Abstract
Herpes simplex viruses (HSVs) cause a latent infection in humans which is mainly associated with characteristic cold sores or fever blisters and genital blisters. Large segments of the world population are suffering from the HSV infection and early diagnosis as well as treatments are needed to avoid further complications. HSV surveillance is very sparse, especially from developing countries including India. The aim of the present study is to develop and evaluate molecular assays for rapid detection and typing of HSV. In the present study, viral DNA was extracted from cerebro-spinal fluid from HSV suspected encephalitis patients. The conventional multiplex PCR for HSV-1 and HSV-2 was optimized and their comparative analysis was done with Real-Time qPCR for detection and typing of HSV. Out of 137 clinical samples, eleven samples (8.03%) were diagnosed as HSV positive by Real-Time qPCR while ten (7.3%) by conventional multiplex PCR which were further typed as subtyping HSV-1 (nine) and HSV-2 (two). Real-Time qPCR is highly sensitive and able to detect 9.4 × 101 to 3.1 × 106 copies/ml of HSV DNA. Conventional PCR was found to be having 99.21% specificity with 100% sensitivity. The positive predictive value was 90.91% whereas negative predictive value was 100%. Logistic regression indicates blisters with pain and skin rash as the most significant symptoms associated with HSV infection. The present study could be applied for rapid, specific, sensitive and cost-effective diagnosis of HSV-1 and HSV-2 thereby helpful in better patient management through early detection and treatment of HSV.
Keywords: Herpes viruses, HSV-1, HSV-2, Encephalitis, Multiplex PCR, Real-time qPCR
Introduction
Herpes Simplex Viruses (HSV) are highly infectious viruses, belong to Herpesviridae family which is further divided into three subfamilies viz. Alpha (α), Beta (β) and Gamma (γ) herpes viruses. There are nine herpes viruses that are documented to infect naturally and exclusively humans hence frequently known as Human Herpes Virus (HHV) [20]. The genome of these viruses are linear, double-stranded DNA (dsDNA) located at the central region of the core but its precise packaging inside the core is not well known. The genomic lengths are 152 kbp and 115 kbp for HSV-1 and HSV-2 respectively. Their genome is confined by 162 capsomers that form a capsid exhibiting icosapentahedral symmetry [9]. The most striking feature of HSV-1 and HSV-2 is its capability to show latency after its primary infection [19]. Both HSV-1 and HSV-2 can cause life-long infections but exhibit no seasonal variation [33]. Both HSV-1 and HSV-2 ubiquitously exist and affect both urban as well as remote populations. HSV-1 generally causes cold sores/fever blisters on the facial region while rarely responsible for severe and fatal encephalitis in immunocompromised individuals and neonates [3, 16]. HSV-2 usually transmitted sexually and there are nearly 500 million cases of infection with the addition of 23 million new infections every year [16]. About 1500–2000 new cases of neonatal herpes are diagnosed every year and due to improper treatment reached up to 60% fatality rate with neurological disorders in survivors [29]. National Center for Health Statistics (NCHS) and Centers for Disease Control and Prevention (CDC) reported, 47.8% HSV-1 seroprevalence and 11.9% HSV-2 seroprevalence among those 14–49 aged during 2015–2016 [11, 18, 30] Seroprevalence of HSV-1 in Asian countries is nearly 50% in the children and 75% in the adult population. HSV-1 contributes influentially to Sexually Transmitted Diseases (STDs) with 6% genital ulcer disease and one out of five genital herpes infections [12]. The complications associated with HSV infection include encephalitis, meningitis, ocular, herpetic whitlow etc. and cause severe infection in neonates, pregnant mothers and immuno-compromised patients. These all complications demand a rapid and accurate diagnostic system for HSV so that the patient could be timely provided with the proper treatment. PCR is reported as a new gold standard diagnostic assay for the detection of HSV infections in the Central Nervous System (CNS) [28]. As HSV often shows a latent infection, lesions may or may not be actively present all the time. In the case of late lesions, PCR results have been found to be more superior in sensitivity as that of culture [22, 32] Markoulatos et al. [17] have developed a PCR to identify herpes DNA in CSF of patients that exhibit viral encephalitis symptoms.
