers in other categories. (F) Genome-wide view of CNA patterns. Each row is a tumor sample: SCLC-red, LUAD-light green, NEPC-blue, CRPC-brown; SC-green, non-SC (NSC)-orange. (G) Visualization of copy number changes that are observed in both lung and prostate SCNC signatures. Each cancer type was analyzed by PLSR independently and then combined. Y-axis represents the mean of the concordant PLSR loadings. See also Table S2.
A pan-cancer predictor of SCNCs reveals unannotated cases
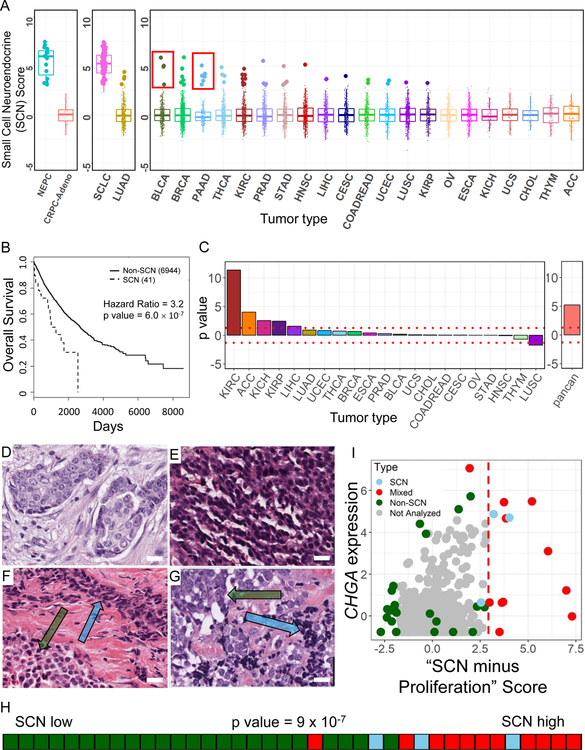
Clinical classification of SCNCs relies on histology and pathology defined by a few sets of morphological and molecular features. To uncover cases missed by these traditional approaches we analyzed purported non-SCN tumors using the gene expression-defined PCA SCN convergence framework (Figure 1A). Through a projection approach, the signature weightings for each gene (Table S3) were used to predict SCN phenotypes across approximately 7000 primary TCGA tumors from 21 different epithelial tumor types (Figure 4A). Boxplots of the first component score reveal a low frequency of primary tumor cases that contain a SCN component (~1%). Except for a subset of low grade non-SCN pancreatic neuroendocrine tumors (PNETs), these samples were high grade tumors (Table S3), consistent with reports in the literature for pathology-defined SCN cases (Oronsky et al., 2017; Watson et al., 2018). Of note, many other high-grade samples had low SCN scores. Thus, SCN versus non-SCN high-grade tumors may need to be addressed differently in the clinic.
Figure 4. Pan-cancer identification of primary tumors with an SCN signature.
(A) Gene expression-based prediction of SCN phenotype in epithelial TCGA patient tumors. Predictions made by projection onto PCv1 from Figure 1A. For each cancer type, samples greater than 3 standard deviations from the mean are highlighted as enlarged data points. Red inset boxes indicate known cases of non-SCN PNETS in the TCGA PAAD cohort (6 of 8; 2 missed are just subthreshold) and SCNCs in BLCA (3 of 4, 1 missed is the next sample subthreshold). Cancer types in rightmost box are left-right sorted based on the average of top 3 scores per cancer type. Lines inside boxplots represent the 25th, 50th, and 75th quantiles. Whiskers extend to 1.5 the interquartile range. (B) Kaplan-Meier overall survival analysis for predicted SCN versus non-SCN cases from TCGA epithelial cancers (samples in panel A right box plus LUAD; PAAD, SCLC, NEPC and CRPC-Adeno not included). P value is calculated controlling for tumor type. (C) P values from a Cox regression survival analysis in individual cancer types using the continuous SCN score (left), and pan-cancer Cox regression using the continuous score, accounting for cancer type (right). (D-G) TCGA BRCA hematoxylin and eosin (H&E) stained diagnostic slides of invasive ductal carcinoma (D; TCGA-D8-A1XD), small cell neuroendocrine carcinoma (E; TCGA-BH-A0HL), mixed tumor with components of invasive ductal carcinoma (lower left, green arrow) and small cell neuroendocrine carcinoma (upper right, blue arrow) (F; TCGA-E9-A245), mixed tumor with components of large cell neuroendocrine carcinoma (upper left, green arrow) and small cell neuroendocrine carcinoma (lower right, blue arrow) (G; TCGA-A1-A0SK). Scale bars in images represent approximately 20 µm. (H) Rug plot and Kolmogorov-Smirnov enrichment p value of breast cases scored by pathologist for SCN features ordered by their proliferation-removed SCN score. (I) Scatter plot of all samples in TCGA BRCA cohort. (x-axis: Proliferation removed SCN score; y-axis: Z-scored Chromogranin A expression. Cases to the right of the dashed red line, x=3, were computationally predicted as SCN-like). See also Figures S3, S4 and Table S3.
We next compared the predicted SCN (referred to as SCN-like) to non-SCN cases, controlling for tumor type and excluding the indolent non-SCN PNET cases, and found a significant decrease in patient overall survival (Figure 4B, Table S3). Pan-cancer association of the SCN phenotype with poor overall survival was robust to changes in the SCN-score threshold, and further supported in the majority of epithelial cancers by survival analysis based on continuous SCN-score (Figure 4C, Table S3). Unlike the traditional classification scheme that creates a dichotomy (Oronsky et al., 2017), our model supports evaluating each cancer sample along a spectrum. Notably, even cancers with SCN scores in the range that are not overtly annotated as SCN by pathology analysis have poorer outcomes.
We next investigated the histological features of the tumors most strongly predicted to be SCN-like. A number of these cases received SCN scores greater than 3 standard deviations above the mean although almost none was diagnosed as SCNCs based on the original pathology reports. To confirm the predictions, we chose samples based on the proliferation-removed SCN score across multiple tissue types for pathologic examination (Gutman et al., 2013). Fourteen of 16 cases were confirmed by the analysis to have SCN or neuroendocrine features (Table S3; Web Resource).
Because SCN breast cancers are considered rare, with a reported incidence of 0.1% (Wang et al., 2014), we next investigated the presence of SCN histology features in a panel of predicted SCN-like BRCA cases. A pathologist thoroughly examined slides of TCGA breast tumor cases covering a full range of SCN scores (Figure 4D–4G; Figure S3A–S3H; Table S3; Web Resource). The gene-expression based SCN score was indeed statistically predictive of BRCA samples with pathology-based SCN features (Figure 4H). Thus, the pan-tissue pathology analysis validated the transcriptome-based SCN signature score as a predictor of tumors with SCN morphology features, and supports an under-diagnosis of SCN cases.
In the pathology analysis, high SCN signature-score BRCA samples were typically called either SCN-positive, or more often as having mixed histology (Table S3). Three of 4 breast cancer subtypes (Basal, Luminal B, Luminal A) displayed regions with SCN pathology, usually in cases with accompanying genetic dysregulation of TP53 and RB1, suggesting a subset of cases for which closer pathologic interrogation will be beneficial to uncover the often focal regions of SCN morphology (Figure S3I, Table S3). The SCN score-high BRCA samples did not uniformly express the traditional SCN markers of CHGA, SYP, and NCAM1 (Figure 4I, Table S3). This finding reinforces the appreciation that heterogeneity in expression limits the use of only a small set of markers in the clinical identification of aggressive SCN signature-positive tumor cases.
REST/NRSF is a transcriptional repressor that negatively regulates neural gene expression. Loss of REST transcriptional repression has been linked to promoting the SCN phenotype in NEPC and REST activation inhibits neuroendocrine differentiation in SCLC (Lim et al., 2017; Zhang et al., 2015). Our analysis supports that REST activity regulates the SCN phenotype in a pan-cancer manner (Figure S3J–S3L). The expression of REST repressional target genes is positively correlated with SCN score across many epithelial and other tumor types, consistent with loss of REST-mediated repression (Figure S3K). The expression of REST itself is somewhat positively correlated with SCN score in the majority of cancers types, downweighing REST’s potential as a direct marker of non-NE tissues in samples with mixed histology. Overall the SCN score and pathology analyses above validate that tumors with SCN-like molecular signatures can be missed by standard pathology examination, and thus may be present more commonly than currently appreciated in cancer types in which SCNCs are considered rare.
Mutations associated with the pan-tissue SCN phenotype
Mutations and loss of TP53 and RB1 are associated with lung and prostate SCNCs (Beltran et al., 2016; George et al., 2015), and contribute to transdifferentiation and SCN-like histology in vivo (Ku et al., 2017). We next sought to identify additional mutations associated with SCN features in a pan-tissue context by directly leveraging the spectrum of SCN signature scores across primary tumors (Figure 4A). First using known cases of SCNC in lung and prostate tissues, we confirmed significant associations of TP53 and RB1 mutations with high SCN score, as well as a significant association of FOXA1 mutation, whose wild-type expression inhibits transition to NEPC (Kim et al., 2017) (Figure S4A, Table S3). In other epithelial cancer types, in addition to TP53 and RB1 mutations, we uncovered SCN score-associated mutations in NRAS (neuroblastoma-RAS) and genes such as OBSCN and BCLAF1 that have been previously associated with tissue specific SCNCs (Figure S4B, Table S3) (Cho et al., 2016; Rudin et al., 2012). In contrast, KRAS mutations were enriched in cases on the non-SCN side of the spectrum. Even in cancer types that are already highly neuronal such as glioblastoma (GBM), TP53 mutations were associated with higher SCN score (Figure S4C, Table S3). These results point to mutations beyond TP53 and RB1 that are common to SCN-like cancers across tissue types, and supports their mutational contribution to the development of SCN phenotypes.
Metastatic non-SCN tumors express the SCN signature profile more strongly than primary non-SCN tumors
Given that SCNCs can arise from epithelial tissues, we investigated the extent of the SCN signature in metastatic adenocarcinomas (MAd) in comparison to primary adenocarcinomas (PAd). For lung, prostate, and bladder tissues, we found that the expression profiles of MAd samples were more similar to SCNCs than were their respective PAds (Figure 5A). MAd samples typically do not express canonical SCN markers, but express increasing levels of other SCN-associated genes and transcription factors (Figure 5B). To determine the SCN signature score of MAd and SCNC samples on a pan-tissue scale, cases from the MET500 dataset (Robinson et al., 2017) were projected onto the SCN framework of Figure 1A (Figure S5A). Plotting the expression levels of the top 50 genes of the SCN signature in prostate cancers visually demonstrated the increased similarity of prostate MAds to prostate SCNCs (Figure S5B). Metastatic non-SCN samples tended to have SCN-score distributions significantly shifted upwards on the SCN spectrum in relation to their respective primary non-SCN samples, in multiple different tissues (Figure 5C).
