Abstract
Infection of the heart muscle with cardiotropic viruses is one of the major aetiologies of myocarditis and acute and chronic inflammatory cardiomyopathy (DCMi). However, viral myocarditis and subsequent dilated cardiomyopathy is still a challenging disease to diagnose and to treat and is therefore a significant public health issue globally. Advances in clinical examination and thorough molecular genetic analysis of intramyocardial viruses and their activation status have incrementally improved our understanding of molecular pathogenesis and pathophysiology of viral infections of the heart muscle. To date, several cardiotropic viruses have been implicated as causes of myocarditis and DCMi. These include, among others, classical cardiotropic enteroviruses (Coxsackieviruses B), the most commonly detected parvovirus B19, and human herpes virus 6. A newcomer is the respiratory virus that has triggered the worst pandemic in a century, SARS-CoV-2, whose involvement and impact in viral cardiovascular disease is under scrutiny. Despite extensive research into the pathomechanisms of viral infections of the cardiovascular system, our knowledge regarding their treatment and management is still incomplete. Accordingly, in this review, we aim to explore and summarize the current knowledge and available evidence on viral infections of the heart. We focus on diagnostics, clinical relevance and cardiovascular consequences, pathophysiology, and current and novel treatment strategies.
Keywords: Viral infections, Myocarditis, Inflammatory cardiomyopathy, Advanced diagnostics, Treatment strategies
1. Introduction
Infectious agents are the major causes of myocarditis and inflammatory cardiomyopathy (DCMi).1–4 The clinical presentation is extremely heterogeneous, the natural history is unpredictable, and prognosis also varies according to the underlying aetiology, environmental factors—most commonly initiated by a virus—and genetic predispositions.5,6 This fact, in conjunction to the lack of non-invasive specific diagnostic methods, makes it an underdiagnosed entity.
To date, several cardiotropic viruses have been implicated as causes of myocarditis and DCMi. The main viruses associated are enteroviruses (EVs), including Coxsackievirus B3 (CVB3), and adenoviruses (ADVs), the most commonly detected parvovirus B19 (B19V), influenza (A, B), human herpesvirus 6 (HHV6), human immunodeficiency virus (HIV), hepatitis C virus (HCV), human cytomegalovirus (CMV), and Epstein–Barr virus (EBV) (Table 1).7,8 All these viruses can cause myocarditis with similar inflammatory features (Figure 1).5,10
Table 1.
List of viral species detected in EMB samples
| Viruses in EMB | Viral genome organization |
|---|---|
| Adenovirus | dsDNA |
| Arenavirus | ssRNA |
| Coronavirus (including Sars-CoV-2) | ssRNA |
| Coxsackievirus (A, B) | ssRNA |
| Cytomegalovirus | dsDNA |
| Dengue virus | ssRNA |
| Echovirus | ssRNA |
| Encephalomyocarditis virus | ssRNA |
| Epstein–Barr virus | dsDNA |
| Hepatitis B virus | dsDNA |
| Hepatitis C virus | ssRNA |
| Herpes simplex virus | dsDNA |
| Human herpesvirus-6 | dsDNA |
| Human immunodeficiency virus | ssRNA |
| Influenza (A, B) virus | ssRNA |
| Measles virus | ssRNA |
| Metapneumovirus | ssRNA |
| Mumps virus | ssRNA |
| Parvovirus B19 | ssDNA |
| Polio virus | ssRNA |
| Rabies virus | ssRNA |
| Respiratory syncytial virus | ssRNA |
| Rubella virus | ssRNA |
| Vaccinia virus | dsDNA |
| Varicella-zoster virus | dsDNA |
| Variola virus | dsDNA |
| Zika virus | ssRNA |
ds, double stranded; ss, single stranded.
Figure 1.
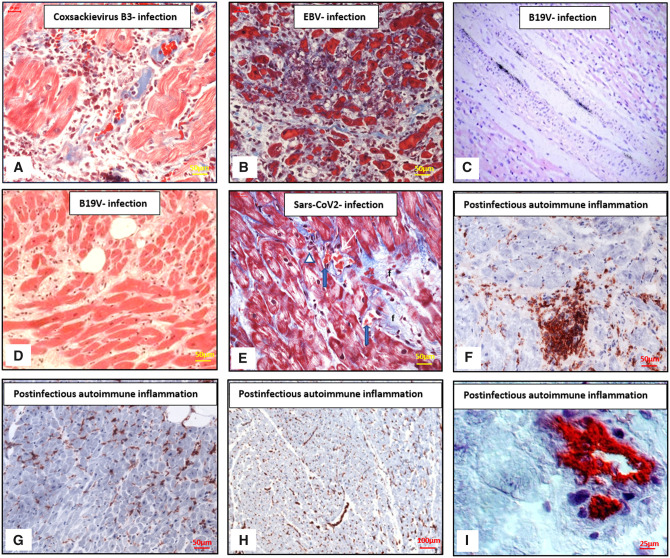
(Immuno-)Histological manifestations of myocarditis and inflammatory cardiomyopathy. (A) CVB3-positive patient, histological analysis of active myocarditis with massively infiltrating cells and myocytolysis. Azan staining. Scale bars: 50 µm. (B) Active myocarditis in a case of EBV infection with dense infiltration of inflammatory cells, necrosis, and dissolution of myocytes in the centre of the panel. Azan stain. Scale bars: 50 µm. (C) Detection of B19V in the endothelial layer of an intramyocardial vessel in the heart (radioactive in situ hybridization, original high-power magnification, haematoxylin, and eosin) obtained at autopsy from an infant who died from myocarditis. Reprinted with permission from Bock et al.9 (D) Enhanced fibrosis in a B19V positive patient with transcriptional activity. H&E stain. Scale bars: 50 µm. (E) Histological analysis in a patient with positive proof of SARS-CoV-2 genomes in EMB. In the periphery of a fibrosis (f) capillaries (white arrow) with sinus-like structure contain aggregated erythrocytes (blue arrows), unstructured protein, and lack endothelial cells. Adjacent some round cells (white triangle). Myocytes distended without signs of damage. Azan stain. Scale bars: 50 µm. (F) Enhanced focal post-infectious autoimmune inflammation, IH staining of focal infiltration of CD3-positive T-lymphocytes. Scale bars: 50 µm. (G) Post-infectious autoimmune inflammation, IH staining of diffuse infiltration of CD45R0-positive T-memory cells. Scale bars: 50 µm. (H) Post-infectious autoimmune inflammation, IH staining of increased HLA-DR isotype—expression. Scale bars: 100 µm. (I) Post-infectious autoimmune inflammation, IH staining of increased VCAM-1 expression. Scale bars: 25 µm. B19V, parvovirus B19; EBV, Epstein–Barr virus; HLA-DR, human leukocyte antigen-DR; IH, immunohistochemistry; VCAM-1, vascular cell adhesion protein 1.
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the causative agent of a cluster of suspicious pneumonia cases in Wuhan, Hubei, China. The incredible fast worldwide spread of the coronavirus disease 2019 (COVID-19) prompted the World Health Organization to declare COVID-19 a pandemic on 11 March 2020. Epidemiological data from the present coronavirus pandemic demonstrate a significant relationship between COVID-19 and cardiovascular disease (CVD). Whether there is direct myocardial damage caused by SARS-CoV-2 or if it is primarily an endothelial disease is currently under investigation.11–14 A recent landmark study by Bailey et al.15 employing human autopsy tissues, human pluripotent stem cell-derived cardiomyocytes, and engineered heart tissues has provided evidence that SARS-CoV-2 directly infects cardiomyocytes and does not infect cardiac macrophages, fibroblasts, or endothelial cells. They also found that infection of cardiomyocytes resulted in cytokine induction, sarcomere disassembly, and cell death. Beyond a broad spectrum of previous clinical studies into the multiple other aspects of COVID-19, these data provide important additional insight into specific SARS-CoV-2 pathology within the heart.
To better understand the frequently unpredictable progression of viral myocarditis and DCMi, one has to address the underlying pathophysiological processes.16,17 Currently, there is consensus that both—immune-mediated and viral cytotoxic mechanisms—play a significant role in this regard.7 Beyond virus cytotoxicity, chronic immune stimulation or autoimmunity in DCMi results from incompletely cleared virus infection, or in response to the preceding virus- or immune-mediated chronic tissue damage, respectively, even in the absence of infectious viral particles.5,6
It is considered possible that at some point in progression, multiple aetiologic types confluence into a common autoimmune pathogenic process that leads to chronic inflammation, tissue remodelling, and fibrosis, ultimately progressing to the clinical phenotype of dilated cardiomyopathy (DCM). A consistent progression from myocarditis to DCM is described in about 30% of myocarditis patients. Any diagnostics started at this time often cannot elucidate the initial causes of the disease.18–24
Understanding the underlying molecular mechanisms is required in order to be able to estimate the prognosis of the patients and is fundamental to proper management and specific treatments.20,25 Viral diagnostics and antiviral treatment should be started early before irreversible myocardial damage has developed.25,26
In this review, we discuss common viral infections and various stages of disease. We assess pathogenesis and mechanisms, clinical relevance and consequences, and outline patient-specific therapeutic options that are based on an accurate diagnosis, covering current and novel treatment strategies.
2. SARS-CoV-2
SARS-CoV-2 is a novel coronavirus that was identified as the cause of COVID-19 in early 2020 (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses27). Infection with SARS-CoV-2 can lead to viral pneumonia and acute respiratory distress syndrome and is accompanied by an increased risk of morbidity and mortality.28 Besides respiratory complications, SARS-CoV-2 can trigger cytokine storm and coagulation abnormalities, leading to thromboembolic events up to multiorgan damage.14,29 Strikingly, there is a strong connection between CVD and severity of COVID-19. Initial clinical data suggested that both, susceptibility and clinical cause are highly dependent on cardiovascular comorbidities.30
2.1 Virological background
SARS-CoV-2 is a membrane-enveloped positive-sense, single-stranded RNA virus with a diameter of ∼80–140 nm. Infection with human coronaviruses mainly results in respiratory and enteric diseases ranging from mild ‘cold-like’ symptoms up to severe life-threatening respiratory pathologies and lung injuries.27 The infection of host cells with SARS-CoV-2 involves specific binding of viral spike (S) protein to the cellular entry receptor angiotensin-converting enzyme 2 (ACE2).31 In addition, fusion of viral particles is dependent on the proteolytic cleavage of the S protein by the host cell surface serine protease TMPRSS2.
Host organism’s innate immune response plays a major role in the cause of COVID-19. Thus, several SARS-CoV-2 accessory proteins have been suggested to affect the innate immune response.32 Abnormal pro-inflammatory cytokine levels and immune cell infiltration have been associated with the severity of tissue damage and morbidity of coronavirus infection.33,34 Aberrant infiltration of pro-inflammatory macrophages, cytotoxic T-cells, and neutrophils has been observed in COVID-19.35,36 Thus, dysregulation of host immune response and elevated cytokine release seem to be crucial factors for the severity of COVID-19.
