Abstract
Treating acute kidney injury (AKI) represents an important unmet medical need both in terms of the seriousness of this medical problem and the number of patients. There is also a large untapped market opportunity in treating AKI. Over the years, there has been much effort in search of therapeutics with minimal success. However, over the same time period, new understanding of the underlying pathobiology and molecular mechanisms of kidney injury have undoubtedly helped the search for new therapeutics. Along this line, carbon monoxide (CO) has emerged as a promising therapeutic agent because of its demonstrated cytoprotective, and immunomodulatory effects. CO has also been shown to sensitize cancer, but not normal cells, to chemotherapy. This is particularly important in treating cisplatin-induced AKI, a common clinical problem that develops in patients receiving cisplatin therapies for a number of different solid organ malignancies. This review will examine and make the case that CO be developed into a therapeutic agent against AKI.
Keywords: Carbon monoxide, Acute kidney injury, Nephrotoxicity, CO-RMs, Organic CO prodrugs
1. Introduction
Acute kidney injury (AKI) is a common, global health concern that manifests as a rapid decline in kidney function that occurs over a 7-day period or less.1–3 Despite this simple and homogeneous definition, AKI is in fact a highly heterogeneous clinical syndrome that is precipitated by different injurious events, often in combination, including dehydration, heart failure, blood loss, major trauma including surgery and crush injury, sepsis and chemical toxicities.2–4 AKI also commonly occurs in association with comorbidities including pre-existing chronic kidney disease (CKD), old age, diabetes and heart failure, all of which increase the risk of AKI and may influence the underlying pathobiology and response to treatment.2–4 AKI from any cause is a strong, independent predictor of mortality, and often results in incomplete recovery, giving rise to CKD, and in some patients, end stage renal disease (ESRD).2–4 Despite the clinical significance of this problem, supportive care, avoiding additional kidney injury, and when possible, removing the initial injurious event, are the primary treatment options. Currently, no therapeutic interventions have been shown to definitively prevent AKI, improve recovery, or improve long-term outcomes after AKI, including CKD, ESRD or death.5–8 In terms of the scale of the problems, AKI has been reported to complicate up to 20% of hospitalizations9,10 with a prevalence rate estimated to be 1.8 in 1000 in the general population in a 2007 study.11 In a 2018 report by MarketWatch, the number of AKI patients in 2016 was estimated to be over 1.5M with the market size being $4.3B in the seven major markets including the US, EU5 (Germany, Spain, Italy, France, and UK), and Japan.12 For these reasons, there is a broad consensus amongst the clinical and basic AKI research communities that development of the first effective disease modifying treatments for AKI will address an important, unmet medical need.13
In this review, we will examine the common causes of AKI, discuss existing efforts in search of therapeutic agents for AKI, and focus on a particularly promising cytoprotective agent, carbon monoxide (CO), with analyses of the current state of the field and future directions of research in this area.
2. Causes and Mechanisms of Acute Kidney Injury
In order to examine the issue of developing possible therapeutics, it is important to first look at the etiology and pathogenic mechanisms of AKI. A number of common pathogenic mechanisms have been identified from (often imperfect) pre-clinical models of AKI.14 These include oxidative stress resulting from the release of cell free hemoglobin into the circulation of patients undergoing cardiopulmonary bypass, and from direct toxicity of radiographic contrast agents for renal tubular epithelial cells; direct renal tubular and vascular cell damage, and secondary inflammatory cell damage resulting from reduction in renal blood flow, ischemia-reperfusion injury (IRI), and microvascular thrombosis that occur during cardiac bypass surgery and with the use of radiographic contrast agents.15,16 In addition, signaling mediated by the release of Damage Associated Molecular Pattern (DAMP) molecules promotes activation of systemic inflammatory responses in patients undergoing cardiac surgery and has been implicated as a mechanism of inflammation associated renal damage in these patients.17 Based on these findings, a number of unsuccessful clinical studies have evaluated the use of a variety of antioxidants and vasodilators as preventive strategies to reduce AKI after cardiac surgery and following administration of iodinated radiographic contrast agents.14 Standard approaches of using steroids and immunosuppressive agents to suppress inflammation are effective in patients with drug-induced allergic interstitial nephritis, which accounts for up to 20% of patients presenting with unexplained AKI,18 but are of no proven benefit for either the prevention or treatment of other forms of AKI. More recently, there has been interest in the use of immunomodulatory therapies targeting exaggerated inflammatory responses, including interference with DAMP signaling, modification of upstream events associated with defects in cellular metabolism and mitochondrial damage, and targeting regulators of cellular apoptosis in patients with AKI.17,19,20 However, many of these have yet to be evaluated in humans, and none has shown significant effects on AKI incidence or outcomes. These studies also indicate the complexity of molecular and pathway-level mechanisms of AKI induction and progression depending on etiological origin, and suggest challenges ahead in developing therapeutics to either prevent or treat AKI, examples of which are discussed below.
2.1. Cardiac Surgery-induced AKI
Cardiac surgery is an established risk factor for AKI: 10-30% of patients undergoing cardiac surgery develop some degree of AKI with 1-3% progressing to severe dialysis-dependent AKI (AKI-D).21,22 AKI after cardiac surgery has been attributed to the effects of nephrotoxins, hypoxia, mechanical trauma, inflammation, cardiopulmonary bypass, and hemodynamic instability.23 Generally accepted practices aimed at reducing the risk of AKI include the choice of IV fluids and vasoactive agents, glucose control, and even remote ischemic preconditioning, which showed beneficial effects in some,24 but not all studies.25 No pharmacologic agents have been shown definitively to either reduce the risk of AKI or treat established AKI after cardiac surgery.
2.2. Delayed Graft Function after Renal Transplantation
Non-immunological AKI occurs after renal transplantation and presents a distinct clinical and therapeutic opportunity for AKI prevention.13 Delayed graft function (DGF) in renal transplant recipients is defined as the occurrence of severe AKI without immunological rejection that requires dialysis within 7 days of the transplant. DGF most commonly occurs after cadaveric donor kidney transplantation, resulting from prolonged ischemic time during and after the donor nephrectomy. As such, DGF shares many of the same pathophysiological mechanisms associated with reduced renal blood flow and ischemia-reperfusion tissue injury that occurs in cardiac surgery and contrast-induced AKI.26 DGF is associated with a reduction in long-term renal transplant survival.27,28 Given the shortage of suitable kidney donors for renal transplantation,29 there is a particular need to develop effective therapies that prevent DGF. Therapeutic interventions to reduce DGF are focused largely on improving regional renal blood flow, decreasing inflammation, and reducing oxidative stress that result from ischemia-reperfusion induced tissue injury.26,30 However, unlike other forms of AKI, therapeutic interventions may be targeted to both the recipient with AKI and to the donor kidney after nephrectomy but before transplantation by modifications to the organ preservation solutions.26 In addition, because of the accessibility and therefore safety of performing percutaneous biopsies of donor kidneys before and after transplantation,31 clinical studies can be extended to include sequential renal biopsies of the kidney before and after transplantation. This provides an unprecedented opportunity to monitor and study the molecular and cellular responses to these therapeutic interventions in the target organ.13
2.2. Chemically-induced AKI
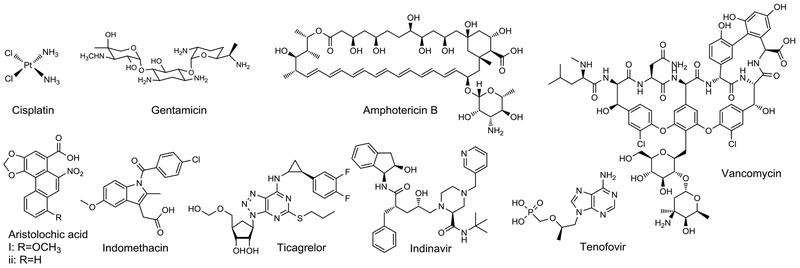
Chemically induced kidney injury is a common cause of AKI. Drug-induced AKI is the second most common cause of hospital-acquired AKI.32 Other chemicals that are known to induce kidney injuries include diagnostic agents, metal ions, toxins in food, nutritional supplements, and the environment. Especially important are AKI induced by drugs such as cisplatin (CP), antibiotics, and iodinated radiographic contrast agents (Figure 1). Other chemicals including the Chinese herbal extract, aristolochic acid (AA), and heavy metals, are less common causes of AKI, but have been included in this review for completeness. Below we discuss each category in the context of potential therapeutic and preventative interventions.
Figure 1.
Compounds known to induce AKI.
2.2.1. Cisplatin
Cisplatin (CP)-induced AKI (CP-AKI) is a common and clinically significant problem that not only promotes AKI and CKD in patients undergoing cancer chemotherapy, but also limits the ability of many patients to complete effective courses of chemotherapy to treat their cancer.13 CP is one of the oldest and still most effective, first-line therapies for a diverse range of solid organ malignancies including testicular,33 ovarian,34 lung,35 head and neck,36 cervical,37 bladder,38 and gastric cancers.39 However, its use is often limited by nephrotoxicity, resulting from the accumulation of CP in the kidney. Second and third generations of CP analogs have been developed that are less nephrotoxic, but none of these has the same anti-tumor efficacy as CP itself.39 Like other forms of AKI, CP-AKI is more common in the elderly and in patients with CKD.39 Mechanisms of injury are thought to result from direct genomic and mitochondrial DNA damage giving rise to increased oxidative stress, inflammation, and tubular cell death.40,41 Cancer patients typically undergo repeated cycles of CP treatment. Hydration with intravenous fluids reduce the rate of AKI, but ~30% of patients still develop AKI, which not only limits their ability to complete long-term treatment regimens required to control the cancer, but also increases the risk of CKD progression in cancer survivors.42 Therapeutic intervention in these patients may not only reduce the rate of AKI and CKD progression after CP treatment, but will also allow patients to complete potentially curative courses of chemotherapy that would otherwise be impossible because of CP-AKI.13 This provides a unique opportunity for therapeutic development, in which the efficacy of therapeutic interventions for CP-AKI might be evaluated using less challenging, but still significant, non-renal primary end-points, including the impact of the therapy on cancer-free survival.13
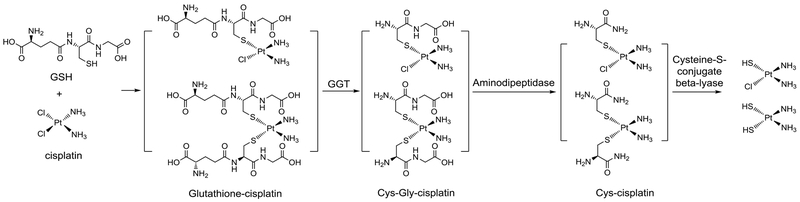
One of the challenges in developing therapeutics for CP-AKI is that many of the approaches that might be used to reduce oxidative stress, inflammation and tubular cell death, also reduce the efficacy of CP as chemotherapy.41 The mechanism of CP-AKI is recognized as a complex multifactorial process, including uptake in the kidney, metabolic activation, and tissue damage. Kidney-specific uptake of CP occurs via highly expressed transporters in renal proximal tubular cells, including organic cation transporters and copper transporter, Ctr1.40 The large surface area of the proximal tubules and high vascular perfusion rates account for the high concentrations of CP found in proximal tubular cells after CP treatment.43 Once internalized, CP forms GSH-CP conjugates with glutathione, which are then metabolized by γ-glutamyl transpeptidase (GGT) and cysteine-S-conjugate β-lyase into the activated form of nephrotoxic Cys-Gly-CP and Cys-CP conjugates. These intermediates end up forming the cytotoxic reactive thiol species (Figure 2).43,44 Short-term cytotoxicity of these cysteine S-CP conjugates is twice as high as free CP (which is not toxic at 50 μM) in confluent LLC-PK1 cells. Of note, glutathione is involved in the usual detoxification mechanism; however, here it also contributes to CP cytotoxicity in non-dividing proximal tubular cells. In contrast, in rapidly-growing cancer cells glutathione abolishes CP’s anticancer effect due to the decreased DNA intercalating activity of the conjugates. Furthermore, it is important to note that γ-glutamyl transpeptidase and cysteine-S-conjugate β-lyase are highly expressed in proximal tubular cells, contributing to the preferential toxicity of CP in the kidney. These fundamental chemistry problems of CP exacerbate the issue of CP-AKI and CP efficacy. Mechanistically, both apoptosis and necrosis are involved in the tissue damage process. The intrinsic and extrinsic pathways of CP-induced apoptosis of kidney cells have been systematically reviewed.40 All such information helps the analysis of the underlying pathology for AKI, and the development of preventative and therapeutic agents.
Figure 2.
Formation of cysteine S-cisplatin conjugates. (Summarized based on related reports43–48 and reviews47,49)
2.2.2. Antibiotic-induced AKI
Aside from drug-induced allergic interstitial nephritis,18 antibiotics, particularly aminoglycosides, are well known for their ototoxicity and nephrotoxicity.50 The nephrotoxicity is mostly attributed to the cationic property of this class of antibiotics, which facilitates their accumulation in the lysosome of proximal tubular cells, leading to disruption of protein synthesis and mitochondrial function,51 and induction of ROS generation.52 Of note, gentamicin-induced ototoxicity and nephrotoxicity are dependent on the circadian pharmacodynamics at the cell, tissue, and organ levels.53 Incidentally, CO achieves its nephroprotective effect partially through regulating the circadian clock.54 This aspect will be discussed in the CO gas section of this review. Nephrotoxicity is also a major issue of the anti-fungal drug amphotericin B.55 The induction of pre-glomerular vasoconstriction due to changes in ion-permeability is believed to be a major mechanism of nephrotoxicity. Another antibacterial drug, vancomycin, which is commonly used for the treatment of multiple-drug resistant bacterial infections, is also known for its nephrotoxicity either alone or in synergy with aminoglycosides, depending on the dose, disease severity, renal function, and co-administration of other nephrotoxic medications.56 Although the mechanism of vancomycin-induced AKI is not fully understood, mitochondrion-related induction of ROS generation is reported to be a major factor in its pathophysiology. Interestingly, lipophilic (such as vitamin E), but not hydrophilic antioxidants (such as vitamin C or N-acetyl cysteine), are effective in inhibiting vancomycin-induced AKI.57
2.2.3. Radiologic Contrast Agent-Induced AKI (CI-AKI)
Iodinated contrast agents, which are used for a variety of different radiological investigations, are the third most common cause of hospital-acquired AKI,58 accounting for more than 10% of cases.59 Approximately 7% of patients being treated with radiologic contrast agents for percutaneous coronary interventions develop AKI, and 0.3% with AKI require dialysis, which carries a >30% risk of death.60 The problem is more pronounced in those with CDK, diabetes, and old age. The pathogenesis of CI-AKI is multifactorial with the mechanism being attributed to direct epithelial cell cytotoxicity through the generation of ROS,59 inflammatory cytokines,61 and from reduced renal arteriolar blood flow and glomerular filtration rate due to high viscosity and osmotic effects.58 The nephrotoxicity problem of contrast agents presents serious problems in diagnosing various diseases in vulnerable populations. A therapeutic agent that can either prevent or treat established AKI is expected to make a significant difference in patient care.
