Abstract
Bilaterally symmetrical primordia of visceral organs undergo asymmetrical morphogenesis leading to typical arrangement of visceral organs in the adult. Asymmetrical morphogenesis within the upper abdomen leads, among others, to the formation of the omental bursa dorsally to the rotated stomach. A widespread view of this process assumes kinking of thin mesenteries as a main mechanism. This view is based on a theory proposed already by Johannes Müller in 1830 and was repeatedly criticized, but some of the most plausible alternative views (initially proposed by Swaen in 1897 and Broman in 1904) still remain to be proven. Here, we analyzed serial histological sections of human embryos between stages 12 and 15 at high light microscopical resolution to reveal the succession of events giving rise to the development of the omental bursa and its relation to the emerging stomach asymmetry. Our analysis indicates that morphological symmetry breaking in the upper abdomen occurs within a wide mesenchymal plate called here mesenteric septum and is based on differential behavior of the coelomic epithelium which causes asymmetric paragastric recess formation and, importantly, precedes initial rotation of stomach. Our results thus provide the first histological evidence of breaking the symmetry of the early foregut anlage in the human embryo and pave the way for experimental studies of left‐right symmetry breaking in the upper abdomen in experimental model organisms.
Keywords: coelom, left‐right symmetry breaking, mesentery, omental bursa, stomach
1. INTRODUCTION
Breaking of morphological left‐right symmetry during early developmental stages is a common characteristic of many higher metazoan organisms (Blum & Ott, 2018). In vertebrates, this process leads to asymmetric arrangement of most visceral organs which develop almost exclusively within the coelomic cavity. Asymmetric development of the embryonic gut, including gut rotation, asymmetric arrangement of liver, pancreas, and spleen, as well as asymmetric arrangements of peritoneal folds, is of interest for developmental biology (Davis et al., 2017; Kurpios et al., 2008) as well as for the medical practice. Therefore, development of the upper abdomen especially of the stomach and the omental bursa (also described as lesser sack) is considered basic knowledge in medical education. Congenital disorders within this area are variable and frequently associated with abnormalities in asymmetric development. These so‐called laterality defects include complete inversion of visceral organs also referred to as situs inversus as well as different forms of heterotaxy or situs ambiguus which in turn includes partially inverted position of visceral organs and right and left isomerism (Blum & Ott, 2018; Lucas et al., 2020; Peeters & Devriendt, 2006). Left isomerism in upper abdomen is associated with polysplenia, abnormalities of the biliary tree with agenesis of gall bladder, and interrupted inferior vena cava, whereas right isomerism is frequently associated with agenesis of spleen and microgastria with a straight stomach positioned in the midline (Bartram et al., 2005; Kessler & Smulewicz, 1973; Mishalany et al., 1982). Understanding of these disorders requires clarification of corresponding developmental processes. Surprisingly, the common view represented by most recent textbooks of anatomy is still based on the description initially provided by Johannes Müller in 1830 (Müller, 1830) and supposes that the originally straight and bilaterally symmetric stomach primordium is suspended in a thin dorsal mesentery and undergoes a clockwise rotation (as seen from the cranial view). This rotation of the stomach is believed to be associated with (or even caused by) the leftwards bending of the peritoneal fold connecting the stomach with the dorsal abdominal wall (the dorsal mesogastrium) on the left side. This scenario has been particularly popular through the ages perhaps because it provides a simply visualized explanation for the view that the dorsal rim of the stomach primordium gives rise to the great curvature (on the left side) and the ventral rim gives rise to the small curvature (on the right side). Progressive bending together with asymmetrical growth of the stomach tissue leads to further rotation within the frontal plane and, thereby, to the adult arrangement of the stomach and surrounding tissues. An important part of this widely represented view is that bending of the dorsal mesentery of the stomach creates a space which gives rise to the omental bursa (cf. Figure S1(a–c)). However, this hypothesis is not based on empirical evidence such as a histological description of closely spaced stages of lower foregut development even though the complex structure of the omental bursa in the adult relies on a sequence of complex morphogenetic events, none of which have been clarified til today.
Wilhelm His suggested in 1880 that the omental bursa develops independently from the rotation of the stomach as a space (recess) emerging in the right side of mesogastrium (His, 1880, cf. Figure S1(d,f)). This observation was supported by further studies (Broman, 1904; Swaen, 1896, 1897) and adapted by some textbooks early on (Keibel & Mall, 1910; s. a. Starck, 1975). According to this view, the development of the omental bursa results from the formation of communicating cavities which separate the primordial stomach from the surrounding tissues. These cavities arise from a single extension of the coelomic cavity and were defined by Broman (1904) according to their position as pneumato‐enteric, hepato‐enteric, as well as mesenterico‐enteric recesses. The mesenterico‐enteric recess is located dorsally to the stomach anlage (Brummer, 1982; Heckl, 1979; Pernkopf, 1922) and later, after pancreas formation in the dorsal mesentery, also separates the stomach from the pancreas. Therefore, it was argued that the mesenterico‐enteric recess gives rise to the pancreatico‐enteric recess (Broman, 1904, 1905; Heckl, 1979), which in turn is involved in mature omental bursa development (Nakamura et al., 2020). In addition, the greater omental recess was most recently defined as an extension of the pancreatico‐enteric recess (Nakamura et al., 2020).
As a mechanism for the autonomous development of the omental bursa Broman proposed that the recess formation is initiated by active invagination of the coelomic epithelium (Broman, 1904, 1938, for a review). Alternatively, it was suggested that the omental bursa develops by fusion of intercellular clefts similar to the process of coelom formation in the early somite stage embryo (Heckl, 1979; Kanagasuntheram, 1957; Figure S1(a) inset), and this view was adapted in a modified form also by several textbooks (Hamilton & Mossman, 1972; Harrison, 1978; Langman, 1975; Standring & Standring, 2016). Still, the evidence for this mechanism has remained controversial and the empirical basis for it is scarce 1979. The third view suggests that the formation of the cavities is caused by growth of an additional mesenterial fold on the right‐side lateral to the main mesentery (Pernkopf, 1922; cf. also Hochstetter, 1888; Figure S1(j,i)).
