Abstract
Skeletal muscle myoblast differentiation involves elaborate signaling networks, including the activity of various ion channels and transporters. Several K+ and Ca2+ channels have been shown to affect myogenesis, but little is known about roles of Cl− channels in the associated processes. Here, we report that the leucine-rich repeat containing family 8 (LRRC8)/volume-regulated anion channel (VRAC) promotes mouse myoblast differentiation. All LRRC8 subunits of heteromeric VRAC were expressed during myotube formation of murine C2C12 myoblasts. Pharmacological VRAC inhibitors, siRNA-mediated knockdown of the essential VRAC subunit LRRC8A, or VRAC activity-suppressing overexpression of LRRC8A effectively reduced the expression of the myogenic transcription factor myogenin and suppressed myoblast fusion while not affecting myoblast proliferation. We found that inhibiting VRAC impairs plasma membrane hyperpolarization early during differentiation. At later times (more than 6 h after inducing differentiation), VRAC inhibition no longer suppressed myoblast differentiation, suggesting that VRAC acts upstream of K+ channel activation. Consequently, VRAC inhibition prevented the increase of intracellular steady-state Ca2+ levels that normally occurs during myogenesis. Our results may explain the mechanism for the thinning of skeletal muscle bundles observed in LRRC8A-deficient mice and highlight the importance of the LRRC8/VRAC anion channel in cell differentiation.
Keywords: calcium, cell differentiation, chloride channel, myogenesis, skeletal muscle, C2C12 myoblasts, hyperpolarization, membrane potential, volume-regulated anion channel (VRAC)
Introduction
Skeletal muscle formation includes the proliferation, differentiation, and fusion of myoblasts into multinucleated myotubes (1, 2). The commitment to terminal differentiation of myoblasts involves a complex system of regulatory signaling pathways (3). This includes the action of ion transport proteins responsible for the hyperpolarization of the plasma membrane (4–8) and an increase of resting cytosolic Ca2+ and oscillatory signaling (9–12). The sequential activation of two distinct K+ channels, the ether-à-go-go Kv10.1 (4) and the inward rectifier Kir2.1 (6), was shown to cause myoblast hyperpolarization from about −10 mV to about −80 mV at the acquisition of fusion-competency. This decrease in turn triggers the activation of T-type voltage-gated Ca2+ channels, resulting in an increase in intracellular Ca2+ concentration necessary for the differentiation of myoblasts into myotubes (3, 11, 13). Several further K+ and Ca2+ channels were implicated in myoblast differentiation, including Kv7.4 (14, 15), TASK2 and TREK1 (16), IP3R1 (17), and store-operated Ca2+ channels (18–21). In contrast, little is known about the role played by anion channels in skeletal myogenesis. However, the thinned muscle bundles observed in LRRC8A-deficient (Lrrc8a−/−) mice implicate volume-regulated LRRC8 anion channels in muscle formation (22).
The volume-regulated anion channel (VRAC)2 is formed by hexameric LRRC8 heteromers (23–25). LRRC8A is the only obligatory subunit and requires conjugation with at least one of the other LRRC8 family members (LRRC8B-E) to form functional plasma membrane channels (24). These channels are ubiquitously expressed in varying subunit configurations in vertebrate cells and mediate the flux of Cl− and organic osmolytes upon activation (25–29). VRAC opens upon osmotic cell swelling by an unknown mechanism. Importantly, it can also be activated under isovolumetric conditions by various signaling pathways (29–32). Besides its role in regulatory volume decrease upon osmotic cell swelling, VRAC has been implicated in various further physiological processes related to cell volume regulation (29). In addition, its impact on membrane potential has been shown to be involved in exocytic insulin release (33, 34) and through its conductance of larger osmolytes such as neurotransmitters it is involved in cell–cell communications such as between astrocytes and neurons (35, 36). The overall physiological importance of LRRC8 channels is demonstrated by the severe phenotypes of Lrrc8a−/− mice deficient in the essential VRAC subunit (22). They exhibit high prenatal and postnatal lethality, growth retardation, curly hair, and defective developments in many organs. Notably, mice lacking LRRC8A appear normal at birth but display significantly thinned skeletal muscle bundles at later ages (22); however, the underlying mechanism is unknown. Recently, two groups independently showed that LRRC8A-dependent VRAC is involved in membrane depolarization and activation of voltage-gated Ca2+ channel–mediated intracellular Ca2+ signaling in pancreatic β-cells (33, 34), processes which are also known to be important during myoblast differentiation and fusion (11, 37).
In this study, we have investigated the role of VRAC in skeletal myogenesis using mouse C2C12 myoblasts. Our results indicate that VRAC contributes to hyperpolarization and intracellular Ca2+ signals, thereby promoting myoblast differentiation.
Results
Pharmacological inhibition of VRAC impairs C2C12 myoblast differentiation and fusion
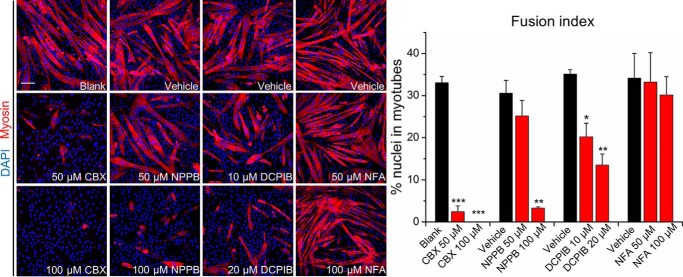
To investigate a putative role of VRAC in myogenesis, we first examined the effects of various pharmacological VRAC inhibitors on myoblast fusion: carbenoxolone (CBX), an inhibitor of VRAC, pannexins, and gap junction–forming connexins (38); 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), a more general Cl− channel inhibitor (30); and 4-(2-butyl-6, 7-dichloro-2-cyclopentyl-indan-1-on-5-yl) oxybutyric acid (DCPIB), a VRAC antagonist with high sensitivity at micromolar concentrations (39). As a control, we included niflumic acid (NFA), a potent inhibitor of Ca2+-activated Cl− channels with medium to low sensitivity for VRAC, which at the used concentrations may only partly inhibit VRAC (30, 40, 41). We induced C2C12 cells to differentiate by reducing the serum in the growth medium from 10% FBS to 2% horse serum for 4 days in the presence of drugs at various concentrations. The VRAC inhibitors CBX, NPPB, and DCPIB significantly reduced C2C12 myoblast differentiation and fusion in a dose-dependent manner, whereas the Ca2+-activated Cl− channel inhibitor NFA did not show any effect (Fig. 1).
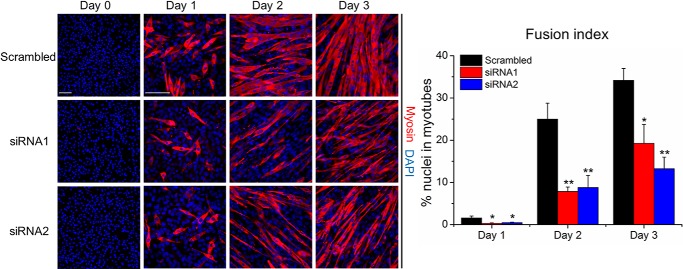
Figure 1.