2.2.4. AKI Induced by Exposure to Toxins in the Environment, Food, and Nutritional Supplements
Heavy metals are common toxic contaminants in the soil and water. The kidney and liver are the main targets of heavy metal-induced toxicity. From an occupational safety point of view, this is an important contemporary issue. In clinical practice, metal-induced kidney toxicity had become less and less of a problem, at least in developed countries, until the recent approval of Trisenox® (arsenic trioxide) for the treatment of acute promyelocytic leukemia (APL), which brought the issue of metal-induced kidney toxicity to the forefront again because of the known nephrotoxicity associated with arsenic trioxide.62,63 Another sad aspect of metal toxicity is the on-going occurrence of intentional metal-poisoning,64–66 unintentional use because of a lack of education of the general population and/or regulatory guidelines governing traditional medicine,67–70 or by pure accident.71,72 Arsenic (As), cadmium (Cd), lead (Pb), and mercury (Hg) are the most prevalent environmental metal toxicants. They are all known to induce nephrotoxicity at different concentrations due to their preferential accumulation in the kidney by receptor-mediated endocytosis in the proximal tubular cells.71 For example, the kidney can accumulate as much as 50% of chronically consumed Cd2+.73 The receptors are primarily found to be GLUT (glucose transporter) 1 and GLUT5 for As74 and DMT1 (divalent metal ion transporter 1) for Cd2+.75 Hg2+ is primarily transported into the proximal tubular cell via amino acid transporters in the form of Hg-Cys and Hg-Hcy conjugates.76 Pb2+ as a mimic of Ca2+ is transported by Ca2+ channels and also via receptor-mediated endocytosis in the form of Pb2+-protein complex.77 Heavy metal-induced nephrotoxicity is mainly attributed to oxidative stress to the proximal tubular cells. For example, 2 μM As2O3 can induce superoxide anion and hydrogen peroxide generation in HEK293 cell through activating the NADPH-oxidase.78 Cd2+ accumulates in mitochondria and disrupts complex III, therefore, inhibiting the respiratory chain and leading to the generation of ROS and induction of apoptosis.75 Other mechanisms, including interaction with protein and DNA, and perturbing critical enzyme activity, are also involved.79 Generally speaking, on the cell level the toxicity of these common heavy metals is in the order of Cd > As ≥ Hg > Pb. Specifically, in human kidney tubular epithelial (HK-2) cells, IC50 value is 1.35 μM for CdCl2;80 13.2 μM for As2O3;81 and 10 μM in MDCK cells for HgCl2,82 and 1.7 mM in HEK 293 cells for Pb(NO3)2.83 In addition, the discrepancy between the results in MDCK and HEK 293 deserves one special note. Although HEK 293 cells are commonly used in studies of kidney toxicity, there has been a report revealing its adrenal neuronal cell nature. As a result, it is suggested that HEK 293 cells are not suitable for in-vitro modeling of typical kidney cells.84
Occupational contact with crystalline silica (SiO2) induces nephrotoxicity or aggravates established renal disease among workers related to mining, sand and stone quarry, mason, and ceramic industry. Although the mechanism has yet to be elucidated, crystalline silica in the renal parenchyma and an autoimmune process due to the activation of macrophages have been suggested to contribute to renal injury.85
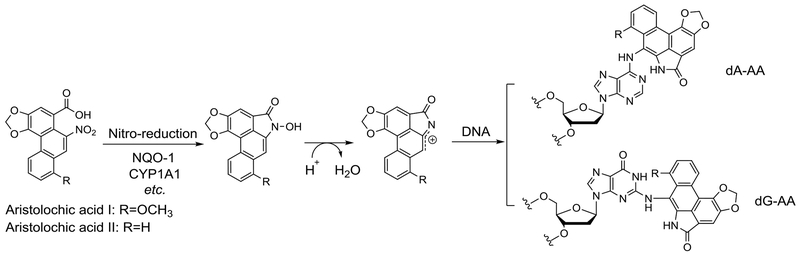
In the field of nutritional supplements and health foods, a notorious case of toxin-induced AKI and CDK is aristolochic acid (AA)-induced nephrotoxicity (AAN).86,87 AA is a natural product found in the Aristolochia species. Since many of the Aristolochia species have been used as traditional herbal medicines in Asian countries for hundreds of years, the extent of the nephrotoxicity problems is yet to be fully understood. The hazard was not well recognized until the report of several consecutive incidents of women in Belgium experiencing severe renal failure after taking the same slimming pills containing Aristolochia species herb. It is also the cause of endemic nephropathy found in the Balkan regions due to AA-contaminated grains. Due to its widespread use and irreversible impact on health, AA has been extensively studied for its nephrotoxicity in vitro and in animal models.88 AA needs metabolic activation in the liver and kidney, which in turn become the primary target organs of AA toxicity. Cumulative AA intoxication can result in acute and chronic lesions including AKI, liver and kidney cancer, and urinary tract cancer. Metabolic activation through reduction of the nitro-group of AA by a reductases such as NAD(P)H quinone dehydrogenase 1 (NQO1) and cytochrome P450 family 1 subfamily A member 1 (CYP1A1) leads to the formation of an aristololactam nitrenium ion,89 which can covalently bind to DNA and act as a mutagen (Figure 3).89,90 Thus, AAN is the outcome of a multi-facet process involving DNA adduct formation, oxidative stress, apoptosis, vasoconstriction, and tubular destruction. All of these factors contribute to the early phase of AKI followed by infiltration of inflammatory cells in renal interstitium, which leads to fibrosis and carcinogenesis.91 It should be noted that compared with in-vitro cell studies that usually required 100-200 μM AA to induce significant toxicity,92 the dosage needed to demonstrate in-vivo toxicity is relatively low (10 mg/kg).93 The increased toxicity in vivo is consistent with the known metabolic activation in vivo. Therefore, in-vitro studies using AA may not be able to fully recapitulate the bioactivation part. Further, the formation of DNA adduct by AA does not seem to induce significant cytotoxicity at a low dosage. On the other hand, Leung and Chan found that AA was able to induce a higher level of RNA modifications than DNA, which may constitute a critical factor of AAN and AA’s carcinogenicity.94 Another issue related to the in-vivo study of AAN is the elevated toxicity in male mice. Much of this gender difference is attributed to the inhibitory effect of female hormone 17β-estradiol on the p53 pathway, leading to reduced renal tubular injury in an AAN mouse model.95 All of these observations have implications for the development of AKI therapy.
Figure 3.
Metabolic activation of AA and formation of DNA adducts.
2.2.5. Miscellaneous Nephrotoxic Agents
Many other agents can also induce kidney damage under the right conditions. For example, non-steroidal anti-inflammatory drugs (NSAIDs), such as indomethacin, can induce nephrotoxicity in patients with impaired renal function due to perturbation of renal hemodynamics through inhibition of prostaglandin synthesis.96 Such inhibition leads to fluid and electrolyte imbalances that can give rise to AKI, nephrotic syndrome, interstitial nephritis, and renal papillary necrosis. Additional examples of nephrotoxic agents include Ticagrelor, an anti-thrombotic drug used for controlling coronary syndrome,97 and lipopolysaccharides (LPS) and bacterial sepsis,98,99 which causes systemic inflammatory response syndrome. Antiretroviral drugs Indinavir and Tenofovir can promote AKI in HIV patients taking antiretroviral therapy. Tenofovir, as a nucleoside/nucleotide reverse transcriptase inhibitor, is associated with inhibition of mitochondrial DNA polymerase γ, which leads to mitochondrial dysfunction and impaired oxidative phosphorylation. This ultimately results in severe proximal and distal tubules tissue damage caused by necrosis, fibrosis, and edema along with lactic acidosis caused by increased lactic acid production.100,101 Protease inhibitor Indinavir’s nephrotoxicity is mainly attributed to its low solubility in less acidic/neutral urine (0.3 mg/ml at pH 5.0, 0.035 mg/ml at pH 6.0, and 0.02 mg/ml at pH7.0), which results in intratubular and intracellular crystals as well as crystalluria, causing chronic interstitial nephritis, interstitial fibrosis, tubular atrophy, multinucleated histiocytes, urinary tract transitional epithelial cells irritation. At this point, it is important to point out that solubility-related kidney injuries are a subject of its own in terms of their scope and mechanism of actions. Many compounds at high enough concentrations, including lactic acid,102 melamine, and cyanuric acid,103 are known to result in injurious events. However, these are beyond the scope of this review.
3. Current Treatments for AKI
There have been a number of studies aimed at developing new treatments either to prevent AKI from occurring as a result of planned, potentially injurious interventions such as cardiac surgery and treatment with nephrotoxic agents, or treatments that have been designed to hasten renal recovery in patients with established AKI.19 Generally speaking, new understandings of the pathogenesis of AKI are the underpinnings of efforts to identify therapeutic targets and to develop therapeutic options. Potential targets include those involved in inflammation, oxidative stress, apoptosis, cellular repair, cytoprotection, fibrosis, cellular metabolism, and mitochondrial function as well as hemodynamics and oxygen delivery. Those agents and approaches that have been tested in clinical trials include trimetazidine,104 forced diuresis with matched hydration during surgery,105 N-acetylcysteine,106,107 HMG Co-A reductase inhibitors (statins),108 NSAIDs,109 thyroid hormones,110 atrial natriuretic peptide (ANP),111,112 and remote ischemic preconditioning.113 There are also others in early stages of clinical development,114,115 including, anti-inflammatory agents (recombinant alkaline phosphatase (RecAP), AB103, ABT-719), immunosuppressant (cyclosporin A), antioxidants (iron chelators, heme arginate), vasodilators (levosimendan), apoptosis inhibitors (p53 siRNA QPI-1002), and tissue repair agents including synthetic oligopeptide THR-184, c-Met agonist ANG-3777 (also known as BB3), and allogeneic mesenchymal stem cells such as AC607. However, despite these ongoing studies, there are no therapeutics available with established efficacy in either preventing or treating established AKI. Clearly, new and innovative efforts are needed to meet this unmet medical need.
4. Carbon Monoxide as an Innovative Therapeutic for AKI
Carbon monoxide (CO) has been studied for well over 100 years, primarily as a tool to assess oxygen bioavailability, but more so as a gas to avoid due to its touted negative effects on the brain and heart. Over the past twenty years, CO has been redefined as a protective molecule, produced endogenously by all cells. Furthermore, there is now remarkable evidence supporting exogenous delivery as a therapeutic in multiple disease pathologies. In recent years, CO has been extensively examined as a therapeutic agent for treating inflammatory conditions and in offering cytoprotection in various organs including kidney,54,116–118 heart,119,120 liver,121,122 brain,123,124 and GI,125–131 among others.132,133 Along this line, CO has especially promising potential in treating various kidney conditions including AKI. Below we discuss the use of carbon monoxide for the prevention and potential treatment of AKI based on extensive preclinical evidence in a number of animal models of AKI, including ischemia reperfusion induced AKI (IRI-AKI) and CP-AKI. CO has also been evaluated in clinical trials of DGF in renal transplants. In the following section, we discuss the use of CO as a therapy and how this might be applied to abrogate many of the common pathophysiologic pathways that are activated, leading to AKI.
CO is an endogenous signaling molecule produced by heme oxygenase-mediated heme degradation to bilirubin, CO, and iron.134 The heme oxygenases (HO) are present in all cells as either an inducible form, HO-1, or as a constitutive form (HO-2). HO-1 is induced by a plethora of stressors, and is cytoprotective acting to promote cellular stability and survival when its level is increased. CO production is an obligatory physiological process and an integral part of red blood cell heme turn over in the spleen with daily production on the scale of about 400 μmol. Indeed during stress such as asthma, infection or sickle cell crises, CO increases in the body as measured in exhaled breath solely due to the increase in HO-1 activity.135 Interest in HO, specifically the HO-1 inducible isoform, has continued to grow beyond its role in heme metabolism, and has expanded into many scientific disciplines, primarily driven by its three products that include not only CO, but also the bile pigments and iron. In the majority of instances, increases in HO-1 and the generation of CO are highly salutary in the resolution of pathophysiologic conditions in the kidney. In fact one of the seminal publications demonstrating the importance of HO-1 was on studying the renoprotective role of HO-1 in a rat model of rhabdomyolysis by Nath et al.135 Since this report, there have been a large number of publications describing the benefits of HO-1 induction in the kidney.136–139 Moreover, a great deal of insight has been made towards understanding the mechanisms of action; and while there have been those that discuss the bile pigments, biliverdin and bilirubin as well as iron, most have been focused on CO.
Although at high doses CO is lethal, it actually represents no more risk and safety issues than most commonly used drugs or even nutrients. All indications are that CO can be developed as a safe therapeutic agent.133,140 For example, CO actually has a higher safety margin than glucose, potassium, calcium, insulin, heparin, nitric oxide, digitalis, and many others.133 The common initial reaction of CO being very toxic and the perception that CO needs to be targeted to the site of action for therapeutic usage largely comes from the consequence of “first impression,” because most people first learned of CO in the context of CO poisoning. Even going beyond the terms of a safety margin, one can look at the safety issue of CO in an absolute sense and find that it is far safer than compounds that we normally associate with the term “toxin.” For example, under normal physiological conditions, less than 2.3% of the hemoglobin in an adult is CO bound (COHb). This number is 8-9% for smokers (two packs a day) and over 12% in newborn babies, while therapeutic efficacy is achieved in the range of 3-9%. The US Food and Drug Administration (FDA) set 14% COHb as a safety threshold for clinical trials in kidney DGF. If one uses 12 g/dl of hemoglobin as a low threshold under normal physiological conditions for a healthy adult, this translates into 7.5 mM in the blood. Thus, 10% COHb is 750 μM, which is safe in humans. In comparison, peak plasma concentrations for Tylenol is >100 μM, naproxen >300 μM, Zantac 1.5 μM, doxorubicin 1-2 μM,141 imatinib 3.5 μM, and 5-fluorouracil >300 μM. In experimental animal models, CO at 3 mg/kg is sufficient to demonstrate efficacy without adverse effect.132 In contrast, the LD50 of botulinum toxin (Botox) is less than 2.1 ng/kg i.v. or i.m., and 13 ng/kg inhaled.142 What is needed for CO to achieve therapeutic effects is well within the range of concentrations that one normally sees for other approved therapeutics and is known to be very safe. With over 100 years of investigation, CO is extremely well understood with respect to its physiological levels and pharmacokinetic behaviors. CO is highly diffusible, distributes throughout the body through partial pressure gradients, and is transported in the blood by reversibly binding to hemoglobin. In addition, it has a solubility of about 1 mM in aqueous solution. The half-life of CO in humans is approximately 3-7 h with no metabolism or tissue accumulation.132 All CO eventually exits the body via the lungs. In fact, exhaled breath has been used not only as an indicator of CO levels in the body, but paradoxically as an indicator of tissue stress, because of the elevated levels of endogenous CO generation by HO-1 under such conditions.134
There have been extensive studies of the mechanism of action of CO for various indications. Some of the identified molecular targets (directly or indirectly) include the soluble form of guanylyl cyclase, mitochondrial oxidases, catalase, nitric oxide synthases, mitogen-activated protein (MAP) kinases, PPARγ, HIF-1α, Nrf2, ion channels, and cystathionine β-synthase (CBS), among others.143–147 The fact that this endogenous signaling molecule has multiple targets is probably part of the reasons for the unique and diverse physiological and therapeutic effects of CO. For example, CO is known to alleviate colitis by suppressing inflammation without the associated depression of the host’s ability to fight off infection.126–131,148,149 Further, CO is cytoprotective in models of doxorubicin-induced cardiotoxicity119,150–152 and yet at the same time sensitizes cancer cells to this chemotherapy by nearly 1000 fold.153 What separates CO from other anti-inflammatory agents such as steroids are the lack of side effects and the inherent ability of CO to befit the needs of the tissue to boost the likelihood of survival. Interestingly, CO can simultaneously block transplant vascular stenosis by arresting vascular smooth muscle cell growth, while promoting motility and proliferation of endothelial cells.154 Similarly, CO blocks the proinflammatory cytokine TNF, while enhancing expression of the anti-inflammatory cytokine IL-10 in macrophages and in tissue.144,155 The pleotropic effect of CO is seen as a unique strength of this endogenous cytoprotective signaling molecule and the pharmacology of CO is very attractive from a development and commercialization standpoint.
4.1. Carbon Monoxide as a treatment for ischemia-reperfusion induced tissue injuries
As with any drug development program, there is a sequence of events that must occur towards commercialization of a medical therapy. First and foremost is proof of concept. After a decade of research demonstrating the benefits of CO in preclinical animal models, kidney transplant was the first indication selected for clinical studies, based on rigorous experimentation in rodent and eventually large animal models.132,143,156 In multiple organ transplant studies, the data sets were similar in that they all showed that CO could prevent IRI and organ rejection.157–160 The cellular and molecular mechanisms in preventing IRI were found to be diverse, and include inhibition in cell death pathways including blockade of caspases and mitochondrion-mediated oxidative injury,161,162 and down-regulation of pro-inflammatory pathways with reduced levels of cytokines, adhesion molecules, and leukocyte activation and infiltration.163,164 Blockade of coagulation and pro-regenerative modalities, particularly in the kidney were clearly evident in animals that were treated with CO. Importantly, inhaled gas, CO-RMs, and CO prodrugs were identical in nearly all scenario’s tested, which has increased confidence in the field as to the medical utility of CO.132,133,163–165 The pleiotropic effects of CO that resulted in kidney protection were both encouraging and challenging in that the central master regulator and molecular target, if there is one, of the observed beneficial effects remains elusive. One common theme is the propensity for CO to bind to heme and modulate tissue oxygen gradients, which are critical in the kidney. Thus, many of the identified signaling pathways revolve around proteins that contain heme and include mitochondrial oxidases, catalase, nitric oxide synthases, and guanylyl cyclase among others.166,167
4.2. Carbon Monoxide Delivery
An ongoing challenge with developing CO as a therapeutic agent is determining the precise dosing regimen and optimal route of delivery. Gas exposures are typically carried out using parts per million (ppm) quantities, but this becomes a challenge for clinicians that are used to drugs delivered as a milligram dose. Given that CO is so highly diffusible among all tissue compartments, the amounts present may be extremely transient, but potentially very important, particularly if the purpose is to keep the COHb level low. While COHb remains the penultimate biomarker for CO from both a safety and therapeutic standpoint, there is debate whether this complex is the ideal measure by which to base pharmacological efficacy. Further, the hemoglobin level, lung capacity, respiratory rate, and physiological state of a patient could also be major factors in affecting the dose of CO that actually enters the systemic circulation as measured by COHb. As noted above, the upper limit for COHb that was approved by the FDA for the human kidney transplant trials was 14%, but the majority of literature reports show that COHb levels at <10% are effective. Earlier efforts in evaluating the therapeutic effects of CO used inhaled CO. Importantly, metal-immobilized carbonyls have also been used as donors, which allows for precise controls of dosage. Such practice was first reported by McKendrick and Snodgrass in 1891 who studied CO from nickel tetracarbonyl and found it to be an antipyretic.168 Incidentally, CO is also used in mining for nickel because of its high affinity for nickel and the ease of separation through distillation of the nickel tetracarbonyl complex, which easily releases CO as well. However, nickel tetracarbonyl is highly toxic. Motterlini and others developed immobilized carbonyls with much-decreased toxicity as metal-based CO-releasing molecules (CO-RMs) using metals such as ruthenium, iron, and manganese.132 Later, other permutations including enzyme-controlled release,169,170 encapsulated metal-based CO-RMs,171–175 and photosensitive organic CO-RMs176–180 were developed. There are also oral forms of CO in a solution being developed by Hillhurst Biopharmaceuticals,181 and organic CO-RMs that require light for activation.177,180,182 Most recently, Wang’s lab developed several hundreds of organic CO prodrugs belonging to different structural classes133,149,156,183 that have diverse delivery properties including tunable CO release rates,184–186 triggered release (pH-,185 esterase-,184,187 and ROS-sensitive188), mitochondrion-targeting,121 dual-triggers,183,187 and delivering more than one payload using a single prodrug.115,183,184,187 CO delivered in various forms has been studied in animal models of kidney injury. The mechanisms that have been described in acute kidney injury involve an intriguing relationship with adenosinergic signaling pathways and maintenance of normal circadian rhythm.54 In the absence of CD39 5’ectonucleotidase or Per-2, CO is ineffective in preventing kidney IRI.54 In a swine model of DGF of a kidney allograft, CO exposure reduced acute tubular necrosis, apoptosis, tissue factor and P-selectin expression while enhancing proliferative repair as measured by phosphorylation of retinol-binding protein and histone H3.157 Below the discussions are divided based on the modality of CO delivery tested in experimental models.