2.2 Cardiovascular involvement
A meta-analysis involving more than 6000 COVID-19 patients indicates an incidence of cardiac injury ranging from 15% to 42% depending on age and disease severity.37 Post-mortem analysis of cardiac tissue of 39 patients who died as a consequence of coronavirus infection, revealed an incidence of 61.5% positive SARS-CoV-2 RNA detection in the heart.38 A recent meta-analysis and literature screening revealed hypertension (28%), diabetes (14%), and CVD (12%) to be the most prevalent comorbidities in COVID-19 patients and thus, independent risk factors for mortality.39 Moreover, a study including 40 SARS-CoV-2 positive patients confirmed the relationship between the presence of COVID-19 and acute cardiac damage.40 However, whether cardiac injury is directly induced by SARS-CoV-2 infection is not clarified yet.41 There are numerous hypotheses assessing the impact of SARS-CoV-2 infection on cardiovascular manifestations. These range from direct myocardial injury by disturbance of the ACE2 signalling, over systemic inflammatory damage (including cytokine storm) to cardiometabolic issues, arrhythmias, and ischaemia.13
Infection has been proven for SARS-CoV-2 in cardiomyocytes and organoids.15 Noteworthy, endotheliitis has been suggested to be involved in SARS-CoV-2-mediated cardiac damage.13,42,43 A study on cardiac autopsy tissue from COVID-19 positive patients identified strong ACE2/TMPRSS2 expression in capillaries of the heart and endotheliitis of small vessels with prevalence of CD4+ and CD68+ inflammatory cells.44 Another post-mortem analysis of nine COVID-19 patients, who died due to cardiogenic shock, revealed the involvement of all compartments of the heart including intramural vessels, conduction tissue, and subepicardial ganglia.45 There is accumulating evidence that SARS-CoV-2 S protein directly interacts with myocardial Toll-Like Receptor (TLR)4 leading to activation of the TLR4 signalling cascade (including pro-inflammatory cytokines and type I interferons) and even to up-regulation of ACE2 surface expression.46
2.3 Myocarditis and inflammatory cardiomyopathy
First detection of SARS-CoV-2 genomes was provided in endomyocardial biopsies (EMBs) of patients with suspected myocarditis or unexplained heart failure.47 Ultrastructural analysis of EMB of a 69-year-old patient positively tested for SARS-CoV-2 identified viral particles within the interstitial cells of the myocardium.48 Cardiac magnetic resonance imaging in patients recently recovered from Sars-CoV-2 infection identified 78% with cardiac involvement and 60% with an ongoing myocardial inflammation.49 Further studies must show how long the effects last. EMB analysis of two patients with a history of upper airway infection of unknown origin and clinical signs of myocarditis revealed positive detection of SARS-CoV-2 genome in combination with elevated inflammatory cell infiltration.50 Since nasopharyngeal swabs tested negative for SARS-CoV-2 in these patients, it is likely that cardiac inflammation development is delayed following previous infection. Further case reports documented left ventricular dysfunction and inflammation of the heart related to direct Sars-CoV-2-infection with a latency period of 4 weeks after the onset of pulmonary symptoms.51 Autopsy cases from COVID-19 victims confirm lymphomononuclear infiltrates in the myocardium with focal necrosis of adjacent myocytes, in the pericardium as well as in intramural vessels with necrosis of the vascular wall.45
2.4 Acute coronary syndrome
As known for other infectious diseases, SARS-CoV-2 is assumed to trigger acute coronary syndrome (ACS). However, the incidence of ASC in COVID-19 patients is still illusive and detailed mechanisms of SARS-CoV-2 contribution remain speculative. Putative involvement of COVID-19 in the development of ACS includes plaque rupture, coronary spasm or micro-thrombi induced by cytokine storm, and endothelial or vascular injury by direct infection of these cells with SARS-CoV-2.52
3. Human B19V
Human parvovirus (B19V) genomes are the most frequently detected viral species in EMBs of patients with suspected heart failure (Figure 2).53–55 Infection with B19V can start during childhood and continues throughout adulthood, such that between 70% and 88% of adults show serologic evidence of past infections (Table 1).
Figure 2.
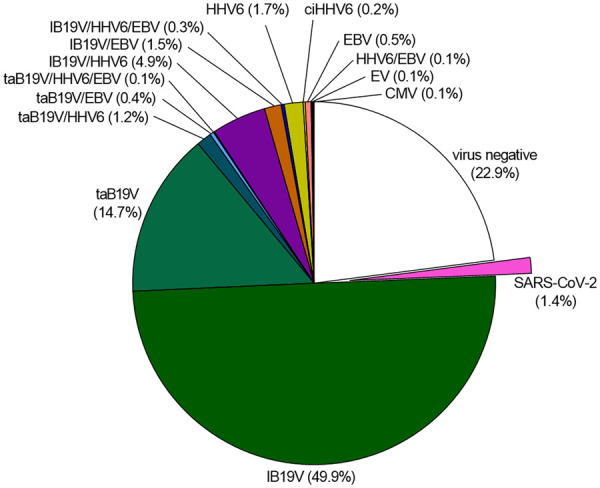
Distribution of viral genomes in EMBs of n = 1132 consecutive patients in 2020 with suspicion of myocarditis or unexplained heart failure. B19V infection is divided into latent (lB19V) and transcriptional active (taB19V) infection. For SARS-CoV-2, n = 364 EMBs were analysed, of which n = 5 (1.4%) were positive for SARS-CoV-2 genomes. B19V, parvovirus B19; ciHHV6, chromosomal integrated human herpesvirus 6; CMV, cytomegalovirus; EBV, Epstein–Barr virus; EMB, endomyocardial biopsy; EV, enterovirus; HHV6, human herpesvirus 6.
3.1 Virological background
B19V is a non-enveloped single-stranded linear DNA virus of 20–24 nm in diameter. Its ∼5.6 kb genome encodes for two major proteins, the non-structural protein (NS1) and VP1/2 protein (capsid protein), and the small accessory 11 and 7.5 kDa proteins of largely unknown function. The NS1 protein transactivates viral transcription and host genes, induces cell cycle arrest and DNA damage response, in order to facilitate viral replication and host cell apoptosis to release viral progeny.56,57 Various molecular mechanisms, such as NS1 induced apoptosis may be responsible for direct cytotoxicity.58
3.2 Cardiovascular involvement
The association of myocardial B19V genome detection to heart diseases is still a matter of controversial discussion.9,55,59 B19V DNA genomes were detected in ∼73% of patients EMBs (Figure 2) and were also found in 55% of healthy donor hearts suggesting no causal relationship.60 However, these studies did not differentiate between latent (inactive) and transcriptional active (positive mRNA) viral infection. In contrast to latent B19V infection, expression of B19V viral mRNA and proteins in the myocardium was demonstrated to be of significance,61 and replicative active B19V in the myocardium is related to adverse clinical outcome.9,54
We identified different cell types belonging to the heterogenous group of bone marrow-derived circulating angiogenic cells with similarities to endothelial and erythroid lineage, to be targets for B19V infection.62 In chronic B19V-associated disease, cardiomyocytes, which are devoid of B19V receptors, are precluded from infection.63 Endothelial dysfunction is a consequence of impaired endothelial regeneration during cardiac B19V infection, leading to impaired coronary microcirculation and results in secondary cardiac myocyte damage.62,64,65 Besides direct cytopathic effects, B19V potentially induces autoimmunity66 possibly triggered by phospholipase activity of VP1u domain.67,68
4. Further cardiotropic viral pathogens
Besides B19V, primary cardiotropic viruses are EV including CVB3 and echoviruses, ADV, the Herpesviridae genus, such as HHV6, EBV, and CMV, all of which may cause or trigger myocarditis and DCMi53,69 (Figure 2). Influenza-(IAV/IBV), HCV, and HIV infections are associated with an increased incidence of cardiac complications.70–72 In addition to the cardiotropic viruses mentioned above, there are sporadic reports, most often as case reports, identifying varicella zoster virus (VZV), Zika virus, Dengue virus, and Chikungunya virus being linked to viral myocarditis. Similar observations have been made for rabies, rubella, mumps, and measles virus.8 In general, proof of viral genomes in the myocardium is independent from the severeness of myocardial dysfunction.53 Whether all of these viruses can be causative for the development of viral myocarditis or just being an incidental finding has to be determined.
4.1 Enteroviruses
EVs (Picornaviridae) are small, single-stranded, positive-sense RNA viruses. Non-enveloped EVs are common human pathogens responsible for lower and upper respiratory tract infections that are transmitted via the faecal–oral route targeting the heart secondarily. Twenty years ago, frequency of enteroviral infection accounts for up to 10% of heart failure patients who underwent EMB. However, recent studies report less frequent finding of CVB-3, which might be associated with regional and temporal patterns.73,74 EV and ADV enter cardiomyocytes via binding the transmembrane Coxsackievirus and adenovirus receptor (CAR), which represents a potential antiviral target.75,76 Direct cardiac damage during acute phase is a consequence of viral replication and impaired cellular translation, induction of apoptosis, and oxidative stress followed by cell lysis.75,77,78 During sub-acute phase of CVB-3 infection, an unbalanced immune response and immune-mediated destruction of cardiac tissue or induction of autoimmune processes may occur.79,80 EV persistence in the myocardium is associated to a significant higher mortality.81 Genotyping revealed a strong correlation between the CCR5 mutation and spontaneous virus clearance with improved outcomes.82
During the chronic phase, CVB-3 might be eliminated or viral persistence may result in the progression to DCM characterized by cytoskeletal disruption and compromised contractility often associated with virus mediated immune response80, 83 (Figure 3).
Figure 3.