VRAC inhibitors impair C2C12 myoblast fusion. Cells were stained with an antibody against myosin (red) and DAPI (nuclei, blue) after 4 days in differentiation medium with indicated concentrations of drugs. The fusion index was calculated as the percentage of nuclei in myotubes (with ≥2 nuclei) among all nuclei. Data are presented as mean ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 compared with the respective controls using one-way ANOVA. Scale bar, 100 μm.
LRRC8A is dispensable for myoblast proliferation but required for normal differentiation
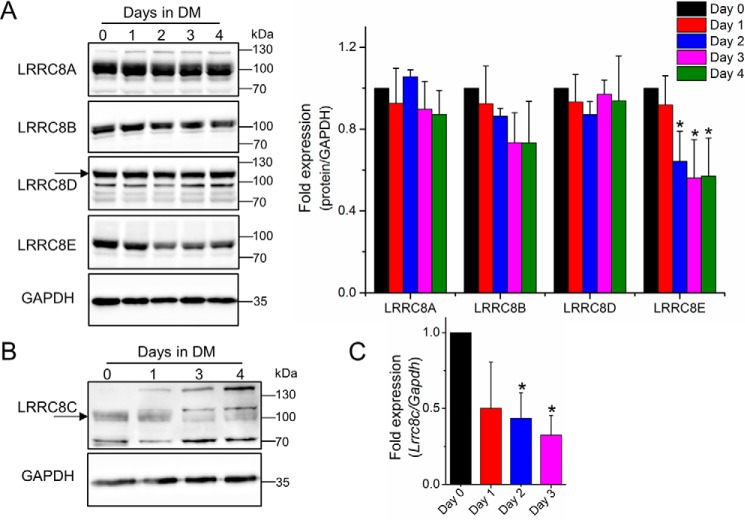
Because the pharmacological data suggest a role for VRAC in myoblast differentiation/fusion, we next aimed at testing its involvement in this process on a molecular biological level. To this end, we first determined the expression of the VRAC subunits during myotube formation. Western blot analysis revealed that undifferentiated C2C12 myoblasts expressed all five LRRC8 paralogues (Fig. 2, A and B). The amounts of LRRC8A and LRRC8D did not change during the first 4 days of cell differentiation. LRRC8B levels showed the tendency to decrease, whereas LRRC8E levels were strongly reduced already after 2 days of myoblast exposure to differentiation medium (Fig. 2A). LRRC8C protein seemed to decrease as well (Fig. 2B), but because the immunodetection was not unambiguous, we performed quantitative PCR, which revealed a significant decrease in Lrrc8c mRNA levels after 2 days of cell differentiation (Fig. 2C).
Figure 2.
Expression profile of LRRC8/VRAC subunits during C2C12 cell differentiation. A, Western blot analysis (left) and quantification (right) of LRRC8A, LRRC8B, LRRC8D, and LRRC8E protein levels. B, Western blot analysis of LRRC8C. Arrows in (A) and (B) indicate specific bands as deduced from published knockout controls. C, quantitative PCR analysis of Lrrc8c mRNA, -fold changes relative to day 0. All data are presented as mean ± S.D. from at least three independent experiments. *, p < 0.05 compared with day 0 using one-way ANOVA. DM, differentiation medium.
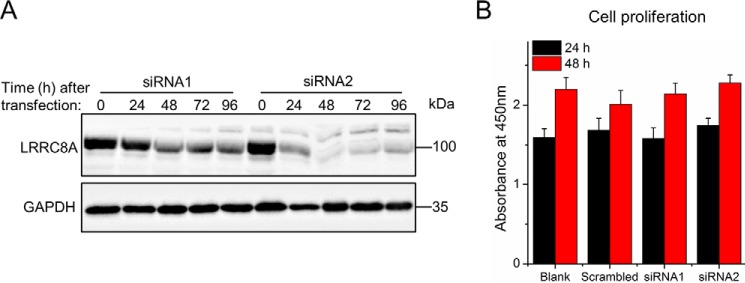
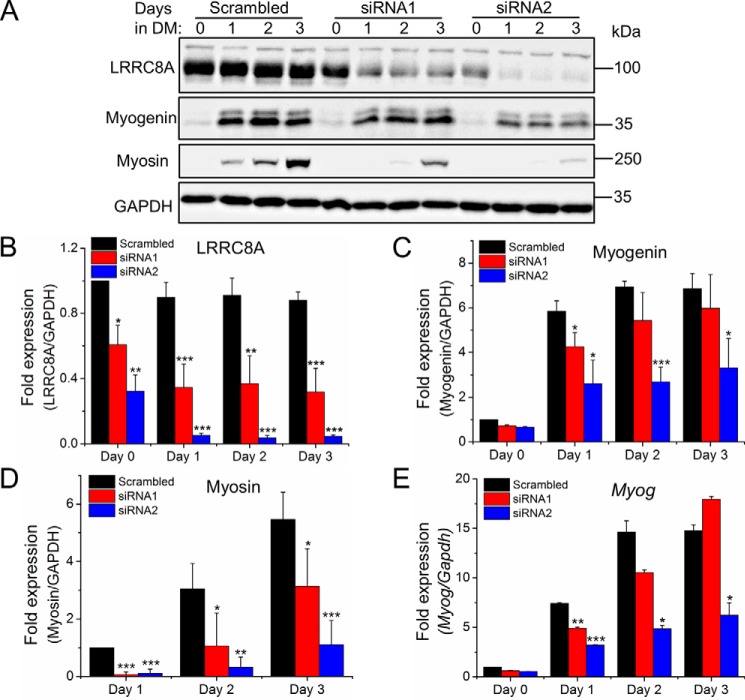
Next, we silenced the expression of the essential VRAC subunit LRRC8A with two individual siRNAs in C2C12 cells, using a scrambled siRNA as control. Western blotting confirmed a robust knockdown of LRRC8A protein, with siRNA2 being more efficient than siRNA1 (Fig. 3A). Notably, siRNA-mediated LRRC8A knockdown did not affect proliferation of C2C12 cells, as shown by the unaffected dehydrogenase activity (Fig. 3B). Hence, we could assess the effect of LRRC8A knockdown on the differentiation and formation of myotubes. Starting 1 day after siRNA transfection, C2C12 cells were exposed to differentiation medium for 3 days. At the start of differentiation induction, the amount of LRRC8A protein was reduced by ∼40 and ∼70% in cells treated with siRNA1 and siRNA2, respectively, and decreased further during the observed time of differentiation (Fig. 4, A and B). Knockdown of LRRC8A significantly reduced the expression of myogenin, an essential myogenic transcription factor (Fig. 4, A, C, and E) and of myosin, another marker of myoblast terminal differentiation (Fig. 4, A and D and Fig. 5) compared with scrambled siRNA. Furthermore, myoblast fusion was drastically diminished by LRRC8A silencing (Fig. 5). Scrambled control for the siRNA did not affect C2C12 differentiation or fusion (data not shown) and the inhibitory effects of siRNA1 and siRNA2 on myoblast differentiation and fusion correlated with their knockdown efficiencies. Collectively, these results suggest that LRRC8A is dispensable for myoblast proliferation but critically involved in myogenic commitment.