This ambiguity in the description of initial omental bursa development now justifies a formal revision of the asymmetric development of the early foregut and its neighboring tissues. We therefore present a detailed histological analysis, including 3D‐reconstructions of the primordial stomach area, especially with respect to the initiation of asymmetric development of the omental bursa. We focused our analysis on embryos between Carnegie stages 12 and 15 (26–38 days of development), which include both embryos immediately prior to the asymmetrical development in the foregut region and embryos displaying obvious asymmetrical arrangement. Elucidation of the earliest steps of omental bursa formation in this way may form the basis for mechanistic and molecular studies of left‐right symmetry breaking. In combination with the recent three‐dimensional reconstruction of the later phases of the omental bursa formation as a consequence of peritoneal recess formation in human embryos between stages 13 and 21 (Nakamura et al., 2020), our study may contribute to overcoming the tradition to describe the development of the omental bursa as the result of a simple rotation of the stomach anlage.
2. MATERIALS AND METHODS
2.1. Embryos
Embryos used for this study are historical specimens and belong either to the Carnegie collection of the Developmental Anatomy Center at the National Museum of Health and Medicine in Silver Spring, MD, USA, or to the Human Embryology Collection (Blechschmidt Collection) of the Centre of Anatomy, University Medical Centre Göttingen, Göttingen, Germany. Images of embryos #8943 and #836 from the Carnegie collection (Gasser et al., 2017) are available at the open source website http://virtualhumanembryo.lsuhsc.edu/. Embryos had been fixed, embedded, sectioned, and histologically stained using different protocols. Details about stage, fixation, embedding can be found in Table 1. Images were acquired using Zeiss Axio Scan.Z1, Zeiss Axioplan 2, or Nikon E800 microscopes and processed as described (de Bakker et al., 2016; Rulle et al., 2018).
Table 1.
Summary of information about embryos used in this study
| Stage | Specimen | Origin | Year | Acquired through | Days of development (estimated) | CRL | Fixative | Staining | Plane of sectioning | Z‐resol |
|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 8505A | CC | 1947 | Miscarriage | 29 | 2.86 | Formol | Hematoxylin and Phloxin | Transversal | 10.32 |
| 12 | 8943 | CC | 1934 | Hysterectomy | 29–31 | 3.58 | Zenker's Formol | Hematoxylin and Eosin | Transversal | 8.22 |
| 12 | 1964_01_11 | EB | 1964 | No information | 29–31 | 6.3 | Bouin | Hematoxylin and Eosin | Transversal | 10 |
| 13 | 836 | CC | 1914 | Hysterectomy | 32 | 4.09 | Mercuric Acid | Alum Cochineal | Transversal | 15 |
| 13 | 1954_09_11 | EB | 1954 | Hysterectomy | 30–33 | 3.75 | Bouin | Hematoxylin and Eosin | Fronto‐transversal | 10 |
| 14 | 1951_08_01 | EB | 1951 | Post mortem dissection | 33 | 3.4 | Bouin | Hematoxylin and Eosin | Transversal | 10 |
| 15 | 3512 | CC | 1921 | Miscarriage | 36 | 6.55 | Formol | Alum Cochineal | Transversal | 10.06 |
| 15 | 721 | CC | 1913 | No information | 36 | 4.79 | Zenker's Formol | Hematoxylin and Eosin | Transversal | 8.69 |
Abbreviations: EB, Blechschmidt Collection; CC, Carnegie collection; CRL (µm), crown‐rump‐length, Z‐resol, Z‐resolution in µm.
2.2. Three‐Dimensional Reconstruction
Photographic images were automatically aligned used Amira® software and subsequently manually adjusted (de Bakker et al., 2016). In the next step aligned images were subjected to manual segmentation of embryonic structures of interest using the open source software “Fiji‐ImageJ‐win64” and the plugin “TrakEM2” (Rulle et al., 2018; Schindelin et al., 2012). Reconstruction models were exported as U3D files, imported in Adobe Acrobat XI pro® (http://www.adobe.com), and converted into 3D‐PDF files. The 3D‐PDF function was used for creating digital sections.
2.3. Terminology
We use the term mesenterico‐enteric recess because this study deals with the early phase of omental bursa formation, when the recess is located dorsal to the stomach (and ventral to the root of the mesentery) as originally defined by Broman (1904) and later used by other authors (Brummer, 1982; Heckl, 1979; Keibel & Mall, 1910; McGaw, 1924; Pernkopf, 1922). In his late review, Broman (1938) uses the term pancreatico‐enteric recess for the late‐forming caudal recess of the omental bursa and omits the term mesenterico‐enteric recess to demonstrate the equivalent (and possibly the origin) of the pancreatico‐enteric recess at early stages in a simplified schematic manner (legend of figure 1 in Broman, 1938, cf. Figure S1 of this study). For later stages of omental bursa development we welcome the use of the term pancreatico‐(lieno)‐enteric recess next to the pancreas (and spleen) developing in the dorsal mesogastrium and the use of the term greater omental recess for the transient cavity within the greater omentum as suggested recently by Nakamura et al. (2020).
2.4. Ethics
The present study was approved by the Ethics Committee of University Medical Center Göttingen (reference no. 30/3/20).
3. RESULTS
Transversal sections of an early‐stage‐12 embryo (Carnegie #8943, cf. Table 1) reveal the caudal foregut as a kite‐shaped lumen located in the midline: its long axis is oriented along the dorso‐ventral axis with the “long” sides of the “kite” positioned ventrally (Figure 1(a–c)). Its epithelial lining is flat to cuboidal dorsally and cuboidal to columnar ventrally. Caudally, transversal sections reveal the hepatic diverticulum and primordia of the gallbladder within the septum transversum (Figure 1(c,d)) and the coelomic cavities to both sides of the foregut to be principally symmetrical. The most dorsal parts of the foregut are located within the dorsal body wall next to the paired aorta and, therefore, a dorsal mesentery is not formed yet at this level. Instead, the foregut is embedded into a mesenchymal plate which faces laterally the epithelial lining of the coelomic cavity, ventrally the heart, the septum transversum, and the hepatic primordium, and dorsally the dorsal body wall. At the level of the septum transversum, the plate appears particularly narrow. Following the description of this mesenchymal plate as a “cloison mesenterique” by Swaen (1897) or "mesenterielle Scheidewand" by Broman (1904), we address this plate here as the mesenteric septum (Figure 1).
Figure 1.