4.2.1. Carbon Monoxide Gas
Understandably, the gaseous form of CO was the first modality tested in various in-vitro and in-vivo studies based on the simplicity of the pharmacology, clinical applicability, and purity. Early studies established 250 ppm as the dose that was very well tolerated, elevated COHb levels, and showed remarkable benefits when animals were exposed for as little as 1 hour. Much of the early work using gaseous CO to offer protection of the kidney studied IRI. Survival of an organ subjected to a period of poor blood flow is dependent on the length it experiences a lack of oxygen. IRI, caused by an infarct, emboli or in the most extreme scenario, anoxia as would exist with an organ destined for transplantation, results in significant tissue injury. This is not only due to the sudden lack of oxygen, but the reperfusion that instills additional devastating damage from a sudden rise in reactive oxygen species generation and alterations in vascular permeability as blood flow is restored. The idea of a gaseous molecule possessing biological functionality has been well known for greater than one hundred years. For centuries, oxygen was thought to be the sole gas molecule central to survival, but this changed dramatically a little over 30 years ago with the discovery of nitric oxide (NO),189,190 which began to unravel the novel concept that endogenous production of a gaseous molecule can impart diverse and critical functional effects on a wide spectrum of biological and pathological processes. Shortly thereafter, and in a complementary series of events, carbon monoxide (CO) and hydrogen sulfide (H2S)191,192,132,134 emerged as the second and third gaseous signaling molecules, respectively, with important bioactive properties. Collectively, these gases launched what became the field of gasotransmitters.153
Ironically, inhaled CO gas was used early on by scientists to assist in the description of hemoglobin and how hemoglobin carries oxygen and carbon dioxide. Since then, CO gas was classified as a toxic entity, lethal to aerobic life and one of the primary pollutants in industrial societies. Despite this classification, CO is now accepted as a fundamental bioactive gaseous mediator that possesses potent cytoprotective functions.132 Over the span of the past two decades, and hundreds of publications describing fruitful discoveries, there is now an enormous understanding of how CO impacts numerous disease processes including AKI.
The effects of CO in the kidney has been evaluated in human clinical trials in the prevention of DGF after renal transplantation. The decision to study DGF in human was based on the vast preclinical evidence including proof of principle data obtained in pigs,157 insight into mechanisms of action, the feasibility of a clinical trial, and straightforward readouts for AKI such as organ function and the amount of time before dialysis could be discontinued. Unfortunately, it was placed on hold and eventually discontinued for reasons related to corporate decision-makers and not at all based on safety or efficacy. Prolong Pharmaceuticals stopped a Phase-2 trial using CO-saturated bovine pegylated hemoglobin in renal transplantation. Pegylated hemoglobin, as a delivery agent can be problematic to the kidney and pegylation can influence nitric oxide levels and therein the regulation of vasomotor tone. It is unclear why the study was withdrawn (www.clinicaltrials.gov; NCT02658162).
The majority of drugs in trials for kidney transplantation, or organ transplantation in general, are focused on modulating the immediate immune response resulting from ischemia reperfusion-induced tissue injury (inflammation and coagulation), and to reduce the incidence of T-cell mediated rejection. Beyond transplantation, the kidney remains a key target organ where CO has been shown to be protective with unique opportunities in areas such as chemotherapy-mediated injury as observed with CP or methotrexate.193,194 With the advent of new agents entering the marketplace, especially within the areas of neurologic and cardiovascular disorders as well as various antivirals and antimicrobials, the incidence of AKI can only increase and this does not include the vast unknown of what occurs in individuals prescribed multidrug therapeutics where drug-drug interactions have not been comprehensively studied. Given the lack of metabolism of CO, it offers a unique ability to protect the kidney against drug toxicities without the likelihood of interactions with other agents. This of course needs to be studied and formally tested as CO may influence the metabolism of various drugs by the kidney or are otherwise sequestered by the kidney during elimination.
When gaseous CO is used, there is the unique need for a reliable fail-safe delivery device, preferably one that bases the dose on body mass and can account for differences in pulmonary functions, e.g. tidal volumes, respiratory rates, and diffusion. Considering the additional challenges of compressed gas cylinders, non-compliance with wearing masks, and concerns for those administering the gas being exposed, gas delivery as a treatment modality is viewed as arduous and logistically difficult. Hence there have been great efforts put forth to provide alternative approaches to CO delivery.
4.2.2. Metal-based CO-RMs
With the demonstrated therapeutic efficacy of CO gas in treating various conditions in the late 1990s, efforts were underway in early 2000, by Motterlini and others, to study metal-based CO-releasing molecules (CO-RMs) in the form of immobilized carbonyls with the aim delivering carefully controlled doses of CO. These compounds vary in the metal used (Mn2+, Fe2+, Ru2+, etc.), ligand properties to modulate release rates and water solubility, and triggers (water, light, and enzymes) needed to initiate CO release. Readers are referred to some excellent reviews summarizing the various CO-RMs, if in-depth knowledge is needed.132,143 Some commonly used CO-RMs include CORM-1, which is Mn2+-based and releases CO in aqueous solution upon light irradiation; CORM-2, which is Ru(II)-based and capable of spontaneously transferring CO to a CO acceptor, such as myoglobin, in aqueous solution without light irradiation;195 and CORM-3, which is also Ru(II)-based with enhanced water solubility when compared with its predecessors.196 These CO-RMs were said to release CO with half-lives ranging from 3.6 min to 98.3 h depending on the solution composition determined by a comparative myoglobin assay.196 On the other hand, CO release from both CORM-2 and CORM-3 has been reported to be highly dependent on the presence of thio-species in the media;197 therefore, CO dose might be difficult to control for in-vivo applications.198 Later, the same group reported water-soluble CORM-A1 based on boranocarbonate decomposition chemistry. Unlike CORM-1~3, CORM-A1 releases CO in aqueous solution with a half-life of 21 min at pH 7.4 and the release can be stimulated at a lower pH with a shorter half-life.199 At the same time, it forms borane as a reductive intermediate, which undergoes reaction with water to generate one molecule of boric acid and hydrogen as the formal byproducts.198 Although CORM-A1 does not contain a transition metal, boron as an ultra-trace element has been termed as “metalloid”. Therefore, CORM-A1 is included in this category for discussion purposes. In 2011, Motterlini’s group disclosed the manganese-based CORM-401, which was shown to release three molecules of CO per CO-RM.200 Recently, the same lab reported a hybrid CO-RM termed HYCO,201 which is comprised of CO linked to a known inducer of HO-1 with the idea being that endogenously generated CO would be additive in the salutary effects along with the exogenously delivered CO. Again, there has been extensive research and reviews on metal-based CO-RMs including iron-, manganese-, and molybdenum-based CO-RMs.132,140,143,202 However, the discussions will be limited to these five CO-RMs because they are the only ones examined in kidney protection-related studies (Table 1).
Table 1.
CORMs studied in kidney protection activity
| CORM No. and Ref. | Chemical Structures | Solubility | Release profile (in PBS, pH 7.4, 37 °C) |
|---|---|---|---|
| CORM-1195 |  |
DMSO Ethanol | Light-dependent Fast release (t1/2 < 1 min) Release ratio:a 1:1 |
| CORM-2195,b |  |
DMSO Ethanol |
Ligand substitution induced; Fast release (t1/2 ≈ 1 min) in the presence of dithionate;b Release ratio:a 0.75:1 |
| CORM-3196,b |  |
H2O | Ligand substitution induced / water-CO shift reaction; Fast (t1/2 ≈ 1 min) in the presence of dithionate;b stable at acidic pH; Release ratio:a 1:1 |
| CORM-A1199 |  |
H2O | pH-dependent, with gaseous CO release (t1/2 ≈ 21 min at pH 7.4); Release ratio a: 1:1 |
| CORM-401 200 |  |
H2O | Ligand substitution induced Fast release (t1/2 < 4 min) ) in the presence of dithionate;b Release ratio a: 3:1 |
These versatile CO donors have been extensively studied for their anti-inflammatory and anti-apoptotic effects in non-cancerous cells such as kidney endothelial cells, hepatocytes and cardiomyocytes; and for their pro-apoptotic effect in cancer cells, dysregulated fibroblasts, and aggressive T cells.132 Studies of the renal-protective effects are generally focused on three areas; general effects on renal microcirculation, protection related to IRI (i.e. organ transplantation), and protection against chemical- or trauma-induced nephrotoxicity.
Stec and co-workers found that in anesthetized C57BL/6J mice, CORM-A1 at a dose of 0.96 μmol per mouse could significantly increase renal blood flow by 33 ± 6% compared to no-treatment controls.205 The result was in agreement with previous findings by Motterlini that CORM-A1 reduced IRI in part by blocking TLR2, 4 and 6.206 Vasodilatory activity was also found with perfusion using 150 μM and 300 μM CORM-2 in isolated rat afferent arterioles. The activity level was comparable with a 10-μM CO solution (saturated in Tyrode solution). The results provide direct evidence of CO’s effect on renal microcirculation and potential reno-protective activity.207 Carretero and co-workers took the studies a step further to understand the mechanism of CO’s effect in renal microcirculation. It was found that CORM-3 at 50 uM could decrease tubuloglomerular feedback (TGF), which is the sensing mechanism that induces constriction of the afferent arteriole upon increased luminal NaCl concentration.208 Later, the same lab revealed that in isolated rabbit afferent arterioles (Af-Art, with glomerulus and adherent tubular segments), microperfusion with 50 μM CORM-3 could attenuate TGF levels by 1/3 to 1/2; while this effect could be blocked with the addition of 1 μM soluble guanylate cyclase inhibitor LY-53583. Such results indicate that the attenuation of TGF by CORM-3 is mediated by activation of the soluble guanylate cyclase (sGC)/cGMP system. Further studies with a cGMP-dependent protein kinase (PKG) inhibitor KT-5823 and phosphodiesterase 2 (PDE2) inhibitor BAY-60-7550 as well as a cAMP analog db-cAMP revealed that CORM-3 could attenuate TGF via activation of PKG (but not PDE2) to activate the cGMP pathway thereby reducing cAMP levels. 209
This pleiotropic activity of CO in the vasculature functioning as both a vasodilator and a vasoconstrictor prompted the question of which factors are involved in the vasoregulatory phenotype observed with CO. Lamon et al. questioned vasodilatory effect of CO based on the lower binding affinity of CO with guanylyl cyclase when compared to NO and the observed increased oxidative stress in vascular endothelial cells in response to CO exposure.203 Since ROS have been implicated in the pathway associated with vasoconstriction, the study utilized CO gas and CORM-3 to show that 0.1~1.0 μM of dissolved CO gas (in sealed vials) corresponds to 10~100 μM CORM-3 in inducing vasoconstriction of rat renal arteries. Both 1.0 μM of dissolved CO gas and 100 μM CORM-3 was found to induce superoxide O2·− production in rat renal interlobular arteries, which correlated with the induction of vasoconstriction while the post-CO-released iCORM-3 showed no effects. Pretreatment with 1 mM of Tempol, a SOD mimetic anti-oxidant, was found to convert the constrictive effect of CO into a dilation effect. Inhibition of O2·− generation by nitric oxide synthase (NOS), (NADPH)-oxidase, and xanthine oxidase (XO) with their corresponding inhibitors L-NAME (1 mM), apocynin (100 μM), and allopurinol (100 μM) could also convert CORM-3’s vasoconstriction effect to that of vasodilation. These studies also showed that 100 nM of bile pigments biliverdin/bilirubin could completely inhibit O2·− generation by rat renal interlobar arteries in response to 100 μM CORM-3 and led to the suggestion that endogenously produced CO functions as a vasodilator. In addition to these findings, it was also intriguing to find that it only required 1% of the dose of CORM-3 for the CO gas to achieve a similar effect. This is also consistent with the findings that only about 1% CO gas recovery from CORM-3 could be detected in physiological buffers with gas chromatography.203 Such findings are consistent with the work by Poole in demonstrating that CORM-3 only releases a small amount of CO, as detected by gas-phase FT-IR, in the buffer in the absence of any sulfur species.204 This aspect is further discussed at the end of this section.
Similar to studies using CO gas, CORMs have also been studied in treating IRI after organ transplantation, which features rapid reactive oxygen species (ROS) generation, leukocyte infiltration, and additional mechanical injury.54 The severity of the IRI is one of the major factors that dictate long-term graft survival. Several CORMs have been studied for their effectiveness in preventing IRI. Perfusion of an isolated rabbit kidney with CORM-3 and CORM-A1 at 50 μM in Celsior solution showed renal protection against damage inflicted by cold storage and IRI. Whereas the spent CO-RM (iCORM) offered no protective effect.210 Similar protection of an isolated porcine kidney was also reported with CORM-3 perfusion at concentrations of 50 μM and 100 μM. Intriguingly, higher doses (200 or 400 μM) and iCORM (50 μM) failed to show protection against IRI.211,212 The dose-dependence in these studies deserves some attention. First, in such an in-vitro experiment, “concentration” of the CO-RM may need to be coupled with the volume of the solution used in order to interpret the data because it might be the amount and duration of the CO that is delivered, not only the “concentration,” that makes the difference. Second, Poole has found that reaction with a thiol/sulfide species is an integral part of how CORM-3 functions.204 All these need to be taken into consideration when interpreting the data.
In a rat kidney transplantation experiment, donor rats pretreated with CORM-2 (8 mg/kg, IP) 18 h before kidney retrieval followed by cold-preservation for 26 h and then transplantation into the recipient rat showed a remarkable increase in kidney survival with normal serum creatinine levels after transplantation; whereas all the rats in the vehicle control and iCORM control groups died of uremia within 3 days after transplantation.213 Similar protective effects were also observed with perfusion of an isolated rat kidney with 1 ml of 100-μM CORM-3 solution followed by cold storage and transplantation into an isogenic rat. Histopathological analyses revealed less necrosis of tubular and glomerular cells in CORM-treated animals compared with controls. In a recent report, CORM-401 was used at 200 μM to perfuse isolated porcine kidney’s preconditioned with 1 h warm ischemia. The treated kidney was reperfused with isogeneic porcine blood. It was found that CORM-401 perfusion reduced urinary protein excretion and kidney injury makers compared to the controls. CORM-401 treatment also improved renal histological characteristics and prevented renal hemorrhage and thrombosis.206 In yet another study of kidney injury using a thermally injured mouse model, CORM-2 (8 mg/kg i.v.) was found to attenuate polymorphonuclear leukocyte infiltration into renal tissue and prevent activation of NF-κB. Such results explain, at least in part, the potent anti-inflammatory effects of CO. It should be noted that CORM-2 is also ruthenium-based. CO release from CORM-2 has also been reported to be thiol-dependent.197 The implications of these new chemistry insights in the interpretation of data needed to be further examined.
