We provide cumulative incidence and prevalence estimates for 8 human herpesviruses in a US pediatric cohort and examine risk factors. Higher salivary bacteriome diversity was associated with longer survival to first detection of human herpesvirus-6 and cytomegalovirus.
Keywords: CMV, early childhood, HHV6, human herpesviruses, prospective cohort, salivary bacteriome
Abstract
Background
The bacteriome is associated with susceptibility to some eukaryotic viruses, but no study has examined associations between the salivary bacteriome and human herpesviruses (HHVs). We provide new prevalence and incidence estimates for salivary herpesviruses detection and estimate associations with bacteriome diversity in young children.
Methods
Salivary samples collected at ages ~2, 8, 12, and 24 months from 153 children participating in the Center for Oral Health Research in Appalachia cohort 2 (COHRA2) were screened for HHVs using the Fast-Track Neuro9 multiplex PCR assay, and for the bacteriome using 16S rRNA amplicon sequencing. We used Cox proportional hazard models to test for associations between the salivary bacteriome and hazards of cytomegalovirus (CMV) and human herpesvirus-6 (HHV6).
Results
CMV, HHV6, and Epstein-Barr virus (EBV) were detected at all visits. Human herpesvirus-7 (HHV7) was first detected at the 8-month visit and herpes simplex virus 1 (HSV1) was only detected at the 12-month visit. Varicella-zoster virus, herpes simplex virus 2, and human herpesvirus-8 were never detected. HHV6 (24-month cumulative incidence: 73.8%) and CMV (24-month cumulative incidence: 32.3%) were detected most frequently. Increasing salivary bacteriome diversity was associated with longer survival to first detection of CMV (hazard ratio [95% CI]: 0.24 [0.12, 0.49]) and HHV6 (hazard ratio [95% CI]: 0.24 [0.13, 0.44]).
Conclusion
CMV, HHV6, EBV, HHV7, and HSV1 were detected in the saliva during the first 2 years of life. Time to first detection of CMV and HHV6 was associated with salivary bacteriome diversity, suggesting a possible interaction between HHVs and the salivary bacteriome.
Humans are the primary host for 8 herpesviruses: herpes simplex virus 1 (HSV1), herpes simplex virus 2 (HSV2), varicella-zoster virus (VZV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), human herpesvirus-6 (HHV6), human herpesvirus-7 (HHV7), and Kaposi’s sarcoma herpesvirus (HHV8). Human herpesviruses (HHVs) can persist indefinitely in their hosts, undergoing cycles of reactivation and latency. Thus, understanding patterns of primary herpesvirus acquisition in healthy children is important for stemming transmission to vulnerable populations, such as immunocompromised [1] and pregnant individuals [2], for whom infection and reactivation can produce severe morbidity and mortality.
In the United States, primary infection with HSV1 [3], CMV [2], and EBV [4] occurs in childhood or adolescence while HHV6 [5] is often acquired before age 2 and HHV7 [1] between the ages of 2 and 5 [6]. Childhood acquisition of these HHVs is driven by oral transmission. Yet few recent US studies have prospectively measured HHV detection in children’s saliva. In a 2005 prospective cohort of 277 US children, the cumulative incidence of HHV6 acquisition was 40% by 12 months and 77% by 24 months of age [7]. In a 1988 study of urine and saliva samples from 75 children <3 years in a single US daycare, the 26-month cumulative incidence of CMV was 51% [8]. Recent prospective studies of HHVs primary acquisition and salivary detection in healthy, young, US children are lacking. Further, we found no prospective estimates of primary EBV, HSV1, or HHV7 detection in the saliva of healthy US children under 2 years of age since 2005.
Pathogen resistance, viral tolerance, stimulation of adaptive immune response, and calibration of the activation threshold of innate immune response by host-associated microbial communities may moderate acquisition, duration, and transmission of viral infections [9–11], including influenza [12, 13] and HIV/AIDS [14]. Bacteria can provide viral binding sites and increase virion stability by forming viral-bacterial complexes, which also may enhance adherence to host cells [15–17]. Despite the importance of herpesvirus and the role of children in maintaining the herpesvirus transmission system, few epidemiological studies have examined whether commensal microbiota influence herpesvirus acquisition and detection in healthy children. We found only one study examining the association between the gut microbiome and HHVs acquisition in healthy children: this Swedish cohort study of 281 children found that Staphylococcus aureus gut colonization reduced the time to CMV seropositivity [18]. Other studies have focused on periodontal bacteria and herpesvirus shedding in adults or immunocompromised children [19–22]. We found no studies investigating the relationship of the oral microbiome with HHV detection in the saliva of healthy children.
