Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a highly metastatic disease. Tumors are poorly immunogenic and immunosuppressive, preventing T cell activation in the tumor microenvironment. Here, we present a microbial-based immunotherapeutic treatment for selective delivery of an immunogenic tetanus toxoid protein (TT856–1313) into PDAC tumor cells by attenuated Listeria monocytogenes. This treatment reactivated preexisting TT-specific memory T cells to kill infected tumor cells in mice. Treatment of KrasG12D,p53R172H, Pdx1-Cre (KPC) mice with Listeria-TT resulted in TT accumulation inside tumor cells, attraction of TT-specific memory CD4 T cells to the tumor microenvironment, and production of perforin and granzyme B in tumors. Low doses of gemcitabine (GEM) increased immune effects of Listeria-TT, turning immunologically cold into hot tumors in mice. In vivo depletion of T cells from Listeria-TT + GEM–treated mice demonstrated a CD4 T cell–mediated reduction in tumor burden. CD4 T cells from TT-vaccinated mice were able to kill TT-expressing Panc-02 tumor cells in vitro. In addition, peritumoral lymph node–like structures were observed in close contact with pancreatic tumors in KPC mice treated with Listeria-TT or Listeria-TT + GEM. These structures displayed CD4 and CD8 T cells producing perforin and granzyme B. Whereas CD4 T cells efficiently infiltrated the KPC tumors, CD8 T cells did not. Listeria-TT + GEM treatment of KPC mice with advanced PDAC reduced tumor burden by 80% and metastases by 87% after treatment and increased survival by 40% compared to nontreated mice. These results suggest that Listeria-delivered recall antigens could be an alternative to neoantigen-mediated cancer immunotherapy.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is often diagnosed at an advanced stage and is therefore difficult to cure. Modern antineoplastic therapies, such as gemcitabine (GEM), provide minimal increased survival benefits (1–4), whereas the combination of GEM and Abraxane or FOLFIRINOX (FOLinic acid, Fluoracil, IRINothecan, Oxaliplatin) further modestly improves survival (5, 6). Therefore, additional and innovative approaches to PDAC treatment are necessary. Immunotherapy with checkpoint inhibitors has shown promising results for some cancers but less so for PDAC (7). PDAC is poorly immunogenic due to its low mutational load and a lack of effective neoantigens (8, 9). Moreover, immune suppression, particularly by myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), prevents T cell activation in the tumor microenvironment (TME) (10–12).
To address these problems, we developed a treatment using an attenuated bacterium Listeria monocytogenes (13) (Listeria) to selectively deliver highly immunogenic recall antigens, such as tetanus toxoid (TT) into tumor cells. Here, the TT functions as a neoantigen alternative that reactivates preexisting TT-specific memory T cells generated during childhood vaccinations. The TT-specific memory cells are in turn attracted to the TT in the TME, where they destroy the infected, now highly immunogenic tumor cells.
Memory T cells are less prone to apoptosis and more efficiently activated than naive T cells in the TME of patients with cancer and tumor-bearing mice (14, 15). Moreover, memory T cells circulate in the blood for life and can be reactivated even in old age when most cancers occur (16–18). Long-lasting memory T cells overcome the problem of age-related loss of naive T cells caused by immunosenescence. To further improve T cell responses against TT, Listeria-TT was combined with low doses of GEM, which alone reduces the immune suppressive MDSC and TAM populations.
In previous studies, we analyzed mechanisms involved in the selective delivery and survival of Listeria in the TME. We showed that Listeria attracts MDSCs (10) and infects MDSCs most likely through C3bi and C1q receptors (19–21). MDSCs are present in large numbers in patients and mice with cancer (11, 22). However, the primary tumor also attracts MDSCs through the production of cytokines and factors (11, 23), and thus, MDSC delivers Listeria to the TME as a Trojan horse (10, 22). Once at the tumor site, Listeria spreads from MDSCs into tumor cells (10) through a cell-to-cell mechanism unique to Listeria (24). Briefly, Listeria accomplishes intercellular spread by abducting the host cell’s actin-based motility components to propel itself into the host cell’s plasma membrane and infect adjacent cells. The membrane-associated bacterial protein ActA is necessary and sufficient for such motility. Whereas Listeria is rapidly eliminated from normal tissues by the immune system, in the TME, Listeria is protected through strong immune suppression. This combination of selective attraction of Listeria-infected MDSCs by the tumor and an immunosuppressed environment enables Listeria to selectivity enter, multiply, and survive in the TME but not in normal tissues (10, 22, 25, 26). This makes Listeria a highly suitable platform for the selective delivery of anticancer agents to the TME (22, 25, 27). This approach is distinctly different from previous clinical trials where Listeria has been used as a vaccine to infect antigen-presenting cells to stimulate T cells against naturally expressed, weak tumor–associated antigens (28). In our approach, Listeria is used to deliver strong childhood vaccine recall antigens into tumor cells, thereby reactivating the preexisting memory T cells to TT, killing the infected tumor cells.
In the current study, we tested the combination of Listeria-TT + GEM in two mouse models of pancreatic cancer, a syngeneic Panc-02 model (29) and a transgenic KrasG12D,p53R172H, Pdx1-Cre (KPC) model (30, 31). Listeria-TT was administered intraperitoneally, and the biodistribution of Listeria-TT and the production of TT in tumor cells were monitored in vivo. TT was delivered to the TME, attracting TT-specific CD4 T cells. This correlated with an over 80% reduction of pancreatic cancer and metastases and a 40% improvement in survival. We demonstrated that these CD4 T cells and not the CD8 T cells were responsible for reduction of the pancreatic cancer in vivo and for killing TT-expressing tumor cells in vitro. The results of this study provide a potential immunotherapeutic treatment for pancreatic and other currently difficult-to-treat cancers and warrants further investigation.
RESULTS
Development and characterization of Listeria-TT
The Listeria construct used to develop Listeria-TT includes pGG34 (32), consisting of a truncated noncytolytic listeriolysin O (LLO) fused to a nontoxic TT856–1313 fragment and a Myc sequence for detection as outlined in Fig. 1A. Secretion of LLO-TT856–1313 protein into the culture medium by the bacteria was detected by Western blotting (Fig. 1B and fig. S1A). In vitro infection of Panc-02 tumor cells with Listeria-TT856–1313 resulted in the expression of TT protein in the tumor cells (Fig. 1C and fig. S1B).
Fig. 1. Development and characterization of the Listeria-TT construct.

(A) Listeria-TT construct. TT856–1313, a nontoxic fragment of the C terminus of TT cDNA (amino acid position 856 to 1313, 52 kDa) was cloned as a fusion protein with a truncated noncytolytic listeriolysin O (LLO, 48kDa) into the Listeria plasmid pGG34, under the control of the LLO promoter (P). A myc tag was included for detection of the TT protein. (B) Western blot detection of TT protein secretion using anti-myc antibody. Complete blot shown in fig. S1A. Lane 1: Negative control (growth medium); lane 2: supernatant of Listeria-TT culture; lane 3: pellet of Listeria-TT culture. (C) Detection of TT protein in tumor cells. Lane 1: Panc-02 tumor cells; lane 2: Panc-02 tumor cells infected with Listeria alone; lane 3: Panc-02 tumor cells infected with Listeria-TT. Complete blot shown in fig. S1B. Anti–β-actin antibody was used to show equal loading of the samples. Abs, antibodies. (D) Mouse Panc-02 tumor cells infected with Listeria-TT. Average of two independent experiments, three wells per group. (E) Mouse Panc-02 tumor cell killing by Listeria alone or Listeria-TT. Average of three independent experiments; three wells per group. (F) Listeria-TT accumulation in tumors and metastases but not in normal tissues. A single high dose of Listeria-TT, 107 CFU/200 μl, was injected intraperitoneally into Panc-02 mice, and Listeria-TT CFUs were measured in tumor, metastatic, and normal tissues at different time points. Averages of a single experiment; n = 3 mice per time point, n = 3 tissues per organ/tumor/metastases. (G) Listeria accumulation in the metastases (mainly in pancreas) after 12 high doses (107 CFU). (H) Listeria or Listeria-TT infection of human Mia-PaCa2 pancreatic tumor cells in vitro. (I) Listeria or Listeria-TT killing human MiaPaCa2 pancreatic tumor cells in vitro. (J) Listeria-accumulation in human pancreatic tumors and metastases in vivo. A single high dose of Listeria-TT, 5 × 107 CFU/200 μl, was injected intraperitoneally in nude mice with MiaPaCa2 tumors in the pancreas, and Listeria-TT CFUs were measured in tumor, metastatic, and normal tissues at different time points. Averages of a single experiment; n = 3 mice per time point, n = 3 tissues per organ/tumor/metastases. (K) Listeria-TT infecting tumor cells and macrophages (M) in Panc-02-dendra-2 tumors of live mice by intravital imaging. Tumor cells are green, macrophages are blue, and Listeria-TT are yellow. In (E) and (I), all groups were compared to untreated (none). ns, not significant. In D,E, and I, statistical significance was determined by Mann-Whitney. *P < 0.05, ***P < 0.001. ****P < 0.0001. GI, gastrointestinal; LN, lymph nodes, BM, bone marrow.
Listeria-TT infection of tumor cells in vitro and in vivo
We previously showed that Listeria can be used as a delivery platform for anticancer agents (22, 25). To ensure that TT fusion to LLO did not alter LLO function, because LLO is essential to escape the vacuole after phagocytosis (33), we tested the delivery function of Listeria-TT and the infection rate of Panc-02 tumor cells cultured with Listeria-TT in comparison to Listeria alone. Panc-02 tumor cells were infected in both cases (Fig. 1D). We also demonstrated that Listeria-TT effectively killed the Panc-02 cells (Fig. 1E). The infection time in vitro was 2 hours, whereas in vivo, a complete treatment cycle takes 14 days. Thus, Listeria has considerably more time to infect tumor cells in vivo than in vitro. We intraperitoneally injected Listeria alone [107 colony-forming units (CFUs)] in mice with peritoneal Panc-02 tumors and metastases (22, 25). Confocal microscopy and quantitative analysis showed that Listeria accumulated in tumors and metastases with variable numbers. Listeria accumulated in tumors with a range of 30 to 135 and in metastases with a range of 24 to 291 Listeria bacteria per square millimeter tumor area compared to 0 to 9 Listeria bacteria in normal spleen (P = 0.001 and P = 0.0001, respectively) of the Panc-02 peritoneal model (fig. S2, A and B). In addition, a picture of the primary tumor, metastases in the pancreas, as well as normal spleen and pancreas (fig. S2C), and hematoxylin and eosin staining (fig. S2, D and E) have been included.
TT is highly immunogenic and may lead to faster elimination of Listeria-TT in vivo than Listeria alone, consequently preventing accumulation in the TME. To evaluate this issue, a single high dose of Listeria-TT (107 CFU) was injected intraperitoneally into Panc-02 mice, and numbers of Listeria-TT were quantified in all tissues, including tumor, metastases, and normal tissues, at different time points (1, 3, and 7 days after injection of the Listeria-TT). As shown in Fig. 1F and table S1, the CFU of Listeria-TT was 10-fold higher in the primary tumor than in the spleen on day 1 (P = 0.0040), whereas this was true for metastases in the pancreas compared to the spleen (7.5-fold) (P = 0.0019) on day 3. By day 7, no Listeria-TT bacteria were found in the spleen or other normal tissues, whereas Listeria-TT was still detectable in liver and pancreas metastases, 1000 and 3875 CFUs per gram tissue (P = 0.0451), respectively. In summary, Listeria-TT accumulated more abundantly in tumors and metastases than in spleen or other normal tissues on days 1 and 3 after injection of the Listeria-TT, whereas on day 7, Listeria-TT was completely absent in normal tissues but still detectable in tumors and metastases. Listeria, even after 12 high doses, was still able to colonize the immune suppressive TME, mainly the metastases, but was eliminated faster in normal tissues that lack immune suppression (Fig. 1G).
The same dose (107 CFU) of Listeria injected intravenously leads to nearly undetectable numbers of CFU of Listeria in tumors and metastases (fig. S3, A to C). This was true not only for metastases in the liver and pancreas of the Panc-02 model but also for metastases in the lungs of the 4T1 breast tumor model (fig. S3B).