Materials and methods
HSV reference strains and their processing
Reference strains of HSV-1 and HSV-2 were kindly provided by Department of Microbiology, All India Institute of Medical Sciences (AIIMS), New Delhi and were used for the optimization of conventional PCR and Real Time PCR. Viral DNA was extracted from an aliquot of Reference strains of HSV-1 and HSV-2 with the help of a Commercial kit (GeneJET™ Viral DNA/RNA Purification Kit, Thermo Fisher Scientific, USA) as per manufacturer’s instructions. DNA was eluted in 50 μl in elution buffer and divided into aliquots. After proper labeling, aliquots were stored at − 80 °C until used. All the extraction steps were done under the sterile conditions in Bio-safety Level II (BSL-II).
Monoplex and multiplex PCR for HSV-1 and HSV-2
Two individual monoplex conventional PCRs were standardized for HSV-1 and HSV-2 by using their respective strain-specific primers. In the current study two forward primers (one for HSV-1 and one for HSV-2) and one common reverse primer for both were taken, details of primers and their respective gene targets are given in Table 1. Different concentrations of primers, dNTPs, MgCl2 and Taq DNA polymerase were used for standardization of the assays. Different conditions of the PCR program including initial denaturation, annealing, extension temperatures and the number of cycles were optimized. The results were visualized by agarose gel (2%) electrophoresis. The optimized monoplex PCRs were combined in multiplex format for the detection of HSV-1 and HSV-2.
Table 1.
Details of the primers used in conventional PCR for detection and typing of HSV
| Primer | Sequence (5′ → 3′) | Gene target | Genome location | GenBank accession No. | Primer length (bp) | References |
|---|---|---|---|---|---|---|
| HSVA | ATGGTGAACATCGACATGTACGG | DNA polymerase catalytic subunit (UL30) |
1558–1580 1795–1817 |
23 | [13] | |
| HSV-1 | CCTCGCGTTCGTCCTCGTCCTCC | DNA polymerase catalytic subunit (UL30) | 2004–2026 | 23 | [13] | |
| HSV-2 | CCTCCTTGTCGAGGCCCCGAAAC | DNA polymerase catalytic subunit (UL30) | 2164–2186 | M16321 | 23 | [13] |
Evaluation of multiplex conventional PCR on clinical samples
Clinical samples
Clinical samples (2 ml CSF) were collected by authorized laboratory personnel from suspected patients at In-Patient Department (IPD) of Pt. B.D. Sharma, PGIMS, Rohtak, Haryana, India from May 2017 to May 2019. The patients were enrolled on the basis of clinical symptoms like fever, blisters, seizure, altered sensorium and hallucination. All Herpes patients (age group 2–80 years of either sex) with clinical history of fever, blisters, altered behavior and hallucinations for more than 6 weeks which are suggestive of herpes as per WHO guidelines. Pregnant women having symptoms of HSV were included in the study. Any specimen collected from a subject already positive HIV patients, immunocompromised patient at the time of specimen collection were excluded from the study. All procedures performed in studies involving human samples were in accordance with the ethical standards of the Institutional Human Ethical Committee (IHEC) of Maharshi Dayanand University, Rohtak, Haryana, India. The samples were processed inside the Biosafety Level II (BSL-II) cabinet on the same day of collection. Aliquots were made and stored at − 80 °C.
Viral DNA extraction
Viral DNA was extracted from the clinical sample by GeneJET™ viral DNA purification kit (Thermo Fisher Scientific, USA), by following the manufacturer’s instructions. The extracted viral DNA was labeled and was stored at − 20 °C until used. It was further amplified in the multiplex PCR with their specific primers to detect the presence of HSV-1 or HSV-2.
