Highlights
-
•
Collision tumour is the concrescence of two neighbouring independent neoplasm occurring in the same site.
-
•
Collision tumours are rare and are reported in various organs.
-
•
Collision tumours in breast are reported in various combinations.
-
•
The treatment of these tumours is not tailored and prognosis depends on the histologic type and pathologic stage of the most aggressive component.
Keywords: Breast, Cancer, Collision, Squamous cell cancer, Invasice ductal cancer, Case report
Abstract
Introduction
Breast cancer is the most common diagnosed cancer among women worldwide. Invasive ductal carcinoma (IDC) is the most common type, on the other hand, squamous cell carcinoma of the skin (SCC) overlying the breast is a rare tumor. The co-presence of two tumor types in one organ is even a rarer entity, termed as collision tumor. Only 3 known cases of collision tumor with breast invasive ductal and skin squamous carcinoma were reported in the literature.
Case presentation
An otherwise medically free 91-year-old, postmenopausal, female presented with left breast fungating mass for four months. Pre-operative core tissue biopsy and incisional skin biopsy revealed two distinct tumor subtypes of invasive ductal carcinoma, positive for progesterone, estrogen receptors and negative for human epidermal growth factor receptor 2, as well as skin squamous cell carcinoma, and axillary lymph node metastasis. Patient underwent left breast modified radical mastectomy and split skin grafting for wound closure. The final histopathology was consistent with grade 2 IDC. The nipple and areola complex were involved by moderately differentiated squamous cell carcinoma. Currently patient on adjuvant hormonal treatment. Follow up showed no local recurrence or distal metastasis.
Conclusion
Collision tumors of the breast with IDC and SCC of the overlying skin is very rare. The surgeon has to be aware of of such entity as the proper peri-operative management should be tailored to target the most aggressive histologic subtype.
1. Introduction
Breast cancer is the most common diagnosed cancer among women worldwide. The most common type of invasive breast cancer is infiltrating ductal carcinoma (IDC), accounting for 70–80 % of all invasive breast cancers. Squamous cell carcinoma (SCC) of the skin overlying the breast is very rare tumor that is diagnosed when more than 90 % of the malignant cells are of the squamous type. Collision tumor is the concrescence of two neighboring independent neoplasm occurring in the same site. Although Collision tumors are rare, they were reported in various organs. These tumors in the breast were reported in various combinations. The treatment of these tumors is not tailored and prognosis depends on the histologic type and pathologic stage of the most aggressive component. In this paper, we present a case of a 91 year old lady who had a 4 month-history of fumigating left breast mass. The histopathological analysis of the biopsy, as well as the post operative tissue diagnosis, were both consistent with invasive ductal carcinoma of the breast and squamous cell carcinoma of the overlying skin, a collision of two tumors that have been only reported in 3 patients in the English literature as far as we know. This case was reported in line with SCARE criteria [1]
2. Case presentation
Our patient is an otherwise medically free 91-year-old postmenopausal female who is known to have hypertension and non-insulin dependent diabetes mellitus. She presented to our hospital in November 2018 with a four month-history of left breast ulcerated and fungating mass, which has gradually progressed in size. The mass was associated with spontaneous bloody left nipple discharge. The patient has no family history of breast or ovarian cancer, and never used hormonal replacement therapy.
Physical examination of the left breast showed ulcerated fungating, measuring 12 × 7 cm in size, with involvement of the nipple-areola complex. The overlying skin had necrosis. The mass was not attached to the chest wall or the underlying muscle (Fig. 1). The right breast and both axillae were unremarkable, with no palpable masses in the supraclavicular fossae. The provisional clinical diagnosis was locally advance left breast cancer.
Fig. 1.
Left breast fungating ulcerating mass 12 × 7 include nipple areolar complex not attached to chest wall, no axillary lymphadenopathy.