We also evaluated the infection and rate of cell death of human pancreatic tumor cells by Listeria. The infection rate of human pancreatic tumor cells Mia-PaCa2 (Fig. 1H) by Listeria (P = 0.0012) and Listeria-TT (P = 0.0022) was 12- to 17-fold higher than that of the mouse pancreatic tumor cells (Fig. 1D), whereas the killing rate overnight was similar in the two models (Fig. 1, E and I), consistent with previous literature (34). To evaluate the clinical application of Listeria, we determined the biodistribution of Listeria in nude mice with human pancreatic tumors. For this purpose, 106 human pancreatic Mia-PaCa2 tumor cells were injected orthotopically in the pancreas of nude mice, and 6 weeks later, when tumors were palpable and 1 cm in diameter, a single high dose of Listeria (107 CFU) was injected intraperitoneally. The numbers of Listeria were quantified in all tissues at different time points as described above. When 1 × 107 CFU of Listeria was injected, the bacteria were hardly detected in tumors, metastases, and normal tissues. Consistent with our results, others have reported lower numbers of CFU of Listeria after injection in nude compared to wild-type mice (35). Therefore, we increased the dose to 5 × 107 CFU of Listeria, which resulted in a fourfold higher number of CFU of Listeria in the human pancreatic tumor (Fig. 1J) compared to the mouse tumors (P = 0.0048) (Fig. 1F). On day 1, the number of CFU of Listeria was very low in tumors and metastases (8.3 × 103 and 3.3 × 103 per gram tissue, respectively). However, on day 3, the number of CFU was high, particularly in metastases (average of 3.3 × 105 per gram tissue). Even on day 7, there were still high numbers of Listeria in the primary tumor (1.6 × 105 CFU of Listeria per gram tissue) and metastases (5 × 104 CFU per gram tissue) (Fig. 1J and table S2). In summary, we have shown here that Listeria efficiently accumulates in human cell line xenograft tumors. To confirm that Listeria-TT was taken up in vivo by tumor cells within the TME of live mice, the Listeria-TT was incubated with anti-Listeria polyclonal antiserum and anti–immunoglobulin G (IgG)–Alexa 680 antibodies. Subsequently, the Alexa 680–labeled Listeria-TT was then injected into the peritoneal cavity of transgenic mice whose macrophages expressed cyan fluorescent protein (CFP) (36) and which had orthotopic, Dendra-2–labeled pancreatic tumors (Panc-02-Dendra). High-resolution intravital multiphoton microscopy of these tumors showed individual Listeria-TT bacteria inside tumor cells and macrophages (Fig. 1K). Outside the tumor cells, aggregates of Listeria were observed. The polyclonal antiserum against Listeria contained agglutinating antibodies and was responsible for the aggregated Listeria observed outside the cells in Fig. 1K. One of the hallmarks of the Listeria we used is that they continuously spread from cell to cell and that TT protein is secreted in and outside the tumor cells. To determine how much of a tumor expressed TT, we performed immunohistochemistry (IHC) on tumors of KPC mice that received a complete treatment cycle as outlined in fig. S4 and quantified how much of the tumor was positive in the TT staining. As shown in Fig. 2 (A and B), TT was secreted in about 80% of the primary tumor, including TT inside tumor cells and, most likely, macrophages. We used TT detection as measurement of success of delivery. We also analyzed all four treatment groups by TT staining, and as expected, TT was only detected in tissues of KPC mice treated with Listeria-TT or Listeria-TT + GEM (Fig. 2C). Detection of TT was stronger in the tumors of Listeria-TT + GEM–treated mice than of Listeria-TT–treated mice (74.5 and 24.0%, respectively, P < 0.0001) (Fig. 2B). Although GEM kills Listeria in vitro, more Listeria bacteria were detected in the tumors of Listeria + GEM–treated mice compared to Listeria alone (98.6 × 103 versus 29.3 × 103 CFU per gram tissue, P = 0.0152) (Fig. 2, D and E).
Fig. 2. Delivery of TT by Listeria in pancreatic tumors in mice.

(A) IHC of KPC mouse tumors 2 days after receiving a complete treatment cycle with Listeria-TT + GEM as outlined in fig. S4 stained for TT. As negative control, the secondary antibody (anti–IgG-HRP conjugated) only was used. (B) TT expression in KPC tumors of LM-TT + GEM− or LM-TT–treated mice was quantified by IHC (averaged per square millimeter tumor area of 10 fields) with n = 3 KPC mice per group. Mann-Whitney, ****P < 0.001. Error bars represent SEM. All groups were compared to LM-TT + GEM. (C) TT expression and CD4 T cells were analyzed in KPC tumors by IHC of Listeria-TT + GEM–treated and control mice (saline, GEM, and LM-TT). Arrows point to TT and CD4 T cells around the neoplastic pancreatic ducts. (D) Effect of GEM on Listeria in vitro. Average of one experiment; n = 3 wells per concentration, three fields per well. (E) Effect of GEM on Listeria in vivo. The CFU of Listeria was counted 1 day after the treatment. Average of two independent experiments; n = 3 mice per group. Mann-Whitney; *P < 0.05, ***P < 0.001, and ****P < 0.0001. LM-TT, Listeria-TT; GEM, gemcitabine. In (D), all groups were compared to untreated Listeria (none).
Listeria-TT reactivates preexisting memory T cells to TT in tumor-bearing mice
The elderly, who represent a majority of cancer patients, have a marked reduction in naïve T cells (37). Therefore, we expect that cancer immunotherapy might be more effective if the need for naïve T cells could be avoided during cancer treatment (38). To address this point, we generated TT-specific memory CD4 and CD8 T cells in young mice through two injections of TT vaccine (TTvacc) 2 weeks before tumor development (Fig. 3A), similar to the TT vaccinations that have been used in humans to generate memory T cells and B cells to TT. Once the tumors and metastases were detected by positron emission tomography (PET) scan, we started with a complete treatment cycle with Listeria-TT + GEM as outlined in fig. S4. After the complete treatment cycle, spleen cells were isolated from the KPC tumor–bearing mice, restimulated with TT protein in vitro, and analyzed for memory T cell responses producing interferon-γ (IFNγ) (Fig. 3B). We found that CD4 and CD8 T cells in mice that received Listeria-TT or Listeria-TT + GEM were strongly activated by TT but not in mice that received saline or GEM alone (P < 0.05 and P < 0.001), indicating the high specificity of CD4 and CD8 memory T cells responsive for the TT protein. Similar CD4 and CD8 T cell responses were observed in the spleen of Panc-02 mice (fig. S3D).
Fig. 3. Generation and recall of memory CD4 and CD8 T cell responses to TT.

(A) Generation of CD4 (left) and CD8 (right) memory T cells to TT by immunization of mice with tetanus vaccine (TTvac) before tumor development as represented by IFNy-producing cells. All groups were compared to TTvacc. (B) Reactivation of TT-specific memory CD4 (left) and CD8 (right) T cells in KPC mice after tumor development through the Listeria-TT + GEM treatment cycle. The y axis represents the number of spleen cells producing IFNγ, and the x axis shows the spleen cell populations depleted or not depleted for CD4 or CD8 T cells. All groups were compared to nondepleted groups. (C) C57BL/6 mice with Panc-02 tumors received one high and multiple low doses of Listeria alone as described in fig. S4. Spleen cells were isolated and analyzed for T cell responses to Survivin protein by ELISPOT. A similar experiment was performed in KPC mice with pancreatic tumors but now received Listeria-TT + GEM. Representative results of two experiments are shown; n = 3 mice per group (pooled), six wells per group. Statistical significance by Mann-Whitney test: **P < 0.01, and ****P < 0.0001. All groups were compared to nondepleted groups. (D) ELISPOT analysis of CD45+ IFNγ-producing immune cells isolated from orthotopic Panc-02 tumors in mice treated with Listeria-TT + GEM. (E) Flow cytometry of IFNγ-producing CD4+ T cells in the CD45+ fraction, isolated from orthotopic Panc-02 tumors. In each group, tumors of five mice were pooled. Mann-Whitney; **P < 0.01, ***P < 0.001, and ****P < 0.0001. The error bars represent SEM.
We also studied endogenous tumor-associated antigen (TAA)–specific T cell responses induced by Listeria. We demonstrated by enzyme-linked immunosorbent spot (ELISPOT) that Listeria generates CD4 and CD8 T cell responses to Survivin (Fig. 3C), a TAA that is expressed by Panc-02 and KPC mice (39, 40). TAAs, including Survivin, are less immunogenic than TT, and their T cell responses are less vigorous compared to TT-specific T cells as seen in Fig. 3 (B and C), which demonstrate that the number of TT-specific IFNγ-producing T cells restimulated with TT protein is six to eight times higher than the number of Survivin-specific IFNγ-producing T cells restimulated with Survivin protein (P = 0.0043).
To evaluate whether Listeria-TT + GEM was able to activate T cells in the tumor-bearing KPC mice, we also analyzed ex vivo T cell responses, without in vitro TT-restimulation. At the end of the treatment cycle, we found that CD4 and CD8 T cell responses, as reflected by their granzyme B and perforin production, improved in pooled spleen cell populations of mice treated with Listeria-TT + GEM compared to the saline mice (table S3A). Perforin increased from 0.25 and 0.26% to 14.27 and 16.70%, and granzyme B increased from 1.6 and 3.1% to 12.17 and 12.60% in CD4 and CD8 T cells, respectively. The production of IFNγ was variable. Listeria or GEM alone also increased the production of perforin in CD4 and CD8 T cells compared to the saline treatments, consistent with previous findings (41). Similar results were found in Panc-02 mice where Listeria-TT + GEM improved T cell responses compared to all other groups (table S3B and fig. S5, A and B). These results indicate that Listeria-TT + GEM treatment was able to activate T cells in tumor-bearing mice. Also, CD4 T cells in Panc-02 mouse tumors receiving Listeria-TT + GEM produced IFNγ (Fig. 3, D and E).
Listeria-TT + GEM turns immunologically “cold” tumors into immunologically “hot” tumors
To visualize the TME and T cells in more detail, the KPC tumors were analyzed by IHC. KPC mice received a full treatment cycle with Listeria-TT + GEM, including the prevaccinations with the TTvacc. As shown in Fig. 2C, CD4 T cells were present in the tumors of KPC mice treated with Listeria-TT but at low numbers in the tumors of saline- or GEM-treated mice. A larger image magnification of the CD4 T cells in the KPC tumors of all four treatment groups is shown in fig. S6 (A to D). The addition of GEM to the Listeria-TT treatment significantly increased the number of CD4 T cells in the tumors of KPC mice compared to all other groups (P = 0.001) (Fig. 4A). CD8 T cells were sparsely present in the tumor areas (fig. S6, E to H). CD4 T cells were found in the tumor areas that expressed TT protein and CD31-positive vessels (fig. S7). In support of T cell trafficking (42, 43), L-selectin, Cxc3, and chemokines Cxcl9 and Cxcl10 were highly up-regulated in KPC tumors of mice treated with Listeria-TT + GEM compared to untreated mice (P < 0.05) (fig. S8). To show that these CD4 T cells in the pancreatic tumors are functional, we evaluated the abundance of perforin and granzyme B, both of which are involved in T cell–mediated tumor cell cytolysis. Here, we demonstrated that both granzyme B and perforin were detected in the pancreatic tumors of Listeria-TT- or Listeria-TT + GEM–treated mice by IHC but not in the tumors of saline or GEM-treated mice (Fig. 4, A and B). CD40 and CD40L were also up-regulated in KPC tumors by Listeria-TT + GEM compared to saline-treated mice (P < 0.05) (fig. S8A).
Fig. 4. CD4 memory T cells migrate to TME and are activated by Listeria-TT + GEM.