Conventional PCR reaction
The master mix of PCR reaction was comprised of 0.2 µM dNTPs, 1.5 mM of MgCl2, 0.2 U/µl of Taq DNA polymerase, 0.2 µM of each primer. The details of the primers used have been described in Table 1. Thermo-cycler conditions were; 3 min of initial denaturation at 95 °C, 40 cycles consisting of denaturation at 95 °C for 30 s (sec.), annealing at 59 °C for 50 s. followed by extension at 72 °C for 1 min. The final extension step of 72 °C for 7 min was performed to get the detectable amplified PCR product. The amplicons were analysed using 2% agarose gel electrophoresis and visualized in a gel documentation system (Gel doc™ XR, BioRad, USA).
Real-Time PCR Reaction
Quantitative amplification of the HSV DNA was done by the help of Geno Sen’s HSV-1 and HSV-2 Real-Time PCR kit for Rotor-Gene 6000 (Corbett Research, Australia) software version Rotor-Gene 1.7.87. The master mix consists of all the necessary reagents including HSV-1 and HSV-2 specific primers and enzymes which help in detecting specific amplicon in fluorescence channel cycling FAM and reference gene on cycling JOE. The kit was provided with two strains having five standard concentrations (S1–S5) having 105 copies/µl - 101 copies/µl respectively which helps in viral load determination. The inhibition control gene was used to determine and control possible PCR inhibition. The Real-Time qPCR program includes 95 °C hold for 10 min followed by 45 cycles of denaturation at 95 °C for 15 s, annealing step at 55 °C for 20 s and extension at 72 °C for 15 s.
Results
Clinical samples
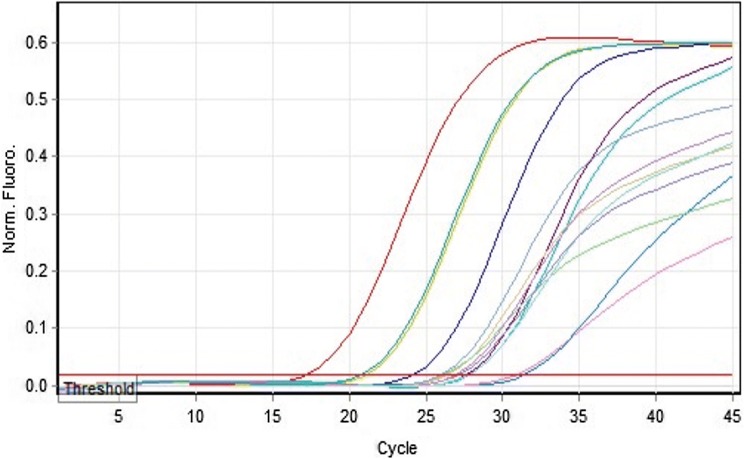
Total 137 clinical samples were collected from the suspected Herpes Simplex Encephalitis (HSE) patients after their written consent. All the collected samples were screened by multiplex conventional PCR, In HSV-1 positive samples, the PCR resulted in amplification of a PCR product which appeared as a band of 469 bp in agarose gel while a band at 391 bp (Fig. 1) was obtained for HSV-2 positive samples. Positive and negative controls were also included in every assay. Out of 137 patient samples, the ten (7.3%) were positive by conventional multiplex PCR while one more sample i.e. total Eleven (8.03%) were positive for HSV by Real-Time qPCR. HSV positive sample were further sub-typed into nine (6.67%) HSV-1 positive and two (1.46%) HSV-2. In the present study, out of total 137 samples, HSV positive samples computing 81.82% (HSV-1) and 18.18% (HSV-2). It was reported in our study that the Real-Time qPCR detect HSV DNA from 9.4 × 101 to 3.1 × 106 copies/ml. All the required clinical details of positive samples were given in Table 2. Quantification of HSV DNA was carried out by drawing a standard curve that includes the standard and clinical sample DNA. The cycle threshold (Ct value) was found in the valid range from 20.89 to 33.77 (Fig. 2). The sensitivity of Geno Sen’s HSV Real-Time PCR RG commercial kit was tested by serial dilution from 107 to 10−1copies/ml and the detection limit was perceived as 90 copies/ml.
