Abstract
Introduction
Micronutrient supplementation is recommended in Ebola Virus Disease (EVD) care; however, there is limited data on its therapeutic effects.
Methods
This retrospective cohort study included patients with EVD admitted to five Ebola Treatment Units (ETU) in Sierra Leone and Liberia during September 2014 to December 2015. A uniform protocol was used to guide ETU care, however, due to supply limitations, only a subset of patients received multivitamins. Data on demographics, clinical characteristics, and laboratory testing was collected. The outcome of interest was facility-based mortality and the primary predictor was multivitamin supplementation initiated within 48 h of admission. The multivitamin formulations included: thiamine, riboflavin, niacin and vitamins A, C, and D3. Propensity score models (PSM) were used to match patients based on covariates associated with multivitamin administration and mortality. Mortality between cases treated and untreated within 48 h of admission were compared using generalized estimating equations to calculate relative risk with bootstrap methods employed to assess statistical significance.
Results
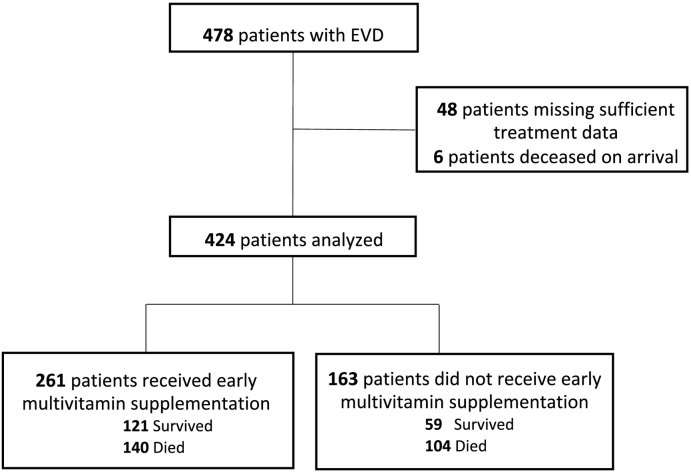
There were 424 patients with EVD who had sufficient treatment data for analysis, of which 261 (61.6%) had daily multivitamins initiated within 48 h of admission. The mean age of the cohort was 30.5 years and 59.4% were female. In the propensity score matched analysis, mortality was 53.5% among patients receiving multivitamins and 66.2% among patients not receiving multivitamins, resulting in a relative risk for mortality of 0.81 (p = 0.03) for patients receiving multivitamins.
Conclusion
Early multivitamin supplementation was associated with lower overall mortality. Further research on the impact of micronutrient supplementation in EVD is warranted.
Keywords: Ebola virus disease, Mortality, Multivitamins, Nutrition, Liberia, Sierra Leone
African relevance
-
•
Outbreaks of Ebola Virus Disease are common in sub-Saharan Africa
-
•
Despite nearly two dozen outbreaks, little data has been collected to date on the effectiveness of various supportive care measures for Ebola Virus Disease
-
•
The results of this study can be used to improve care for patients with Ebola Virus Disease in the current outbreak in DRC as well as future outbreaks
Introduction
The 2014–16 Ebola virus disease (EVD) epidemic in West Africa resulted in >28,000 infections and over 11,000 deaths, with populations in Liberia, Sierra Leone, and Guinea most affected [1]. Among patients treated outside of Africa during the epidemic, mortality was 18.5% [2], approximately half that of the lowest reported mortality from any of the Ebola treatment units (ETUs) in Africa [[3], [4], [5], [6], [7]]. Patients with EVD cared for in high-income countries (HICs) received extensive supportive care measures, many of which were not consistently available in the African setting [8]. Although the impacts of supportive care strategies on EVD mortality in low-resource settings are potentially promising, the roles of specific components of that supportive care remain largely unstudied.
Micronutrients are a common component of supportive care in EVD [2,3,5,[9], [10], [11], [12], [13]], and international guidelines call for supplementation when micronutrient deficiencies are present [14,15]. This includes the use of multivitamins, which have been associated with clinical improvement in other infectious diseases, such as tuberculosis, dengue and HIV [[16], [17], [18], [19]]. Multivitamins also provide cofactors that have been shown to have clinical benefits in sepsis [[20], [21], [22], [23]]. Furthermore, research on vitamin supplementation demonstrates the greatest benefits in populations with a low baseline nutritional status [24], such as in sub-Saharan Africa [25,26], This prior evidence suggest that the use of low-cost multivitamins could yield clinical benefits in patients with EVD; however, no data currently exists on the impact of multivitamin supplementation on patient-centered outcomes.
During the 2014–16 EVD epidemic, International Medical Corps (IMC) established and operated five ETUs in Liberia and Sierra Leone, which cared for 478 patients diagnosed with EVD. Clinical, epidemiologic, and laboratory data were gathered on most patients, resulting in a robust, multi-national database to investigate the roles of specific management strategies on patient outcomes [27]. This study evaluated the impact of early multivitamin supplementation on mortality among patients with EVD admitted to the five IMC ETUs.