KPC mice were treated with saline, GEM, Listeria-TT, or Listeria-TT + GEM as outlined in fig. S4. Three days after the last treatment, the pancreas with tumors including lymph node–like structures (LNS) were excised and processed for TT, CD4, perforin, and granzyme B staining by IHC. (A) Quantification of CD4 T cells in KPC tumors and the production of perforin and granzyme B in the KPC tumors and LNS. CD4 T cell numbers were counted in the tumor areas of all treatment groups. Also, the number of perforin and granzyme B–producing cells was counted in the tumor areas and LNS of all treatment groups. In each group, 4 to 10 fields were measured, and the number of cells per 1 mm2 was calculated. All groups were compared to the saline group. Results of three mice per group were averaged. Mann-Whitney; *P < 0.05, **P < 0.01, and ***P < 0.001. (B) Production of perforin and granzyme B in KPC tumors by IHC. (C) Representative IHC of CD4 T cells in the KPC tumors and the presence of LNS were analyzed in all treatment groups. (D) Production of perforin and granzyme B in KPC LNS, by IHC.
Large lymph node–like structures (LNS) were frequently observed in close contact with the KPC tumors of mice treated with Listeria-TT or Listeria-TT + GEM and less frequently and smaller in size in KPC mice treated with GEM or saline (Listeria-TT or Listeria-TT + GEM, five of five mice; GEM, two of five mice; saline, one of five mice; Fig. 4C). Higher magnification images of a LNS are shown in fig. S7D. A significant increase in the production of perforin and granzyme B was observed in the LNS of Listeria-TT + GEM–treated mice compared to the saline control group (P = 0.01) (Fig. 4, A and D, and fig. S7, E to H). We also analyzed the T cells in the LNS for the production of IFNγ and memory phenotype by flow cytometry. Briefly, the CD45+ lymphocytes (81.2%) from the LNS of Listeria-TT–treated mice were gated, followed by gating of CD4 (40.4%) or CD8 (52.0%) T cells and by gating of the IFNγ+ CD4 (3.28%) or CD8 (2.91%) T cells (Fig. 5A). Within these IFNγ+ CD4 and IFNγ+ CD8 T cell populations, 54 and 55%, respectively, exhibited a memory phenotype (CD44+CD62L−). This was supported by RNA-scope, which showed both CD4 and CD8 T cells expressing ifnγ (fig. S9) in the LNS of Listeria-TT- and Listeria-TT + GEM–treated KPC mice.
Fig. 5. Listeria-TT + GEM activates immune responses in primary tumors and LNS.

(A) Isolation and flow cytometry analysis of CD4 and CD8 T cells in a LNS of a mouse that received Listeria-TT. The CD45+ cells were gated, followed by CD3CD4 or CD3CD8 gating, followed by gating of CD3CD4 and CD3CD8 T cells producing intracellular IFNγ, followed by staining to identify T cells with memory phenotype (CD44+CD62L−). (B) RNA-seq analysis of immune responses and tumor cell death pathways in pancreatic tumors of KPC mice in response to Listeria-TT + GEM treatment and control groups. The results of two mice in each group were averaged. The heatmap was generated by unbiased hieratical clustering at the following statistical parameters. Statistical parameters for the paired-wise comparison: P < 0.05. The signature contains 20 genes. (C) Flow cytometric analysis of changes in MDSC (CD11b+Gr1+) in blood and tumors and TAMs (CD11b+F4/80+) in tumors of Panc-02 mice in response to the Listeria-TT + GEM treatment. All groups were compared to the saline group. The gating strategy of MDSCs and TAMs is shown in fig. S13 (A and B). Average of a single experiment of MDSCs in blood with n = 3 mice per group and the average of two independent experiments of MDSCs and TAMs in tumors with n = 3 mice per group. The error bars represent SEM. Mann-Whitney *P < 0.05, ***P < 0.001, ****P < 0.0001.
To confirm the IHC observations, we performed RNA sequencing (RNA-seq) analyses of pancreatic tumors of KPC mice with Listeria-TT + GEM–treated and control groups. As shown in Fig. 5B, the expression of CD4 but less of CD8 genes was up-regulated in the tumors of Listeria-TT + GEM–treated KPC mice (P < 0.05). Moreover, multiple granzymes were expressed, including A and B (P < 0.05), whereas perforin was less abundantly expressed than granzyme B, in the tumors of Listeria-TT + GEM− and Listeria-TT–treated mice (P > 0.05). Also, genes involved in tumor cell apoptotic pathways, such as multiple caspases, were up-regulated compared to the saline-treated mice (P < 0.05). Major histocompatibility complex (MHC) class II genes were up-regulated in the tumors of KPC mice treated with Listeria-TT + GEM or Listeria-TT alone (P < 0.05) (Fig. 5B). The expression of MHC class II in tumors of Listeria-TT + GEM–treated KPC mice was confirmed by IHC (fig. S10).
In addition, we compared the RNA-seq data of KPC tumors with and without LNS for immune function. The difference in expression of immune-related genes between the saline and Listeria-TT + GEM group was considerably larger when LNS were present (up to nine-fold increase in expression, P < 0.05) (fig. S11A), compared to the saline and Listeria-TT + GEM group of tumors without LNS (up to fivefold increase in expression, P < 0.05) (Fig. 5B). Together, the results indicated that Listeria-TT + GEM treatment altered cold tumors to immunologically hot tumors, promoting a strong accumulation of CD4 T cells in the KPC tumors, as well as the production of perforin and granzyme B, and that LNS may have played a role here.
MDSCs in tumors are more immune suppressive than in blood or normal tissues
MDSCs were analyzed in tumors, blood, and normal tissues (spleen) of different tumor models for their production of immune suppressive cytokines by flow cytometry. In the 4T1 breast tumor model, we found that the production of interleukin-6 (IL-6) (P = 0.0001), IL-10 (P = 0.001), and transforming growth factor–β (TGF-β) (P = 0.01) was significantly higher in monocytic MDSC of tumors than of blood or normal tissue (fig. S12A). This was also true for the granulocytic MDSC but much less pronounced compared to the monocytic MDSC. In addition, in the Panc-02 model, we found that IL-10 (P = 0.01) and TGF-β (P = 0.05) were significantly higher in monocytic MDSC of tumors than of blood or normal tissues (fig. S12B), whereas IL-6 production was higher in the monocytic MDSC of tumors than of blood or normal tissues, but this was not significant (P > 0.05). No significant differences in the production of immune suppressive cytokines were observed in the granulocytic MDCS, with an exception for TGF-β, which showed a small but significant increase (P = 0.05) in the tumor compared to the spleen (fig. S12B).
Listeria-TT and GEM alter the TME
In Panc-02 tumor–bearing mice, GEM treatment reduced the MDSC population in the blood by 80 to 90% (P = 0.0001) and in primary tumors by 50% (P = 0.001), compared to the saline group (Fig. 5C). Similarly, the TAM population in primary tumors was decreased by 67% compared to the saline group (P = 0.0001) (fig. 5C). Moreover, for the residual MDSC and TAM cells, the production in pancreatic metastases of factors involved in immune suppression, including IL-10, IL-6, and the transcription factor MARCO, were reduced in mice treated with Listeria-TT + GEM (table S3, C and D, and fig. S13, A and B), whereas there was increased production by these cells of tumor necrosis factor–α (TNF-α), involved in tumor cell killing, and expression of CD80, involved in T cell activation. These findings indicate that Listeria TT + GEM altered the TME.
Listeria-TT + GEM reduces advanced and early pancreatic cancer in KPC and Panc-02 mice and improves survival
After evaluating the effect of Listeria-TT + GEM on immune responses in the TME, we also evaluated the therapeutic effect on pancreatic cancer in KPC mice. For this purpose, young KPC mice (6 to 8 weeks of age) were prevaccinated twice with the TTvacc 1 week apart before tumor development. Once the tumors and metastases appeared (3.5 to 5 months of age) as confirmed by PET scan analysis, the KPC mice were treated with Listeria-TT + GEM as outlined in fig. S4, and the standardized uptake value (SUV)max, a measure of the metabolic activity and growth/size of tumors and metastases, was evaluated. Significant reductions in SUVmax for PDAC tumors from 1.9 to 0.4 (P = 0.01) and for liver metastases from 2.1 to 0.3 (P = 0.01) were observed in the mice receiving Listeria-TT + GEM treatment (Fig. 6A). Small changes in the SUVmax of tumors and metastases in the saline and GEM control groups were not statistically significant (P > 0.05) nor was the 50% decrease in the SUVmax of the Listeria monocytogenes (LM)-TT group (Fig. 6A). A representative example of a pancreatic KPC tumor in each group is shown in fig. S8F.
Fig. 6. Listeria-TT + GEM reduces advanced pancreatic cancer in KPC mice.
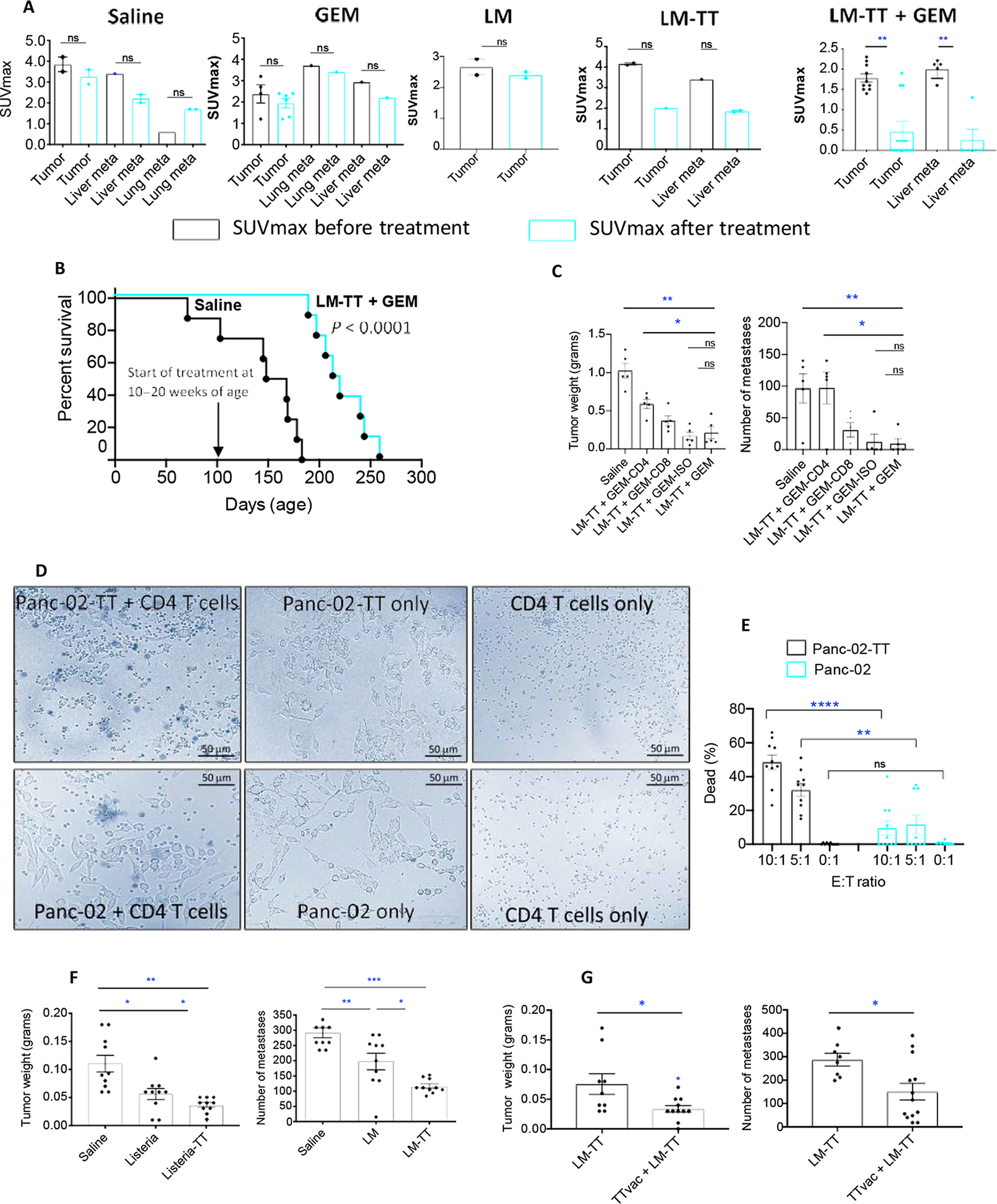
KPC mice received the combined treatment as outlined in fig. S4. (A) Effect of treatment on advanced pancreatic cancer in KPC mice. Averages are shown for a single experiment with five mice in the Listeria-TT + GEM group and two mice in each other treatment group. Listeria-TT + GEM was started at age 3 to 5.5 months, after tumors and metastases were verified through PET scan. SUVmax of tumors and metastases was measured before (black bar) and after treatment (cyan blue bar). (B) KPC mice of 10 to 20 weeks of age were treated with Listeria-TT + GEM or saline as described in fig. S4, with two high doses of Listeria-TT, 1 week apart. At the end of treatments, mice were monitored until death, and survival was assessed, when defined clinical end points were reached. This was one experiment with eight mice per group. Significant differences were determined by the Mantel-Cox test P < 0.0001. (C) Mice with Panc-02 tumors and metastases were treated with anti-CD4 and anti-CD8 antibodies during Listeria-TT +GEM treatment. The antibodies (300 μg/200 μl) were administered intraperitoneally every third day (five doses total). Appropriate isotype antibodies were used as negative control. At the conclusion of the experiment, the tumor weight and number of metastases were determined. n = 7 mice per group. Mann-Whitney; *P < 0.05 and **P < 0.01. All mice were compared to the LM-TT + GEM group. (D) Example of CD4 T cells of TT-vaccinated mice destroying TT-expressing Panc-02 tumor cells in vitro. Effector (CD4 T cells) and target (tumor cells) were mixed at ratios 10:1, 5:1, and 0:1 and cultured for 3 hours. Percentage of dead TT-expressing was compared to non–TT-expressing tumor cells by trypan blue. Percentage of dead TT- and non–TT-expressing tumor cells in the presence and absence of TT-specific memory T cells was compared. (E) Results were quantified and analyzed by Mann-Whitney test. **P < 0.01 and ****P < 0.0001. Each dot represents the percentage of dead tumor cells per field. Ten fields spread over 10 wells per group were analyzed, and the results were averaged within each group. (F) Panc-02 mice with advanced pancreatic cancer received the Listeria-TT ± TTvac as outlined in fig. S4, and tumor weight and number of metastases were measured. (G) Panc-02 mice received Listeria-TT with and without prior TTvacc; at end point, tumors were weighted and the number of metastases was counted. The results of two experiments were averaged with n = 8 to 9 mice per group and analyzed by Mann-Whitney test; *P < 0.05, **P < 0.01, and ***P < 0.001. Error bars represent SEM.