An ultrasound of the left breast revealed 3.6 × 3 × 3.5 cm heterogeneous lesion with increased peripheral vascularity in the retroareolar region reaching the skin that is in keep with ulcerative mass (Fig. 2), with suspicious left axillary lymphadenopathy. Mammogram of the left breast showed 6.6 × 5.5 cm irregular, dense mass in the left retroareolar region that is associated with microcalcifications (Fig. 3). Computed tomography scan of the chest, abdomen and pelvis with contrast revealed left retroareolar mass with suspicious left axillary lymph nodes and no evidence of metastasis in the lungs and liver (Fig. 4).
Fig. 2.
Ultrasound of the left breast shows large heterogeneous lesion with increased peripheral vascularity at the retroareolar region reaching skin keeping with ulcerative mass; measures 3.6 × 3 × 3.5 cm. Associated with skin thickening.
Fig. 3.
Mammogram shows left retroareolar region 6.6 × 5.5 cm irregular, dense mass associated with faintly seen microcalcifications.
Fig. 4.
Computed tomography of chest, abdomen and pelvis revealed Left retro-areolar mass invading the skin with suspicious left axillary lymph nodes.
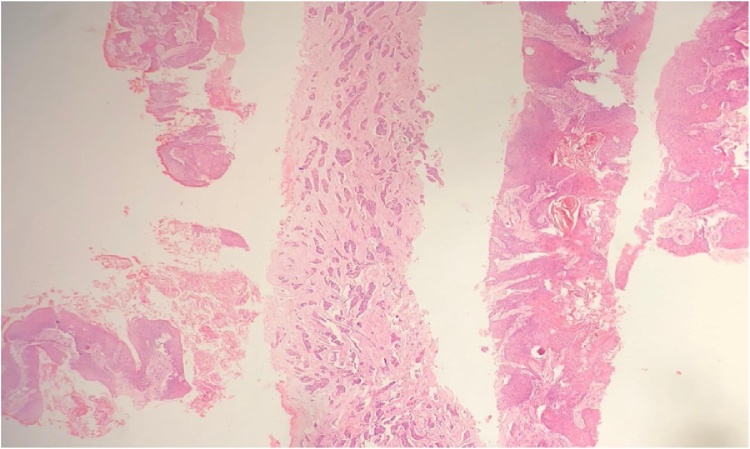
Tru cut biopsy of left breast mass revealed grade 2 invasive ductal carcinoma with irregular islands of well differentiated squamous cell carcinoma (Fig. 5). Incisional biopsy involving the skin showed well differentiated invasive squamous cell carcinoma, keratinizing type; stained positive for CK 5/6 (Fig. 6, Fig. 7). Immunohistochemistry profile of the IDC was positive for estrogen receptor (ER), progesterone receptor (PR), and negative for human epidermal growth factor receptor-2 (HER2), E-Cadherin was positive, and Ki67: 6% (Fig. 8, Fig. 9)
Fig. 5.
showing grade 2 invasive ductal carcinoma, and irregular islands of invasive well differentiated squamous cell carcinoma in different cores (H&E).