Fig. 1.
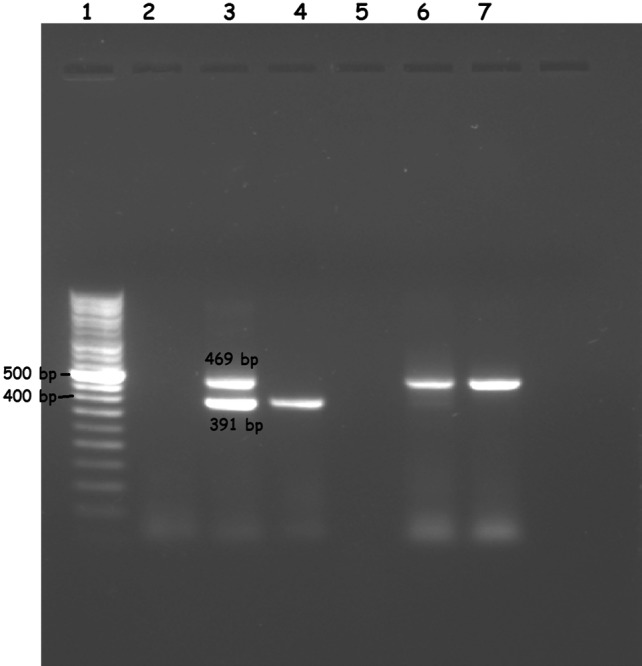
Multiplex subtyping of conventional PCR on clinical samples. Examination of PCR product in 2% (w/v) agarose gel electrophoresis. Lane 1 1 kb ladder, Lane 2 negative control, Lane 3 positive control, Lane 4–7 CLINICAL samples; Lane 4 show HSV-2 positive while Lane 6,7 show HSV-1 positive samples
Table 2.
Symptom profile of patients recruited in sample collection
| Participant id | Age (in years) | Gender | Residential status | Conventional PCR | Real-time qPCR | Subtype | Copy number (copies/ml) |
|---|---|---|---|---|---|---|---|
| HPS 5 | 65 | Female | Rural | Positive | Positive | HSV-2 | 2.2 × 103 |
| HPS 27 | 20 | Female | Rural | Negative | Positive | HSV-2 | 9.4 × 101 |
| HPS 46 | 10 | Male | Rural | Positive | Positive | HSV-1 | 2.5 × 104 |
| HPS 47 | 19 | Male | Rural | Positive | Positive | HSV-1 | 6.8 × 104 |
| HPS 48 | 38 | Female | Urban | Positive | Positive | HSV-1 | 7.2 × 104 |
| HPS 49 | 70 | Male | Rural | Positive | Positive | HSV-1 | 2.6 × 104 |
| HPS 50 | 7 | Male | Rural | Positive | Positive | HSV-1 | 8.7 × 104 |
| HPS 51 | 5 | Male | Urban | Positive | Positive | HSV-1 | 4.1 × 104 |
| HPS 52 | 7 | Female | Urban | Positive | Positive | HSV-1 | 4.5 × 104 |
| HPS 89 | 2 | Male | Rural | Positive | Positive | HSV-1 | 3.1 × 106 |
| HPS 137 | 65 | Female | Rural | Positive | Positive | HSV-1 | 6.3 × 104 |
Fig. 2.