We also tested Listeria-TT + GEM therapeutically in a Panc-02 mouse peritoneal model (for more details, see Materials and Methods) with early and advanced pancreatic cancer as outlined in fig. S4. We found nearly complete elimination of early-stage pancreatic cancer in mice treated with Listeria-TT + GEM (fig. S8, C and G) and a significant reduction in primary (85%; P < 0.001) and metastatic tumor burdens (89%; P < 0.0001) in Panc-02 mice with advanced pancreatic cancer and treated with Listeria-TT + GEM or Listeria-TT alone (fig. S8D) compared to the saline group (fig. S8, D and H).
Last, we tested Listeria-TT + GEM in mice with advanced orthotopically generated Panc-02 tumors and metastases. Similarly, mice were prevaccinated with the TTvacc at young age (6 to 8 weeks of age) before tumor development. Fourteen days after injection of Panc-02 tumor cells into the pancreas, when tumors and metastases were developed (0.5 to 1 cm), treatment with Listeria-TT + GEM and control groups was started and continued for 2 weeks, as outlined in fig. S4. Two days after the last treatment, mice were euthanized and analyzed for tumor weight and number of metastases. A significant reduction was observed in the weight of the primary tumors (68%; P < 0.05) and in the number of liver metastases (95%; P < 0.01) of Listeria-TT + GEM–treated compared to the saline group (fig. S8, E and I).
To validate the clinical value of these efficacy studies, we analyzed the survival of KPC mice treated with Listeria-TT + GEM or saline. Mice were immunized with the TTvacc and Listeria-TT + GEM; however, two instead of one high dose of Listeria-TT was administered 1 week apart, to deliver more TT to the TME. The Listeria-TT treatments were started when the mice were 10 to 20 weeks of age, and tumors were palpable. As shown in Fig. 6B, KPC mice treated with Listeria-TT + GEM lived 2 months longer than the untreated mice, which was significant (P < 0.0001).
In vivo depletion of T cells demonstrates CD4 T cell–mediated reduction of tumors and metastases through Listeria-TT + GEM treatment
Here, we analyzed whether CD4 T cells could be responsible for the reduction in tumor weight and number of metastases in mice treated with Listeria-TT + GEM. For this purpose, we depleted CD4 and CD8 in vivo, as described previously (44). Briefly, young C57BL/6 mice (6 to 8 weeks of age) were prevaccinated with the TTvacc before tumor development. Ten days after injection of the Panc-02 tumor cells, when tumors were 0.5 to 1 cm in diameter, mice were treated with Listeria-TT + GEM in the presence of anti-CD4 or anti-CD8 antibodies administered every third day (300 μg per dose, for five doses in total). As shown in Fig. 6C, the average tumor weight in mice treated with Listeria-TT + GEM plus CD4 antibodies was significantly increased by 64% compared to the Listeria-TT + GEM alone group (P = 0.0159), whereas the tumors in mice depleted for CD8 T cells were increased by 43% compared to the Listeria-TT + GEM alone group, but this was not statistically significant (P = 0.2063). As expected, the tumors in mice treated with Listeria-TT + GEM plus the isotype control (negative control) were not statistically significant different from the tumor in mice treated with Listeria-TT + GEM only (P > 0.9999, Mann-Whitney). Similarly, the number of metastases in mice treated with Listeria-TT + GEM plus CD4 antibodies significantly increased by 92% compared to mice treated with Listeria-TT + GEM alone (P = 0.0435), whereas the number of metastases in mice treated with Listeria-TT + GEM plus antibodies to CD8 T cells was increased by 74%, but this was statistically not significant (P = 0.1746) (Fig. 6C). In addition, here, the number of metastases in mice treated with Listeria-TT + GEM plus the isotype control was not significantly different compared to mice treated with Listeria-TT + GEM only (P > 0.999). Flow cytometry analysis of the spleen confirmed that CD4 and CD8 T cells were efficiently depleted in vivo (fig. S11B). In summary, these results indicate that CD4 T cells contributed to the reduction of tumors and metastases in vivo.
CD4 T cells from TT-vaccinated mice killing TT-expressing tumor cells in vitro
To demonstrate that the TT-specific T cells were able to destroy TT-expressing tumor cells, we first developed a tumor cell line expressing TT by stably transfecting Panc-02 cells with pcDNA3.1-TT, followed by culture in the presence of neomycin to select for tumor cells containing the pcDNA3.1-TT plasmid. Expression of TT was shown by immunofluorescence (IF) (fig. S14A). The Panc-02-TT tumor cells also express MHC class II (fig. S14B), which is required for T cell–mediated tumor cell killing. Using this TT-expressing Panc-02 tumor cell line, we performed in vitro assays to evaluate whether CD4 T cells of TT-vaccinated mice were able to destroy the TT-expressing tumor cells. Briefly, C57BL/6 mice were immunized with the TTvacc twice, 1 week apart to generate the TT-specific memory T cells. One week after the last immunization, spleen cells were isolated, and CD4 T cells were collected through magnetic bead technology. Subsequently, these CD4 T cells were mixed with tumor cells at various ratios of 10:1, 5:1, and 0:1 and incubated for 3 hours at 37°C and 5% CO2. We compared the destruction of Panc-02 cells expressing TT by CD4 T cells with Panc-02 cells lacking the expression of TT. Trypan blue was used to discriminate between live and dead tumor cells. We found that the percentage of dead Panc-02 tumor cells expressing TT was significantly higher than the percentage of Panc-02 cells not expressing TT (P = 0.0001) (Fig. 6, D and E). In addition, a significant difference was observed in the percentage of dead tumor cells expressing TT in the presence compared to in the absence of CD4 T cells (P = 0.0001) (Fig. 6, D and E). However, without TT expression, no significant difference was observed in the percentage of dead tumor cells with or without CD4 T cells (P = 0.8518) (Fig. 6, D and E). In summary, we have shown here that CD4 T cells of TT-vaccinated mice destroy TT-expressing Panc-02 cells in vitro.
TT contributes to reduction in pancreatic cancer
Next, we examined to what extent TT contributes to reduction of the pancreatic cancer through two different modalities. First, we compared the effect of Listeria-TT treatment to Listeria-alone treatment on advanced pancreatic cancer in two different mouse tumor models, Panc-02 and KPC. Mice that received Listeria-TT also received the TTvacc before tumor development. As shown in Fig. 6F, the tumor weight and the number of metastases were significantly lower in the mice treated with Listeria-TT compared to Listeria alone (P < 0.05). A similar trend was observed in the transgenic KPC mice. Listeria-TT reduced the SUVmax of tumors and metastases by 50%, whereas hardly an effect was observed by Listeria alone (Fig. 6A).
Second, we evaluated the effect of the TT vaccination on the efficacy of the Listeria-TT treatment. For this purpose, Listeria-TT was administered with and without prior TT vaccinations in the Panc-02 model. As shown in Fig. 6G, the number of metastases and tumor weight were significantly less in the TT-vacccinated + Listeria-TT–treated mice compared to the mice treated with Listeria-TT alone (Mann-Whitney P < 0.05). In conclusion, TT contributed to the reduction of the pancreatic cancer.
Safety of Listeria-TT
We next tested the safety of intraperitoneally injected Listeria in multiple mouse tumor models. A dose-limiting toxicity study with Listeria-TT showed that the median lethal dose (LD50) of Listeria-TT was 2 × 108 CFU (fig. S8B). Our treatment studies used a dosage of 107 CFU of Listeria-TT, which is well below the LD50.
We also analyzed potential toxicity by pathological examination 2 days after treatment of C57BL/6 mice with Listeria-TT + GEM. Although a mild increase in leukocytes was observed in the spleen, liver, and lungs, no pathological effects of Listeria-TT + GEM were observed (table S4). In addition, we compared liver functions of the treated C57Bl/6 mice. A small increase in the aminotransferase concentration was observed in the plasma from Listeria-TT + GEM–treated mice compared to the saline group, whereas alanine aminotransferase amounts were similar between the two groups (table S5). These results indicate safety of intraperitoneally injected Listeria in mouse models.
DISCUSSION
The success of cancer immunotherapies such as checkpoint inhibitors and chimeric antigen receptor (CAR) T cells in PDAC has been underwhelming, due to low immunogenicity of PDAC tumors, strong immune suppression, and inefficient activation of naïve T cells in the TME (8, 9, 11, 12, 14, 15). Moreover, low neoantigen expression results in poor attraction of neoantigen-specific T cells (45, 46). We addressed these problems by using TT as an alternative for neoantigens, and GEM was used to reduce immune suppression in the TME. In this study, we demonstrated the effectiveness of a tumor-targeting attenuated Listeria, in combination with GEM, in treating animal models of early and advanced pancreatic cancer. Our treatment strategy offers the advantages of selective delivery of a high abundance of immunogenic TT protein to the TME and inside tumor cells, altering the function of macrophages and MDSCs in the TME to promote immune stimulation. TT, now used as tumor antigen, could be an alternative for those patients who lack neoantigen expression. Moreover, we showed the contribution of TT to reducing pancreatic cancer using different treatment modalities. For example, Listeria-TT was significantly more effective than Listeria (P = 0.05) alone in reducing tumor size in the Panc-02 model, and omitting the TTvacc before Listeria-TT treatments resulted in a significant weaker effect on metastases and tumors compared to mice that received the TTvacc before the Listeria-TT treatments P = 0.05).
We also observed beneficial effects of GEM on Listeria or Listeria-TT treatments. For example, GEM significantly increased the number of Listeria bacteria, when compared to Listeria alone (P = 0.05), and significantly increased the amounts of TT protein in the KPC tumors, when compared to the Listeria-TT treatment alone (P = 0.0001). This also correlated with significantly more CD4 T cells in the KPC tumors of mice treated with Listeria-TT + GEM compared to Listeria-TT (P = 0.01). In addition, GEM reduced the MDSC population, which down-regulates L-selectin on T cells, a gene involved in T cell trafficking. l-selectin was strongly up-regulated (P < 0.05) in tumors of KPC mice treated with Listeria-TT + GEM.