Figure 3.
LRRC8A knockdown does not affect myoblast proliferation. A, Western blot analysis of LRRC8A expression after siRNA transfection. B, quantitative analysis of C2C12 cell proliferation by measuring dehydrogenase activity at the indicated time points (hours) after siRNA transfection. Cells were plated at the same initial intensities and incubated overnight before transfection. Data are presented as mean ± S.D. from three independent experiments.
Figure 4.
LRRC8A knockdown impairs myoblast differentiation. A, Western blot analysis of LRRC8A, myogenin, and myosin. DM, differentiation medium. B–D, quantification of Western blot analysis. -Fold changes of LRRC8A (B) and myogenin (C) expression are normalized to day 0 with scrambled control. -Fold changes of myosin (D) expression are normalized to day 1 with scrambled control. E, quantitative PCR analysis of myogenin (Myog) on the indicated day of differentiation. -Fold changes of Myog expression are normalized to day 0 with scrambled control. All data are presented as mean ± S.D. from at least three independent experiments. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 compared with the respective controls using one-way ANOVA.
Figure 5.
LRRC8A knockdown impairs myoblast fusion. C2C12 cells were stained with an anti-myosin antibody (red) and DAPI (nuclei, blue) on the indicated day of differentiation. The fusion index was calculated as the percentage of nuclei in myotubes (with ≥2 nuclei) among all nuclei. Data are presented as mean ± S.D. from three independent experiments. *, p < 0.05 and **, p < 0.01 compared with the respective controls using one-way ANOVA. Scale bars, 100 μm.
VRAC activity promotes myoblast differentiation
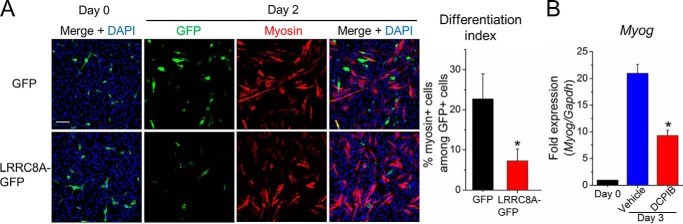
To further assess the role of VRAC during myogenesis, we overexpressed LRRC8A in C2C12 cells. Overexpression of LRRC8A alone, without another LRRC8, has previously been shown to suppress endogenous VRAC currents (23, 24). C2C12 myoblasts were transfected with plasmid DNA encoding LRRC8A fused to GFP (LRRC8A-GFP) or GFP alone. The next day, when expression of LRRC8A-GFP was already observable (Fig. 6A), the cells were induced to differentiate by serum withdrawal. 2 days later, more than 20% of GFP-expressing control cells were positive for the differentiation marker myosin, whereas this ratio was significantly lower with only ∼7% myosin-positive cells among the LRRC8A-GFP-expressing cells (Fig. 6A). This inhibition of C2C12 myoblast differentiation by LRRC8A overexpression corroborates the notion that the role for LRRC8A indeed lies in its requirement for VRAC activity. Consistently, DCPIB significantly reduced the myogenin mRNA (Myog) expression after 3 days of cell differentiation (Fig. 6B), in agreement with DCPIB impairing myoblast fusion (Fig. 1).
Figure 6.
Decreased VRAC activity inhibits myoblast differentiation. A, C2C12 cells transfected with plasmid DNA for GFP or LRRC8A-GFP expression (green) were stained with an anti-myosin antibody (red) and DAPI (nuclei, blue) on the indicated day of differentiation. The differentiation index was calculated as the percentage of myosin+ cells among GFP+ cells. More than 300 GFP+ cells were calculated for each group. Scale bar, 100 μm. B, quantitative PCR analysis of myogenin (Myog) after 3 days of cell differentiation in the presence of 20 μm DCPIB or of vehicle (DMSO) alone. -Fold changes of Myog expression are relative to day 0. All data are presented as mean ± S.D. from three independent experiments. *, p < 0.05 compared with the respective controls using a two-tailed unpaired t test.
VRAC is required for myoblast hyperpolarization and subsequent increased [Ca2+]i
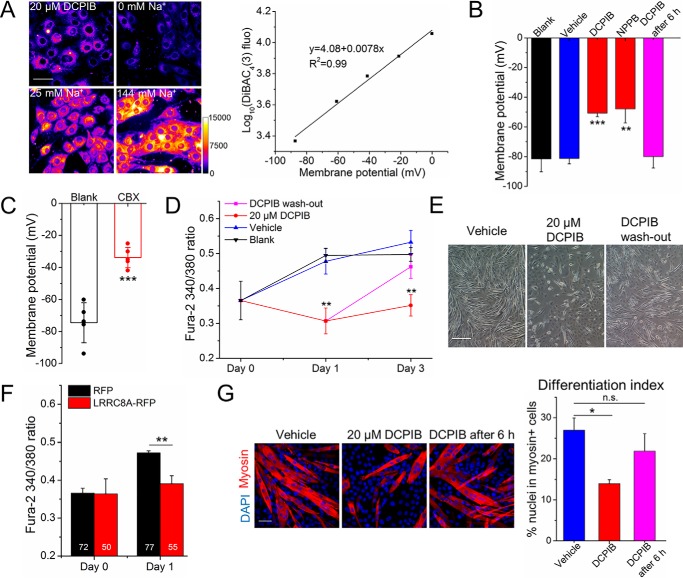
Upon induction of differentiation, myoblasts sequentially hyperpolarize because of the activation of two K+ channels, ether-à-go-go (4) and Kir2.1 (5, 6). This hyperpolarization is completed within the first 6 h of differentiation (7, 42). It induces a small, but sustained, inward Ca2+ current, sufficient to cause a detectable steady-state increase in intracellular Ca2+ ([Ca2+]i) (11, 13). To investigate whether VRAC influences the membrane potential during myogenic differentiation, we first established a protocol using the potentiometric fluorescent probe DiBAC4(3) (7, 43) to measure the plasma membrane potential of differentiating myoblasts (Fig. 7A). After 6–8 h of differentiation, C2C12 cells possessed the normal average resting membrane potential of −80 mV in the presence of vehicle (0.1% DMSO) but only −50 mV and −47 mV in the presence of 20 μm DCPIB and 100 μm NPPB, respectively (Fig. 7B). Electrophysiological recordings of C2C12 myoblasts after 20–24 h of differentiation showed a resting membrane potential of −75 mV (Fig. 7C). However, in the presence of 100 μm CBX, the resting membrane potential was found to be −34 mV. These data suggest that VRAC inhibition impaired the normal hyperpolarization. We next examined whether this impinges on the changes of resting cytosolic Ca2+ concentration [Ca2+]i during myoblast differentiation. Using the ratiometric Ca2+-sensitive fluorescence dye Fura-2, we observed the expected increase of steady-state [Ca2+]i in C2C12 cells in differentiation medium (Fig. 7D). In the presence of DCPIB, but not of the vehicle DMSO, this increase was abolished (Fig. 7D). When DCPIB was removed after 24 h from the differentiation medium, Ca2+ levels increased (Fig. 7D) and myoblast fusion proceeded normally (Fig. 7E). Consistently, VRAC suppression by overexpression of RFP-labeled LRRC8A (LRRC8A-RFP) prevented the increase of [Ca2+]i (Fig. 7F). Notably, VRAC activity seemed to be required predominantly during the first 6 h of cell differentiation, because the addition of DCPIB after this time did not affect the resting membrane potential of C2C12 myoblasts (Fig. 7B) or their differentiation (Fig. 7G).