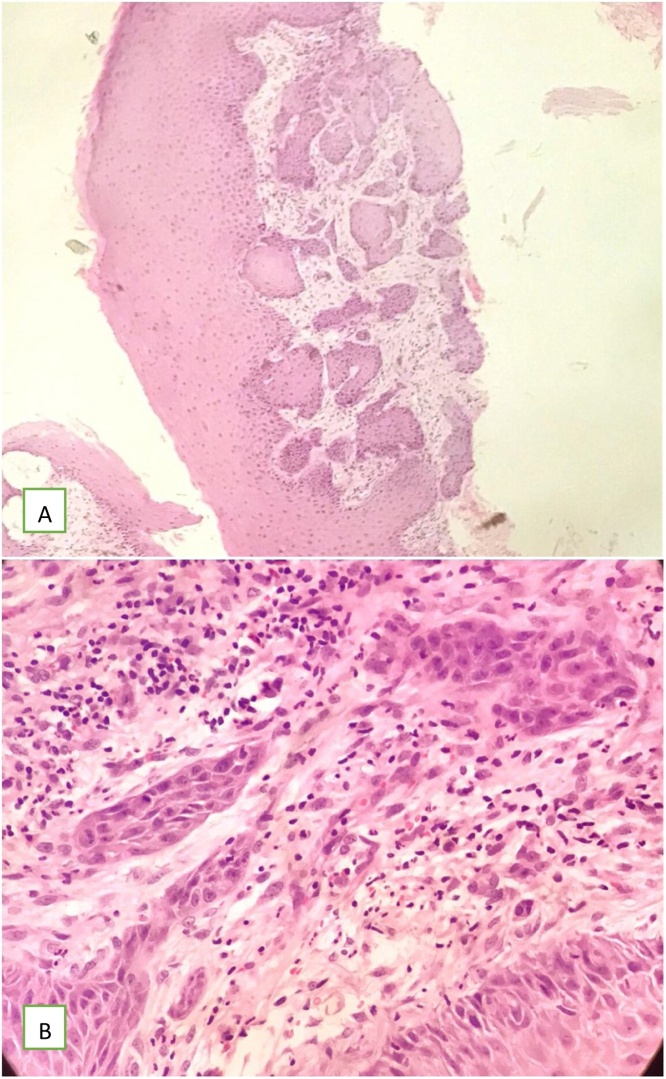
Fig. 6.
Core showing nests of squamous cell carcinoma infiltrating deeply in the dermis. (a) original magnification 10X (b) Higher magnification of squamous cell carcinoma component infiltrating the dermis with surrounding desmoplastic reaction. (H&E, original magnification 40X).
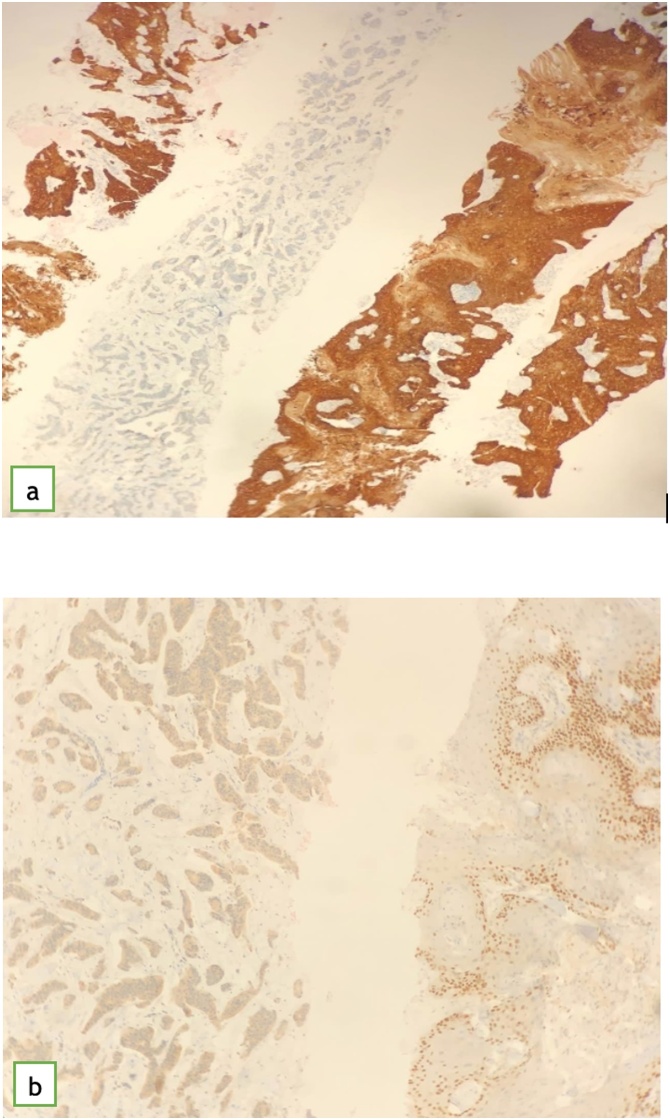
Fig. 7.
showing (a) CK5/6 featuring positive strong diffuse staining in squamous cell carcinoma and completely negative staining in invasive ductal carcinoma. (b) showing p63 which highlights the basal layer of squamous cell carcinoma and it is negative in the invasive ductal carcinoma.