The mesenteric septum of an early stage 12 human embryo (Carnegie #8943). (a–d) Transversal sections. Intersecting arrows in A indicate anatomical axes. Asterisks mark the thickened coelomic epithelium. An overview image of the embryo with black lines indicating the position of the transversal sections is shown in the upper right corner of (d). (e, f) Magnification of the mesenteric septum and the developing foregut. Squared segments are shown at higher magnification in (b) and (c), respectively. Arrowheads indicate mitotic cells in the coelomic epithelium. White arrow points to the irregularly shaped basal side of the epithelium in (e) and black arrow points to the regular continuous line corresponding to the intact basal membrane in (f). Abbreviations: d, dorsal; v, ventral; l, left; r, right; ccc, common coelomic cavity (corresponds to intra‐embryonic coelom with exception of cavities formed by recesses); ao, aorta; sv, sinus venosus; st, septum transversum; ms, mesenteric septum; fg, foregut; mg, midgut; hd, hepatic diverticulum; and gb, gallbladder. Scale bar in (d) 100 µm for (a–d) and 20 µm for (e) and (f)
Within the mesenteric septum, no clear signs of cavity formation are seen (Figure 1(b), (c) and (e), (f)) but the epithelial lining of the coelomic cavity lateral to it varies in height dramatically: the cells of the medial (visceral) part of the coelomic epithelium are cuboidal to columnar, whereas cells of lateral (parietal) part display a predominantly squamous morphology. The thickening of the visceral lining is especially pronounced at the level of the lung primordia, the hepatic diverticulum, and the gallbladder (Figure 1(a–d)); here, it appears (pseudo)stratified but does not show signs of invagination found at later stages (s. below). The pseudostratified stretches of the epithelium display numerous mitotic figures apically (cf. Figure 1(e,f)) while the line formed by the basal side of epithelium displays an irregular shape indicating a discontinuous basement membrane.
Analysis of two late‐stage‐12 embryos (#8505A; #EB 1964_01_11) reveals an advanced morphology at the level of the caudal foregut despite their marked differences in CRL (Table 1): the caudal foregut is straight and narrow in transversal sections, aligned along the dorso‐ventral axis, and almost completely contained in the mesenteric septum (Figure 2(a–d), here embryo #8505A). The cranio‐caudal extent of its straight shape and its position in the midline is seen in 3D reconstructions (Figure 7(a,b)). At the level cranial to the septum transversum the foregut epithelium shows bilateral epithelial thickenings with a close topographical relationship to the early lung buds (Figure 2(a)). Caudally, transversal sections reveal hepatic cords within the septum transversum at levels below the epithelial bud of the hepatic diverticulum, which appears as an outgrowth of the caudal foregut (Figure 2(d)).
Figure 2.

Asymmetric bud‐like thickening in the coelomic epithelium of the mesenteric septum as a first sign of symmetry breaking of a stage‐12 human embryo (Carnegie #8505A). (a–d) Transversal sections of the embryo (anatomical axes oriented as in Figure 1). the position of the transversal sections is indicated in the overview image in (d). Bars in (c) mark the position of the frontal sections shown in Figure 7(a) and (b). Abbreviations: ccc, common coelomic cavity; lb, lung bud; rv, right ventricle; lv, left ventricle; sv, sinus venosus; st, septum transversum; ms, mesenteric septum; and hd, hepatic diverticulum. Scale bar in (d): 100 µm for (a–d)
Figure 7.

Foregut asymmetry between stages 12 (a–c) and 13 (d–f): The asymmetrically arranged mesenteric septum precedes the emergence of the pneumato‐enteric recess. (a, b) Frontal views of 3D reconstructions of a stage‐12 embryo (Carnegie #8505A); (b) is, in addition, rotated counter‐clockwise in the transversal plane to show the invagination in the coelomic epithelium (arrow). (c) Right lateral view of the visceral coelomic epithelial covering at stage 12. (d) Frontal view of 3D reconstruction of the coelomic cavity and the pneumato‐enteric recess at stage 13 (embryo Carnegie #836). Arrowhead in (c) corresponds to the narrow invagination on the left side seen in Figure 4. (e) Asymmetrical arrangement of coelomic epithelium and coelomic cavities seen in the frontal section of 3D reconstruction of the same stage 13 embryo. Formation of paramesenteric fold (f) on the right side (cf. Figure S1). (f) Right lateral view of the visceral coelomic epithelial covering at stage 13 demonstrating the narrow opening of the emerging pneumato‐enteric recess (white arrow). Stomach (s) and lung bud (lb) are in green; coelomic epithelium (ce) in azure blue; common coelomic cavity (ccc) in turquoise; pneumato‐enteric recess (per) in teal; surface of section through epithelium in gray, and through common coelomic cavity (ccc), in turquoise
The mesenteric septum appears in cranial sections as a wide plate which becomes narrow caudally. The visceral epithelial lining of the coelom is mostly columnar and displays, in addition, at levels caudal to the lung buds a multilayered or pseudostratified thickening which gives rise bilaterally to bud‐like structures reaching into the mesenchymal tissue towards the foregut (Figure 2(c)). These bud‐like thickenings are found in many consecutive sections, measuring along the cranio‐caudal axis about 40 µm on the left side and about 50 µm on the right side; their cranial border corresponds to the cranial border of the septum transversum. The 3D reconstruction reveals that these thickenings correspond to bilateral folds which reach into the mesenchymal core of the mesenteric septum ventrally, and in the frontal views display a step‐like shape formed by the basal side of thickened coelomic epithelium (Figure 7(a,b)). The apical sides of the coelomic epithelia display rather smooth surfaces with exception of the right ventral side where it also reveals a step‐like shape with a small indentation. Transversal sections at corresponding levels reveal at the right side a slight invagination of the coelomic epithelium (Figure 2(c)). Mesenchymal cells within the mesenteric septum are densely packed with exception of areas basal to the bud‐like thickening as well as to the foregut epithelium. As before, no signs of cavity or cleft formation are seen in the mesenchyme of the mesenteric septum.
At the early stage 13 (#EB 1954_09_11), the mesenteric septum has lengthened further in the dorso‐ventral plane with the effect that the foregut is now completely surrounded by the mesenteric septum and a dorsal mesentery becomes distinct (Figure 3(a–c)). Numerous irregularly shaped intercellular spaces within the mesenchyme of the mesenteric septum are seen. Some of these spaces are bordered by flat cells which display the morphology of embryonic mesenchyme whereas others are formed by endothelial‐like cells and contain blood cell precursors (Figure 3(f), cf. also below). The coelomic epithelium covering the mesenteric septum displays several stretches of marked thickening, especially dorsally. On the right side, the coelomic epithelium shows a deep latero‐medial invagination and forms a short recess (extending dorso‐caudally and ventro‐cranially) reaching the level of the lung bud (Figure 3). On the left side, in contrast, the thickening of the coelomic epithelium does not form an invagination. However, the left visceral coelomic epithelium forms a narrow recess ventrally at a level just above the septum transversum (not shown).