Our study begins to fill this gap. Using longitudinally collected samples from 153 children we (1) estimate prevalence and cumulative incidence rates of salivary detection of 8 HHVs and (2) examine associations between salivary herpesvirus detection and the early-life salivary bacteriome. Cumulative incidences of HHV6 and CMV were high by age 2, consistent with other prospective studies. Further, more diverse salivary bacteriomes were associated with longer time to first detection of salivary CMV and HHV6.
MATERIALS AND METHODS
Study Cohort
The Center for Oral Health Research in Appalachia cohort 2 (COHRA2) [23] recruited 1172 pregnant white women from Pennsylvania and West Virginia and followed their children prospectively. At in-person visits, mothers and infants underwent oral examinations and saliva sample collection by trained, calibrated dental professionals. The protocols for the clinical examination and regular calibrations are described elsewhere [23]. Sociodemographic, medical history, and behavioral information was collected via in-person or telephone interviews at ~6-month intervals. The study protocol was approved by the Institutional Review Boards at the University of Pittsburgh and the University of West Virginia. Mothers provided informed consent for themselves and their children.
This analysis was limited to Pennsylvanian children born between June 2014 and May 2016 who had completed at least 3 research visits by June 2017 and whose saliva samples were collected exclusively using OMNIgene·Discover kits (OM-505, DNA Genotek) (Supplementary Text). These inclusion criteria resulted in 153 children with samples from the 2-month, first tooth, 12-month, and 2-year visits (Supplementary Figure 1). For most children, the first tooth visit occurred between 6 and 9 months of age. However, 4 children had first tooth visits which occurred after their 12-month visit (average of 2.5 months after their 12-month visit). Therefore, we grouped data for these 4 by when the visit occurred rather than by the visit number according to protocol; hereafter the first tooth visit is referred to instead as the 8-month visit. Of the 153 participants included, 65 had data from the 24-month visit.
Virus Detection
We detected HHVs using the Fast-Track Neuro9 multiplex PCR assay (Fast-Track Diagnostics [FTD], Sliema, Malta). Viral nucleic acids were extracted from 200 µL thawed saliva using the Qiagen MinElute Virus Kit. We screened 20 µL of this elute for 16S DNA to ensure no bacterial contamination. Subsequently, 40 µL of elute was used in the Fast-Track Diagnostics Neuro9 kit. A cutoff of 35 PCR cycles was used to determine positive/negative viral samples.
Salivary Microbiome
Bacterial DNA was extracted from a separate aliquot of saliva, and library preparation and sequencing of the 16S rRNA amplicon was performed by the Michigan Microbial Systems Molecular Biology Laboratory using previously validated protocols [24]. Briefly, the V4 variable region was amplified from extracted DNA using barcoded dual-index primers and sequenced using the Illumina MiSeq [24]. Of 516 samples, 94 were submitted in duplicate and each plate was submitted with a positive mock community control and 2 negative controls (Supplementary Text). Raw sequence files are publicly available at PRJNA650552; phenotype data for the COHRA2 study are available on dbGaP upon application.
Bioinformatics
Reads were processed to amplicon sequence variants (ASVs) using DADA2 (version 1.14.1) and the Human Oral Microbiome Database (HOMD) version 15.2 (Supplementary Text). We excluded samples with <750 reads (n = 7 samples lost, 2 with duplicate samples available). We compared the Bray-Curtis dissimilarity index between samples submitted in duplicate to identify problematic replicates and visually compared abundance plots (Supplementary Text). Reads from non-problematic replicates were summed together. Salivary bacteriome alpha diversity was measured using the Shannon index, which takes into account the presence and abundance of bacterial taxa.
Covariates
We included as covariates potential confounders identified from the literature. Time invariant covariates included maternal education at prenatal visit, maternal age at prenatal visit, and route of delivery. Time variant covariates included breastfeeding status, maternal employment, household income, and maternal gingivitis status (categorized as no gingivitis, localized gingivitis, and generalized gingivitis). Gingival disease and associated bacteria have previously been reported to be associated with herpesvirus detection in adult saliva [20–22].
Statistical Analysis
We used Cox proportional hazard models to test for associations between the time-varying salivary microbiome and time to salivary herpesvirus detection while controlling for potential confounders. We tested for differences in the abundance of taxa by detection of the most common HHVs using ALDEx2 [25] separately at the genus and species level, independently at each visit (Supplementary Text).
RESULTS
Study Population
The mothers of the 153 participating children were white and between 20 and 43 years of age (mean = 30; SD = 4.6 years). Most mothers had more than a high school education (Table 1); 73.9% held a bachelor’s or more advanced degree. Most women had vaginal deliveries (79.7%) (Table 1). Breastfeeding decreased and maternal reports of child antibiotic use increased as children aged (Table 2).
Table 1.