Amplification curve for RT-qPCR detecting HSV. Normalized fluorescence is the ∆Rn showing variation in fluorescence signal of reporter dye (FAM) normalized to the fluorescence signal of internal dye (JOE). The curve was plotted between ∆Rn and cycle of amplification. Ct is the cycle at which the fluorescence meets the threshold in the amplification curve
Sensitivity and specificity
The drawn HSV standard curve was linear having 3 × 103 - 3 × 107 copies/ml and the slope value comes out to be − 3.388 as shown in Fig. 3. The linear regres-sion coefficient was 0.998 and the efficiency of Real-Time qPCR was 97.2%. The specificity and sensitivity of the conventional PCR were found to be 99.21% and 100% respectively. The Positive Predictive Value (PPV) was 90.91% whereas Negative Predictive Value (NPV) was 100%. Further, the disease prevalence in the present study comes out to be 7.30%.
Fig. 3.
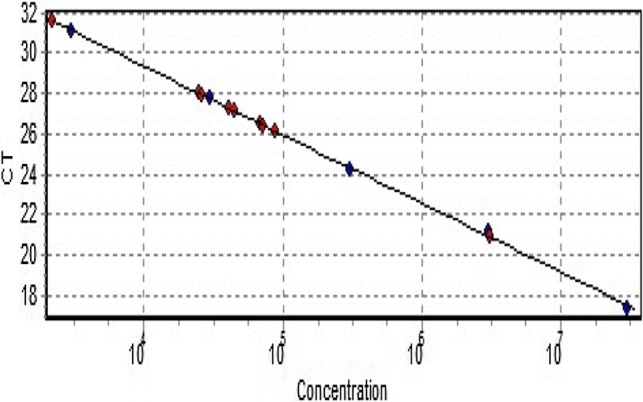
The graph showing standard curve of Ct-value vs concentration. DNA copy number is denoted as concentration. Red dots represent the samples and blue dots denote the standards all forming a straight line
Demographics
In the present study. it was observed that symptoms like fever, altered sensorium, fatigue and weakness account for a very high percentage in the total number of cases as well as the HSV-PCR positive cases but none of these symptoms have significant P value (P < 0.05). Binary logistic regression was done through SPSS software which calculates the odds ratios, 95% Confidence Interval (CI) and P values of all the symptoms (Table 3). The blisters, pain in blisters and skin rashes are significantly found in the HSV positive cases. Although the sample data is less in the current study it was observed that more cases of HSV-1 were detected than HSV-2 infection. In this study, the locality (i.e. Rural/Urban), age and gender of the patient does not show any association with the number and severity of the infection.
Table 3.
Logistics regression analysis of different clinical symptoms with positive HSV samples as outcome variable
| Symptoms | Total samples (n = 137) | HSV positive (n = 11) | OR | 95% CI (lower–upper) | P value |
|---|---|---|---|---|---|
| Fever | 96 (70.0%) | 8 (72.7%) | 1.152 | 0.290–4.579 | 0.841 |
| Headache | 58 (42.3%) | 8 (72.7%) | 3.556 | 0.901–14.035 | 0.070 |
| Blisters | 20 (14.6%) | 5 (45.4%) | 6.167 | 1.675–22.708 | 0.006* |
| Blister pain | 15 (10.9%) | 4 (36.4%) | 5.974 | 1.510–23.638 | 0.011* |
| Seizures | 53 (38.7%) | 3 (27.3%) | 0.570 | 0.144–2.252 | 0.423 |
| Skin Rash | 21 (15.3%) | 5 (45.4%) | 3.929 | 1.033–14.939 | 0.045* |
| Irritation | 31 (22.6%) | 5 (45.4%) | 2.000 | 0.546–7.326 | 0.295 |
| Fatigue and weakness | 90 (65.7%) | 10 (90.9%) | 0.518 | 0.149–1.802 | 0.301 |
| Altered sensorium | 94 (68.6%) | 10 (90.9%) | 2.171 | 0.448–10.505 | 0.335 |
| Altered consciousness | 48 (35.1%) | 4 (36.4%) | 1.190 | 0.331–4.275 | 0.790 |
| Hallucination | 28 (20.4%) | 4 (36.4%) | 1.515 | 0.375–6.127 | 0.560 |
OR odd ratio, CI confidence interval
*Statistically significant P value calculated by using SPSS software; P value significant if P < 0.05
Discussion
HSV is one of the leading sources of sporadic viral encephalitis and high morbidity and mortality due to HSV complications demand a rapid and specific diagnostic method [24, 27]. The documented data on HSV prevalence especially in North India is very sparse which poses a hurdle in the patient management and treatment. The encephalitis and meningitis in patients can be suspected on the basis of abnormality in Magnetic Resonance Imaging (MRI) findings and Computed Tomography (CT) scan reports but the exact cause can be detected by brain biopsy, which is very risky but believed to be gold standard in such cases until the introduction of PCR as diagnostic technique [14, 26]. Brain biopsy is suggested in severe cases facing dreadful symptoms and where treatment is not effective. Apart from brain biopsy sample, CSF obtained from lumbosacral puncture can also be used as a sample but it contains many inhibitory factors which retard the growth of virus in cell culture. Thus for CSF samples, PCR is a promising alternative for detecting the presence of HSV-2 [15, 23, 32].