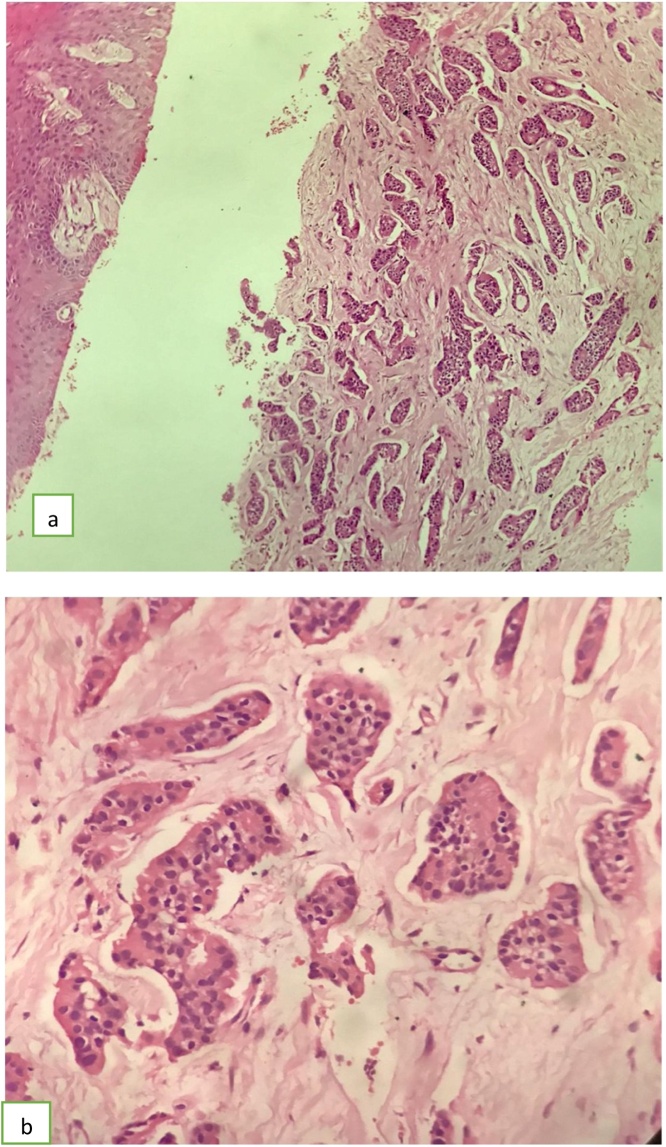
Fig. 8.
Core showing infiltrating ductal carcinoma. (a) H&E, original magnification 20X, (b) Higher magnification 40X.
Fig. 9.
showing (a) ER stain: strong diffuse positivity in the invasive ductal carcinoma. (b) PR stain: strong diffuse positivity in invasive ductal carcinoma. (c) HER2: 2+ Negative result.
Our diagnosis was left breast cT4bN1M0 with concomitant squamous cell carcinoma of the skin overlying the left breast. The patient was prepared and underwent left breast modified radical mastectomy and split skin grafting from left thigh on December 17, 2018, by breast surgeon and plastic surgeon.