Methods
This retrospective multisite cohort study utilized data collected at five ETUs operated by IMC between September 15, 2014 and December 31, 2015 in Liberia and Sierra Leone [27]. The ETUs were setup in areas identified as needing access to care in the affected communities distinct from pre-existing health centers and were comprised of organized triage, treatment and staff areas and housed all supplies needed to care for patients in the outbreak setting. ETUs at Makeni, Lunsar and Margibi were standard, white tents, while ETUs at Kambia and Bong were constructed with aluminum roofs, with either brick (Kambia) or blue-tarp walls (Bong) [28]. The Sierra Leone Ethics and Scientific Review Committee and the University of Liberia and Rhode Island Hospital Institutional Review Boards provided ethical approval for this study.
All patients admitted to the five study ETUs with a final diagnosis of EVD during the months of operation were eligible for inclusion. Patients who were deceased at time of arrival, confirmed to be negative for EVD by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) testing, or missing data on multivitamin treatment were excluded from analysis.
All admitted patients were treated by trained response providers using standardized guidelines, developed by IMC in consultation with local health authorities (Appendix) [14,29]. The guidelines included daily supplementation with multivitamins for all patients. However, due to variations in the availability of multivitamins over the course of the epidemic, actual care varied. Two primary multivitamin formulations were used in the IMC ETUs, based on availability. The first, manufactured by Medopharm Private Limited, Chennai, India, included Vitamin A (2500 IU), B1 (1 mg), B2 (0.5 mg), B3 (7.5 mg), C (15 mg), and D3 (300 IU), while the second, manufactured by CSPC Ouyi Pharmaceutical Co, Shijiazhuang, China, included Vitamin A (800 IU), B1 (0.5 mg), B2 (0.5 mg), B3 (7.5 mg), and D3 (200 IU). In addition to the multivitamins, the IMC guidelines also recommended Vitamin A (1000 retinol units on Day 1 and 2) and Vitamin C (500 mg three times daily) supplementation, though actual care varied based on supply availability.
Admittance to the EVD-confirmed ward of the ETUs required positive RT-PCR laboratory confirmation. Ebola virus RT-PCR cycle threshold (CT) values, which are inversely proportional to viral load, were obtained from the United States Naval Medical Research Center Mobile Laboratory for ETUs in Liberia and Public Health England and the Nigerian/European Mobile Laboratory for ETUs in Sierra Leone using previously published procedures [30]. Malaria testing, when performed, utilized the commercially available BinaxNow™ rapid diagnostic test (RDT), which identifies four Plasmodium species: Falciparum, Malariae, Vivax and Ovale.
As reported previously, trained providers collected data on demographics and baseline clinical signs/symptoms at triage on standardized forms [27]. Clinical characteristics and treatment data were recorded one to six times daily (median 3 times daily) on all admitted patients based on human resource availability using standardized forms, while final disposition and diagnosis were recorded on standardized discharge forms, as described previously [27,30]. All data forms were digitized and filed into a unified relational electronic database [27]. Data quality was measured using Lot Quality Assurance Sampling, which demonstrated 99% consistency with the source records [27,31].
Statistical analyses were undertaken using R version 3.3.3 [32]. Descriptive analyses were performed for all variables using frequencies with percentages, medians with interquartile ranges (IQR), or means with standard deviations (SD) as appropriate.
The outcome of interest was observed mortality during ETU care. Due to the high immediate post-admission mortality risks documented in the study ETUs [30], and the resulting potential survivor bias, the primary predictor variable utilized was early oral multivitamin treatment, defined as initiation within 48 h of admission. Multivitamin treatment was coded as dichotomous in the primary analysis. Time of multivitamin initiation and duration of treatment were summarized. Univariate analyses compared demographic and clinical characteristics between groups treated and not treated with multivitamins and also between patients suffering mortality and those who survived, using Pearson X2 or Fisher's exact tests for categorical variables and by Mann-Whitney or t-tests for continuous variables, as appropriate.
Propensity score development and modeling
To control for confounding, a propensity score model (PSM) was used to match and compare patients treated and not treated with multivitamins during the first 48 h of ETU care [33]. Variables were chosen for inclusion in the PSM based on their univariate relationship to multivitamin exposure or survival in either the study cohort or prior literature [33].
Variables of gender, abnormal bleeding, malaria RDT results and triage CT values were modeled as categorical, while age was modeled utilizing a cubic spline to control for the known quadratic relationship between age and survival in EVD [30]. After fitting the PSM, a common support interval approach to prevent over-extrapolation was used in which all observations, with a probability of receiving treatment less than the minimum in the treated cohort and greater than the maximum in the control cohort, were filtered out [34]. After filtering for the common support interval, two patients were removed. Each multivitamin treated patient was matched to an untreated patient with the nearest propensity score and exact CT value and malaria testing results (due to the high correlation of those factors with the outcome of interest) [5,30,35,36]. Matching was done with replacement for the untreated cohort. Covariate balance was assessed before and after matching based on the standardized bias, to ensure balance between treatment and control cohorts. Following 1:1 matching, with 76 unique controls, the probability of mortality between cases treated and not treated with multivitamins was assessed. Mortality outcomes between cases treated and not treated with multivitamins were compared using propensity-matched generalized estimating equations (GEE) to calculate relative risks (RR). Bootstrap methods with 1000 iterations were used to calculate p-values and characterize statistically significant differences between groups.
Since demographic and clinical data were available for all included patients, only laboratory data was missing. As laboratory data was not missing at random (patients who died were more likely to lack laboratory results), multiple imputation was not utilized. Instead, CT values were categorized as: >22 (low viral load), ≤22 (high viral load), and missing, to prevent the introduction of bias (cut-points were based on those used previously in the literature) [37]. Similarly, results for malaria RDTs were coded as positive, negative or missing.