Figure 7.
VRAC contributes to myoblast hyperpolarization and subsequently increased [Ca2+]i. A, calibration for measuring the resting membrane potential with the fluorescence probe DiBAC4(3). Left, representative images of C2C12 cells stained with DiBAC4(3) after differentiation for 6–8 h with or without DCPIB. Different external sodium concentrations and gramicidin were used to set the membrane potential. Fluorescence intensities are depicted in color code, as indicated by the calibration bar. Scale bar, 50 μm. Right, logarithm of the mean fluorescence intensity plotted against the calculated membrane potential (see “Experimental procedures”) and the best linear regression fit, which was used to calculate the membrane potentials in (B). B, average resting membrane potential of C2C12 myoblasts measured after 6–8 h in differentiation medium (blank) supplemented with 20 μm DCPIB, 100 μm NPPB or vehicle (DMSO) only; or DCPIB was added after 6 h. C, resting membrane potential of C2C12 cells measured after 20–24 h of differentiation in the presence or absence of 100 μm CBX by whole-cell patch clamp recording. Symbols represent values of individual cells. D, Fura-2 ratios in C2C12 cells measured on the indicated day of differentiation in the presence or absence of DCPIB. For DCPIB washout, DCPIB-containing medium was replaced by DCPIB-free medium after 24 h. E, differential interference contrast images of C2C12 cells after 3 days in differentiation medium in the presence of DCPIB (for the complete time or only for 24 h in the case of washout) or vehicle (DMSO) only. Scale bar, 200 μm. F, Fura-2 ratios in C2C12 cells expressing RFP or LRRC8A-RFP on the indicated day of differentiation. Numbers in bars indicate the total number of cells for each group. G, C2C12 cells were stained with an anti-myosin antibody (red) and DAPI (nuclei, blue) after 3 days in differentiation medium in the presence of 20 μm DCPIB, vehicle only, or with DCPIB added only 6 h after start of cell differentiation. The differentiation index was calculated as the percentage of nuclei in myosin+ cells among all nuclei. Scale bar, 50 μm. All data are presented as mean ± S.D. from three independent experiments. n.s., not significant. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 compared with the respective controls using one-way ANOVA (B and G) or a two-tailed unpaired t test (C, D, and F).
In summary, our results suggest that activation of VRAC activity contributes to the hyperpolarization of myoblasts and the rise in intracellular Ca2+, thereby supporting myoblast differentiation and fusion.
Discussion
Functional VRAC is required for normal myoblast differentiation
The thinned skeletal muscle bundles displayed by Lrrc8a−/− mice (22) suggest that lack of LRRC8A may lead to dysfunction of myoblast proliferation, differentiation, or fusion into multinucleated myotubes. Here, we show that consistent with a previous report (44), inhibition of the LRRC8A-containing anion channel VRAC does not impair the proliferation of C2C12 myoblasts. But we find that indeed VRAC inhibition does impair the differentiation and fusion of C2C12 cells. A role for LRRC8A in adipocyte differentiation was reported previously (45). In that context, LRRC8A was proposed to function independent of VRAC activity by regulating insulin-PI3K-AKT2-GLUT4 signaling through a physical interaction with the Cav1-IRS1-IR inhibitor GRB2 (45). We find that not only knocking down LRRC8A, but also pharmacological VRAC inhibitors and even VRAC current-suppressing overexpression of LRRC8A, impair myoblast differentiation and fusion. This demonstrates that not only the presence of LRRC8A, but also indeed the activity of VRAC plays a crucial role during myogenesis.
The molecular mechanism of VRAC activation is still unknown. Although experimentally this channel is often activated by osmotic cell swelling, various pathways seem to exist that can open VRAC under isotonic conditions (29–32). These include the involvement of signaling by integrins (46) and RhoA (47), which also play important roles in myogenesis (3). The pathways leading to the activation of VRAC during myoblast differentiation remain to be explored.
VRAC promotes myoblast hyperpolarization
When human myoblasts are induced to differentiate, the activation of an ether-à-go-go K+ channel rapidly hyperpolarizes myoblasts from −8 mV to approximately −35 mV (4, 5), coinciding with a cell-cycle arrest (48). Shortly thereafter, the resting membrane potential of myoblasts drops further to approximately −75 mV, because of the activation of the inward-rectifying K+ channel Kir2.1 (5, 6, 37). Hyperpolarization to similar values was shown for C2C12 myoblasts (49), as we also observed here. It was reported that the Kir2.1 channel is active at the plasma membrane already after 6 h of cell differentiation (7, 42). We found that myoblasts did not fully hyperpolarize, but remained at an intermediate resting potential when VRAC was inhibited, consistent with a function of VRAC before that of Kir2.1. In agreement with a role of VRAC in the early phase of myoblast differentiation, volume-activated Cl− currents drastically decrease during differentiation of C2C12 cells (50). We also observed significantly decreased expression of LRRC8C and LRRC8E after 2 days of cell differentiation. However, it is questionable if this underlies the reduction of VRAC currents, as the protein levels of the other paralogues, especially of LRRC8A, remained unchanged.
It may seem surprising that the anion channel VRAC contributes to membrane hyperpolarization, because in other situations it is associated with depolarization, such as during pancreatic insulin release where its activity triggers the early opening of voltage-gated Ca2+ channels (33, 34). Before the induction of differentiation, C2C12 myoblasts were shown to express very low level of the Na+-K+-2Cl− cotransporter NKCC1, which is then up-regulated during differentiation (51). Thus, undifferentiated C2C12 possibly has a low intracellular Cl− concentration. This would lead to an influx of chloride and hence membrane hyperpolarization upon VRAC activation within the first several hours of myoblast differentiation. Alternatively, VRAC could indirectly affect the membrane potential by modulating the activity of Kir2.1 during myoblast differentiation. It may influence regulatory pathways, such as those leading to the dephosphorylation of Kir2.1 at tyrosine-242 (42) or Cdo signaling (52).
In conclusion, we show that the volume-regulated anion channel VRAC, by controlling hyperpolarization and cytosolic Ca2+ signals, plays a critical role in myoblast differentiation. Our findings highlight the hitherto unknown importance of Cl− channels in cell differentiation. Given that hyperpolarization is generally necessary for stem cell differentiation (8, 53), VRAC may have roles in other differentiation processes, such as osteoblastogenesis (54, 55).