Maternal Characteristics at Prenatal Visit and Birth Delivery Route for 153 Mother-Infant Pairs From the Pennsylvania Site of the Center for Oral Health Research in Appalachia 2 (COHRA2) Study
| N = 153 | |
|---|---|
| Variable | Mean (SD) or N (%) |
| Maternal age at prenatal visit | 30.3 (4.64) |
| Child sex | |
| Female | 67 (43.8%) |
| Male | 86 (56.2%) |
| Maternal prenatal education | |
| High school education or less | 20 (13.1%) |
| More than high school | 133 (86.9%) |
| Birth delivery route | |
| Missing | 1 (0.65%) |
| Don’t know | 1 (0.65%) |
| Vaginal | 122 (79.7%) |
| C-section | 29 (19.0%) |
| Employment status at prenatal visit | |
| Full-time employment | 74 (48.4%) |
| Other | 45 (29.4%) |
| Part-time employment | 34 (22.2%) |
Table 2.
Viral, Microbial, and Behavioral Characteristics by Time Over the First 2 Years of Life Among 153 Mother-Infant Pairs From the Pennsylvania Site of the Center for Oral Health Research in Appalachia 2 (COHRA2) Study
| 2-mo Visit | 8-mo Visit | 12-mo Visit | 24-mo Visit | |
|---|---|---|---|---|
| N = 153 | N = 151 | N = 147 | N = 65 | |
| Variable | N (%) | N (%) | N (%) | N (%) |
| Child age, mean (SD) in months | 2.00 (0.34) | 8.23 (1.48) | 11.7 (0.77) | 23.7 (0.78) |
| CMV: Detection | 4 (2.6%) | 26 (17.2%) | 29 (19.7%) | 11 (16.9%) |
| EBV: Detection | 6 (3.9%) | 3 (2.0%) | 6 (4.1%) | 7 (10.8%) |
| HHV6: Detection | 4 (2.6%) | 23 (15.2%) | 53 (36.1%) | 47 (72.3%) |
| HHV7: Detection | 0 (0.0%) | 4 (2.7%) | 9 (6.1%) | 16 (24.6%) |
| HSV1: Detection | 0 (0.0%) | 0 (0.00%) | 2 (1.4%) | 0 (0.0%) |
| Shannon index, mean (SD) | 2.02 (0.52) | 2.85 (0.38) | 3.18 (0.38) | 3.69 (0.32) |
| Currently breastfeedinga | ||||
| No | 43 (28.1%) | 68 (45.0%) | 89 (60.5%) | 50 (76.9%) |
| Yes | 110 (71.9%) | 83 (55.0%) | 58 (39.5%) | 15 (23.1%) |
| Household income (yearly) | ||||
| <$50 000 | 41 (26.8%) | 38 (25.2%) | 39 (26.5%) | 22 (33.8%) |
| $50 000-$99 999 | 51 (33.3%) | 52 (34.4%) | 48 (32.7%) | 16 (24.6%) |
| ≥$100 000 | 59 (38.6%) | 58 (38.4%) | 58 (39.5%) | 25 (38.5%) |
| Missing | 2 (1.31%) | 3 (1.99%) | 2 (1.36%) | 2 (3.08%) |
| Maternal employment status | ||||
| Otherb | 73 (47.7%) | 45 (29.8%) | 48 (32.7%) | 24 (36.9%) |
| Part-time employment | 30 (19.6%) | 39 (25.8%) | 34 (23.1%) | 17 (26.2%) |
| Full-time employment | 50 (32.7%) | 67 (44.4%) | 65 (44.2%) | 24 (36.9%) |
| Mother’s gingivitis status at visit | ||||
| None | 22 (14.4%) | 23 (15.2%) | 21 (14.3%) | 6 (9.2%) |
| Localized | 98 (64.1%) | 101 (66.9%) | 100 (68.0%) | 55 (84.6%) |
| Generalized | 33 (21.6%) | 27 (17.9%) | 26 (17.7%) | 4 (6.2%) |
| Maternal report of child antibiotics | ||||
| Antibiotics <30 d before visit | 4 (2.6%) | 8 (5.3%) | 2 (1.4%) | 0 (0.00%) |
| Antibiotics >30 d before visit | 2 (1.3%) | 24 (15.9%) | 30 (20.4%) | 23 (35.4%) |
| No antibiotics reported or no call data within 180 d of visit | 147 (96.1%) | 119 (78.8%) | 115 (78.2%) | 42 (64.6%) |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV6, human herpesvirus-6; HHV7, human herpesvirus-7; HSV1, herpes simplex virus 1.
aSubjects were marked as breastfeeding if the child’s reported age (in months and weeks) at breastfeeding cessation was at or before the visit date. Some individuals failed to report an age of child at breastfeeding cessation, for these individuals, the midpoint between the last phone call at which breastfeeding was reported and the first phone call without breastfeeding reported was used as the date of breastfeeding cessation.
bOther employment includes all women who answered that they were neither full- nor part-time employed, including those who were unemployed or were students.