Three sensitivity analyses were performed using propensity-matched generalized estimating equations as described in the primary analysis. The first compared mortality outcomes between patients treated with vitamin A without multivitamins to those treated with vitamin A and multivitamins. The second compared those treated with vitamin C without multivitamins to those treated with vitamin C and multivitamins. The final sensitivity analysis assessed mortality between those treated and not treated with early multivitamin supplementation and matched on a broad selection of pharmacologic agents which included paracetamol, omeprazole, vitamin A, vitamin C, Coartem, and cefixime, as well as the other covariates used in the primary model. In all sensitivity analyses, covariate balance was assessed before and after matching based on the standardized bias.
Results
In the overall population, 478 patients with EVD presented to the study ETUs during operation, of which 424 had sufficient data for analysis (Fig. 1). The mean age was 30.5 (SD: 18.7) years and 59.4% were female. The median duration of care was 8 (IQR: 5, 13) days. There was larger proportion of patients from Sierra Leone ETUs versus those from those operated in Liberia. Table 1 provides data on the proportion of patients who developed clinical signs and symptoms during ETU care. CT values were available for 281 patients, of which 159 (37.5%) had a high viral load (CT value ≤ 22).
Fig. 1.
Study population.
Table 1.
Characteristics overall cohort.
| n (%) or mean (±SD) | |
|---|---|
| Age | 30.5 (±18.7) |
| Sex | |
| Female | 253 (59.4) |
| Male | 171 (40.3) |
| Country | |
| Liberia | 134 (31.6%) |
| Sierra Leone | 290 (68.4%) |
| CT values | |
| High viral load | 159 (37.5) |
| Low viral load | 122 (28.8) |
| Missing | 143 (33.7) |
| Clinical characteristics | |
| Anorexia | 342 (80.7) |
| Any bleeding | 198 (46.9) |
| Coma | 42 (9.9) |
| Confusion | 56 (13.4) |
| Diarrhea | 363 (85.6) |
| Dysphagia | 248 (58.7) |
| Dyspnea | 204 (48.3) |
| Fever | 325 (76.7) |
| Stomach pain | 326 (76.9) |
| Vomiting | 325 (76.7) |
SD = Standard Deviation, CT = Cycle Threshold.
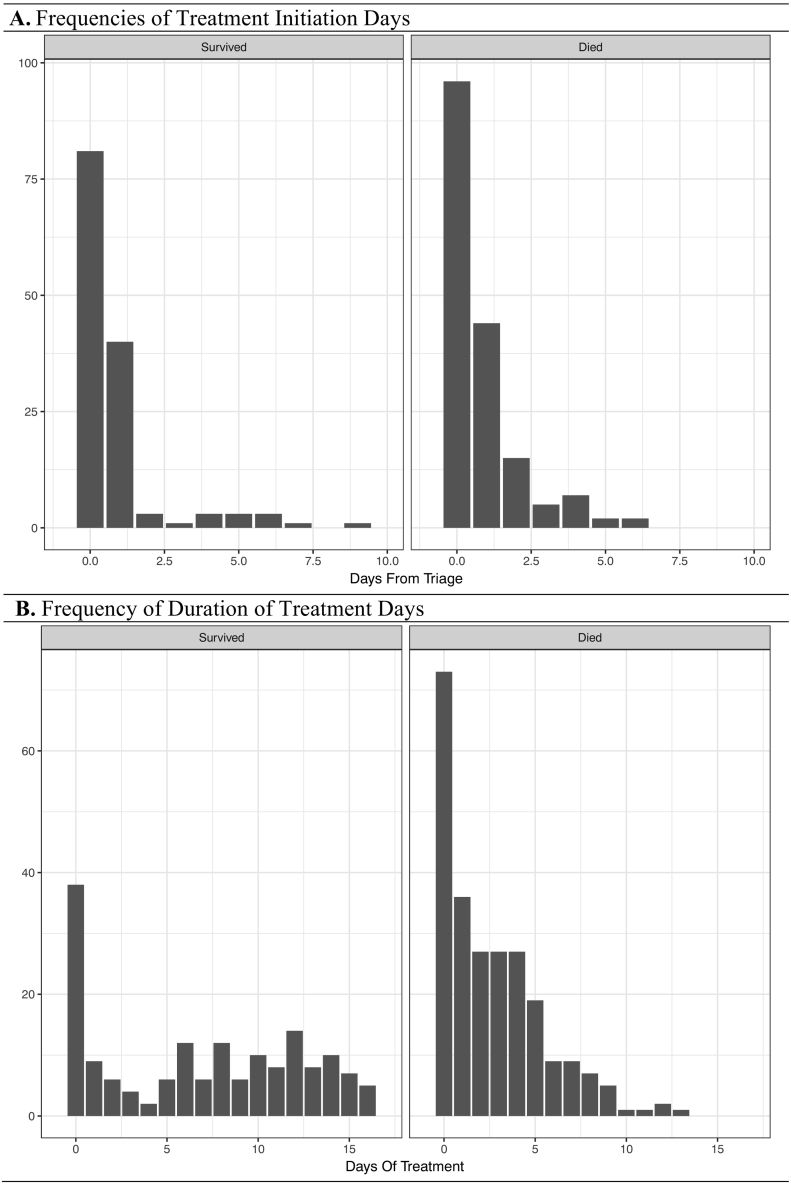
During ETU care 313 (73.8%) patients were treated with multivitamins among which 261 (61.6%) patients had daily multivitamins initiated during the first 48 h of care. Median length of treatment with multivitamins for all cases was 5 (IQR: 3, 10) days. Frequency distributions for the time to initiation and duration of treatment with multivitamins are shown in Fig. 2. Patients receiving multivitamins during the first 48 h were significantly more likely to be female and have low CT values, and were significantly less likely to have symptoms/signs of anorexia, bleeding, confusion and fever during ETU care (Table 2). Co-treatment within 48 h of care with multivitamin and vitamin A or multivitamin and vitamin C occurred in 248 (58.9%) and 230 (54.2%) patients, respectively.
