Abstract
Objective:
Radiation therapy (RT) may improve outcomes for patients with oligometastatic cancer. We sought to determine if there are long-term survivors treated with definitive RT for recurrent or oligometastatic gynecological cancer (ROMGC), and to evaluate the clinical and disease characteristics of these patients.
Methods:
We performed a landmark analysis in 48 patients with ROMGC who survived for ≥5 years following definitive RT of their metastasis. Patient characteristics were extracted from the medical record. DFS was modeled using the Kaplan-Meier method.
Results:
This cohort included 20 patients (42%) with ovarian cancer, 16 (33%) with endometrial cancer, 11 (23%) with cervical cancer, and one (2%) with vaginal cancer. The sites of ROMGC were the pelvic (46%), para-aortic (44%), supraclavicular (7%), mediastinal (4%), axillary (4%) lymph nodes and the lung (5.5%). Median total RT dose and fractionation were 62.1 Gy and 2.1 Gy/fraction; one patient was treated with SBRT. 32 patients (67%) received chemoradiation; these patients had higher rates of median DFS than those treated with RT alone (93 vs. 34 months, P=0.05). At median follow-up of 11.7 years, 11 (23%) patients had progression of disease. 20 (42%) patients had died, 9 (19%) died from non-gynecologic cancer and 8 (17%) from gynecologic cancer (three were unknown). 25 (52%) patients were alive and disease- free (10 initially had endometrial cancer [63% of these patients], eight had cervical cancer [73%], six had ovarian cancer [30%], one had vaginal cancer [100%]).
Conclusions:
Long-term survival is possible for patients treated with definitive RT for ROMG, however randomized data are needed to identify which patients derive the most benefit.
Keywords: Gynecologic neoplasms, Neoplasm metastasis, Radiotherapy
INTRODUCTION
The standard treatment for patients with metastatic gynecological cancer is systemic therapy using multi-agent chemotherapy.1 Despite incremental increases in progression-free survival, 5-year survival rates for metastatic patients remain low: 17.6% for cervical cancer, 17.8% for uterine cancer, and 30.3% for ovarian cancer.2,3 Novel targeted systemic agents have shown promise for metastatic gynecological cancer, with several studies demonstrating extended survival in patients receiving these treatments.1,4,5 Advanced radiation therapy (RT) techniques similarly show promise for patients with metastatic gynecological cancer, however no prospective studies exist to demonstrate their effects on survival. Thus, further studies are needed to investigate the role of RT in metastatic gynecological cancer.
A subset of cancer patients who recur have oligometastatic disease, which is the presence of a limited number of metastases that may be treated with curative intent. In non-gynecological cancers, studies have shown that definitive RT for oligometastatic disease leads to prolonged survival.6 For recurrent or oligometastatic gynecological cancer (ROMGC), retrospective studies have shown that definitive radiation treatment of ROMGC leads to greater than 80% local control.7 Additionally, following definitive RT for oligometastatic cervical cancer, Ning et al. showed a median progression-free survival (PFS) and overall survival (OS) of 22 and 51 months, respectively, which compares favorably to outcomes in patients treated with systemic therapy.8 Another study demonstrated a median OS of 50 months after stereotactic body radiation therapy (SBRT) for oligometastatic ovarian cancer.9 Yegya-Raman et al. performed a systematic review of studies analyzing SBRT as definitive treatment for oligometastatic disease from all gynecological cancer types, and similarly showed a median OS of 20 to 60 months following RT.7 These data suggest that local consolidative control of ROMGC using RT contributes to a prolonged survival as compared to historical survival rates in metastatic patients.
Following multi-disciplinary discussion at our institution, we have chosen to treat some ROMGC patients with curative-intent RT. We subsequently found that some of these patients were “cured” of their cancer; for example, we provide two case reports of patients with long-term survival following definitive RT for ROMGC in Figure 1. While the aforementioned studies and our anecdotal experience demonstrate promising outcomes in ROMGC patients treated with definitive RT, the longevity of such survival and characteristics of patients with this long-term survival is unknown. Thus, we sought to determine the curability of definitive RT for ROMGC, and to evaluate the clinical and disease characteristics of patients with prolonged survival.
Figure 1:
Two case reports of patients with long-term survival following definitive radiation therapy (RT) for recurrent or oligometastatic gynecological cancer (ROMGC).
(a) Patient A is a 59 year-old female diagnosed with stage IA endometrioid adenocarcinoma of the uterus in 2003. She was treated with hysterectomy. In 2005, surveillance imaging showed two enlarged common iliac lymph nodes measuring 3.1 cm and 1.8 cm. Biopsy confirmed metastatic adenocarcinoma. She was treated with definitive IMRT (66Gy in 30 fractions) to the pelvic lymph nodes with weekly cisplatin and 4 cycles of adjuvant carboplatin/taxol. Six months later, surveillance imaging showed complete resolution of the metastatic lymph nodes. Surveillance imaging in 2011 continued to show no evidence of disease. She was without evidence of disease at her last follow-up in 2021.