Salivary Herpesvirus Detection
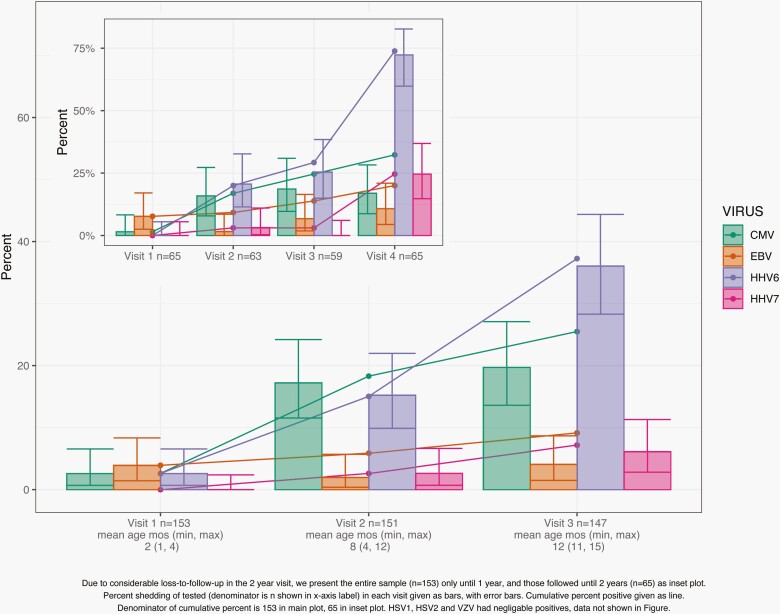
We detected viral DNA for CMV, EBV, HHV6, HHV7, and HSV1 but not for HSV2, VZV, or Kaposi’s sarcoma herpesvirus. HSV1 was detected in only 2 samples, both at the 12-month visit (Table 2). The 12-month cumulative incidence was 37.3% for HHV6 detection and 25.5% for CMV detection; 12-month cumulative incidence was lower for EBV (9.2%) and HHV7 (7.2%) (Figure 1). Among the 65 children with a 24-month sample, the 24-month cumulative incidence was 73.8% for HHV6 detection, 32.3% for CMV detection, 24.6% for HHV7 detection, and 20% for EBV detection. A majority of children with HHV6 or CMV detected at the 8-month visit also had viral DNA detected at the 12-month visit (HHV6: 83%; CMV: 62%) (Supplementary Figure 2). Over the course of follow-up, 33% of the 153 children had >1 HHV detected (Supplementary Tables 1 and 2).
Figure 1.
Cumulative incidence (lines) and prevalence (bars) estimates for the 4 most commonly detected herpesviruses in a sample of 153 children from the Pennsylvania site of the Center for Oral Health Research in Appalachia 2 study: HHV6, CMV, HHV7, and EBV. HSV1 was only detected in 2 samples from the 12-month time point, data not shown. No HSV2, VZV, or Kaposi’s sarcoma herpesvirus was detected. Inset graph shows cumulative incidence and prevalence data for the 65 individuals for whom 24-month salivary samples were available; otherwise, data are for all 153 individuals for whom salivary samples up to and including the 12-month visit were available. Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV6, human herpesvirus-6; HHV7, human herpesvirus-7; HSV1, herpes simplex virus 1; HSV2, herpes simplex virus 1; VZV, varicella-zoster virus.
Associations With Salivary Bacteriome Diversity
The salivary bacteriome increased in diversity as children aged, transitioning from Streptococcus- and Veillonella dominated at 2 months to a more evenly distributed community including oral commensals Neisseria, Fusobacterium, and Haemophilus (Figure 2). Breastfeeding was associated with lower Shannon index in the 2- and 8-month visits (Supplementary Figure 3). The salivary bacteriome of children with antibiotic use reported ≤30 days before a visit was less diverse than those with no antibiotic use, however, this was not true if antibiotic use was >30 days before the visit (Supplementary Figure 3). Antibiotic use was not associated with detection of any HHVs in bivariate analyses. Diversity was higher in children from lower-income households at the 2-month visit; this association was insignificant after stratifying by breastfeeding (Supplementary Figure 3).
Figure 2.
(A) Relative abundance plots of bacteriome salivary composition over time (facets correspond to visits). Each bar is a sample, and the colors correspond to taxonomic classifications displayed in the legend, colors are stacked from the top down in the order they appear in the legend. Highly abundant species are displayed in darker hues of the genus color. Genera with a mean relative abundance of 1% (ie, <0.01) were collapsed to an “Other” category—displayed in gray. (B) Violin plots with captive box plots showing the distribution of Shannon index from both rarefied and non-rarefied samples over time (facets correspond to visits).