On the basis of TT expression in the tumors (Fig. 2C), it seems that Listeria accumulated more around the pancreatic ducts in the KPC tumors. It has been reported that phospholipases A2 and B are produced in the pancreatic duct in bile products (47), which is critical for the survival of Listeria (48, 49), which may explain the higher expression of TT around the pancreatic ducts.
Although ELISPOT data showed that both CD4 and CD8 T cells were activated by TT in vitro in the spleen of KPC mice treated with Listeria-TT + GEM, we observed predominantly CD4 T cells by IHC, whereas CD8 T cells hardly infiltrated the tumors. These CD4 T cells produced 10-fold higher concentrations of IFNγ in Listeria-TT + GEM–treated mice compared to the saline mice. In addition, the LNS contained both CD4 and CD8 T cells, but only the CD4 T cells accumulated efficiently in the tumor areas. In humans, the TTvacc is known to predominantly activate CD4 T cells (50). Moreover, depletion of T cells in vivo of mice treated with Listeria-TT + GEM demonstrated that CD4 T cells contributed to the reduction of tumors and metastases in vivo and that CD4 T cells isolated from TT-vaccinated mice destroy TT-expressing tumor cells in vitro.
We observed well-developed LNS in close contact with the tumors in KPC mice treated with Listeria-TT or Listeria-TT + GEM. Chronic infections with bacteria lead to trafficking of T cells into the circulation and the formation of LNS in different tissues where the infection develops (43). The formation of LNS and T cell trafficking is generated through the production of chemokines and activation of their ligands and selectins on T cells (51). We found that the Cxcr3 receptor and its chemokines Cxcl9 and Cxcl10 were highly up-regulated by Listeria-TT + GEM compared to the saline group (P < 0.05) in KPC tumors and possibly participated in T cell trafficking to the KPC tumors. Both Cxcl9 and Cxcl10 are induced by IFNγ (52, 53), which is highly up-regulated by the Listeria-based immunizations. Perforin and granzyme B were detected in LNS of Listeria-TT + GEM–treated mice.
Listeria-based immunotherapies for pancreatic cancer have also been reported by others. For instance, KPC mice in the early stage of PDAC (6 to 8 weeks of age) that were immunized with Listeria double deleted for ActA and InlB (LADD) (54), expressing the Kras12GD mutation, combined agents that reduce immune suppression, demonstrated significantly improved survival compared to untreated mice (P = 0.002), but no improvement was shown when started at 8 to 12 weeks of age (55). In contrast, our treatment with Listeria-TT + GEM improved the survival of KPC mice significantly (P < 0.0001), even when treatments were started at an advanced stage of cancer (10 to 20 weeks of age). Other interesting studies with Listeria-based immunotherapy showed improvement in T cell activation in the TME and reduction in tumor burden in mouse models of breast, skin, and cervical cancer (56).
Listeria has also been tested in clinical trials in patients with various cancers, including pancreatic cancer, showing safety and tumor-specific T cell responses. However, the trials have only modestly improved patient survival (28). As mentioned earlier, the concept of Listeria previously tested in clinical trials is completely different from our concept. The Listeria used in the clinical trials is based on a “classical concept,” where the delivery of tumor-specific antigens into dendritic cells is used to stimulate naïve and memory T cells to these antigens, which are naturally expressed by the tumors. Our concept is based on colonizing and changing the TME by Listeria and the delivery of a highly immunogenic childhood vaccine antigen TT into tumor cells by Listeria, thus reactivating preexisting memory T cells to TT. This modest survival using the classical concept in clinical trials may be partly the result of the deletion of ActA and InlB of in the Listeria to improve safety and consequently removing its ability to spread and multiply in vivo and partly the result of administering Listeria intravenously. As demonstrated in this study, Listeria administered intravenously hardly reaches pancreatic and breast cancer tumors and metastases, whereas when administered intraperitoneally, Listeria colonizes the TME abundantly, delivers TT to the TME and inside tumor cells attracting CD4 T cells, and infects/alters MDSCs and TAMs. In multiple biodistribution studies in mice with pancreatic and breast cancer, we found that Listeria was rapidly eliminated most likely by the immune system in blood (10, 22, 25), because the blood lacks immune suppression, whereas the TME is heavily immune suppressed. We found that MDSC in tumors were more immune suppressive than in blood or normal tissues. The immune suppression was more pronounced in monocytic than in granulocytic MDSCs, which is consistent with the literature (57). Thus, when Listeria bacteria are injected intraperitoneally, they directly infect the immune suppressive MDSCs, which then migrate to the TME, resulting in spread of the Listeria through the TME.
Last, this study also has limitations and raised additional questions. First, more Listeria bacteria were found after treatment with Listeria + GEM compared to Listeria alone. One potential reason for this outcome is that GEM reduces immune suppression and thus may restrict bacterial survival to the TME and inside tumor cells, or GEM may have induced more necrosis creating a more hypoxic environment, which favors Listeria survival (58). This needs to be analyzed in more detail. In addition, our results show that CD4 T cells were activated by Listeria-TT + GEM in the LNS and correlated with a decrease in the number of metastases and tumor growth, as well as improved survival. However, tumor-draining lymph nodes were found to correlate with immune suppression, a more metastatic phenotype, and poor outcomes in patients with PDAC (59). It is possible that the activated CD4 T cells in our study migrated from the LNS to the tumor and then killed the tumor cells. It has been suggested by others that these peritumoral LNS allows more efficient T cell activation because of lack of immune suppression that is present in the primary tumors (60). Last, to progress into clinical trials, the safety of intraperitoneal injections of Listeria in patients needs to be explored. Intraperitoneal injections are required for delivery of Listeria selectively to tumors and metastases (Listeria injected intravenously is immediately eliminated in blood and hardly reaches tumors). Because intravenous delivery is more common than intraperitoneal delivery, retraining of clinicians regarding route of treatment will be required.
In summary, our Listeria recall antigen concept demonstrated that TT is delivered by Listeria in relatively high concentrations in the pancreatic tumors. This results in the attraction of TT-specific CD4 T cells, which produce IFNγ, perforin, and granzyme B in the TME. This contributes to reduction in tumor burden and destroying TT-expressing tumor cells in vitro. This concept has been presented in fig. S15.
Last but not least, elderly patients with cancer react less efficiently to vaccines than young adults (61, 62), which is also true for cancer immunotherapy (38, 63), due to a marked reduction in naïve T cells later in life (37). We believe that our approach of reactivating memory T cells generated during childhood overcomes the need for naïve T cells at older age, potentially making this therapeutic strategy particularly relevant to patients with pancreatic cancer with an average age of 70 years (64). This may lead to new treatment modalities against pancreatic cancer, where other types of therapies fail.
MATERIALS AND METHODS
Study design
The study was initiated to deliver childhood vaccine antigen TT (as a neoantigen alternative) selectively to tumors and metastases through Listeria infection and to reactivate TT-specific memory T cells generated earlier in life to destroy the infected tumor cells now expressing TT antigen. GEM was added to reduce immune suppression. We investigated the effect of Listeria-TT and GEM in various mouse models of pancreatic cancer. Listeria-TT and GEM were injected intraperitoneally, resulting in the accumulation of Listeria and TT in tumors and metastases. We demonstrated a considerable improvement in TT-specific memory CD4 T cells in the pancreatic tumors, tumor burden, and survival. All experiments involving mice have been subjected to rigorous review by the Institutional Animal Care and Use Committee (IACUC) and through grant applications. Sample sizes and end points were selected on the basis of our published experience with Listeria infection. The Listeria-TT batch used in this study was subjected to the following quality controls such as purity, concentration, stability, expression of TT and LLO, infection and kill of tumor cells, LD50, and sequencing the prfA gene. Sample sizes and end points were selected on the basis of our published experience with Listeria infection. Wherever possible, preliminary mouse experiments were performed to determine requirement for sample sizes, considering the available resources and ethical use of the animals. Female and male mice were assigned randomly to the experimental groups. The samples have been coded and randomized. All the processing and analysis steps have been performed in a blinded manner; identity of samples has been revealed only at the step of results interpretation. Our study achieved robust and unbiased results because we are mostly relying on technical methodologies that have been extensively validated (tumor development and therapeutic treatment, ELISPOT, flow cytometry, in vitro killing of tumor cell assay, RNA-seq, RNAscope, IHC, and PET scan). Sample size and experimental replication are provided in the legends of each figure. Outliers were not excluded in this study. Resources of antibodies, bacterial strains, recombinant proteins and chemicals, commercial assays, cell lines, primers and probes, and equipment are provided in table S6.
Animal care
C57BL/6 and Balcb/C mice 6 to 8 weeks of age, used to generate the Panc-02 and 4T1 models, respectively, were obtained from Charles River. KPC mice (30) were generated in the laboratories of C.G. and S.K.L., as described previously (25) and are available from The Jackson Laboratory. C57Bl/6N-Tg(Cfms-gal4-vp16)-(UAS-eCFP) (36) were maintained in the laboratory of J. Condeelis. Nude mice (Nu/J, strain 002019) were purchased from Jackson Laboratories and used for generating the xenograft model with the human pancreatic tumor cell line Mia-PaCa2. Male and female mice were used in this study. All mice were housed in the animal husbandry facility at Albert Einstein College of Medicine according to the Association and Accreditation of Laboratory Animal Care guidelines and kept under biosafety level-2 (BSL-2) conditions as required for Listeria treatments. All KPC mice were genotyped by Transnetyx. IACUC animal protocols 20170711 and 00001313 were used in this study.
Cell lines
The Panc-02 cell line was derived from a methylcholanthreneinduced ductal adenocarcinoma growing in a C57BL/6 female mouse (65). Panc-02 cells were cultured in McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, nonessential amino acids, 1 mM sodium pyruvate, and penicillin-streptomycin (100 U/ml). The Panc-02 cell line expressing Dendra-2 was developed in the laboratory of C.G. Briefly, Panc-02 cells were transfected with pDendra2C (ClonTech), and positive cells were selected using neomycin and fluorescence-activated cell sorting (FACS). The TT-expressing Panc-02 tumor cell line was generated earlier in the laboratory of C.G. Briefly, Panc-02 cells were transfected with pcDNA3.1-TT and then cultured in neomycin to select for tumor cells containing the pcDNA-3.1-TT plasmid. Expression of TT and MHC class II was demonstrated by IF. The KPC tumor cell line (provided by V.B., Memorial Sloan Kettering Cancer Center, NY and also available via Ximbio) was derived from a pancreatic tumor of a transgenic KPC mouse (KrasG12D,p53R172H, Pdx1-Cre) (66). The 4T1 cell line was derived from a spontaneous mammary carcinoma in a BALB/c mouse (67). Various 4T1 sublines have been generated with different patterns of metastases (68), which is provided to our laboratory by F. Miller (Karmanos Institute, Wayne State University). The 4T1 cell line used in this study is highly aggressive, metastasizing predominantly to the mesenteric lymph nodes and less frequently to the diaphragm, portal liver, spleen, and kidneys (13). The Mia-PaCa2 cell line was purchased from American Type Culture Collection (CRL-1420).
Listeria and Listeria-TT
In this study, an attenuated L. monocytogenes (Listeria) was used as the vehicle for delivery of TT856–1313 (69) to the TME and inside tumor cells. The Listeria plasmid pGG-34, expressing the noncytolytic truncated form of LLO under control of the hemolysin promoter (Phly) and the positive regulatory factor A (prfA), with mutations to further reduce pathogenicity, have been described elsewhere (32). Listeria-TT856–1313 was developed in our laboratory (Fig. 1A). Briefly, TT856–1313 (69) was cloned by polymerase chain reaction into pCR2.1 using primers containing XHoI and XMaI restriction sites and a myc tag for detection of TT. (PrF5′: CTC GAG TCA ACA CCA ATT CCA TTT; PrR5′: CCC GGG TTA TAG ATC TTC TTC TGA AAT TAG TTT TTG TTC TGT CCA TCC TTC ATC TGT). Subsequently, the pCR2.1-XHoI-TT856–1313-Myc-XmaI and pGG34 plasmids were digested with XHoI and XMaI, and the XHoI-TT856–1313-myc-XMaI DNA fragment was cloned into the pGG34 vector and then electroporated into the Listeria background strain XFL7 (32). The Listeria-TT856–1313 was characterized by DNA sequencing and by evaluating the secretion of TT856–1313 into the culture medium (Fig. 1B). The TT856–1313 fragment contains both mouse and human immunodominant T cell epitopes (50, 70).