Experimental procedures
Cell culture and drugs
C2C12 mouse skeletal muscle myoblasts (American Type Culture Collection, CRL-1772; kindly provided by P. Knaus, Freie Universität, Berlin, Germany) were maintained in growth medium (DMEM supplemented with 10% FBS, 100 units ml−1 penicillin, and 100 μg ml−1 streptomycin) at 5% CO2 at 37 °C. To induce myogenic differentiation, cells at 90–100% confluency were rinsed with Dulbecco's PBS and then switched to differentiation medium (DMEM supplemented with 2% horse serum, 100 units ml−1 penicillin, and 100 μg ml−1 streptomycin). Upon induction of differentiation, medium containing the following drugs or vehicle if applicable (DMSO from PAN-Biotech) was replaced daily: CBX (Sigma-Aldrich, C4790), NFA (Sigma-Aldrich, N0630); NPPB (Tocris Bioscience, 0593), and DCPIB (Tocris Bioscience, 1540). For stock solutions, NFA, NPPB, and DCPIB were dissolved in DMSO, CBX in water.
Immunofluorescence staining
After the indicated time in differentiation medium, C2C12 cells growing on coverslips were rinsed with PBS and fixed in 4% paraformaldehyde/PBS for 15 min at room temperature. Cells were subsequently permeabilized in 0.2% Triton X-100/PBS for 20 min and blocked in 3% BSA/PBS for 1 h. Then, cells were incubated overnight at 4 °C with a monoclonal anti-myosin antibody (clone MF20; Developmental Studies Hybridoma Bank) in blocking buffer (0.28 μg ml−1). After washing with PBS, cells were incubated with Alexa Fluor 555–labeled anti-mouse Ig (1:1000; Molecular Probes) for 1 h at room temperature, stained with DAPI (1:1000; Sigma-Aldrich) for 10 min, and mounted with Roti-Mount FluorCare (Carl Roth). Images were acquired with a DMi8 fluorescence microscope using a 20× or 40× objective (Leica Microsystems).
Western blotting
Cells were collected with a cell scraper on ice and lysed in precooled RIPA buffer (150 mm NaCl, 50 mm Tris, pH 8.0, 5 mm EDTA, pH 8.0, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) containing protease inhibitor cocktails (Roche). Total protein amount was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of protein (20 μg per lane) were separated by 10% SDS-PAGE and transferred onto nitrocellulose membrane (Macherey & Nagel). Blotted membranes were subsequently blocked in 5% skim milk in TBS-T (20 mm Tris, pH 7.6, 150 mm NaCl, and 0.02% Tween-20) for 1 h at room temperature, incubated with primary antibodies overnight at 4°C, and stained with HRP-conjugated secondary antibodies for 40 min at room temperature. Primary antibodies were rabbit anti-LRRC8A-E subunits (1 μg ml−1; kindly provided by T. J. Jentsch, FMP and MDC Research, Berlin, Germany) (24, 56), mouse anti-myogenin (clone F5D, 1 μg ml−1; Developmental Studies Hybridoma Bank), anti-myosin (clone MF20, 0.28 μg ml−1; Developmental Studies Hybridoma Bank), rabbit anti-GAPDH (14C10, 1:2500; Cell Signaling Technology). Secondary antibodies were goat anti-mouse and goat anti-rabbit (1:5000; Jackson ImmunoResearch Laboratories). Signals were detected using an enhanced chemiluminescence reagent (HRP juice; PJK GmbH) and a ChemiSmart5000 digital imaging system (Vilber-Lourmat). Densitometric quantification was performed with the Fiji software (57).
Cell transfection
For siRNA experiments, C2C12 myoblasts were transfected with 15 nm siRNA (scrambled siRNA: a nontargeting negative control siRNA; Lrrc8a siRNA1: sense, CCU UGU AAG UGG GUC ACC ATT; Lrrc8a siRNA2: sense, GAU CGA CAC CAG UAC AAC UTT; Thermo Fisher Scientific) using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. For overexpression, 2 μg ml−1 of plasmid DNA of pEGFP-N1-LRRC8A, pmRFP-N1-LRRC8A (kindly provided by T. J. Jentsch) (24) or expression vectors pEGFP-N1, pmRFP-N1 (Clontech) was transfected into cells using FuGENE 6 (Promega) according to the manufacturer's instructions. Cells were induced to differentiate 1 day after transfection.
Cell proliferation assay
C2C12 myoblasts were seeded at 5000 cells per well in 96-well plates and transfected with siRNA or plasmids the next day. Cell viability was evaluated with the Cell Counting Kit-8 (Sigma-Aldrich) at 24 and 48 h post transfection. The absorbance of the water-soluble formazan dye produced from tetrazolium salt WST-8 by cellular dehydrogenase activity was measured at 450 nm using a microplate reader (Biochrom).
Quantitative real-time PCR
Total RNA was isolated from C2C12 cells with a NucleoSpin RNA Kit (Macherey & Nagel). SuperScript II Reverse Transcriptase (Invitrogen), Oligo (dT)20 Primer (Invitrogen), and 1 μg of total RNA as template were used for cDNA synthesis. To assess gene expression, standard quantitative PCR was conducted with Power SYBR Green PCR Master Mix (Applied Biosystems) on StepOnePlus Real-Time PCR System (Applied Biosystems). Results were analyzed with the comparative cycle threshold CT (ΔΔCT) method by using Gapdh as the reference gene. Primers included Lrrc8c-F, 5′-TCC TTT TCT GCG GAT ACC CT-3′; Lrrc8c-R, 5′-AAC TCG GTC ACC GGA ATC AT-3′; Myog-F, 5′-CCA AGG TCT CCT GTG CTG ATG-3′; Myog-R, 5′-TTG GCA AAA CCA CAC AAT GC-3′; Gapdh-F, 5′-TGC GAC TTC AAC AGC AAC TC-3′; Gapdh-R, 5′-GCC TCT CTT GCT CAG TGT CC-3′.
Fluorescence measurement of plasma membrane potential
Membrane potential measurements using the fluorescent bis-oxonol type plasma membrane potential indicator DiBAC4(3) were performed as described previously (7, 43). C2C12 myoblasts growing in 8-well chambers (Sarstedt) were washed twice and then incubated at 37 °C in imaging buffer (containing in mm: 144 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, pH 7.4) with DiBAC4(3) (1 μm; Molecular Probes) for 30 min. After incubation, measurements were performed at room temperature on a DMi8 fluorescence microscope (Leica Microsystems) with a 63×/1.40 NA oil-immersion objective. DiBAC4(3) fluorescence images were acquired at 16-bit, 4 × 4 binning, and 50-msec exposure with a FITC filter set (Ex: 480/40, Dc: 505, Em: 527/30) and an OcraFlash 4.0 camera (Hamamatsu). Calibration for each experiment was performed by adding gramicidin (20 μg ml−1; Sigma-Aldrich) to isotonic imaging buffer containing varying ratios of Na+ and N-methyl-d-glucamine, maintaining [Na+] + [N-methyl-d-glucamine] = 144 mm, in individual wells of 8-well chambers. The membrane potential (Em) was calculated with the Nernst equation (Equation 1):
| (Eq. 1) |
where R is the universal gas constant, T = 298 K, z = 1, F is the Faraday constant, [K+]o = 5 mm; [Na+]i + [K+]i is assumed to be 150 mm.