Time to First Detection of HHV6 and CMV Is Positively Associated With Decreased Salivary Bacteriome Diversity
We used multivariable Cox proportional hazard models and a martingale counting process to analyze time to first positive salivary test for HHV6 and CMV only, as the viral prevalence was too small for the other detected viruses (Table 2). We excluded 2 individuals whose delivery method was unknown. Household income was strongly associated with maternal education (P < .001) and exhibited some missingness, so we included only maternal education in the main model. Because the number of children with recent antibiotic use was low (n = 14 child visits), antibiotic use was not included in the main models. Both of these variables were included in sensitivity analyses.
Only children who were negative for the virus at the baseline visit (2-month visit) were included in the time-to-acquisition analysis (n = 147 for HHV6 and n = 146 for CMV) (Supplementary Figure 1). Because only 65 saliva samples were available at the 2-year visit, data were censored after the 12-month visit. Children without 12-month visit data but with 8-month visit data were censored at the midpoint between their 8-month visit and 365 days from their 2-month visit. Increasing salivary bacteriome diversity, as measured by the Shannon Index, was associated with longer survival to first detection of CMV (HR [95% CI]: 0.24 [0.12, 0.49]) and HHV6 (HR [95% CI]: 0.24 [0.13, 0.44]). No other variables were significantly associated with time to first CMV detection although maternal gingivitis had a large, nonsignificant association with shorter survival to first CMV detection (localized vs none: HR [95% CI]: 1.72 [0.5, 5.94]; generalized vs none: HR [95% CI]: 1.65 [0.38, 7.29]). HHV6 detection occurred earlier among children whose mothers were employed part time compared to unemployed, and maternal education beyond high school was associated with longer time to first HHV6 detection (Table 3). In the sensitivity analyses, antibiotic use and household income were not associated with time to CMV or HHV6 detection and other associations remained similar (Supplementary Text; Supplementary Figure 4). As a sensitivity analysis, we limited our sample to the 65 children who attended a 24-month visit and censored at the 24-month visit. Bacteriome diversity remained associated with longer time to first CMV (HR [95% CI]: 0.27 [0.09, 0.76]) and HHV6 detection (HR [95% CI]: 0.25 [0.13, 0.47]) (Supplementary Figure 4). However, a global test of the Schoenfeld residuals suggested the proportional hazards assumption was violated in the HHV6 model.
Table 3.
Associations Between Time to First Detection of CMV (n = 146) and HHV6 (n = 147) and Salivary Microbiome Diversity in the First Year of Life Among Pennsylvanian Children Participating in the Center for Oral Health Research in Appalachia 2 (COHRA2) Study
| CMV | HHV6 | |||
|---|---|---|---|---|
| N = 146a | N = 147b | |||
| Variable | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
| Shannon index | 0.28 (0.15, 0.53)*** | 0.24 (0.12, 0.49)*** | 0.29 (0.17, 0.51)*** | 0.24 (0.13, 0.44)*** |
| Currently breastfeeding (ref = not currently) | 0.81 (0.40, 1.62) | 0.68 (0.3, 1.55) | 1.68 (0.96, 2.95)+ | 1.67 (0.88, 3.19) |
| Vaginal delivery (ref = C-section) | 0.69 (0.31, 1.53) | 0.67 (0.29, 1.56) | 1.87 (0.84, 4.15) | 2.00 (0.87, 4.58) |
| Baby sex male (ref = female) | 0.98 (0.49, 1.95) | 0.98 (0.46, 2.06) | 1.19 (0.68, 2.09) | 0.95 (0.52, 1.73) |
| Maternal employment (ref = other) | ||||
| Part time | 0.53 (0.19, 1.46) | 0.64 (0.22, 1.89) | 1.23 (0.63, 2.41) | 2.48 (1.15, 5.35)* |
| Full time | 0.67 (0.32, 1.43) | 0.67 (0.28, 1.64) | 0.5 (0.26, 0.98)* | 0.77 (0.37, 1.6) |
| Maternal education more than high school | 0.88 (0.34, 2.28) | 0.9 (0.29, 2.78) | 0.53 (0.26, 1.06)+ | 0.45 (0.20, 1.01)+ |
| Maternal gingivitis (ref = none) | ||||
| Localized | 1.65 (0.49, 5.54) | 1.72 (0.5, 5.94) | 0.67 (0.33, 1.35) | 0.57 (0.27, 1.18) |
| Generalized | 2.62 (0.71, 9.72) | 1.65 (0.38, 7.29) | 1.15 (0.5, 2.64) | 1.06 (0.36, 3.09) |
| Maternal age at prenatal visit | 0.94 (0.87, 1.02) | 0.92 (0.84, 1.01)+ | 0.99 (0.94, 1.06) | 1.02 (0.95, 1.09) |
Abbreviations: CMV, cytomegalovirus; HHV6, human herpesvirus-6, HR, hazard ratio.
aOf the 153 original mother infant pairs included in this study 2 mothers did not report a delivery method. An additional 5 children were positive for CMV at the first visit for which salivary bacteriome data was available, and so were not at risk for developing CMV.
bOf the 153 original mother infant pairs included in this study 2 mothers did not report a delivery method. An additional 4 children were positive for HHV6 at the first visit for which salivary bacteriome data was available, and so were not at risk for developing HHV6.