(b) Patient B is a 51 year-old female diagnosed with stage IB adenocarcinoma of the cervix in 2004. She was treated with chemoradiation. In 2010, surveillance imaging showed two enlarged para-tracheal lymph nodes measuring 2.5cm and 1.7cm. Biopsy confirmed metastatic adenocarcinoma of cervical origin. She was treated with definitive IMRT (66Gy in 30 fractions) to the mediastinal lymph nodes with weekly cisplatin. Six months later, surveillance imaging showed stable treated para-tracheal lymph nodes. Surveillance PET scan in 2011 showed resolution of the metastatic lesion with no other sites of disease. She was without evidence of disease at her last follow-up in 2020.
MATERIALS AND METHODS
Patient selection
We performed a retrospective landmark analysis on 48 patients with ROMGC and who survived for at least five years following RT to their metastasis. We used the landmark method to determine the prolonged survival and possible “cure” following treatment of ROMGC. The five-year landmark timepoint was chosen as previous studies have shown that median overall survival of ROMGC patients approaches five years.8–10 Patients were included if their ROMGC was diagnosed at the time of their primary tumor diagnosis (distant site) or at the time of recurrence (regional or distant site). Patients with regional recurrence, such as in the pelvic lymph nodes, were included as these patients are eligible for ongoing oligometastatic clinical trials. Patients with vaginal recurrences were excluded due to the previously established curative treatments for these patients. The definition of “oligometastatic” was consistent with the definition used in the SABR-COMET clinical trial: up to five metastatic lesions with a maximum of three in one organ.6 All gynecological malignancies and histologies were included. The study was approved by the institutional review board.
To be eligible for inclusion, all patients received definitive RT at our institution between 2000 and 2015. Definitive RT was defined as an equivalent dose in 2 Gy fractions (EQD2) ≥ 55 Gy using conventional or hypofractionated radiation techniques. Some patients received chemotherapy as part of their ROMGC treatment regimen. Patients were excluded if they had surgical resection of their ROMGC prior to receiving RT.
Treatment
Of the 48 patients eligible for inclusion in our study, 45 (94%) had ROMGC in a nodal site, including pelvic, paraaortic, supraclavicular, and mediastinal lymph nodes, and three (6%) had ROMGC in a solid organ (lung). Nodal ROMGC sites were treated with 2D/3DRT or intensity-modulated RT (IMRT) to a median dose of 62 Gy (interquartile range [IQR] 54.6 – 65.1 Gy) to the gross tumor volume (GTV) in 2.0 – 2.2 Gy per fraction. For a majority of patients, a lower dose of 45 – 50 Gy in 1.8 – 2.0 Gy per fraction was used to treat the clinical target volume (CTV). The CTV was defined as the entire involved nodal echelon and at-risk adjacent lymphatics as determined by the treating radiation oncologist. The CTV received a 0.5 – 1 cm expansion to create the planning target volume (PTV) to take into account variations in daily treatment set-up.
Of the three patients with ROMGC in the lung, two were treated with 3DRT and one was treated with SBRT. Both patients treated with 3DRT received a total dose of 52.8 Gy given in 2.4 Gy per fraction for 22 fractions. The GTV received a 5 mm margin to create the CTV and the CTV received a 5 mm margin to create the PTV. For the patient who received SBRT, the total dose was 37.5 Gy given in 12.5 Gy per fraction for 3 fractions. The GTV received a 5 mm margin to create the PTV.
Patients received chemotherapy before (induction), during (concurrent), and after (adjuvant) definitive radiation treatment of their ROMGC. Induction chemotherapy was defined as chemotherapy administered within three months of the RT start date. Adjuvant chemotherapy was defined as planned chemotherapy administered within two months of the RT completion date. Acute radiation and chemoradiation treatment-related toxicity was graded according to the Common Terminology Criteria for Adverse Events v4.0.
Following definitive RT completion, patients were followed with surveillance imaging or physical exam every three months for two years, then every six months for two years, and then annually.
Study endpoints
The primary endpoint was disease-free survival (DFS) after radiation treatment of the patient’s ROMGC. Disease progression was defined as presence of a local, regional, or distant recurrence as identified on imaging (CT, PET/CT, MRI), with a majority confirmed on pathology review. In cases without pathology confirmation, the progression of disease was confirmed independently by a board-certified radiologist, radiation oncologist, and gynecologic oncologist. Local recurrence was defined as development of new disease within the treated ROMGC radiation field. Regional recurrence was defined as development of new disease in a lymphatic or visceral site adjacent to the treated ROMGC radiation field. Distant recurrence was defined as development of new disease outside of the treated ROMGC radiation field and its adjacent structures. The time to disease progression was calculated from the date of ROMGC radiation treatment completion to the date of the imaging study showing progression. The secondary endpoint was overall survival (OS) after radiation treatment of the patient’s ROMGC. Date of death was extracted from the medical record or from online obituary records. The cause of death was determined from the medical record and coded as from gynecological cancer, non-gynecological cancer, or unknown. The time to death was calculated from the date of ROMGC radiation treatment completion to the date of death.