Figure 5. Metastases across multiple tissue types have increased expression of SCN features.
(A) Projection of lung, prostate, bladder normal tissue, primary and metastatic non-SCN tumor samples, and SCN tumor samples onto the PCA framework of Figure 1A. Centroids and 80% confidence regions for the indicated groups are displayed. Here, the pancreatic, cervix, stomach, and thymus blue samples are those annotated with a neuroendocrine (NE)-related term in Robinson et al., 2017. (B) Heatmap of the expression of canonical SCN marker genes (top) and key SCN transcription factor genes (bottom) for prostate normal, primary adeno, metastatic adeno, and metastatic SCN. Samples ordered from left to right by the sum z-score of the genes displayed, as indicated at the top of the heatmaps. (C) Pan-cancer projections onto framework of Figure 1A. Data are from TCGA normal samples, TCGA primary tumors, MET500 metastatic tumors, SCLC tumors (George et al., 2015), and CRPC and NEPC tumors (Beltran et al., 2016). Plotted are the PCV1 values, representing SCN score. The SKIN.met cohort has two sources, the TCGA and MET500 databases. Wilcoxon-Mann-Whitney p values are shown comparing primary (orange) and metastatic (red) cases. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Lines inside boxplots represent the 25th, 50th, and 75th quantiles. Whiskers extend to 1.5 the interquartile range. See also Figure S5.
Because the SCN score contains both neuronal and proliferation components (Figure 1E), we sought to deconvolute these components in the primary, metastatic, and SCN samples. While a proliferation signal is a contributing component, metastatic cases have a significant increase in the expression of the SCN program even when the proliferation component is removed (Figure S5C). Furthermore, this signature deconvolution separates low grade PNET cases, which have a low-proliferation score (red box Figure S5C), from pancreatic SCN cases, consistent with reports that these cancers are distinct (Yachida et al., 2012). Additional cases of low grade PNETs, as well as neuroendocrine tumors of the rectum and small intestine (Alvarez et al., 2018), both fell in the same pattern of having low proliferation but high neuroendocrine features (Figure S5D). Taken together, these results support that MAds derive elements of their aggressive phenotypes from both a proliferation program and from a program associated with the neuronal programs of SCNCs.
Hematopoietic cancers share expression profiles and drug sensitivities with SCNCs.
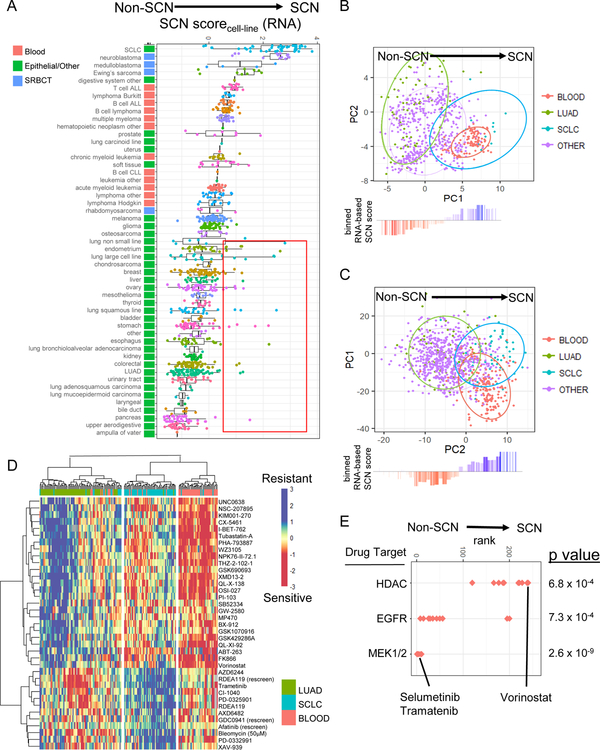
We next leveraged the concordance of SCN tumors and cell lines (Figures S1–S2, Figure 3) and the availability of drug screening data to gain insight into potential therapeutic vulnerabilities of SCNCs. Training on LUAD and SCLC cell lines, we scored all remaining CCLE cell lines using a gene expression- and PLSR-based SCN score (Figure 6A, Table S4). Consistent with the tumor findings, i) known SCN cell lines had higher SCN scores than most epithelial lines, and ii) the epithelial cases included a few cell lines that were not annotated as small cell but nonetheless had a strong SCN score (red box in Figure 6A). Unexpectedly, hematopoietic cancer cell lines had higher SCN expression signatures in comparison to the non-blood epithelial cancers (Figure 6A), which was likewise observed when the same analysis was performed on tumor data (Figure S6A, Table S4).
Figure 6. Blood cancers have SCN-like gene and protein expression profiles and drug sensitivities.
(A) Cell line gene expression SCN score (component 1 score of projection on PLSR of LUAD and SCLC lines). Z-score adjusted across all cell line samples together. Cancer types top-bottom sorted based on average SCN score per type. Red box: Non-SCN annotated epithelial lines with strong SCN score. Lines inside boxplots represent the 25th, 50th, and 75th quantiles. Whiskers extend to 1.5 the interquartile range. (B) Protein profile of blood cell lines, from projection onto PCA of LUAD and SCLC lines. (C) Drug sensitivity profile of blood cell lines, from projection onto PCA of IC50 values for LUAD and SCLC lines. Ellipses represent 80% confidence regions. In panels B and C, waterfall plots show binned gene expression SCN score of projected cell lines. (D) Heatmap of drugs with differential sensitivity between LUAD and SCLC cell lines (t-test p value < 0.0001). Hierarchical clustering is done on IC50 measurements for LUAD, SCLC, and blood lines. (E) Enrichment of drug targets for drugs more effective in LUAD or SCLC lines. Kolmogorov Smirnov test p values. See also Figures S6–S7 and Table S4.
We next analyzed protein expression signatures using reverse-phase protein array measurements across cell lines from various tissues (Li et al., 2017). PCA well segregated SCLC and LUAD cell lines (Figure 6B). Further supporting the unanticipated similarity in SCNCs and blood cancers, blood cancers as a group had projection-based protein profiles highly similar to SCLC (Figure 6B, Table S4), with increased expression of proteins such as BCL2, and regulators of cell cycle such as ATM, CHK1, and E2F1, which are candidate therapeutic targets in SCLC (Doerr et al., 2017) (Figure S6B, Table S4).
The similarity between SCNCs and blood cancers was seen again in large-scale drug sensitivity data (Iorio et al., 2016). Analogous to the expression-based analysis, PCA was performed on the matrix of all drug sensitivity IC50 values across LUAD and SCLC cell lines. This unsupervised approach revealed a sensitivity/resistance profile for SCLC cases (Figure 6C). Parallel to the protein-based analysis, blood cancers projected into the SCLC drug sensitivity space, while epithelial tissue cancer types projected into the LUAD space (Figure 6C). Hierarchical clustering based on the drugs with top differential sensitivity between LUAD and SCLC lines also supported the drug sensitivity similarities between SCLC and blood cancers (Figure 6D). Compared to LUAD lines, SCLC lines were more sensitive to drugs that inhibit histone deacetylation such as Vorinostat (Figure 6E), and more resistant to drugs that target EGFR or components of ERK/MAPK signaling pathways, such as Trametinib and Selumatinib (Figure 6E). Taken together, these findings support that hematopoietic cancers have similarities to SCNCs that range from expression profiles to drug sensitivity-based phenotype profiles.
Small-round-blue cell tumors share drug sensitivities with lung SCN and blood cancers
In addition to blood cancers, our gene signature analysis revealed that small-round-blue cell tumor (SRBCT) cell lines have high SCN gene expression signature scores (Figure 6A). These similarities between SRBCTs and SCNCs were also observed in protein profile (Figure S7A) and drug sensitivity profile analysis (Figure S7B).
Drugs with high differential sensitivity both in SCLC versus LUAD and in blood versus non-blood comparisons potentially act through a shared SCN-blood mechanism. The top drugs with such a pan-SCN-blood sensitivity profile included FK866 and THZ-2–102-1, to which SRBCTs also displayed sensitivity (Figure S7C, Table S4). FK866 is one of several NAMPT inhibitors shown to have indications for efficacy in both SCLC and neuronal cancers (Cole et al., 2017; Watson et al., 2009). THZ-2–102-1, a CDK7 inhibitor, targets components of transcriptional regulation and has been shown to be highly effective in MYCN-amplified neuroblastoma (Chipumuro et al., 2014).
Of note, blood cancers and the majority of, but not all, SCNCs grow in suspension in vitro. Nevertheless, SCLC suspension and SCLC adherent lines are intermingled in their PCA-based drug sensitivity profiles, and both are distinct from LUAD (Figure S7D). The parallel drug sensitivities of SCLC and blood cancers (R = 0.68) was maintained when the analysis was restricted to the 31% of SCLC lines in the drug sensitivity database that grow adherent in culture (R =0.69; n=20 adherent, n=6 semi-adherent, n=38 suspension) (Figure S7D). Furthermore, SRBCTs largely grow adherent in culture (n=50 adherent, n=6 semi-adherent, n=6 suspension). Taken together, these results support that the commonalities in drug sensitivities among blood, SCN and SRBCT cancers are not solely due to suspension culture characteristics (Fig S7D).
An expression-based SCN classifier is predictive of sensitivity to SCN targeting drugs in non-SCN epithelial cancers across tissues
Comparing gene expression and drug profiles in cell lines we found that increased SCN expression score generally correlated with increased sensitivity to SCN-targeting drugs (Figure S7E). This correlation had an overall combined p value of 1.4 × 10−5, and reached statistical significance in four individual epithelial tissue types. In addition, tissues with a greater mean SCN-expression signature also had drug sensitivity profiles that were closer to that of SCLC (Figure S7F). Thus, as epithelial cancers from various tissues develop SCN gene expression programs, they become increasingly susceptible to a similar panel of SCN-targeting drugs. These results support that the small cell phenotype exists along an expression signature-defined spectrum that has influence on therapeutic vulnerabilities in individual cancers.