Monoclonal antibody-based immunoassays (Mab), Western Blot and Enzyme Immuno Assay (EIA) are the serological methods used for diagnosis of HSV but are highly prone to contamination, slow, comparatively high expenditure and required fluorescent microscope [1, 8]. Further, the Kalon test (commercial EIA) is a promising diagnostic assay for HSV with good sensitivity and specificity but it fails to detect new seroconversions and is limited to latent infections only [31]. Loop-mediated Isothermal Amplification (LAMP) is an emerging economical diagnostic method for some common viral infections like influenza [25]. A reliable and sensitive LAMP assay was developed for HSV-1 mainly for the low budget laboratories [21]. Recent studies reflect that PCR is the gold standard diagnostic method for HSV detection in CSF samples [1, 4, 6, 7].
Rapid diagnosis not only helps in patients’ therapeutic management but also helps in identifying the patients that could possibly transmit the infection to others. HSV infection is also common in neonates due to intrapartum contact with infected maternal genital secretions either new or recurrence of latent infection [2, 5, 10]. In the present study, symptoms like mild to high-grade fever and headache are observed in more than 72% of the HSV positive cases but do not show significant P value. Although, fatigue along with weakness and altered sensorium was noticed in maximum HSV positive cases alike fever but was very common in HSV negative encephalitis patients hence do not act as a key indicator of HSV infection. Further, hallucination and seizure that were reported as significant symptoms of encephalitis but in our study, these symptoms did not show significant correlation (P > 0.05) with HSV infection. The painful blisters and skin rashes which were observed merely 10–15% in total cases but nearly 45% of HSV positive patients were complaining about some kind of blisters or skin rashes that provides a very strong indication (P < 0.05) of HSV in encephalitis patients. However, some blisters were fluid-filled in several patients but any kind of bleeding is absent.
The present study concludes conventional multiplex PCR as a rapid, sensitive, specific and cost-effective method of detection of HSV infection from CSF of encephalitis patients. The presence or history of blisters, Blister pain, and skin rashes hold a significant correlation with the presence of HSV infection in encephalitis patients. Early and accurate diagnosis facilitates health workers to supply an effective treatment to patients and lowers the morbidity plus mortality risk due to HSV encephalitis.
Acknowledgements
The authors are thankful to Department of Microbiology, All India Institute of Medical Sciences (AIIMS), New Delhi for kindly endow with Standard strainsof HSV and Pt. B.D. Sharma, PGIMS, Rohtak for providing clinical samples and to Maharshi Dayanand University, Rohtak, for providing University Research Scholarship to Divya Dhull.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human samples were in accordance with the ethical standards of the Institutional Human Ethical Committee (IHEC) of Maharshi Dayanand University, Rohtak, Haryana, India (Letter No. IHEC/112/04.07.2017).