+P < .10, *P < .05, ***P < .001. P value from Wald test of coefficient in Cox-proportional hazard model.
Detection of CMV Predicts Longer Time to Higher Bacteriome Diversity
We predicted time to first salivary bacteriome sample with a Shannon index of ≥3 using positive HHV6 and CMV status as the main predictors, controlling for all the other variables included in the previous multivariable models and censoring children at the 12-month visit. Individuals with Shannon index ≥3 at the first available visit (n = 5) were excluded. CMV detection was associated with longer times to Shannon index ≥3 (HR [95% CI]: 0.36 [0.14, 0.95]). Breastfeeding status was also associated with longer time to Shannon index ≥3 (HR [95% CI]: 0.54 [0.35, 0.82]) and vaginal delivery was marginally associated with faster times to Shannon index ≥3 (HR [95% CI]: 1.58 [0.93, 2.66]). When only the 65 children with 24-month data were included, the direction of the associations with CMV and breastfeeding were consistent but nonsignificant and the global test of Schoenfeld residuals suggested violation of the proportional hazard assumption.
No Significant Associations Between Bacterial Taxa and Detection of HHV6 or CMV
No individual bacterial species or genera were significantly associated with HHV6 or CMV detection at any visit after Benjamini-Hochberg correction for multiple testing (Supplementary Text; Supplementary Tables 3 and 4).
HHV6 and CMV Shedding Was Not Associated With Reported Childhood Illness
Using data from phone calls that occurred within a 6-month window prior to in-person visits, we examined associations between HHV6 or CMV and responses to questions about whether the child had experienced a cold, diarrhea, or eczema since the previous interview, or reports that the child had a fever or rash in response to an open-ended question about other illnesses in the same time period. More children with HHV6 detected had a maternal report of fever or eczema in the 6-month window prior to the visit than those who did not, however, these differences were not statistically significant (Supplementary Figure 5).
DISCUSSION
Our analysis of 153 Appalachian children followed from age 2 months through 2 years increases our understanding of the frequency and correlates of salivary herpesvirus detection in early childhood. We provide cumulative incidence estimates for HHV6, CMV, HHV7, EBV, and HSV1 among White, Appalachian children ages 2-24 months and report a novel finding: increased diversity of the salivary bacteriome is associated with increased time to salivary detection of CMV and HHV6.
Our cumulative incidence and prevalence estimates of HHV6 and CMV DNA detection at 1 and 2 years of age are consistent with previous prospective [7, 8] and cross-sectional [26] reports. In our study, HHV7 detection occurred later and less frequently than HHV6 detection, with a prevalence of 25% by the 24-month visit. This is consistent with a previous cross-sectional report on HHV6 and HHV7 DNA detection prevalence in blood among those aged 7-12 months (HHV6 53%; HHV7 18%) vs 13-24 months (HHV6 54%; HHV7 26%) [27]. EBV detection in our sample was relatively rare but increased to 10% prevalence in the 24-month visit, consistent with evidence that EBV seroprevalence increases gradually with age, and has seroconversion peaks in later childhood (2-4 years) and adolescence (14-18 years) in developed countries [28–30]. HSV1, however, was detected in only 2 of our study children. This may reflect recent a recent transition in HSV1 epidemiology, with declining seropositivity and oral transmission in childhood [31, 32]. A key limitation of our incidence and prevalence estimates is the sampling regime for saliva samples. Since individuals could have shed undetected between visits, outcome misclassification is possible. Moreover, our ability to draw conclusions about the duration of herpesvirus shedding is limited. Additionally, saliva samples were not taken in response to symptom presentation, which limits inference on the specific timing of viral acquisition.
The novel association of higher diversity of the salivary bacteriome with longer times to first detection of CMV and HHV6 is consistent with previously reported associations between eukaryotic viruses and the microbiome (reviewed in Domínguez-Díaz et al) [9]. Bacterial communities and their emergent properties, such as diversity, have been hypothesized to impact innate and adaptive immunity, and HHVs also modulate immune responses, so host immune functioning could confound or mediate our observed associations [33, 34]. Further, in vitro experiments and observational studies in periodontal patients have found direct, biochemical interactions or associations between specific bacteria and HHV which might explain our observed associations [35–37].