Fig. 2.
Multivitamin treatment initiation and duration times stratified by outcome.
Table 2.
Patient characteristics stratified by treatment status.
| Multivitamin (−) (n = 163) n (%) or mean (±SD) |
Multivitamin (+) (n = 261) n (%) or mean (±SD) |
p value | |
|---|---|---|---|
| Age | 31 (±18) | 30.2 (±19.2) | 0.693 |
| Sex | |||
| Female | 85 (52.1) | 168 (64.4) | 0.014 |
| Male | 78 (47.9) | 93 (35.6) | |
| CT values | |||
| High viral load | 33 (20.2) | 126 (48.3) | <0.001 |
| Low viral load | 49 (30.1) | 73 (28.0) | |
| Missing results | 81 (49.7) | 62 (23.8) | |
| Clinical characteristics | |||
| Anorexia | 119 (73.6) | 163 (62.5) | 0.015 |
| Any bleeding | 60 (37.4) | 61 (23.4) | 0.003 |
| Coma | 1 (1.2) | 3 (1.5) | 0.791 |
| Confusion | 16 (10.4) | 10 (4.2) | 0.022 |
| Diarrhea | 112 (69.3) | 185 (70.9) | 0.735 |
| Dysphagia | 73 (44.8) | 97 (37.5) | 0.143 |
| Dyspnea | 57 (35) | 78 (29.9) | 0.280 |
| Fever | 138 (84.7) | 182 (70.1) | 0.000 |
| Stomach pain | 104 (64.4) | 159 (61.3) | 0.519 |
| Vomiting | 102 (62.6) | 151 (58.2) | 0.374 |
SD = Standard Deviation, CT = Cycle Threshold.
Overall, 244 (57.5%) patients died during ETU care. As reported previously, there were no significant differences in mortality outcomes between the five ETU sites [30]. Viral load on admission was strongly correlated with mortality: among patients who died, 44.7% of cases had a CT value ≤ 22 while only 16.8% of cases had a CT value > 22 (p < 0.001). Patients who died were significantly more likely to have developed bleeding, diarrhea, dysphagia and dyspnea during ETU care (Table 3).
Table 3.
Patient characteristics stratified by mortality status.
| Discharged alive (n = 180) n (%) or mean (±SD) |
Suffered mortality (n = 244) n (%) or mean (±SD) |
p value | |
|---|---|---|---|
| Age | 28.7 (±15.3) | 31.8 (+20.8) | 0.080 |
| Sex | |||
| Female | 108 (60) | 145 (59.4) | 0.906 |
| Male | 72 (40) | 99 (40.6) | |
| CT values | |||
| High viral load | 50 (27.8) | 109 (44.7) | <0.001 |
| Low viral load | 81 (45.0) | 41 (16.8) | |
| Missing results | 49 (27.2) | 94 (38.5) | |
| Clinical characteristics | |||
| Anorexia | 86 (47.8) | 119 (48.8) | 0.840 |
| Any bleeding | 32 (17.8) | 61 (25.4) | 0.057 |
| Coma | 0 (0) | 1 (0.8) | 0.158 |
| Confusion | 5 (3.3) | 13 (5.7) | 0.231 |
| Diarrhea | 93 (51.7) | 152 (62.3) | 0.029 |
| Dysphagia | 43 (24.4) | 90 (36.9) | 0.006 |
| Dyspnea | 39 (21.7) | 80 (32.8) | 0.010 |
| Fever | 133 (73.9) | 183 (75.4) | 0.723 |
| Stomach pain | 91 (50.6) | 134 (55.3) | 0.332 |
| Vomiting | 86 (48.3) | 115 (47.5) | 0.872 |
SD = Standard Deviation, CT = Cycle Threshold.
Observed mortality was 53.6% among patients treated with multivitamins within 48 h of admission and 63.8% among patients not treated within 48 h of admission. For patients who died, median survival time was 4 (IQR: 2, 6) days among cases not receiving multivitamins within 48 h of ETU admission and 5 (IQR: 4, 7) days among those receiving multivitamins.