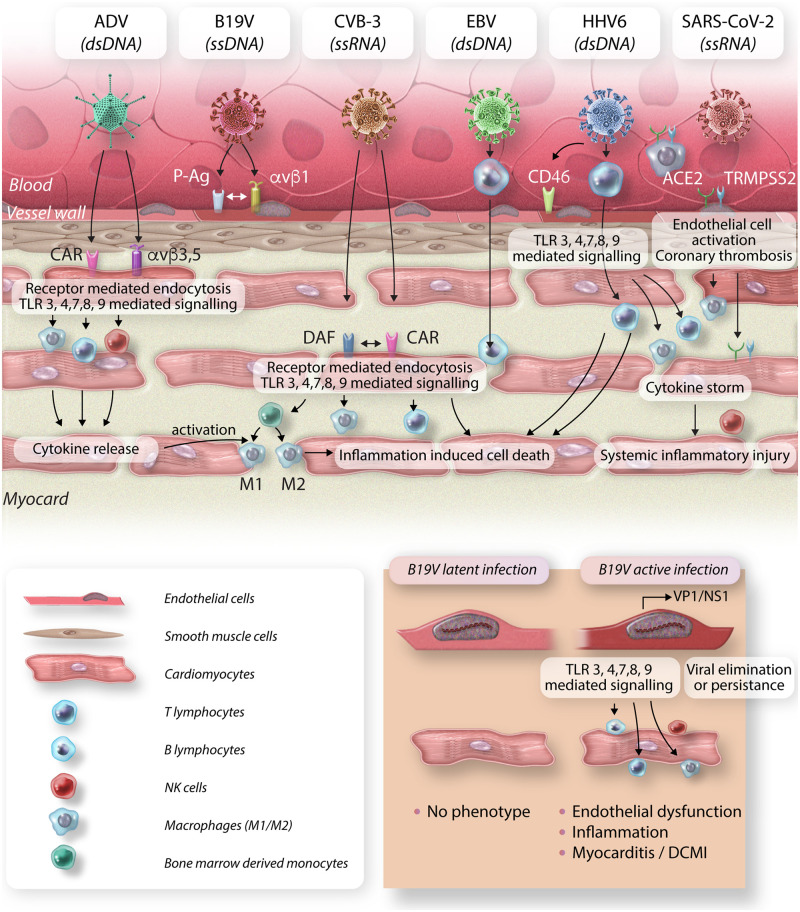
Most abundant cardiotropic viruses and their target cells in the heart. CVB-3 and ADV enter cardiomyocytes via binding the transmembrane CAR. In addition, decay-accelerating factor serves as CVB-3 receptor. Integrins (αvβ3 and αvβ5) promote ADV internalization. B19V targets endothelial cells by binding to erythrocyte P antigen and integrin αvβ1 as co-receptor. EBV efficiently infects resting human B lymphocytes, whereas HHV6 primarily targets CD4+ T lymphocytes. Using CD46 as cellular receptor, HHV6 can directly infect endothelial cells and subsequently enter adjacent tissues. SARS-CoV-2 cellular entry involves specific binding to the ACE2 receptor as well as proteolytic cleavage by the host cell surface serine protease TMPRSS2. For SARS-CoV-2, several cardiac targets including vascular endothelial cells and cardiomyocytes are proposed. Moreover, pulmonary-derived macrophages are suggested carrying the virus into the myocardium. As a consequence of viral infection, TLR3, 4, 7, 8, and 9 signalling cascade is initiated, followed by infiltration of several inflammatory cells including T and B lymphocytes, natural killer cells and bone-marrow derived monocytes, which differentiate into M1 and M2 macrophages. B19V infection can be differentiated into latent infection without myocardial damage and active infection characterized by VP1 and/or NS1 mRNA detection. The later can result in severe endothelial dysfunction, followed by immune cell infiltration and development of DCMi. ds, double stranded; ss, single stranded.
4.2 Human herpesviruses
HHV6 (subtype A and B), as the most frequently found herpesvirus in the myocardium (Figure 2), primarily infects CD4+ T lymphocytes.84,85 HHV6 is a double stranded enveloped DNA virus with a genome of ∼170 kb that encodes for various viral proteins, including a viral DNA polymerase, further proteins and microRNAs (miRNAs), that are involved in the control of viral latency, host cell cycle and evasion of immune response. Infection is usually acquired during childhood in the absence of clinical symptoms or it may manifest as Exanthema subitum and results in lifelong persistence with a seroprevalence of >90%.84 Re-activation after latency occurs by unknown mechanisms and is mostly asymptomatic in immunocompetent individuals while leading to sub-acute clinical symptoms in the immunocompromised patients. Clinical relevance of HHV6 infection of the myocardium has been shown in particular for paediatric patients after heart transplantation.86 There is strong evidence that co-infection with other viruses, in particular with B19V, contributes to cardiac dysfunction since exclusive cardiac infection with HHV6 occurs only rarely.87
The HHV6 genome may integrate into the telomere region of somatic cells or germ line cells [chromosomally integrated HHV6 (ciHHV6)]. The prevalence of ciHHV6 is ∼0.8–1.5% of HHV-6-positive EMBs.88
4.3 CMV, EBV, and VZV
Only few case reports describe findings of CMV, EBV, and VZV in the myocardium that are associated with a pathological phenotype.69,89,90 Molecular mechanism of CMV and EBV infection of the myocardium remain to be elucidated, however pathophysiological effects most probably result from immune-mediated damage or endothelial dysfunction as a consequence of CMV replication (Figure 3).
4.4 Hepatitis C virus
Accumulating evidence suggests that HCV, a globally widespread RNA virus that mainly affects the liver, may also play a role in the pathogenesis of heart diseases including myocarditis and DCM.72,91 Besides hepatitis C, chronic HCV infection is associated with various extrahepatic manifestations, like glomerulonephritis, myositis, and others. Extrahepatic manifestations are believed to be due to the lymphotropism of HCV with accumulation of circulating immune complexes, modulation of host immune response, and activation of autoimmune responses.92 In recent multicentric studies, Matsumori et al.72,93 identified a significant higher seroprevalence of anti-HCV antibodies in patients suffering from myocarditis, DCM, and heart failure than in the general population. Additionally, HCV RNA genomes could be also detected in anti-HCV positive sera and EMBs from patients with myocarditis and DCM. The pathogenesis of HCV-induced myocarditis and DCM is still poorly investigated; however, a recent study provides evidence that of mononuclear cells a major target of HCV could be leukocytes and especially CD68 positive monocytes/macrophages.94 These cells induced by HCV infection may cause inflammation in the organs including the heart muscle leading to myocarditis, DCM, and other cardiomyopathies.
5. Clinical presentation
Myocarditis und DCMi present with heterogeneous clinical signs and symptoms, ranging from subclinical disease to refractory cardiogenic shock with substantial morbidity and mortality.17,95 A virus-specific phenotype of myocardial diseases does not exist. Patients present with uncharacteristic complaints, such as angina, dyspnoea, fatigue, reduced physical ability, or arrhythmias in the presence of a preserved or impaired systolic or diastolic ventricular function.96 A viral infection of the respiratory or the gastrointestinal tract, may precede the onset of cardiac symptoms, although the occurrence of such a viral syndrome is highly variable. In acute disease, sudden onset of chest pain, dyspnoea, and heart failure with normal or enlarged ventricular chambers, ventricular arrhythmias, and abnormal ST-elevation changes in the presence of elevated cardiac enzymes are highly suspicious for an acute viral myocarditis, if a coronary artery disease has been excluded.1,2,4,10
6. Diagnostics of viral infections
The initial evaluation of acute myocarditis and DCMi includes a detailed history and physical examination in which possible features suggestive of aetiology may provide clues.
Cardiac serum biomarkers, specifically troponin I and troponin T, can help to confirm the diagnosis, but lack sensitivity. Other inflammatory serum markers, including white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels, may be elevated in acute myocarditis, but are neither sensitive nor specific in terms of determining the presence or absence of active myocardial inflammation with or without viral infection.8,97 Serologic testing has often been used in the past to identify pathogens in viral myocarditis. However, these methods lack direct correlation between viral infection and myocarditis.10,98
Electrocardiographic findings in myocarditis patients include T-wave and ST-segment changes, including ST-segment elevation mimicking acute myocardial infarction. However, these changes are neither sensitive nor specific for the diagnosis of myocarditis and DCMi. Echocardiography is a valuable tool in detecting global or regional wall motion abnormalities, with myocardial strain patterns adding a special value.10
Magnet resonance imaging (MRI) with extracellular contrast agent can be valuable for mapping tissue hyperaemia associated with the intense inflammatory response of acute myocarditis. Imaging techniques, such as MRI provide accurate non-invasive tissue characterization but not genesis clarification of an infectious agent because they cannot detect or quantify different viral types and loads or subtypes of immune response.99
EMB is the gold- standard method to distinguish directly infectious agent-mediated from multiple types of immune-mediated injury of tissue and can provide specific aetiologic information with significant consequences for management and differential therapy.1,8,10,97 The 2013 European Society of Cardiology position paper recommends characterization of cardiac inflammation and infection by immunohistochemistry and viral analysis using quantitative PCR methods (real-time PCR and nested PCR with reverse transcription).
Viral presence does not always satisfy the Dallas criteria of myocarditis perhaps due to different timepoints in diagnosing. Therefore, beside histological and immunohistological evaluation, molecular analysis of EMB is a prerequisite to establish viral infection and persistence.100,101
State-of-the-art molecular virological diagnostics of EMBs for pathogen detection should not be restricted to the PCR proof of viral RNA or DNA genomes alone, but further include the quantification of viral loads and transcriptional activity.1,53 Recent data show that testing of replicative status is clinically relevant and is, therefore, a prerequisite for further therapeutic decisions.54,61 Additionally, virus genotypes and variants may be detected by next-generation sequencing.102miRNAs are important epigenetic regulators of the immune response in the heart. Epigenetic factors influence the expression of different genes as well as the genetic susceptibility to the development of myocarditis and DCMi.103,104 A panel of miRNAs in serum provides a new non-invasive diagnostic perspective to identify patients with unexplained heart failure, who should undergo an EMB due to intramyocardial inflammation and/or viral persistence.105
The expression of eight miRNAs was significantly increased in samples from patients with advanced heart failure and viral persistence with or without inflammation.106 Thus, miRNAs can serve as a non-invasive, additional tool for indication of EMB decision making.