Figure 3.

Early development of the pneumato‐enteric recess in an early‐stage‐13 embryo (#EB 1954_09_11). (a–c) Fronto‐transversal sections. Arrows point to the emerging right‐sided recess. The position of the fronto‐transversal sections is indicated in the overview image in (c). (d) High magnification of the squared segment in (c). Note the wide intercellular spaces within the mesenteric septum. Asterisk points to intercellular spaces formed by mesenchymal cells. Hash marks a blood vessel. Abbreviations: ao, aorta; ms, mesenteric septum; lb, lung bud; fg, foregut; and ccc, common coelomic cavity. Scale bar in (d): 100 µm for (a–c); 20 µm for (d)
Transversal sections of a late‐stage‐13 embryo (Carnegie # 836) reveal several derivatives of the foregut and a wide mesenteric septum. In particular, the respiratory primordia are now separated from the dorsally located digestive anlagen (the prospective esophagus) and form a distinct tracheal primordium and a left and a right primary bronchus (Figure 4(a)). Caudally, the digestive tube shows an oval cross‐section with its longer axis aligned with the dorso‐ventral axis, and this represents the prospective stomach (Figure 4(c,d)). The epithelial covering of the mesenteric septum displays a mainly flat to cuboidal shape cranially (Figure 4(a,b)) and a high columnar shape further caudally (Figure 4(c,d)). Whereas the esophagus of stage 13 is located in the embryonic midline in the center of the mesenteric septum (Figure 4(a)), the stomach primordium is shifted slightly to the left side and displays a minor inclination relative to the sagittal plane. Here, at levels of the stomach and the caudal esophagus, the mesenchymal core of the mesenteric septum contains a slender semilunar cavity, which extends about 200 µm caudally from the level just caudal to the lung buds and up to 200 µm in the transversal plane. Due to this close topographical relationship with the respiratory and digestive anlagen, the cavity is addressed here as the pneumato‐enteric recess as initially indicated by Broman (1904). The pneumato‐enteric recess opens into the right coelomic cavity at the level just cranial to the gastroduodenal junction (Figure 4(e)) and is oriented here along the dorso‐ventral axis on the right side of the stomach primordium (Figure 4(d)); at more cranial levels, the cavity is shifted towards the midline ventral to the esophagus (Figure 4(a,b)). The epithelial lining of the pneumato‐enteric recess varies in thickness as follows (Figure 5): whereas its lateral wall is covered by squamous cells similar to the parietal coelomic epithelium, in its dorsal and medial wall the epithelial lining displays a high columnar morphology with a partially (pseudo)stratified structure and frequent mitotic figures (Figure 5(b,c)). Moreover, the right epithelial layer of the stomach runs parallel to the medial covering of the pneumato‐enteric recess, whereas the left layer displays a strong convexity (Figure 4(c,d)). The left side of the mesenteric septum does not display a cavity but the common coelomic cavity itself forms a cleft‐like narrow invagination on the left side of the stomach with a shape mirroring the shape of the right‐sided pneumato‐enteric cavity in the transversal sections shown (arrowheads in Figure 4(c,d)). The visceral coelomic epithelial lining on the left side is also columnar and displays a thickening reaching into the mesenchymal tissue (Figure 4(c)). 3D reconstructions of the coelomic epithelium reveal that this thickening corresponds to the tangential view of the fold caused by the step‐like narrowing of the mesenteric septum (Figure 5(d)). Different from stage 12, this step‐like arrangement is located on the right side further caudally as compared to the left and is now partially separated from the rest by the pneumato‐enteric recess (Figure 7) hence revealing the emerging asymmetry of the mesenteric septum. Mesenchymal cells within the mesenteric septum are densely packed. However, less dense areas are located near the ventral part of the foregut derivatives and many clefts lined by squamous cells are seen (Figure 5(b)). Consecutive sections indicate that these clefts, which are lined by thin endothelial cells and contain blood cells, are anlagen of mesenteric blood vessels (Figure 5(a,c)).
Figure 4.

Fully developed pneumato‐enteric recess in a stage‐13 embryo (Carnegie #836) next to an almost symmetrical stomach anlage. (a–e) Transversal sections of the embryo (anatomical axes are oriented as in Figure 1). Arrowhead in (c) points to the narrow invagination of the coelomic epithelium on the left side (cf. Figure 7). Position of the transversal sections is indicated in the overview image in (e). Abbreviations: ao, aorta; lb, lung bud; ms, mesenteric septum; pm, paramesentery; per, pneumato‐enteric recess; ra, right atrium; la, left atrium; rv, right ventricle; lv, left ventricle; sv, sinus venosus; st, septum transversum; and hc, hepatic cords. Scale bar in (e): 200 µm for (a–e)
Figure 5.

Different epithelial configurations in the lining of the pneumato‐enteric recess shown in Figure 4 as signs of local morphogenetic epithelial activity. Sections are aligned according to the orientation of the longest cross‐sectional diameter of the recess. Intersecting arrows in A indicate anatomical axes. Arrows point to the endothelial covering of blood vessels; and hashes mark the cleft which corresponds to the lumen of the blood vessel. Arrowhead in (b) shows a mitotic figure. Black square brackets mark the height of columnar (pseudostratified) and flat stretches of the epithelium, respectively. Scale bar in (d): 20 µm for (a–c)
At stages 14 (embryo #EB 1951_08_01) and 15 (embryos Carnegie #3512, and # 721), foregut differentiation is principally the same as at stage 13. The mesenteric septum, however, is now increasingly partitioned to encompass esophagus, lung primordium, and stomach whereby indentations form between, and separate, these organ anlagen (Figure 6). The liver increases rapidly in volume and occupies most of the space between the ventral and the dorsal body walls. The common coelomic cavity is, therefore, shifted toward the posterior body wall forming several narrow coelomic clefts. The stomach displays an asymmetric morphology in transversal sections with the long axis inclined to the left of the dorso‐ventral axis (Figure 6(b–d)). This inclination gradually increases reaching 40–45° (Figure 6(b–d)). Due to its position between primordia, coelomic recess formation can now be subdivided as follows: the pneumato‐enteric recess proper is still oriented along the dorso‐ventral axis on the right side of the stomach and the distal esophagus primordium and reaches the level of the bronchial buds (Figure 6(a)). At more caudal level, the recess is located between the stomach and the liver, thus corresponding to the hepato‐enteric recess, and it forms an additional cleft on the left side located dorsal to the stomach and caudal to it, thus corresponding to the mesenterico‐enteric recess (Figure 6(c, d)). The common cavity formed by these recesses opens on the right side into the common coelomic cavity dorsal to the liver (Figure 6(d)). The caudal end of the recess is oriented perpendicularly to the dorso‐ventral axis and is now located dorsal to the duodenum primordium. The recess separates the mesenchymal tissue dorsal to the stomach and the liver into the dorsal mesogastrium and an additional mesenteric connection between the dorsal body wall tissue and the liver which we call paramesentery in analogy to the terms "Nebengekröse" and "Mesolaterale" introduced by Broman (1904) and Swaen (1897), respectively. Whereas the epithelial lining of the recess in the stage‐14 embryo (#EB 1951_08_01) displays a differential cell morphology with a higher epithelium and spike‐like basal extensions (not shown), the epithelial lining of the recesses at stage 15 reveals a more homogenous epithelial morphology.