Propensity matched analysis
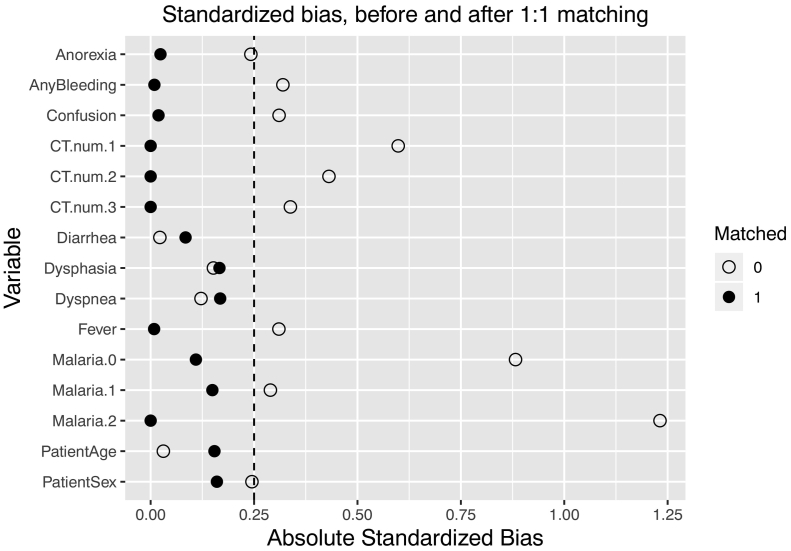
Propensity matching achieved covariate balance among all predictors associated with either multivitamin administration, mortality, or both (Fig. 3), allowing for a fair comparison between groups. In the propensity score matched analysis, mortality was 53.5% among patients receiving multivitamins and 66.2% among patients not receiving multivitamins, with a risk ratio (RR) of 0.81 (p = 0.03) for mortality when multivitamin treatment was initiated within the first 48 h of ETU care.
Fig. 3.
Covariate balance among predictors before and after propensity score matching.
In a propensity score matched analysis comparing patients receiving both vitamin A and early multivitamin supplementation to patients receiving vitamin A without early multivitamins, there was no significant difference in the relative risk of mortality between treatment groups (RR = 0.92, p = 0.70). In a separate vitamin C sensitivity analysis, significantly lower mortality risk was observed in patients treated with both early multivitamins and vitamin C as compared to those receiving vitamin C and no early multivitamins (RR = 0.64, p < 0.001). In a third sensitivity analysis matching on a broad selection of medications to control for confounding by other treatments received, patients treated with early multivitamins as compared to those not treated still had a reduced risk of mortality (RR = 0.74, p = 0.03).
Discussion
The current report is the first to demonstrate the clinical impact of multivitamin supplementation on clinical outcomes in patients with EVD in a resource-limited setting. The results demonstrate that patients with EVD who were treated with early oral multivitamin supplementation had lower facility-based mortality than those who were not treated with early oral multivitamin supplementation, even after adjusting for potential confounders using propensity score matching. Given these findings, in conjunction with the low-cost of multivitamins, this supportive therapy should be considered for use in future EVD outbreaks.
Although nutritional status impacts immune function and micronutrient supplementation is a common component of supportive care in EVD [2,3,5,[9], [10], [11], [12], [13],38,39], there are no other published reports investigating the impacts of multivitamins in the treatment of EVD patients under outbreak conditions. However data from other infectious disease states provides support for the provision of multivitamins in EVD. Multivitamins have been associated with clinical improvement in the care of patients with tuberculosis and HIV [[16], [17], [18]]. A randomized, placebo-controlled trial conducted in Tanzania with multivitamins composed of vitamins B-complex, C, and E, found that HIV-infected pregnant women not on HIV treatment who received multivitamin supplementation had significantly improved immune responses, reduced HIV disease progression, and death [17]. An additional placebo-controlled trial utilizing micronutrient supplements (vitamins A, B-complex, C, E and selenium) found a significant reduction in TB recurrence risk among HIV-negative patients [19]. Vitamin C has shown benefits in upper respiratory infections and severe pneumonias [16,40], and evidence also demonstrates that treatment with vitamins C is associated with improved hemodynamics and survival in severe sepsis [20], which has similar pathophysiology to EVD [21,22]. Vitamins D suppresses T-cell activation and genes involved in cell proliferation and differentiation; down-regulates the production of pro-inflammatory cytokines; and plays multiple roles in antimicrobial defense and immune regulation [41,42]. Given this body of evidence there is biological plausibility for the improved mortality observed in the current data among patients with EVD who received early multivitamin supplementation.
In sensitivity analysis controlling for a broad range of pharmacologic treatments, similar benefits were found with early multivitamin supplementation. In the sensitivity analyses evaluating patients also treated with vitamin C, the addition of multivitamins still resulted in significantly reduced mortality. In assessing the addition of multivitamins to patients already treated with vitamin A, however, there was no significant differences in mortality identified. This may indicate that vitamin A is a key micronutrient responsible for the improved outcomes observed, as the magnitude of the early multivitamin effect on mortality was mitigated, yet not fully negated, in the vitamin A sensitivity models. The potential benefits of vitamin A therapies in EVD are supported by prior literature on vitamin A showing that it is essential for innate and adaptive immunity – being integral in the maintenance of epithelial integrity and differentiation and function in cellular immune response [[43], [44], [45]]. Given the limited power to detect significant differences, these sensitivity analyses should be considered exploratory. However, they do highlight the need for further research pertaining to micronutrient supplementation in EVD care and suggest vitamin A should be an important focus of future studies.
The World Health Organization guidelines for viral hemorrhagic fever state that patients do not need micronutrient supplementation if adequate fortified nutrition is administered [14]. However, vitamin A is recommended for children under five years of age who have not received vitamin A supplementation in the past six months [14]. The Médecins Sans Frontières guidelines recommend supplementation only if micronutrient deficiency is present [15]. Given the high baseline prevalence of malnutrition and specifically micronutrient deficiencies that exist in sub-Saharan African populations [25,26], and particularly the west African populations that were most affected during the 2014–2016 outbreak [16,46,47], empiric treatment with multivitamins during outbreaks occurring in the African context is currently warranted, unless future research demonstrates harms or futility with such approaches.