Statistical analyses
Patient characteristics and outcomes were summarized with N (%) for categorical variables and median (interquartile range) for continuous variables. Actuarial rates of DFS and OS were calculated using the Kaplan-Meier method. For DFS, disease progression and death were scored as events, with patients censored at date of progression or death. For OS, patients were censored at last follow-up or death. Log-rank tests were used to evaluate potential differences between groups. Statistical analyses were conducted using SPSS, Version 26.0 (IBM Corp., NY, USA).
RESULTS
Patient and clinical characteristics
Forty-eight patients survived for five years following definitive RT for their ROMGC and were included in our analysis. A majority of patients (20 patients, 42%) had ovarian cancer as their primary tumor diagnosis; 16 (33%) had endometrial cancer, 11 (23%) had cervical cancer, and 1 (2%) had vaginal cancer. A majority of tumors were adenocarcinoma histology (35%). Sixteen patients (33%) were Stage I at primary tumor diagnosis, three (6%) were Stage II, 23 (48%) were Stage III, and five (10%) were Stage IV. For treatment of their primary tumor, 39 patients (81%) received surgery, 32 (67%) received systemic therapy, and 14 (29%) received RT. The remaining clinical characteristics for the primary tumor treatment are described in Table 1.
Table 1:
Patient and primary tumor treatment characteristics (N=48).
| N (%) | |
|---|---|
| Median age at diagnosis (years) (IQR) | 52 (47–62) |
| Race/Ethnicity | |
| White, Non-Hispanic | 35 (72.9%) |
| Hispanic | 9 (18.8%) |
| Black, Non-Hispanic | 3 (6.3%) |
| Asian, Non-Hispanic | 1 (2.1%) |
| Diagnosis | |
| Ovarian | 20 (41.7%) |
| Endometrial | 16 (33.3%) |
| Cervical | 11 (22.9%) |
| Vaginal | 1 (2.1%) |
| Histology | |
| Ovarian | |
| Serous carcinoma | 10 (20.8%) |
| Adenocarcinoma | 3 (6.3%) |
| Mullerian carcinoma | 2 (4.2%) |
| Clear cell carcinoma | 2 (4.2%) |
| Mixed carcinoma | 2 (4.2%) |
| Sarcoma | 1 (2.1%) |
| Endometrial | |
| Adenocarcinoma | 11 (22.9%) |
| Mullerian carcinoma | 2 (4.2%) |
| Clear cell carcinoma | 2 (4.2%) |
| Serous carcinoma | 1 (2.1%) |
| Cervical | |
| Squamous cell carcinoma | 6 (12.5%) |
| Adenocarcinoma | 3 (6.3%) |
| Mixed carcinoma | 2 (4.2%) |
| Vaginal | |
| Squamous cell carcinoma | 1 (2.1%) |
| Stage | |
| I | 16 (33.3%) |
| II | 3 (6.3%) |
| III | 23 (47.9%) |
| IV | 5 (10.4%) |
| Unknown | 1 (2.1%) |
| Grade | |
| 1 | 5 (10.4%) |
| 2 | 9 (18.8%) |
| 3 | 21 (43.8%) |
| Unknown | 13 (27.1%) |
| Surgical resection | |
| No | 9 (18.8%) |
| Yes | 39 (81.3%) |
| Systemic therapy | |
| No | 16 (33.3%) |
| Yes | 32 (66.7%) |
| Systemic therapy agent | |
| Carboplatin-Paclitaxel | 19 (59.4%) |
| Carboplatin-Cyclophosphamide | 3 (9.4%) |
| Carboplatin | 3 (9.4%) |
| Cisplatin-Paclitaxel | 2 (6.3%) |
| Cisplatin | 18 (56.3%) |
| Topotecan | 1 (3.1%) |
| Radiation therapy | |
| No | 34 (70.8%) |
| Yes | 14 (29.2%) |
| External-beam radiation site | |
| Whole pelvis | 13 (87.7%) |
| Extended-field | 7 (46.7%) |
| Radiation technique | |
| Brachytherapy | 7 (50%) |
| Intensity-modulated radiation therapy | 5 (35.7%) |
| 2D/3D | 4 (28.6%) |
| Unknown | 1 (7.1%) |
| Median external-beam radiation dose (Gy) (IQR) | 50.0 (42.5–69.4) |
| Median external-beam radiation dose per fraction (Gy) (IQR) | 2.0 (1.8–2.0) |
The median time to ROMGC diagnosis was 28 months (IQR 13 – 47). The median number of prior courses of systemic therapy was 0 (IQR 0 – 1). Forty-one patients (85%) had one involved organ site at time of ROMGC diagnosis and seven (15%) had two involved organ sites. The most common sites of ROMGC were in the pelvic (46%) and para-aortic (44%) lymph nodes (LN) (Table 2). There were eight (15%) ROMGC lesions in distant nodal sites (two in mediastinal LNs, two in axillary LNs, four in supraclavicular LNs) and three (6%) ROMGC lesions in a solid organ (all in lung). A majority of patients (73%) were treated with intensity-modulated radiation therapy (IMRT) or volumetric-modulated arc therapy (VMAT). Twelve patients (13%) were treated with 2D/3DRT and one patient (2%) was treated with SBRT. The median total radiation dose was 62 Gy (IQR 54.6 – 65.1) given in 2.1 Gy per fraction (IQR 2.0 – 2.2). Chemotherapy was administered for thirty-two patients (67%). Seven (22%) patients had induction chemotherapy, 23 (72%) had concurrent chemotherapy, and two (6%) had adjuvant chemotherapy (Table 2).