Figure 6.

Increasing stomach asymmetry correlates with the expansion of the mesenterico‐enteric recess. (a–d) Transversal sections of a stage‐15 embryo (embryo Carnegie #3512). Anatomical axes are oriented as in Figure 1. Asterisk marks the lumen connecting the common coelomic cavity with the developing omental bursa. Position of the transversal sections is indicated in the overview image in (d). Abbreviations: ccc, common coelomic cavity; a, aorta; lb, lung bud; e, esophagus; per, her, and mer/per, (right) pneumato‐enteric, hepato‐enteric, and mesenterico‐enteric (pancreatico‐enteric) recesses, respectively; s, stomach; d, duodenum; dm, dorsal mesogastrium; hc, hepatic cords; and pm, paramesentery. Scale bar in (d): 50 µm for (a–d)
3D reconstruction at stage 15 (embryo Carnegie #3512) reveals that the overall shape of the stomach mirrors now its adult morphology with small and greater curvatures which are now oriented obliquely to the sagittal axis (Figure 8). The recesses, now suitably addressed as the primordium of the omental bursa, form a unitary cavity which is elongated in the cranio‐caudal direction, and in its caudal part forms a dorsal extension toward the right side; here, it opens into the common coelomic cavity, whereas on the left side it follows the emerging greater curvature of the stomach.
Figure 8.

Ventral and dorsal views of isolated stomach and omental bursa anlagen, the latter of which is composed of the pneumato‐enteric (per), the mesenterico‐enteric (mer), and the hepato‐enteric (her) recesses, at stage 15 and derived from the 3D reconstructed embryo shown in Figure 6 (Carnegie #3512). The omental bursa is located right and dorso caudal to the stomach which displays significant left‐right asymmetry with major and minor curvatures on its left and right sides, respectively. Stomach (s) and esophagus (e) are presented in green, omental bursa primordia in teal
4. DISCUSSION
The present analysis reveals that the emerging asymmetry of the human foregut and its related mesenteries takes place within a wide mesenchymal plate initially named “cloison mesenterique” by Swaen (1897) or “mesenterielle Scheidewand” by Broman (1904) and referred to here as mesenteric septum. Confirming and supporting the conclusion proposed recently by Nakamura and colleagues (Nakamura et al., 2020) our data complements this view of omental bursa development by focusing on initial morphological steps of symmetry breaking within the mesenteric tissue.
4.1. The mesenteric septum of Swaen as a “bedding” for emerging recess formation
The mesenteric septum of Swaen (1897) emerges at stage 12 (i.e., just after the foregut has closed at stage 11), separates left and right coelomic cavities, and is the “mesenchymal bedding” of the (asymmetric) recess development. In addition, it provides a flexible matrix for the specific development of esophagus, stomach, and lung tissue. Still straight and bilaterally symmetric at stage 12, the foregut shifts its caudal part to the left side simultaneously with the appearance of a slender semilunar cavity, the pneumato‐enteric recess, on the right side at stage 13. Initial development of the pneumato‐enteric recess is related to left‐right differences in the visceral coelomic covering of the mesenteric septum at stage 12 and subsequent invagination of the coelomic epithelium along the right side of distal foregut and lung bud at stage 13; this is followed by subdivision of the recess into hepato‐enteric, pneumato‐enteric, and mesenterico‐enteric recesses, respectively, at stages 14 and 15. Crucially, the asymmetric morphogenesis of the coelomic lining of the mesenteric septum precedes bending and rotation of the stomach. On the right side, the mesenteric septum gives rise also to the paramesentery, which connects the emerging liver directly to the dorsal body wall and provides (1) an “environment” for the development of the vena cava and its connection to the liver and (2) a right wall of the superior recess of the mature omental bursa.
4.2. Differential development of the coelomic epithelium
Our analysis of the stage 12 embryo revealed the bilateral symmetrical thickening of the mesenteric septum with a bud‐like shape in transverse sections and the asymmetrical invagination of the coelomic lining of the mesenteric septum on the right side. This invagination displays, however, a complex morphology and is related to the asymmetry of the mesenteric septum revealing a step‐like arrangement on the right side ventrally (s. 3D reconstruction of the early‐stage‐12 embryo in Figure 7). Initial asymmetrical arrangement is also seen in historical specimens such as Carnegie embryo #5923 (O'Rahilly & Müller, 1987), embryo I (Swaen, 1897), and embryo ES23/2 (Brummer, 1982). Here, too, the differential arrangement of coelomic epithelium is followed by the emergence of an unambiguous morphological asymmetry at stage 13 and characterized by development of the pneumato‐enteric recess. However, according to Broman (1904) the pneumato‐enteric recess emerges bilaterally, i.e., a short pneumato‐enteric recess develops with a short time delay also on the left side and disappears soon, an observation which was supported recently by the description of a transient small recess between stages 17 and 19 (Aben & de Bakker, 2012). The persistence of a left pneumato‐enteric recess, if it forms at all, is probably of short duration since the vast majority of reported embryos at stages comparable to Carnegie stage 13 display a right pneumato‐enteric recess, only (cf. Table 2). As bud‐like structures are present in the coelomic lining on both sides of the mesenteric septum at stage 12, it could be speculated that the development or maintenance of the recess on the left side is "successfully" inhibited as an early step of asymmetrical development in most embryos.
Table 2.