This study must be interpreted with limitations. The multivitamin supplementation data were not drawn from patients who were randomized to either a treatment or a control allocation. Although a propensity score matched analysis was employed to control for confounding, it cannot control for unmeasured or missing variables. However, it should be noted that the decision to withhold multivitamins was based mostly on supply limitations, as they were a standard part of the clinical protocol at all five sites, reducing the likelihood of confounding by indication. Although rigorous data acquisition methods were used, which provided a high-quality database for analysis, there was some missing laboratory information for CT values and malaria RDT results. To minimize any bias derived from the missing laboratory data, the models were run such that cases were matched exactly on the status of those variables. Although less important from a programmatic and outbreak response perspective, the present data does not allow us to determine which specific components of the multivitamin supplementation accounted for the benefits observed. Future laboratory and clinical research would have utility in further investigating the biochemical mechanisms of micronutrient supplementation in reducing mortality for patients with EVD. Finally, all studied patients were treated at IMC facilities, which may threaten the generalizability of the findings. However, as the IMC guidelines utilized to guide clinical care at all sites were based on other international guidelines in widespread use and the resource constraints experienced by IMC were common at most ETUs during the 2014–2016 epidemic, these data are likely generalizable to future EVD outbreaks in low-resource settings of sub-Saharan Africa.
Conclusion
The magnitude of the West Africa epidemic and the ongoing 2018–2019 outbreak in the Democratic Republic of Congo and Uganda [48], point-up the threats of EVD globally and the imperative need for advancement of understanding of pragmatic and impactful treatment strategies. The current data, which is derived from a large, multisite, epidemic population, is the first available on multivitamin supplementation in an outbreak setting from a high-risk population in Africa and demonstrates that early treatment resulted in lower overall mortality in adjusted analyses. Given these findings, in conjunction with the low-cost for supplementation, and the demonstrated safety of multivitamins in prior infectious disease research [49,50], supportive therapy with multivitamins should be further studied and considered for use in future EVD outbreaks occurring in populations with high burdens of nutritional deficiencies.
Dissemination of results
Results of this study were shared with the International Medical Corps team currently responding to the EVD outbreak in the Democratic Republic of Congo.
Authors' contribution
Authors contributed as follow to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting of the work, or revising it critically for important intellectual content: DY and ARA contributed 25% each; TL and ACL 10%each; SMP 15%; and JLP, DKC, SBK, MM, FS, MAS and LL 2% each. All authors approved the version to be published and agreed to be accountable for all aspects of the work.
Conflict of interest
The authors declare no conflict of interest. All authors had full access to all study data and had final responsibility for the decision to submit for publication. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the views of International Medical Corps or any governmental bodies or academic organizations. This work was supported by the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases [grant number R03AI132801]. The funding source had no involvement in the design or conduct of the study or the decision to submit for publication.
Acknowledgments
We would like to thank International Medical Corps and the Governments of Liberia, Sierra Leone, and Guinea for contributing data for this research. We also thank all the generous institutional, corporate, foundation, and individual donors who placed their confidence and trust in International Medical Corps and made its work during the Ebola epidemic possible. We thank the United States Naval Medical Research Center, Public Health England, the European Union Mobile Laboratory, and the Nigerian Laboratory for providing laboratory support to International Medical Corps Ebola Treatment Units in Liberia and Sierra Leone and making their data available for this research. Finally, we thank all the International Medical Corps staff in Liberia and Sierra Leone, including the data collection officers at each Ebola treatment unit, without whom this data would not be available for analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.afjem.2019.11.001. Additional data may be accessed through the Ebola Data Platform at https://www.iddo.org/research-themes/ebola.
Appendix A. Supplementary data
Supplementary material. IMC guidelines on Ebola virus disease management.