7. Treatment options
Symptomatic heart failure therapy may improve clinical symptoms and hemodynamic situation. However, a specific antiviral or anti-inflammatory therapy is not covered by this.17
7.1 SARS-CoV-2
Several antiviral therapies are currently being investigated for patients with COVID-19, including strategies to prevent viral entry into the host cell (e.g. chloroquine and hydroxychloroquine), protease inhibitors (lopinavir-ritonavir and darunavir), RNA polymerase inhibitors (remdesivir), and anti-cytokine agents [e.g. interleukin (IL)-6 receptor antagonists], all of which relate to general treatment strategies.107
Negative results were obtained for clinical trials of newly developed HIV protease inhibitors, such as lopinavir/ritonavir [Randomized Evaluation of COVid-19 thERapY (RECOVERY) Trial] and darunavir/cobicistat for COVID-19, with no significant impact on mortality or length of hospital stay.108 Clinical trials with ribavirin against MERS showed high levels of toxicity.109 In a Phase 3 clinical trial, remdesivir was not associated with clinical improvement.110 Chloroquine or hydroxychloroquine does not seem to show significant improvement in mortality. In COVID-19, elevated IL-6 levels have been correlated with increased mortality, sparking interest in the use of tocilizumab—a recombinant, monoclonal antibody against the IL-6 receptor—for COVID-19 therapy. A randomized, placebo-controlled trial in patients with severe COVID-19 demonstrated that tocilizumab did not reduce mortality or intubation rates.111 Convalescent plasma from recovered COVID-19 patients contains naturally produced antibodies that can provide temporary protection against the worst effects of the disease. Synthetic anti-SARS-CoV-2 antibody cocktails are highly enriched specific antibodies against the SARS-CoV-2 S glycoprotein that prevent cell entry. The antibody cocktails are currently being clinically tested as part of the RECOVERY Collaborative Group Trials. As COVID-19 triggers a pro-coagulatory state that increases the risk for thromboembolic events, first studies indicate an improved outcome under antithrombotic treatment.112,113
7.2 EV, ADV, and B19V
Spontaneous enteroviral clearance is associated with significant improvement of LVEF while persistence leads to progressive heart failure and is associated with significantly higher risk of death.17,74 A non-randomized study was started treating EV and ADV positive patients with interferon-ß (IFN-ß). Upon IFN-ß treatment complete elimination of EV and ADV genome was proved by follow-up EMBs after finishing of the antiviral therapy.81 Virus clearance was paralleled by an improvement of mean LVEF and an amelioration of heart failure symptoms and improvement of survival. Thereafter, a Phase 2 study—betaferon in a chronic viral cardiomyopathy—trial was initiated.114 Patients with symptoms of heart failure and biopsy-proven EV, ADV, and/or B19V genomes were randomly assigned to double-blinded treatment. Compared to the placebo, virus elimination and/or virus load reduction was higher in the IFN-ß groups. IFN-ß treatment was associated with significant improvement on NYHA functional class improvement and in quality of life. IFN-β treatment has proven less effective in clearing B19V infection. However, no differentiation between latent B19V infection and viral transcriptional activity was made in this study. In a pilot study, endothelial dysfunction improved with treatment of IFN-ß due to suppression of viral replicative intermediates, suggesting that this treatment option may improve endothelial viability.63 Innovative therapy and prevention strategies to control B19V transcriptional activity are currently under investigation. Telbivudine is an antiviral nucleoside analogue reverse transcriptase inhibitor with pleiotropic immunomodulatory effects that has been described to be effective in retroviral and pararetroviral (hepatitis B virus) infections by preventing dysregulation of BIRC3 and thus suppresses induction of apoptotic pathways.115 Clinical improvement and reduction of transcriptional activity has been shown after Telbivudine treatment in a non-randomized study.116 Intravenous immunoglobulin therapy did not result in clinical improvement of B19V-associated chronic DCM, however, transcriptional activity was not evaluated.117
7.3 HIV, HCV, and HHV6
Patients with HIV-associated myocarditis or DCMI are treated by antiretroviral therapy with clear survival benefits although with cardiac side effects of medication.118,119 Patients with HCV-associated DCMi were treated by combination therapies employing ombitasvir, paritaprevir, ritonavir, and dasabuvir.70,120
Treatment with aciclovir, ganciclovir, or valaciclovir might be considered for herpesvirus infections, although their efficacy has not been directly evaluated in patients with myocarditis. Persistently high loads of HHV6 genomes in blood cells or tissues confirm the presence of ciHHV6. Elimination of the chromosomally integrated virus is impossible, but the transcriptional activity of ciHHV6 may be reduced by treatment with valganciclovir.88
7.4 Post-viral autoimmunity
Myocardial inflammation or systemic autoimmunity persisting despite virus elimination warrants immunosuppressive treatment, in order to prevent later immune-mediated myocardial injury. However, viral genomes have to be excluded prior to immunosuppressive therapy as analysis of patients with DCMi showed that patients with persistent viruses did not improve or even deteriorated upon immunosuppressive therapy, while virus-negative patients improved significantly.121,122 Treatment approaches for these patients with post-infectious chronic myocarditis/inflammatory cardiomyopathy consist of corticosteroids, azathioprine, or cyclosporine A, in addition to optimal heart failure medication.123
TH17 cell response seems to be one of the keys in the progression to chronic damage, cardiac fibrosis, and loss of cardiac function in autoimmune processes.5 The potential of an anti-IL-17 therapy still needs to be evaluated.
The Phase 3, multicentre double-blind, placebo-controlled, randomized-withdrawal study RHAPSODY provided evidence of the potential efficacy and safety of rilonacept, an IL-1α and β inhibitor in chronic pericarditis.124 This agent may also be considered as a potential therapeutic option for post-viral inflammation processes.
8. Perspectives
8.1 Prophylaxis
Whereas conventional antiviral vaccine development methods125 have proven efficient against SARS-CoV-2, the most recent virus of immense medical impact, novel, and entirely RNA-based vaccines have yielded exceptionally good results against this agent.126–128 The revolutionary method successfully used to develop the BioNTec© and Moderna© vaccine was never before employed at scale, and indeed the RNA modification/stabilization/purification methods129,130 as well as the associated nanoparticle delivery tools131 are of recent origin. Importantly, as emphasized by the authors of the landmark paper reporting the results of the BioNTec© vaccine trial,126 they could start the development of the vaccination RNA sequence immediately after the publication of the genome sequence of the new virus,28 which was derived soon after the recognition of COVID-19 as a new disease entity.132,133 Speed and adaptation to entirely new or variant viruses, which unfortunately are most likely to emerge in the future, are significant advantages beyond the current pandemic.134
8.2 New treatment strategies
Whereas prophylaxis was and will of course always be superior to any possible treatment, a spectrum of novel nucleic acid-based therapeutics against molecular targets that cannot be sufficiently or optimally addressed using traditional small molecule drugs or antibodies, has recently successfully entered the clinical arena. In the field of cardiovascular medicine,135,136 several large-scale clinical trials have proven clinical efficacy of RNA-targeted therapeutics for gene silencing (ASO antisense oligonucleotides; RNA interference-inducing siRNAs). Long-acting ASO and/or siRNA molecules lower apo(a), PCSK9, apoCIII, ANGPTL3, or transthyretin (TTR) for the prevention and treatment of patients with atherosclerotic CVD or TTR amyloidosis. Further approaches of interest are miRNA-modulating and epigenetic therapies, as well as methods based on CRISPR-Cas systems. The latter are of particular interest for the field of virology, too, since they are highly adaptable to essentially all viruses and their individual key molecular therapeutic targets. While below, we focus on SARS-CoV-2, all other cardiotropic viruses are amenable to the same strategies.
It is also important to note that the incidence of cardiovascular/myocardial infections with several viruses is known to be highly variable over decades. Since detection of myocardial viral infections is far more difficult compared to systemic ones, this epidaemia-like rise and fall of viruses, such as CVB3 could only be detected by large-scale in-depth myocardial diagnostics, which are not commonly conducted.74 This is an unfortunate situation since at times CVB3 or ADVs caused a large fraction of all heart failure cases among children and adults, whereas their incidence is currently low. Conversely, if a large epidemic or even pandemic with a highly cardiotropic virus ‘free’ of systemic signs on infection would be rapidly spreading, clinical recognition of this wave could be critically delayed until a rather high number of heart failure cases arises in a population without recognizable risk factors. In fact, this was the way by which the first viral myocarditis/heart failure ‘outbreak’ with CVB3 was discovered in the small city of Coxsackie, in New York state. On the other hand, recent CRISPR-based technological breakthroughs including massively multiplexed nucleic acid detection using the CARMEN-Cas13 system137 now enable more comprehensive virome screening than prior PCR-based approaches.
8.2.1 CRISPR-based methods
A recent landmark paper reported the development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza.138 The authors demonstrate a CRISPR-Cas13-based strategy, prophylactic antiviral CRISPR in human cells (PAC-MAN), for viral inhibition that can degrade RNA from both SARS-CoV-2 sequences and live influenza A viruses in human lung epithelial cells. Importantly, their bioinformatic analysis showed that a group of only six crRNAs can target more than 90% of all coronaviruses. They conclude that with the development of a safe and effective system for respiratory tract delivery, PAC-MAN has the potential to become an important pan-coronavirus inhibition strategy.
8.2.2 ASO- and RNAi-based methods
siRNA molecules for silencing nucleocapsid phosphoprotein and surface glycoprotein gene of SARS-CoV-2 have been designed.139,140 Other groups141,142 have determined the structural landscape of SARS-CoV-2 RNA and regulatory untranslated regions of SARS-CoV-2 and other coronaviruses. They found ASOs targeting the structural elements and FDA-approved drugs inhibiting the SARS-CoV-2 RNA-binding proteins dramatically reduced SARS-CoV-2 infection in cells derived from human liver and lung tumours. These studies shed light on ASO candidate therapeutics.
Cardiomyocyte-targeted RNAi has been investigated to inhibit cardiotropic viruses143,144 including human CVB3145,146 and human ADV147 in cardiomyocytes. Of note, B19V may be transactivated by adenoviral helper functions in vascular endothelial cells148 illustrating the impact of intercurrent viral co-infections upon clinical course. In addition to directly antiviral approaches, RNAi was also evaluated regarding its potential to suppress pathogenic cardiac inflammation.149 RNAi against a single cellular target was able to block multiple interacting pro-inflammatory and profibrotic pathways in cardiac fibroblasts. Successful clinical translation of these approaches, as well as of recombinant expression of virus receptor traps150 critically depends on the availability of clinically safe and efficient drug delivery systems (Figure 4).
Figure 4.
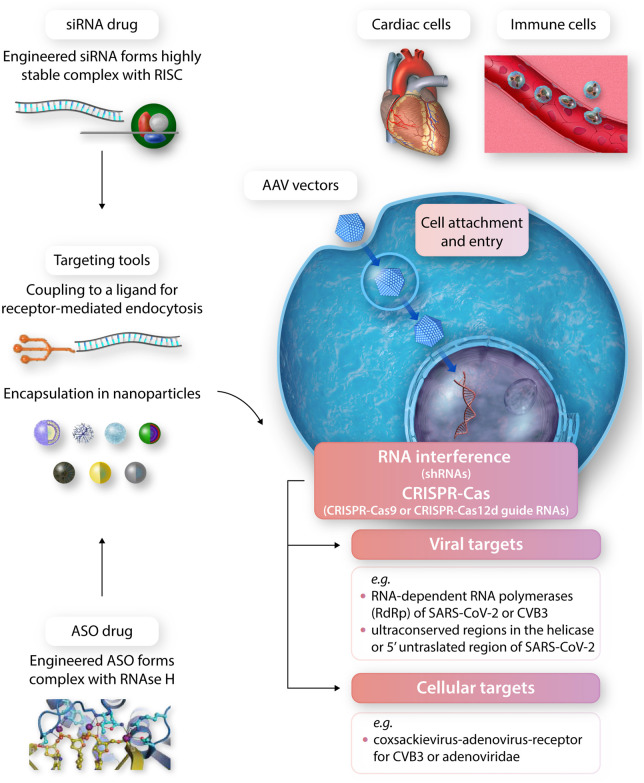
Innovative antiviral strategies. The continuing need for the development of innovative antiviral strategies is strikingly illustrated by the catastrophic SARS-CoV-2 pandemic, which suddenly arose by transmission of an animal virus to man and is difficult to control, amongst other problems, due to sequential accumulation of mutations. The recent introduction of novel therapeutic approaches based on biological antiviral defence systems (RNA interference, CRISPR-Cas) or antisense drugs (ASOs) is most welcome in this context. Although technically demanding, RNAi and ASO drugs have entered cardiovascular clinical practice when the key problem of their liver-directed targeting was solved by ligand-coupling and nanoparticle encapsulation (to the left). Further development of ASO, RNAi 141, 142 and CRISPR-Cas 140, 163 antiviral drugs justifies major efforts since essentially any viral or cellular target (examples are given for Cosackieviruses and SARS-CoV-2) may be addressed by these highly flexible tools once efficient delivery to the affected tissue is enabled. In that regard, a recent pioneering study by Bailey et al.15 is of interest. Decades after similar work on CVB3 myocarditis in humans, this article dealing with SARS-CoV-2 finds similarly restricted cellular tropism (cardiomyocytes but not cardiac macrophages, fibroblasts, or endothelial cells) and mechanistic sequelae of SARS-CoV-2 infection (innate immune activation with cytokine induction, sarcomere disassembly, and cell death). Whereas recombinant AAV vectors (to the right) were successfully employed for RNAi and anti-miR therapy of myocardial disorders in animal models, this approach has not yet entered the clinical arena. Global efforts, significantly driven by the current pandemic, are currently being devoted to fully exploit the clinical potential of these new antiviral strategies.