Summary of reported data concerning early left‐right asymmetry within mesenteric septum at stages between first signs of asymmetry in its coelomic covering (stage 12) and formation of pneumato‐enteric recess (stage 13)
| Embryo (serial no.) | Reference | Size (mm) | Stage | Right side | Left side |
|---|---|---|---|---|---|
| Carnegie #8505A | Present report | 2.86 | 12 | Invagination | Flat |
| ES23/2 | Brummer, 1982 | 3 | 12 | Invagination | Flat |
| 118/330/8 | Brummer, 1982 | 3 | 12 | Invagination | Flat |
| Pi1 | Pernkopf, 1922 | 3.2 | 12 | Invagination | Shallow groove |
| Embryo I | Broman, 1904 | 3.4 | 12 | Invagination | Invagination (L<R) |
| "3.8 mm" | Swaen, 1897 | 3.8 | 12 | Invagination | Shallow groove |
| Embryo II | Broman, 1904 | 3 | 13 | PER | PER (L<R) |
| H.86 | Kanagasuntheram, 1957 | 3.8 | 13 | PER | Shallow groove |
| EB 1954_09_11 | Present report | 3.8 | 13 | PER | Shallow groove |
| Embryo 10 (Strahl) | Keibel and Elze, 1908 | 4 | 13 | PER | ? |
| "4 mm" | Kanagasuntheram, 1957 | 4 | 13 | PER | Flat |
| Carnegie #836 | Present report | 4.09 | 13 | PER | Shallow groove |
| No3 | Pernkopf, 1922 | 4.3 | 13 | PER | Shallow groove |
| 118/329/3 | Brummer, 1982 | 4‐4.5 | 13 | PER | PER (L<R) |
| Ha4 | Pernkopf, 1922 | 4.9 | 13 | PER | Shallow groove |
| "5 mm" | Kanagasuntheram, 1957 | 5 | 13 | PER | Flat |
| Millard | Kanagasuntheram, 1957 | 5 | 13 | PER | Flat |
| Kyoto #04953 | Nakamura et al., 2020 | 7.1 | 13 | PER | ? |
| No6 | Pernkopf, 1922 | 5.4 | 13 / 14 | PER | Shallow groove |
Abbreviations: EB, embryo from Blechschmidt Collection; ?, not described; PER, pneumato‐enteric recess; L<R, significant difference in size between right and left pneumato‐enteric recesses.
4.3. Possible mechanisms of initial omental bursa development
The initiation of (unilateral) recess formation by way of invagination into the mesenteric septum and occurring prior to both dorsal mesentery formation and asymmetric arrangement of the stomach indicates that among the proposed mechanisms of the omental bursa formation, simple kinking of the dorsal mesentery leading to the rotation of stomach (cf. Figure S1(a–c)) can no longer be seen to be a plausible sequence of events. This suggestion is in line with the description of omental bursa morphogenesis as a consequence of recess formation (Nakamura et al., 2020). In addition, our data does not support other proposals, either, such as coelomic cleft formation with subsequent fusion within the mesenteric septum (Heckl, 1979; Kanagasuntheram, 1957; cf. inset in Figure S1(a)): coelomic clefts form typically from epitheloid mesodermal condensationwhile (bland) widened intercellular spaces within the mesenteric septum are formed by elongated mesenchymal cells. Instead, our data is in line with the mechanism of active local invagination of the coelomic epithelium into the mesenteric septum as proposed early on by Broman in 1904. Specifically, the epithelial lining of the mesenteric septum reveals signs of morphogenetic activation and local bud formation within a (pseudo)stratified epithelium, high mitotic activity, and signs of degradation of the basement membrane as can also be seen, for example, in lung branching morphogenesis (Moore et al., 2005). Importantly, differential epithelial activation seen within different parts of the lining of the emerging recess (cf. Figure 5) may indicate the direction of its expansion and further subdivisions (Figure 8). Interestingly, the epithelial sheet of the midgut mesentery in mouse and chicken embryos displays similar left‐right differences (Davis et al., 2008) which were proposed to be involved in the midgut rotation.
As an alternative to the active epithelial growth model, an asymmetrical growth of the peritoneal fold has been proposed as a possible scenario for omental bursa formation (Pernkopf, 1922). According to this hypothesis, the formation of paramesentery is an effect of the descending peritoneal fold investing the right lung primordia (Figure S1(j,i), see also Figure 9 b1, b4). A topological consequence of this suggestion is the early left‐right asymmetry of the mesenteric septum, i.e., the emergence of the additional fold on the right side extending caudally further than compared to the left. Indeed, some signs of the expected asymmetry are revealed by our 3D reconstruction of the stage‐13 embryo (Figure 7). However, a further analysis of available human and other mammalian embryos is required to clarify whether both scenarios may, indeed, not be mutually exclusive since asymmetric growth of the fold requires local coelomic epithelium activity at the tip of the emerging recess.
Figure 9.