Validation of shared SCN and blood susceptibilities based on gene dependencies
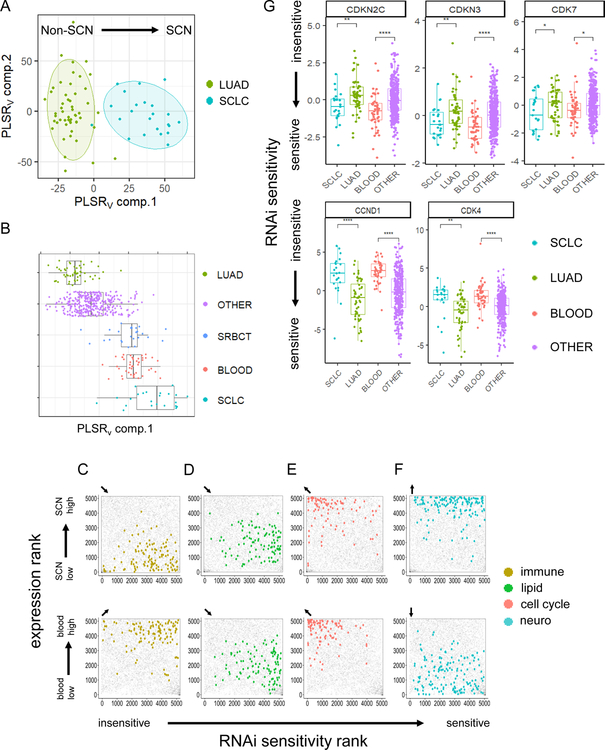
To validate the finding that SCN and blood cancers shared drug susceptibility, we analyzed a genome-scale RNA-interference (RNAi; shRNA-based) functional screen across a large panel of cell lines (Tsherniak et al., 2017). We first trained a prediction model on the gene dependency data between LUAD and SCLC cell lines (Figure 7A). A PLSR-based dependency prediction approach had 92% cross-validation accuracy for predicting the identity of lung cell lines that have concordant annotation and gene expression signatures (see STAR Methods). Applying the prediction model to the remaining cell types showed that blood and SRBCT lines share gene dependencies with the SCLC lines (Figure 7B). In reverse, using a blood versus non-blood RNAi sensitivity framework as the predictor confirmed that SCNCs and SRBCT’s have more blood-like dependencies (Figure S8A–S8B). Thus, the RNAi data validates our drug-based findings that SCNCs, hematopoietic cancers, and SRBCTs have shared susceptibilities.
Figure 7. Validation of shared vulnerabilities based on genome-scale functional RNAi screens.
(A) Varimax-rotated PLSR model trained on the genome-scale RNAi sensitivity values for LUAD and SCLC cell lines. Ellipses represent 80% confidence regions. (B) Prediction of RNAi sensitivity profile for blood, SRBCTs, and all other cell lines in the dataset. SCN sensitivity score based on projection to the varimax PLSR component 1 from panel A (which included LUAD and SCLC). (C-F) Comparison of gene set expression rank to gene set sensitivity rank for cell line SCLC versus LUAD (top) and blood versus non-blood (bottom) for gene sets containing selected keywords. Gene set RRHO scatter plots are subcategorized and colored by immune (C), lipid (D), cell cycle (E), and neuro (F) gene sets, with all other gene sets colored gray. Arrows in top left corner of individual panels indicate direction of significance (q < 0.01) by Kolmogorov-Smirnov test (diagonal arrows indicate significance in both expression and sensitivity directions; Benjamani-Hochberg correction). (G) Select targets with differential SCLC versus LUAD and blood versus non-blood RNAi sensitivity. The y-axis (RNAi sensitivity) is the published Demeter score. Student’s t-test p values. * p < 0.1, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Lines inside boxplots represent the 25th, 50th, and 75th quantiles. Whiskers extend to 1.5 the interquartile range. See also Figure S8 and Table S5.
Investigating biological pathways revealed that SCN and blood cancer cell lines shared susceptibility to disruption of immune pathways and lipid and sterol metabolism (Figure 7C–D, Figure S8C). Notably, this susceptibility enrichment was not exclusively tied to elevated (nor depressed) gene expression. In fact, SCN cell lines have lower expression of immune genes but remain sensitive to their disruption. In contrast, SCN and blood lines share increased expression of cell cycle genes, but both had decreased sensitivity to the knockdown of this gene category (Figure 7E). Although SCNCs had high expression of neural genes (Figure 1), and a few distinct neural gene sets did show genetic sensitivity to knockdown (Table S5, Figure 7F upper graph), there was no substantial enrichment of susceptibility to knockdown of neural genes – supporting that many components of the upregulated neuronal gene programs promote phenotypes distinct from the regulation of cell survival (Figure 7F). These expression versus sensitivity results highlight that cells are not particularly dependent on genes with elevated expression. Furthermore, even the knockdown of genes with reduced expression can have functional consequences.
While our transcriptome, proteome, drug sensitivity, and gene susceptibility analyses above uncovered many similarities between SCNCs and blood cancers (Figure S8C–S8D), naturally there were also many differences, especially at the gene expression level with blood cancers expressing more immune-annotated genes, and SCNCs expressing more neuro-annotated genes (Figure S8E).
We further investigated dependencies on protein targets of interest currently in clinical trials for various cancer types. We focused on CDKs and CDK antagonists, which had strong differential sensitivities in the LUAD versus SCLC, and blood versus non-blood comparisons. CCND1 and CDK4 knockdown were more effective in LUAD compared to either SCLC or blood cancers, while knockdown of CDKN2C, a CDK4 inhibitor, was more effective in SCLC and blood cancers (Figure 7G). The CCND1-CDK4 complex has been shown to inhibit phospho-RB to promote cell cycle progression, and loss of RB function is a hallmark in the development of the SCN phenotype (Figure 3G & Figure S4A–B). These results predict that epithelial cancers could use de-differentiation to an SCN phenotype as a resistance mechanism to CDK4 inhibition. This finding is consistent with RB loss as a resistance mechanism to CDK4 antagonism observed in preclinical models of liver cancer and glioblastoma, and in patients with metastatic breast cancer (Bollard et al., 2017; Condorelli et al., 2018). Notably, CDK7 knockdown was more effective in SCLC and blood cancers, paralleling the drug sensitivity finding for THZ-2–102-1, a CDK7 inhibitor (Figure 7G, Figure S7C). Thus, CDK7 sensitivity provides a specific example of a previously documented sensitivity in both SCNCs and blood cancers (Cayrol et al., 2017; Christensen et al., 2014), that is a part of the wider panel of shared sensitivities revealed by our pan-cancer and functional screen analysis.
Concordant gene expression-based drug sensitivity profiles in SCN and blood tumors
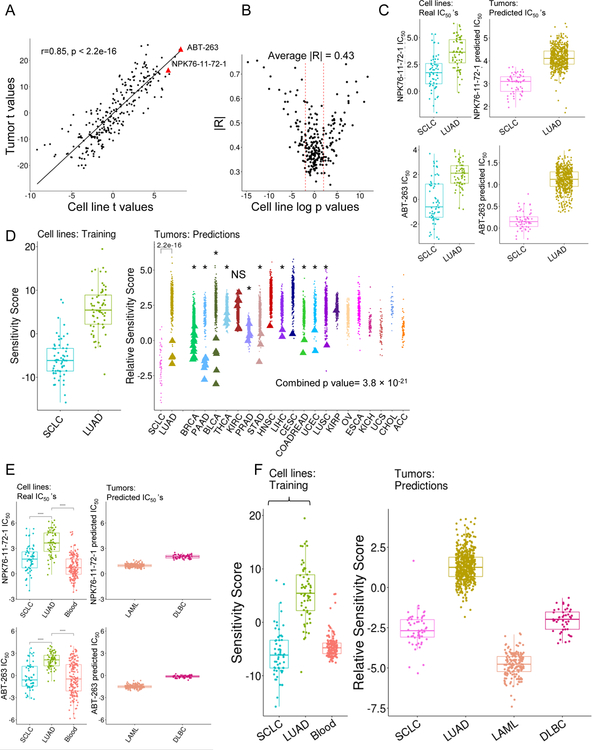
We next investigated if the gene expression profiles associated with drug susceptibilities are present in primary tumors. We built an elastic net (ENET)-based predictor of drug sensitivities in tumors trained on the LUAD and SCLC cell line gene expression profiles. In general, drugs that were differentially potent in LUAD versus SCLC cell lines were likewise predicted to be differentially potent in LUAD versus SCLC tumors (Figure 8A–8B). This result included a drug that targets BCL2 (ABT-263), which is often overexpressed in SCLC as a resistance mechanism to etoposide treatment (van Meerbeeck et al., 2011); and the PLK inhibitor NPK76-II-72–1 (Figure 8C, red dots Figure 7A). To determine the global sensitivity status of individual tumors, ENET-predicted tumor sensitivities were projected onto the PCA drug sensitivities of LUAD and SCLC cell lines (as shown in Figure 8C). We found that epithelial tumors with previously unannotated SCN features (based on gene expression profiles, Figure 4A) typically had drug sensitivity profiles more similar to annotated SCLC tumors than to LUAD tumors (Figure 8D). Ten of 11 tumor types had a significant association of expression-based predicted SCN status to predicted SCN-like sensitivity (KS test-based; Figure 8D, Table S6). These results support that the expression profiles associated with drug sensitivities in vitro are reflective of the in vivo tumor setting.
Figure 8. Tumors recapitulate SCN and blood cell line sensitivity signatures.
(A) Scatter plot of t-values from t-test of true IC50 values of SCLC versus LUAD in cell lines (x-axis) and predicted IC50 values in tumors (y–axis). (B) Comparison of cross validation model R2 values (cell line-based) to signed log p values of cell line SCLC versus LUAD true drug sensitivities (IC50). R2 values are from true cell line drug sensitivities versus cross validation-based predicted cell line sensitivities. Dashed lines are p value 0.05 (log p value = 1.3). (C) Real and predicted sensitivities for NPK76-II-72–1 and ABT-263. Y-axis is the log IC50. (D) Projection of RNA-seq, ENET-based predicted sensitivities of epithelial tumors onto PCA framework of SCLC and LUAD cell lines. More negative “Relative Sensitivity Scores” correspond to more SCN-like drug sensitivity profiles. Asterisks (*) denote individual tumor type significance by Kolmogorov Smirnov test, NS = not significant. SCLC and LUAD tumor predicted sensitivity compared via a Wilcoxon-Mann-Whitney test (p < 2.2 × 10 −16). Combined p value calculated with Stouffer’s test. (E) Real and predicted sensitivities of NPK76-II-72–1 and ABT-263 for blood tumors. Y-axis is the log IC50. (F) Projection of ENET-based sensitivities of epithelial tumors and blood tumors onto PCA framework of SCLC and LUAD cell lines (open bracket); combined p value from Stouffer’s test. More negative “Relative Sensitivity Scores” correspond to more SCN-like drug sensitivity profiles. Lines inside boxplots represent the 25th, 50th, and 75th quantiles. Whiskers extend to 1.5 the interquartile range. See also Table S6.