Informed consent
Patient information sheet and detailed informed consent were taken from each patient before collecting sample.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abraham A, Babu M, Kavitha S, Jesudason M, Sridharan G (2009) A molecular method for typing Herpes simplex virus isolates as an alternative to immunofluorescence methods. Indian J Med Microbiol. http://www.ijmm.org/text.asp?2009/27/1/22/45163. [PubMed]
- 2.Anzivino E, Fioriti D, Mischitelli M, Bellizzi A, Barucca V, Chiarini F, et al. Herpes simplex virus infection in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention. Virol J. 2009 doi: 10.1186/1743-422X-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arduino PG, Porter SR. Oral and perioral herpes simplex virus type 1 (HSV-1) infection: review of its management. Oral Dis. 2006 doi: 10.1111/j.1601-0825.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- 4.Aryee EAN, Bailey RL, Natividad-Sancho A, Kaye S, Holland MJ. Detection, quantification and genotyping of herpes simplex virus in cervicovaginal secretions by real-time PCR: a cross sectional survey. Virol J. 2005 doi: 10.1186/1743-422X-2-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker DA. Consequences of herpes simplex virus in pregnancy and their prevention. Curr Opin Infect Dis. 2007 doi: 10.1097/QCO.0b013e328013cb19. [DOI] [PubMed] [Google Scholar]
- 6.Behzad-Behbahani A, Abbas BB, Abdolvahab A, Gholamali YP, Roshanak B, Mahmood R (2003) Clinical signs as a guide for performing HSV-PCR in correct diagnosis of herpes simplex virus encephalitis. Neurol India. http://www.neurologyindia.com/text.asp?2003/51/3/341/1164. [PubMed]
- 7.Bhullar SS, Chandak NH, Purohit HJ, Taori GM, Daginawala HF, Kashyap RS. Determination of viral load by quantitative real-time PCR in herpes simplex encephalitis patients. Intervirology. 2014;57:1–7. doi: 10.1159/000351521. [DOI] [PubMed] [Google Scholar]
- 8.Burrows J, Nitsche A, Bayly B, Walker E, Higgins G, Kok T. Detection and subtyping of Herpes simplex virus in clinical samples byLightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiol. 2002 doi: 10.1186/1471-2180-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng N, Trus BL, Belnap DM, Newcomb WW, Brown JC, Steven AC. Handedness of the herpes simplex virus capsid and procapsid. J Virol. 2002 doi: 10.1128/jvi.76.15.7855-7859.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enright AM, Prober CG. Neonatal herpes infection: diagnosis, treatment and prevention. Semin Neonatol. 2002 doi: 10.1053/siny.2002.0115. [DOI] [PubMed] [Google Scholar]
- 11.Herpes simplex virus In: World Health Organization. 2017. Available from: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus/. Accessed 5 Sept 2019
- 12.Khadr L, Harfouche M, Omori R, Schwarzer G, Chemaitelly H, Abu-Raddad LJ. The epidemiology of herpes simplex virus type 1 in Asia: systematic review, meta-analyses, and meta-regressions. Clin Infect Dis. 2019 doi: 10.1093/cid/ciy562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura H, Shibata M, Kuzushima K, Nishikawa K, Nishiyama Y, Morishima T. Detection and direct typing of herpes simplex virus by polymerase chain reaction. Med Microbiol Immunol. 1990 doi: 10.1007/BF00195248. [DOI] [PubMed] [Google Scholar]
- 14.Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016 doi: 10.3988/jcn.2016.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014 doi: 10.1186/1743-422X-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008 doi: 10.2471/blt.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markoulatos P, Georgopoulou A, Siafakas N, Plakokefalos E, Tzanakaki G, Kourea-Kremastinou J. Laboratory diagnosis of common herpesvirus infections of the central nervous system by a multiplex PCR assay. J Clin Microbiol. 2001 doi: 10.1128/JCM.39.12.4426-4432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuillan G, Kruszon-Moran D, Flagg EW, Paulose-Ram R (2018) Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49. United States, 2015–2016. NCHS data brief [PubMed]