The final histopathology showed left breast invasive ductal carcinoma grade 2, with tumor size of 4.6 × 4.2 × 3.9 cm, with evidence of lymphovascular invasion, nipple and areola complex are involved by moderately differentiated squamous cell carcinoma, all surgical margins are negative for malignancy, and no in situ component. The axillary lymph nodes revealed 11 out of 25 positive for metastasis (ductal), and immunohistochemistry was ER positive, PR positive, HER2 negative. The pathological stage was pT2N2a. Post-operative course: Vacuum Assisted Closure (VAC) dressing was applied for few days, after that, she was discharged with good condition with viable skin graft (Fig. 5). She did not receive adjuvant chemotherapy because she was unfit. She was started on letrozole 2.5 mg orally once daily, and calcium with vitamin D supplements. She received post-operative radiotherapy on the left chest wall and regional lymph nodes (50 GY/25 fraction). The patient has been following up for 18 months so far, and her disease still under control with no evidence of local or systemic recurrence.
3. Discussion
Globally, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in women. In the United States, breast cancer is the most commonly diagnosed cancer and the second most common cause of cancer death in women [2]. The most common type of invasive breast cancer is infiltrating ductal carcinoma, accounting for 70–80 percent of invasive breast lesions. It is also termed infiltrating carcinoma of no special type or infiltrating carcinoma not otherwise specified (NOS).
Squamous cell carcinoma (SCC) of the skin overlying the breast is an uncommon tumor that is diagnosed when more than 90 % of the malignant cells are of the squamous type. It has low incidence in breast, constituting less than 0.1 % of all breast carcinomas [3]. Clinical and radiologic appearances are not specific, and tumors are usually hormone receptor negative [4]. The prognosis for this type of breast cancer is still a subject of controversy as some reports suggest that it is aggressive, with an outcome comparable to that of poorly differentiated breast adenocarcinoma [5,6].
Metaplastic SCC refers to carcinoma that shows dominant areas of non-glandular squamous differentiation; other components could include ductal cells, spindle cells, chondrocytes, osteocytes, and striated muscle cells [7]. The broad range of microscopic appearances of breast metaplastic SCCs has resulted in significant diagnostic challenges. Clinical and radiological signs of metaplastic SCC are also non-specific [8].
Collision tumors are clinical entities in which there are two histologically distinct tumor subtypes present in the same organ. The presence of carcinoma and other tumors in same breast as a finding of composite tumor is extremely rare, and there have been few reports of collision tumor consisting of invasive ductal carcinoma admixed with breast lymphoma, chronic lymphocytic leukemia, phyllodes tumor and lactating adenoma.
Collision tumor with infiltrating ductal carcinoma and squamous cell carcinoma of the breast is even rarer and to the best of our knowledge, only 3 similar cases have been reported in the English literature [9,10].
Histopathologic sections from the left breast mass of our patient show solid infiltrative nests and glands of pleomorphic atypical cells with occasional mitotic figures, consistent with invasive ductal carcinoma. The immunohistochemical profile shows positive staining for E-Cadherin (confirming ductal origin) as well as strong nuclear staining of Estrogen and Progesterone receptors (ER and PR) in more than 98 % of tumor cells. HER2 showed equivocal (2+) result. Within the same container another core showed infiltrating nests and islands of well-differentiated keratinizing squamous cell carcinoma. Additionally, an incisional biopsy received from the left breast skin lesion showed focally dysplastic squamous epithelial skin lining with underlying invasive well-differentiated keratinizing squamous cell carcinoma, with deep infiltration into the dermis, surrounded by desmoplastic stromal reaction.
There are multiple factors in this case that favor the possibility of being two different (collision/composite) tumors rather than a metaplastic invasive mammary carcinoma with squamous differentiation. The first reason is that the invasive ductal carcinoma and the irregular islands of invasive well differentiated squamous cell carcinoma are seen separately in the cores with no evident connection between the two components (Fig. 5). Immunohistochemical stains for CK5/6 and P63 (which are considered myoepithelial markers) showed strong diffuse staining for CK5/6 in the squamous cell carcinoma which also showed focal basal staining for P63. While the invasive ductal carcinoma component showed completely negative result (Fig. 7). CD34 stain was not preformed because no spindle cell component seen. The second reason is that metaplastic breast carcinoma usually presents as a high grade tumor with triple negative immunohistochemical profile. This is not the case in our patient, since this tumor is of low grade and strongly positive for hormonal receptors (ER and PR). The third reason is the presence of dysplasia in the overlying skin which suggests that the squamous component is primary synchronous skin squamous cell carcinoma and not a metaplastic breast carcinoma with squamous differentiation [9,11].