References
- 1.WHO Ebola situation reports. 2017. http://apps.who.int/ebola/ebola-situation-reports Available from:
- 2.Uyeki TM, Mehta AK, Davey RT, Jr, Liddell AM, Wolf T, Vetter P. Clinical management of Ebola virus disease in the united states and Europe. N Engl J Med. 2016;374(7):636–646. doi: 10.1056/NEJMoa1504874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansumana R, Jacobsen KH, Sahr F, Idris M, Bangura H, Boie-Jalloh M. Ebola in Freetown area, Sierra Leone–a case study of 581 patients. N Engl J Med. 2015;372(6):587–588. doi: 10.1056/NEJMc1413685. [DOI] [PubMed] [Google Scholar]
- 4.Bah EI, Lamah MC, Fletcher T, Jacob ST, Brett-Major DM, Sall AA. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372(1):40–47. doi: 10.1056/NEJMoa1411249. [DOI] [PubMed] [Google Scholar]
- 5.Hunt L, Gupta-Wright A, Simms V, Tamba F, Knott V, Tamba K. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015;15(11):1292–1299. doi: 10.1016/S1473-3099(15)00144-9. [DOI] [PubMed] [Google Scholar]
- 6.Qin E, Bi J, Zhao M, Wang Y, Guo T, Yan T. Clinical features of patients with Ebola virus disease in Sierra Leone. Clin Infect Dis. 2015;61(4):491–495. doi: 10.1093/cid/civ319. [DOI] [PubMed] [Google Scholar]
- 7.Schieffelin JS, Shaffer JG, Goba A, Gbakie M, Gire SK, Colubri A. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371(22):2092–2100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bausch DG, Feldmann H, Geisbert TW, Bray M, Sprecher AG, Boumandouki P. Outbreaks of filovirus hemorrhagic fever: time to refocus on the patient. J Infect Dis. 2007;196(Suppl. 2):S136–S141. doi: 10.1086/520542. [DOI] [PubMed] [Google Scholar]
- 9.Barry M, Traore FA, Sako FB, Kpamy DO, Bah EI, Poncin M. Ebola outbreak in Conakry, Guinea: epidemiological, clinical, and outcome features. Med Mal Infect. 2014;44(11−12):491–494. doi: 10.1016/j.medmal.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Damkjaer M, Rudolf F, Mishra S, Young A, Storgaard M. Clinical features and outcome of Ebola virus disease in pediatric patients: a retrospective case series. J Pediatr. 2017;182(e1):378–381. doi: 10.1016/j.jpeds.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Levine AC, Shetty PP, Burbach R, Cheemalapati S, Glavis-Bloom J, Wiskel T. Derivation and internal validation of the Ebola prediction score for risk stratification of patients with suspected Ebola virus disease. Ann Emerg Med. 2015;66(3):285–293. doi: 10.1016/j.annemergmed.2015.03.011. (e1) [DOI] [PubMed] [Google Scholar]
- 12.Mupapa K, Massamba M, Kibadi K, Kuvula K, Bwaka A, Kipasa M. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis. 1999;179(Suppl. 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 13.Shao X, Ren W, Zhou F. Clinical presentation and care of patients with Ebola virus disease in the China Ebola treatment unit, Liberia. Jpn J Infect Dis. 2017;70(1):32–37. doi: 10.7883/yoken.JJID.2015.597. [DOI] [PubMed] [Google Scholar]
- 14.WHO Clinical management of patients with viral haemorrhagic fever: a pocket guide for front-line health workers: interim emergency guidance for country adaptation. 2016. https://apps.who.int/iris/bitstream/handle/10665/205570/9789241549608_eng.pdf;jsessionid=0D79676E24CB76B743D7F84962BB24F0?sequence=1 Available from:
- 15.Sterk E. Medicines Sans Frontieres publication. 2008. Filovirus haemorrhagic fever guideline, 2008. [Google Scholar]
- 16.Ahmed S, Finkelstein JL, Stewart AM, Kenneth J, Polhemus ME, Endy TP. Micronutrients and dengue. Am J Trop Med Hyg. 2014;91(5):1049–1056. doi: 10.4269/ajtmh.14-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudfeld CR, Buchanan A, Ulenga N, Spiegelman D, Mtisi E, Hertzmark E. Effectiveness of a multivitamin supplementation program among HIV-infected adults in Tanzania. AIDS. 2019;33(1):93–100. doi: 10.1097/QAD.0000000000002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHenry MS, Dixit A, Vreeman RC. A systematic review of nutritional supplementation in HIV-infected children in resource-limited settings. Journal of the International Association of Providers of AIDS Care (JIAPAC) 2015;14(4):313–323. doi: 10.1177/2325957414539044. [DOI] [PubMed] [Google Scholar]
- 19.Villamor E, Mugusi F, Urassa W, Bosch RJ, Saathoff E, Matsumoto K. A trial of the effect of micronutrient supplementation on treatment outcome, T cell counts, morbidity, and mortality in adults with pulmonary tuberculosis. J Infect Dis. 2008;197(11):1499–1505. doi: 10.1086/587846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care. 2015;19:418. doi: 10.1186/s13054-015-1131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roddy P, Colebunders R, Jeffs B, Palma PP, Van Herp M, Borchert M. Filovirus hemorrhagic fever outbreak case management: a review of current and future treatment options. J Infect Dis. 2011;204(Suppl. 3):S791–S795. doi: 10.1093/infdis/jir297. [DOI] [PubMed] [Google Scholar]