As discussed above, CP is a commonly used anticancer drug, however, with severe and sometimes dose-limiting kidney toxicity. There is a great deal of interest in identifying preventative or therapeutic agents that can be used in conjunction with CP. In this case, there are two basic requirements: i) the agent needs to be able to attenuate CP’s nephrotoxicity; and ii) the agent must not reduce the efficacy of CP as a chemotherapeutic in treating cancer. With the known ability of CO to inhibit and/or sensitize cancer cells toward chemotherapy,106,214–224 including CP-resistant cancer cells,225 CO delivered in any form represents an attractive agent for this purpose. Motterlini and co-workers reported in 2006 the first study of the protective effects of metal-based CO-RMs in drug-related kidney injury.226 CORM-3 at a concentration of 10 μM completely abolished CP (50 μM) induced caspase-3 activity and cell detachment of renal tubule epithelial cells (LLC-PK1), which was proposed to indicate suppression of apoptosis.226 Animal studies in male Wistar rats also revealed that pretreatment with 10 mg/kg CORM-3, 24 h before CP (7.5 mg/kg) administration followed by daily treatment with the same CORM-3 dosage for 3 days completely prevented CP-induced increases in plasma urea and creatinine, which are considered hallmarks of AKI. In all the experiments, the iCORM-3 control showed no effect. Han et al. also reported that a 3-h pretreatment with CORM-3 attenuated CP-induced (50 μM) cytotoxicity in LLC-PK1 and HK2 cells. Statistically significant results were observed with CORM-3 at concentrations ranging from 50-200 μM for LLC-PK1 cells and 12.5-200 μM for HK2 cells. Experiments were also performed on the renal clear cell cancer cell lines Caki-1 and Caki-2, which are resistant to CP. Protective effects were observed with CORM-3 at 200 μM and 100-200 μM, respectively, followed by treatment with 200 μM of CP for 24 h.227 At the molecular level, CORM-3 pretreatment attenuated CP-induced increases in TNF mRNA and oxidative stress signaling molecules, including Erk1/2, JNK, and p38. In LLC-PK1 and HK-2 cell, but not in CP-resistant cells, CORM-3 reduced expression of the apoptotic marker cleaved-caspase 3. Among all the factors, oxidative stress and inflammation induced by CP are said to be the likely targets for CO in the observed protection against AKI. It is important to note that overexpression of HO-1 ameliorated CP-induced apoptosis in vitro while HO-1 deficiency resulted in higher vulnerability to CP-AKI in vivo. Further, suppression of TNF also significantly suppressed the induction of other cytokines elevated by CP-AKI228 and ameliorated CP nephrotoxicity in vivo.229 All indications point toward CO offering a special opportunity to treat AKI induced by CP and others.
Poisoning by heavy metals such as lead was reported to benefit from administration of CORM-A1.230 In an animal study using rats poisoned by 100 mg/kg lead acetate (p.o. 3 months), CORM-A1 treatment (0.1 mg/kg/d) for 3 months restored serum urea from 51.13±3.12 mg/dl to a similar level of the non-poisoned control group (32.75±1.60 mg/dl). Intracellular glutathione (GSH) of the kidney tissue was also restored from 2.98±0.18 μmol/g to the level comparable with the non-poisoned control (4.86±0.21μmol/g). Serum creatinine and malondialdehyde levels were also significantly decreased by CORM-A1 treatment compared with the poisoned group (2.49±0.14 mg/dl and 0.83±0.03 for the poisoned group and control group respectively). CORM-A1 was also shown to prevent elevations of inflammatory cytokines (TNF, IL-1β) and caspase-3. The low dose of CORM-A1 (0.1 mg/kg/d) used, as compared to animal model studies using CORM-3 (10 mg/kg), is also interesting, but probably can be explained by the low yield of CO release from CORM-3.203,204 Of note, the salutary effects of CORM-A1 were comparable with 10 mg/kg/d L-NAME and 3 mg/kg/d NaHS in all tested kidney function-related biochemical markers.230
With all the promising results of CO-RMs, it seems that there is a need for additional mechanistic chemistry work to clarify issues related to active species, release kinetics, and the effect of medium, among others. For example, Ru(II)-based CO-RMs such as CORM-3 had been reported to have very strong antimicrobial effects while exerting minimal mammalian cytotoxicity. For a long time, the observed effect was attributed to the CO released from CORM-3. However, a recent study by Poole and co-workers suggested that CORM-3 releases minimal amounts of CO under the conditions studied and CO is unlikely to be the reason for the observed biological activity, at least in some of the studies including the reported antimicrobial activities.204 Instead, Poole suggested that direct reaction between ruthenium ion and protein thiols are most likely the reason for the reported antimicrobial activity of CORM-3.204 The same chemistry work also yielded information on medium-dependent CO-release profile for CORM-3, which is different from previously reported numbers. The report went on to conclude that “these results necessitate a major reappraisal of the biological effects of CORM-3 and related CORMs.” Along a similar line, Ru(II)-based CORM-2 has also been reported to react with cysteine thiol chemically;231 CORM-2 and the CO-released by-product iCORM-2 have been reported with significant toxicologic issues in various cell lines.232,233 These findings can be a significant contributing factor to the observed CO-release kinetics,197 and biological activities.204,232 There are also other reported findings, suggesting the need to look at the chemistry of metal-based CO-RMs in greater detail.234,235,225 Especially important is the need to delineate the CO release kinetics and required release-conditions, de-convolute the effects of CO and the metal “carrier” under various conditions, understand the effect of the medium on the observed biological effect of CO-RMs with special attention to possible interactions at the molecular level. As with any drug discovery efforts, more studies are needed in assessing the drug-like properties of the metal-based CO-RMs and other CO donors including the organic CO prodrugs from our own lab to be discussed in the following section.149,156,234 Collectively these reports suggest the need for greater interactions and collaborations among chemists, biologists, and pharmaceutical scientists.
4.2.3. Organic CO Prodrugs
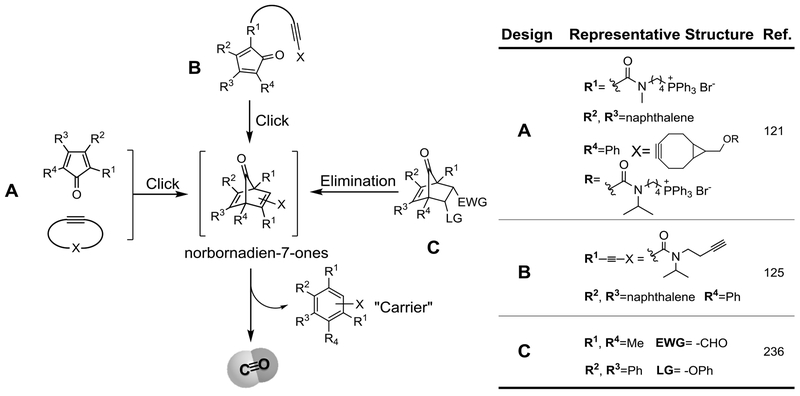
With the success of CO gas and metal-based CO-RMs in demonstrating CO’s efficacy in treating various conditions, a consensus has emerged that there is an urgent need to start addressing issues such as drug-like properties, developability, and safety of CO-delivering modalities. Along this line, Wang’s lab at Georgia State University has developed a large number (hundreds) of organic CO prodrugs, belonging to different structural scaffolds.133,149,156,183 The search for organic prodrugs is based on the premise that the pharmaceutical industry has extensive experience in developing small organic molecules as therapeutic agents, which should minimize the degree of uncertainties in later stage development. Further, organic molecules offer a very high degree of structural diversity, which is important for medicinal chemistry optimization work, pipeline building, and diversity of structural scaffolds. With such thinking in mind, the Wang lab set out to develop CO prodrugs with tunable CO release rate, controlled/triggered release, ability to target, and ability to deliver more than one payload using a single prodrug. The design principle is based on the ability for substituted norbornadien-7-ones to undergo a cheletropic reaction to extrude CO, leaving behind an aromatic side product (Figure 4). With this key concept in mind, precursors to the norbornadien-7-ones include compounds capable of intermolecular Diels-Alder reactions (A), intramolecular Diels-Alder reactions (B), and elimination reactions (C). Because the chemistry has been extensively reviewed,133,149,156,183 only a brief overview of the design principles is described here. Specifically, scaffolds A and B capitalize on the inter/intramolecular Diels-Alder (DA) reaction to trigger CO release under physiological conditions. Both approaches allow for tuning the CO release rates within a wide range (2 min to days).125,185,186 Tuning the CO release half-life of the bimolecular CO prodrugs relies on the diene-dienophile pairs and the electronic effect of the substitution because the first step is rate-limiting. On the other hand, for the intramolecular system, the CO release rate relies on both electronic and entropic effects to tune the Diels-Alder reaction rates. The bimolecular CO prodrugs allowed for the development of a new concept of “enrichment triggered CO release” by conjugating both components with a mitochondria-targeting moiety to achieve targeted CO delivery with improved biological outcomes in vitro and in vivo.121 Based on the intramolecular approach (B), an esterase-activated CO prodrug and a cascade prodrug system for the co-delivery of CO and another payload have also been devised.115,184 The last approach (C) leverages on an elimination reaction to generate norbornadien-7-ones for CO release from norborn-2-en-7-ones. It should be noted that the Larsen lab237 and the Wang lab both reported prodrug systems that take advantage of this elimination approach for CO prodrug design. This approach allows for the synthesis of pH-,185,237 ROS-,188 and esterase-sensitive CO prodrugs.187 It is also interesting to note that the release rate of the pH-sensitive ones can be adjusted based on the leaving group ability and can be quantitatively predicted using the Hammett constant of the substituents on the phenol leaving group.185
Figure 4.
The general design and representative compounds of the organic CO prodrugs.
Along with the CO prodrugs developed by the Wang group discussed above, other transition metal-free organic CO donors also been reported (Figure 5). Larson and coworkers have reported an approach based on norborn-2-en-7-one chemistry (1).238 The elimination of the HBr under basic conditions results in the formation of the norbornadienone, which undergo cheletropic loss of CO and aromatization to form the byproduct. Compound 1 showed in-vitro vascular dilatory effects with an EC50 of about 1.6 μM. There are also extensive studies using photo-activated organic CO donors. For example, the carboxylic acid derivatives of fluorescein (2)182 and borondipyrromethene difluoride (BODIPY) (3)177 were shown to release CO in physiological aqueous solution under visible light exposure at wavelengths of 500 nm and 500-720 nm respectively. Exposure with 470 nm light could trigger the release of the two equivalents of CO from the α-diketone derivatives 4. However, possibly due to the hydration of the diketone, compound 4 failed to release CO in aqueous solution and needed to be encapsulated in pluronic micelles to facilitate CO release in aqueous solution.178 Berreau and coworkers developed a series of 3-hydroxyflavone derivatives 5 to deliver CO in biological scenarios with visible light irradiation under aerobic conditions.239 The further modified compound 5-2 could respond to intracellular thio-species/oxygen/light to induce CO release in an ‘AND’ logical gate fashion.240 By tethering a triphenylphosphinium (TPP) moiety, compound 5-3 could target mitochondria and release CO with light irradiation.241
Figure 5.
Other reported organic CO donors.
These photo-sensitive CO donors feature “real-time” CO release upon light exposure, which can be externally controlled in a spatial and temporal manner. Furthermore, CO release usually results in fluorescent changes of the donor molecule, which can be harnessed to monitor the location and the efficiency of the CO release.242 The feasibility and versatility of structural modifications to achieve different targeting/triggering mechanisms also underscores the flexibility of the organic CO donor approach.
The organic prodrugs are the “newcomers” in this field. However, their efficacy has been pharmacologically validated in multiple animal models including colitis,125 systemic inflammation,187 chemically induced liver injury,121,187 vasorelaxation,237 and importantly in the kidney, IRI-AKI.54 Specifically, one prodrug (BW-AQ-101, t1/2 2 min) has been shown to attenuate IRI-AKI in a mouse model using male C57BL/6 mice. At 100 mg/kg (i.p., the equivalent of 7 mg of CO/kg), the prodrug was able to substantially reduce serum creatinine level by 60% and blood urea nitrogen (BUN) level by 50%, when compared against the untreated group. In comparison, similar results were observed in the group exposed to 250 ppm of CO gas for 1 h. The dosage used in this IRI model was much higher than in models of colitis (15 mg/kg) and systemic inflammation (7.5-15 mg/kg). The EC50 value of the prodrug in the liver injury model was much lower (0.4 mg/kg). However, the results from the IRI-AKI studies cannot be directly compared to the other models because the prodrug used in the liver injury model was specifically targeted to the mitochondria where the site of action is.121 With a non-targeted prodrug, the EC50 was about 10 mg/kg when studied in lipopolysaccharide (LPS)-induced systemic inflammation and liver injury model in mice.187 It is not readily clear why the dosage needed in the kidney IRI model was higher. One possible explanation may lie with the CO release half-life. The kidney IRI model used a fast-releasing prodrug (t1/2 2 min), while all the other studies used prodrugs that have t1/2 in the range of 1-2 h. Some direct comparative studies may help clarify the question the optimal t1/2 is for a given indication.
Although the number of CO prodrugs studied in IRI-AKI is limited, there is much promise because of the unique properties of these organic CO prodrugs. Much more work is needed to understand the optimal conditions of CO prodrugs to use for IRI-AKI. As described in the section on metal-based CO-RMs, the drug-like property issue needs to be part of the design strategy for organic CO prodrugs as well.156 The hydrophobicity of the molecules used necessitates special formulations for in vivo studies. Efforts are needed and are underway to address these pharmaceutical issues through formulation and other approaches.
4.2.4. Others Forms of CO Delivery
In addition to gaseous CO, metal-based CO-RMs, and the organic CO prodrugs, there are also other forms of CO delivery. For example, Hillhurst Biopharmaceuticals developed a proprietary CO oral formulation, which has shown efficacy in various animal models181 including kidney IRI and sickle cell disease.54 As mentioned earlier, ProLong Pharmaceuticals has studied the use of pegylated bovine hemoglobin for delivering CO (www.clinicaltrials.gov). There are also others who have prepared organic CO-RMs that release CO upon photolysis.176–180,243,244 Each of the above modalities has been extensively studied and found to effectively deliver CO and modulate cellular function. The abundance of data available demonstrating CO’s efficacy, the structural diversity of CO donors, and the availability of multiple delivery modalities all give much hope to the possibility of developing CO-based therapeutics for AKI and other organ injuries.
5. Cellular studies, animal models, and clinical trials
From the descriptions above and from published literature reports, all the indications are that CO is a very promising therapy for a wide range of indications, including kidney injury. However, there is still the question of how to correlate the results from cellular studies, small animal models, and large animal models, with the ultimate question being how to extrapolate to humans. Allometric scaling alone is a big enough problem in any drug discovery and development project because of differences in biology, metabolism, and pharmacological responses. This challenge is amplified with a gaseous molecule as the active ingredient because of its volatility and the difficulties in controlling dose and half-life. Further, understandably, CO’s half-life in vivo is dependent on the CO exposure level, basal metabolic rates, and hemoglobin levels, and hemoglobin from varying animal species may have different relative affinity to CO.245 There are other challenges in translating available results to studies in large animals and humans.
The first challenge is the question of concentration, potency and dosage. This should be a relatively straightforward question under normal circumstances. However, with CO it is not. In cellular studies using gaseous CO, the dose is measured in ppm and exposure time, which does not truly give “concentration” and “dosage” information. When metal-based CO-RMs were used in literature reports, concentration information was available only for the donor. However, given the new findings on the low CO release yield and its dependence on media and thio species,197,204,246 the “concentration” of CO is expected to be hard to assess for some of the metal-based CORMS. There are even questions raised regarding the biological responses observed from studies using CO-RMs, especially those from Ru-based CO-RMs,204 which constitute a large percentage of the studies using CO-RMs. Additionally, release half-life from a gas donor significantly impacts the sustained concentration of the gas molecule.247 There has been very little study of this aspect using metal-based CO-RMs. With photo-sensitive CO donors, the stoichiometry and release rates are far more defined. However, photo-sensitive CO donors are not readily applicable in animal models, at least not on a large scale. All these significantly complicate the issue of translating successful results from cellular and small animal studies to human. Further, there is the real issue related to the inactive components that accumulate after CO release. They are simply not the same molecule, without the CO present. In fact, the iCORMs are entirely different molecules in and of themselves, and this needs to be more clearly understood in terms of toxicology and pharmacology and demonstrating CO effects versus non-specific responses to the carrier molecule.