The treatment of the collision tumors of breast is lacking guidelines and tailored treatment. The prognosis depends on the histopathologic subtype and pathologic stage of the more aggressive tumor subtype. Hence the management strategy should be planned to appropriately tackle the most aggressive subtype. Lymph node metastasis in SCC occurs in approximately 54 % of cases, give rise to more distant metastasis and has bad prognosis [9]. In our case, the patient had axillary lymph node metastasis of IDC origin and not SCC indicating the indolent behavior of SCC component compared to IDC.
4. Conclusion
In conclusion, one has to be aware that collision tumors occur in breast and identifying the histologic subtype with the pathologic staging are both important to plan the treatment and predict the prognosis. The collision tumors of breast with IDC and SCC of skin over the breast is very rare and as far as to our knowledge goes, there are 4 reported cases of its kind in the English literature.
Declaration of Competing Interest
We declare no conflict of interest.
Funding
No funding was required.
Ethical approval
Case reports do not require ethical approval as per our institute protocol.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Study conception and design: Alawami, Al-Faraj, El Sayed
Acquisition of data: Alawami
Analysis and interpretation of data: Alawami, Al Duhileb, Al-Faraj
Drafting of manuscript: Alawami, AlOmran, Al Duhileb
Critical revision: Alawami, AlOmran, Al-Faraj, El Sayed, Al Duhileb.
Registration of research studies
Not applicable
Guarantor
Alawami, Al Duhileb
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 Statement: Updating Consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Rosen P.R. Lippincott Williams & Wilkins, 2001; Philadelphia, PA: 1997. Rosen’s Breast Pathology; pp. 455–461. [Google Scholar]
- 4.Tayeb K., Saadi I., Kharmash M. Primary squamous cell carcinoma of the breast: report of three cases [French] Cancer Radiother. 2002;6:366–368. doi: 10.1016/s1278-3218(02)00258-5. [DOI] [PubMed] [Google Scholar]
- 5.Behranwala K.A., Nasiri N., Abdullah N. Squamous cell carcinoma of the breast:clinicopathologic implications and outcome. Eur. J. Surg. Oncol. 2003;29(386) doi: 10.1053/ejso.2002.1422. [DOI] [PubMed] [Google Scholar]
- 6.Cornog J.L., Mobini J., Steiger E. Squamous carcinoma of the breast. Am. J. Clin. Pathol. 1971;55 doi: 10.1093/ajcp/55.4.410. [DOI] [PubMed] [Google Scholar]
- 7.Elston C.W., Ellis I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002;41:154. [PubMed] [Google Scholar]
- 8.Lakhani S.R., Ellise I.O., Schnitt S.J., editors. WHO Classification of Tumors of the Breast. 4th edition. IARC; Lyon, France: 2012. pp. 85–87. [Google Scholar] [Google Scholar]
- 9.Sentani K., Tashiro T., Oue N., Yasuiw Synchronous Squamous cell carcinoma of the breast and Invasive lobular carcinoma. APMIS. 2007;115:1422–1425. doi: 10.1111/j.1600-0463.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 10.Mallik D. Invasive ductal carcinoma of breast and squamous cell carcinoma of anterior chest wall - A rare collision. Clin. Case Rep. 2020;8(9):1618–1621. doi: 10.1002/ccr3.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geetha R., Kalyani R., Srinivas M.V., Shakthidasan C. A rare collision tumour of infiltrating ductal carcinoma and squamous cell carcinoma of skin overlying breast: a case report. J. Clin. Diagn. Res. 2015;9(1):XD06–XD08. doi: 10.7860/JCDR/2015/10437.5464. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]