8.2.3 Non-coding RNA (ncRNA) targets
ncRNAs including miRNAs are deeply involved in the host cells’ innate antiviral immune response.151 There are multiple targets for human miRNAs on SARS-CoV-2 RNA, most of which are located in the 5' and 3' untranslated regions. Mutations of the viral genome that result in the creation or loss of miRNA-binding sites may therefore have substantial effects on the pathogenicity of SARS-CoV-2.152 Thus, Alam et al.153 have shown that human miRNA-122, a previously known cofactor of another RNA virus, HCV, whose genome it binds as a prerequisite for pathogenesis, can also bind the RNA genome of SARS-CoV-2 with high affinity. This opens the possibility of using RNA-based drugs against HCV, such as Miravirsen, to treat COVID-19.
Relevance of miRNAs for the clinical course of infections has also been documented for human cardiomyopathies associated with B19V154 or CVB3. In the latter, differential cardiac miRNA expression closely predicted the clinical course. The most highly expressed miRNAs associated with rapid progression and an adverse outcome could possibly constitute RNAi targets.
8.2.4 Remaining challenges
We recently discussed that the high potential of CRISPR and other nucleic acid drugs needs to be weighed against potential risks, but from the clinical practice viewpoint the delivery issue of the nucleic acid drugs to target organs is only partially solved. Current nanoparticle vehicles employing the Gal-NAc system have efficiently delivered ASO and RNAi drugs to the liver as documented in several landmark trials in the cardiovascular field.135 Progress has also been achieved towards aerosol delivery of nucleic acid drugs to the lung including a combination treatment using an inhalable GapmeR oligonucleotide and recombinant ACE2 for COVID-19.155,156
Biologically efficient delivery of nucleic acid therapeutics (siRNAs) to the myocardium has been achieved by recombinant expression from AAV viral vectors in animal models.157,158 Whereas this delivery approach has not yet entered the clinical arena.159,160 AAV-based as well as non-viral delivery of a broad spectrum of novel antiviral nucleic acids drugs would thus become available for treatment trials of viral cardiomyopathies. In summary and synopsis with a recent comprehensive review by Le et al.161 on nucleic acid-based technologies targeting coronaviruses, it is evident that possible clinical success of any nucleic acid drug is critically dependent on the technological challenge of efficient and focused drug delivery.
8.3 Need for highly versatile antiviral tools
Importantly, the above new therapeutic approaches offer extremely high versatility to adapt to essentially any coding or non-coding, viral or host cell, molecular target. Further, their large-scale production will follow similar (i.e. RNA, DNA, and XNA) synthetic pathways, enabling massive up-scaling of therapeutics production if required.
The current pandemic, originating from transmission of a mutated animal virus to man, has heightened concerns and awareness that amongst the vast number of animal viruses others may cross the species barrier to humans.162,163 Therefore, foresighted expansion of our antiviral arsenal appears warranted.
The combination of genetic factors that increase susceptibility to cardiomyopathy combined with acquired causes of cardiomyopathy, such as viral infection and/or autoimmunity, may be an explanation for the variable penetrance and severity of DCMi. The availability of novel techniques and novel insights into pathophysiology will help to address knowledge gaps in the future. Efficacy of therapeutic approaches needs to be evaluated in large, controlled, randomized trials to facilitate the development of personalized treatment options.
Acknowledgements
Figures were created at the Institute for cardiac diagnostic and therapy (IKDT) Berlin, Germany. For excellent work, we thank Dr G. Aleshcheva and Prof. U. Gross (IKDT Berlin, Germany).
Conflict of interest: None declared.
Funding
This work was supported by grants of ERA-Net on Cardiovascular Diseases (ERA-CVD; JTC2016-40-158) and a ProFIT grant of the Investitionsbank Berlin/cofunded of EFRE (No. 10169096, 10169098, 10169028).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
This article is part of the Spotlight Issue on Cardiovascular Immunology.
References
- 1.Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss H-P, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 2.Sagar S, Liu PP, Cooper LT.. Myocarditis. Lancet 2012;379:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultheiss HP, Fairweather DL, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A, Mazzanti A, McMurray J, Priori SG.. Dilated cardiomyopathy. Nat Rev Dis Prim 2019;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blauwet LA, Cooper LT.. Myocarditis. Prog Cardiovasc Dis 2010;52:274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bracamonte-Baran W, Čiháková D.. Cardiac autoimmunity: myocarditis. Adv Exp Med Biol 2017;1003:187–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannata A, Artico J, Gentile P, Merlo M, Sinagra G.. Myocarditis evolving in cardiomyopathy: when genetics and offending causes work together. Eur Heart J Suppl 2019;21:B90–B95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose NR. Viral myocarditis. Curr Opin Rheumatol 2016;28:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leone O, Pieroni M, Rapezzi C, Olivotto I.. The spectrum of myocarditis: from pathology to the clinics. Virchows Arch 2019;475:279–301. [DOI] [PubMed] [Google Scholar]
- 9.Bock C-T, Klingel K, Kandolf R.. Human parvovirus B19–associated myocarditis. N Engl J Med 2010;362:1248–1249. [DOI] [PubMed] [Google Scholar]
- 10.Sinagra G, Anzini M, Pereira NL, Bussani R, Finocchiaro G, Bartunek J, Merlo M.. Myocarditis in clinical practice. Mayo Clin Proc 2016;91:1256–1266. [DOI] [PubMed] [Google Scholar]
- 11.Hanna R, Dalvi S, Sălăgean T, Pop ID, Bordea IR, Benedicenti S.. Understanding COVID-19 pandemic: molecular mechanisms and potential therapeutic strategies. An evidence-based review. J Inflamm Res 2021;14:13–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV III, Kwon DH, Singh T, Tilton JC, Tsai EJ, Tucker NR, Barnard J, Loscalzo J.. COVID-19 and cardiovascular disease: from bench to bedside. Circ Res 2021;128:1214–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqi HK, Libby P, Ridker PM.. COVID-19 - a vascular disease. Trends Cardiovasc Med 2021;31:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokolowska M, Lukasik ZM, Agache I, Akdis CA, Akdis D, Akdis M, Barcik W, Brough HA, Eiwegger T, Eljaszewicz A, Eyerich S, Feleszko W, Gomez-Casado C, Hoffmann-Sommergruber K, Janda J, Jiménez-Saiz R, Jutel M, Knol EF, Kortekaas Krohn I, Kothari A, Makowska J, Moniuszko M, Morita H, O'Mahony L, Nadeau K, Ozdemir C, Pali-Schöll I, Palomares O, Papaleo F, Prunicki M, Schmidt-Weber CB, Sediva A, Schwarze J, Shamji MH, Tramper-Stranders GA, van de Veen W, Untersmayr E.. Immunology of COVID-19: mechanisms, clinical outcome, diagnostics, and perspectives-A report of the European Academy of Allergy and Clinical Immunology (EAACI). Allergy 2020;75:2445–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey AL, Dmytrenko O, Greenberg L, Bredemeyer AL, Ma P, Liu J, Penna V, Winkler ES, Sviben S, Brooks E, Nair AP, Heck KA, Rali AS, Simpson L, Saririan M, Hobohm D, Stump WT, Fitzpatrick JA, Xie X, Zhang X, Shi P-Y, Hinson JT, Gi W-T, Schmidt C, Leuschner F, Lin C-Y, Diamond MS, Greenberg MJ, Lavine KJ.. SARS-CoV-2 infects human engineered heart tissues and models COVID-19 myocarditis. JACC Basic Transl Sci 2021;6:331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung G, Luo H, Qiu Y, Yang D, McManus B.. Myocarditis. Circ Res 2016;118:496–514. [DOI] [PubMed] [Google Scholar]
- 17.Schultheiss HP, Khl U, Cooper LT.. The management of myocarditis. Eur Heart J 2011;32: 2616–2625. [DOI] [PubMed] [Google Scholar]
- 18.Kawai C, Matsumori A.. Dilated cardiomyopathy update: infectious-immune theory revisited. Heart Fail Rev 2013;18:703–714. [DOI] [PubMed] [Google Scholar]
- 19.Heymans S, Eriksson U, Lehtonen J, Cooper LT.. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol 2016;68:2348–2364. [DOI] [PubMed] [Google Scholar]
- 20.Monda E, Palmiero G, Rubino M, Verrillo F, Amodio F, Di Fraia F, Pacileo R, Fimiani F, Esposito A, Cirillo A, Fusco A, Moscarella E, Frisso G, Russo MG, Pacileo G, Calabrò P, Scudiero O, Caiazza M, Limongelli G.. Molecular basis of inflammation in the pathogenesis of cardiomyopathies. Int J Mol Sci 2020;21:6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Root-Bernstein R, Fairweather D.. Unresolved issues in theories of autoimmune disease using myocarditis as a framework. J Theor Biol 2015;375:101–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corsten MF, Schroen B, Heymans S.. Inflammation in viral myocarditis: friend or foe? Trends Mol Med 2012;18:426–437. [DOI] [PubMed] [Google Scholar]
- 23.Esfandiarei M, McManus BM.. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol Mech Dis 2008;3:127–155. [DOI] [PubMed] [Google Scholar]
- 24.Elamm C, Fairweather D, Cooper LT.. Republished: pathogenesis and diagnosis of myocarditis. Postgrad Med J 2012;88:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultheiss H-P, Escher F.. Myocarditis: treatment of myocarditis. ESC CardioMed. Oxford, UK: Oxford University Press; 2018. [Google Scholar]
- 26.Kühl U, Schultheiss H-P.. Myocarditis: early biopsy allows for tailored regenerative treatment. Dtsch Arztebl Int 2012;109:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorbalenya AE. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP.. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry M-C, Bois MC, Lin PT, Maleszewski JJ, Stone JR.. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 2020;41:3827–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S.. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thoms M, Buschauer R, Ameismeier M, Koepke L, Denk T, Hirschenberger M, Kratzat H, Hayn M, Mackens-Kiani T, Cheng J, Straub JH, Stürzel CM, Fröhlich T, Berninghausen O, Becker T, Kirchhoff F, Sparrer KMJ, Beckmann R.. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science 2020;369:1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M.. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci 2020;257:118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, Zhang Z.. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020;26:842–844. [DOI] [PubMed] [Google Scholar]