In animal model studies, COHb is commonly used as the measure of CO exposure. This gives a solid parameter to correlate results from different routes of administration of various forms of CO. However, there are issues that are not well defined. How high does the COHb level need to be to initiate the protective response? For instance, is there a threshold that needs to be reached to activate a signaling pathway? How long does the elevated COHb level need to be maintained? Is COHb the ideal measure of CO exposure or is there a more specific biomarker? As reviewed by Romao et al.,248 an extreme example was a study done in dogs, that showed that 80% COHb was not lethal when generated by partially replacing the circulating blood with ex-vivo CO-loaded red blood cells. On the other hand, when inhalation of air with 13% CO was used, all the dogs died within 1 h after reaching a similar COHb level (54~90%). Such results indicate that a relatively high level of COHb does not compromise the O2-carrying capacity of the blood to a life-threating level, and yet the high level of dissolved CO, in plasma and not the COHb, is probably what contributes to CO toxicity.249 Furthermore, in animal models, species differences are another major consideration. As suggested by Jilma et al. in their clinical studies, therapeutic effects could be affected by distinct differences between small and large animals such as rodents and primates in terms of hemoglobin’s affinity constant for CO and O2, half-life of COHb, saturation levels of COHb with the same CO gas concentration, as well as the basic physiological differences between species including heart and respiratory rates.245 Further, assessing efficacy will depend on the readouts that are chosen and the stimulus used. Jilma et al. chose endotoxin and measured four cytokines tested over a limited kinetics where no effects of CO were observed. In contrast Mazzola et al.,250 tested in a similar endotoxin model while they also observed a lack of effect on the same cytokines measured by Jilma, yet they observed striking effects on abrogating endotoxin-induced respiratory derangement which was not measured by Jilma. Though there have been limited studies on this subject, available data indeed suggest the need for much more work. For example, in a nonhuman primate model of lung inflammation in male cynomolgus macaques,251 inhalation of 500 ppm CO gas for 6 h after LPS administration reduced LPS-induced TNF-α release in bronchoalveolar lavage fluid by about 10% and neutrophilia by 67%. However, unlike pretreatment with budesonide, a corticosteroid, IL-6 and IL-8 were not affected by 500 ppm CO inhalation. In this study, the CO dosage (500 ppm for 6 h) was higher than one can achieve in human studies: COHb level of the cynomolgus macaques reached 34% with 500 ppm CO for 6 h and 25% with 250 ppm CO for 6 h respectively from a baseline of about 4.4%. Of note, 34% COHb level is higher than the suggested toxic levels in humans (>25%) and was well tolerated in the cynomolgus macaques without clinical signs of CO toxicity or poisoning. Intriguingly, only the 500 ppm CO exposure group showed significant differences in decreasing pulmonary neutrophilia, while the 250 ppm CO exposure only showed marginal effects. In comparison, 3-9% COHb was sufficient to elicit biological responses in many rodent models.54,121,252 In a human clinical study of LPS-induced systemic inflammation (n=13), 1 h inhalation of 10 or 50 ppm CO gas did not induce a significant increase in COHb (less than 1%). However, a single inhaled dose of 500 ppm CO for 1 h led to a peak COHb level of 9%, which was also achieved by inhaling 250 ppm CO for 2 h. These dosages were well tolerated by the participants without any significant effect on vital parameters. In efficacy studies, CO inhalation was followed by immediate 2 ng/kg intravenous bolus of LPS infusion, leading to inflammatory responses, as indicated by changes in the level of cytokines such as TNF-α, IL-6, IL-8, and IL-10. However, there were no significant differences observed between the CO-treatment group and controls. It was suggested by the authors that the sample size, individual variations, LPS dosage, CO concentration, mode of administration, the gender of the subjects should be further investigated before drawing any conclusion.245 All such results suggest the need to study species differences when considering pharmacokinetics and pharmacodynamics as related to efficacy.
In humans, CO gas inhalation with various dosing protocols and concentrations (10-1500 ppm) has been extensively studied for safety. However, there are limited studies in pharmacological efficacy with conclusive findings. Summarizing these clinical trials goes beyond the scope of this review article on AKI. Therefore, only a few examples are given to present a sense of the “state of the art” and challenges ahead with a focus on those that bear relevance to inflammation and organ protection. In the area of safety assessment, an early phase-I clinical trial ( NCT00122694) studied inhalation of 100-125 ppm CO gas for 2 h/day for 4 consecutive days in patients with chronic obstructive pulmonary disease (COPD). Such exposure led to a median COHb peak level of 2.6-3.1%, which was well tolerated.253 In 2018, a phase-I clinical trial ( NCT02425579) of sepsis-induced acute respiratory distress syndrome (ARDS) confirmed that inhalation of 100-200 ppm CO gas for 90 min for 5 consecutive days was well-tolerated without administration-associated adverse events. Median peak COHb levels increased from 1.97% (placebo air-treated) to 3.48-4.9%. The results also support the accuracy of the Coburn-Forster-Kane equation in predicting COHb levels. Incidentally, a significant reduction in circulating mitochondrial DNA levels was observed with dependency on CO dosage.254 There are other studies that also indicate safety ( NCT01214187) in humans when exposed to 100-200 ppm CO 2 h daily, twice weekly for 12 weeks; although the maximal COHb level increase was only 1.2%, lower than the placebo air group (2.62%).255 Such results suggest that higher levels of exposures may be needed for reproducible outcomes. Importantly, CO was used in Phase II human clinical trial in kidney transplantation by Ikaria in 2007 ( NCT00531856), but was never completed. The allowable limit for COHb was 14% and no adverse events were noted (personal communication). One case study provides some telltale signs of CO’s beneficial effect in human kidney IRI. Specifically, an unexpectedly excellent outcome in an individual kidney transplant was achieved from a CO-poisoned donor despite the prolonged exposure to warm ischemia (~100 min). The authors suggested possible attribution of the outcome to CO pre-conditioning.256 Of course, one has to be cautious in interpreting the results of a single case. However, the many documented cases of successful kidney transplantation using CO-poisoned donors lend further credence to the possibility of CO’s beneficial effects in kidney IRI.257–260
From these studies, one can clearly see that more work is needed in translating success in small animal models to humans. Such work should start with examining the interplay among CO dosage, duration of treatment, and frequency of treatment as well as a threshold level of COHb needed for a given indication, first in animal models and then allometric scaling to humans. Naturally, these are critical issues to examine in the course of developing a therapy for AKI based on CO.
6. Conclusions
Overall, AKI is a significant clinical problem with no therapeutics available to either prevent or treat kidney injury. Although there is a consensus as to the underlying pathologies including oxidative stress, mitochondrial damage, and inflammation, agents that focus on each factor alone do not seem to prevent and/or attenuate disease progression. In recent years, CO delivered in different forms has emerged as a promising agent with demonstrated therapeutic efficacy in animal models of AKI. We believe that the pleiotropic effect of CO is a key factor for its success and also presents challenges in understanding the molecular mechanism(s) of action. Further, because of the physical characteristics of CO, much work is needed to understand the correlation of dosage, delivery form, pharmacokinetics, and pharmacodynamics. At this time, even the question of the best parameter to monitor as an indicator of CO exposure is not a foregone conclusion. Much more work is needed in this promising field. Meaningful progress can only come through collaborations among biologists, chemists, clinicians, and pharmaceutical scientists to ultimately bring CO to patients.
Acknowledgments:
CO-related work in the BW lab is partially funded by the National Institutes of Health (R01DK119202), an endowment set up with the help of the Georgia Research Alliance, and internal resources at Georgia State University. CO-related work in the LEO lab was supported by NIH awards R43-GM125430, and Department of Defense award W81XWH-16-0464 to LEO. LEO is an adjunct professor at Aston University, UK and a scientific consultant for Hillhurst Biopharmaceuticals. Work on AKI in the MDC lab is funded by grants from the National Institutes of Health (R01DK112688), and the Department of Defense (W81XWH-17-1-0610).
References:
- 1.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. [DOI] [PubMed] [Google Scholar]
- 2.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nature reviews Nephrology. 2014;10:193–207. [DOI] [PubMed] [Google Scholar]
- 3.Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerda J, Chawla LS. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14:607–625. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet (London, England). 2012;380:756–766. [DOI] [PubMed] [Google Scholar]
- 5.Billings FTt, Shaw AD. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract. 2014;127:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okusa MD, Molitoris BA, Palevsky PM, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Faubel S, Kellum JA, Wald R, Chertow GM, Levin A, Waikar SS, Murray PT, Parikh CR, Shaw AD, Go AS, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene TH, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA. Design of clinical trials in acute kidney injury: a report from an NIDDK workshop--prevention trials. Clin J Am Soc Nephrol. 2012;7:851–855. [DOI] [PubMed] [Google Scholar]
- 7.Weisbord SD, Palevsky PM. Design of Clinical Trials in Acute Kidney Injury: Lessons from the Past and Future Directions. Semin Nephrol. 2016;36:42–52. [DOI] [PubMed] [Google Scholar]
- 8.Molitoris BA, Okusa MD, Palevsky PM, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene TH, Faubel SG, Kellum JA, Wald R, Chertow GM, Levin A, Waikar SS, Murray PT, Parikh CR, Shaw AD, Go AS, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA. Design of clinical trials in AKI: a report from an NIDDK workshop. Trials of patients with sepsis and in selected hospital settings. Clin J Am Soc Nephrol. 2012;7:856–860. [DOI] [PubMed] [Google Scholar]
- 9.Patel SS, Palant CE, Mahajan V, Chawla LS. Sequelae of AKI. Best Pract Res Clin Anaesthesiol. 2017;31:415–425 and references cited therein. [DOI] [PubMed] [Google Scholar]
- 10.Meersch M, Volmering S, Zarbock A. Prevention of acute kidney injury. Best Pract Res Clin Anaesthesiol. 2017;31:361–370 and references cited therein. [DOI] [PubMed] [Google Scholar]
- 11.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. [DOI] [PubMed] [Google Scholar]
- 12.MarketWatch. Global Acute Kidney Injury (AKI) Epidemiology Forecast Report 2016-2027: Total Incident Population in 7MM Found to be 1,516,635 in 2016 -. ResearchAndMarkets.com; 2018.
- 13.Zuk A, Palevsky PM, Fried L, Harrell FE Jr., Khan S, McKay DB, Devey L, Chawla L, de Caestecker M, Kaufman JS, Thompson BT, Agarwal A, Greene T, Okusa MD, Bonventre JV, Dember LM, Liu KD, Humphreys BD, Gossett D, Xie Y, Norton JM, Kimmel PL, Star RA. Overcoming Translational Barriers in Acute Kidney Injury: A Report from an NIDDK Workshop. Clin J Am Soc Nephrol. 2018;13:1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skrypnyk NI, Siskind LJ, Faubel S, de Caestecker MP. Bridging translation for acute kidney injury with better preclinical modeling of human disease. Am J Physiol Renal Physiol. 2016;310:F972–F984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehran R, Dangas GD, Weisbord SD. Contrast-Associated Acute Kidney Injury. N Engl J Med. 2019;380:2146–2155. [DOI] [PubMed] [Google Scholar]
- 16.Chew STH, Hwang NC. Acute Kidney Injury After Cardiac Surgery: A Narrative Review of the Literature. J Cardiothorac Vasc Anesth. 2019;33:1122–1138. [DOI] [PubMed] [Google Scholar]
- 17.Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C, Acute Dialysis Quality Initiative Consensus XWG. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J Am Soc Nephrol. 2016;27:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moledina DG, Perazella MA. Drug-Induced Acute Interstitial Nephritis. Clin J Am Soc Nephrol. 2017;12:2046–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulse M, Rosner MH. Drugs in Development for Acute Kidney Injury. Drugs. 2019;79:811–821. [DOI] [PubMed] [Google Scholar]
- 20.Gonsalez SR, Cortês AL, Silva RCd, Lowe J, Prieto MC, Silva Lara Ld. Acute kidney injury overview: From basic findings to new prevention and therapy strategies. Pharmacol Ther. 2019;200:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu J, Chen R, Liu S, Yu X, Zou J, Ding X. Global Incidence and Outcomes of Adult Patients With Acute Kidney Injury After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth. 2016;30:82–89. [DOI] [PubMed] [Google Scholar]
- 23.Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10:500–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce B, Bole I, Patel V, Brown DL. Clinical Outcomes of Remote Ischemic Preconditioning Prior to Cardiac Surgery: A Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2017;6:pii: e004666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie J, Zhang X, Xu J, Zhang Z, Klingensmith NJ, Liu S, Pan C, Yang Y, Qiu H. Effect of Remote Ischemic Preconditioning on Outcomes in Adult Cardiac Surgery: A Systematic Review and Meta-analysis of Randomized Controlled Studies. Anesth Analg. 2018;127:30–38. [DOI] [PubMed] [Google Scholar]
- 26.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11:2279–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mannon RB. Delayed Graft Function: The AKI of Kidney Transplantation. Nephron. 2018;140:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahl D, Haddad Z, Datoo A, Qazi YA. Delayed graft function in kidney transplantation. Curr Opin Organ Transplant. 2019;24:82–86. [DOI] [PubMed] [Google Scholar]
- 29.McCormick F, Held PJ, Chertow GM. The Terrible Toll of the Kidney Shortage. J Am Soc Nephrol. 2018;29:2775–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannon RB. Acute Kidney Injury in Kidney Transplants: New Insights. Nephron. 2019:1–4. [DOI] [PubMed] [Google Scholar]
- 31.Henderson LK, Nankivell BJ, Chapman JR. Surveillance protocol kidney transplant biopsies: their evolving role in clinical practice. Am J Transplant. 2011;11:1570–1575. [DOI] [PubMed] [Google Scholar]
- 32.Brown JR, Rezaee ME, Marshall EJ, Matheny ME. Hospital Mortality in the United States following Acute Kidney Injury. Biomed Res Int. 2016;2016:4278579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerwing M, Jacobsen C, Dyshlovoy S, Hauschild J, Rohlfing T, Oing C, Venz S, Oldenburg J, Oechsle K, Bokemeyer C, von Amsberg G, Honecker F. Cabazitaxel overcomes cisplatin resistance in germ cell tumour cells. J Cancer Res Clin Oncol. 2016;142:1979–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Senduny FF, Badria FA, El-Waseef AM, Chauhan SC, Halaweish F. Approach for chemosensitization of cisplatin-resistant ovarian cancer by cucurbitacin B. Tumour Biol. 2016;37:685–698. [DOI] [PubMed] [Google Scholar]
- 35.Ceribelli A, Pino MS, Gelibter AJ, Milella M, Cecere FL, Caterino M, Facciolo F, Mirri A, Cognetti F. Sequential chemotherapy in nonsmall-cell lung cancer: cisplatin and gemcitabine followed by docetaxel. Cancer. 2007;109:727–731. [DOI] [PubMed] [Google Scholar]