Another limitation of our study is that we have no measure of out-of-home childcare. CMV shedding has been associated with out-of-home childcare [26], although HHV6 acquisition has not [7]. An association of the oral microbiome with out-of-home childcare is biologically plausible, although one study reported no association [38]. We used maternal employment status as a proxy for out-of-home childcare, but residual confounding likely remains. Additionally, some studies have found associations of socioeconomic status with incidence of HHVs [2, 39], as well as with salivary bacteriome composition [40]. While we controlled for maternal education, employment status, and household income, residual confounding by socioeconomic factors not captured by these variables is a possible explanation for the observed association. Studies assessing immune function, absolute abundance of specific bacteria, and these confounders will be required to sort out what mechanisms explain our observation.
We did not find any significant associations between maternal report of childhood illness and detection of CMV or HHV6 DNA in children’s saliva, unlike Zerr et al [7]. This likely reflects limitations in our assessment of childhood illnesses, which were not designed to assess herpesvirus symptoms, such as rash and fever. Childhood illness was based on maternal report of child’s experienced illnesses since the time of the last interview, so the time window of potential illness report was likely too broad to accurately capture symptoms of primary herpesvirus acquisition. Medical record data were not available for more careful ascertainment of childhood illness or inclusion of other pertinent clinical data, such as childhood vaccinations.
In addition to the aforementioned limitations, our sample was limited to White, Appalachian children. Bacteriome diversity and composition can vary by both ethnicity and geography [40], as can HHV incidences [39]. Thus, our results may not be generalizable to other populations. Despite these limitations, our study has some key strengths. We tested for several herpesvirus simultaneously in a population-based cohort, allowing us to provide new estimates of HHV6, HHV7, CMV, and EBV in this age group. The longitudinal design allowed us to estimate both prevalence and cumulative incidence. We provide results of a novel investigation into associations between the salivary bacteriome and detection of salivary CMV and HHV6 DNA. Further investigations into the relationships between oral microbiota and herpesvirus acquisition in healthy children are warranted.
Supplementary Material
Notes
Financial support. This work was supported by the National Institute of Dental and Craniofacial Research at the National Institutes of Health [R01DE014899, F31 DE029992 to F.B.] and the National Human Genome Research Institute at the National Institutes for Health [T32 HG00040 to F.B.].
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dewhurst S, Skrincosky D, van Loon N. Human herpesvirus 7. Expert Rev Mol Med 1997; 1(2):1–10. [DOI] [PubMed] [Google Scholar]
- 2.Cannon MJ. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol 2009; 46:S6–10. [DOI] [PubMed] [Google Scholar]
- 3.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet 2001; 357(9267):1513–8. [DOI] [PubMed] [Google Scholar]
- 4.Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein-Barr virus infection in U.S. children ages 6–19, 2003–2010. PLoS One 2013; 8(5):e64921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okuno T, Takahashi K, Balachandra K, et al. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol 1989; 27(4):651–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cermelli C, Fabio G, Montorsi M, Sabbatini AM, Portolani M. Prevalence of antibodies to human herpesviruses 6 and 7 in early infancy and age at primary infection. New Microbiol 1996; 19(1):1–8. [PubMed] [Google Scholar]
- 7.Zerr D, Meier A, Selke S, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med 2005; 352:768–76. [DOI] [PubMed] [Google Scholar]
- 8.Adler SP. Molecular epidemiology of cytomegalovirus: viral transmission among children attending a day care center, their parents, and caretakers. J Pediatr 1988; 112(3):366–72. [DOI] [PubMed] [Google Scholar]
- 9.Domínguez-Díaz C, García-Orozco A, Riera-Leal A, Padilla-Arellano JR, Fafutis-Morris M. Microbiota and its role on viral evasion: is it with us or against us? Front Cell Infect Microbiol 2019; 9:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson CM, Pfeiffer JK. Viruses and the microbiota. Annu Rev Virol 2014; 1:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N, Ma W-T, Pang M, Fan Q-L, Hua J-L. The commensal microbiota and viral infection: a comprehensive review. Front Immunol 2019; 10:1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KH, Gordon A, Shedden K, et al. The respiratory microbiome and susceptibility to influenza virus infection. PLoS One 2019; 14(1):e0207898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe HM, Meliopoulos VA, Iverson A, et al. Direct interactions with influenza promote bacterial adherence during respiratory infections. Nat Microbiol 2019; 4(8):1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koay WLA, Siems LV, Persaud D. The microbiome and HIV persistence: implications for viral remission and cure. Curr Opin HIV AIDS 2018; 13(1):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe HM, Rosch JW. Close encounters of the viral kind: cross-kingdom synergies at the host–pathogen interface. BioEssays 2019; 41(12):1900128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagan T, Cortese M, Rouphael N, et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell 2019; 178:1313–28.