As in the cell line empirical-sensitivity data case, patient blood cancers shared predicted-drug sensitivity profiles with SCN tumors. These shared profiles included the BCL2 inhibitor ABT-263, which is in a class of drugs approved to treat chronic lymphoblastic leukemia (CLL), and is in development for other hematological malignancies (Hantel et al., 2018; Seymour et al., 2018) (Figure 8E–8F). Thus, we observe a similarity between SCNCs and blood cancers that spans gene and protein expression, and drug sensitivity and gene vulnerability profiles.
Discussion
The highly convergent molecular profiles of SCN tumors reported here match well-documented pathology findings of related morphologies and biomarkers across different tissue types (Klimstra et al., 2015; Rickman et al., 2017). The strength of these shared profiles allowed us to define a molecular classifier that identified cases with SCN or neuroendocrine features across numerous purportedly non-SCN primary tumors. These cases may not have been reported in the original pathology due to the fact that their SCN component is typically focal and largely unexpected in epithelial tumors. Survival analysis supported the aggressiveness of these SCN-like epithelial cancers (Chen et al., 2018). As such, screening for and targeting the SCN-like phenotype early in individual patient cases could have clinical benefit.
The implications of identifying this spectrum of SCNCs is further apparent in functional data on drug response and gene dependency. We found three categories of tumors to generally share expression and sensitivity features with pathology-defined SCN cases: SCN-like epithelial cancers, SRBCTs, and hematopoietic malignancies. Epithelial tumor types in which SCN tumors rarely occur but have poor prognosis (Inno et al., 2016), present statistical challenges in advancing care through clinical trials, and thus cross-tissue learning supported by shared molecular profiles will likely be required. Our findings thus support and guide efforts to identify rare SCNC cases and develop therapies that will target SCNCs from multiple tissue sites of origin.
The finding that small molecules preferentially effective in SCNs are also effective in hematopoietic malignancies was initially unexpected. However, there are several lines of phenotypic and molecular evidence in support of the sensitivity profile similarity. SCNCs such as those of the lung and prostate as well as blood cancers are among the most highly undifferentiated cancers, and are characterized by upregulation of stem-like signaling pathways (Malta et al., 2018; Smith et al., 2018). In culture, SCNCs display some phenotypic characteristics of hematopoietic cancer cell lines, such as a propensity to grow in suspension. In patients, SCN tumors downregulate genes involved in adhesion function compared to non-SCN tumors, corresponding with the widespread nature of SCN metastases (Wong et al., 2009). However, both adherent SCN and suspension SCN cell lines display similar drug sensitivity profiles to blood cancers, supporting that their drug sensitivity profiles are not defined solely by their suspension characteristic. SCNCs and lymphomas also share histological similarities. Namely, the small round blue cell phenotype seen in both tumor types can make distinguishing them a challenge despite their notably different origin, often requiring staining for more specific molecular markers (De Las Casas et al., 2004). Importantly, case reports have warned of the misdiagnosis of hematopoietic cancers as SRBCTs due to both morphological and histological similarities. For example, CD99, a marker used in the diagnosis of Ewing’s sarcoma, is frequently highly expressed in hematopoietic malignancies (Lucas et al., 2001; Ozdemirli et al., 1998).
The commonalities between SCNCs and blood cancers in functionally-defined susceptibilities provide guidance and avenues for hematopoietic malignancy therapies to inform SCN therapies, and vice versa. Importantly, the sensitivity profiles demonstrate predicted concordance in tumor data. Our analysis of the functional screens and previous studies point to the disruption of epigenetic regulators through use of HDAC inhibitors (Platta et al., 2007) or to the targeting of antiapoptotic factors such as BCL2 (Lochmann et al., 2018) as approaches to target both blood and SCNCs. CDK7 inhibitors have been shown to be effective in SCLCs, MYCN-driven SRBCTs, and blood cancers (Cayrol et al., 2017; Chipumuro et al., 2014; Christensen et al., 2014) – matching the cancer types that we co-classified in our sensitivity profiling analysis. Future work thus should investigate whether other therapeutic approaches successful in one cancer type can be applied to the cancer types reported here to have parallel sensitivity profiles. For example, CDK7 or HDAC inhibitors in combination with BCL2 inhibition are reported leads for treating lymphomas and leukemias, respectively (Cyrenne et al., 2017), and thus warrant testing in SCNCs.
In analysis of gene dependencies, we found that cell cycle-related CDKs were more effective in non-SCN and non-blood cancers. In contrast, knockdown or inhibition of CDK7, which regulates transcription but not the cell cycle (Lapenna and Giordano, 2009), was more effective in SCNCs and blood cancers. Disruption of CDK7 has been shown to interfere with RNA Polymerase II pausing, allowing concomitant decrease of super-enhancer driven strong transcription of sets of oncogenes (Nilson et al., 2015). This concept of de-amplifying the effect of multiple oncogenic transcription factors offers an approach to target cancers of distinct cell types (e.g. SCNCs, SRBCTs, blood cancers) that may upregulate different TFs, but through the same molecular mechanism, as previously postulated (Nilson et al., 2015). Our analysis also uncovered similarities for further therapeutic investigation. For example, both SCN and blood cancers share elevated sensitivities to targeting lipid pathways, which are generally known to regulate cancer cell signaling and metastasis (Pascual et al., 2017).
The cell of origin for SCNCs is not universally defined. SCNCs have been alternatively reported to arise from primary normal neuroendocrine cell precursors in some cases and transdifferentiation from adenocarcinoma in others (Watson et al., 2015). The cell of origin may vary depending on factors such as whether the SCNCs arise de novo or as a resistance mechanism to therapeutics (Feng et al., 2017; Rickman et al., 2017). In either case, the transdifferentiation-linked path to an SCN cancer converges, and thus leads to molecular profiles and phenotypes increasingly independent of the tissue of origin. This model of convergence is supported by our demonstration that normal prostate and lung cells can be reprogrammed into prostate and lung SCNCs, respectively, using a common set of small cell cancer-associated genetic perturbations (Park et al., 2018). Despite using distinct cell types as the starting material, the resulting tumor models had highly similar molecular profiles that matched the overlapping profiles of patient prostate and lung SCNCs. Combination therapies that both treat non-SCNCs and prevent escape towards SCNCs may help curtail the incidence of the disease. The pan-tissue scope of shared SCN susceptibilities supports the potential for pan-cancer therapy development to impact a large number of otherwise aggressive, metastatic, and treatment-resistant cancers.
Web Resource: SCN Signatures
The web resource at https://systems.crump.ucla.edu/scn/ can be used to 1) predict samples that have SCN features based on RNAseq data by projection to the varimax-rotated PCA in Figure 1A; 2) predict relative drug sensitivities of samples that may have SCN features using the ENET model trained on SCLC and LUAD cell line data (Figure 8); and 3) view high-power pathology images of SCN regions of the tumors investigated in Figure 4 and Table S3.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Thomas G. Graeber (TGraeber@mednet.ucla.edu).
ABBREVIATIONS
TCGA cancer type abbreviations are found at https://gdc.cancer.gov/resources-tcga-users/tcga-code-tables/tcga-study-abbreviations
METHOD DETAILS
Data Acquisition and Processing
Gene expression data of primary tumors was obtained from the TCGA (https://xenabrowser.net/hub/), of SCLC from (George et al., 2015), of human NEPC and CRPC from (Beltran et al., 2016), of the MET500 data which contains non SCN and SCN metastatic samples across multiple cancers from (Robinson et al., 2017), of all lung lines in the CCLE from (Barretina et al., 2012). To minimize batch effects, we uniformly processed the raw FASTQs through a single analysis pipeline (TOIL) (Vivian et al., 2017). CCLE lung cell lines were processed through TOIL when comparing to TCGA samples which were also processed through TOIL. Another version of the CCLE expression data on all cell lines was obtained in a format pre-processed through Salmon (Patro et al., 2017) (https://ocg.cancer.gov/ctd2-data-project/translational-genomics-research-institute-quantified-cancer-cell-line-encyclopedia), and all analyses done on CCLE cell lines only were performed using this data from this processing pipeline (Figure 6A). The CLCGP lung cancer microarray gene expression dataset used to build the ARACNe network was downloaded from www.uni-koeln.de/med-fak/clcgp. For RNAseq, upper quartile normalized expression values were transformed to log2 (x + 1).
The 450 K methylation array data for cell lines was obtained from Iorio et al. (2016), for human SCLC tumors from Mohammad et al. (2015), and for cancers in the TCGA from Hoadley et al. (2018) in processed form. The reduced representation bisulfite sequencing (RRBS) data for human NPEC and CRPC tumors was obtained from Beltran et al. (2016) in FASTQ format (dbGAP:phs000909.v1.p1) and aligned to hg38 using bwameth (Pedersen et al., 2014). Methylation metrics were called using MethylDackel, which groups cytosines into one of three sequence contexts: CpG, CHG, or CHH. Only cytosines in CpG context were used for downstream analysis.
Drug Sensitivity data was obtained processed as IC50 values from the Genomics of Drug Sensitivity in Cancer website (https://www.cancerrxgene.org). RNAi sensitivity data (Achilles version 2.20.2) was obtained processed (Demeter1 scores) from the Depmap website (https://depmap.org/portal/)
Cell line annotation was performed by harmonizing the annotation of the CCLE, GDSC, and Demeter datasets. Cell line annotations were cross checked with ATCC (https://www.atcc.org/), Cellosaurus (https://web.expasy.org/cellosaurus/), and DSMZ (https://www.dsmz.de/) when possible. When discrepancies arose between these 3 online sources and the primary annotation, we defaulted to the online sources, which have stringent analysis pipelines. Lines with problematic annotation as defined by Cellosaurus were left out of the analyses. Tumor and cell line annotations used are in Table S7 Cell line culture growth characteristics (e.g. adherent vs suspension) were obtained from the Dependency Map database (https://depmap.org/portal/) and (Iorio et al., 2016).