- 36.Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, Dalby M, Mistry P, Sen M, O’Toole L, Al Booz H, Dyker K, Moleron R, Whitaker S, Brennan S, Cook A, Griffin M, Aynsley E, Rolles M, De Winton E, Chan A, Srinivasan D, Nixon I, Grumett J, Leemans CR, Buter J, Henderson J, Harrington K, McConkey C, Gray A, Dunn J, De EHPVTG. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet (London, England). 2019;393:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu H, Luo H, Zhang W, Shen Z, Hu X, Zhu X. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther. 2016;10:1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin JF, Lin YC, Tsai TF, Chen HE, Chou KY, Hwang TI. Cisplatin induces protective autophagy through activation of BECN1 in human bladder cancer cells. Drug Des Devel Ther. 2017;11:1517–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh S Cisplatin: The first metal based anticancer drug. Bioorg Chem. 2019;88:102925. [DOI] [PubMed] [Google Scholar]
- 40.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. [DOI] [PubMed] [Google Scholar]
- 41.Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, Arsenijevic N. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 2019;26:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latcha S, Jaimes EA, Patil S, Glezerman IG, Mehta S, Flombaum CD. Long-Term Renal Outcomes after Cisplatin Treatment. Clin J Am Soc Nephrol. 2016;11:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Townsend DM, Deng M, Zhang L, Lapus MG, Hanigan MH. Metabolism of Cisplatin to a nephrotoxin in proximal tubule cells. J Am Soc Nephrol. 2003;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Hanigan MH. Role of cysteine S-conjugate beta-lyase in the metabolism of cisplatin. J Pharmacol Exp Ther. 2003;306:988–994. [DOI] [PubMed] [Google Scholar]
- 45.Nagar R, Khan AR, Poonia A, Mishra PK, Singh S. Metabolism of cisplatin in the organs of Rattus norvegicus: role of Glutathione S-transferase P1. Eur J Drug Metab Pharmacokinet. 2015;40:45–51. [DOI] [PubMed] [Google Scholar]
- 46.Berners-Price SJ, Kuchel PW. Reaction of cis- and trans-[PtCl2(NH3)2] with reduced glutathione inside human red blood cells, studied by 1H and 15N-[1H] DEPT NMR. J Inorg Biochem. 1990;38:327–345. [DOI] [PubMed] [Google Scholar]
- 47.Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31:15–25. [DOI] [PubMed] [Google Scholar]
- 48.Ishikawa T, Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993;268:20116–20125. [PubMed] [Google Scholar]
- 49.Cooper AJ, Pinto JT. Cysteine S-conjugate beta-lyases. Amino Acids. 2006;30:1–15. [DOI] [PubMed] [Google Scholar]
- 50.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43:1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olbricht CJ, Fink M, Gutjahr E. Alterations in lysosomal enzymes of the proximal tubule in gentamicin nephrotoxicity. Kidney Int. 1991;39:639–646. [DOI] [PubMed] [Google Scholar]
- 52.Walker PD, Barri Y, Shah SV. Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail. 1999;21:433–442. [DOI] [PubMed] [Google Scholar]
- 53.Blunston MA, Yonovitz A, Woodahl EL, Smolensky MH. Gentamicin-induced ototoxicity and nephrotoxicity vary with circadian time of treatment and entail separate mechanisms. Chronobiol Int. 2015;32:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Correa-Costa M, Gallo D, Csizmadia E, Gomperts E, Lieberum JL, Hauser CJ, Ji X, Wang B, Camara NOS, Robson SC, Otterbein LE. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc Natl Acad Sci U S A. 2018;115:E2302–E2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anaissie EJ, Vartivarian SE, Abi-Said D, Uzun O, Pinczowski H, Kontoyiannis DP, Khoury P, Papadakis K, Gardner A, Raad, II, Gilbreath J, Bodey GP. Fluconazole versus amphotericin B in the treatment of hematogenous candidiasis: a matched cohort study. Am J Med. 1996;101:170–176. [DOI] [PubMed] [Google Scholar]
- 56.Filippone EJ, Kraft WK, Farber JL. The Nephrotoxicity of Vancomycin. Clin Pharmacol Ther. 2017;102:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ocak S, Gorur S, Hakverdi S, Celik S, Erdogan S. Protective effects of caffeic acid phenethyl ester, vitamin C, vitamin E and N-acetylcysteine on vancomycin-induced nephrotoxicity in rats. Basic Clin Pharmacol Toxicol. 2007;100:328–333. [DOI] [PubMed] [Google Scholar]
- 58.Sadat U Radiographic contrast-media-induced acute kidney injury: pathophysiology and prophylactic strategies. ISRN Radiol. 2013;2013:496438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quintavalle C, Brenca M, De Micco F, Fiore D, Romano S, Romano MF, Apone F, Bianco A, Zabatta MA, Troncone G, Briguori C, Condorelli G. In vivo and in vitro assessment of pathways involved in contrast media-induced renal cells apoptosis. Cell Death Dis. 2011;2:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC, Rumsfeld JS, Spertus JA. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knebel F, Schimke I, Eddicks S, Walde T, Ziebig R, Schattke S, Baumann G, Borges AC. Does contrast echocardiography induce increases in markers of myocardial necrosis, inflammation and oxidative stress suggesting myocardial injury? Cardiovasc Ultrasound. 2005;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khairul I, Wang QQ, Jiang YH, Wang C, Naranmandura H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget. 2017;8:23905–23926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de The H Differentiation therapy revisited. Nat Rev Cancer. 2018;18:117–127. [DOI] [PubMed] [Google Scholar]
- 64.Dias D, Bessa J, Guimaraes S, Soares ME, Bastos Mde L, Teixeira HM. Inorganic mercury intoxication: A case report. Forensic Sci Int. 2016;259:e20–24. [DOI] [PubMed] [Google Scholar]
- 65.Caine B Timeline: The death of Eric Miller of Raleigh and the murder trial of his wife Ann. The News & Observer 2018;July 19. [Google Scholar]
- 66.Yancey-Bragg N California engineer poisoned his colleague for 18 months, police say. He’s charged with attempted murder. USA Today. 2019:April 4. [Google Scholar]
- 67.Tang G, Tu X, Feng P. Lead Poisoning Caused by Traditional Chinese Medicine: A Case Report and Literature Review. Tohoku J Exp Med. 2017;243:127–131. [DOI] [PubMed] [Google Scholar]
- 68.Kim H, Hughes PJ, Hawes EM. Adverse events associated with metal contamination of traditional chinese medicines in Korea: a clinical review. Yonsei Med J. 2014;55:1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coghlan ML, Maker G, Crighton E, Haile J, Murray DC, White NE, Byard RW, Bellgard MI, Mullaney I, Trengove R, Allcock RJ, Nash C, Hoban C, Jarrett K, Edwards R, Musgrave IF, Bunce M. Combined DNA, toxicological and heavy metal analyses provides an auditing toolkit to improve pharmacovigilance of traditional Chinese medicine (TCM). Sci Rep. 2015;5:17475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marginean CO, Melit LE, Moldovan H, Lupu VV, Marginean MO. Lead poisoning in a 16-year-old girl: a case report and a review of the literature (CARE compliant). Medicine. 2016;95:e4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petracca M, Scafa F, Boeri R, Flachi D, Candura SM. Imported occupational lead poisoning: report of four cases. Med Lav. 2013;104:428–433. [PubMed] [Google Scholar]
- 72.Staff-Writer. Mercury Poisoning Kills Lab Chemist. Science. 1997:June 11. [Google Scholar]
- 73.Yang H, Shu Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int J Mol Sci. 2015;16:1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zalups RK. Reductions in renal mass and the nephropathy induced by mercury. Toxicol Appl Pharmacol. 1997;143:366–379. [DOI] [PubMed] [Google Scholar]
- 75.Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals. 2010;23:783–792. [DOI] [PubMed] [Google Scholar]
- 76.Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev. 2000;52:113–143. [PubMed] [Google Scholar]
- 77.Orr SE, Bridges CC. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sasaki A, Oshima Y, Fujimura A. An approach to elucidate potential mechanism of renal toxicity of arsenic trioxide. Exp Hematol. 2007;35:252–262. [DOI] [PubMed] [Google Scholar]
- 79.Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z. Toxic Effect of Acute Cadmium and Lead Exposure in Rat Blood, Liver, and Kidney. Int J Environ Res Public Health. 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang L, Mu X, Fu J, Zhou Z. In vitro cytotoxicity assay with selected chemicals using human cells to predict target-organ toxicity of liver and kidney. Toxicol In Vitro. 2007;21:734–740. [DOI] [PubMed] [Google Scholar]
- 81.Li S, Zhao J, Huang R, Steiner T, Bourner M, Mitchell M, Thompson DC, Zhao B, Xia M. Development and Application of Human Renal Proximal Tubule Epithelial Cells for Assessment of Compound Toxicity. Curr Chem Genom Transl Med. 2017;11:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bracken WM, Sharma RP, Bourcier DR. Cellular distribution of inorganic mercury and its relation to cytotoxicity in bovine kidney cell cultures. J Toxicol Environ Health. 1984;13:865–877. [DOI] [PubMed] [Google Scholar]
- 83.Moghimi Benhangi H, Ahmadi S, Hakimi M, Molafilabi A, Faraji H, Mashkani B. Protective effects of isatin and its synthetic derivatives against iron, copper and lead toxicity. Toxicol In Vitro. 2019;54:232–236. [DOI] [PubMed] [Google Scholar]
- 84.Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–871. [DOI] [PubMed] [Google Scholar]
- 85.Ghahramani N Silica nephropathy. Int J Occup Environ Med. 2010;1:108–115. [PubMed] [Google Scholar]
- 86.Yang B, Xie Y, Guo M, Rosner MH, Yang H, Ronco C. Nephrotoxicity and Chinese Herbal Medicine. Clin J Am Soc Nephrol. 2018;13:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu J, Liu X, Fan J, Chen W, Wang J, Zeng Y, Feng X, Yu X, Yang X. Bardoxolone methyl (BARD) ameliorates aristolochic acid (AA)-induced acute kidney injury through Nrf2 pathway. Toxicology. 2014;318:22–31. [DOI] [PubMed] [Google Scholar]
- 88.Anandagoda N, Lord GM. Preventing aristolochic acid nephropathy. Clin J Am Soc Nephrol. 2015;10:167–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stiborova M, Frei E, Arlt VM, Schmeiser HH. Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy. Mutat Res. 2008;658:55–67. [DOI] [PubMed] [Google Scholar]
- 90.Priestap HA, de los Santos C, Quirke JM. Identification of a reduction product of aristolochic acid: implications for the metabolic activation of carcinogenic aristolochic acid. J Nat Prod. 2010;73:1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jadot I, Decleves AE, Nortier J, Caron N. An Integrated View of Aristolochic Acid Nephropathy: Update of the Literature. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu FY, Wu TS, Chen TW, Liu BH. Aristolochic acid I induced oxidative DNA damage associated with glutathione depletion and ERK1/2 activation in human cells. Toxicol In Vitro. 2011;25:810–816. [DOI] [PubMed] [Google Scholar]
- 93.Pozdzik AA, Salmon IJ, Debelle FD, Decaestecker C, Van den Branden C, Verbeelen D, Deschodt-Lanckman MM, Vanherweghem JL, Nortier JL. Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int. 2008;73:595–607. [DOI] [PubMed] [Google Scholar]
- 94.Leung EM, Chan W. Comparison of DNA and RNA Adduct Formation: Significantly Higher Levels of RNA than DNA Modifications in the Internal Organs of Aristolochic Acid-Dosed Rats. Chem Res Toxicol. 2015;28:248–255. [DOI] [PubMed] [Google Scholar]
- 95.Shi M, Ma L, Zhou L, Fu P. Renal Protective Effects of 17beta-Estradiol on Mice with Acute Aristolochic Acid Nephropathy. Molecules. 2016;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Whelton A Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106:13S–24S. [DOI] [PubMed] [Google Scholar]
- 97.Park IS, Lee SB, Song SH, Seong EY, Kim IY, Rhee H, Kim MJ, Lee DW. Ticagrelor-induced acute kidney injury can increase serum concentration of statin and lead to concurrence of rhabdomyolysis. Anatol J Cardiol. 2018;19:225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stasi A, Intini A, Divella C, Franzin R, Montemurno E, Grandaliano G, Ronco C, Fiaccadori E, Pertosa GB, Gesualdo L, Castellano G. Emerging role of Lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol Dial Transplant. 2017;32:24–31. [DOI] [PubMed] [Google Scholar]
- 99.Tang Y, Wang C, Wang Y, Zhang J, Wang F, Li L, Meng X, Li G, Li Y, Wang L. Isoliquiritigenin attenuates LPS-induced AKI by suppression of inflammation involving NF-kappaB pathway. Am J Transl Res. 2018;10:4141–4151. [PMC free article] [PubMed] [Google Scholar]
- 100.Berns JS, Kasbekar N. Highly active antiretroviral therapy and the kidney: an update on antiretroviral medications for nephrologists. Clin J Am Soc Nephrol. 2006;1:117–129. [DOI] [PubMed] [Google Scholar]
- 101.Izzedine H, Harris M, Perazella MA. The nephrotoxic effects of HAART. Nat Rev Nephrol. 2009;5:563–573. [DOI] [PubMed] [Google Scholar]
- 102.Bellomo R Bench-to-bedside review: lactate and the kidney. Crit Care. 2002;6:322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dalal RP, Goldfarb DS. Melamine-related kidney stones and renal toxicity. Nat Rev Nephrol. 2011;7:267–274. [DOI] [PubMed] [Google Scholar]
- 104.Mirhosseni A, Farahani B, Gandomi-Mohammadabadi A, Keyvani H, Biglari-Abhari M, Davari A, Vazirizade-Mahabadi MH, Savaj S. Preventive Effect of Trimetazidine on Contrast-Induced Acute Kidney Injury in CKD Patients Based on Urinary Neutrophil Gelatinase-associated Lipocalin (uNGAL): A randomized Clinical Trial. Iran J Kidney Dis. 2019;13:191–197. [PubMed] [Google Scholar]
- 105.Arbel Y, Ben-Assa E, Puzhevsky D, Litmanowicz B, Galli N, Chorin E, Halkin A, Sadeh B, Konigstein M, Bassat OK, Steinvil A, Bazan S, Banai S, Finkelstein A. Forced diuresis with matched hydration during transcatheter aortic valve implantation for Reducing Acute Kidney Injury: a randomized, sham-controlled study (REDUCE-AKI). European heart journal. 2019:doi: 10.1093/eurheartj/ehz1343. [DOI] [PubMed] [Google Scholar]
- 106.Yan Y, Du C, Li G, Chen L, Yan Y, Chen G, Hu W, Chang L. CO suppresses prostate cancer cell growth by directly targeting LKB1/AMPK/mTOR pathway in vitro and in vivo. Urol Oncol. 2018;36:312, e311–312.e318. [DOI] [PubMed] [Google Scholar]
- 107.Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-Acetylcysteine in Preventing Acute Kidney Injury After Cardiac Surgery: A Meta-Analysis Study. J Invest Surg. 2018;31:14–23. [DOI] [PubMed] [Google Scholar]
- 108.Lewicki M, Ng I, Schneider AG. HMG CoA reductase inhibitors (statins) for preventing acute kidney injury after surgical procedures requiring cardiac bypass. Cochrane Database Syst Rev. 2015:Cd010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bell S, Rennie T, Marwick CA, Davey P. Effects of peri-operative nonsteroidal anti-inflammatory drugs on post-operative kidney function for adults with normal kidney function. Cochrane Database Syst Rev. 2018;11:Cd011274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nigwekar SU, Strippoli GF, Navaneethan SD. Thyroid hormones for acute kidney injury. Cochrane Database Syst Rev. 2013:Cd006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nigwekar SU, Navaneethan SD, Parikh CR, Hix JK. Atrial natriuretic peptide for preventing and treating acute kidney injury. Cochrane Database Syst Rev. 2009:Cd006028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamada H, Doi K, Tsukamoto T, Kiyomoto H, Yamashita K, Yanagita M, Terada Y, Mori K. Low-dose atrial natriuretic peptide for prevention or treatment of acute kidney injury: a systematic review and meta-analysis. Crit Care. 2019;23:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ghaemian A, Yazdani J, Azizi S, Farsavian AA, Nabati M, Malekrah A, Dabirian M, Espahbodi F, Mirjani B, Mohsenipouya H, Heshmatian J. Remote ischemic preconditioning to reduce contrast-induced acute kidney injury in chronic kidney disease: a randomized controlled trial. BMC Nephrol. 2018;19:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gallagher KM, O’Neill S, Harrison EM, Ross JA, Wigmore SJ, Hughes J. Recent early clinical drug development for acute kidney injury. Expert Opin Investig Drugs. 2017;26:141–154. [DOI] [PubMed] [Google Scholar]
- 115.De La Cruz K, Benoit SL, Pan Z, Maier R, Ji X, Wang B. Click, Release, and Fluoresce: A Chemical Strategy for a Cascade Prodrug System for Codelivery of Carbon Monoxide, a Drug Payload, and a Fluorescent Reporter. Org Lett. 2018;20 897–900. [DOI] [PubMed] [Google Scholar]
- 116.Uddin MJ, Pak ES, Ha H. Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress. Korean J Physiol Pharmacol. 2018;22:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng Y, Rong J. Therapeutic Potential of Heme Oxygenase-1/carbon Monoxide System Against Ischemia-Reperfusion Injury. Curr Pharm Des. 2017;23:3884–3898. [DOI] [PubMed] [Google Scholar]