Table 2:
Recurrent or oligometastatic gynecological cancer treatment characteristics (N=48).
| N (%) | |
|---|---|
| Median time to ROMGC (months) (IQR) | 28 (13–47) |
| Median number of prior courses of systemic therapy (IQR) | 0 (0–1) |
| Number of oligometastatic sitesa | |
| 1 | 41 (85.4%) |
| 2 | 7 (14.6%) |
| Sites of first recurrence (N=55) | |
| Para-aortic lymph node | 21 (43.8%) |
| External iliac lymph node | 9 (16.4%) |
| Internal iliac lymph node | 1 (1.8%) |
| Common iliac lymph node | 5 (9.1%) |
| Inguinal lymph node | 5 (9.1%) |
| Pre-sacral lymph node | 1 (1.8%) |
| Obturator lymph node | 1 (1.8%) |
| Supraclavicular lymph node | 4 (7.3%) |
| Mediastinum lymph node | 2 (3.6%) |
| Axillary lymph node | 2 (3.6%) |
| Lung | 3 (5.5%) |
| Median size of ROMGC (cm) (IQR) | 1.8 (1.3–3.0) |
| Radiation therapy technique | |
| IMRT/VMAT | 35 (72.9%) |
| 2D/3D | 12 (12.5%) |
| SBRT | 1 (2.1%) |
| Median external-beam radiation dose (Gy) (IQR) | 62 (54.6–65.1) |
| Median external-beam radiation dose per fraction (Gy) (IQR) | 2.1 (2.0–2.2) |
| Systemic therapy | |
| No | 16 (33.3%) |
| Yes | 32 (66.7%) |
| Systemic therapy timing | |
| Induction | 7 (21.9%) |
| Concurrent | 23 (71.9%) |
| Adjuvant | 2 (6.3%) |
| Systemic therapy agent | |
| Carboplatin-Paclitaxel | 11 (34.4%) |
| Carboplatin-Interferon | 1 (3.1%) |
| Carboplatin | 1 (3.1%) |
| Paclitaxel | 2 (6.3%) |
| Cisplatin-Interferon | 1 (3.1%) |
| Cisplatin | 16 (50%) |
Abbreviations: Recurrent or oligometastatic gynecological cancer, ROMGC; Intensity-modulated radiation therapy, IMRT; Volumetric-modulated arc therapy, VMAT; Stereotactic body radiation therapy, SBRT.
Number of oligometastatic lesions at first ROMGC diagnosis.
Survival endpoints
Twenty-four patients (50%) had disease recurrence after receiving definitive RT for their ROMGC (Table 3); median time to recurrence was 28 months (IQR 16 – 54). The initial gynecological cancer profile of the patients who recurred was as follows: three had cervical cancer (27% of these patients), five had endometrial cancer (31% of all endometrial cancer patients), and 16 had ovarian cancer (80% of these patients). Six (25%) of the patients with recurrence had local (in-field) failure, 14 (58%) had regional failure, and four (17%) had simultaneous regional and distant failure. Of the six patients with local failure, all were treated with total RT doses in the 62 – 68 Gy range at 2.06 – 2.33 Gy per fraction. Five of these patients had primary ovarian cancer (three with ovarian serous carcinoma, one with ovarian mullerian carcinoma, one with ovarian mucinous adenocarcinoma) and one had primary endometrial endometrioid adenocarcinoma. Salvage treatment varied between combinations of resection (13%), systemic therapy (75%), and additional courses of RT (29%).
Table 3:
Survival characteristics following treatment of recurrent or oligometastatic gynecological cancer (N=48).
| N (%) | |
|---|---|
| Number of patients with failure | 24 (50%) |
| Median time to first failure (months) (IQR) | 28 (16–54) |
| Site of failurea | |
| Local | 6 (25%) |
| Regional | 14 (58.3%) |
| Regional/Distant | 4 (16.7%) |
| Salvage treatmentb | |
| Surgery | 3 (12.5%) |
| Systemic therapy | 18 (75%) |
| Radiation therapy | 7 (29.2%) |
| Status at last follow-up | |
| No evidence of disease | 25 (52.1%) |
| Progression of disease | 3 (6.3%) |
| Death | 20 (41.7%) |
| Cause of death | |
| Non-gynecological cancer | 9 (45%) |
| Gynecological cancer | 8 (42.1%) |
| Unknown | 3 (15.8%) |
Local failure was defined as recurrence within the treated ROMGC radiation field. Regional failure was defined as recurrence in a lymphatic or visceral site adjacent to the radiation field. Distant failure was defined as recurrence outside of the treated ROMGC radiation field and its adjacent structures.