- 36.Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, Debnath O, Thürmann L, Kurth F, Völker MT, Kazmierski J, Timmermann B, Twardziok S, Schneider S, Machleidt F, Müller-Redetzky H, Maier M, Krannich A, Schmidt S, Balzer F, Liebig J, Loske J, Suttorp N, Eils J, Ishaque N, Liebert UG, von Kalle C, Hocke A, Witzenrath M, Goffinet C, Drosten C, Laudi S, Lehmann I, Conrad C, Sander L-E, Eils R.. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat Biotechnol 2020;38:970–979. [DOI] [PubMed] [Google Scholar]
- 37.Fu L, Liu X, Su Y, Ma J, Hong K.. Prevalence and impact of cardiac injury on COVID‐19: a systematic review and meta‐analysis. Clin Cardiol 2021;44:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss H-P, Blankenberg S, Püschel K, Westermann D.. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol 2020;5:1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fathi M, Vakili K, Sayehmiri F, Mohamadkhani A, Hajiesmaeili M, Rezaei-Tavirani M, Eilami O.. The prognostic value of comorbidity for the severity of COVID-19: a systematic review and meta-analysis study. PLoS One 2021;16:e0246190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saleh A, Matsumori A, Abdelrazek S, Eltaweel S, Salous A, Neumann F-J, Antz M.. Myocardial involvement in coronavirus disease 19. Herz 2020;45:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libby P. The heart in COVID-19: primary target or secondary bystander? JACC Basic Transl Sci 2020;5:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libby P, Lüscher T.. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020;41:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker RC. COVID-19-associated vasculitis and vasculopathy. J Thromb Thrombolysis 2020;50:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maccio U, Zinkernagel AS, Shambat SM, Zeng X, Cathomas G, Ruschitzka F, Schuepbach RA, Moch H, Varga Z.. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine 2021;63:103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Del Nonno F, Frustaci A, Verardo R, Chimenti C, Nicastri E, Antinori A, Petrosillo N, Lalle E, Agrati C, Ippolito G.. Virus-negative myopericarditis in human coronavirus infection: report from an autopsy series. Circ Heart Fail 2020;13:doi: 10.1161/CIRCHEARTFAILURE.120.007636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aboudounya MM, Heads RJ.. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediators Inflamm 2021;2021:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escher F, Pietsch H, Aleshcheva G, Bock T, Baumeier C, Elsaesser A, Wenzel P, Hamm C, Westenfeld R, Schultheiss M, Gross U, Morawietz L, Schultheiss H.. Detection of viral SARS‐CoV‐2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail 2020;7:2440–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E.. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail 2020;22:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E.. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenzel P, Kopp S, Göbel S, Jansen T, Geyer M, Hahn F, Kreitner K-F, Escher F, Schultheiss H-P, Münzel T.. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res 2020;116:1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pietsch H, Escher F, Aleshcheva G, Baumeier C, Morawietz L, Elsaesser A, Schultheiss H-P.. Proof of SARS-CoV-2 genomes in endomyocardial biopsy with latency after acute infection. Int J Infect Dis 2021;102:70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H.. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuhl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP.. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with ‘idiopathic’ left ventricular dysfunction. Circulation 2005;111:887–893. [DOI] [PubMed] [Google Scholar]
- 54.Kuhl U, Lassner D, Dorner A, Rohde M, Escher F, Seeberg B, Hertel E, Tschope C, Skurk C, Gross UM, Schultheiss H-P, Poller W.. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res Cardiol 2013;108:372. [DOI] [PubMed] [Google Scholar]
- 55.Verdonschot J, Hazebroek M, Merken J, Debing Y, Dennert R, Brunner-La Rocca H-P, Heymans S.. Relevance of cardiac parvovirus B19 in myocarditis and dilated cardiomyopathy: review of the literature. Eur J Heart Fail 2016;18:1430–1441. [DOI] [PubMed] [Google Scholar]
- 56.Bachelier K, Biehl S, Schwarz V, Kindermann I, Kandolf R, Sauter M, Ukena C, Yilmaz A, Sliwa K, Bock C-T, Klingel K, Böhm M.. Parvovirus B19-induced vascular damage in the heart is associated with elevated circulating endothelial microparticles. PLoS One 2017;12:e0176311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duechting A, TschöPe C, Kaiser H, Lamkemeyer T, Tanaka N, Aberle S, Lang F, Torresi J, Kandolf R, Bock C-T.. Human parvovirus B19 NS1 protein modulates inflammatory signaling by activation of STAT3/PIAS3 in human endothelial cells. J Virol 2008;82:7942–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu T-C, Wu W-J, Chen M-C, Tsay GJ.. Human parvovirus B19 non-structural protein (NS1) induces apoptosis through mitochondria cell death pathway in COS-7 cells. Scand J Infect Dis 2004;36:570–577. [DOI] [PubMed] [Google Scholar]
- 59.Stewart GC, Lopez-Molina J, Gottumukkala R, Rosner GF, Anello MS, Hecht JL, Winters GL, Padera RF, Baughman KL, Lipes MA.. Myocardial parvovirus B19 persistence: lack of association with clinicopathologic phenotype in adults with heart failure. Circ Heart Fail 2011;4:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hjalmarsson C, Liljeqvist J-Å, Lindh M, Karason K, Bollano E, Oldfors A, Andersson B.. Parvovirus B19 in endomyocardial biopsy of patients with idiopathic dilated cardiomyopathy: foe or bystander? J Card Fail 2019;25:60–63. [DOI] [PubMed] [Google Scholar]
- 61.Pietsch H, Escher F, Aleshcheva G, Lassner D, Bock C-T, Schultheiss H-P.. Detection of parvovirus mRNAs as markers for viral activity in endomyocardial biopsy-based diagnosis of patients with unexplained heart failure. Sci Rep 2020;10:22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidt-Lucke C, Zobel T, Schrepfer S, Kuhl U, Wang D, Klingel K, Becher PM, Fechner H, Pozzuto T, Van Linthout S, Lassner D, Spillmann F, Escher F, Holinski S, Volk H-D, Schultheiss H-P, Tschope C.. Impaired endothelial regeneration through human parvovirus B19-infected circulating angiogenic cells in patients with cardiomyopathy. J Infect Dis 2015;212:1070–1081. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt-Lucke C, Spillmann F, Bock T, Kühl U, Van Linthout S, Schultheiss H-P, Tschöpe C.. Interferon beta modulates endothelial damage in patients with cardiac persistence of human parvovirus B19 infection. J Infect Dis 2010;201:936–945. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt-Lucke C, Zobel T, Escher F, Tschope C, Lassner D, Kuhl U, Gubbe K, Volk H-D, Schultheiss H-P.. Human parvovirus B19 (B19V) up-regulates CXCR4 surface expression of circulating angiogenic cells: implications for cardiac ischemia in B19V cardiomyopathy. J Infect Dis 2018;217:456–465. [DOI] [PubMed] [Google Scholar]
- 65.Escher F, Kuhl U, Sabi T, Suckau L, Lassner D, Poller W, Schultheiss H-P, Noutsias M.. Immunohistological detection of Parvovirus B19 capsid proteins in endomyocardial biopsies from dilated cardiomyopathy patients. Med Sci Monit 2008;14:CR333–CR338. [PubMed] [Google Scholar]
- 66.Magro CM, Crowson AN, Dawood M, Nuovo GJ.. Parvoviral infection of endothelial cells and its possible role in vasculitis and autoimmune diseases. J Rheumatol 2002;29:1227–1235. [PubMed] [Google Scholar]
- 67.Kerr JR. The role of parvovirus B19 in the pathogenesis of autoimmunity and autoimmune disease. J Clin Pathol 2016;69:279–291. [DOI] [PubMed] [Google Scholar]
- 68.Bogomolovas J, Šimoliūnas E, Rinkūnaitė I, Smalinskaitė L, Podkopajev A, Bironaitė D, Weis C-A, Marx A, Bukelskienė V, Gretz N, Grabauskienė V, Labeit D, Labeit S.. A novel murine model of parvovirus associated dilated cardiomyopathy induced by immunization with VP1-unique region of parvovirus B19. Biomed Res Int 2016;2016:1627184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss H-P, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA.. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol 2003;42:466–472. [DOI] [PubMed] [Google Scholar]
- 70.Poller W, Kaya Z, Muche M, Kasner M, Skurk C, Kappert K, Tauber R, Escher F, Schultheiss H-P, Epple H-J, Landmesser U.. High incidence of cardiac dysfunction and response to antiviral treatment in patients with chronic hepatitis C virus infection. Clin Res Cardiol 2017;106:551–556. [DOI] [PubMed] [Google Scholar]
- 71.Frustaci A, Petrosillo N, Francone M, Verardo R, Ippolito G, Chimenti C.. Biopsy-proven autoimmune myocarditis in HIV-associated dilated cardiomyopathy. BMC Infect Dis 2014;14:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsumori A, Matoba Y, Sasayama S.. Dilated cardiomyopathy associated with hepatitis C virus infection. Circulation 1995;92:2519–2525. [DOI] [PubMed] [Google Scholar]
- 73.Bouin A, Gretteau P-A, Wehbe M, Renois F, N'Guyen Y, Lévêque N, Vu MN, Tracy S, Chapman NM, Bruneval P, Fornes P, Semler BL, Andreoletti L.. Enterovirus persistence in cardiac cells of patients with idiopathic dilated cardiomyopathy is linked to 5’ terminal genomic RNA-deleted viral populations with viral-encoded proteinase activities. Circulation 2019;139:2326–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.KüHl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss H-P.. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 2005;112:1965–1970. [DOI] [PubMed] [Google Scholar]
- 75.Pauschinger M, Phan MD, Doerner A, Kuehl U, Schwimmbeck PL, Poller W, Kandolf R, Schultheiss HP.. Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation 1999;99:889–895. [DOI] [PubMed] [Google Scholar]
- 76.Pauschinger M, Bowles NE, Fuentes-Garcia FJ, Pham V, KüHl U, Schwimmbeck PL, Schultheiss H-P, Towbin JA.. Detection of adenoviral genome in the myocardium of adult patients with idiopathic left ventricular dysfunction. Circulation 1999;99:1348–1354. [DOI] [PubMed] [Google Scholar]
- 77.Peischard S, Ho HT, Theiss C, Strutz-Seebohm N, Seebohm G.. A kidnapping story: how Coxsackievirus B3 and its host cell interact. Cell Physiol Biochem 2019;53:121–140. [DOI] [PubMed] [Google Scholar]
- 78.Garmaroudi FS, Marchant D, Hendry R, Luo H, Yang D, Ye X, Shi J, McManus BM.. Coxsackievirus B3 replication and pathogenesis. Future Microbiol 2015;10:629–653. [DOI] [PubMed] [Google Scholar]
- 79.Gauntt CJ, Arizpe HM, Higdon AL, Wood HJ, Bowers DF, Rozek MM, Crawley R.. Molecular mimicry, anti-coxsackievirus B3 neutralizing monoclonal antibodies, and myocarditis. J Immunol 1995;154:2983–2995. [PubMed] [Google Scholar]