- 19.Novak N, Peng WM. Dancing with the enemy: the interplay of herpes simplex virus with dendritic cells. Clin Exp Immunol. 2005 doi: 10.1111/j.1365-2249.2005.02927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellett PE, Roizman B. The Herpesviridae: a brief introduction. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields virology. Philadelphia: Lippincott, Williams & Wilkins; 2006. pp. 2479–2499. [Google Scholar]
- 21.Pourhossein B, Soleimanjahi H, Behzadian F, Khansarinejad B. Loop-Mediated Isothermal Amplification (LAMP) for the Rapid Diagnosis of Herpes Simplex Virus Type 1 (HSV-1) Iranian Journal of Virology. 2011;5(1):1–5. doi: 10.21859/isv.5.1.1. [DOI] [Google Scholar]
- 22.Ratnam S, Severini A, Zahariadis C, Petric M, Romanowski B. The diagnosis of genital herpes—beyond culture: an evidence-based guide for the utilization of polymerase chain reaction and herpes simplex virus type-specific serology. Can J Infect Dis Med Microbiol. 2007 doi: 10.1155/2007/505364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roa PL, Alonso R, De Egea V, Usubillaga R, Muñoz P, Bouza E. PCR for detection of herpes simplex virus in cerebrospinal fluid: alternative acceptance criteria for diagnostic workup. J Clin Microbiol. 2013 doi: 10.1128/JCM.00950-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saraya AW, Wacharapluesadee S, Petcharat S, Sittidetboripat N, Ghai S, Wilde H, et al. Normocellular CSF in herpes simplex encephalitis. BMC Res Notes. 2016 doi: 10.1186/s13104-016-1922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma V, Chaudhry D, Kaushik S. Evaluation of clinical applicability of reverse transcription-loop-mediated isothermal amplification assay for detection and subtyping of Influenza A viruses. J Virol Methods. 2018 doi: 10.1016/j.jviromet.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steiner I, Budka H, Chaudhuri A, Koskiniemi M, Sainio K, Salonen O, et al. Viral meningoencephalitis: a review of diagnostic methods and guidelines for management. Eur J Neurol. 2010 doi: 10.1111/j.1468-1331.2010.02970.x. [DOI] [PubMed] [Google Scholar]
- 27.Steiner I, Schmutzhard E, Sellner J, Chaudhuri A, Kennedy PGE. EFNS-ENS guidelines for the use of PCR technology for the diagnosis of infections of the nervous system. Eur J Neurol. 2012 doi: 10.1111/j.1468-1331.2012.03808.x. [DOI] [PubMed] [Google Scholar]
- 28.Strick LB, Wald A. Diagnostics for herpes simplex virus: is PCR the new gold standard? Mol Diagn Ther. 2006 doi: 10.1007/BF03256439. [DOI] [PubMed] [Google Scholar]
- 29.Sukhbir SK. Neonatal herpes simplex infection. ISRN Infect Dis. 2013 doi: 10.5402/2013/473053. [DOI] [Google Scholar]
- 30.Suzich JB, Cliffe AR. Strength in diversity: understanding the pathways to herpes simplex virus reactivation. Virology. 2018 doi: 10.1016/j.virol.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Dyck E, Buvé A, Weiss HA, Glynn JR, Brown DWG, De Deken B, et al. Performance of commercially available enzyme immunoassays for detection of antibodies against herpes simplex virus type 2 in African populations. J Clin Microbiol. 2004 doi: 10.1128/JCM.42.7.2961-2965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wald A, Huang M, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV Isolation in cell culture. J Infect Dis. 2003 doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 33.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001 doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]