Infection of tumor cells in vitro
The in vitro infectivity of Panc-02 tumor cells was assessed as described previously (34). Briefly, 106 cells/ml were infected with 107 CFU of Listeria or Listeria-TT for 1 hour at 37°C in culture medium and then incubated with gentamicin (50 μg/ml) for 1 hour to kill extracellular Listeria. Last, cells were washed with phosphate-buffered saline (PBS) and lysed in water, and serial dilutions were plated onto LB agar to quantify the infection rate the next day.
Evaluation of cell death
As described previously (34), tumor cell killing by Listeria or Listeria-TT was determined in vitro as follows. Panc-02 cells (3 × 103) plated in 96-well plates were infected with 107 CFU per well of Listeria or Listeria-TT for 2 hours at 37°C, and then, gentamicin (50 μg/ml) was added. Live and dead cells were counted the next day using Trypan blue staining. A similar experiment was performed with human MiaPaCa2 pancreatic tumor cells described for Panc-02 cells above.
Biodistribution of Listeria-TT
C57BL/6 mice were injected with 106 Panc-02 mouse pancreatic tumor cells as described above and, 14 days later, injected with a single large dose 107 CFU of Listeria-TT. Mice were euthanized at various time points after the injection (as indicated in the figure), and metastases, tumors, and normal tissues were dissected, weighed, and analyzed for CFU of Listeria-TT, as described previously (25). In a separate study, Panc-02 tumors were generated, then treated with 12 high doses of Listeria (107 CFU) and, 2 days after the last treatment, analyzed for CFU of Listeria in tumor and normal tissues.
Nude mice were orthotopically injected with 106 Mia-Paca2 human pancreatic tumor cells in the pancreas and, 6 weeks later, with a single large dose of 5 × 107 CFU of Listeria. Mice were euthanized at various time points (as indicated in the figure) after the injection, and metastases, tumors, and normal tissues were dissected, weighed, and analyzed for CFU of Listeria, as described previously (25).
Dose-limiting toxicity
C57BL/6 mice were injected intraperitoneally with various doses of Listeria-TT, and survival was evaluated over the next 20 days, as described previously (25). Briefly, varying doses of Listeria-TT (1 × 107 to 4 × 108, as indicated in fig. S8B) were injected 14 days after tumor cell injection (when tumors were palpable (0.5 to 1 cm), and survival was monitored over the next 20 days.
Tumor development
Panc-02 tumor cells were injected into the mammary fat pad (peritoneal cavity model) (105) or orthotopically into the pancreas (106) of C57BL/6 mice. When injected into the mammary fad pat, a relatively small primary tumor develops at the place of injection (peritoneal membrane), and tumor cells metastasize via the peritoneal cavity to other organs, predominantly to the pancreas and less abundantly to the liver, mesenchymal lymph nodes along the gastrointestinal tract, and the diaphragm (22); however, when injected into the pancreas tumor cells, a primary tumor develops in the pancreas, and tumor cells metastasize to the liver only (71). In transgenic KPC mice (KrasG12D/+;LSL-Trp53R172H/+), multiple primary tumors develop spontaneously in the pancreas, metastasizing to the liver and lungs (25, 30).
Orthotopic Panc-02 or KPC model
Orthotopic Panc-02 or KPC tumors were generated in C57BL/6 mice as described previously (72). Briefly, mice were anesthetized with ketamine/xylazine (respectively, 100 mg and 10 mg/kg, ip), the hair was removed at the location of the spleen, and the skin was sterilized with Betadine followed by 70% alcohol. The animal was covered with gauze sponge surrounding the incision site. A 1-cm incision was made in the abdominal skin and muscle just lateral to the midline and directly above the spleen/pancreas to allow visualization. The spleen/pancreas was gently retracted and positioned to allow injection of 106 Panc-02 or KPC tumor cells directly into the pancreas, from the tail all the way to the head of the pancreas. To prevent leakage of injected cell suspension, the injection site was tied off after tumor cell injections with dissolvable suture. The spleen and pancreas were then replaced within the abdominal cavity, and both muscle and skin layers were closed with sutures. After recovery from surgery, mice were monitored and weighed daily. A palpable tumor appeared within 2 weeks in the pancreas, and metastases in the liver began to develop within 2 to 4 weeks.
Protocol for treatment of Panc-02 and KPC mice with Listeria-TT + GEM
A detailed rationale for this treatment protocol and schematic view of all treatments are shown in fig. S4. Panc-02 mice (peritoneal model) (22, 25): To generate memory T cells to TT, C57BL/6 mice were immunized twice intramuscularly with the TTvacc (the same used for childhood vaccinations, 0.3 μg/50 μl) 1 week apart. Subsequently, Panc-02 tumor cells (105/100 μl) were injected into mammary fat pad. A single high dose of Listeria-TT (107 CFU) was injected intraperitoneally either 3 to 5 days after tumor cell injection when tumors were 1 to 3 mm (early pancreatic cancer) or 10 to 14 days after tumor cell injection when tumors were 5 to 10 mm (advanced pancreatic cancer).
Panc-02 mice (orthotopic model) (73): In addition, Panc-02 tumor cells (106/50 μl) were injected into pancreas, and 10 to 14 days later, when tumors were 5 to 10 mm, a single high dose of Listeria-TT (107 CFU) was injected intraperitoneally. Three days later, GEM (Gemizan, Zuventus) treatment was started (1.2 mg per mouse, every 3 days) and continued for 14 days (six doses in total). Concomitantly, low doses of Listeria-TT were administered daily for 2 weeks (14 doses in total). All mice were euthanized 2 days after the last treatment and visually analyzed for tumor weight and the number of metastases, as described previously (22). KPC mice (transgenic model), age 3.5 to 5 months, received the same treatment described above after verification of the presence of tumors and metastases by PET scan.
Survival
KPC mice were treated with TTvacc and Listeria-TT + GEM as outlined in fig. S4. At the end of treatments, mice were monitored without any further treatment until they succumbed spontaneously or were terminated upon appearance of severe premorbid symptoms requiring euthanasia as specified by our approved animal use protocol.
In vivo depletion of T cells
CD4 and CD8 T cells were depleted from C57BL/6 mice with orthotopic Panc-02 tumors during Listeria-TT + GEM treatment. Briefly, 10 to 14 days after tumor cell injection (when tumors were about 5 mm2), mice were treated with Listeria-TT + GEM and with 300 μg of anti-CD4 (clone GK1.5, catalog no. BE0003–1, BioXCell) or anti-CD8 (clone YTS169.4, catalog no. BE0117, BioXCell) antibodies (five injections every third day). All mice were euthanized 2 days after the last anti-CD4 or anti-CD8 treatment and analyzed for tumor weight and number of metastases. As control, isotype-matched rat antibodies against HRPN were used (clone LTF-2, catalog no. BE0090, BioXCell).
Intravital multiphoton imaging
Panc-02 tumor cells (106) expressing Dendra-2 were injected into the pancreas of transgenic mice [C57BL/6N-Tg(Cfms-gal4-vp16)-(UAS-eCFP)] (36) in which the macrophages were labeled with CFP. Three weeks later, when a pancreatic tumor developed and on the same day as imaging, Listeria-TT was labeled ex vivo with Alexa 680 by incubation with rabbit anti-Listeria polyclonal antiserum (dilution 1:200) and anti-rabbit IgG-Alexa 680 (dilution 1:250). Listeria-TT-Alexa-680 was injected intraperitoneally, and 4 hours later, when Listeria-TT had accumulated in the TME and infected tumor cells, mice were anesthetized using isoflurane. Pancreatic tumors were externalized through a small incision (~0.7 cm) through the skin and peritoneal wall and stabilized for imaging using previously published protocols (74). Multiphoton intravital microscopy was performed using a custom-built two-laser multiphoton microscope (75). Tumors were imaged with a femtosecond laser set to 880 nm for excitation of CFP or the green form of Dendra-2, and an optical parametric oscillator set to 1240 nm for excitation of Alexa 680. Z-stacks to a depth of 45 μm were acquired with a 1-μm slice interval. During the course of imaging, the animal was placed in a heated chamber maintained at physiological temperature. Mice were monitored for heart rate, breathing rate, pulse distension, breath distension, and oxygen saturation using a pulse oximeter (MouseSTAT, Kent Scientific).
PET scan
PET was performed as described previously (76). Briefly, the effect of the Listeria-TT + GEM treatment on the tumor and metastases was monitored by microPET (Siemens Imaging System) before and after treatment. For this purpose, mice were injected with the positron emitter [18F]-labeled deoxyglucose. The uptake of F-fluorodeoxyglucose 18F-FDG by tumors and metastases was quantified by microPET as described previously (22). Increased FDG uptake reflects increased metabolic activity of cancer cells, expressed as SUVmax.
Flow cytometry
Immune cells from spleens, blood, or metastases of mice were isolated as described previously (73). Anti-CD45 antibodies were used to identify the leukocyte population in tumors and metastases. Anti-CD3 and anti-CD8 antibodies were used to identify CD8 T cells, anti-CD3 and anti-CD4 to identify CD4 T cells, anti-CD11b and anti-Gr1 to identify MDSC, and anti-CD11b and anti-F4/80 to identify TAM. To detect intracellular cytokine production, the cells were incubated with GolgiPlug (1 μg/ml) for 6 hours and then treated with Cytofix/Cytoperm according to the manufacturer’s instructions, before staining with antibodies to intracellular cytokines, IFNγ, granzyme B, perforin, IL-6, IL-10, TNF-α, and other markers, such as the T cell response inhibitor MARCO, the T cell activation marker CD69, or the costimulator CD80. Appropriate isotype controls were included for each sample. A total of 50,000 to 100,000 cells were acquired by the LSR-II FACS system (Beckton and Dickinson) and analyzed using FlowJo 7.6 software. Cell debris and dead cells were excluded from the analysis based on scatter signals and use of the Fixable Blue or Green Live/Dead Cell Stain Kit.
ELISPOT
Spleen cells were isolated from treated and control Panc-02 and KPC mice for ELISPOT (catalog no. 551083, BD Biosciences) analysis, as described previously (13). To detect T cell responses to TT, 105 spleen cells were incubated with purified TT protein (5 μg/ml). The frequency of IFNγ-producing spleen cells was measured 72 hours later using an ELISPOT reader (CTL Immunospot S4 analyzer). To determine the frequency of IFNγ-producing CD4 and CD8 T cells, spleen cells were depleted for CD4 and CD8 T cells using magnetic bead depletion techniques according to the manufacturer’s instructions. A similar protocol was used for T cells isolated from Panc-02 orthotopic tumors.
In addition, T cell responses to TAA Survivin were analyzed by ELISPOT as described previously (15). Briefly, spleen cells were isolated from Listeria-TT + GEM–treated and control KPC mice for ELISPOT. To detect T cell responses to Survivin, 2 × 105 spleen cells were incubated with Survivin protein (50 μg/ml) (MGAPALPQIWQLYLKNYRIATF KNWPFLEDCACTPERMAEAGFIHCPTENEPDLAQCFFCFKELEGWEPDDNPIEEHRKHSPGCAFLTVKKQMEELTVSEFLKLDRQRAKNKIAKETNNKQKEFEETAKTTRQSIEQLAA) (catalog no. LS-G22824/147580, LSBio) for 72 hours. After the 72-hour incubation, the frequency of IFNγ-producing spleen cells was measured using an ELISPOT reader as described above. To determine the frequency of IFNγ-producing CD4 and CD8 T cells, spleen cells were depleted for CD4 and CD8 T cells using magnetic bead depletion techniques as described above.