Some patients were treated with more than one salvage option.
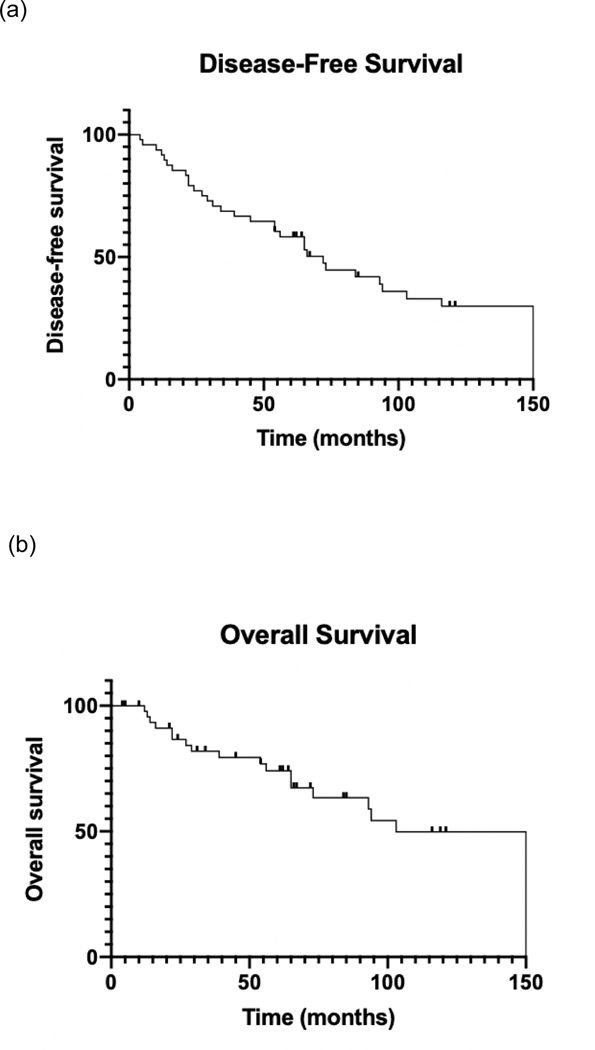
The median follow-up was 11.7 years from completion of definitive RT for ROMGC. The median DFS and OS for all patients following ROMGC treatment completion was 73 (range: 4 – 212) and 200 months (range: 61 – 212), respectively (Figure 2). Patients who received any sequence of chemotherapy with RT had longer median DFS than those treated with RT alone (93 vs. 34 months, P=0.05; Figure 3). At the last follow-up, 11 (22.9%) patients had progression of disease, three of whom were alive (one cervical cancer patient [9%] and two ovarian cancer patients [10%]). Twenty (42%) patients had died at the last follow-up; nine (45%) died of non-gynecological cancer causes, eight (42%) died of gynecological cancer (one endometrial cancer patient [6%], one cervical cancer patient [9%], and six ovarian cancer patients [30%]), and three (16%) had unknown cause of death. Of the nine who died from non- gynecological cancer, five died of non-cancer-related reasons and four died of second primary cancers (two had acute myeloblastic leukemia, one had bladder cancer, one had colon cancer).
Figure 2:
(a) Disease-free survival (DFS) and (b) overall survival (OS) following definitive radiation treatment (RT) of recurrent or oligometastatic gynecological cancer (ROMGC). Time is measured in months from completion of ROMGC treatment.
Figure 3:
Disease-free survival (DFS) following radiation alone versus chemoradiation for recurrent or oligometastatic gynecological cancer (ROMGC). Time is measured in months from completion of ROMGC treatment.
Twenty-five (52%) patients were alive and had no evidence of disease at long-term follow-up. The initial gynecological cancer profile of these 25 patients was as follows: 10 had endometrial cancer (63% of all endometrial cancer patients), eight had cervical cancer (73% of these patients), six had ovarian cancer (30% of these patients), one had vaginal cancer (100% of these patients). Ten were initially treated for para-aortic LN ROMGC (48% of all para-aortic LN ROMGC patients), eight for pelvic LN ROMGC (36% of these patients), six for supra-diaphragmatic LN ROMGC (75% of these patients), and two for lung ROMGC (67% of these patients).
Toxicity
Among the 48 patients, the most common toxicity was nausea (25 patients, 52%) (Table 4). Sixteen patients (33%) experienced Grade 2 toxicities. One patient (2%) experienced a Grade 3 toxicity during chemoradiation and was hospitalized for neutropenia. None of the reported toxicities resulted in radiation treatment breaks. There were no Grade 4 or 5 toxicities observed.