- 118.Abe T, Yazawa K, Fujino M, Imamura R, Hatayama N, Kakuta Y, Tsutahara K, Okumi M, Ichimaru N, Kaimori JY, Isaka Y, Seki K, Takahara S, Li XK, Nonomura N. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab Invest. 2017;97:468–477. [DOI] [PubMed] [Google Scholar]
- 119.Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim HH, Choi S. Therapeutic Aspects of Carbon Monoxide in Cardiovascular Disease. Int J Mol Sci. 2019;19:doi: 10.3390/ijms19082381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng Y, Ji X, Yu B, Ji K, Gallo D, Csizmadia E, Zhu M, de la Cruz LKC, Choudhary MR, Chittavong V, Pan Z, Yuan Z, Otterbein LE, Wang B. Enrichment-triggered Prodrug Activation Demonstrated through Mitochondria-targeted Delivery of Doxorubicin and Carbon Monoxide. Nature Chem. 2018;10:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun J, Guo E, Yang J, Yang Y, Liu S, Hu J, Jiang X, Dirsch O, Dahmen U, Dong W, Liu A. Carbon monoxide ameliorates hepatic ischemia/reperfusion injury via sirtuin 1-mediated deacetylation of high-mobility group box 1 in rats. Liver Transpl. 2017;23:510–526. [DOI] [PubMed] [Google Scholar]
- 123.Che X, Fang Y, Si X, Wang J, Hu X, Reis C, Chen S. The Role of Gaseous Molecules in Traumatic Brain Injury: An Updated Review. Front Neurosci. 2018;12:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Choi YK, Maki T, Mandeville ET, Koh SH, Hayakawa K, Arai K, Kim YM, Whalen MJ, Xing C, Wang X, Kim KW, Lo EH. Dual effects of carbon monoxide on pericytes and neurogenesis in traumatic brain injury. Nat Med. 2016;22:1335–1341. [DOI] [PubMed] [Google Scholar]
- 125.Wang P, Yao L, Zhou LL, Liu YS, Chen MD, Wu HD, Chang RM, Li Y, Zhou MG, Fang XS, Yu T, Jiang LY, Huang ZT. Carbon Monoxide Improves Neurologic Outcomes by Mitochondrial Biogenesis after Global Cerebral Ischemia Induced by Cardiac Arrest in Rats. Int J Biol Sci. 2016;12:1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hegazi RAF, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plev SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1–dependent pathway. J Exp Med. 2005;202:1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, Matsuoka KM, Sepulveda AR, Li FL, Otterbein LE, Plevy SE. An Anti-Inflammatory Role for Carbon Monoxide and Heme Oxygenase-1 in Chronic Th2-Mediated Murine Colitis. J Immunol. 2011;186:5506–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Steiger C, Uchiyama K, Takagi T, Mizushima K, Higashimura Y, Gutmann M, Hermann C, Botov S, Schmalz HG, Naito Y, Meinel L. Prevention of colitis by controlled oral drug delivery of carbon monoxide. J Control Release. 2016;239:128–136. [DOI] [PubMed] [Google Scholar]
- 129.Takagi T, Naito Y, Mizushima K, Akagiri S, Suzuki, Hirata I, Omatsu T, Handa O, Kokura S, Ichikawa H, Yoshikawa T. Inhalation of Carbon Monoxide Ameliorates TNBS-Induced Colitis in Mice Through the Inhibition of TNF-α Expression. Digest Dis Sci. 2010;55:2797–2804. [DOI] [PubMed] [Google Scholar]
- 130.Takagi T, Naito Y, Uchiyama K, Suzuki T, Hirata I, Mizushima K, Tsuboi N, Hayashi N, Handa O, Ishikawa T, Yagi N, Kokura S, Ichikawa H, Yoshikawa T. Carbon Monoxide Liberated from Carbon Monoxide-Releasing Molecule Exerts an Anti-inflammatory Effect on Dextran Sulfate Sodium-Induced Colitis in Mice. Digest Dis Sci. 2011;56:1663–1671. [DOI] [PubMed] [Google Scholar]
- 131.Uddin MJ, Jeong SO, Zheng M, Chen Y, Cho GJ, Chung HT, Joe Y. Carbon monoxide attenuates dextran sulfate sodium-induced colitis via inhibition of GSK-3beta signaling. Oxid Med Cell Longev. 2013;2013:210563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. [DOI] [PubMed] [Google Scholar]
- 133.Ji X, Damera K, Zheng Y, Yu B, Otterbein LE, Wang B. Toward Carbon Monoxide-based Therapeutics: Critical Drug Delivery and Developability Issues. J Pharm Sci. 2016;105:406–416 and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. [DOI] [PubMed] [Google Scholar]
- 135.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Drummond HA, Mitchell ZL, Abraham NG, Stec DE. Targeting Heme Oxygenase-1 in Cardiovascular and Kidney Disease. Antioxidants. 2019;8:doi: 10.3390/antiox8060181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Y, Zhang Z, Liu Y, Jing H, Ke X, Dong F. Use of carbon monoxide breath test to assess red blood cell lifespan in newly diagnosed multiple myeloma patients. J Breath Res. 2019:in press, DOI: 10.1088/1752-7163/ab1082c1012. [DOI] [PubMed] [Google Scholar]
- 138.Paredi P, Biernacki W, Invernizzi G, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide levels elevated in diabetes and correlated with glucose concentration in blood: a new test for monitoring the disease? Chest. 1999;116:1007–1011. [DOI] [PubMed] [Google Scholar]
- 139.Biernacki WA, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide in patients with lower respiratory tract infection. Respir Med. 2001;95:1003–1005. [DOI] [PubMed] [Google Scholar]
- 140.Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Investig Drugs. 2005;14:1305–1318. [DOI] [PubMed] [Google Scholar]
- 141.Barpe DR, Rosa DD, Froehlich PE. Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur J Pharm Sci. 2010;41:458–463. [DOI] [PubMed] [Google Scholar]
- 142.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285:1059–1070. [DOI] [PubMed] [Google Scholar]
- 143.Motterlini R, Foresti R. Biological signaling by carbon monoxide and carbon monoxide-releasing molecules. Am J Physiol Cell Physiol. 2017;312:C302–c313. [DOI] [PubMed] [Google Scholar]
- 144.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. [DOI] [PubMed] [Google Scholar]
- 145.Haschemi A, Chin BY, Jeitler M, Esterbauer H, Wagner O, Bilban M, Otterbein LE. Carbon monoxide induced PPARgamma SUMOylation and UCP2 block inflammatory gene expression in macrophages. PLoS One. 2011;6:e26376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bilban M, Bach FH, Otterbein SL, Ifedigbo E, d’Avila JC, Esterbauer H, Chin BY, Usheva A, Robson SC, Wagner O, Otterbein LE. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610. [DOI] [PubMed] [Google Scholar]
- 147.Zuckerbraun BS, Chin BY, Bilban M, d’Avila JC, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. Faseb J. 2007;21:1099–1106. [DOI] [PubMed] [Google Scholar]
- 148.Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, Kaczmarek E, Lee PJ, uckerbraun BS, Flavell R, Soares MP, Otterbein LE. Macrophages sense and kill bacteria through carbon monoxide–dependent inflammasome activation. J Clin Invest. 2014;124:4926–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ji X, Wang B. Strategies toward Organic Carbon Monoxide Prodrugs. Acc Chem Res. 2018;51:1377–1385. [DOI] [PubMed] [Google Scholar]
- 150.Musameh MD, Green CJ, Mann BE, Motterlini R, Fuller BJ. CO liberated from a carbon monoxide-releasing molecule exerts a positive inotropic effect in doxorubicin-induced cardiomyopathy. J Cardiovasc Pharmacol. 2010;55:168–175. [DOI] [PubMed] [Google Scholar]
- 151.Soni H, Pandya G, Patel P, Acharya A, Jain M, Mehta AA. Beneficial effects of carbon monoxide-releasing molecule-2 (CORM-2) on acute doxorubicin cardiotoxicity in mice: role of oxidative stress and apoptosis. Toxicol Appl Pharmacol. 2011;253:70–80. [DOI] [PubMed] [Google Scholar]
- 152.Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2–8. [DOI] [PubMed] [Google Scholar]
- 153.Wegiel B, Hanto DW, Otterbein LE. The social network of carbon monoxide in medicine. Trends Mol Med. 2013;19:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. [DOI] [PubMed] [Google Scholar]
- 155.Uddin MJ, Li CS, Joe Y, Chen Y, Zhang Q, Ryter SW, Chung HT. Carbon Monoxide Inhibits Tenascin-C Mediated Inflammation via IL-10 Expression in a Septic Mouse Model. Mediators Inflamm. 2015;2015:613249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ji X, Wang B. Toward carbon monoxide based therapeutics: carbon monoxide in a pill. Pharm Pat Anal. 2017;6:171–177. [DOI] [PubMed] [Google Scholar]
- 157.Hanto DW, Maki T, Yoon MH, Csizmadia E, Chin BY, Gallo D, Konduru B, Kuramitsu K, Smith NR, Berssenbrugge A, Attanasio C, Thomas M, Wegiel B, Otterbein LE. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in Swine. Am J Transplant. 2010;10:2421–2430. [DOI] [PubMed] [Google Scholar]
- 158.Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE, Murase N. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am J Transplant. 2005;5:282–291. [DOI] [PubMed] [Google Scholar]
- 159.Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, Nalesnik MA, Otterbein LE, Murase N. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol. 2004;287:F979–989. [DOI] [PubMed] [Google Scholar]
- 160.Nakao A, Kimizuka K, Stolz DB, Seda Neto J, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Bauer AJ, Nalesnik MA, Otterbein LE, Geller DA, Murase N. Protective effect of carbon monoxide inhalation for cold-preserved small intestinal grafts. Surgery. 2003;134:285–292. [DOI] [PubMed] [Google Scholar]
- 161.Shi J, Yu JB, Liu W, Wang D, Zhang Y, Gong LR, Dong SA, Liu DQ. Carbon monoxide alleviates lipopolysaccharide-induced oxidative stress injury through suppressing the expression of Fis1 in NR8383 cells. Exp Cell Res. 2016;349:162–167. [DOI] [PubMed] [Google Scholar]
- 162.Huang Y, Ye Z, Ma T, Li H, Zhao Y, Chen W, Wang Y, Yan X, Gao Y, Li Z. Carbon monoxide (CO) modulates hydrogen peroxide (H2O2)-mediated cellular dysfunction by targeting mitochondria in rabbit lens epithelial cells. Exp Eye Res. 2018;169:68–78. [DOI] [PubMed] [Google Scholar]
- 163.Sun B, Sun Z, Jin Q, Chen X. CO-releasing molecules (CORM-2)-liberated CO attenuates leukocytes infiltration in the renal tissue of thermally injured mice. Int J Biol Sci. 2008;4:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AM, Serhan CN. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J Immunol. 2013;190:6378–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ozaki KS, Kimura S, Murase N. Use of carbon monoxide in minimizing ischemia/reperfusion injury in transplantation. Transplant Rev (Orlando). 2012;26:125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zuckerbraun BS, Chin BY, Wegiel B, Billiar TR, Czsimadia E, Rao J, Shimoda L, Ifedigbo E, Kanno S, Otterbein LE. Carbon monoxide reverses established pulmonary hypertension. J Exp Med. 2006;203:2109–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sarady JK, Zuckerbraun BS, Bilban M, Wagner O, Usheva A, Liu F, Ifedigbo E, Zamora R, Choi AM, Otterbein LE. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. Faseb j. 2004;18:854–856. [DOI] [PubMed] [Google Scholar]
- 168.McKendrick JG, Snodgrass W. On the physiological action of carbon monoxide of nickel. Br Med J. 1891;1:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Romanski S, Stamellou E, Jaraba JT, Storz D, Kramer BK, Hafner M, Amslinger S, Schmalz HG, Yard BA. Enzyme-triggered CO-releasing molecules (ET-CORMs): evaluation of biological activity in relation to their structure. Free Radic Biol Med. 2013;65:78–88. [DOI] [PubMed] [Google Scholar]
- 170.Stamellou E, Storz D, Botov S, Ntasis E, Wedel J, Sollazzo S, Kramer BK, van Son W, Seelen M, Schmalz HG, Schmidt A, Hafner M, Yard BA. Different design of enzyme-triggered CO-releasing molecules (ET-CORMs) reveals quantitative differences in biological activities in terms of toxicity and inflammation. Redox Biol. 2014;2:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yin H, Fang J, Liao L, Nakamura H, Maeda H. Styrene-maleic acid copolymer-encapsulated CORM2, a water-soluble carbon monoxide (CO) donor with a constant CO-releasing property, exhibits therapeutic potential for inflammatory bowel disease. J Control Release. 2014;187:14–21. [DOI] [PubMed] [Google Scholar]
- 172.Hasegawa U, van der Vlies AJ, Simeoni E, Wandrey C, Hubbell JA. Carbon monoxide-releasing micelles for immunotherapy. J Am Chem Soc. 2010;132:18273–18280. [DOI] [PubMed] [Google Scholar]
- 173.Pierri AE, Huang PJ, Garcia JV, Stanfill JG, Chui M, Wu G, Zheng N, Ford PC. A photoCORM nanocarrier for CO release using NIR light. Chem Commun (Camb). 2015;51:2072–2075. [DOI] [PubMed] [Google Scholar]
- 174.Dordelmann G, Pfeiffer H, Birkner A, Schatzschneider U. Silicium dioxide nanoparticles as carriers for photoactivatable CO-releasing molecules (PhotoCORMs). Inorg Chem. 2011;50:4362–4367. [DOI] [PubMed] [Google Scholar]
- 175.Matson JB, Webber MJ, Tamboli VK, Weber B, Stupp SI. A Peptide-Based Material for Therapeutic Carbon Monoxide Delivery. Soft Matter. 2012;8:2689–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Antony LA, Slanina T, Sebej P, Solomek T, Klan P. Fluorescein analogue xanthene-9-carboxylic acid: a transition-metal-free CO releasing molecule activated by green light. Org Lett. 2013;15:4552–4555. [DOI] [PubMed] [Google Scholar]
- 177.Palao E, Slanina T, Muchova L, Solomek T, Vitek L, Klan P. Transition-Metal-Free CO-Releasing BODIPY Derivatives Activatable by Visible to NIR Light as Promising Bioactive Molecules. J Am Chem Soc. 2016;138:126–133. [DOI] [PubMed] [Google Scholar]
- 178.Peng P, Wang C, Shi Z, Johns VK, Ma L, Oyer J, Copik A, Igarashi R, Liao Y. Visible-light activatable organic CO-releasing molecules (PhotoCORMs) that simultaneously generate fluorophores. Org Biomol Chem. 2013;11:6671–6674. [DOI] [PubMed] [Google Scholar]
- 179.Anderson SN, Richards JM, Esquer HJ, Benninghoff AD, Arif AM, Berreau LM. A Structurally-Tunable 3-Hydroxyflavone Motif for Visible Light-Induced Carbon Monoxide-Releasing Molecules (CORMs). ChemistryOpen. 2015;4:590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Popova M, Soboleva T, Ayad S, Benninghoff AD, Berreau LM. Visible-Light-Activated Quinolone Carbon-Monoxide-Releasing Molecule: Prodrug and Albumin-Assisted Delivery Enables Anticancer and Potent Anti-Inflammatory Effects. J Am Chem Soc. 2018;140:9721–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Belcher JD, Gomperts E, Nguyen J, Chen C, Abdulla F, Kiser ZM, Gallo D, Levy H, Otterbein LE, Vercellotti GM. Oral carbon monoxide therapy in murine sickle cell disease: Beneficial effects on vaso-occlusion, inflammation and anemia. PLoS One. 2018;13:e0205194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Antony LA, Slanina T, Sebej P, Solomek T, Klan P. Fluorescein analogue xanthene-9-carboxylic acid: a transition-metal-free CO releasing molecule activated by green light. Org Lett. 2013;15:4552–4555. [DOI] [PubMed] [Google Scholar]
- 183.Ji X, Aghoghovbia RE, De La Cruz LKC, Pan Z, Yang X, Yu B, Wang B. Click and Release: A High-Content Bioorthogonal Prodrug with Multiple Outputs. Org Lett. 2019;21:3649–3652. [DOI] [PubMed] [Google Scholar]
- 184.Ji X, Ji K, Chittavong V, Aghoghovbia RE, Zhu M, Wang B. Click and Fluoresce: A Bioorthogonally Activated Smart Probe for Wash-Free Fluorescent Labeling of Biomolecules. J Org Chem. 2017;82:1471–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pan Z, Ji X, Chittavong V, Li W, Ji K, Zhu M, Zhang J, Wang B. Organic CO-prodrugs: Structure CO-release rate relationship Studies. Chem Eur J 2017;23:9838–9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang D, Viennois E, Ji K, Damera K, Draganov A, Zheng Y, Dai C, Merlin D, Wang B. A Click-and-Release Approach to CO Prodrugs. Chem Commun. 2014;50:15890–15893. [DOI] [PubMed] [Google Scholar]
- 187.Ji X, Pan Z, Li C, Kang T, De La Cruz LKC, Yang L, Yuan Z, Ke B, Wang B. Esterase-Sensitive and pH-Controlled Carbon Monoxide Prodrugs for Treating Systemic Inflammation. J Med Chem. 2019;62:3163–3168. [DOI] [PubMed] [Google Scholar]
- 188.Pan Z, Zhang J, Chittavong V, Ji X, Wang B. Organic CO Prodrugs Activated by Endogenous ROS. Org Lett. 2018;20:8–11. [DOI] [PubMed] [Google Scholar]
- 189.Culotta E, Koshland DEJ. NO news is good news. Science 1992;258:1862–1864. [DOI] [PubMed] [Google Scholar]
- 190.Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li PL, Ritter JK. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int J Mol Sci 2018;19:pii: E2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zheng Y, Yu B, De La Cruz LK, Roy Choudhury M, Anifowose A, Wang B. Toward Hydrogen Sulfide Based Therapeutics: Critical Drug Delivery and Developability Issues. Med Res Rev. 2018;38:57–100. [DOI] [PubMed] [Google Scholar]
- 192.Powell CR, Dillon KM, Matson JB. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem Pharmacol. 2018;149:110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int J Mol Sci. 2019;20:doi: 10.3390/ijms20123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Severin MJ, Campagno RV, Brandoni A, Torres AM. Time evolution of methotrexate-induced kidney injury: A comparative study between different biomarkers of renal damage in rats. Clin Exp Pharmacol Physiol. 2019:doi: 10.1111/1440-1681.13122. [DOI] [PubMed] [Google Scholar]