QUANTIFICATION AND STATISTICAL ANALYSIS
PCA/PLSR
Log2 transformed upper quartile normalized expression of coding genes was used to perform unsupervised principal component analysis (PCA). This method uncovers latent components which are a linear combinations of the features that most strongly vary across the datasets. PCA was performed centered and unscaled using the prcomp function in R. Partial Least Squares Regression (PLSR) is a supervised version of PCA that seeks to find the latent vectors that maximize the covariance of the input variables (e.g. gene expression) and the response (e.g. phenotypes). Varimax rotation of the PCA (PCAv) or PLSR loadings (PLSRv) was performed on 2 components, without Kaiser normalization, using the R varimax package. Projections onto varimax-rotated PCA/PLSR frameworks were done by multiplication of the original projected sample scores by the varimax rotation matrix. When applicable (e.g. Figure 1A) TCGA cancers (LUAD, PRAD, and BLCA) were randomly down sampled to more closely match the number of samples in data sets from George et al. and Beltran et al.
Small Cell Neuroendocrine (SCN) and Proliferation Scores
Tumors:
For RNAseq data using patient tumors, “SCN score” i s the PC1 score after projection onto the Figure 1A varimax PCA framework. Because of the nature of PCA, this score is determined as a linear combination of weights that includes every coding gene, and hence is not strictly dependent on solely one subset of genes. After projection onto this framework, the score was either z-scored in each individual tumor type as in Figure 4A (to highlight outlier samples), or left un-zscored to place cancers on a common scale as in Figure 5A, 5C, and S6A. For the two left boxes in Figure 4A, SCN scores are z-normalized w.r.t CRPC (leftmost box) and LUAD (middle box). For samples in the right box, SCN score is the value of PCv1 from projection onto Figure 1A, z-score normalized by cancer type. Samples greater than 3 standard deviations from the mean in the z-scored analysis were deemed “SCN-like” (e.g. Figure 4A–B).
As SCNCs are highly proliferative and display neuroendocrine features we sought to de-convolve these two influences on SCN score. To that end we created a “SCN minus proliferation score”. A list of proliferation genes was generated from the union of three lists of proliferation genes published by Benporath et al, Cyclebase (https://cyclebase.org/), and KEGG cell cycle genes. PCA was performed using only these proliferation genes, and the absolute value of each gene’s Pearson correlation to PC1 was calculated. A ROC curve was created using two classes with the pROC package to choose a threshold cutoff (Youden’s J statistic) for genes highly correlated with proliferation. Correlated genes above threshold and all annotated proliferation genes from the original list were removed. PCA and GSEA-squared analysis (see below) was then redone. “SCN minus proliferation score” is thus the PC1 score after sample projection onto the varimax PCA of the samples used in Figure 1A with this new gene list. A “Proliferation Only Score” was also created using the union of genes in the three lists and those removed using the ROC curve method. These proliferation-related genes were used to create another varimax PCA of the samples in Figure 1A. Proliferation Score is the varimax PC1 score after projection onto this framework. The proliferation-removed score was used in the analysis of the breast cancer slides to highlight samples that had a high probability of having neuroendocrine features. This method additionally has utility in distinguishing between SCN samples which have both neuroendocrine and proliferative features, from the indolent primitive neuroectodermal tumors which have neuroendocrine features but lack a proliferative signature. The proliferation-removed score was used in Figure 4I, S3I, S5C–D. For Figure 4I and S3J, the proliferation-removed score was z-scored, since only BRCA tumors were involved in these panels. For Figure S5D, both the x and y-axes were left not z-score normalized to place all samples on a common scale.
Cell Lines:
For cell lines, RNA gene expression and RNAi sensitivity based SCN scores were calculated by projection onto the varimax PLSR framework of the SCLC/LUAD dichotomy. For both these data types, SCN Score is the “component 1” score after projection onto this framework. Samples with annotation not concordant with their expression-based predictions, from a linear-discriminant Leave-one-out Cross Validation analysis using the first three components of an expression PCA, were removed in the RNAi susceptibility-based PLSR. For protein data, the “SCN score” is the PC1 score of a sample upon projection onto the protein data-based framework of the SCLC/LUAD dichotomy (e.g. Figure 6B). For drug sensitivity, as the distinction between non-SCN (LUAD) and SCN cases (SCLC) is on PC2, the drug sensitivity “SCN score” is the PC2 score of a sample after projection onto that framework (e.g. Figure 6C).
Transcription Factor Analysis
ARACNe (Lachmann et al., 2016) network connections were created using all genes, and then the network nodes were restricted to 1675 transcription factors (TFs) by combining all TF gene sets in the GO gene ontology. One network was built for each of lung (CLCGP et al., 2013), prostate (Beltran et al., 2016), and bladder (TCGA), using a balanced set of samples from the SCN and adenocarcinoma groups when possible, with default settings. VIPER analysis (Alvarez et al., 2016) was performed using the msviper function from R package viper, with a minimum network size of 10. The combined p value across the three tissues was calculated using Stouffer’s method by converting two-way p values from msviper into one-way p values using the two2one and sumz functions from the metap package in R.
GSEA-squared
Gene Set Enrichment Analysis (Subramanian et al., 2005) was done on pre-ranked lists of genes using the MSigDB C5 gene sets and Kolmogorov-Smirnov (KS)-based statistics. In order to identify categories of genesets that could be enriched or de-enriched, all individual words in the genesets were collected and their frequencies were tabulated. Words with frequencies <5 or >500 were excluded. All genesets were then ranked by their NES value. Using genesets containing each particular word, all the individual words were then ranked by their KS test p value using ks.test.2 at (https://github.com/franapoli/signed-ks-test). Top words with small p values were considered categories of interest, such as ‘immune’or ‘neuron’. A manual curation of other top words related to the categories, and inspection of gene sets containing these words, was done to group related gene sets. The keywords used for each category are listed below:
| Category | Keywords |
|---|---|
| Neuro | NEURO, SYNAP, VOLTAGE, AXON, CEREBRAL, CORTEX |
| Cell cycle | CELL_CYCLE, MITOTIC, DNA_REPLICATION, CHROMOSOME_SEGREGATION, SPINDLE, CELL_DIVISION |
| Splicing | SPLICING, SPLICESOME, SPLICESOMAL |
| Immune | INFLAM, IMMUNE, IMMUNITY, INTERLUEKIN, LEUKOCYTE |
| Adhesion | ADHESION, ADHERENS |
All gene sets were then ranked by their Normalized Enrichment Score (NES), and KS tests were performed to assess the distribution of gene set categories using ks.test.2. This second application of KS test-based enrichment analysis led to the coining of ‘GSEA-squared,’ enrichment analysis first on genes,and then on geneset categories. For multiple-hypothesis testing of KS tests of enrichment in Figures 7C–7F, each category’s one-way p values were corrected using the Benjamani-Hochberg method placing them within the full list of keywords.
REST Analysis
REST/NRSF is transcriptional repressor and restricts neural gene expression. We used an aggregate measure of REST activity by calculating an inferred “REST Activity Score” for each sample from RNAseq data. “REST Activity Score” for individual samples was determined using the gene set NRSF_01 from MSIGDB (http://software.broadinstitute.org/gsea/msigdb/geneset_page.jsp?geneSetName=NRSF_01) which contains genes that have a 3’ UTR motif that matches annotation for the REST transcription factor. In this model a higher “REST Activity Score” corresponds to de-repression of REST target genes, and hence conditions that support more neural gene expression, For each sample, a “single sample gene set enrichment score” (ssGSEA) was determined using the V$NRSF_01 gene set. REST gene expression and “REST Activity Score” were correlated to “SCN Score “ in individual cancer types using the Pearson’s correlation (Barbie et al., 2009). Possibilities for the discrepancy between REST activity and REST expression is that REST function can be lost in multiple ways such as by mutation, (Mahamdallie et al., 2015), or by truncation/alternative splicing that abrogates its function (Chen and Miller, 2018) – both of which would not require concordant changes in transcription levels.
Rank Rank Hypergeometric Overlap (RRHO)
Rank Rank Hypergeometric Overlap was performed for ‘signature overlap analysis’ using the online tool and the R package RRHO, with step size 100 for expression data and gene-based methylation data, and 2000 for probe-based methylation data such as that used in the ‘signature overlap analysis’ (Plaisier et al., 2010).
Methylation analysis
Methylation levels were expressed as β-values, indicating the overall proportion of methylation at each particular site [methylated / (methylated+umethylated)]. PCAs were performed centered and unscaled on the entire data matrix. The IlluminaHumanMethylation450k.db package was used to provide annotation information on the location of the probe in relation to regulatory elements. Tissue-agnostic enhancer locations were provided by the IlluminaHumanMethylation450k.db package (Tim Triche, Jr., 2017), which informatically determines enhancer probes using ENCODE data. For the probe-based methylation data, PLSR was run individually on lung and bladder tissues, regressing against the phenotype of SCN or non-SCN. Thus, extreme values in the PLSR loadings represent sites with differential methylation between SCN and non-SCN tumors. Non-SCN samples for lung and bladder were down-sampled to more closely match the number of SCN samples. Signature overlap analysis was then performed using RRHO on probe-level data. Prostate, which was sequenced by reduced-representation bisulfite sequencing (RRBS) was not included for site level analysis due to much fewer sites measured by the microarray platform used for bladder and lung. To test for the importance of open-sea regions, which are typically distant from the transcriptional start site (TSS) rather than CG island regions (TSS proximal), in contributing to the SCN versus non-SCN distinction, the absolute value of the loadings was ranked, and a 1-sided KS test was performed against a background of all sites. To incorporate prostate data with the lung and bladder 450K array data, the number of sites was reduced to sites represented across both platforms. Sites were then further collapsed to genes by matching probes to genes using IlluminaHumanMethylation450k annotation. Probes that matched to multiple genes based on the Illumina annotation were removed. Averaged methylation values for each gene were then ranked by PLSR loadings on each tissue type. Genes were ranked by averaged lung, prostate, bladder PLSR component 1 loadings, and GSEA was performed on this ranked list. The gene-based summarization of methylation data was also performed on the lung cell lines and PLSR was performed. The equivalent methylation data format from tumors was projected to the cell line framework.
CNA analysis
TCGA SNP6.0 Affymetrix derived seg files were downloaded from the GDC repository. Cell line seg files were created using RAWCOPY from the .cel files with default settings (Mayrhofer et al., 2016). Seg files were inputted into GISTIC2.0 to obtain both thresholded calls and continuous log2 CNA values mapped to genes. PCA was performed uncentered and unscaled on the continuous log2 CNA data. IGV was used for visualization. For lung, a random sample of LUAD samples and all SCLC samples were used. For prostate, the samples were subset to include only one sample from each patient. One region containing highly focal CNAs on Chromosome 1 was removed by inspection of prostate PCA loadings, because it vastly dominated the top components’ loadings of the PCA analysis and was determined to be a likely technical artifact. To determine consistently amplified or deleted regions in SCN cases of lung and prostate SCN samples, we performed PLSR on lung and prostate samples together or independently, regressing on phenotype in each case (1 and 0 representing SCN and non-SCN samples respectively). To determine consistent regions of amplifications and deletions, we evaluated the loadings of the individual PLSR analyses (consistent regions were defined by being commonly positive or commonly negative in the CNA PLSR loading values). The loadings for consistent regions were averaged, non-consistent regions were set to 0.