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanada S, Pirzadeh M, Carver KY, Deng JC. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol 2018; 9:2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvalho-Queiroz C, Johansson MA, Persson J-O, et al. Associations between EBV and CMV seropositivity, early exposures, and gut microbiota in a prospective birth cohort: a 10-year follow-up. Front Pediatr 2016; 4:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slots J. Herpesviral-bacterial interactions in periodontal diseases. Periodontol 2000 2010; 52:117–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contreras A, Slots J. Herpesviruses in human periodontal disease. J Periodontal Res 2000; 35:3–16. [DOI] [PubMed] [Google Scholar]
- 21.Zhu C, Li F, Wong MCM, et al. Association between herpesviruses and chronic periodontitis: a meta-analysis based on case-control studies. PLoS One 2015; 10(12):e0144319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamma JJ, Contreras A, Slots J. Herpes viruses and periodontopathic bacteria in early‐onset periodontitis. J Clin Periodontol 2001; 28(9):879–85. [DOI] [PubMed] [Google Scholar]
- 23.Neiswanger K, McNeil DW, Foxman B, et al. Oral health in a sample of pregnant women from Northern Appalachia (2011–2015). Int J Dent 2015; 2015:469312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 2013; 79(17):5112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandes AD, Macklaim JM, Linn TG, Reid G, Gloor GB. ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-Seq. PLoS One 2013; 8(7):e67019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stowell JD, Mask K, Amin M, et al. Cross-sectional study of cytomegalovirus shedding and immunological markers among seropositive children and their mothers. BMC Infect Dis 2014; 14:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall CB, Caserta MT, Schnabel KC, et al. Characteristics and acquisition of human herpesvirus (HHV)-7 infections in relation to infection with HHV-6. J Infect Dis 2006; 193(8):1063–9. [DOI] [PubMed] [Google Scholar]
- 28.Smatti MK, Al-Sadeq DW, Ali NH, et al. Epstein–Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol 2018; 8:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins CD, Swerdlow AJ, Macsween KF, et al. A study of risk factors for acquisition of Epstein-Barr virus and its subtypes. J Infect Dis 2007; 195:474–82. [DOI] [PubMed] [Google Scholar]
- 30.Pariente M, Bartolomé J, Lorente S, Crespo MD. [Age distribution of serological profiles of Epstein-Barr virus infection: review of results from a diagnostic laboratory]. Enferm Infecc Microbiol Clin 2007; 25:108–10. [DOI] [PubMed] [Google Scholar]
- 31.Ayoub HH, Chemaitelly H, Abu-Raddad LJ. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: model-based predictions. BMC Med 2019; 17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertz GJ, Rosenthal SL, Stanberry LR. Is herpes simplex virus type 1 (HSV-1) now more common than HSV-2 in first episodes of genital herpes? Sex Transm Dis 2003; 30:801–2. [DOI] [PubMed] [Google Scholar]
- 33.Sehrawat S, Kumar D, Rouse BT. Herpesviruses: harmonious pathogens but relevant cofactors in other diseases? Front Cell Infect Microbiol 2018; 8:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014; 157(1):121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapoor A, Fan Y-H, Arav-Boger R. Bacterial muramyl dipeptide (MDP) restricts human cytomegalovirus replication via an IFN-β-dependent pathway. Sci Rep 2016; 6:20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai K, Inoue H, Tamura M, et al. The periodontal pathogen Porphyromonas gingivalis induces the Epstein–Barr virus lytic switch transactivator ZEBRA by histone modification. Biochimie 2012; 94(3):839–46. [DOI] [PubMed] [Google Scholar]
- 37.Passariello C, Gigola P, Testarelli L, et al. Evaluation of microbiota associated with Herpesviruses in active sites of generalized aggressive periodontitis. Ann Stomatol (Roma) 2017; 8(2):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Man WH, Clerc M, de Steenhuijsen Piters WAA, et al. Loss of microbial topography between oral and nasopharyngeal microbiota and development of respiratory infections early in life. Am J Respir Crit Care Med 2019; 200(6):760–70. [DOI] [PubMed] [Google Scholar]
- 39.Jansen MAE, van den Heuvel D, Bouthoorn SH, et al. Determinants of ethnic differences in cytomegalovirus, Epstein-Barr virus, and herpes simplex virus type 1 seroprevalence in childhood. J Pediatr 2016; 170:126–34.e6. [DOI] [PubMed] [Google Scholar]
- 40.Renson A, Jones HE, Beghini F, et al. Sociodemographic variation in the oral microbiome. Ann Epidemiol 2019; 35:73–80.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.