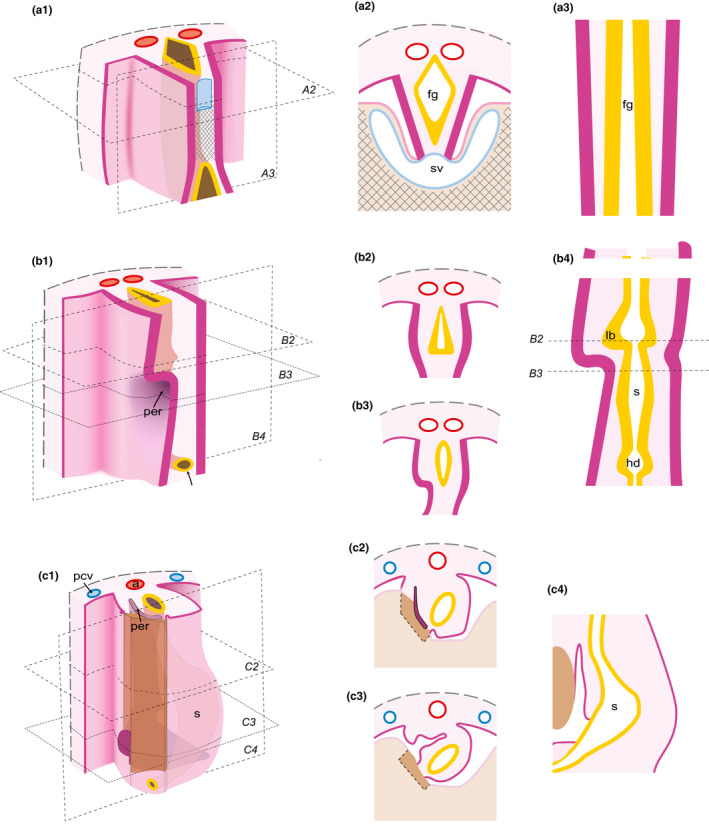
Steps of omental bursa development inferred from the results of the present study. Related areas are shown in anterolateral views (a1; b1; c1) and transversal (a2; b2; b3; c2; c3) and frontal (a3; b4; c4) sections. (a1‐a3) Initial symmetrical arrangement of the foregut and its mesenteries. (b1‐b4) Beginning of left‐right asymmetry in the mesenteric septum. (c1‐c4) Asymmetric enlargement of recesses and corresponding displacement of the stomach primordium. Structures located ventrally (including especially septum transversum and liver primordia as well as the sinus venosus) are removed in a1 to enable the view into the coelomic cavity. Abbreviations: s, stomach; lb, lung bud; hd, hepatic diverticulum; a, aorta; and sv, sinus venosus. Crosshatched lines illustrate septum transversum. Epithelial covering of the coelom, pink; sectioned coelomic epithelium, magenta; mesenchymal tissue of the digestive system and the neighboring posterior body wall, rose; aorta, red; veins, blue; foregut with lung and hepatic buds, yellow; and liver primordium tissue, brown. The levels of transversal and frontal sections are shown in a1, b1, and c1, respectively
Further analysis of the development of the omental bursa should include also mechanistic and molecular studies. Specifically, a new analysis of initial mechanisms leading to the morphological left‐right asymmetry of abdominal organs may include functional studies of genes asymmetrically expressed in the lateral plate mesoderm and next to the differing coelomic epithelial lining immediately prior to foregut closure (i.e., at stages equivalent to stage 11 in the human). Promising candidates for such molecular studies may be the homeobox genes Pitx2 (Campione et al., 1999), Islet‐1 (Yuan & Schoenwolf, 2000), or cytoskeleton‐associated genes such as Daam2 (Welsh et al., 2013), as well as the analysis of the role of extracellular substances such as hyaluronic acid in asymmetric morphogenesis (Davis et al., 2008; Kurpios et al., 2008).
5. CONCLUSIONS
The present analysis supports the idea of an autonomous development of the omental bursa as the result of recess formation as proposed initially by Swaen (1897) and Broman (1904) and as most recently confirmed by Nakamura et al. (2020). This recess formation occurs in the mesenteric septum, here defined as the broad connection between the foregut and the dorsal body wall. Our study indicates, however, that the initial steps of the recess formation involve both differential activity of coelomic epithelium and asymmetric morphogenesis of the mesenteric septum, hence, incorporating views of Broman (1904) and Pernkopf (1922). Crucially, recess formation precedes the rotation of the stomach and, therefore, represents the earliest morphological left‐right asymmetry in the human digestive system. Furthermore, specifically oriented enlargement of recesses close to the stomach (and lung bud) epithelium suggests the omental bursa to be a major factor for the differential growth (“rotation”) of the stomach. Very much in line with the recent study by Nakamura et al. (2020) on later stages our results call for a principal modification of textbook schematics for early gut development (Figure 9), which would (1) adhere as much as possible to the dynamic morphology of both the septum transversum and the mesenteric septum, (2) address the differential appearance of the coelomic epithelium, and (3) refer to defined (Carnegie) stages of human embryos; representing mesenteries as thin lines and the liver as a largely isolated structure may thus be avoided. Correct presentation of omental bursa development may, in turn, contribute to a better understanding of mechanisms of developmental abnormalities in the upper abdomen.
Supporting information
Figure S1
ACKNOWLEDGMENTS
The authors would like to thank Hannes Sydow for his technical assistance, John Cork, Ph.D, and Raymond F. Gasser, PhD (New Orleans) for the images of the embryos #8943 and #836 from the Carnegie collection (http://virtualhumanembryo.lsuhsc.edu/). The De Snoo–van’t Hoogerhuijs Foundation is gratefully acknowledged for their financial support. We are grateful to Stefan Elsberger for his help in creating Figure 9. Open access funding enabled and organized by Projekt DEAL.
Schäfer T, Stankova V, Viebahn C, de Bakker B, Tsikolia N. Initial morphological symmetry breaking in the foregut and development of the omental bursa in human embryos. J Anat.2021;238:1010–1022. 10.1111/joa.13344
REFERENCES
- Aben, K. & de Bakker, B.S. (2012) The embryonic development of the pneumato‐enteric recess. Amsterdam: Amsterdam University College. [Google Scholar]
- Bartram, U. , Wirbelauer, J. & Speer, C.P. (2005) Heterotaxy syndrome – Asplenia and polysplenia as indicators of visceral malposition and complex congenital heart disease. Biology of the Neonate, 88, 278–290. [DOI] [PubMed] [Google Scholar]
- Blum, M. & Ott, T. (2018) Animal left‐right asymmetry. Current Biology, 28, R301–R304. [DOI] [PubMed] [Google Scholar]
- Broman, I. (1904) Die Entwicklungsgeschichte der Bursa omentalis und aehnlicher Rezessbildungen bei den Wirbeltieren. Wiesbaden: Bergmann. [Google Scholar]
- Broman, I. (1905) Über die Entwickelung der Mesenterien der Leberligamente und der Leberform bei den Lungenfischen. Jena, Germany: Fischer. [Google Scholar]
- Broman, I. (1938) Warum wird die Entwicklung der Bursa omentalis in Lehrbüchern fortwährend unrichtig beschrieben? Anatomischer Anzeiger, 86, 195–202. [Google Scholar]
- Brummer, G. (1982) The development of the omental bursa. Acta Anatomica, 113, 281–295. [PubMed] [Google Scholar]
- Campione, M. , Steinbeisser, H. , Schweickert, A. , Deissler, K. , van Bebber, F. , Lowe, L.A. et al. (1999) The homeobox gene Pitx2: mediator of asymmetric left‐right signaling in vertebrate heart and gut looping. Development, 126, 1225–1234. [DOI] [PubMed] [Google Scholar]