Integrated CNA (iCNA) score (Graham et al., 2017) for each sample was defined as:
Pathology analysis
To evaluate the molecular profile (gene expression)-based SCN predictions, a pathologist evaluated the available TCGA hematoxylin and eosin (H&E) stained histology slide images located at the Cancer Digital Slide Archive (http://cancer.digitalslidearchive.net/) (Gutman et al., 2013). Typically, each TCGA case has sections from formalin-fixed paraffin-embedded (FFPE) tissue and from frozen tissue. To avoid artifacts associated with frozen sections, only FFPE sections were used for histology classification. For the pan-cancer histology analysis, 16 tumor samples with high proliferation-removed SCN signature scores from multiple tissue types were analyzed by a pathologist. Representative images of neuroendocrine and non-neuroendocrine regions were obtained by taking screen shots. Cases with no SCN region identified may be explained by tumors with focal SCN, and pathology calls being performed on tissue not adjacent to the tissue used for sequencing.
From the breast cancer (BRCA) cohort we ranked samples by their proliferation-removed SCN score, and sampled to cover a full range of proliferation-removed SCN scores. Thirty-eight samples were selected to include 1) the majority of SCN high cases, 2) a random sampling of tumors with middling SCN scores, 3) tumors with low SCN score, and 4) samples with high expression levels of at least one of four traditional SCN markers, CHGA, SYP, NCAM1, and TTF1. Each digital slide was divided into 4 roughly equal quadrants to ensure that the slide was examined evenly across different regions. In each quadrant, 20 non-contiguous areas were examined at low, medium and high powers to determine the histologic features of the tumor. Representative images were obtained at the indicated magnification by taking screen shots. Cases were classified as mixed tumors when two different histologic types (most commonly, invasive ductal carcinoma and small cell neuroendocrine carcinoma) co-existed. For a case to be classified as a mixed tumor, the minor component (usually, small cell neuroendocrine carcinoma) needed to occupy a substantial area of the tumor. Specifically, we required that the tumor cells of the minor histology should coalesce in a region that is equal to or larger than 2 high power fields. To determine if pathology based-SCN positive cases were enriched in samples with high SCN score, samples were rank ordered by SCN score and a Kolmogorov-Smirnov enrichment test was performed on pathology-based SCN status (Table S3). The Web Resource contains a pathology page with detailed histology images (http://systems.crump.ucla.edu/scn/). For Table S3, grade and stage information were obtained from the TCGA Pan-Cancer Clinical Data Resource (https://gdc.cancer.gov/about-data/publications/PanCan-Clinical-2018).
Survival analysis
For the pan-cancer analysis, Cox regressions were performed based on SCN-like/non-SCN status (SCN-like status = SCN z-score of 3 and above), controlling for cancer type using the coxph function in R. Various thresholds of the SCN score were used for the pan-cancer survival analysis to make Table S3. Survival analysis based on a continuous scale was performed using the PCv1-based SCN score determined by projection onto the Figure 1A PCA plot for epithelial cancers, and controlling for cancer type for the pan-cancer analysis, using the coxph function in R. Wald-test p values were reported.
Mutation analysis
Mutations were assembled from the MutsigCV2 calls for each tumor type from firebrowse.org. The gene list used was the union of recurrent mutations (mutsigcv2 q-value < 0.1) from all cancers in the TCGA. To determine the association of mutations with the neuroendocrine phenotype a generalized linear model (GLM) was used. In the analysis of cancers with known SCN cases (Figure S4A) a logistic regression was conditioned on the SCN score and the tissue type on the combined data from “prostate” (consisting of NEPC, CRPC, PRAD) and “lung” (SCLC, LUAD). For the pan-epithelial cancer analysis (Figure S4B) the logistic regression was conditioned on the PCv1-based SCN score (projection onto Figure 1A) and cancer type using all epithelial cancers in the TCGA. In the individual cancer cases (Figure S4C) a Wilcoxon rank sum test on neuroendocrine score of mutant vs non-mutant was used. Multiple hypothesis correction was performed with the Benjamani-Hochberg method.
RPPA analysis
RPPA data was obtained from (Li et al., 2017). As this data has missing values, imputation was performed using probabilistic PCA, using the ppca and completeObs functions in the pcaMethods package (Stacklies et al., 2007). LUAD and SCLC cell lines were processed together in one batch without other cell lines so as not to intermix test and training sets. Missing value imputation for all other cell lines were performed together in one batch. Prior to each imputation we removed all proteins with greater than 25% missing values in that batch. Samples were projected onto the PCA of the imputed values SCLC and LUAD (Figure 6B). The SRBCT group in the protein data consisted of neuroblastoma, medulloblastoma, Ewing’s sarcoma, and rhabdomyosarcoma.
Drug sensitivity analysis
Drug sensitivity data (log IC50 values) of 255 small molecules across a wide panel of cancer cell lines from multiple tissue types was obtained from the GDSC (Iorio et al., 2016). For LUAD and SCLC cell lines, differential sensitivity to each drug was calculated using the Student’s t-test. These drugs were then ranked by the t-test statistic. Annotation on drug target and target pathway was obtained from the pharmacogenomics screen published by Iorio et al. From the annotation on drug targets, a list of genes or biological targets was obtained. For each target, a KS test was performed (bootstrap n = 1000) on the t-test ranked list of drugs that contained that target in its annotation, resulting in a list of targets significantly enriched or de-enriched for small cell cancer sensitivity. Missing values in the drug sensitivity data were imputed using the weighted average of k-nearest neighbors (n = 3). For SCLC and LUAD samples that were used as training data, k-Nearest Neighbors (kNN) imputation was performed on the lung samples alone. kNN imputation was then then performed on all other cell lines together, excluding the SCLC and LUAD samples. PCA was performed on the imputed matrix of drug sensitivity log IC50 values across SCLC and LUAD cell lines using all drugs in the screen. We then projected the drug data for all lines onto the PCA defined on lung SCLC and LUAD, including cancer of hematological and neuroectodermal origin. Projected points (all lines excluding lung SCLC and LUAD) in the drug sensitivity plot were annotated by their expression projection values. These values were binned along the x-axis and mean expression projection values were summarized in the corresponding waterfall plot (eg. Figure 6B, Figure 6C). Combined p values were obtained by the weighted-Stouffer test with the one way null hypothesis that the Pearson correlation is less than zero; using the lower tail of the t-distribution for values outputted by cor.test in R (lung small cell not included). The square root of individual group sample sizes were used as weights to stabilize the variance of the mean in this calculation (Zaykin, 2011). The SRBCT group in the drug sensitivity data consisted of neuroblastoma, medulloblastoma, Ewing’s sarcoma, and rhabdomyosarcoma.
Gene Dependency
shRNA data was taken from the Achilles Project (Tsherniak et al., 2017). Demeter gene dependency scores were used. A lower Demeter score indicates sensitivity to downregulation of that gene. PLSR was performed on 1) LUAD and SCLC lines, and 2) blood and non-blood lines (all cell lines except SRBCT, LUAD, or SCLC lines). The SRBCT group in the gene dependency data consisted of neuroblastoma, medulloblastoma, Ewing’s sarcoma, rhabdomyosarcoma, and merkel cell carcinoma. Varimax rotation was performed on 2 components. Other cell lines were projected onto the lung or blood PLSR frameworks using the varimax-rotated loadings. Student’s t-test was performed on LUAD versus SCLC lines, and blood versus non-blood lines, producing two gene lists, each ranked by p value representing differential RNAi sensitivity in the two above comparisons. RRHO was performed on these two ranked lists of genes, and on their corresponding GSEA-analyzed genesets ranked by NES score. For each gene, the sum of the LUAD versus SCLC rank and blood versus non-blood rank for each gene was calculated, and the list was co-ranked using this sum. Enrichment analysis (GSEA) was performed on the co-ranked list of genes using the MSigDB C2: KEGG, C2: Reactome, and C5: all GO gene sets. GSEA-squared (see above) was performed by ranking individual words by their signed KS test p value using ks.test.2. The keywords used for each category are listed below:
| Category | Keywords |
|---|---|
| Telomere | TELOMERE |
| Immune | INFLAM, IMMUNE, IMMUNITY, INTERLUEKIN, LEUKOCYTE |
| Cell cycle | CELL_CYCLE, MITOTIC, DNA_REPLICATION, CHROMOSOME_SEGREGATION, SPINDLE, CELL_DIVISION |
| Lipids | COA, LIPID, STEROL |
| Neuro | NEURO, SYNAP, VOLTAGE, AXON, CEREBRAL, CORTEX |
Tumor drug sensitivity prediction
Tumor drug sensitivity prediction were performed using elastic nets (ENET) with the caret package in R, using cell line RNAseq and IC50 drug sensitivity data. For each drug two distinct models were created. 1) Using the 1000 most variable genes at the RNA level to predict drug sensitivity in the SCLC and LUAD cell lines (used in Figure 8A–8D), and 2) using 1000 of the most variable genes across all cell lines (used in Figure 8E–8F). This resulted in a total of 510 models (255 drugs each). For the models used in Figure 8A–8D, model cross-validation performance improves for drugs with significant true differential sensitivities, showing that the models are built on relevant gene expression features. Using the predicted values, tumors were projected onto the PCA of real drug sensitivity values shown in Figure 6C. The relative sensitivity scores are the PC2 values from this projection.
DATA AND SOFTWARE AVAILABILITY
Public Data Resources
Data from the CCLE (expression) and GDSC (drug sensitivity and methylation) databases were downloaded from the Broad Institute and Cancer X Gene resources (http://www.broadinstitute.org/ccle; http://www.cancerrxgene.org/downloads), respectively.
Supplementary Material
Table S1. Pan-cancer convergence of SCN carcinoma. Related to Figure 1.
Table S2. SCNCs of lung and prostate origin share DNA copy number alteration patterns. Related to Figure 3.
Table S3. Pan-cancer identification of primary tumors with an SCN signature. Related to Figure 4.
Table S4. Blood cancers have SCN-like gene and protein expression profiles and drug sensitivities. Related to Figure 6.