Table 4:
Acute toxicities following definitive radiation therapy of recurrent or oligometastatic gynecological cancer.
| Toxicity Type | Toxicity Grade, N (%) | ||
|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |
| Nausea | 25 (52.1%) | 3 (6.3%) | - |
| Dermatitis | 6 (12.5%) | 4 (8.3%) | - |
| Fatigue | 7 (14.6%) | - | - |
| Pain | 4 (8.3%) | 2 (4.2%) | - |
| Urinary dysfunction | 6 (12.5%) | 1 (2.1%) | - |
| Appetite loss | 2 (4.2%) | - | - |
| Dysphagia | 2 (4.2%) | - | - |
| Neutropenia | 2 (4.2%) | 1 (2.1%) | 1 (2.1%) |
| Thrombocytopenia | 2 (4.2%) | - | |
DISCUSSION
This study investigated the possibility for long-term survival following definitive RT for recurrent or oligometastatic gynecological cancer. A majority of patients had pelvic or para-aortic ROMGC lesions, and were treated with combined IMRT and chemotherapy. Patients with endometrial cancer and cervical cancer had lower rates of recurrence following treatment of their ROMGC and better rates of long-term survival as compared with patients with ovarian cancer. Notably, we found that patients who received chemotherapy with RT had longer survival than those who received RT alone. Following ROMGC treatment, half of patients, with varying primary cancer types and sites of ROMGC, remained disease-free through our long-term median follow-up of 11.7 years. Thus, long-term survival seems to be achievable in ROMGC patients.
Previous studies across gynecological malignancy types have similarly shown favorable outcomes and acceptable toxicities from treating ROMGC patients with definitive RT. One study analyzed ROMGC following 2D/3DRT, and found that definitive RT was associated with improved locoregional control.11 Yegya-Raman et al.’s systematic review of SBRT for ROMGC showed that definitive RT results in ≥80% local control in a majority of studies, with disease progression most commonly occurring outside the RT field.7 Additionally, several analyses have shown that definitive RT for ROMGC was well-tolerated with low incidence of grade 3 or higher toxicities and minimal late toxicities.7,12,13 Follow-up in these studies ranged from 5 to 55 months.7,11 Our study is the first with significantly long median follow-up (140 months) and demonstrates that definitive RT for ROMGC is well-tolerated and leads to prolonged disease-free survival (≥5 years) for some patients.
It is difficult to directly compare the results of the aforementioned studies with ours as they studied all patients with ROMGC regardless of survival status and focused on SBRT as the definitive modality whereas our patients were selected for good survival performance and the majority were treated with longer treatment regimens using conventionally-fractionated IMRT. However, as the field of radiation oncology adapts to new technologies and modalities, it is worth discussing the optimal radiation fractionation and field size for ROMGC patients. The advantages of SBRT are clear: short treatment duration, excellent local control, and minimal grade 3 or greater toxicities.7 One disadvantage of using SBRT in nodal ROMGC is the lack of coverage of the entire nodal basin at risk, which may have consequences as gynecological malignancies tend to harbor microscopic disease in nodal distributions. In our study, a majority were treated with RT covering the ROMGC site as well as the entire nodal basin involved by the metastatic lesion, resulting in a 75% local control rate within the treated area. In some SBRT retrospective studies, a subset of patients who received SBRT alone had recurrences in the at-risk nodal basin as compared to patients who received upfront elective nodal RT in addition to SBRT had no at-risk nodal failures.8,14,15 It is unknown whether salvage of at-risk nodal basin failures has comparable survival to upfront elective nodal RT combined with ablative treatment of the ROMGC lesion, thus future studies are needed to investigate the optimal ROMGC radiation technique and target.
Patients with ROMGC represent a heterogeneous cohort with differing cancer histologies, sites of metastasis, number of metastases, and salvage therapy options. Accordingly, careful selection for patients who may have survival benefit from definitive RT for their ROMGC is important. Prior studies have suggested that smaller size of ROMGC is associated with better outcomes following definitive RT.7 Additionally, patients with nodal or lung ROMGC who are treated with definitive RT have longer survival,7,13 as supported by our study’s patient population consisting of all nodal and lung ROMGC patients. The primary cancer type may also be an important consideration as our study and others have demonstrated better outcomes among cervical cancer and endometrial cancer patients.16 Finally, prior studies have shown no difference in DFS based on the number of ROMGC lesions (up to five) at diagnosis.8,12 All together, the current data from this and prior studies suggest that aggressive treatment of ROMGC patients with varying primary cancer types, sites of ROMGC, and number of metastases may be worthwhile. In our current practice, we carefully select ROMGC patients for such aggressive treatment, with the following examples of our RT approach:
Recurrent or oligometastatic lymph node: We use conventional radiation fractionation for these patients in order to include an elective regional nodal volume to prevent adjacent recurrences. We typically treat to 45 Gy in 25 fractions to a CTV including the regional nodal basin with a simultaneous integrated boost to 50 Gy to the gross lymph node. This is typically followed by a sequential boost to the GTV to 60–66 Gy depending on the lesion size and location.
Recurrent or oligometastatic lesion in solid organs, such as lung, liver, adrenal glands, or bone: We use hypofractionated RT or SBRT for these patients. The dose and fractionation depend on lesion location and adjacent structures.