- 195.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–24. [DOI] [PubMed] [Google Scholar]
- 196.Motterlini R, Mann BE, Johnson TR, Clark JE, Foresti R, Green CJ. Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr Pharm Des. 2003;9:2525–2539. [DOI] [PubMed] [Google Scholar]
- 197.McLean S, Mann BE, Poole RK. Sulfite species enhance carbon monoxide release from CO-releasing molecules: implications for the deoxymyoglobin assay of activity. Anal Biochem. 2012;427:36–40. [DOI] [PubMed] [Google Scholar]
- 198.Klein M, Neugebauer U, Schmitt M, Popp J. Elucidation of the CO-Release Kinetics of CORM-A1 by Means of Vibrational Spectroscopy. Chemphyschem. 2016;17:985–993. [DOI] [PubMed] [Google Scholar]
- 199.Motterlini R, Sawle P, Hammad J, Bains S, Alberto R, Foresti R, Green CJ. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005;19:284–286. [DOI] [PubMed] [Google Scholar]
- 200.Crook SH, Mann BE, Meijer AJ, Adams H, Sawle P, Scapens D, Motterlini R. [Mn(CO)4{S2CNMe(CH2CO2H)}], a new water-soluble CO-releasing molecule. Dalton Trans. 2011;40:4230–4235. [DOI] [PubMed] [Google Scholar]
- 201.Motterlini R, Nikam A, Manin S, Ollivier A, Wilson JL, Djouadi S, Muchova L, Martens T, Rivard M, Foresti R. HYCO-3, a dual CO-releaser/Nrf2 activator, reduces tissue inflammation in mice challenged with lipopolysaccharide. Redox Biol. 2019;20:334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ji X, Damera K, Zheng Y, Yu B, Otterbein LE, Wang B. Toward Carbon Monoxide–Based Therapeutics: Critical Drug Delivery and Developability Issues. Journal of Pharmaceutical Sciences. 2016;105:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lamon BD, Zhang FF, Puri N, Brodsky SV, Goligorsky MS, Nasjletti A. Dual pathways of carbon monoxide-mediated vasoregulation: modulation by redox mechanisms. Circ Res. 2009;105:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Southam HM, Smith TW, Lyon RL, Liao C, Trevitt CR, Middlemiss LA, Cox FL, Chapman JA, El-Khamisy SF, Hippler M, Williamson MP, Henderson PJF, Poole RK. A thiol-reactive Ru(II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO-releasing molecule-3. Redox Biol. 2018;18:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ryan MJ, Jernigan NL, Drummond HA, McLemore GR, Jr., Rimoldi JM, Poreddy SR, Gadepalli RS, Stec DE. Renal vascular responses to CORM-A1 in the mouse. Pharmacol Res. 2006;54:24–29. [DOI] [PubMed] [Google Scholar]
- 206.Bhattacharjee RN, Richard-Mohamed M, Sun Q, Haig A, Aboalsamh G, Barrett P, Mayer R, Alhasan I, Pineda-Solis K, Jiang L, Alharbi H, Saha M, Patterson E, Sener A, Cepinskas G, Jevnikar AM, Luke PPW. CORM-401 Reduces Ischemia Reperfusion Injury in an Ex Vivo Renal Porcine Model of the Donation After Circulatory Death. Transplantation. 2018;102:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Botros FT, Navar LG. Interaction between endogenously produced carbon monoxide and nitric oxide in regulation of renal afferent arterioles. Am J Physiol Heart Circ Physiol. 2006;291:H2772–2778. [DOI] [PubMed] [Google Scholar]
- 208.Ren Y, D’Ambrosio MA, Wang H, Liu R, Garvin JL, Carretero OA. Heme oxygenase metabolites inhibit tubuloglomerular feedback (TGF). Am J Physiol Renal Physiol. 2008;295:F1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ren Y, D’Ambrosio MA, Wang H, Falck JR, Peterson EL, Garvin JL, Carretero OA. Mechanisms of carbon monoxide attenuation of tubuloglomerular feedback. Hypertension. 2012;59:1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sandouka A, Fuller BJ, Mann BE, Green CJ, Foresti R, Motterlini R. Treatment with CO-RMs during cold storage improves renal function at reperfusion. Kidney Int. 2006;69:239–247. [DOI] [PubMed] [Google Scholar]
- 211.Bagul A, Hosgood SA, Kaushik M, Nicholson ML. Carbon monoxide protects against ischemia-reperfusion injury in an experimental model of controlled nonheartbeating donor kidney. Transplantation. 2008;85:576–581. [DOI] [PubMed] [Google Scholar]
- 212.Hosgood SA, Bagul A, Kaushik M, Rimoldi J, Gadepalli RS, Nicholson ML. Application of nitric oxide and carbon monoxide in a model of renal preservation. Br J Surg. 2008;95:1060–1067. [DOI] [PubMed] [Google Scholar]
- 213.Caumartin Y, Stephen J, Deng JP, Lian D, Lan Z, Liu W, Garcia B, Jevnikar AM, Wang H, Cepinskas G, Luke PP. Carbon monoxide-releasing molecules protect against ischemia-reperfusion injury during kidney transplantation. Kidney Int. 2011;79:1080–1089. [DOI] [PubMed] [Google Scholar]
- 214.Nemeth Z, Csizmadia E, Vikstrom L, Li M, Bisht K, Feizi A, Otterbein S, Zuckerbraun B, Costa DB, Pandolfi PP, Fillinger J, Dome B, Otterbein LE, Wegiel B. Alterations of tumor microenvironment by carbon monoxide impedes lung cancer growth. Oncotarget. 2016;7:23919–23932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wegiel B, Gallo D, Csizmadia E, Harris C, Belcher J, Vercellotti GM, Penacho N, Seth P, Sukhatme V, Ahmed A, Pandolfi PP, Helczynski L, Bjartell A, Persson JL, Otterbein LE. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013;73:7009–7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Vitek L, Gbelcova H, Muchova L, Vanova K, Zelenka J, Konickova R, Suk J, Zadinova M, Knejzlik Z, Ahmad S, Fujisawa T, Ahmed A, Ruml T. Antiproliferative effects of carbon monoxide on pancreatic cancer. Dig Liver Dis. 2014;46:369–375. [DOI] [PubMed] [Google Scholar]
- 217.Kourti M, Jiang WG, Cai J. Aspects of Carbon Monoxide in Form of CO-Releasing Molecules Used in Cancer Treatment: More Light on the Way. Oxid Med Cell Longev. 2017;2017:9326454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li Y, Dang J, Liang Q, Yin L. Carbon monoxide (CO)-Strengthened cooperative bioreductive anti-tumor therapy via mitochondrial exhaustion and hypoxia induction. Biomaterials. 2019;209:138–151. [DOI] [PubMed] [Google Scholar]
- 219.Wang Y, Liu Z, Wang H, Meng Z, Wang Y, Miao W, Li X, Ren H. Starvation-amplified CO generation for enhanced cancer therapy via an erythrocyte membrane-biomimetic gas nanofactory. Acta Biomater. 2019;92:241–253. [DOI] [PubMed] [Google Scholar]
- 220.Kourti M, Westwell A, Jiang W, Cai J. Repurposing old carbon monoxide-releasing molecules towards the anti-angiogenic therapy of triple-negative breast cancer. Oncotarget. 2019;10:1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kawahara B, Moller T, Hu-Moore K, Carrington S, Faull KF, Sen S, Mascharak PK. Attenuation of Antioxidant Capacity in Human Breast Cancer Cells by Carbon Monoxide through Inhibition of Cystathionine beta-Synthase Activity: Implications in Chemotherapeutic Drug Sensitivity. J Med Chem. 2017;60:8000–8010. [DOI] [PubMed] [Google Scholar]
- 222.Pinto MN, Chakraborty I, Sandoval C, Mascharak PK. Eradication of HT-29 colorectal adenocarcinoma cells by controlled photorelease of CO from a CO-releasing polymer (photoCORP-1) triggered by visible light through an optical fiber-based device. J Control Release. 2017;264:192–202. [DOI] [PubMed] [Google Scholar]
- 223.Chakraborty I, Jimenez J, Mascharak PK. CO-Induced apoptotic death of colorectal cancer cells by a luminescent photoCORM grafted on biocompatible carboxymethyl chitosan. Chem Commun. 2017;53:5519–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shao L, Gu YY, Jiang CH, Liu CY, Lv LP, Liu JN, Zou Y. Carbon monoxide releasing molecule-2 suppresses proliferation, migration, invasion, and promotes apoptosis in non-small cell lung cancer Calu-3 cells. Eur Rev Med Pharmacol Sci. 2018;22:1948–1957. [DOI] [PubMed] [Google Scholar]
- 225.Kawahara B, Ramadoss S, Chaudhuri G, Janzen C, Sen S, Mascharak PK. Carbon monoxide sensitizes cisplatin-resistant ovarian cancer cell lines toward cisplatin via attenuation of levels of glutathione and nuclear metallothionein. J Inorg Biochem. 2019;191:29–39. [DOI] [PubMed] [Google Scholar]
- 226.Tayem Y, Johnson TR, Mann BE, Green CJ, Motterlini R. Protection against cisplatin-induced nephrotoxicity by a carbon monoxide-releasing molecule. Am J Physiol Renal Physiol. 2006;290:F789–794. [DOI] [PubMed] [Google Scholar]
- 227.Yoon YE, Lee KS, Lee YJ, Lee HH, Han WK. Renoprotective Effects of Carbon Monoxide-Releasing Molecule 3 in Ischemia-Reperfusion Injury and Cisplatin-Induced Toxicity. Transplant Proc. 2017;49:1175–1182. [DOI] [PubMed] [Google Scholar]
- 228.Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int Suppl. 2004:S56–61. [DOI] [PubMed] [Google Scholar]
- 229.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Abdel-Zaher AO, Abd-Ellatief RB, Aboulhagag NA, Farghaly HSM, Al-Wasei FMM. The interrelationship between gasotransmitters and lead-induced renal toxicity in rats. Toxicol Lett. 2019;310:39–50. [DOI] [PubMed] [Google Scholar]
- 231.Seixas JD, Chaves-Ferreira M, Montes-Grajales D, Goncalves AM, Marques AR, Saraiva LM, Olivero-Verbel J, Romao CC, Bernardes GJ. An N-Acetyl Cysteine Ruthenium Tricarbonyl Conjugate Enables Simultaneous Release of CO and Ablation of Reactive Oxygen Species. Chemistry. 2015;21:14708–14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Winburn IC, Gunatunga K, McKernan RD, Walker RJ, Sammut IA, Harrison JC. Cell damage following carbon monoxide releasing molecule exposure: implications for therapeutic applications. Basic Clin Pharmacol Toxicol. 2012;111:31–41. [DOI] [PubMed] [Google Scholar]
- 233.Czibik G, Sagave J, Martinov V, Ishaq B, Sohl M, Sefland I, Carlsen H, Farnebo F, Blomhoff R, Valen G. Cardioprotection by hypoxia-inducible factor 1 alpha transfection in skeletal muscle is dependent on haem oxygenase activity in mice. Cardiovasc Res. 2009;82:107–114. [DOI] [PubMed] [Google Scholar]
- 234.Santos-Silva T, Mukhopadhyay A, Seixas JD, Bernardes GJ, Romao CC, Romao MJ. Towards improved therapeutic CORMs: understanding the reactivity of CORM-3 with proteins. Curr Med Chem. 2011;18:3361–3366. [DOI] [PubMed] [Google Scholar]
- 235.Nobre LS, Jeremias H, Romao CC, Saraiva LM. Examining the antimicrobial activity and toxicity to animal cells of different types of CO-releasing molecules. Dalton Trans. 2016;45:1455–1466. [DOI] [PubMed] [Google Scholar]
- 236.Ji X, De La Cruz LKC, Pan Z, Chittavong V, Wang B. pH-Sensitive metal-free carbon monoxide prodrugs with tunable and predictable release rates. Chemical Communications. 2017;53:9628–9631. [DOI] [PubMed] [Google Scholar]
- 237.Kueh JTB, Stanley NJ, Hewitt RJ, Woods LM, Larsen L, Harrison JC, Rennison D, Brimble MA, Sammut IA, Larsen DS. Norborn-2-en-7-ones as physiologically-triggered carbon monoxide-releasing prodrugs. Chem Sci 2017;8:5454–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kueh JTB, Stanley NJ, Hewitt RJ, Woods LM, Larsen L, Harrison JC, Rennison D, Brimble MA, Sammut IA, Larsen DS. Norborn-2-en-7-ones as physiologically-triggered carbon monoxide-releasing prodrugs. Chem Sci. 2017;8:5454–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Anderson SN, Richards JM, Esquer HJ, Benninghoff AD, Arif AM, Berreau LM. A Structurally-Tunable 3-Hydroxyflavone Motif for Visible Light-Induced Carbon Monoxide-Releasing Molecules (CORMs). ChemistryOpen. 2015;4:590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Soboleva T, Esquer HJ, Benninghoff AD, Berreau LM. Sense and Release: A Thiol-Responsive Flavonol-Based Photonically Driven Carbon Monoxide-Releasing Molecule That Operates via a Multiple-Input AND Logic Gate. J Am Chem Soc. 2017;139:9435–9438. [DOI] [PubMed] [Google Scholar]
- 241.Soboleva T, Esquer HJ, Anderson SN, Berreau LM, Benninghoff AD. Mitochondrial-Localized Versus Cytosolic Intracellular CO-Releasing Organic PhotoCORMs: Evaluation of CO Effects Using Bioenergetics. ACS Chem Biol. 2018;13:2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Soboleva T, Berreau LM. Tracking CO release in cells via the luminescence of donor molecules and/or their by-products. Isr J Chem. 2019;59:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Poloukhtine A, Popik VV. Mechanism of the cyclopropenone decarbonylation reaction. A density functional theory and transient spectroscopy study. J Phys Chem A. 2006;110:1749–1757. [DOI] [PubMed] [Google Scholar]
- 244.Slanina T, Sebej P. Visible-light-activated photoCORMs: rational design of CO-releasing organic molecules absorbing in the tissue-transparent window. Photochem Photobiol Sci. 2018;17:692–710. [DOI] [PubMed] [Google Scholar]
- 245.Mayr FB, Spiel A, Leitner J, Marsik C, Germann P, Ullrich R, Wagner O, Jilma B. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2005;171:354–360. [DOI] [PubMed] [Google Scholar]
- 246.Desmard M, Foresti R, Morin D, Dagouassat M, Berdeaux A, Denamur E, Crook SH, Mann BE, Scapens D, Montravers P, Boczkowski J, Motterlini R. Differential antibacterial activity against Pseudomonas aeruginosa by carbon monoxide-releasing molecules. Antioxid Redox Signal. 2012;16:153–163. [DOI] [PubMed] [Google Scholar]
- 247.Zheng Y, Yu B, Ji K, Pan Z, Chittavong V, Wang B. Esterase-Sensitive Prodrugs with Tunable Release Rates and Direct Generation of Hydrogen Sulfide. Angew Chem Int Ed Engl. 2016;55:4514–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Romao CC, Blattler WA, Seixas JD, Bernardes GJ. Developing drug molecules for therapy with carbon monoxide. Chem Soc Rev. 2012;41:3571–3583. [DOI] [PubMed] [Google Scholar]
- 249.Goldbaum LR, Ramirez RG, Absalon KB. What is the mechanism of carbon monoxide toxicity? Aviat Space Environ Med. 1975;46:1289–1291. [PubMed] [Google Scholar]
- 250.Mazzola S, Forni M, Albertini M, Bacci ML, Zannoni A, Gentilini F, Lavitrano M, Bach FH, Otterbein LE, Clement MG. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J. 2005;19:2045–2047. [DOI] [PubMed] [Google Scholar]
- 251.Mitchell LA, Channell MM, Royer CM, Ryter SW, Choi AM, McDonald JD. Evaluation of inhaled carbon monoxide as an anti-inflammatory therapy in a nonhuman primate model of lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L891–897. [DOI] [PubMed] [Google Scholar]
- 252.Ling K, Men F, Wang WC, Zhou YQ, Zhang HW, Ye DW. Carbon Monoxide and Its Controlled Release: Therapeutic Application, Detection, and Development of Carbon Monoxide Releasing Molecules (CORMs). J Med Chem. 2018;61:2611–2635. [DOI] [PubMed] [Google Scholar]
- 253.Bathoorn E, Slebos DJ, Postma DS, Koeter GH, van Oosterhout AJ, van der Toorn M, Boezen HM, Kerstjens HA. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J. 2007;30:1131–1137. [DOI] [PubMed] [Google Scholar]
- 254.Fredenburgh LE, Perrella MA, Barragan-Bradford D, Hess DR, Peters E, Welty-Wolf KE, Kraft BD, Harris RS, Maurer R, Nakahira K, Oromendia C, Davies JD, Higuera A, Schiffer KT, Englert JA, Dieffenbach PB, Berlin DA, Lagambina S, Bouthot M, Sullivan AI, Nuccio PF, Kone MT, Malik MJ, Porras MAP, Finkelsztein E, Winkler T, Hurwitz S, Serhan CN, Piantadosi CA, Baron RM, Thompson BT, Choi AM. A phase I trial of low-dose inhaled carbon monoxide in sepsis-induced ARDS. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Rosas IO, Goldberg HJ, Collard HR, El-Chemaly S, Flaherty K, Hunninghake GM, Lasky JA, Lederer DJ, Machado R, Martinez FJ, Maurer R, Teller D, Noth I, Peters E, Raghu G, Garcia JGN, Choi AMK. A Phase II Clinical Trial of Low-Dose Inhaled Carbon Monoxide in Idiopathic Pulmonary Fibrosis. Chest. 2018;153:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bojakowski K, Gaciong Z, Grochowiecki T, Szmidt J. Carbon monoxide may reduce ischemia reperfusion injury: a case report of complicated kidney transplantation from a carbon monoxide poisoned donor. Transplant Proc. 2007;39:2928–2929. [DOI] [PubMed] [Google Scholar]
- 257.Wood DM, Chan WL, Dargan PI. Using drug-intoxicated deaths as potential organ donors: impression of attendees at the American college of medical toxicology 2014 annual scientific meeting. J Med Toxicol. 2014;10:360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Busche MN, Knobloch K, Herold C, Kramer R, Vogt PM, Rennekampff HO. Solid organ procurement from donors with carbon monoxide poisoning and/or burn--a systematic review. Burns. 2011;37:814–822. [DOI] [PubMed] [Google Scholar]
- 259.Siskind E, Jonsson J. Successful Kidney Transplant From Donor With Carbon Monoxide Poisoning. Transplantation. 2018;102:e365. [DOI] [PubMed] [Google Scholar]
- 260.Hebert MJ, Boucher A, Beaucage G, Girard R, Dandavino R. Transplantation of kidneys from a donor with carbon monoxide poisoning. N Engl J Med. 1992;326:1571. [DOI] [PubMed] [Google Scholar]