Table S5. Validation of shared vulnerabilities based on genome-scale functional RNAi screens. Related to Figure 7.
Table S6. Tumors recapitulate SCN and blood cell line sensitivity signatures. Related to Figure 8.
Table S7. Related to STAR Methods.
KEY RESOURCES TABLE
Significance.
SCNCs are aggressive and histologically similar across tissue types, and no effective treatment modalities are available. Lung and prostate adenocarcinomas can transdifferentiate to SCNCs in response to targeted therapy. This has important consequences in that SCNCs, once considered rare, may become increasingly common with the emergence of resistance cases from targeted therapies. Here we define molecular signatures for SCNCs, and we find that SCNCs share similar drug and RNAi vulnerabilities with blood cancers. Our results guide the detection of SCN-like cases in the clinic, and support the exploration of treatments for SCNCs that mimic treatments for blood cancers.
Highlights.
Pan-tissue convergent molecular programs in small cell neuroendocrine (SCN) cancers
Poorer prognosis for tumors with high SCN signatures across epithelial cancers
Drug sensitivity profiles are shared between SCN cancers and blood cancers
SCN cancers and hematological malignancies have common functional dependencies
Acknowledgements
We thank members of TGG and ONW labs for helpful discussion. We thank Weihong Yan for assistance in creating the web resource and pathology image webpages. TGG and ONW are supported by the NIH NCI R01 CA222877, the UCLA SPORE in Prostate Cancer (NIH NCI P50 CA092131), the W.M. Keck Foundation, and the UCLA Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Hal Gaba Director’s Fund for Cancer Stem Cell Research. BNG and TGG are supported by NIH NCI R01 CA208303, UC Tobacco-Related Disease Research Program (TRDRP; 26IP-0036). JH is supported by NIH NCI R01 CA205001. KMS is supported by the UCLA Medical Scientist Training Program (NIH NIGMS T32 GM008042) and the Systems and Integrative Biology Training Grant at UCLA (NIH T32 GM008185). BAS is supported by a Prostate Cancer Foundation Young Investigator Award. Biostatistics supported by the National Center for Advancing Translational Sciences UCLA CTSI Grant UL1TR000124. JWP is supported by UCLA Broad Stem Cell Research Center postdoctoral fellowship and an NIH NCI K99/R00 Pathway to Independence Award K99 CA218731.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
ONW currently has consulting, equity, and/or board relationships with Trethera Corporation, Kronos Biosciences, Sofie Biosciences, and Allogene Therapeutics. None of these companies contributed to or directed any of the research reported in this article.
References
- Alanee S, Moore A, Nutt M, Holland B, Dynda D, El-Zawahry A, and McVary KT (2015). Contemporary Incidence and Mortality Rates of Neuroendocrine Prostate Cancer. Anticancer Res 35, 4145–4150. [PubMed] [Google Scholar]
- Alvarez MJ, Shen Y, Giorgi FM, Lachmann A, Ding BB, Ye BH, and Califano A (2016). Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat. Genet 48, 838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez MJ, Subramaniam PS, Tang LH, Grunn A, Aburi M, Rieckhof G, Komissarova EV, Hagan EA, Bodei L, Clemons PA, et al. (2018). A precision oncology approach to the pharmacological targeting of mechanistic dependencies in neuroendocrine tumors. Nat. Genet 50, 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, Schinzel AC, Sandy P, Meylan E, Scholl C, et al. (2009). Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 462, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV, Sonkin D, et al. (2012). The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BVSK, Varambally S, et al. (2016). Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med 22, 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard J, Miguela V, Ruiz de Galarreta M, Venkatesh A, Bian CB, Roberto MP, Tovar V, Sia D, Molina-Sánchez P, Nguyen CB., et al. (2017). Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut 66, 1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M (2018). org.Hs.eg.db: Genome wide annotation for Human. R package version 3.6.0
- Cayrol F, Praditsuktavorn P, Fernando TM, Kwiatkowski N, Marullo R, Calvo-Vidal MN, Phillip J, Pera B, Yang SN, Takpradit K, et al. (2017). THZ1 targeting CDK7 suppresses STAT transcriptional activity and sensitizes T-cell lymphomas to BCL2 inhibitors. Nat. Commun 8, 14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-L, and Miller GM (2018). Alternative REST Splicing Underappreciated. ENeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Zhang Y, Gibbons DL, Deneen B, Kwiatkowski DJ, Ittmann M, and Creighton CJ (2018). Pan-Cancer Molecular Classes Transcending Tumor Lineage Across 32 Cancer Types, Multiple Data Platforms, and over 10,000 Cases. Clin. Cancer Res [DOI] [PMC free article] [PubMed]
- Chipumuro E, Marco E, Christensen CL, Kwiatkowski N, Zhang T, Hatheway CM, Abraham BJ, Sharma B, Yeung C, Altabef A, et al. (2014). CDK7 Inhibition Suppresses Super-Enhancer-Linked Oncogenic Transcription in MYCN-Driven Cancer. Cell 159, 1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SY, Choi M, Ban H-J, Lee CH, Park S, Kim H, Kim Y-S, Lee YS, and Lee J-Y (2016). Cervical small cell neuroendocrine tumor mutation profiles via whole exome sequencing. Oncotarget 8, 8095–8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CL, Kwiatkowski N, Abraham BJ, Carretero J, Al-Shahrour F, Zhang T, Chipumuro E, Herter-Sprie GS, Akbay EA, Altabef A, et al. (2014). Targeting Transcriptional Addictions in Small Cell Lung Cancer with a Covalent CDK7 Inhibitor. Cancer Cell 26, 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLCGP et al. (2013). A Genomics-Based Classification of Human Lung Tumors. Sci. Transl. Med 5, 209ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J, Guiot M-C, Gravel M, Bernier C, Shore GC, and Roulston A (2017). Novel NAPRT specific antibody identifies small cell lung cancer and neuronal cancers as promising clinical indications for a NAMPT inhibitor/niacin co-administration strategy. Oncotarget 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli R, Spring L, O’Shaughnessy J, Lacroix L, Bailleux C, Scott V, Dubois J, Nagy RJ, Lanman RB, Iafrate AJ, et al. (2018). Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 29, 640–645. [DOI] [PubMed] [Google Scholar]
- Cyrenne BM, Lewis JM, Weed JG, Carlson KR, Mirza FN, Foss FM, and Girardi M (2017). Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood 130, 2073–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Casas LE, Gokden M, Mukunyadzi P, White P, Baker SJ, Hermonat PL, You H, Korourian S, Malak SF, and Miranda RN (2004). A morphologic and statistical comparative study of small-cell carcinoma and non-Hodgkin’s lymphoma in fine-needle aspiration biopsy material from lymph nodes. Diagn. Cytopathol 31, 229–234. [DOI] [PubMed] [Google Scholar]
- Doerr F, George J, Schmitt A, Beleggia F, Rehkämper T, Hermann S, Walter V, Weber J-P, Thomas RK, Wittersheim M, et al. (2017). Targeting a non-oncogene addiction to the ATR/CHK1 axis for the treatment of small cell lung cancer. Sci. Rep 7, 15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N, Yin Y, He Y, and Huang J (2017). Alternative Splicing Provides a Novel Molecular Mechanism for Prostatic Small-cell Neuroendocrine Carcinoma. Eur. Urol 71, 79–80. [DOI] [PubMed] [Google Scholar]
- George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, Leenders F, Lu X, Fernández-Cuesta L, Bosco G, et al. (2015). Com prehensive genomic profiles of small cell lung cancer. Nature 524, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NA, Minasyan A, Lomova A, Cass A, Balanis NG, Friedman M, Chan S, Zhao S, Delgado A, Go J, et al. (2017). Recurrent patterns of DNA copy number alterations in tumors reflect metabolic selection pressures. Mol. Syst. Biol 13, 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, Cobb J, Somanna D, Park Y, Wang F, Kurc T, Saltz JH, Brat DJ, Cooper LAD, and Kong J (2013). Cancer Digital Slide Archive: an informatics resource to support integrated in silico analysis of TCGA pathology data. J. Am. Med. Inform. Assoc 20, 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantel A, Wynne J, Lacayo N, Khaw SL, Rubnitz J, Mullighan C, Schmidt M, Zhou Y, Ross JA, Rosenwinkel L, et al. (2018). Safety and Efficacy of the BCL Inhibitors Venetoclax and Navitoclax in Combination with Chemotherapy in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Clin. Lymphoma Myeloma Leuk 18, S184–S185. [Google Scholar]
- Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, Shen R, Taylor AM, Cherniack AD, Thorsson V, et al. (2018). Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 173, 291–304.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inno A, Bogina G, Turazza M, Bortesi L, Duranti S, Massocco A, Zamboni G, Carbognin G, Alongi F, Salgarello M, et al. (2016). Neuroendocrine Carcinoma of the Breast: Current Evidence and Future Perspectives. The Oncologist 21, 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio F, Knijnenburg TA, Vis DJ, Bignell GR, Menden MP, Schubert M, Aben N, Gonçalves E, Barthorpe S, Lightfoot H, et al. (2016). A Landscape of Pharmacogenomic Interactions in Cancer. Cell 166, 740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jin H, Zhao JC, Yang YA, Li Y, Yang X, Dong X, and Yu J (2017). FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene 36, 4072–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimstra DS, Beltran H, Lilenbaum R, and Bergsland E (2015). The Spectrum of Neuroendocrine Tumors: Histologic Classification, Unique Features and Areas of Overlap. Am. Soc. Clin. Oncol. Educ. Book 35, 92–103. [DOI] [PubMed] [Google Scholar]
- Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, Goodrich MM, Labbé DP, Gomez EC, Wang J, et al. (2017). Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn Max.C. from J.W., Steve Weston, Andre Williams, Chris Keefer, Allan Engelhardt, Tony Cooper, Zachary Mayer, Brenton Kenkel, the R. Core Team, Michael Benesty,Reynald Lescarbeau, Andrew Ziem, Luca Scrucca, Yuan Tang and Can Candan. (2016). caret: Classification and Regression
- Lachmann A, Giorgi FM, Lopez G, and Califano A (2016). ARACNe-AP: gene network reverse engineering through adaptive partitioning inference of mutual information. Bioinformatics 32, 2233–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapenna S, and Giordano A (2009). Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov 8, 547–566. [DOI] [PubMed] [Google Scholar]