In patients deemed appropriate to receive definitive RT for ROMGC, it is essential to consider concomitant chemotherapy. Our study showed acceptable acute toxicities and better DFS in patients who received chemotherapy alongside definitive RT, and a prior study from our institution showed no difference in survival based on the timing of chemotherapy (induction, concurrent, or adjuvant) in oligometastatic cervical cancer patients.8 There is a paucity of literature studying chemoradiation for ROMGC, however one phase I study of chemotherapy with SBRT showed acceptable toxicities.17 Thus, this is a critical area of further investigation. Furthermore, the combination of immunotherapy or targeted therapy with definitive RT for ROMGC should also be further investigated given the promise of these systemic therapies in advanced ovarian, cervical, and endometrial cancers.18–21
There has been limited representation of gynecological cancer patients on prospective oligometastatic studies.22 Our results and the prior retrospective studies discussed in this paper demonstrate minimal toxicities and favorable survival outcomes, leading to prolonged survival in a subset of patients. A prospective study is warranted to evaluate which ROMGC patients derive a survival benefit from definitive RT. Several ongoing phase I/II clinical trials exist studying SBRT in ROMGC,23–26 with some incorporating systemic therapy.27–31 These studies are a great starting point; however, there are no current randomized phase III studies studying ROMGC and additional questions remain unknown, including the optimal RT technique and target for ROMGC as well as optimal follow-up strategies for these patients.
Our study has several limitations. First, this was a single-institution retrospective review, which limits the generalizability of our results. Despite this limitation, our sample size was comparable to prior studies of oligometastatic gynecological cancer. Second, we utilized a landmark design of our analysis by identifying a carefully selected, well-performing cohort in order to assess the potential for long-term survival. Another disadvantage of the landmark design is the unanswered question of significance of our findings as related to the entire ROMGC patient cohort. Thus, our cohort cannot be used to determine the survival rates for all patients receiving definitive treatment of ROMGC. However, the aim of our study was to determine the possibility of long-term survival following definitive RT for ROMGC patients, which we were effectively able to determine given the moderate sample size and long median follow-up of over 11 years.
CONCLUSIONS
Long-term survival is achievable in some patients with recurrent or oligometastatic gynecological cancer. Definitive radiation therapy may be an essential component of treatment for ROMGC and contribute to the long-term survival in these patients. Chemotherapy is another essential aspect of treatment, and should be considered alongside definitive RT. Patients with different primary cancer types and sites of ROMGC may all benefit from aggressive treatment of oligometastatic disease, thus randomized clinical trials are needed to identify patients who have a survival advantage following definitive RT for ROMGC. Then, we may be able to treat such patients with an unprecedented goal for metastatic gynecological cancer patients: cure.
Research Highlights:
We studied recurrent or oligometastatic gynecological cancer (ROMGC) survivors following receipt of definitive RT.
48 patients were studied: 20 ovarian cancer, 16 endometrial cancer, 11 cervical cancer, 1 vaginal cancer.
Patients who received chemoradiation had better median DFS than those treated with RT alone (93 vs. 34 months, P=0.05).
25 patients (52%) were alive and disease-free at median follow-up of 11.7 years.
Acknowledgments
Funding: This work was supported in part by Cancer Center Support (Core) grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Footnotes
Prior presentation: This work was presented as a poster at the 63rd Annual ASTRO Scientific Meeting on October 25, 2021.
Data Sharing Statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tewari KS, Sill MW, Long HJ 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SEER 5-Year Relative Survival Rates, 2011–2017. National Cancer Insitutute;2018. [Google Scholar]
- 3.Bodurka-Bevers D, Morris M, Eifel PJ, et al. Posttherapy surveillance of women with cervical cancer: an outcomes analysis. Gynecol Oncol. 2000;78(2):187–193. [DOI] [PubMed] [Google Scholar]
- 4.Moore KN, Secord AA, Geller MA, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):636–648. [DOI] [PubMed] [Google Scholar]
- 5.Chan JK, Tian C, Teoh D, et al. Survival after recurrence in early-stage high-risk epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;116(3):307–311. [DOI] [PubMed] [Google Scholar]
- 6.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. (1474–547X (Electronic)). [DOI] [PubMed] [Google Scholar]