- 80.Fairweather DL, Frisancho-Kiss S, Rose NR.. Viruses as adjuvants for autoimmunity: evidence from Coxsackievirus-induced myocarditis. Rev Med Virol 2005;15:17–27. [DOI] [PubMed] [Google Scholar]
- 81.Kühl U, Lassner D, von SJ, Poller W, Schultheiss H-P.. Interferon-beta improves survival in enterovirus-associated cardiomyopathy. J Am Coll Cardiol 2012;60:1295–1296. [DOI] [PubMed] [Google Scholar]
- 82.Lassner D, Siegismund CS, Kühl U, Rohde M, Stroux A, Escher F, Schultheiss H-P.. CCR5del32 genotype in human enteroviral cardiomyopathy leads to spontaneous virus clearance and improved outcome compared to wildtype CCR5. J Transl Med 2018;16:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sin J, Mangale V, Thienphrapa W, Gottlieb RA, Feuer R.. Recent progress in understanding coxsackievirus replication, dissemination, and pathogsenesis. Virology 2015;484:288–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clark DA. Human herpesvirus 6. Rev Med Virol 2000;10:155–173. [DOI] [PubMed] [Google Scholar]
- 85.Escher F, Kühl U, Gross U, Westermann D, Poller W, Tschöpe C, Lassner D, Schultheiss H-P.. Aggravation of left ventricular dysfunction in patients with biopsy-proven cardiac human herpesvirus A and B infection. J Clin Virol 2015;63:1–5. [DOI] [PubMed] [Google Scholar]
- 86.Das BB, Prusty BK, Niu J, Huang M-L, Zhu H, Eliassen E, Kuypers JM, Jerome KR.. Detection of parvovirus B19 and human herpesvirus 6 in pediatric dilated cardiomyopathy: impact after heart transplantation. Ann Pediatr Cardiol 2020;13:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vallbracht KB, Schwimmbeck PL, Kühl U, Rauch U, Seeberg B, Schultheiss H-P.. Differential aspects of endothelial function of the coronary microcirculation considering myocardial virus persistence, endothelial activation, and myocardial leukocyte infiltrates. Circulation 2005;111:1784–1791. [DOI] [PubMed] [Google Scholar]
- 88.Kühl U, Lassner D, Wallaschek N, Gross UM, Krueger GRF, Seeberg B, Kaufer BB, Escher F, Poller W, Schultheiss H-P.. Chromosomally integrated human herpesvirus 6 in heart failure: prevalence and treatment. Eur J Heart Fail 2015;17:9–19. [DOI] [PubMed] [Google Scholar]
- 89.Ioannou A, Tsappa I, Metaxa S, Missouris CG.. Ventricular fibrillation following varicella zoster myocarditis. Case Rep Cardiol 2017;2017:1017686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kytö V, Vuorinen T, Saukko P, Lautenschlager I, Lignitz E, Saraste A, Voipio-Pulkki L-M.. Cytomegalovirus infection of the heart is common in patients with fatal myocarditis. Clin Infect Dis 2005;40:683–688. [DOI] [PubMed] [Google Scholar]
- 91.Ilyas SZ, Tabassum R, Hamed H, Rehman SU, Qadri I.. Hepatitis C virus-associated extrahepatic manifestations in lung and heart and antiviral therapy-related cardiopulmonary toxicity. Viral Immunol 2017;30:633–641. [DOI] [PubMed] [Google Scholar]
- 92.Galossi A, Guarisco R, Bellis L, Puoti C.. Extrahepatic manifestations of chronic HCV infection. J Gastrointestin Liver Dis 2007;16:65–73. [PubMed] [Google Scholar]
- 93.Matsumori A, Shimada T, Chapman NM, Tracy SM, Mason JW.. Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail 2006;12:293–298. [DOI] [PubMed] [Google Scholar]
- 94.Haykal M, Matsumori A, Saleh A, Fayez M, Negm H, Shalaby M, Bassuony S.. Diagnosis and treatment of HCV heart diseases. Expert Rev Cardiovasc Ther 2021;19:493–499. [DOI] [PubMed] [Google Scholar]
- 95.Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, Friedrich MG, Klingel K, Lehtonen J, Moslehi JJ, Pedrotti P, Rimoldi OE, Schultheiss H-P, Tschöpe C, Cooper LTJ, Camici PG.. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail 2020;13:e007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caforio ALP, Marcolongo R, Basso C, Iliceto S.. Clinical presentation and diagnosis of myocarditis. Heart 2015;101:1332–1344. [DOI] [PubMed] [Google Scholar]
- 97.Japp AG, Gulati A, Cook SA, Cowie MR, Prasad SK.. The diagnosis and evaluation of dilated cardiomyopathy. J Am Coll Cardiol 2016;67:2996–3010. [DOI] [PubMed] [Google Scholar]
- 98.Mahfoud F, Gärtner B, Kindermann M, Ukena C, Gadomski K, Klingel K, Kandolf R, Böhm M, Kindermann I.. Virus serology in patients with suspected myocarditis: utility or futility? Eur Heart J 2011;32:897–903. [DOI] [PubMed] [Google Scholar]
- 99.Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, Noutsias M, Schultheiss H-P, Kühl U.. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology 2008;246:401–409. [DOI] [PubMed] [Google Scholar]
- 100.Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G, Bruneval P, Burke M, Butany J, Calabrese F, d'Amati G, Edwards WD, Fallon JT, Fishbein MC, Gallagher PJ, Halushka MK, McManus B, Pucci A, Rodriguez ER, Saffitz JE, Sheppard MN, Steenbergen C, Stone JR, Tan C, Thiene G, van der Wal AC, Winters GL.. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol 2012;21:245–274. [DOI] [PubMed] [Google Scholar]
- 101.Thiene G, Bruneval P, Veinot J, Leone O.. Diagnostic use of the endomyocardial biopsy: a consensus statement. Virchows Arch 2013;463:1–5. [DOI] [PubMed] [Google Scholar]
- 102.Takeuchi S, Kawada J-I, Okuno Y, Horiba K, Suzuki T, Torii Y, Yasuda K, Numaguchi A, Kato T, Takahashi Y, Ito Y.. Identification of potential pathogenic viruses in patients with acute myocarditis using next-generation sequencing. J Med Virol 2018;90:1814–1821. [DOI] [PubMed] [Google Scholar]
- 103.Siegismund CS, Rohde M, Kühl U, Escher F, Schultheiss HP, Lassner D.. Absent microRNAs in different tissues of patients with acquired cardiomyopathy. Genomics Proteomics Bioinformatics 2016;14:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Corsten MF, Papageorgiou A, Verhesen W, Carai P, Lindow M, Obad S, Summer G, Coort SLM, Hazebroek M, van Leeuwen R, Gijbels MJJ, Wijnands E, Biessen EAL, De Winther MPJ, Stassen FRM, Carmeliet P, Kauppinen S, Schroen B, Heymans S.. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ Res 2012;111:415–425. [DOI] [PubMed] [Google Scholar]
- 105.Aleshcheva G, Pietsch H, Escher F, Schultheiss H-P.. MicroRNA profiling as a novel diagnostic tool for identification of patients with inflammatory and/or virally induced cardiomyopathies. ESC Heart Fail 2021;8:408–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuehl U, Lassner D, Gast M, Stroux A, Rohde M, Siegismund C, Wang X, Escher F, Gross M, Skurk C, Tschoepe C, Loebel M, Scheibenbogen C, Schultheiss H-P, Poller W.. Differential cardiac microRNA expression predicts the clinical course in human enterovirus cardiomyopathy. Circ Heart Fail 2015;8:605–618. [DOI] [PubMed] [Google Scholar]
- 107.Liuzzo G, Patrono C.. Re-purposed antiviral drugs without a purpose in COVID-19: a valuable lesson for clinicians. Eur Heart J 2021;42:882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Horby PW, Mafham M, Bell JL, Linsell L, Staplin N, Emberson J, Palfreeman A, Raw J, Elmahi E, Prudon B, Green C, Carley S, Chadwick D, Davies M, Wise MP, Baillie JK, Chappell LC, Faust SN, Jaki T, Jefferey K, Lim WS, Montgomery A, Rowan K, Juszczak E, Haynes R, Landray MJ.. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2020;396:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB.. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 2020;323:1824–1836. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C.. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen Y-D, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med 2020;383:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian CD, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH, Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fröhlich GM, Jeschke E, Eichler U, Thiele H, Alhariri L, Reinthaler M, Kastrati A, Leistner DM, Skurk C, Landmesser U, Günster C.. Impact of oral anticoagulation on clinical outcomes of COVID-19: a nationwide cohort study of hospitalized patients in Germany. Clin Res Cardiol 2021;110:1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schultheiss HP, Piper C, Sowade O, Waagstein F, Kapp JF, Wegscheider K, Groetzbach G, Pauschinger M, Escher F, Arbustini E, Siedentop H, Kuehl U.. Betaferon in chronic viral cardiomyopathy (BICC) trial: effects of interferon-β treatment in patients with chronic viral cardiomyopathy. Clin Res Cardiol 2016;105:763–773. [DOI] [PubMed] [Google Scholar]
- 115.Zobel T, Bock C-T, Kühl U, Rohde M, Lassner D, Schultheiss H-P, Schmidt-Lucke C.. Telbivudine reduces parvovirus B19-induced apoptosis in circulating angiogenic cells. Viruses 2019;11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schultheiss H-P, Bock T, Pietsch H, Aleshcheva G, Baumeier C, Fruhwald F, Escher F.. Nucleoside analogue reverse transcriptase inhibitors improve clinical outcome in transcriptional active human parvovirus B19-positive patients. J Clin Med 2021;10:1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hazebroek MR, Henkens MTHM, Raafs AG, Verdonschot JAJ, Merken JJ, Dennert RM, Eurlings C, Abdul Hamid MA, Wolffs PFG, Winkens B, Brunner-La Rocca H-P, Knackstedt C, van Paassen P, van Empel VPM, Heymans SRB.. Intravenous immunoglobulin therapy in adult patients with idiopathic chronic cardiomyopathy and cardiac parvovirus B19 persistence: a prospective, double-blind, randomized, placebo-controlled clinical trial. Eur J Heart Fail 2021;23:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Remick J, Georgiopoulou V, Marti C, Ofotokun I, Kalogeropoulos A, Lewis W, Butler J.. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation 2014;129:1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ntusi N, O’Dwyer E, Dorrell L, Wainwright E, Piechnik S, Clutton G, Hancock G, Ferreira V, Cox P, Badri M, Karamitsos T, Emmanuel S, Clarke K, Neubauer S, Holloway C.. HIV-1-related cardiovascular disease is associated with chronic inflammation, frequent pericardial effusions, and probable myocardial edema. Circ Cardiovasc Imaging 2016;9:e004430. [DOI] [PubMed] [Google Scholar]
- 120.Wong GLH, Seto WK, Wong VWS, Yuen MF, Chan HLY.. Review article: long-term safety of oral anti-viral treatment for chronic hepatitis B. Aliment Pharmacol Ther 2018;47:730–737. [DOI] [PubMed] [Google Scholar]
- 121.Frustaci A, Chimenti C, Calabrese F, Pieroni M, Thiene G, Maseri A.. Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunologic profile of responders versus nonresponders. Circulation 2003;107:857–863. [DOI] [PubMed] [Google Scholar]
- 122.Frustaci A, Russo MA, Chimenti C.. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 2009;30:1995–2002. [DOI] [PubMed] [Google Scholar]
- 123.Escher F, Kühl U, Lassner D, Poller W, Westermann D, Pieske B, Tschöpe C, Schultheiss H-P.. Long-term outcome of patients with virus-negative chronic myocarditis or inflammatory cardiomyopathy after immunosuppressive therapy. Clin Res Cardiol 2016;105:1011–1020. [DOI] [PubMed] [Google Scholar]