- Davis, A. , Amin, N.M. , Johnson, C. , Bagley, K. , Ghashghaei, H.T. & Nascone‐Yoder, N. (2017) Stomach curvature is generated by left‐right asymmetric gut morphogenesis. Development, 144, 1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, N.M. , Kurpios, N.A. , Sun, X. , Gros, J. , Martin, J.F. & Tabin, C.J. (2008) The chirality of gut rotation derives from left‐right asymmetric changes in the architecture of the dorsal mesentery. Developmental Cell, 15, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bakker, B.S. , de Jong, K.H. , Hagoort, J. , de Bree, K. , Besselink, C.T. , de Kanter, F.E. et al. (2016) An interactive three‐dimensional digital atlas and quantitative database of human development. Science, 354(6315), 1019–1027. [DOI] [PubMed] [Google Scholar]
- Gasser, R.F. , Cork, R.J. , Noe, A. , Lockett, E. , Wilson, E. (2017) The Virtual Human Embryo—The endownment for human development, inc.: Computer Imaging Laboratory. New Orleans, LA: Louisiana State University Health Sciences Center. [Google Scholar]
- Hamilton, W.J. & Mossman, H.W. (1972) Hamilton, Boyd and Mossman's Human embryology. Prenatal development of form and function, 4th edition. London, UK: Macmillan Press. [Google Scholar]
- Harrison, R.G. (1978) Clinical embryology. London: Academic Press. [Google Scholar]
- Heckl, E. (1979) Development of the omental bursa of the sheep and its significance for the topography of the abdominal‐cavity. Zentralblatt für Veterinärmedizin Reihe C, 8, 10–39. [DOI] [PubMed] [Google Scholar]
- His, W. (1880) Anatomie menschlicher Embryonen. Leipzig, Germany: Vogel. [Google Scholar]
- Hochstetter, F. (1888) Über das Gekröse der hinteren Hohlvene. Anatomischer Anzeiger, 3, 965–974. [Google Scholar]
- Kanagasuntheram, R. (1957) Development of the human lesser sac. Journal of Anatomy, 91, 188–206. [PMC free article] [PubMed] [Google Scholar]
- Keibel, F. & Elze, C. (1908) Normentafel zur Entwicklungsgeschichte des Menschen. Jena, Germany: Gustav Fischer. [Google Scholar]
- Keibel, F. & Mall, F.P. (1910) Manual of human embryology. Philadelphia & London: J. B: Lippincott Company. [Google Scholar]
- Kessler, H. & Smulewicz, J.J. (1973) Microgastria associated with agenesis of the spleen. Radiology, 107, 393–396. [DOI] [PubMed] [Google Scholar]
- Kurpios, N.A. , Ibanes, M. , Davis, N.M. , Lui, W. , Katz, T. , Martin, J.F. et al. (2008) The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proceedings of the National Academy of Sciences of the United States of America, 105, 8499–8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman, J. (1975) Medical embryology human development, normal and abnormal, 3rd edition. Baltimore, MD: Williams & Wilkins. [Google Scholar]
- Lucas, J.S. , Davis, S.D. , Omran, H. & Shoemark, A. (2020) Primary ciliary dyskinesia in the genomics age. The Lancet. Respiratory Medicine, 8, 202–216. [DOI] [PubMed] [Google Scholar]
- McGaw, W.H. (1924) A stage in the development of the serous cavities. Anatomical Record, 28(2), 105–129. [Google Scholar]
- Mishalany, H. , Mahnovski, V. & Woolley, M. (1982) Congenital asplenia and anomalies of the gastrointestinal tract. Surgery, 91, 38–41. [PubMed] [Google Scholar]
- Moore, K.A. , Polte, T. , Huang, S. , Shi, B. , Alsberg, E. , Sunday, M.E. et al. (2005) Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Developmental Dynamics, 232, 268–281. [DOI] [PubMed] [Google Scholar]
- Müller, J. (1830) Uber den Ursprung der Netze und ihr Verhältnis zum Peritonealsacke beim Menschen, aus anat. Untersuchungen an Embryonen. Meckels Arch. Anat. Physiol, 395–411. [Google Scholar]
- Nakamura, T. , Yamada, S. , Funatomi, T. , Takakuwa, T. , Shinohara, H. & Sakai, Y. (2020) Three‐dimensional morphogenesis of the omental bursa from four recesses in staged human embryos. Journal of Anatomy, 237, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly, R. & Müller, F. (1987) Developmental stages in human embryos. Washington: Carnegie Institution of Washington. [Google Scholar]
- Peeters, H. & Devriendt, K. (2006) Human laterality disorders. European Journal of Medical Genetics, 49, 349–362. [DOI] [PubMed] [Google Scholar]
- Pernkopf, E. (1922) Die Entwicklung der Form des Magendarmkanales beim Menschen. Zeitschrift für Anatomie und Entwicklungsgeschichte, 64, 96–275. [Google Scholar]
- Rulle, A. , Tsikolia, N. , de Bakker, B. , Drummer, C. , Behr, R. & Viebahn, C. (2018) On the enigma of the human neurenteric canal. Cells Tissues Organs, 205, 256–278. [DOI] [PubMed] [Google Scholar]
- Schindelin, J. , Arganda‐Carreras, I. , Frise, E. , Kaynig, V. , Longair, M. , Pietzsch, T. et al. (2012) Fiji: an open‐source platform for biological‐image analysis. Nature Methods, 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring, S. & Standring, S. (2016) Gray's anatomy the anatomical basis of clinical practice (41nd ed.). Elsevier. [Google Scholar]
- Starck, D. (1975). Embryologie. Ein Lehrbuch auf allgemein biologischer Grundlage. 3. Auflage. Stuttgart, Germany: Georg Thieme Verlag. [Google Scholar]
- Swaen, A. (1896) Recherches sur le développement du foie du tube digestif de l'arrière‐cavité du péritoine et du mésentère. J. Anat. Physiol., Paris, 32, 1–84. [Google Scholar]
- Swaen, A. (1897) Recherches sur le développement du foie, du tube digestif, de l'arrière‐cavité du péritoine et du mésentère II. J. Anat. Physiol., Paris, 33, 222–258. [Google Scholar]
- Welsh, I.C. , Thomsen, M. , Gludish, D.W. , Alfonso‐Parra, C. , Bai, Y. , Martin, J.F. et al. (2013) Integration of left‐right Pitx2 transcription and Wnt signaling drives asymmetric gut morphogenesis via Daam2. Developmental Cell, 26, 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, S. & Schoenwolf, G. (2000) Islet‐1 marks the early heart rudiments and is asymmetrically expressed during early rotation of the foregut in the chick embryo. The Anatomical Record, 260, 204–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