- 7.Yegya-Raman N, Cao CD, Hathout L, et al. Stereotactic body radiation therapy for oligometastatic gynecologic malignancies: A systematic review. Gynecol Oncol. 2020;159(2):573–580. [DOI] [PubMed] [Google Scholar]
- 8.Ning MS, Ahobila V, Jhingran A, et al. Outcomes and patterns of relapse after definitive radiation therapy for oligometastatic cervical cancer. Gynecol Oncol. 2018;148(1):132–138. [DOI] [PubMed] [Google Scholar]
- 9.Mills M, Reddy AV, Reshko LB, Richardson KM, Kersh CR. Clinical Outcomes of Stereotactic Body Radiation Therapy for Extracranial Oligometastatic Ovarian Cancer. Int J Radiat Oncol Biol Phys. 2019;105(1):E569. [Google Scholar]
- 10.Macchia G, Lazzari R, Colombo N, et al. A Large, Multicenter, Retrospective Study on Efficacy and Safety of Stereotactic Body Radiotherapy (SBRT) in Oligometastatic Ovarian Cancer (MITO RT1 Study): A Collaboration of MITO, AIRO GYN, and MaNGO Groups. Oncologist. 2020;25(2):e311–e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sole CV, Calvo FA, Lizarraga S, et al. Single-Institution Multidisciplinary Management of Locoregional Oligo-Recurrent Pelvic Malignancies: Long-Term Outcome Analysis. Ann Surg Oncol. 2015;22 Suppl 3:S1247–1255. [DOI] [PubMed] [Google Scholar]
- 12.Reddy AV, Mills MN, Reshko LB, Martin Richardson K, Kersh CR. Stereotactic Body Radiation Therapy in Oligometastatic Uterine Cancer: Clinical Outcomes and Toxicity. Cancer Invest. 2020;38(8–9):522–530. [DOI] [PubMed] [Google Scholar]
- 13.Franceschini D, De Rose F, Franzese C, et al. Predictive Factors for Response and Survival in a Cohort of Oligometastatic Patients Treated With Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. 2019;104(1):111–121. [DOI] [PubMed] [Google Scholar]
- 14.Seo YS, Kim MS, Cho CK, et al. Stereotactic body radiotherapy for oligometastases confined to the para-aortic region: clinical outcomes and the significance of radiotherapy field and dose. Cancer Invest. 2015;33(5):180–187. [DOI] [PubMed] [Google Scholar]
- 15.Higginson DS, Morris DE, Jones EL, Clarke-Pearson D, Varia MA. Stereotactic body radiotherapy (SBRT): Technological innovation and application in gynecologic oncology. Gynecol Oncol. 2011;120(3):404–412. [DOI] [PubMed] [Google Scholar]
- 16.Reshko LB, Baliga S, Crandley EF, et al. Stereotactic body radiation therapy (SBRT) in recurrent, persistent or oligometastatic gynecological cancers. Gynecol Oncol. 2020;159(3):611–617. [DOI] [PubMed] [Google Scholar]
- 17.Kunos CA, Sherertz TM, Mislmani M, et al. Phase I Trial of Carboplatin and Gemcitabine Chemotherapy and Stereotactic Ablative Radiosurgery for the Palliative Treatment of Persistent or Recurrent Gynecologic Cancer. Front Oncol. 2015;5:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamarin D, Burger RA, Sill MW, et al. Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J Clin Oncol. 2020;38(16):1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung HC, Ros W, Delord JP, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2019;37(17):1470–1478. [DOI] [PubMed] [Google Scholar]
- 20.Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol. 2020;38(26):2981–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin LL, Lakomy DS, Ning MS, Simpkins F, Jhingran A. Combining novel agents with radiotherapy for gynecologic malignancies: beyond the era of cisplatin. Int J Gynecol Cancer. 2020;30(4):409–423. [DOI] [PubMed] [Google Scholar]
- 22.Zhang TW, Palma D, D’Souza D, Velker V, Mendez LC. Stereotactic Ablative Radiotherapy for Recurrent or Metastatic Gynecological Cancer: Extending Lives? Curr Treat Options Oncol. 2020;21(7):58. [DOI] [PubMed] [Google Scholar]
- 23.Fisher C. Stereotactic Body Radiation Therapy in Treating Patients With Recurrent Primary Ovarian or Uterine Cancer. (NCT03325634). [Google Scholar]
- 24.Colbert L. Single Fraction or Multi-fraction Palliative Radiation Therapy for the Improvement of Quality of Life in Patients With Metastatic Gynecologic Cancers. (NCT04516135). [Google Scholar]
- 25.Verkooijen HM. The MOMENTUM Study: The Multiple Outcome Evaluation of Radiation Therapy Using the MR-Linac Study (MOMENTUM). (NCT04075305). [Google Scholar]
- 26.Robinson C. MRI-Guided Stereotactic Body Radiation Therapy (SBRT) for Ovarian Cancer. (NCT02582931). [Google Scholar]
- 27.Waggoner S, Carboplatin Gemcitabine Hydrochloride, and Stereotactic Body Radiation Therapy in Gynecological Cancer. (NCT01652794). [Google Scholar]
- 28.Lee LJ. Durvalumab, Tremelimumab + Radiotherapy in Gynecologic Cancer. (NCT03277482). [Google Scholar]
- 29.Lin LL. Stereotactic Body Radiation Therapy, Tremelimumab and Durvalumab in Treating Participants With Recurrent or Metastatic Cervical, Vaginal, or Vulvar Cancers. (NCT03452332). [Google Scholar]
- 30.Denys H. Study of Pembrolizumab, Radiation and Immune Modulatory Cocktail in Cervical/Uterine Cancer (PRIMMO). (NCT03192059). [Google Scholar]
- 31.Ahmed K. SBRT and Atezolizumab in the Management of Recurrent, Persistent, or Metastatic Cervical Cancer. (NCT03614949). [Google Scholar]