Electrophysiological measurement of plasma membrane potential
Whole-cell patch clamp recordings of the resting membrane potential were performed at room temperature with a MultiClamp 700B (Axon Instruments, Molecular Devices) and electrodes with an average resistance of 4 MOhm (range: 3.3–4.8 MOhm) in a submerged chamber containing the external solution containing (in mm): 144 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, pH 7.4, with NaOH, 329 mOsm. C2C12 myoblasts were visualized using IR differential interference contrast optics and an IR video camera (PIKE F-145B, Allied Vision). Recordings were filtered at 10 kHz and sampled at 20 kHz with a Digidata 1550A (Axon Instruments, Molecular Devices). The internal solution consisted of (in mm): 125 potassium gluconate, 5 KCl, 1 EGTA, 2 Na2ATP, 2 MgATP, 0.3 Na2GTP, 10 sodium phosphocreatine, 10 HEPES, pH 7.25, with KOH, 280 mOsm. Recorded traces were corrected for liquid junction potential. The resting membrane potential was determined from the first 15 s of each recorded trace to avoid membrane potential changes because of cytosol washout.
Cytosolic Ca2+ imaging
C2C12 cells growing on glass-bottom dishes (MatTek) were loaded with mix of Fura-2 AM (5 μm; Invitrogen) and Pluronic F-127 (0.02%; Invitrogen) in culture medium at 37°C for 30 min and then washed with imaging buffer containing (in mm): 145 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, pH 7.4. Imaging was performed at room temperature on a DMi8 microscope (Leica Microsystems) equipped with a 63×/1.40 NA oil-immersion objective and an OcraFlash 4.0 camera (Hamamatsu). Samples were excited with an Optoscan monochromator (Cairn Research) at 340 or 380 nm; emission was recorded using a Fura-2 filter set (Dc: 410, Em: 510/84; AHF analysentechnik) with the Winfluor software. At the end of each experiment, Rmax was achieved by the addition of 1 μm ionomycin. No significant variation of Rmax was observed between experiments.
Image processing and quantitative analysis
For both DiBAC4(3) and Fura-2 ratiometric images, the measurement of mean fluorescence intensities of regions of interest was carried out with Fiji. The mean fluorescence intensity of background near the region of interest was subtracted. Fluorescence images of five random fields were analyzed for each sample per experiment. The membrane potential or 340/380 ratio (Ca2+ imaging) from each field per sample was compiled and depicted as mean ± S.D.
Statistical analysis
All data are presented as mean ± S.D.; p values between two groups were determined by a two-tailed unpaired Student's t test. For three or more groups, a one-way analysis of variance (ANOVA) with Bonferroni's post hoc test was performed. p values are indicated according to convention: *, p < 0.05, **, p < 0.01, and ***, p < 0.001, n.s. = not significant.
Author contributions
L. C. and T. M. B. formal analysis; L. C. and T. M. B. investigation; L. C. visualization; L. C., T. M. B., U. K., and T. S. methodology; L. C. and T. S. writing-original draft; L. C. and T. S. writing-review and editing; L. C. and T. S. conceptualization; U. K. and T. S. resources; U. K. and T. S. supervision; T. S. funding acquisition; T. S. project administration.
Acknowledgments
We thank Thomas Jentsch and Petra Knaus for providing antibodies, plasmids, and the C2C12 cell line; Lisa von Kleist, Antje Buttgereit, Benjamin König, and Jerome Jatzlau for experimental support; and Petra Knaus, Sigmar Stricker, Xiaoyan Wei and all group members of the Stauber lab for scientific discussions.
This work was supported by the Oversea Study Program of Guangzhou Elite Project JY201624 Fellowship (to L. C.). The research group is supported by the German Federal Ministry of Education and Research BMBF Grant 031A314 (to T. S.). The authors declare that they have no conflicts of interest with the contents of this article.
- VRAC
- volume-regulated anion channel
- ANOVA
- analysis of variance
- CBX
- carbenoxolone
- DCPIB
- 4-(2-butyl-6, 7-dichloro-2-cyclopentyl-indan-1-on-5-yl) oxybutyric acid
- DiBAC4(3)
- bis-(1, 3-dibarbituric acid)-trimethine oxanol
- LRRC8
- leucine-rich repeat containing 8
- NFA
- niflumic acid
- NPPB
- 5-nitro-2-(3-phenylpropylamino) benzoic acid
- RFP
- red fluorescent protein.
References
- 1. Kang J. S., and Krauss R. S. (2010) Muscle stem cells in developmental and regenerative myogenesis. Curr. Opin. Clin. Nutr. Metab. Care 13, 243–248 10.1097/MCO.0b013e328336ea98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bentzinger C. F., Wang Y. X., and Rudnicki M. A. (2012) Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 4, a008342 10.1101/cshperspect.a008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hindi S. M., Tajrishi M. M., and Kumar A. (2013) Signaling mechanisms in mammalian myoblast fusion. Sci. Signal. 6, re2 10.1126/scisignal.2003832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bijlenga P., Occhiodoro T., Liu J. H., Bader C. R., Bernheim L., and Fischer-Lougheed J. (1998) An ether-à-go-go K+ current, Ih-eag, contributes to the hyperpolarization of human fusion-competent myoblasts. J. Physiol. 512, 317–323 10.1111/j.1469-7793.1998.317be.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu J. H., Bijlenga P., Fischer-Lougheed J., Occhiodoro T., Kaelin A., Bader C. R., and Bernheim L. (1998) Role of an inward rectifier K+ current and of hyperpolarization in human myoblast fusion. J. Physiol. 510, 467–476 10.1111/j.1469-7793.1998.467bk.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fischer-Lougheed J., Liu J. H., Espinos E., Mordasini D., Bader C. R., Belin D., and Bernheim L. (2001) Human myoblast fusion requires expression of functional inward rectifier Kir2.1 channels. J. Cell Biol. 153, 677–686 10.1083/jcb.153.4.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konig S., Hinard V., Arnaudeau S., Holzer N., Potter G., Bader C. R., and Bernheim L. (2004) Membrane hyperpolarization triggers myogenin and myocyte enhancer factor-2 expression during human myoblast differentiation. J. Biol. Chem. 279, 28187–28196 10.1074/jbc.M313932200 [DOI] [PubMed] [Google Scholar]
- 8. Sundelacruz S., Levin M., and Kaplan D. L. (2009) Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. 5, 231–246 10.1007/s12015-009-9080-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Przybylski R. J., MacBride R. G., and Kirby A. C. (1989) Calcium regulation of skeletal myogenesis. I. Cell content critical to myotube formation. In Vitro Cell. Dev. Biol. 25, 830–838 10.1007/BF02623667 [DOI] [PubMed] [Google Scholar]