- 22.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151(6):1229–1238. doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn SO, Meissner K, Mayes LM, Bartels K. Vitamin C in sepsis. Curr Opin Anaesthesiol. 2018;31(1):55–60. doi: 10.1097/ACO.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;i6583:356. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirth JP, Rohner F, Woodruff BA, Chiwile F, Yankson H, Koroma AS. Anemia, micronutrient deficiencies, and malaria in children and women in Sierra Leone prior to the Ebola outbreak - findings of a cross-sectional study. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harika R, Faber M, Samuel F, Kimiywe J, Mulugeta A, Eilander A. Micronutrient status and dietary intake of iron, vitamin a, iodine, folate and zinc in women of reproductive age and pregnant women in Ethiopia, Kenya, Nigeria and South Africa: a systematic review of data from 2005 to 2015. Nutrients. 2017;9(10):1096. doi: 10.3390/nu9101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roshania R, Mallow M, Dunbar N, Mansary D, Shetty P, Lyon T. Successful implementation of a multi-country Ebola virus disease clinical surveillance and data collection system in West Africa: findings and lessons learned. Glob Health Sci Pract. 2016;4(3):394–409. doi: 10.9745/GHSP-D-16-00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters JL, Cho DK, Aluisio AR, Kennedy SB, Massaquoi MB, Sahr F. Environmental temperature and case fatality of patients with Ebola virus disease in Sierra Leone and Liberia, 2014–2015: a retrospective cohort study. Trop Med Int Health. 2019;24(1):23–30. doi: 10.1111/tmi.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MSF . 2008. Filovirus haemorrhagic fever guideline. [Google Scholar]
- 30.Skrable K, Roshania R, Mallow M, Wolfman V, Siakor M, Levine AC. The natural history of acute Ebola virus disease among patients managed in five Ebola treatment units in West Africa: a retrospective cohort study. PLoS Negl Trop Dis. 2017;11(7) doi: 10.1371/journal.pntd.0005700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biedron C, Pagano M, Hedt BL, Kilian A, Ratcliffe A, Mabunda S. An assessment of lot quality assurance sampling to evaluate malaria outcome indicators: extending malaria indicator surveys. Int J Epidemiol. 2010;39(1):72–79. doi: 10.1093/ije/dyp363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Team RC . R Foundation for Statistical Computing; Vienna, Austria: 2016. R: a language and environment for statistical computing; p. 2017. [Google Scholar]
- 33.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dehejia RH, Wahba S. Causal effects in nonexperimental studies: reevaluating the evaluation of training programs. J Am Statist Assoc. 1999;94(448):1053–1062. [Google Scholar]
- 35.Cournac JM, Karkowski L, Bordes J, Aletti M, Duron S, Janvier F. Rhabdomyolysis in Ebola virus disease. Results of an observational study in a treatment center in Guinea. Clin Infect Dis. 2015;62(1):19–23. doi: 10.1093/cid/civ779. [DOI] [PubMed] [Google Scholar]
- 36.Waxman M, Aluisio AR, Rege S, Levine AC. Characteristics and survival of patients with Ebola virus infection, malaria, or both in Sierra Leone: a retrospective cohort study. Lancet Infect Dis. 2017;17(6):654–660. doi: 10.1016/S1473-3099(17)30112-3. [DOI] [PubMed] [Google Scholar]
- 37.Group PIW A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016;375(15):1448–1456. doi: 10.1056/NEJMoa1604330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liddell AM, Davey RT, Jr, Mehta AK, Varkey JB, Kraft CS, Tseggay GK. Characteristics and clinical management of a cluster of 3 patients with Ebola virus disease, including the first domestically acquired cases in the United States. Ann Intern Med. 2015;163(2):81–90. doi: 10.7326/M15-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyon GM, Mehta AK, Varkey JB, Brantly K, Plyler L, McElroy AK. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371(25):2402–2409. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 40.Patel VS, Sampat V, Espey MG, Sitapara R, Wang H, Yang X. Ascorbic acid attenuates hyperoxia-compromised host defense against pulmonary bacterial infection. Am J Respir Cell Mol Biol. 2016;55(4):511–520. doi: 10.1165/rcmb.2015-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selvaraj P, Harishankar M, Afsal K. Vitamin D: immuno-modulation and tuberculosis treatment. Can J Physiol Pharmacol. 2015;93(5):377–384. doi: 10.1139/cjpp-2014-0386. [DOI] [PubMed] [Google Scholar]
- 42.Jirapongsananuruk O, Melamed I, Leung DY. Additive immunosuppressive effects of 1, 25-dihydroxyvitamin D3 and corticosteroids on TH1, but not TH2, responses. J Allergy Clin Immunol. 2000;106(5):981–985. doi: 10.1067/mai.2000.110101. [DOI] [PubMed] [Google Scholar]
- 43.Brown CC, Noelle RJ. Seeing through the dark: new insights into the immune regulatory functions of vitamin A. Eur J Immunol. 2015;45(5):1287–1295. doi: 10.1002/eji.201344398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villamor E, Fawzi WW. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18(3):446–464. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larange A, Cheroutre H. Retinoic acid and retinoic acid receptors as pleiotropic modulators of the immune system. Annu Rev Immunol. 2016;34:369–394. doi: 10.1146/annurev-immunol-041015-055427. [DOI] [PubMed] [Google Scholar]
- 46.FAO Grave food security concerns following the Ebola outbreak in Liberia, Sierra Leone and Guinea : FAO in Emergencies. 2017. http://www.fao.org/emergencies/resources/documents/resources-detail/en/c/248112/ Available from:
- 47.Stanturf JA, Goodrick SL, Warren ML, Jr, Charnley S, Stegall CM. Social vulnerability and Ebola virus disease in rural Liberia. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WHO Situation report on the Ebola outbreak in North Kivu (July 23 2019) 2019. https://apps.who.int/iris/bitstream/handle/10665/326015/SITREP_EVD_DRC_20190721-eng.pdf?ua=1 Available from:
- 49.Visser ME, Durao S, Sinclair D, Irlam JH, Siegfried N. Micronutrient supplementation in adults with HIV infection. Cochrane Database Syst Rev. 2017;5 doi: 10.1002/14651858.CD003650.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baum MK, Campa A, Lai S, Martinez SS, Tsalaile L, Burns P. Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral-naive, HIV-infected adults in Botswana: a randomized clinical. Trial. 2013;310(20):2154–2163. doi: 10.1001/jama.2013.280923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material. IMC guidelines on Ebola virus disease management.