- Lee J-K, Lee J, Kim S, Kim S, Youk J, Park S, An Y, Keam B, Kim D-W, Heo DS, et al. (2017). Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 35, 3065–3074. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao W, Akbani R, Liu W, Ju Z, Ling S, Vellano CP, Roebuck P, Yu Q, Eterovic AK, et al. (2017). Characterization of Human Cancer Cell Lines by Reverse-phase Protein Arrays. Cancer Cell 31, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JS, Ibaseta A, Fischer MM, Cancilla B, O’Young G, Cristea S, Luca VC, Yang D, Jahchan NS, Hamard C, et al. (2017). Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 545, 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochmann TL, Floros KV, Naseri M, Powell KM, Cook W, March RJ, Stein GT, Greninger P, Maves YK, Saunders LR, et al. (2018). Venetoclax Is Effective in Small-Cell Lung Cancers with High BCL-2 Expression. Clin. Cancer Res 24, 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas DR, Bentley G, Dan ME, Tabaczka P, Poulik JM, and Mott MP (2001). Ewing sarcoma vs lymphoblastic lymphoma. A comparative immunohistochemical study. Am. J. Clin. Pathol 115, 11–17. [DOI] [PubMed] [Google Scholar]
- Mahamdallie SS, Hanks S, Karlin KL, Zachariou A, Perdeaux ER, Ruark E, Shaw CA, Renwick A, Ramsay E, Yost S, et al. (2015). Mutations in the transcriptional repressor REST predispose to Wilms tumor. Nat. Genet 47, 1471–1474. [DOI] [PubMed] [Google Scholar]
- Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, Kamińska B, Huelsken J, Omberg L, Gevaert O, et al. (2018). Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 173, 338–354.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoux N, Gettinger SN, O’Kane G, Arbour KC, Neal JW, Husain H, Evans TL, Brahmer JR, Muzikansky A, Bonomi PD, et al. (2018). EGFR -Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J. Clin. Oncol. JCO.18.01585 [DOI] [PMC free article] [PubMed]
- Mayrhofer M, Viklund B, and Isaksson A (2016). Rawcopy: Improved copy number analysis with Affymetrix arrays. Sci. Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meerbeeck JP, Fennell DA, and De Ruysscher DKM (2011). Small-cell lung cancer. Lancet Lond. Engl 378, 1741–1755. [DOI] [PubMed] [Google Scholar]
- Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, and Getz G (2011). GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol 12, R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad HP, Smitheman KN, Kamat CD, Soong D, Federowicz KE, Van Aller GS, Schneck JL, Carson JD, Liu Y, Butticello M, et al. (2015). A DNA Hypomethylation Signature Predicts Antitumor Activity of LSD1 Inhibitors in SCLC. Cancer Cell 28, 57–69. [DOI] [PubMed] [Google Scholar]
- Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen C-C, Wongvipat J, Ku S-Y, Gao D, Cao Z, et al. (2017). SOX2 promotes lineage plasticity and antiandrogen resistance in TP53 - and RB1 -deficient prostate cancer. Science 355, 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal R, Schweizer M, Kryvenko ON, Epstein JI, and Eisenberger MA (2014). Small cell carcinoma of the prostate. Nat. Rev. Urol 11, 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN, Moran T, et al. (2015). RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat. Commun 6, 6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson KA, Guo J, Turek ME, Brogie JE, Delaney E, Luse DS, and Price DH (2015). THZ1 Reveals Roles for Cdk7 in Co-transcriptional Capping and Pausing. Mol. Cell 59, 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg J, et al. (2015). Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol 16, e435–e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oronsky B, Ma PC, Morgensztern D, and Carter CA (2017). Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia N. Y. N 19, 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemirli M, Fanburg-Smith JC, Hartmann DP, Shad AT, Lage JM, Magrath IT, Azumi N, Harris NL, Cossman J, and Jaffe ES (1998). Precursor B-Lymphoblastic lymphoma presenting as a solitary bone tumor and mimicking Ewing’s sarcoma: a report of four cases and review of the literature. Am. J. Surg. Pathol 22, 795–804. [DOI] [PubMed] [Google Scholar]
- Park JW, Lee JK, Sheu KM, Wang L, Balanis NG, Nguyen K, Smith BA, Cheng C, Tsai BL, Cheng D, et al. (2018). Reprogramming normal human epithelial tissues to a common, lethal neuroendocrine cancer lineage. Science 362, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS-O, Berenguer A, Prats N, Toll A, Hueto JA, et al. (2017). Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45. [DOI] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, and Kingsford C (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BS, Eyring K, De S, Yang IV, and Schwartz DA (2014). Fast and accurate alignment of long bisulfite-seq reads. ArXiv14011129 Q-Bio
- Plaisier SB, Taschereau R, Wong JA, and Graeber TG (2010). Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res 38, e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platta CS, Greenblatt DY, Kunnimalaiyaan M, and Chen H (2007). The HDAC Inhibitor Trichostatin A Inhibits Growth of Small Cell Lung Cancer Cells. J. Surg. Res 142, 219–226. [DOI] [PubMed] [Google Scholar]
- Rickman DS, Beltran H, Demichelis F, and Rubin MA (2017). Biology and evolution of poorly differentiated neuroendocrine tumors. Nat. Med 23, 1–10. [DOI] [PubMed] [Google Scholar]
- Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA, Akbani R, et al. (2018). Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 174, 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, and Müller M (2011). pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, Wu Y-M, Lonigro RJ, Vats P, Cobain E, Everett J, Cao X, Rabban E, Kumar-Sinha C, Raymond V, et al. (2017). Integrative clinical genomics of metastatic cancer. Nature 548, 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohart F, Gautier B, Singh A, and Lê Cao K-A (2017). mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol 13, e1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, Bergbower EA, Guan Y, Shin J, Guillory J, et al. (2012). Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet 44, 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, Owen C, Gerecitano J, Robak T, De la Serna J, et al. (2018). Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med 378, 1107–1120. [DOI] [PubMed] [Google Scholar]
- Smith BA, Balanis NG, Nanjundiah A, Sheu KM, Tsai BL, Zhang Q, Park JW, Thompson M, Huang J, Witte ON, et al. (2018). A Human Adult Stem Cell Signature Marks Aggressive Variants across Epithelial Cancers. Cell Rep 24, 3353–3366.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacklies W, Redestig H, Scholz M, Walther D, and Selbig J (2007). pcaMethods--a bioconductor package providing PCA methods for incomplete data. Bioinforma. Oxf. Engl 23, 1164–1167. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, and Lander ES (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur I, and Taipale J (2016). The role of enhancers in cancer. Nat. Rev. Cancer 16, 483–493. [DOI] [PubMed] [Google Scholar]
- Therneau TM, and Grambsch PM (2000). Modeling Survival Data: Extending the Cox Model (Springer Science & Business Media; ). [Google Scholar]
- Triche Tim Jr. <Ttriche@Usc.Edu> (2017). IlluminaHumanMethylation450k.db
- Tsherniak A, Vazquez F, Montgomery PG, Weir BA, Kryukov G, Cowley GS, Gill S, Harrington WF, Pantel S, Krill-Burger JM, et al. (2017). Defining a Cancer Dependency Map. Cell 170, 564–576.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi J, Robert L, Paraiso K, Galvan C, Sheu KM, Lay J, Wong DJL, Atefi M, Shirazi R, Wang X, et al. (2018). Multi-stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell 33, 890–904.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian J, Rao AA, Nothaft FA, Ketchum C, Armstrong J, Novak A, Pfeil J, Narkizian J, Deran AD, Musselman-Brown A, et al. (2017). Toil enables reproducible, open source, big biomedical data analyses. Nat. Biotechnol 35, 314–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wei B, Albarracin CT, Hu J, Abraham SC, and Wu Y (2014). Invasive neuroendocrine carcinoma of the breast: a population-based study from the surveillance, epidemiology and end results (SEER) database. BMC Cancer 14, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GA, Ahmed Y, Picardo S, Chew S, Cobbe S, Mahony C, Crotty J, Wallis F, Shelly MJ, Kiely P, et al. (2018). Unusual Sites of High-Grade Neuroendocrine Carcinomas: A Case Series and Review of the Literature. Am. J. Case Rep 19, 710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M, Roulston A, Belec L, Billot X, Marcellus R, Bedard D, Bernier C, Branchaud S, Chan H, Dairi K, et al. (2009). The Small Molecule GMX1778 Is a Potent Inhibitor of NAD+ Biosynthesis: Strategy for Enhanced Therapy in Nicotinic Acid Phosphoribosyltransferase 1-Deficient Tumors. Mol. Cell. Biol 29, 5872–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PA, Arora VK, and Sawyers CL (2015). Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 15, 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis, 2016 (Springer-Verlag New York; ). [Google Scholar]
- Wong YNS, Jack RH, Mak V, Henrik M, and Davies EA (2009). The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970–2004. BMC Cancer 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, DeMarzo AM, et al. (2012). Small Cell and Large Cell Neuroendocrine Carcinomas of the Pancreas are Genetically Similar and Distinct From Well-differentiated Pancreatic Neuroendocrine Tumors: Am. J. Surg. Pathol 36, 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Denny SK, Greenside PG, Chaikovsky AC, Brady JJ, Ouadah Y, Granja JM, Jahchan NS, Lim JS, Kwok S, et al. (2018). Intertumoral Heterogeneity in SCLC Is Influenced by the Cell Type of Origin. Cancer Discov 8, 1316–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaykin DV (2011). Optimally weighted Z-test is a powerful method for combining probabilities in meta-analysis. J. Evol. Biol 24, 1836–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Coleman IM, Brown LG, True LD, Kollath L, Lucas JM, Lam H-M, Dumpit R, Corey E, Chery L, et al. (2015). SRRM4 Expression and the Loss of REST Activity May Promote the Emergence of the Neuroendocrine Phenotype in Castration-Resistant Prostate Cancer. Clin. Cancer Res 21, 4698–4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pan-cancer convergence of SCN carcinoma. Related to Figure 1.
Table S2. SCNCs of lung and prostate origin share DNA copy number alteration patterns. Related to Figure 3.
Table S3. Pan-cancer identification of primary tumors with an SCN signature. Related to Figure 4.
Table S4. Blood cancers have SCN-like gene and protein expression profiles and drug sensitivities. Related to Figure 6.
Table S5. Validation of shared vulnerabilities based on genome-scale functional RNAi screens. Related to Figure 7.
Table S6. Tumors recapitulate SCN and blood cell line sensitivity signatures. Related to Figure 8.
Table S7. Related to STAR Methods.
Data Availability Statement
Public Data Resources
Data from the CCLE (expression) and GDSC (drug sensitivity and methylation) databases were downloaded from the Broad Institute and Cancer X Gene resources (http://www.broadinstitute.org/ccle; http://www.cancerrxgene.org/downloads), respectively.