In vitro killing assay of tumor cells
CD4 T cells of TT-vaccinated mice were analyzed for their capacity of killing TT-expressing Panc-02 tumor cells in vitro. C57BL/6 mice were immunized with the TTvacc twice 1 week apart as described in fig. S4. One week after the last immunization, CD4 T cells were isolated from the spleen using magnetic bead technology and added to the tumor cells in Effector:Target (E:T) ratios of 10:1, 5:1, and 0:1. Tumor cells were seeded at 4000 cells/100 μl per well in RPMI 1640, 10% FBS, and penicillin-streptomycin on day 1, and CD4 T cells were added to the tumor cells at day 2. Plates were centrifuged for 5 min at 400g and kept in the CO2 incubator at 37°C for 3 hours. Trypan blue was added to discriminate alive and dead tumor cells. The number of live tumor cells in wells containing CD4 T cells was determined by light microscopy and compared to the number of live tumor cells without CD4 T cells. The results using Panc-02 tumor cells expressing TT (Panc-02-TT) were compared to those obtained with Panc-02 tumor cells lacking TT (Panc-02). Ten wells were analyzed in each group, and the results were averaged. The percentage of dead tumor cells was determined using the formula described below
Statistical significance was determined using the Mann-Whitney test.
Western blotting
Expression of TT protein in tumor cells was analyzed by Western blotting as described previously (44). Briefly, cells were lysed in radioimmunoprecipitation assay buffer containing protease inhibitors, and proteins were separated on 4 to 12% gradient SDS-polyacrylamide gels and then electro-transferred to a polyvinylidene difluoride membrane. Membranes were incubated with rabbit anti-myc IgG, followed by horseradish peroxidase (HRP)–conjugated goat anti-rabbit IgG. Detection was with a chemiluminescence detection kit. Antibody to β-actin was used to ensure equal loading.
IHC and IF
Tumors were dissected from pancreas and immediately fixed with buffered formalin, and the tissue was embedded in paraffin. Sections (5 μm) were sliced and placed on slides and then deparaffinized at 60°C for 1 hour, followed by xylene, an ethanol gradient (100 to 70%), water, and PBS. Slides were then incubated for 30 min in 3% hydrogen peroxide followed by boiling in citrate buffer for 20 min. Once the slides were cooled, washed, and blocked with 5% goat serum, the sections were incubated with primary antibodies, such as anti-CD4 (1:100 dilution), anti-CD8α (1:400 dilution), anti-perforin (1:300 dilution), anti-granzyme (1:200 dilution), anti-CD31 (1:100 dilution), anti-TT (1:50 dilution), or anti-MHC class II (1:100 dilution; catalog no. 14-5321-82, Invitrogen), followed by incubation with secondary antibody (mouse anti-goat IgG-HRP) and SignalStain Boost IHC Detection Reagent (catalog no. 8114S, Cell Signaling Technology). Subsequently, the slides were incubated with 3,3′-diaminobenzidine (catalog no. SK-4100, Vector Laboratories), counterstained with hematoxylin, dehydrated through an ethanol gradient (70 to 100%) and xylene, and mounted with Permount. The slides were scanned with a three-dimensional Histech P250 High Capacity Slide Scanner to acquire images and quantification data. Secondary antibodies without primary antibodies were used as negative control.
KPC tumors were digested using Gentlemacs and Collagenase and Dispase as described previously (22), and tumor cells were isolated by magnetic beads (CD45-negative fraction). The CD45-negative fraction was stained with anti–MHC class II–fluorescein isothiocyanate (FITC) antibodies (dilution 1:100; catalog no. 11-5321-82, Invitrogen) and analyzed by a ZOE Fluorescent Cell Imager (Bio-Rad).
In addition, Panc-02 tumor cells stably transfected with pcDNA3.1-TT were cultured in neomycin-containing media (300 μg/ml) and examined for TT expression. Cultured cells were fixed with 4% paraformaldehyde for 10 min and then incubated with primary antibodies to TT (goat anti-TT) (dilution1:50) for 30 min, followed by secondary antibodies (anti-goat IgG-Cy3) (dilution 1: 200) for 30 min. Nontransfected Panc-02 were used as negative control. The TT-expressing Panc-02 tumor cells were also analyzed for MHC class II expression (rabbit anti–MHC class II–FITC) (dilution 1:100; catalog no. 11-5321-82, Invitrogen).
RNA sequencing
Sample preparation for RNA-seq
Total RNA was isolated from the metastases and tumor tissues from two mice per group, treated with deoxyribonuclease, and evaluated using the Agilent Bioanalyzer. The purified high-quality total RNA was submitted to the Epigenetics Shared Facility (ESF) for assay and analysis. An RNA-seq assay using 25 ng of RNA was performed and validated by the ESF.
Whole-transcriptome library preparation and high-throughput RNA-seq
ESF personnel performed ribosomal RNA (rRNA) depletion using a Ribo-Zero rRNA Removal Kit and reverse transcription using random hexamers and oligo(dT) to synthesize double-stranded cDNA. Libraries were prepared with adapters and barcodes for multiplex sequencing and sequenced using the Illumina HiSeq 2500. Raw FASTQ files were trimmed for adapter sequences using quart. FASTQ file sequence quality was evaluated using FastQC (77), and read coverage was determined using the RSeQC package (78).
RNA-seq data analysis
We generated a total of 150 RNA-seq runs. For each run, we obtained at least 20 million reads, with >70% of them aligned to the mouse genome. We used the Bowtie/Tophat/Cufflinks/Cuffdiff software suite (79–81), applying the Tuxedo protocol. The RNA-seq analysis was performed on tumors from transgenic KPC mice treated with Listeria-TT + GEM or saline (control group) and on orthotopic KPC tumors of mice treated with Listeria-TT + GEM, Listeria-TT, GEM, or saline, as outlined in fig. S4.
RNAscope
RNA in situ hybridization (RNAscope, ACD Bio-Techne) was performed on formalin-fixed paraffin-embedded 5-μm sections using the RNAScope Multiplex Fluorescent V2 Assay according to manufacturer’s instructions. Probes used were directed against mouse CD4 (catalog no. 406841), mouse CD8 (catalog no. 401681-C2), and anti-mouse IFNγ (catalog no. 311391-C3). The Fluorophore Opal 520 (C1; catalog no. FP1494001KT, Akoya Biosciences), Opal 570 (C2; catalog no. FP1488001KT, Akoya Biosciences), and Opal 690 (C3; catalog no. FP1497001KT, Akoya Biosciences) were used for detection, 4′,6-diamidino-2-phenylindole was used as counterstain, and ProLong Gold Antifade Mountant (catalog no. P36930, Thermo Fisher Scientific) was used as a mounting media. The slides were then imaged at a Leica SP5 confocal microscope. Images were acquired using an oil objective of 40× (numerical aperture = 1.25) with a 4× zoom.
Statistics
To statistically compare the effects of Listeria-TT + GEM on the growth of metastases and tumors or on immune responses in the mouse models, the Mann-Whitney test was applied using Prism. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Values of P < 0.05 were considered statistically significant. The type of statistical test was determined by Prism. To statistically compare the effects of Listeria-TT + GEM on immunological responses and efficacy, a nonparametric Mann-Whitney (two-tailed) test was used. To statistically compare the effects of Listeria-TT + GEM in a survival study, a Kaplan-Meier curve was generated using Mantel-Cox. ****P < 0.0001.
Supplementary Material
Acknowledgments:
We thank H. Zhang, Department of Pathology, Albert Einstein College of Medicine for providing outstanding training and support regarding the IHC and image analysis.
Funding:
This work was supported by the Pancreatic Cancer Action Network (PCAN) 422247 (C.G.); a private donation of J. and M. Spatz (C.G.); and another private donation of S. Bigelsen (C.G.), Loki Therapeutics (C.G.), NCI Administrative Supplement 3P30CA013330-44S3 (J. Condeelis and C.G.), and NCI Cancer Center support P30CA013330 (Flow Cytometry Core, Pathology Core, MicroPET Core, Genetics Core, and Imaging Core) (funding through the Albert Einstein Cancer Center).
Competing interests:
C.G. is the inventor of the Listeria-Recall Antigen Technology, which was developed in her laboratory, and is described in a patent application 11213577 (granted in the United States and Japan). The patent is licensed to Loki Therapeutics. C.G. is a stockholder of Loki Therapeutics and is an employee of Albert Einstein College of Medicine. J.C. and J.C.M. are members of the scientific advisory board of Loki Therapeutics. All other authors declare that they have no competing interests.
Data and materials availability:
All data associated with this study are present in the paper or Supplementary Materials.
REFERENCES AND NOTES
- 1.Kulke MH, Blaszkowsky LS, Ryan DP, Clark JW, Meyerhardt JA, Zhu AX, Enzinger PC, Kwak EL, Muzikansky A, Lawrence C, Fuchs CS, Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J. Clin. Oncol 25, 4787–4792 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Maitra A, Hruban RH, Pancreatic cancer. Annu. Rev. Pathol 3, 157–188 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W; National Cancer Institute of Canada Clinical Trials Group, Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A Phase III trial of the National Cancer Institute of Canada clinical trials group. J. Clin. Oncol 25, 1960–1966 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber MN, A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J. Clin. Oncol 9, 491–498 (1991). [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M, Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J. Clin. Oncol 29, 4548–4554 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valsecchi ME, Diaz-Canton E, de la Vega M, Littman SJ, Recent treatment advances and novel therapies in pancreas cancer: A review. J. Gastrointest. Cancer 45, 190–201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thind K, Padrnos LJ, Ramanathan RK, Borad MJ, Immunotherapy in pancreatic cancer treatment: A new frontier. Therap. Adv. Gastroenterol 10, 168–194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher TN, Schreiber RD, Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, Wells DK, Cary CIO, Grbovic-Huezo O, Attiyeh M, Medina B, Zhang J, Loo J, Saglimbeni J, Abu-Akeel M, Zappasodi R, Riaz N, Smoragiewicz M, Kelley ZL, Basturk O; `Australian Pancreatic Cancer Genome Initiative, Gonen M, Levine AJ, Allen PJ, Fearon DT, Merad M, Gnjatic S, Iacobuzio-Donahue CA, Wolchok JD, DeMatteo RP, Chan TA, Greenbaum BD, Merghoub T, Leach SD, Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH, Gravekamp C, Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br. J. Cancer 108, 2281–2290 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostrand-Rosenberg S, Sinha P, Myeloid-derived suppressor cells: Linking inflammation and cancer. J. Immunol 182, 4499–4506 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Nagaraj S, Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol 9, 162–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SH, Castro F, Gonzalez D, Maciag PC, Paterson Y, Gravekamp C, Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. Br. J. Cancer 99, 741–749 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia H, Wang W, Crespo J, Kryczek I, Li W, Wei S, Bian Z, Maj T, He M, Liu RJ, He Y, Rattan R, Munkarah A, Guan JL, Zou W, Suppression of FIP200 and autophagy by tumor-derived lactate promotes naïve T cell apoptosis and affects tumor immunity. Sci. Immunol 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahangir A, Chandra D, Quispe-Tintaya W, Singh M, Selvanesan BC, Gravekamp C, Immunotherapy with Listeria reduces metastatic breast cancer in young and old mice through different mechanisms. Onco. Targets. Ther 6, e1342025 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz-Winnenthal H, Pietsch DH, Schimmack S, Bonertz A, Udonta F, Ge Y, Galindo L, Specht S, Volk C, Zgraggen K, Koch M, Buchler MW, Weitz J, Beckhove P, Chronic pancreatitis is associated with disease-specific regulatory T-cell responses. Gastroenterology 138, 1178–1188 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L, Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med 3, 10 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng JF, Willett CG, Fernandez-del Castillo C, Ryan DP, Clark JW, Zhu AX, Rattner DW, Winkelmann JL, Warshaw AL, Patients undergoing treatment for pancreatic adenocarcinoma can mount an effective immune response to vaccinations. Pancreatology 5, 67–74 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Dominguez C, Carrasco-Marin E, Leyva-Cobian F, Role of complement component C1q in phagocytosis of Listeria monocytogenes by murine macrophage-like cell lines. Infect. Immun 61, 3664–3672 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croize J, Arvieux J, Berche P, Colomb MG, Activation of the human complement alternative pathway by Listeria monocytogenes: Evidence for direct binding and proteolysis of the C3 component on bacteria. Infect. Immun 61, 5134–5139 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drevets DA, Sawyer RT, Potter TA, Campbell PA, Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect. Immun 63, 4268–4276 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quispe-Tintaya