- 124.Klein AL, Imazio M, Brucato A, Cremer P, LeWinter M, Abbate A, Lin D, Martini A, Beutler A, Chang S, Fang F, Gervais A, Perrin R, Paolini JF.. RHAPSODY: rationale for and design of a pivotal Phase 3 trial to assess efficacy and safety of rilonacept, an interleukin-1α and interleukin-1β trap, in patients with recurrent pericarditis. Am Heart J 2020;228:81–90. [DOI] [PubMed] [Google Scholar]
- 125.Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I, Berghmans P-J, Kimmel M, Van Damme P, de Hoon J, Smith W, Stephenson KE, De Rosa SC, Cohen KW, McElrath MJ, Cormier E, Scheper G, Barouch DH, Hendriks J, Struyf F, Douoguih M, Van Hoof J, Schuitemaker H.. Interim results of a Phase 1-2a trial of Ad26.COV2.S covid-19 vaccine. N Engl J Med 2021;384:1824–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RWJ, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Teo SP. Review of COVID-19 mRNA vaccines: BNT162b2 and mRNA-1273. J Pharm Pract 2021;doi: 10.1177/08971900211009650. [DOI] [PubMed] [Google Scholar]
- 128.Desmond A, Offit PA.. On the Shoulders of Giants - from Jenner’s cowpox to mRNA covid vaccines. N Engl J Med 2021;384:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karikó K, Muramatsu H, Ludwig J, Weissman D.. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res 2011;39:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D.. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther 2008;16:1833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, Wagner W, Granados A, Greenhouse J, Walker M, Willis E, Yu J-S, McGee CE, Sempowski GD, Mui BL, Tam YK, Huang Y-J, Vanlandingham D, Holmes VM, Balachandran H, Sahu S, Lifton M, Higgs S, Hensley SE, Madden TD, Hope MJ, Karikó K, Santra S, Graham BS, Lewis MG, Pierson TC, Haynes BF, Weissman D.. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017;543:248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Combes AJ, Courau T, Kuhn NF, Hu KH, Ray A, Chen WS, Chew NW, Cleary SJ, Kushnoor D, Reeder GC, Shen A, Tsui J, Hiam-Galvez KJ, Muñoz-Sandoval P, Zhu WS, Lee DS, Sun Y, You R, Magnen M, Rodriguez L, Im KW, Serwas NK, Leligdowicz A, Zamecnik CR, Loudermilk RP, Wilson MR, Ye CJ, Fragiadakis GK, Looney MR, Chan V, Ward A, Carrillo S, Matthay M, Erle DJ, Woodruff PG, Langelier C, Kangelaris K, Hendrickson CM, Calfee C, Rao AA, Krummel MF, UCSF COMET Consortium. Global absence and targeting of protective immune states in severe COVID-19. Nature 2021;591:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Izda V, Jeffries MA, Sawalha AH.. COVID-19: a review of therapeutic strategies and vaccine candidates. Clin Immunol 2021;222:108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Landmesser U, Poller W, Tsimikas S, Most P, Paneni F, Lüscher TF.. From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases. Eur Heart J 2020;41:3884–3899. [DOI] [PubMed] [Google Scholar]
- 136.Poller W, Dimmeler S, Heymans S, Zeller T, Haas J, Karakas M, Leistner D-M, Jakob P, Nakagawa S, Blankenberg S, Engelhardt S, Thum T, Weber C, Meder B, Hajjar R, Landmesser U.. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J 2018;39:2704–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fozouni P, Son S, Díaz de León Derby M, Knott GJ, Gray CN, D'Ambrosio MV, Zhao C, Switz NA, Kumar GR, Stephens SI, Boehm D, Tsou C-L, Shu J, Bhuiya A, Armstrong M, Harris AR, Chen P-Y, Osterloh JM, Meyer-Franke A, Joehnk B, Walcott K, Sil A, Langelier C, Pollard KS, Crawford ED, Puschnik AS, Phelps M, Kistler A, DeRisi JL, Doudna JA, Fletcher DA, Ott M.. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell 2021;184:323–333.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abbott TR, Dhamdhere G, Liu Y, Lin X, Goudy L, Zeng L, Chemparathy A, Chmura S, Heaton NS, Debs R, Pande T, Endy D, La Russa MF, Lewis DB, Qi LS.. Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. Cell 2020;181:865–876.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Idris A, Davis A, Supramaniam A, Acharya D, Kelly G, Tayyar Y, West N, Zhang P, McMillan CLD, Soemardy C, Ray R, O'Meally D, Scott TA, McMillan NAJ, Morris KV.. A SARS-CoV-2 targeted siRNA-nanoparticle therapy for COVID-19. Mol Ther 2021;29:2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sajid MI, Moazzam M, Cho Y, Kato S, Xu A, Way JJ, Lohan S, Tiwari RK.. siRNA therapeutics for the therapy of COVID-19 and other coronaviruses. Mol Pharm 2021;18:2105–2121. [DOI] [PubMed] [Google Scholar]
- 141.Baldassarre A, Paolini A, Bruno SP, Felli C, Tozzi AE, Masotti A.. Potential use of noncoding RNAs and innovative therapeutic strategies to target the 5’UTR of SARS-CoV-2. Epigenomics 2020;12:1349–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun L, Li P, Ju X, Rao J, Huang W, Ren L, Zhang S, Xiong T, Xu K, Zhou X, Gong M, Miska E, Ding Q, Wang J, Zhang QC.. In vivo structural characterization of the SARS-CoV-2 RNA genome identifies host proteins vulnerable to repurposed drugs. Cell 2021;184:1865–1883.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Poller W, Tank J, Skurk C, Gast M.. Cardiovascular RNA interference therapy: the broadening tool and target spectrum. Circ Res 2013;113:588–602. [DOI] [PubMed] [Google Scholar]
- 144.Poller W, Hajjar R, Schultheiss H-P, Fechner H.. Cardiac-targeted delivery of regulatory RNA molecules and genes for the treatment of heart failure. Cardiovasc Res 2010;86:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Werk D, Pinkert S, Heim A, Zeichhardt H, Grunert H-P, Poller W, Erdmann VA, Fechner H, Kurreck J.. Combination of soluble coxsackievirus-adenovirus receptor and anti-coxsackievirus siRNAs exerts synergistic antiviral activity against coxsackievirus B3. Antiviral Res 2009;83:298–306. [DOI] [PubMed] [Google Scholar]
- 146.Stein EA, Pinkert S, Becher PM, Geisler A, Zeichhardt H, Klopfleisch R, Poller W, Tschöpe C, Lassner D, Fechner H, Kurreck J.. Combination of RNA interference and virus receptor trap exerts additive antiviral activity in coxsackievirus B3-induced myocarditis in mice. J Infect Dis 2015;211:613–622. [DOI] [PubMed] [Google Scholar]
- 147.Eckstein A, Grössl T, Geisler A, Wang X, Pinkert S, Pozzuto T, Schwer C, Kurreck J, Weger S, Vetter R, Poller W, Fechner H.. Inhibition of adenovirus infections by siRNA-mediated silencing of early and late adenoviral gene functions. Antiviral Res 2010;88:86–94. [DOI] [PubMed] [Google Scholar]
- 148.Guan W, Wong S, Zhi N, Qiu J.. The genome of human parvovirus b19 can replicate in nonpermissive cells with the help of adenovirus genes and produces infectious virus. J Virol 2009;83:9541–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tank J, Lindner D, Wang X, Stroux A, Gilke L, Gast M, Zietsch C, Skurk C, Scheibenbogen C, Klingel K, Lassner D, Kühl U, Schultheiss H-P, Westermann D, Poller W.. Single-target RNA interference for the blockade of multiple interacting proinflammatory and profibrotic pathways in cardiac fibroblasts. J Mol Cell Cardiol 2014;66:141–156. [DOI] [PubMed] [Google Scholar]
- 150.Pinkert S, Westermann D, Wang X, Klingel K, DöRner A, Savvatis K, Größl T, Krohn S, TschöPe C, Zeichhardt H, Kotsch K, Weitmann K, Hoffmann W, Schultheiss H-P, Spiller OB, Poller W, Fechner H, Prevention of cardiac dysfunction in acute coxsackievirus B3 cardiomyopathy by inducible expression of a soluble coxsackievirus-adenovirus receptor. Circulation 2009;120:2358–2366. [DOI] [PubMed] [Google Scholar]
- 151.Schmidt N, Lareau CA, Keshishian H, Ganskih S, Schneider C, Hennig T, Melanson R, Werner S, Wei Y, Zimmer M, Ade J, Kirschner L, Zielinski S, Dölken L, Lander ES, Caliskan N, Fischer U, Vogel J, Carr SA, Bodem J, Munschauer M.. The SARS-CoV-2 RNA-protein interactome in infected human cells. Nat Microbiol 2021;6:339–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Henzinger H, Barth DA, Klec C, Pichler M.. Non-coding RNAs and SARS-related coronaviruses. Viruses 2020;12:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alam T, Lipovich L.. miRCOVID-19: potential targets of human miRNAs in SARS-CoV-2 for RNA-based drug discovery. Noncoding RNA 2021;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kuhl U, Rohde M, Lassner D, Gross UM, Escher F, Schultheiss HP.. miRNA as activity markers in Parvo B19 associated heart disease. Herz 2012;37:637–643. [DOI] [PubMed] [Google Scholar]
- 155.Crooke ST, Baker BF, Crooke RM, Liang X-H.. Antisense technology: an overview and prospectus. Nat Rev Drug Discov 2021;20:427–453. [DOI] [PubMed] [Google Scholar]
- 156.Wolfreys A, Kilgour J, Allen AD, Dudal S, Freke M, Jones D, Karantabias G, Krantz C, Moore S, Mukaratirwa S, Price M, Tepper J, Cauvin A, Manetz S, Robinson I.. Review of the technical, toxicological, and PKPD considerations for conducting inhalation toxicity studies on biologic pharmaceuticals-the outcome of a Cross-Industry Working Group Survey. Toxicol Pathol 2021;49:261–285. [DOI] [PubMed] [Google Scholar]
- 157.Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskämper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss H-P, Hajjar RJ, Poller WC.. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation 2009;119:1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Domenger C, Grimm D.. Next-generation AAV vectors-do not judge a virus (only) by its cover. Hum Mol Genet 2019;28:R3–R14. [DOI] [PubMed] [Google Scholar]
- 159.Poller W, Fechner H, Kühl U, Pauschinger M, Schultheiss HP.. New therapeutics targets in chronic viral cardiomyopathy. Ernst Schering Res Found Workshop 2006;55:287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hinkel R, Ramanujam D, Kaczmarek V, Howe A, Klett K, Beck C, Dueck A, Thum T, Laugwitz K-L, Maegdefessel L, Weber C, Kupatt C, Engelhardt S.. AntimiR-21 prevents myocardial dysfunction in a pig model of ischemia/reperfusion injury. J Am Coll Cardiol 2020;75:1788–1800. [DOI] [PubMed] [Google Scholar]
- 161.Le TK, Paris C, Khan KS, Robson F, Ng W-L, Rocchi P.. Nucleic acid-based technologies targeting coronaviruses. Trends Biochem Sci 2021;46:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morens DM, Fauci AS.. Emerging pandemic diseases: how we got to COVID-19. Cell 2020;182:1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Latinne A, Hu B, Olival KJ, Zhu G, Zhang L, Li H, Chmura AA, Field HE, Zambrana-Torrelio C, Epstein JH, Li B, Zhang W, Wang L-F, Shi Z-L, Daszak P. Origin and cross-species transmission of bat coronaviruses in China. bioRxiv Prepr Serv Biol 2020. 10.1101/2020.05.31.116061. [DOI] [PMC free article] [PubMed] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.