- 10. Przybylski R. J., Szigeti V., Davidheiser S., and Kirby A. C. (1994) Calcium regulation of skeletal myogenesis. II. Extracellular and cell surface effects. Cell Calcium 15, 132–142 10.1016/0143-4160(94)90052-3 [DOI] [PubMed] [Google Scholar]
- 11. Bijlenga P., Liu J. H., Espinos E., Haenggeli C. A., Fischer-Lougheed J., Bader C. R., and Bernheim L. (2000) T-type a1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc. Natl. Acad. Sci. U.S.A. 97, 7627–7632 10.1073/pnas.97.13.7627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakanishi K., Kakiguchi K., Yonemura S., Nakano A., and Morishima N. (2015) Transient Ca2+ depletion from the endoplasmic reticulum is critical for skeletal myoblast differentiation. FASEB J. 29, 2137–2149 10.1096/fj.14-261529 [DOI] [PubMed] [Google Scholar]
- 13. Konig S., Béguet A., Bader C. R., and Bernheim L. (2006) The calcineurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development 133, 3107–3114 10.1242/dev.02479 [DOI] [PubMed] [Google Scholar]
- 14. Iannotti F. A., Barrese V., Formisano L., Miceli F., and Taglialatela M. (2013) Specification of skeletal muscle differentiation by repressor element-1 silencing transcription factor (REST)-regulated Kv7.4 potassium channels. Mol. Biol. Cell 24, 274–284 10.1091/mbc.e11-12-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iannotti F. A., Silvestri C., Mazzarella E., Martella A., Calvigioni D., Piscitelli F., Ambrosino P., Petrosino S., Czifra G., Bíró T., Harkany T., Taglialatela M., and Di Marzo V. (2014) The endocannabinoid 2-AG controls skeletal muscle cell differentiation via CB1 receptor-dependent inhibition of Kv7 channels. Proc. Natl. Acad. Sci. U.S.A. 111, E2472–E2481 10.1073/pnas.1406728111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Afzali A. M., Ruck T., Herrmann A. M., Iking J., Sommer C., Kleinschnitz C., Preuße C., Stenzel W., Budde T., Wiendl H., Bittner S., and Meuth S. G. (2016) The potassium channels TASK2 and TREK1 regulate functional differentiation of murine skeletal muscle cells. Am. J. Physiol. Cell Physiol. 311, C583–C595 10.1152/ajpcell.00363.2015 [DOI] [PubMed] [Google Scholar]
- 17. Antigny F., Konig S., Bernheim L., and Frieden M. (2014) Inositol 1,4,5 trisphosphate receptor 1 is a key player of human myoblast differentiation. Cell Calcium 56, 513–521 10.1016/j.ceca.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 18. Darbellay B., Arnaudeau S., König S., Jousset H., Bader C., Demaurex N., and Bernheim L. (2009) STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J. Biol. Chem. 284, 5370–5380 10.1074/jbc.M806726200 [DOI] [PubMed] [Google Scholar]
- 19. Woo J. S., Cho C. H., Kim D. H., and Lee E. H. (2010) TRPC3 cation channel plays an important role in proliferation and differentiation of skeletal muscle myoblasts. Exp. Mol. Med. 42, 614–627 10.3858/emm.2010.42.9.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antigny F., Koenig S., Bernheim L., and Frieden M. (2013) During post-natal human myogenesis, normal myotube size requires TRPC1- and TRPC4-mediated Ca2+ entry. J. Cell Sci. 126, 2525–2533 10.1242/jcs.122911 [DOI] [PubMed] [Google Scholar]
- 21. Zanou N., Schakman O., Louis P., Ruegg U. T., Dietrich A., Birnbaumer L., and Gailly P. (2012) Trpc1 ion channel modulates phosphatidylinositol 3-kinase/Akt pathway during myoblast differentiation and muscle regeneration. J. Biol. Chem. 287, 14524–14534 10.1074/jbc.M112.341784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar L., Chou J., Yee C. S., Borzutzky A., Vollmann E. H., von Andrian U. H., Park S. Y., Hollander G., Manis J. P., Poliani P. L., and Geha R. S. (2014) Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J. Exp. Med. 211, 929–942 10.1084/jem.20131379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiu Z., Dubin A. E., Mathur J., Tu B., Reddy K., Miraglia L. J., Reinhardt J., Orth A. P., and Patapoutian A. (2014) SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157, 447–458 10.1016/j.cell.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Voss F. K., Ullrich F., Münch J., Lazarow K., Lutter D., Mah N., Andrade-Navarro M. A., von Kries J. P., Stauber T., and Jentsch T. J. (2014) Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344, 634–638 10.1126/science.1252826 [DOI] [PubMed] [Google Scholar]
- 25. König B., and Stauber T. (2019) Biophysics and structure-function relationships of LRRC8-formed volume-regulated anion channels. Biophys. J. 116, 1185–1193 10.1016/j.bpj.2019.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stauber T. (2015) The volume-regulated anion channel is formed by LRRC8 heteromers—molecular identification and roles in membrane transport and physiology. Biol. Chem. 396, 975–990 10.1515/hsz-2015-0127 [DOI] [PubMed] [Google Scholar]
- 27. Jentsch T. J. (2016) VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat. Rev. Mol. Cell Biol. 17, 293–307 10.1038/nrm.2016.29 [DOI] [PubMed] [Google Scholar]
- 28. Strange K., Yamada T., and Denton J. S. (2019) A 30-year journey from volume-regulated anion currents to molecular structure of the LRRC8 channel. J. Gen. Physiol. 151, 100–117 10.1085/jgp.201812138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen L., König B., Liu T., Pervaiz S., Razzaque Y. S., and Stauber T. (2019) More than just a pressure release valve: Physiological roles of volume-regulated LRRC8 anion channels. Biol. Chem. 10.1515/hsz-2019-0189 [DOI] [PubMed] [Google Scholar]
- 30. Okada Y., Okada T., Sato-Numata K., Islam M. R., Ando-Akatsuka Y., Numata T., Kubo M., Shimizu T., Kurbannazarova R. S., Marunaka Y., and Sabirov R. Z. (2019) Cell volume-activated and volume-correlated anion channels in mammalian cells: Their biophysical, molecular, and pharmacological properties. Pharmacol. Rev. 71, 49–88 10.1124/pr.118.015917 [DOI] [PubMed] [Google Scholar]
- 31. Pedersen S. F., Okada Y., and Nilius B. (2016) Biophysics and physiology of the volume-regulated anion channel (VRAC)/volume-sensitive outwardly rectifying anion channel (VSOR). Pflügers Arch. 468, 371–383 10.1007/s00424-015-1781-6 [DOI] [PubMed] [Google Scholar]