W, Chandra D, Jahangir A, Harris M, Casadevall A, Dadachova E, Gravekamp C, Nontoxic radioactive Listeria(at) is a highly effective therapy against metastatic pancreatic cancer. Proc. Natl. Acad. Sci. U.S.A 110, 8668–8673 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH, Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21, 822–835 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portnoy DA, Auerbuch V, Glomski IJ, The cell biology of Listeria monocytogenes infection: The intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol 158, 409–414 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandra D, Selvanesan BC, Yuan Z, Libutti SK, Koba W, Beck A, Zhu K, Casadevall A, Dadachova E, Gravekamp C, 32-Phosphorus selectively delivered by listeria to pancreatic cancer demonstrates a strong therapeutic effect. Oncotarget 8, 20729–20740 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou S, Gravekamp C, Bermudes D, Liu K, Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 18, 727–743 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh M, Quispe-Tintaya W, Chandra D, Jahangir A, Venkataswamy MM, Ng TW, Sharma-Kharkwal S, Carreno LJ, Porcelli SA, Gravekamp C, Direct incorporation of the NKT-cell activator α-galactosylceramide into a recombinant Listeria monocytogenes improves breast cancer vaccine efficacy. Br. J. Cancer 111, 1945–1954 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forbes NS, Coffin RS, Deng L, Evgin L, Fiering S, Giacalone M, Gravekamp C, Gulley JL, Gunn H, Hoffman RM, Kaur B, Liu K, Lyerly HK, Marciscano AE, Moradian E, Ruppel S, Saltzman DA, Tattersall PJ, Thorne S, Vile RG, Zhang HH, Zhou S, McFadden G, White paper on microbial anti-cancer therapy and prevention. J. Immunother. Cancer 6, 78 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zorde Khvalevsky E, Gabai R, Rachmut IH, Horwitz E, Brunschwig Z, Orbach A, Shemi A, Golan T, Domb AJ, Yavin E, Giladi H, Rivkin L, Simerzin A, Eliakim R, Khalaileh A, Hubert A, Lahav M, Kopelman Y, Goldin E, Dancour A, Hants Y, Arbel-Alon S, Abramovitch R, Shemi A, Galun E, Mutant KRAS is a druggable target for pancreatic cancer. Proc. Natl. Acad. Sci. U.S.A 110, 20723–20728 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA, Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Qiu W, Sahin F, Iacobuzio-Donahue CA, Garcia-Carracedo D, Wang WM, Kuo CY, Chen D, Arking DE, Lowy AM, Hruban RH, Remotti HE, Su GH, Disruption of p16 and activation of Kras in pancreas increase ductal adenocarcinoma formation and metastasis in vivo. Oncotarget 2, 862–873 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y, Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol 167, 6471–6479 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Pamer EG, Immune responses to Listeria monocytogenes. Nat. Rev. Immunol 4, 812–823 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Castro F, Paterson Y, Gravekamp C, High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 69, 5860–5866 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emmerling P, Finger H, Bockemuhl J, Listeria monocytogenes infection in nude mice. Infect. Immun 12, 437–439 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ovchinnikov DA, van Zuylen WJ, DeBats CE, Alexander KA, Kellie S, Hume DA, Expression of Gal4-dependent transgenes in cells of the mononuclear phagocyte system labeled with enhanced cyan fluorescent protein using Csf1r-Gal4VP16/UAS-ECFP double-transgenic mice. J. Leukoc. Biol 83, 430–433 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inamatsu T, Suzuki K, Hashimoto W, Sato K, Differential age-change in the numbers of CD4+CD45RA+DC4+CD29+ T cell subsets in human peripheral blood. Mech. Ageing Dev 63, 57–68 (1992). [DOI] [PubMed] [Google Scholar]
- 38.Gravekamp C, The impact of aging on cancer vaccination. Curr. Opin. Immunol 23, 555–560 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook N, Frese KK, Bapiro TE, Jacobetz MA, Gopinathan A, Miller JL, Rao SS, Demuth T, Howat WJ, Jodrell DI, Tuveson DA, Gamma secretase inhibition promotes hypoxic necrosis in mouse pancreatic ductal adenocarcinoma. J. Exp. Med 209, 437–444 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres MP, Rachagani S, Souchek JJ, Mallya K, Johansson SL, Batra SK, Novel pancreatic cancer cell lines derived from genetically engineered mouse models of spontaneous pancreatic adenocarcinoma: Applications in diagnosis and therapy. PLOS ONE 8, e80580 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu T, Tomogane M, Miyashita M, Ukimura O, Ashihara E, Low dose gemcitabine increases the cytotoxicity of human Vγ9Vδ2 T cells in bladder cancer cells in vitro and in an orthotopic xenograft model. Onco. Targets. Ther 7, e1424671 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groom JR, Luster AD, CXCR3 in T cell function. Exp. Cell Res 317, 620–631 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slaney CY, Kershaw MH, Darcy PK, Trafficking of T cells into tumors. Cancer Res. 74, 7168–7174 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, Zhou J, Hayakawa Y, Karaolis DK, Gravekamp C, STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol. Res 2, 901–910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N, Hruban C, Guthrie VB, Rodgers K, Naidoo J, Kang H, Sharfman W, Georgiades C, Verde F, Illei P, Li QK, Gabrielson E, Brock MV, Zahnow CA, Baylin SB, Scharpf RB, Brahmer JR, Karchin R, Pardoll DM, Velculescu VE, Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 7, 264–276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Efremova M, Finotello F, Rieder D, Trajanoski Z, Neoantigens generated by individual mutations and their role in cancer immunity and immunotherapy. Front. Immunol 8, 1679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schone A, Jungst D, Meyer G, Hernandez-Richter T, Fischer S, Effects of phospholipase A2, free fatty acids and 2-lysolecithin on the crystallization of cholesterol in gallbladder bile. Eur. J. Clin. Invest 30, 715–721 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Portnoy DA, Chakraborty T, Goebel W, Cossart P, Molecular determinants of Listeria monocytogenes pathogenesis. Infect. Immun 60, 1263–1267 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C, Zhang M, Ju J, Nietfeldt J, Wise J, Terry PM, Olson M, Kachman SD, Wiedmann M, Samadpour M, Benson AK, Genome diversification in phylogenetic lineages I and II of Listeria monocytogenes: Identification of segments unique to lineage II populations. J. Bacteriol 185, 5573–5584 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reece JC, Geysen HM, Rodda SJ, Mapping the major human T helper epitopes of tetanus toxin. The emerging picture. J Immunol 151, 6175–6184 (1993). [PubMed] [Google Scholar]
- 51.Stein JV, Nombela-Arrieta C, Chemokine control of lymphocyte trafficking: A general overview. Immunology 116, 1–12 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farber JM, Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukoc. Biol 61, 246–257 (1997). [PubMed] [Google Scholar]
- 53.Qian C, An H, Yu Y, Liu S, Cao X, TLR agonists induce regulatory dendritic cells to recruit Th1 cells via preferential IP-10 secretion and inhibit Th1 proliferation. Blood 109, 3308–3315 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW Jr., Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc. Natl. Acad. Sci. U.S.A 101, 13832–13837 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, Rucki AA, Gunderson AJ, Coussens LM, Brockstedt DG, Dubensky TW Jr., Hassan R, Armstrong TD, Jaffee EM, A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology 146, 1784–1794. e6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toussaint B, Chauchet X, Wang Y, Polack B, Le Gouëllec A, Live-attenuated bacteria as a cancer vaccine vector. Expert Rev. Vaccines 12, 1139–1154 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Kumar V, Patel S, Tcyganov E, Gabrilovich DI, The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 37, 208–220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, Balestrino D, Loh E, Gripenland J, Tiensuu T, Vaitkevicius K, Barthelemy M, Vergassola M, Nahori MA, Soubigou G, Regnault B, Coppee JY, Lecuit M, Johansson J, Cossart P, The Listeria transcriptional landscape from saprophytism to virulence. Nature 459, 950–956 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Fink DM, Steele MM, Hollingsworth MA, The lymphatic system and pancreatic cancer. Cancer Lett. 381, 217–236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goc J, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC, Characteristics of tertiary lymphoid structures in primary cancers. Onco. Targets. Ther 2, e26836 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McElhaney JE, Meneilly GS, Lechelt KE, Bleackley RC, Split-virus influenza vaccines: Do they provide adequate immunity in the elderly? J. Gerontol 49, M37–M43 (1994). [DOI] [PubMed] [Google Scholar]
- 62.Effros RB, Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine 25, 599–604 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Castro F, Leal B, Denny A, Bahar R, Lampkin S, Reddick R, Lu S, Gravekamp C, Vaccination with Mage-b DNA induces CD8 T-cell responses at young but not old age in mice with metastatic breast cancer. Br. J. Cancer 101, 1329–1337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. www.cancer.org/cancer/pancreatic-cancer/causes-risks-prevention/risk-factors.html.
- 65.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP Jr., Schabel FM Jr., Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 44, 717–726 (1984). [PubMed] [Google Scholar]
- 66.Moral JA, Leung J, Rojas LA, Ruan J, Zhao J, Sethna Z, Ramnarain A, Gasmi B, Gururajan M, Redmond D, Askan G, Bhanot U, Elyada E, Park Y, Tuveson DA, Gonen M, Leach SD, Wolchok JD, DeMatteo RP, Merghoub T, Balachandran VP, ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 579, 130–135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aslakson CJ, Miller FR, Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 52, 1399–1405 (1992). [PubMed] [Google Scholar]
- 68.Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P, Lowen D, Javni J, Miller FR, Slavin J, Anderson RL, A novel orthotopic model of breast cancer metastasis to bone. Clin. Exp. Metastasis 17, 163–170 (1999). [DOI] [PubMed] [Google Scholar]
- 69.Medaglini D, Ciabattini A, Spinosa MR, Maggi T, Marcotte H, Oggioni MR, Pozzi G, Immunization with recombinant Streptococcus gordonii expressing tetanus toxin fragment C confers protection from lethal challenge in mice. Vaccine 19, 1931–1939 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Rice J, Buchan S, Stevenson FK, Critical components of a DNA fusion vaccine able to induce protective cytotoxic T cells against a single epitope of a tumor antigen. J. Immunol 169, 3908–3913 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Partecke LI, Sendler M, Kaeding A, Weiss FU, Mayerle J, Dummer A, Nguyen TD, Albers N, Speerforck S, Lerch MM, Heidecke CD, von Bernstorff W, Stier A, A syngeneic orthotopic murine model of pancreatic adenocarcinoma in the C57/BL6 mouse using the Panc02 and 6606PDA cell lines. Eur. Surg. Res 47, 98–107 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Qiu W, Su GH, Development of orthotopic pancreatic tumor mouse models. Methods Mol. Biol 980, 215–223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Selvanesan BC, Meena K, Beck A, Meheus L, Lara O, Rooman I, Gravekamp C, Nicotinamide combined with gemcitabine is an immunomodulatory therapy that restrains pancreatic cancer in mice. J. Immunother. Cancer 8, e001250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Entenberg D, Pastoriza JM, Oktay MH, Voiculescu S, Wang Y, Sosa MS, Aguirre-Ghiso J, Condeelis J, Time-lapsed, large-volume, high-resolution intravital imaging for tissue-wide analysis of single cell dynamics. Methods 128, 65–77 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Entenberg D, Wyckoff J, Gligorijevic B, Roussos ET, Verkhusha VV, Pollard JW, Condeelis J, Setup and use of a two-laser multiphoton microscope for multichannel intravital fluorescence imaging. Nat. Protoc 6, 1500–1520 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H, Over-expression of facilitative glucose transporter genes in human cancer. Biochem. Biophys. Res. Commun 170, 223–230 (1990). [DOI] [PubMed] [Google Scholar]
- 77.Smallwood SA, Lee HJ, Angermueller C, Krueger F, Saadeh H, Peat J, Andrews SR, Stegle O, Reik W, Kelsey G, Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat. Methods 11, 817–820 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang L, Wang S, Li W, RSeQC: Quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L, Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol 28, 511–515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lien WH, Guo X, Polak L, Lawton LN, Young RA, Zheng D, Fuchs E, Genome-wide maps of histone modifications unwind in vivo chromatin states of the hair follicle lineage. Cell Stem Cell 9, 219–232 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L, Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc 7, 562–578 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or Supplementary Materials.