- 32. König B., Hao Y., Schwartz S., Plested A. J., and Stauber T. (2019) A FRET sensor of C-terminal movement reveals VRAC activation by plasma membrane DAG signaling rather than ionic strength. Elife 8, e45421 10.7554/eLife.45421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kang C., Xie L., Gunasekar S. K., Mishra A., Zhang Y., Pai S., Gao Y., Kumar A., Norris A. W., Stephens S. B., and Sah R. (2018) SWELL1 is a glucose sensor regulating β-cell excitability and systemic glycaemia. Nat. Commun. 9, 367 10.1038/s41467-017-02664-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stuhlmann T., Planells-Cases R., and Jentsch T. J. (2018) LRRC8/VRAC anion channels enhance β-cell glucose sensing and insulin secretion. Nat. Commun. 9, 1974 10.1038/s41467-018-04353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mongin A. A. (2016) Volume-regulated anion channel—a frenemy within the brain. Pflügers Arch. 468, 421–441 10.1007/s00424-015-1765-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang J., Vitery M. D. C., Chen J., Osei-Owusu J., Chu J., and Qiu Z. (2019) Glutamate-releasing SWELL1 channel in astrocytes modulates synaptic transmission and promotes brain damage in stroke. Neuron 102, 813–827.e6 10.1016/j.neuron.2019.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu J. H., König S., Michel M., Arnaudeau S., Fischer-Lougheed J., Bader C. R., and Bernheim L. (2003) Acceleration of human myoblast fusion by depolarization: Graded Ca2+ signals involved. Development 130, 3437–3446 10.1242/dev.00562 [DOI] [PubMed] [Google Scholar]
- 38. Benfenati V., Caprini M., Nicchia G. P., Rossi A., Dovizio M., Cervetto C., Nobile M., and Ferroni S. (2009) Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels (Austin) 3, 323–336 10.4161/chan.3.5.9568 [DOI] [PubMed] [Google Scholar]
- 39. Decher N., Lang H. J., Nilius B., Brüggemann A., Busch A. E., and Steinmeyer K. (2001) DCPIB is a novel selective blocker of ICl,swell and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br. J. Pharmacol. 134, 1467–1479 10.1038/sj.bjp.0704413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Friard J., Tauc M., Cougnon M., Compan V., Duranton C., and Rubera I. (2017) Comparative effects of chloride channel inhibitors on LRRC8/VRAC-mediated chloride conductance. Front. Pharmacol. 8, 328 10.3389/fphar.2017.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sato-Numata K., Numata T., Inoue R., and Okada Y. (2016) Distinct pharmacological and molecular properties of the acid-sensitive outwardly rectifying (ASOR) anion channel from those of the volume-sensitive outwardly rectifying (VSOR) anion channel. Pflügers Arch. 468, 795–803 10.1007/s00424-015-1786-1 [DOI] [PubMed] [Google Scholar]
- 42. Hinard V., Belin D., Konig S., Bader C. R., and Bernheim L. (2008) Initiation of human myoblast differentiation via dephosphorylation of Kir2.1 K+ channels at tyrosine 242. Development 135, 859–867 10.1242/dev.011387 [DOI] [PubMed] [Google Scholar]
- 43. Dall'Asta V., Gatti R., Orlandini G., Rossi P. A., Rotoli B. M., Sala R., Bussolati O., and Gazzola G. C. (1997) Membrane potential changes visualized in complete growth media through confocal laser scanning microscopy of bis-oxonol-loaded cells. Exp. Cell Res. 231, 260–268 10.1006/excr.1996.3469 [DOI] [PubMed] [Google Scholar]
- 44. Liu T., and Stauber T. (2019) The volume-regulated anion channel LRRC8/VRAC is dispensable for cell proliferation and migration. Int. J. Mol. Sci. 20, E2663 10.3390/ijms20112663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Y., Xie L., Gunasekar S. K., Tong D., Mishra A., Gibson W. J., Wang C., Fidler T., Marthaler B., Klingelhutz A., Abel E. D., Samuel I., Smith J. K., Cao L., and Sah R. (2017) SWELL1 is a regulator of adipocyte size, insulin signalling and glucose homeostasis. Nat. Cell Biol. 19, 504–517 10.1038/ncb3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neveux I., Doe J., Leblanc N., and Valencik M. L. (2010) Influence of the extracellular matrix and integrins on volume-sensitive osmolyte anion channels in C2C12 myoblasts. Am. J. Physiol. Cell Physiol. 298, C1006–C1017 10.1152/ajpcell.00359.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carton I., Trouet D., Hermans D., Barth H., Aktories K., Droogmans G., Jorgensen N. K., Hoffmann E. K., Nilius B., and Eggermont J. (2002) RhoA exerts a permissive effect on volume-regulated anion channels in vascular endothelial cells. Am. J. Physiol. Cell Physiol. 283, C115–C125 10.1152/ajpcell.00038.2001 [DOI] [PubMed] [Google Scholar]
- 48. Walsh K., and Perlman H. (1997) Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 7, 597–602 10.1016/S0959-437X(97)80005-6 [DOI] [PubMed] [Google Scholar]
- 49. Pietrangelo T., Fioretti B., Mancinelli R., Catacuzzeno L., Franciolini F., Fanò G., and Fulle S. (2006) Extracellular guanosine-5′-triphosphate modulates myogenesis via intermediate Ca2+-activated K+ currents in C2C12 mouse cells. J. Physiol. 572, 721–733 10.1113/jphysiol.2005.102194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Voets T., Wei L., De Smet P., Van Driessche W., Eggermont J., Droogmans G., and Nilius B. (1997) Downregulation of volume-activated Cl− currents during muscle differentiation. Am. J. Physiol. 272, C667–C674 10.1152/ajpcell.1997.272.2.C667 [DOI] [PubMed] [Google Scholar]
- 51. Mandai S., Furukawa S., Kodaka M., Hata Y., Mori T., Nomura N., Ando F., Mori Y., Takahashi D., Yoshizaki Y., Kasagi Y., Arai Y., Sasaki E., Yoshida S., Furuichi Y., Fujii N. L., Sohara E., Rai T., and Uchida S. (2017) Loop diuretics affect skeletal myoblast differentiation and exercise-induced muscle hypertrophy. Sci. Rep. 7, 46369 10.1038/srep46369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leem Y. E., Jeong H. J., Kim H. J., Koh J., Kang K., Bae G. U., Cho H., and Kang J. S. (2016) Cdo regulates surface expression of Kir2.1 K+ channel in myoblast differentiation. PLoS One 11, e0158707 10.1371/journal.pone.0158707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang M., and Brackenbury W. J. (2013) Membrane potential and cancer progression. Front. Physiol. 4, 185 10.3389/fphys.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sundelacruz S., Levin M., and Kaplan D. L. (2008) Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One 3, e3737 10.1371/journal.pone.0003737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sacco S., Giuliano S., Sacconi S., Desnuelle C., Barhanin J., Amri E. Z., and Bendahhou S. (2015) The inward rectifier potassium channel Kir2.1 is required for osteoblastogenesis. Hum. Mol. Genet. 24, 471–479 10.1093/hmg/ddu462 [DOI] [PubMed] [Google Scholar]
- 56. Planells-Cases R., Lutter D., Guyader C., Gerhards N. M., Ullrich F., Elger D. A., Kucukosmanoglu A., Xu G., Voss F. K., Reincke S. M., Stauber T., Blomen V. A., Vis D. J., Wessels L. F., Brummelkamp T. R., Borst P., Rottenberg S., and Jentsch T. J. (2015) Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 34, 2993–3008 10.15252/embj.201592409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J. Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., and Cardona A. (2012) Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]